Abstract
Two N-terminal ends of human type XVIII collagen chains have recently been identified. The two chains have different signal peptides and variant N-terminal noncollagenous NC1 domains of 493 (NC1-493) and 303 (NC1-303) amino acid residues, respectively, but share 301 residues of their NC1 domains as well as the collagenous and C-terminal noncollagenous portions of the molecule. Antibodies were produced against the NC1 region common to both human α1(XVIII) chain variants and against NC1 sequences specific to the long variant and were used in combination with in situ hybridization to localize this collagen in a number of human tissues. They were also used for Western blotting, which resulted in detection of overlapping high-molecular weight bands above the 200-kd standard in a kidney extract. Heparin lyase II and heparin lyase III digestions of kidney and placenta extracts indicated that at least in these tissues, type XVIII collagen contains heparin sulfate glycosaminoglycan side chains. Type XVIII collagen was found to be a ubiquitous basement membrane component, occurring prominently at vascular and epithelial basement membranes throughout the body. Comparison of the expression of the NC1-493 and NC1-303 variants revealed marked differences. The short variant was found in most conventional basement membranes, including blood vessels and the various epithelial structures, and around muscular structures. The long variant was expressed very strongly in liver, where it was virtually the only variant in the liver sinusoids, and it occurred only in minor amounts elsewhere. Thus, the 192 N-terminal residues specific to the long variant apparently confer some functional property needed above all in the liver sinusoids, but also at certain other locations.
Type XVIII collagen is an extracellular matrix protein recently identified by cDNA cloning. 1-4 Elucidation of the complete mouse chain revealed it to be homologous with the previously identified type XV collagen, 5-7 and it has been suggested that type XV and XVIII collagens together form a subgroup of MULTIPLEXINs (multiple triple-helix domains with interruptions) within the collagen family. 2 The mouse type XVIII collagen differs strikingly from type XV by the presence of variant polypeptide forms characterized by three N-terminal noncollagenous domains of differing lengths. 8,9 Interestingly, the longest α1(XVIII) chain form has a motif of 10 cysteine residues homologous to the extracellular part of the frizzled receptors involved in the Wingless signaling pathway in Drosophila. 10
We have recently reported also the full-length human type XVIII collagen cDNAs that encode 1516- or 1336-amino acid residue α1(XVIII) chains. 11 The two chains have different signal peptides and variant N-terminal noncollagenous NC1 domains of 493 (NC1-493) and 303 (NC1-303) amino acid residues, respectively, but share the last 301 residues of their NC1 domains, a 688-residue collagenous sequence (COL1 to COL10) with nine interruptions (NC2 to NC10), and a 312-residue noncollagenous carboxyl-terminal domain (NC11). The amino acid sequences of the human and previously characterized mouse α1(XVIII) chains exhibit an overall identity of 79%, the highest homology being observed in their last 184 residues, corresponding to a 20-kd proteolytic fragment called endostatin that is released from the C terminus of the α1(XVIII) collagen chain and has been shown to inhibit endothelial cell proliferation, angiogenesis, and tumor growth. 12
Our recent Northern analysis of several human adult and fetal tissues shows that human type XVIII collagen is expressed in several tissues in a variant-specific manner. 11 The NC1-493 variant mRNAs were mainly seen in liver, whereas other tissues contained only minor or undetectable levels of the mRNAs. Northern hybridizations with a probe specific to the NC1-303 variant virtually lacked the adult liver signal, but they revealed clear hybridization to heart, kidney, placenta, prostate, ovary, skeletal muscle, and small intestine mRNAs and faint hybridization to mRNAs from several other tissues.
Because it is not known which cells are responsible for the synthesis of type XVIII mRNAs, we performed in situ hybridization experiments using human fetal and selected adult tissue samples. These and immunostaining experiments demonstrated that type XVIII collagen is located ubiquitously in basement membrane (BM) zones, its expression pattern being almost identical to that of the α1 and α2 chains of type IV collagen, with only a few exceptions. Marked differences were found in the location of the variant type XVIII chains. Furthermore, results were obtained with respect to glycosylation of this collagen.
