Abstract
In acute liver injury induced by the injection of CCl4, cell death has been attributed to the necrosis of hepatocytes in the centrilobular area. In the present study, we re-examined the hepatic injury evoked by CCl4 in rats and explored the possibility that apoptosis may also contribute to its pathogenesis. Apoptotic hepatocytes were identified and quantified by light and electron microscopy, the in situ immunohistochemical labeling of nuclear DNA fragmentation, flow cytometry, and DNA gel electrophoresis. We found that a substantial number of hepatocytes underwent apoptosis. Apoptotic changes were also observed in ballooned hepatocytes. Apoptotic hepatocytes increased in number at 3 hours and peaked at 6 hours after the CCl4 injection. Apoptotic bodies were sequestrated in the adjacent hepatocytes and sinusoidal cells. Double staining of the cells with immunostaining for phagocytes and terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling staining for labeling of DNA fragmentation showed that the majority of apoptotic hepatocytes were phagocytosed by Kupffer cells and macrophages. The results indicated that apoptosis occurs in the ballooned and injured hepatocytes of the centrilobular area. What occurs after CCl4 administration may be important in reducing inflammation, shortening the course of acute hepatic injury, and preventing the development of fibrosis.
Cell death is thought to take place by at least two distinct processes, apoptosis and necrosis. 1-4 The hepatotoxic heliotrine 1,1-dichloroethylene, dimethylnitrosamine, and thioacetamide cycloheximide can induce both necrosis and apoptosis of liver cells, 5-9 implying that both mechanisms of cell death are involved in hepatotoxic injury. The well-defined model of necrosis induced by the injection of carbon tetrachloride (CCl4) is widely used in studies of the mechanisms of hepatic injury. 10-12 CCl4 is known to cause hepatic centrilobular necrosis followed by hepatic fibrosis. It is not known whether both mechanisms of cell death occur in response to CCl4. This agent has recently been used to induce necrosis in a control experiment for studies on apoptosis. 7,13-15
The ballooning of hepatocytes is one of the earliest, most frequent, and most conspicuous changes seen in liver injured by CCl4 administration. However, it is also observed in such conditions as viral infection, alcoholic hepatitis, biliary obstruction, starvation, choline deficiency, hypoxia, scurvy, yellow fever, and radiation injury. 16 Although the relationship between the ballooning changes and necrosis and the fate of the ballooned hepatocytes have long been debated, the conventional explanation of cell ballooning is that it is a forerunner of necrosis. 17,18 Thus, studies to determine the fate of the ballooned cell are viewed as important for understanding the underlying mechanisms of the aforementioned diseases.
Our objective was to re-examine the liver injury produced by the injection of CCl4 in rats and to determine whether apoptosis may coexist with necrosis as an additional underlying mechanism for toxicity. We used the analysis of DNA fragmentation by gel electrophoresis and flow cytometry to qualify and quantify apoptosis in the liver, and the terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) assay to identify apoptotic cells among injured hepatocytes at the level of the single cell in situ. 19,20 We further characterized the appearance time of the ballooned hepatocytes and proposed a novel mechanism in which the ballooned cells found their way by apoptosis in this model. Our evidence for the existence of apoptosis in this model actually challenges the conventional view of necrosis induced by CCl4.
Materials and Methods
Rats
Male Wistar rats 7 to 9 weeks old were obtained from Nippon Biological Supplier (Tokyo, Japan) and were bred in the animal facilities at Tokyo Medical and Dental University. Each experimental group was composed of five rats. Animals were sacrificed by anesthesia with ether. The liver of each animal was examined at 3, 6, 12, 24, 48, 72, and 96 hours after a single intraperitoneal injection of 0.3 ml/kg of CCl4 (50% vol/vol in liquid paraffin). The control groups consisted of either untreated animals (0 hours) or those that received an injection of liquid paraffin.
Light and Electron Microscopy
The liver of each control and treated animal was perfused through the portal vein with 1.5% glutaraldehyde in 0.062 mol/L cacodylate buffer (pH 7.4) containing 1% sucrose. Blocks from the fixed tissue were immersed in 0.1 mol/L phosphate-buffered (pH 7.4) 1% osmium tetroxide for 2 hours and then dehydrated and embedded in Poly/Bed 812 (Polysciences Inc., Warrington, PA). Semithin sections (0.5 μm) were stained with toluidine blue for evaluation by light microscopy. Thin sections were doubly stained with uranyl acetate and lead citrate for examination by an electron microscope (JEOL-100 CX) operated at 100 kV.
Preparation of Samples for Flow Cytometry
Hepatocyte suspensions were prepared using the method described by Dolbeare et al 21 with slight modification. Briefly, the liver was removed without perfusion. The tissue blocks were dispersed through stainless mesh in RPMI 1640 culture medium (Life Technologies, Inc., Grand Island, NY). The cell suspensions were pelleted at 500 × g for 5 minutes, fixed in 70% ethanol for 2 hours, digested in 0.05% collagenase II (Life Technologies, Inc.) at room temperature for 10 minutes, processed with 0.5 mg/ml RNase at 37°C for 20 minutes, resuspended in 50 ng/ml propidium iodide solution, and incubated for 2 hours. The stained cells were analyzed by flow cytometry using FACS Calibur (Becton Dickinson, Mountain View, CA). Data are expressed as percentage of apoptotic cells. To prevent digestion of apoptotic cells by Kupffer cells, all steps were performed at 4°C unless otherwise indicated.
DNA Gel Electrophoresis
Isolated cells were counted, pelleted, resuspended in 10 mmol/L ethylenediaminetetraacetic acid and 50 mmol/L Tris-HCl (pH 8.0) containing 0.5% sodium lauryl sarkosinate and 0.5 mg/ml proteinase K, and incubated for 60 minutes at 50°C. Then, 10 mmol/L ethylenediaminetetraacetic acid containing 0.25% bromphenol blue and 40% sucrose was mixed with each DNA extract. The individual extracts were loaded into the wells of a 2% agarose gel containing 3 μg/ml of ethidium bromide. Electrophoresis was performed in 40 mmol/L Tris-HCl containing 40 mmol/L acetic acid and 1 mmol/L ethylenediaminetetraacetic acid.
DNA TUNEL
The livers were perfused through their portal veins with saline for 20 seconds and phosphate-buffered 4% paraformaldehyde for 2 minutes. Slices from the livers were immersed in the same fixative for 6 hours at 4°C and then embedded in paraffin. Sections 4 μm thick were collected on glass slides coated with poly-l-lysine. The nuclear DNA fragmentation of apoptotic cells was labeled in situ using the TUNEL method 19,20 as follows. The sections were first deparaffinized and treated for 15 minutes with 20 μg/ml proteinase K (Boehringer Mannheim, Mannheim, Germany) in 0.1 mol/L Tris-HCl buffer (pH 7.4). They were then treated with 2% H2O2 for 5 minutes and then incubated with 0.3 U/μl terminal deoxynucleotidyl transferase buffer (Life Technologies, Inc.) and 0.04 nmol/μl biotinylated dUTP (Boehringer Mannheim) in terminal deoxynucleotidyl transferase buffer (Life Technologies, Inc.) at 37°C for 60 minutes. The sections were incubated for 10 minutes with 2% bovine serum albumin followed by 30 minutes in peroxidase-conjugated streptavidin (DAKO, Carpinteria, CA) diluted 1:300 with phosphate-buffered saline. Peroxidase activity in the sections was visualized by adding 0.025% 3,3-diaminobenzidine tetrahydrochloride in 0.05 mol/L Tris-HCl buffer (pH 7.4) solution containing 0.01% H2O2 for 5 minutes. To examine the relationship between apoptotic hepatocytes and macrophages, some sections already stained by the TUNEL method were further processed for double staining (TUNEL and ED1 or ED2 or OX-6 monoclonal antibody staining) by the peroxidase anti-peroxidase complex method in a manner similar to that described above. In brief, those sections treated with the primary antibody (Ab) were successively incubated with goat immunoglobulin to mouse immunoglobulin G (DAKO) (diluted 1:50) and with mouse peroxidase anti-peroxidase complex (1:100 dilution) and then exposed to the 3,3-diaminobenzidine tetrahydrochloride solution for visualization.
Immunohistochemistry
Selected sections were immunostained using the streptavidin-biotin-peroxidase complex method with ED1, ED2 (Serotec, Kidlington, Oxford, UK), and OX-6 (Cedarlane, Hornby, Ontario, Canada) monoclonal Abs to identify Kupffer cells/macrophages and major histocompatibility complex (MHC) class II antigen (Ag)+ cells. These sections were first pretreated with 0.3% H2O2 in methanol and then incubated with normal goat serum (1:5 dilution). They were incubated overnight with ED1, ED2, or OX-6 Abs (diluted 1:1000), rinsed in phosphate-buffered saline, incubated for 30 minutes with biotinylated goat anti-mouse immunoglobulin (DAKO) (1:600 dilution), then rinsed again in phosphate-buffered saline, and finally incubated for 30 minutes with peroxidase-conjugated streptavidin (1:300 dilution). All steps were performed at room temperature. Tissue peroxidase activity was visualized by a 5-minute exposure to 3,3-diaminobenzidine tetrahydrochloride solution. Some sections were counterstained with hematoxylin.
Identification of Apoptotic Hepatocytes and Apoptotic Ballooned Hepatocytes
Apoptosis was identified by both light and electron microscopy if the cells exhibited any of the following morphological features: the formation of sharply delineated, uniformly finely granular masses or crescents; nuclear fragments; condensed cytoplasm; apoptotic bodies; and phagocytosis of apoptotic cells by adjacent cells. 1-5 DNA fragmentation of apoptotic cells was identified by both positive staining from the TUNEL method and clearly visible nuclear fragments or sharp and condensed chromatic masses or crescents in the nuclei.
Cell Counting
We used the method described by Bouwens et al 22 to quantify the cell number. For each rat, a total tissue area of approximately 8 mm2 (130 fields, 0.0625 mm2 per field) was counted from a random sample of six sections. The paraffin sections stained by the TUNEL method and the semithin (0.5-μm) sections stained with toluidine blue were evaluated by light microscopy at ×250. The number of apoptotic hepatocytes that exhibited both TUNEL-positive staining and the clear, sharply condensed chromatic masses or crescents in nucleus or nuclear fragments were quantified. Only those ballooned cells with nuclei were counted in the semithin sections. The number of ballooned cells per mm2 was quantified. Those cells with condensed and crescent-shaped chromatic caps and/or nuclear fragmentation and apoptotic bodies were classified as apoptotic ballooned cells. The percentage of apoptotic ballooned hepatocytes in nucleated ballooned cells was analyzed by counting 400 nucleated cells in each rat.
Statistical Analysis
Data are expressed as mean ± SD. The significance of the difference between groups was evaluated by the two-tailed Student’s t-test. Differences between treated groups and control groups were analyzed using the t-test for paired data. A level of P < 0.05 was considered statistically significant.
Results
Morphology
Ballooning of the hepatocytes with appearance of pale, foamlike cytoplasm was first observed at the midzonal region of the lobule 3 hours after CCl4 administration (Figure 1A) ▶ . The ballooned hepatocytes were characterized by dilation of the endoplasmic reticulum (Figure 1B) ▶ . At this stage, apoptotic nuclei characterized by chromatin condensation were found sporadically both in ballooned (Figure 1 ▶ , A and B) and nonballooned hepatocytes (Figure 1C) ▶ . Focal apoptosis was evident in the lobule at 6 hours after CCl4 injection. Apoptotic ballooned hepatocytes with nuclear fragments (Figure 1D) ▶ and apoptotic bodies (Figure 1E) ▶ were frequently observed. By 6 to 12 hours after CCl4 administration, individual necrotic cells were observed in the intermediate area of the lobule (Figure 2A ▶ , hepatocytes; Figure 2B ▶ , ballooned hepatocytes). Necrotic cells showed less electron density in both the cytoplasm and the nucleus. The ballooned hepatocytes formed a circle that surrounded the centrilobular zone of the lobule (Figure 2C) ▶ . Some nuclei of the ballooned cells were blebbing at this time (Figure 2D) ▶ ; the blebs appeared to be shed into the dilated perinuclear space. The heterochromatin also became condensed and margined. Fragments of the condensed nuclei were often separated from the cells and phagocytosed by adjacent cells (Figure 2E) ▶ .
Figure 1.
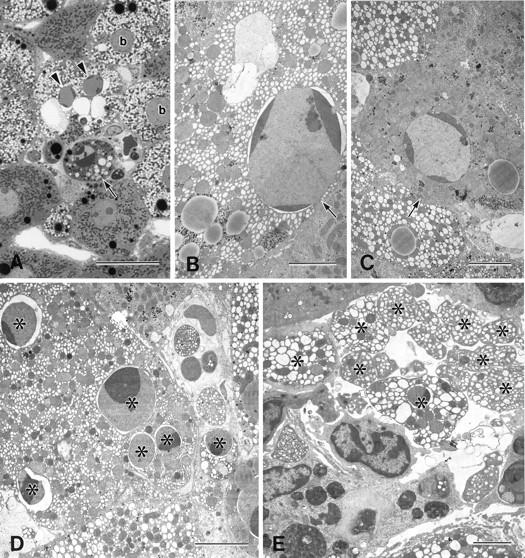
Light and electron micrographs of rat liver 3 to 6 hours after the injection of CCl4. A: Ballooned hepatocytes (b) showed a pale, foamlike cytoplasm. Apoptotic hepatocytes with (B) or without (C) ballooned changes were observed in the lobule. Apoptotic foci in the midzonal area were composed of apoptotic hepatocytes with (arrowheads) and without (arrow) ballooned changes (A). Nuclear fragments (D*) and apoptotic bodies (E*), from the apoptotic ballooned cells were often observed. A, toluidine blue staining. Bars: A, 20 μm; B to E, 5 μm.