Materials and Methods
Preparation of Probes for in Situ Hybridization
Probes corresponding to two regions of the human α1(XVIII) chain mRNAs were used. HuL8.2-E/P, specific to the long variant, was prepared by subcloning a 492-bp EcoRI/PstI insert of HuL8.2 11 to plasmid Bluescript SK (Stratagene, La Jolla, CA). The plasmid HuL8.2-E/P was linearized with EcoRI, and 35S-UTP-labeled antisense RNA was obtained with T3 RNA polymerase and the Riboprobe Combination System in vitro translation kit (Promega, Madison, WI). Labeled sense RNA was prepared correspondingly, except that HuL8.2-E/P was linearized with PstI and T7 RNA polymerase was used.
HL8-E/S, a probe detecting all variants, was prepared by subcloning a 272-bp EcoRI/SacI insert of HL8 11 to plasmid Bluescript SK (Stratagene). The plasmid HL8-E/S was linearized with EcoRI, and the 35S-UTP-labeled antisense RNA was obtained with T3 RNA polymerase. The sense RNA was obtained by linearizing the plasmid HL8-E/S with SacI and by using the T7 RNA polymerase. The probes in the sense orientation served as controls for nonspecific hybridization.
Tissue Sections and in Situ Hybridization Analysis
Several normal placentas, tissues from two 20-gestational week fetuses with Down’s syndrome, and a 19-gestational week fetus with pulmonary adenoid malformation were obtained from legal abortions. Normal skin, skeletal muscle, and liver samples from adult persons were also obtained. Some of the samples were fixed in 10% formalin and embedded in paraffin for routine histology. For in situ hybridization, part of each sample was frozen in liquid nitrogen for cryosections, and another part was fixed in 4% paraformaldehyde and embedded in paraffin.
The in situ hybridizations of paraffin sections were carried out according to Hogan et al 13 and Hoeffler et al, 14 with minor modifications. 15 The only difference compared with our previous procedure 15 was that washing in 2× standard saline citrate at 45°C was followed by washing in 0.2× standard saline citrate at 37°C (twice for 15 minutes each), after which the slides were dehydrated in 30, 50, 75, and 96% ethanol with 0.3 mol/L ammonium acetate and air dried. For autoradiography, the slides were dipped into Kodak NTB-3 nuclear track emulsion diluted 1:1 in 1% glycerol at 45°C and exposed for 8 to 12 days at 4°C. They were then developed in Kodak D-19 developer for 5 minutes at room temperature, fixed for 5 minutes, and counterstained with Gill’s hematoxylin no. 1 (Sigma Chemical Co., St. Louis, MO) and eosin (Orion, Espoo, Finland). The cryostat sections were treated by the same procedure as previously described. 15
Expression of Polypeptide Fragments in E. coli for Antigen Production
A 700-bp cDNA fragment, QH48, corresponding to the common region of the NC1 domain of human type XVIII collagen, was generated by polymerase chain reaction using HL4 11 as a template and primers H18-HIS-4 and H18-HIS-8 (see below). The fragment was subcloned after KpnI/HindIII digestion into the vector pQE-41 (Qiagen, Inc., Santa Clarita, CA), which can be used to express in-frame dihydrofolate reductase (DHFR) fusion proteins with an N-terminal His-tag (clone QH48) and transformed into the Escherichia coli strain M15. The QH48 fragment was expressed as suggested by Qiagen; the bacterial culture was pelleted and resuspended in 6 mol/L guanidine-HCl, 0.5 mol/L NaCl, 20 mmol/L Tris-HCl, pH 7.9; frozen in −70°C; and lysed at room temperature for 1 hour. The suspension was centrifuged for 20 minutes at 12,000 × g, the supernatant was lightly sonicated, and 5 mmol/L imidazole was added, after which it was applied to a 0.75 × 5 cm ProBond column (Invitrogen, Leek, the Netherlands) pre-equilibrated with 8 mol/L urea, 0.5 mol/L NaCl, and 20 mmol/L Tris-HCl, pH 7.9. The denatured polypeptides bound to the column were allowed to renature by means of a slow stepwise decrease in the concentration of urea for 2 hours in the buffer of 0.5 mol/L NaCl and 20 mmol/L Tris-HCl, pH 7.9. The polypeptides were eluted using a stepwise imidazole gradient from 0 to 1 mol/L in 0.5 mol/L NaCl and 20 mmol/L Tris-HCl, pH 7.9. The eluted fractions were monitored at A280 and verified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie staining. The fractions containing the human type XVIII collagen-derived polypeptide were dialyzed against 1× phosphate-buffered saline (PBS) and concentrated by ultrafiltration (molecular weight cut of 10 kd, Millipore, Bedford, MA).