Figure 2.
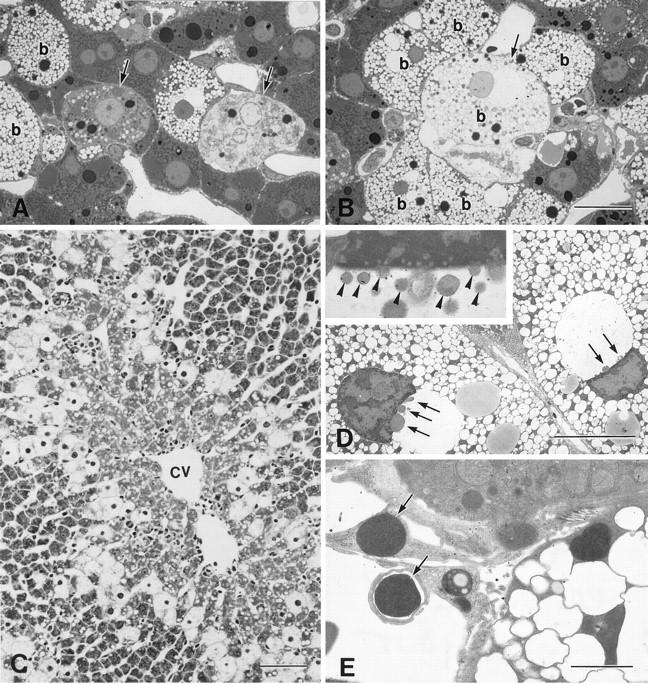
Light and electron micrographs of the liver 6 to 12 hours after the injection of CCl4. A: Necrotic cells (arrows) were found near the ballooned cells 6 hours after the injection of CCl4. B: A few ballooned hepatocytes (b, arrow) were undergoing necrosis. C: Ballooned cells encircled the centrilobular area 12 hours after the injection (cv, central vein). D: Nuclear blebbing (arrows) was observed in many of the ballooned cells, and the nuclei of the ballooned cells appeared crescent shaped. Marginated heterochromatin was apparent. D, inset: Higher magnification of the ballooned cell with nuclear blebbing, the blebs (arrowheads) protruded and detached from nucleus. E: The nuclei or nuclear fragments with highly condensed chromatin (arrows) found outside the cell were phagocytosed by the nearby cells. A and B, toluidine blue staining; C, hematoxylin and eosin staining. Bars: A and B, 20 μm; C, 80 μm; D, 10 μm; E, 2 μm.
By 24 hours, some hepatocytes in the centrilobular zone of the lobule showed apoptosis (Figure 3 ▶ , A and B). The dilation of the cisternae in the ballooned hepatocytes progressively increased until the cisternae occupied most of the cytoplasm (Figure 3C) ▶ .
Figure 3.
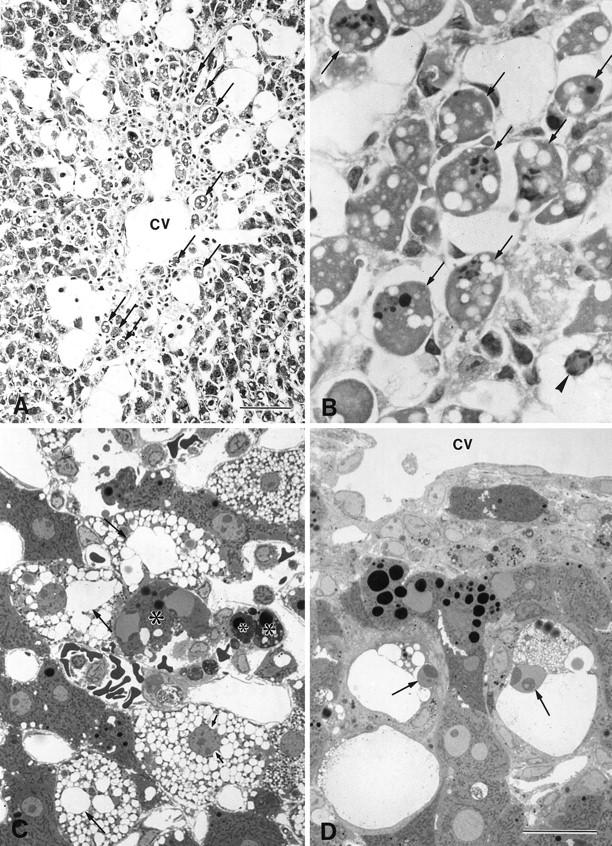
Light micrographs of the liver 24 to 72 hours after the injection of CCl4. A: Many centrilobular hepatocytes (arrows) were seen to undergo apoptosis (cv, central vein). B: Same image as in A, with a higher magnification. A ballooned hepatocyte (arrowhead) exhibited typical condensed chromatic masses in its nucleus. C: Apoptotic cells and apoptotic bodies (*) also formed. The dilation of the cisternae increased progressively, and the nucleus was indented by the dilated endoplasmic reticulum (arrows). D: Ballooned hepatocytes that appeared near the central vein (cv) showed shrinkage and heterochromatin condensation (arrows). A and B, hematoxylin and eosin staining; C and D, toluidine blue staining. Bars: A, 40 μm; B to D, 20 μm.
By 48 hours, most of the hepatocytes in the centrilobular area had disappeared as a result of both induced apoptosis and necrosis. By 72 hours, ballooned hepatocytes lay near the central veins of the lobule and showed apoptotic changes. Shrinkage of the apoptotic cells was significant: they were half the original size of the ballooned cells (Figure 3D) ▶ .
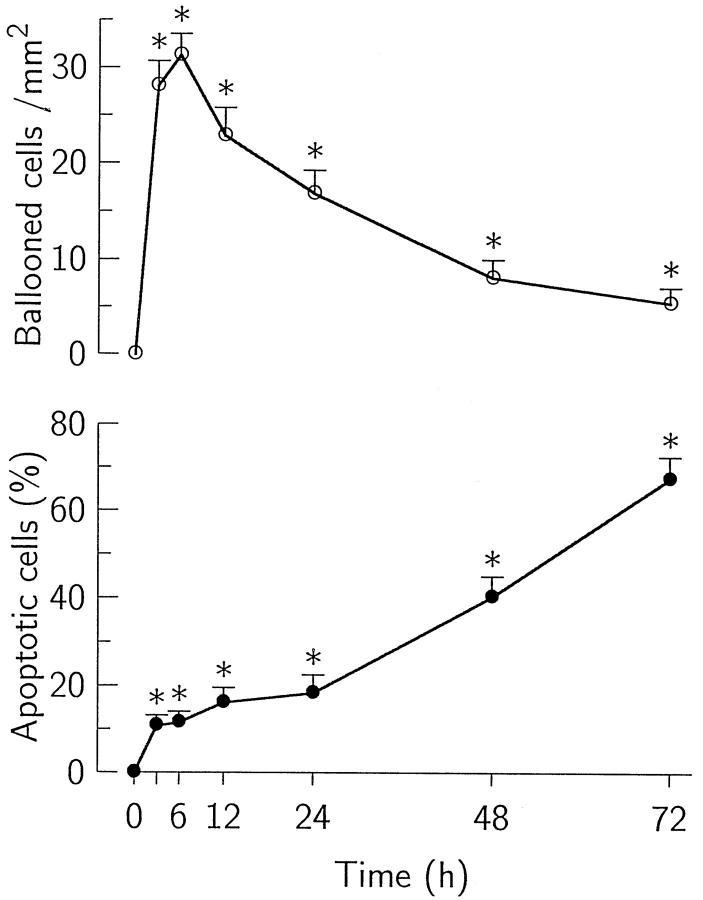
The ballooned hepatocytes were first identified at 3 hours after CCl4 injection; they increased rapidly in number, reaching a maximum at 6 hours, and decreased thereafter. The percentage of apoptotic ballooned cells continued to increase for up to 3 days after the administration of CCl4 (Figure 4) ▶ . Apoptotic bodies were also frequently incorporated in the neighboring cells, such as ballooned hepatocytes (Figure 5A) ▶ , hepatocytes (Figure 5B) ▶ , Kupffer cells (Figure 5C) ▶ , stellate cells (Figure 5D) ▶ , and endothelial cells.
Figure 4.
Number of ballooned hepatocytes and percentage of apoptotic ballooned hepatocytes at various time points after the injection of CCl4. The number of ballooned hepatocytes (○) reached a maximum at 6 hours and then decreased. The percentage of apoptotic ballooned hepatocytes (•) increased for up to 3 days after the injection. Values are means ± SD. *P < 0.05 compared with untreated controls.
Figure 5.
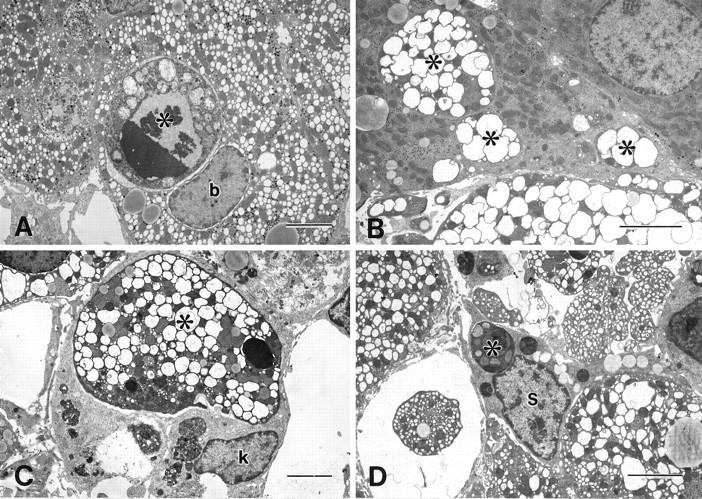
Electron micrographs of the liver 6 hours after the injection of CCl4. Apoptotic bodies (*) of hepatocytes were phagocytosed by ballooned hepatocytes (A, b), hepatocytes (B), Kupffer cells (C, k), and stellate cells (D, s). Bar, 5 μm.
Flow Cytometry and DNA Gel Electrophoresis
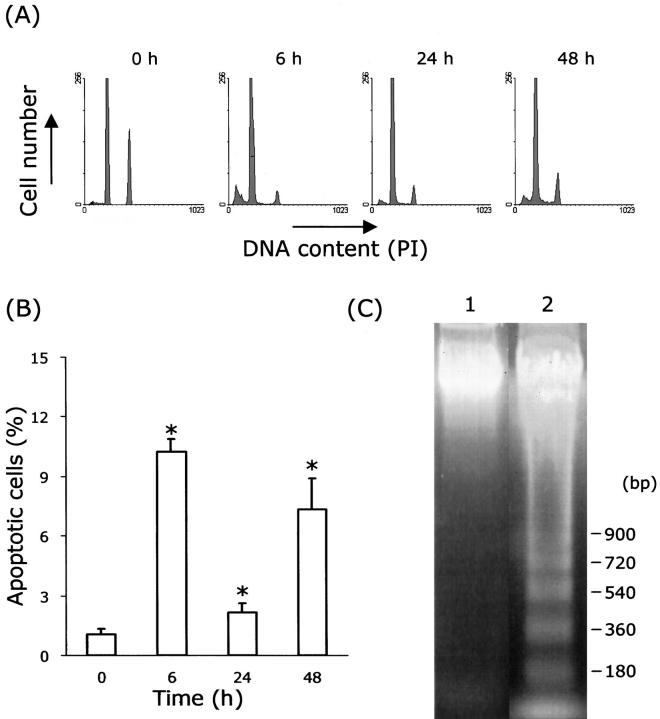
To quantify the apoptotic cells, low-molecular weight DNA was extracted from the ethanol-fixed apoptotic cells, and this had fractionated DNA content. The fluorescence of cells stained with propidium iodide was measured with a FACS Calibur flow cytometer. The number of apoptotic cells increased after the administration of CCl4; most of these cells occurred in sub-G0/G1 phase (Figure 6A) ▶ , and their percentage peaked at 6 hours after the injection of CCl4. Treated groups showed statistical significance compared with control (Figure 6B ▶ ; P < 0.05). No ladder was observed in the control cells; however, a ladder was observed at 6 hours after injection of CCl4 (Figure 6C) ▶ .
Figure 6.
Flow cytometry and DNA gel electrophoresis of hepatocytes from CCl4-treated rat liver. A: Histograms of DNA content at various time points after the CCl4 treatment. Each peak (6 h, 24 h, 48 h) shows an accumulation of sub-G0/G1, G0/G1, and G2/M, respectively. B: Percentage of apoptotic cells is obtained from analysis of flow cytometry. Values are means ± SD. *P < 0.05 compared with 0 hours. C: DNA fragmentation image. Lane 1, 0 hours; Lane 2, 6 hours after injection of CCl4. PI, propidium iodide.