A 430-bp fragment QH67, corresponding to the region specific to the long NC1 domain, was amplified as above, except that the template was a genomic subclone HE1.2 of the human COL18A1 gene (H. Elamaa et al, unpublished data) and the primers were H18-HIS-6 and H18-HIS-7 (see below). This polymerase chain reaction product was subcloned into the vector pQE-41, and the clone QH67 was expressed and purified as for QH48 above.
Antibodies to the fusion proteins QH48 and QH67 were raised by conventional methods. For immunization, 300 μl of purified fusion protein solution was injected into rabbits subcutaneously with complete Freund’s adjuvant (Sigma Chemical Co.) followed by booster injections with incomplete Freund’s adjuvant (Sigma Chemical Co.) at intervals of 14 days. The sera were tested by Western blotting using crude bacterial cell lysates from the expressed clones, QH48.18 and QH1415, encoding type XVIIII collagen sequences without the DHFR leader sequences (see below). Oligonucleotides used were the following (KpnI and HindIII restriction sites are underlined): H18-HIS-4, 5′-ATAAGCTTACGTGGAGACAGAATC-3′; H18-HIS-8, 5′-ATGGTACCCAGCCTCTTCTTCC-3′; H18-HIS-6, 5′-ATAAGCTTAGGGCCCCGTGAGTGG-3′; and H18-HIS-7,5′-ATGGTACCCCGGAATGGTTCCA-3′.
Affinity Purification and Characterization of Human Type XVIII Collagen-Specific Antibodies
Two additional bacterial expression constructs were made to aid affinity purification of the sera. The expression clone QH48.18 was the same as QH48, except that the insert was subcloned into the vector pQE-31 (Qiagen, Inc.), which can be used to express polypeptides with an N-terminal His-tag without the DHFR leader. A slightly longer construct than QH67, a 550-bp fragment QH1415.7, was produced using the primers H18-HIS-14 and H18-HIS-15 (H18-HIS-14, 5′-ATGGTACCCTGGTTCAATAATGAGG-3′; H18-HIS-15, 5′-ATAAGCTTATGAAGATGGTGGTGGC-3′; KpnI and HindIII restriction sites are underlined) and subcloned into the vector pQE-31.
The fragments were expressed and purified as above, except that an additional purification step was included for the QH1415.7 fragment. This was dialyzed in 20 mmol/L piperazine, pH 5.5, after the ProBond column and applied to a HiTrap Q anion-exchange column according to the manufacturer’s instructions (Pharmacia, Uppsala, Sweden), and the bound polypeptides were eluted with a stepwise gradient of NaCl from 0 to 1 mol/L in 20 mmol/L piperazine, pH 5.5. The positive fractions of QH48.18 and QH1415.7 after the ProBond and HiTrap Q column, respectively, were pooled and dialyzed in 0.5 mol/L NaCl, 0.2 mol/L NaHCO3, pH 8.6, after which they were coupled to CNBr-Sepharose (Pharmacia) according to the manufacturer’s protocol. The identities of the isolated polypeptides were confirmed by sequencing their N-terminal ends using automated Edman degradation with an Applied Biosystems model 477A protein sequencer (Department of Medical Biochemistry, University of Oulu, Finland).
For affinity purification of the type XVIII collagen-specific antibodies, the antisera were diluted 1:1 with PBS, pH 7.4, and applied to the respective columns, which were subsequently washed with 2 mol/L NaCl-PBS, pH 7.4. The bound antibody molecules were eluted with 150 mmol/L glycine-HCl, pH 2.5, and then with 100 mmol/L triethylamine, pH 11.0. The fractions containing protein were detected at A280, immediately neutralized with 2 mol/L Tris-HCl, pH 7.5, pooled, and concentrated (molecular weight cut of 100 kd, Millipore) with 1× PBS.