TUNEL and Immunohistochemistry
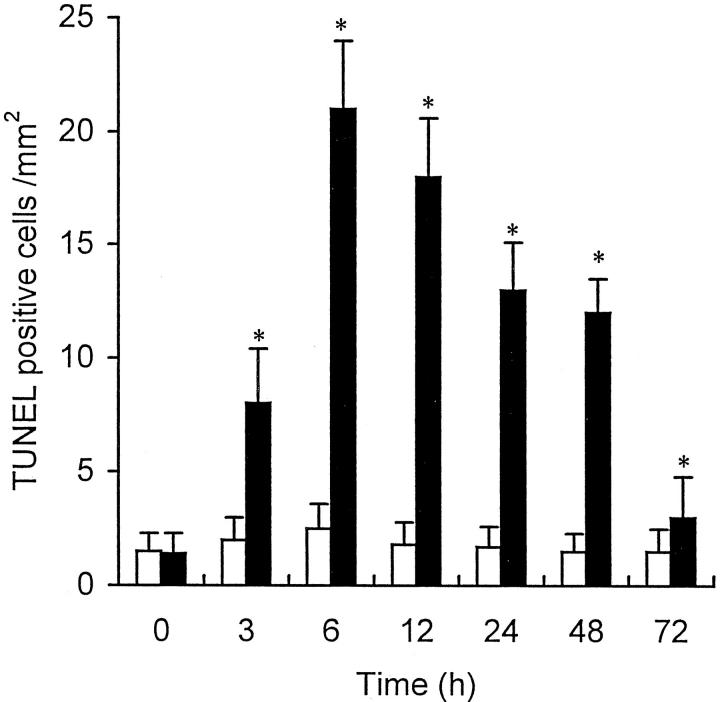
As expected, few TUNEL-positive cells were observed in the liver of the control rats. However, two types of dying cells were identified in the experimental animals: TUNEL-positive cells with (type I; Figure 7 ▶ , A to C) and those without (type II; Figure 7 ▶ , D and E) the classic morphological features of apoptosis (ie, sharply delineated masses or crescents of condensed chromatin). Some necrotic cells that were TUNEL negative exhibited a weakly and nonspecifically stained nucleus and cytoplasm (Figure 7F) ▶ . TUNEL-positive apoptotic cells (type I cells) were first found scattered in the lobules 3 hours after the administration of CCl4. By 6 to 12 hours after the administration, the TUNEL-positive cells were distributed in foci in the intermediate area of the lobule, and by 24 to 48 hours, apoptotic foci were restricted to the centrilobular area. The distribution of these cells coincided with that of the phagocytes, which were also detected as Kupffer cells and macrophages by immunoreaction with ED1 and ED2 Abs (Figure 7 ▶ , G and H). Double staining showed that the majority of the apoptotic cells were in contact with, surrounded by, or situated within, the ED1+ or ED2+ Kupffer cells and/or macrophages that were expressing MHC class II Ag labeled by OX-6 Ab (Figure 7 ▶ , I and J). The number of TUNEL-positive apoptotic cells increased and peaked at 6 hours and then decreased rapidly from 48 hours after CCl4 administration (Figure 8) ▶ . Numerous Kupffer cells/macrophages incorporated the highly fragmented and dying cells. This increase of apoptotic cells differed significantly from the findings in the placebo-treated animals (P < 0.05).
Figure 7.
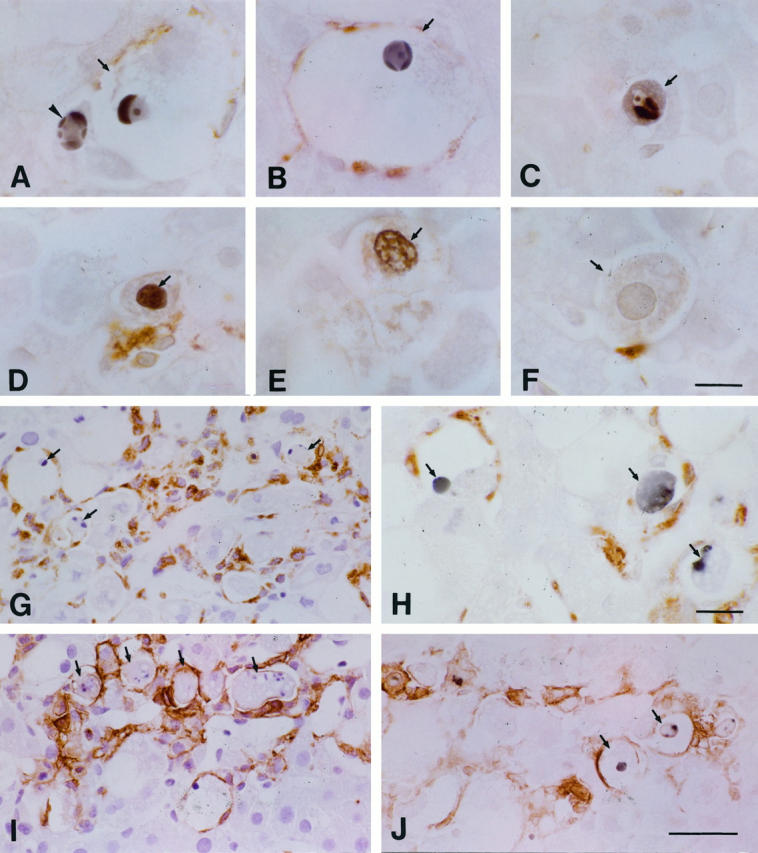
Immunostaining of the liver after the injection of CCl4. A and B: Hepatocytes with (arrows) and without (arrowhead) ballooned changes were identified both by TUNEL-positive staining and by their condensed chromatic masses or crescents. C: An apoptotic body (arrow) was seen in a hepatocyte. D and E: Hepatocytes (arrows) with TUNEL-positive staining but without highly condensed chromatic masses or crescents were present. F: A hepatocyte (arrow) appeared without TUNEL-positive staining (or its cytoplasm was only weakly and nonspecifically stained). G: Many apoptotic hepatocytes (arrows) were phagocytosed or were surrounded by ED1+ cells (brown). H: TUNEL+ cells (arrows) were surrounded by ED2+ cells (brown). I: Similarly to G, many of the apoptotic cells had incorporated with MHC class II Ag+ cells. J: TUNEL-positive cells (arrows) were surrounded by MHC class II Ag+ cells (brown). Bars: A to F, 10 μm; H, 15 μm; G, I, and J, 40 μm.
Figure 8.
Number of TUNEL-positive cells per mm2 in situ obtained at various time points after the injection of CCl4 or liquid paraffin alone. The number of TUNEL-positive cells increased rapidly and peaked at 6 hours in the CCl4-treated animals. ▪, administered CCl4; □, administered liquid paraffin. Values are means ± SD. *P < 0.05 compared with CCl4 (0 hours) or liquid paraffin treatment.
Discussion
It is well documented that necrosis in the centrilobular zone is believed to be a major cause of CCl4-induced acute liver injury. However, both necrosis and apoptosis can be caused by drugs and toxins. 23 We investigated the possibility that apoptosis has a role in the development of the CCl4-induced liver injury. The observations of the present study provide evidence for apoptosis of hepatocytes in the necrotic zone and, for the first time, for apoptosis of ballooned cells in acute liver injury. Confirmation of this process was based on the morphological data by light and electron microscopy, including cell shrinkage; chromatin condensation; formation of apoptotic bodies; phagocytosis by neighboring cells; and the presence of DNA fragmentation by flow cytometry, DNA gel electrophoresis, and TUNEL. These features differ significantly from other forms of cell death in which swelling of cytoplasmic organelles and bursting of the cytoplasmic and nuclear materials are typically seen. 1-4 Overall, these results indicate that apoptosis may be an important component of liver lesion progression.
Physiologically, TUNEL positivity represents a criterion by which to identify apoptosis. Pathologically, a TUNEL-positive reaction can appear in both apoptosis and necrosis. 20 Rink et al 24 demonstrated two types of TUNEL-positive cells in brain injury by light and electron microscopy: one type displayed the morphological features of necrotic cell death, and the other type displayed the morphological features of classic apoptotic cell death. The former were interpreted as necrotic, and the latter apoptotic. A recent report mentioned that centrilobular hepatocytes were labeled by TUNEL staining after a single dose of CCl4. 14 It remains to be determined whether the cells labeled by TUNEL staining are necrotic, whether apoptosis also occurs after CCl4, and whether it might contribute to liver injury regression. Data from the present study suggest that apoptosis, in addition to necrotic cell death, occurred after CCl4 administration. To make quantitative data of apoptosis more precise, it is necessary to differentiate between apoptosis and necrosis by combining TUNEL positivity and the nuclear morphology of dying cells. 20 In response to the TUNEL staining, the TUNEL-positive cells were of two types. Type I cells, with TUNEL positivity of the nucleus and the accepted morphology, such as condensed and margined, crescent-shaped chromatic caps, were apoptotic. Type II cells, with features consistent with necrotic cell death, were necrotic. The manifestation of type I TUNEL-positive cells in the necrotic zone indicated that apoptosis represents one of the mechanisms of cell death after CCl4-induced liver injury.
The fate of the ballooned hepatocytes was not clearly established by previous studies. 16,17 Ballooned cells formed within 6 hours after a single dose of CCl4 and decreased in number thereafter. Ballooned hepatocytes may undergo more profound hydrolytic changes, such as necrosis (Figure 2B) ▶ . In our model, however, there were relatively few necrotic ballooned cells, whereas numerous ballooned cells were undergoing apoptosis. Some of the ballooned hepatocytes exhibited nucleoplasmic blebs that were shed into either the dilated cisternae of endoplasmic reticulum or the perinuclear space at the same time that the nuclei shrank and formed crescents. Although nucleoplasmic blebbing may partially explain the nuclear pyknosis, the mechanism underlying the blebbing phenomenon remains unknown. In response to the elimination of the hepatocytes by apoptosis and necrosis in the centrilobular zone, the cuff of ballooned cells (Figure 2C) ▶ moved nearer the centrilobular veins. Based on a count of 400 ballooned cells, we found that, in contrast to the decrease in number of ballooned cells per mm2, the percentage of apoptotic ballooned cells increased up to 72 hours. The apoptosis of ballooned cells is mainly responsible for their decrease and disappearance (Figure 3D) ▶ . Thus, it is tempting to consider that the ultimate fate of the ballooned cell in this model was apoptosis.
Surprisingly, numerous hepatocytes in the centrilobular zone, the so-called necrotic zone, were seen to undergo apoptosis in the 24 to 48-hour period after the administration of CCl4 (Figure 3 ▶ , A and B). The quantification of apoptotic cells does suggest that the high counts truly reflect a high cell loss, and that the injured cells that constituted up to half the lobule had disappeared in response to both apoptosis and necrosis. Furthermore, proliferating peripheral cells had replaced this band of ballooned and centrilobular cells 72 hours after CCl4 administration. The lobule was completely recovered by 4 days after the CCl4 injection.
The issue of the percentage of cells undergoing apoptosis and ballooning in vivo in CCl4-induced injury is more difficult to accurately calculate, because the loss of plasma membrane integrity, the hallmark of cell necrosis, made it impossible to estimate total hepatocytes (including necrotic cells). Instead, the numbers of TUNEL-positive cells and ballooned cells per mm2 are of significant value in this study. Regarding quantification of apoptotic ballooned cells among the total ballooned cells, it is obvious that the percentage of apoptotic ballooned cells is suitable, because there are few necrotic ballooned cells, and these can be neglected.
Apoptosis appears to be more advantageous than necrosis for removing injured hepatocytes. The latter leads to liberation of the proteins and DNA and to the formation of oxygen radicals, cytokines, and other inflammatory mediators, all of which may trigger potential secondary lethal responses within the organism. Extensive necrosis can damage the tissue structure and result in fibrosis. During the hepatic injury, apoptotic cells were found sporadically in the lobule at 3 hours after the administration of CCl4, and focal apoptosis was evident in the intermediate area at 6 to 12 hours and in the centrilobular area at 24 to 72 hours. Thus, the occurrence and distribution of apoptotic cells is shifted from that of acute injury to a resolution phase. Given that the duration of apoptosis in an individual cell is less than 3 hours, 25 our results indicate that cell loss by apoptosis occurs fairly rapidly and that this can result in the shortening of the time course of the injury. It thus seems logical that a lack of apoptosis can contribute to a more persistent or chronic inflammatory response that, in turn, leads to fibrosis. This is especially true, given that a single injection of CCl4 does not induce fibrosis, whereas injections given twice a week for several weeks do so. 26
The mechanisms for recognizing and eliminating apoptotic cells need to be emphasized. Kupffer cells/macrophages, together with neighboring hepatocytes, stellate cells, and endothelial cells, take part in clearing out apoptotic hepatocytes, findings that agree with our previous results. 27 The majority of phagocytes stained positively as both MHC class II (Ia) Ag+ and ED1+ or ED2+ Kupffer cells and macrophages. Because numerous apoptotic cells were surrounded by Kupffer cells and macrophages (Figure 7 ▶ , G and I), it is suggested that migration of the cells into the hepatic lobule is important for recognizing and phagocytosing the apoptotic bodies.