One-milliliter aliquots of the isopropyl-β-d-thiogalactopyranoside-induced bacterial cell cultures of DHFR, QH48, H48.18, QH67 and QH1415.7 were centrifuged, suspended in 200 μl of 20 mmol/L sodium phosphate, pH 7.2, and treated for analysis under reduced conditions by 12% SDS-PAGE followed by staining with Coomassie Brilliant Blue and Western blotting onto nitrocellulose filters. The affinity-purified antibodies were used at dilutions of 1 μg/ml for 2 hours at room temperature and detected with a horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (Bio-Rad, Richmond, CA) and enhanced chemiluminescence detection reagents (Amersham Corp., Buckinghamshire, UK) as recommended. The antibodies were also characterized by using a kidney tissue sample. Thirty micrograms of Human Kidney Protein Medley sample (Clontech, Palo Alto, CA) with 5% 2-mercaptoethanol was boiled for 5 minutes, applied to a reduced 7% SDS-PAGE, and treated for Western blotting using the type XVIII collagen antibodies. The immunosignals were detected using enhanced chemiluminescence as above.
Characterization of the Glycosaminoglycan Side Chains Attached to Type XVIII
Crude proteoglycan fractions were isolated from a normal human 10-gestational week placenta and a kidney obtained after surgical transplantation of a patient with congenital nephrosis, Finnish type, as described. 16 Shortly, 0.83 g of placenta and 0.33 g of kidney were homogenized in 10 ml of 4 mol/L guanidine-HCl, 4% 3-((3-cholamidopropyl)dimethylammonio)-1-propanesulfonate (CHAPS, Boehringer Mannheim), 0.1 mol/L sodium acetate, pH 5.8, with proteinase inhibitors (Complete EDTA-free, Boehringer Mannheim) for 24 hours at 4°C. The suspensions were then centrifuged at 12,000 × g for 15 minutes, and the supernatants were dialyzed overnight in 8 mol/L urea, 0.1 mol/L NaCl, and 0.1% CHAPS, pH 7.5. The crude proteoglycan fractions were isolated by anion-exchange chromatography (Q-Sepharose, Pharmacia) in the urea buffer. The samples flowed through were discarded, and all of the materials bound to the column were eluted in a single step using 8 mol/L urea, 1.5 mol/L NaCl, 0.1% CHAPS, pH 7.5; dialyzed against distilled water; and lyophilized. Samples corresponding to 200 and 85 mg of the placenta and kidney starting material, respectively, were dissolved in 100 μl of 60 mmol/L sodium acetate and 50 mmol/L Tris-HCl, pH 8.0 (for chondroitinase ABC), 50 mmol/L sodium phosphate, pH 7.1 (for heparin lyase II), or 50 mmol/L NaCl, 50 mmol/L Tris-HCl, pH 7.3 (for heparin lyase III with collagenase). Twenty-five milliunits of chondroitinase ABC (Boehringer Mannheim) or 4 mIU of heparin lyase II or III (Sigma Chemical Co.) were added to 27-μl samples in the buffers mentioned above and incubated at 37°C for overnight. After chondroitinase ABC and heparine lyase II digestions, 10 μl of 4× sodium dodecyl sulfate sample buffer and 1 μl of β-mercaptoethanol were added. Bacterial collagenase digestions were performed after heparin lyase III digestions. For that, 1 μl 0.5 mol/L CaCl2 and 10 U of bacterial collagenase (chromatographically purified; Worthington Biochemical Corp., Freehold, NJ) were added in a reaction volume of 40 μl and incubated at 37°C for additional 4 hours, after which sodium dodecylsulfate sample buffer and β-mercaptoethanol were added as above. Control digestions were treated in the same way but without added enzymes. Each of the digestions was divided into two SDS-PAGE samples for Coomassie Brilliant Blue staining or Western blotting, which were run in a 7% denatured SDS-PAGE gel. Anti-all human (hu) XVIII and anti-long huXVIII antibodies were used as above in enhanced chemiluminescence detection.