Regarding the induction of necrosis or apoptosis by CCl4, one possibility is that there might be a dose dependency; CCl4 is capable of causing cell necrosis and will often induce apoptosis if the injury is milder and less severe. The dose of CCl4 that we used (0.15 ml/kg) is lower than that used by others. 28 A second possibility is that apoptotic cells were digested by neighboring cells after tissue removal, in which case there would be an underestimation of the level of apoptosis; this cannot be ruled out, although the chance was minimized in this study, because the tissues were perfusion fixed. A third is that, unlike other organs, liver contains professional phagocytes: Kupffer cells, which normally represent 70 to 80% of the tissue macrophages and may accelerate elimination of apoptotic cells.
The mechanisms of drug-induced liver cell injury have recently been reviewed, 23,29 and several toxins described can damage DNA by oxidation or alkylation, and ultimately lead to apoptotic cell death. The events that trigger apoptosis in CCl4-induced acute hepatic injury are as yet unknown. CCl4-induced toxicity in isolated hepatocytes is mediated by a direct solvent injury to cell membranes. 30 However, nonparenchymal cells such as Kupffer cells are activated by the release of cytokines, which may contribute to pathophysiologic processes culminating in hepatocyte apoptosis after toxic injury to the liver. 28,31 We have found that the concentration of apoptotic cells is highest in regions enriched in macrophages, and it is possible that within these regions macrophages are initiating and/or propagating these apoptotic events (Figure 7 ▶ , H and J). Pretreatment with the Kupffer cell inhibitor gadolinium chloride prevented cell death due to CCl4. 32 The induction of apoptosis by macrophage is of interest, particularly considering recent reports that apoptotic occurrence is consistent with macrophage cytocidal activity, 33-36 and that the aging neutrophils 37 and those cells from a regressing tissue 33-36 are engulfed before the death of the tissue and subsequently acquire the morphology typical of apoptosis. Confirmed mediators of macrophage cytotoxicity currently include tumor necrosis factor α, nitric oxide, and reactive oxygen intermediates. 36,38-42
Acknowledgments
We thank Yoshihiro Kokubo and Akira Masuda for technical assistance.
Footnotes
Address reprint requests to Dr. Jialan Shi, Division 1, Department of Anatomy, School of Medicine, Tokyo Medical and Dental University, 1-5-45 Yushima, Bunkyo-ku, Tokyo 113, Japan. E-mail: j.shi.ana1@med.tmd.ac.jp.
Supported by Grant C08457002 from the Japanese Ministry of Education, Science, Sports and Culture.
References
- 1.Wyllie AH, Kerr JFR, Currie AR: Cell death: the significance of apoptosis. Int Rev Cytol 1980, 6:251-306 [DOI] [PubMed] [Google Scholar]
- 2.Majno G, Joris I: Apoptosis, oncosis, and necrosis. Am J Pathol 1995, 146:3-15 [PMC free article] [PubMed] [Google Scholar]
- 3.Hockenbery D: Defining apoptosis. Am J Pathol 1995, 146:16-19 [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz SM, Bennett MR: Death by any other name. Am J Pathol 1995, 147:229-234 [PMC free article] [PubMed] [Google Scholar]
- 5.Kerr JFK: An electron-microscope study of liver cell necrosis due to heliotrine. J Pathol 1969, 97:557-562 [DOI] [PubMed] [Google Scholar]
- 6.Reynolds ES, Kanz MF, Chieco P, Moslen MT: 1,1-Dichloroethylene: an apoptotic hepatotoxin? Environ Health Perspect 1984, 57:313-320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pritchard DJ, Butler WH: Apoptosis: the mechanism of cell death in dimethylnitrosamine-induced hepatotoxicity. J Pathol 1989, 158:253-260 [DOI] [PubMed] [Google Scholar]
- 8.Ledda-Columbano GM, Coni P, Curto M, Giacomini L, Faa G, Oliverio S, Piacentini M, Columbano A: Induction of two different modes of cell death, apoptosis and necrosis, in rat liver after a single dose of thioacetamide. Am J Pathol 1991, 139:1099-1109 [PMC free article] [PubMed] [Google Scholar]
- 9.Ledda-Columbano GM, Coni P, Faa G, Manenti G, Columbano A: Rapid induction of apoptosis in rat liver by cycloheximide. Am J Pathol 1992, 140:545-549 [PMC free article] [PubMed] [Google Scholar]
- 10.Camps J, Bargallo T, Gimenez A, Alie S, Caballeria J, Pares A, Joven J, Masana L, Rodes J: Relationship between hepatic lipid peroxidation and fibrogenesis in carbon tetrachloride-treated rats: effect of zinc administration. Clin Sci 1992, 83:695-700 [DOI] [PubMed] [Google Scholar]
- 11.Parola M, Leonarduzzi G, Biasi F, Emanuele A, Biocca ME, Poli G, Dianzani MU: Vitamin E dietary supplementation protects against carbon tetrachloride-induced chronic liver damage and cirrhosis. Hepatology 1992, 16:1014-1021 [DOI] [PubMed] [Google Scholar]
- 12.Liu SL, Esposti SD, Yao T, Diehl AM, Zern MA: Vitamin E therapy of acute CCl4-induced hepatic injury in mice is associated with inhibition of nuclear factor kappa B binding. Hepatology 1995, 22:1474-1481 [PubMed] [Google Scholar]
- 13.Fukuda K, Kojiro M, Chiu JF: Demonstration of extensive chromatin cleavage in transplanted morris hepatoma 7777 tissue: apoptosis or necrosis? Am J Pathol 1993, 142:935-946 [PMC free article] [PubMed] [Google Scholar]
- 14.Grasl-Kraupp B, Ruttkay-Nedecky B, Koudelka K, Bursch W, Schulte-Hermann R: In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis and autolytic cell death: a cautionary note. Hepatology 1995, 21:1465-1468 [DOI] [PubMed] [Google Scholar]
- 15.Columbano A, Endoh T, Denda A, Noguchi O, Nakae D, Hasegawa K, Ledd∼a-Columbano GM, Zedda AI, Konishi Y: Effects of cell proliferation and cell death (apoptosis and necrosis) on the early stages of rat hepatocarcinogenesis. Carcinogenesis 1996, 17:395-400 [DOI] [PubMed] [Google Scholar]
- 16.Phillips MJ, Poucell S, Patterson J, Valencia P: Viral hepatitis. Phillips MJ Poucell S Patterson J Valencia P eds. The Liver: An Atlas and Text of Ultrastructural Pathology. 1987, :pp 37-100 Raven Press, New York [Google Scholar]
- 17.Alison MR, Sarraf CE: Liver cell death: patterns and mechanisms. Gut 1994, 35:577-581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheuer PJ: Viral hepatitis. ed 3 MacSween RNM Anthony PP Scheuer PJ Burt AD Portmann BC eds. Pathology of the Liver, 1994, :pp 243-267 Churchill Livingstone, Edinburgh [Google Scholar]
- 19.Gavriele Y, Sherman Y, Ben-Sasson SA: Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 1992, 119:493-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ansari B, Coates PJ, Greenstein BD, Hall PA: In situ end-labeling detects DNA strand breaks in apoptosis and other physiological and pathological states. J Pathol 1993, 170:1-8 [DOI] [PubMed] [Google Scholar]
- 21.Dolbeare F, Gratzner H, Pallavicini MG, Gray JW: Flow cytometric measurement of total DNA content and incorporated bromodeoxyuridine. Proc Natl Acad Sci USA 1983, 80:5573-5577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouwens L, Baekeland M, De Zanger R, Wisse E: Quantitation, tissue distribution and proliferation kinetics of Kupffer cells in normal rat liver. Hepatology 1986, 6:718-722 [DOI] [PubMed] [Google Scholar]
- 23.Corcoran GB, Fix L, Jones DP, Moslen MT, Nicotera P, Oberhammer FA, Buttyan R: Contemporary issues in toxicology. Apoptosis: molecular control point in toxicity. Toxicol Appl Pharmacol 1994, 128:169-181 [DOI] [PubMed] [Google Scholar]
- 24.Rink A, Fung KM, Trojanowski JQ, Lee VMY, Neugebauer E, McIntosh TK: Evidence of apoptotic cell death after experimental traumatic brain injury in the rat. Am J Pathol 1995, 147:1575-1583 [PMC free article] [PubMed] [Google Scholar]
- 25.Bursch W, Paffe S, Putz B, Barthel G, Schult-Hermann R: Determination of the length of the histological stages of apoptosis in normal liver and in altered hepatic foci of rats. Carcinogenesis 1990, 11:847-853 [DOI] [PubMed] [Google Scholar]
- 26.Bhunchet E, Eishi Y, Wake K: Contribution of immune response to the hepatic fibrosis induced by porcine serum. Hepatology 1996, 23:811-817 [DOI] [PubMed] [Google Scholar]
- 27.Shi J, Fujieda H, Kaneko M, Wake K: Phagocytosis of apoptotic hepatocytes and neutrophils by sinusoidal cells in the rat liver. Wisse E Knook DL Wake K eds. Cells of the Hepatic Sinusoid. 1995, vol 5.:pp 25-28 Kupffer Cell Foundation, Leiden, The Netherlands, [Google Scholar]
- 28.Czaja MJ, Xu J, Alt E: Prevention of carbon tetrachloride-induced rat liver injury by soluble tumor necrosis factor receptor. Gastroenterology 1995, 108:1849-1854 [DOI] [PubMed] [Google Scholar]
- 29.Corcoran GB, Ray SD: Contemporary issues in toxicology. The role of the nucleus and other compartments in toxic cell death produced by alkylating hepatotoxicants. Toxicol Appl Pharmacol 1992, 113:167-183 [DOI] [PubMed] [Google Scholar]
- 30.Berger ML, Bhatt H, Combes B, Estabrook RW: CCl4-induced toxicity in isolated hepatocytes: the importance of direct solvent injury. Hepatology 1986, 6:36-45 [DOI] [PubMed] [Google Scholar]
- 31.Leist M, Gantner F, Naumann H, Bluethmann H, Vogt K, Brigelius-Flohe R, Nicotera P, Volk H-D, Wendel A: Tumor necrosis factor-induced apoptosis during the poisoning of mice with hepatotoxins. Gastroenterology 1997, 112:923-934 [DOI] [PubMed] [Google Scholar]
- 32.Edwards MJ, Keller BJ, Kauffman FC, Thurman RG: The involvement of Kupffer cells in carbon tetrachloride toxicity. Toxicol Appl Pharmacol 1993, 119:275-279 [DOI] [PubMed] [Google Scholar]
- 33.Lang RA, Lustig M, Francois F, Sellinger M, Plesken H: Apoptosis during macrophage-dependent ocular tissue remodeling. Development 1994, 120:3395-3403 [DOI] [PubMed] [Google Scholar]
- 34.Lang RA, Bishop JM: Macrophages are required for cell death and tissue remodeling in the developing mouse eye. Cell 1993, 74:453-462 [DOI] [PubMed] [Google Scholar]
- 35.Cui S, Reichner JS, Mateo RB, Albina JE: Activated murine macrophages induce apoptosis in tumor cells through nitric oxide-dependent or -independent mechanisms. Cancer Res 1994, 54:2462-2467 [PubMed] [Google Scholar]
- 36.Aliprantis AO, Diez-Roux G, Mulder LCF, Zychlinsky A, Lang RA: Do macrophages kill through apoptosis? Immunol Today 1996, 17:573-576 [DOI] [PubMed] [Google Scholar]
- 37.Shi J, Fujieda H, Kokubo Y, Wake K: Apoptosis of neutrophils and their elimination by Kupffer cells in rat liver. Hepatology 1996, 24:1256-1263 [DOI] [PubMed] [Google Scholar]
- 38.Bour ES, Ward LK, Cornman GA, Isom HC: Tumor necrosis factor-α-induced apoptosis in hepatocytes in long-term culture. Am J Pathol 1996, 148:485-495 [PMC free article] [PubMed] [Google Scholar]
- 39.Leist M, Gantner F, Bohlinger I, Germann PG, Tiegs G, Wendel A: Murine hepatocyte apoptosis induced in vitro and in vivo by TNF-α requires transcriptional arrest. J Immunol 1994, 153:1778-1787 [PubMed] [Google Scholar]
- 40.Buttke TM, Sandstrom PA: Oxidative stress as a mediator of apoptosis. Immunol Today 1994, 15:7-10 [DOI] [PubMed] [Google Scholar]
- 41.Jacobson MD: Reactive oxygen species and programmed cell death. Trends in Biochem Sci 1996, 21:83-86 [PubMed] [Google Scholar]
- 42.Leist M, Gantner F, Bohlinger I, Tiegsa G, Germann PG, Wendel A: Tumor necrosis factor-induced hepatocyte apoptosis precedes liver failure in experimental murine shock models. Am J Pathol 1995, 146:1220-1234 [PMC free article] [PubMed] [Google Scholar]