Immunofluorescence Staining of Tissues
Tissue sections from the heart, kidney, liver, lung, and pancreas, from an apparently healthy 17-gestational week male fetus and from two 10-gestational week placentas obtained from therapeutic abortions were analyzed. The mature tissues analyzed included samples from normal adult skin, liver, kidney, and striated neck muscle.
Tissue sections for indirect immunofluorescence staining were immediately frozen in liquid nitrogen and cut into 5-μm cryosections on SuperFrost Plus glass slides (Menzel Gläser). The sections were fixed in precooled ethanol for 10 minutes at −20°C, and unspecific antibody binding was blocked by incubation with 3% bovine serum albumin in PBS, pH 7.3, or with 5% fat-free milk powder in PBS, pH 7.3, for 60 minutes at room temperature followed by overnight incubation at 4°C with a specific antibody. The antibodies used were those against QH48.18 and QH1415.7 at concentrations of 10 μg/ml or 20 μg/ml in 3% bovine serum albumin/PBS, pH 7.3, respectively; a monoclonal antibody to type IV collagen diluted 1:100 (DAKO Corp., Copenhagen, Denmark) as a marker for BMs; a monoclonal antibody to CD34 diluted 1:25 (Novocastra Laboratories Ltd., Newcastle, UK) as a marker for endothelial cells; a monoclonal antibody to α-smooth muscle actin (Sigma Chemical Co.) as a marker for smooth muscle cells; and a monoclonal antibody to vascular endothelial growth factor receptor, FLT4 diluted 1:200 (a generous gift from Professor Kari Alitalo, Haartman Institute, University of Helsinki, Finland), as a marker for lymphatic vessels. After thorough washing with PBS, pH 7.3, a tetrarhodamine isothiocyanate-conjugated polyclonal swine anti-rabbit antibody (DAKO Corp.) or rabbit anti-mouse antibody (DAKO Corp.) was applied, and the samples were incubated for 60 minutes in the dark at room temperature. A fluorescein isothiocyanate-conjugated goat anti-mouse antibody (DAKO Corp.) was used for the double-immunofluorescence stainings. The slides were then mounted with Glysergel (DAKO Corp.) and examined under an epifluorescence microscope (Leitz Aristoplan) equipped with filters for tetrarhodamine isothiocyanate and fluorescein isothiocyanate fluorescence. Control sections were stained with the secondary antibody alone. The specificity of the stainings was further demonstrated by blocking the immunostainings by incubating the affinity-purified type XVIII antibodies overnight at 4°C with solutions of the corresponding human type XVIII collagen-derived polypeptides, ie, QH48.18 and QH1415.7, and using this mixture for additional immunostainings. For better histological analysis, frozen sections from all the tissues examined were stained with hematoxylin and eosin by routine methods.
To reduce the possibility of the epitopes recognized by type XVIII collagen-specific antibodies being masked in tissues, some of the sections were pretreated with 6 mol/L urea, 0.1 mol/L glycine, pH 3.5, for 1 hour at 4°C, with 10 U/ml bacterial collagenase (Worthington Biochemical Corp.) for 2 hours at 37°C, with 5% hyaluronidase (Sigma Chemical Co.) for 15 minutes at 37°C, with 0.2 U chondroitinase ABC (Boehringer Mannheim) at 37°C overnight, with 0.2 U N-glycosidase (Boehringer Mannheim) at 37°C for overnight, or with 0.7 U heparin lyase II and III (Sigma Chemical Co.) at 37°C for overnight before blocking by incubation with 3% bovine serum albumin in PBS.
Results
Preparation, Affinity Purification, and Characterization of Two Polyclonal Antibodies to Human Type XVIII Collagen
The construct QH48, corresponding to all type XVIII variants, and QH67, corresponding to the long variant, were expressed as DHFR fusion proteins in E. coli (Figure 1) ▶ , and the purified recombinant proteins were used to immunize rabbits. The ensuing sera were affinity purified using type XVIII polypeptide fragments expressed without the DHFR leader sequences to isolate type XVIII-specific antibodies designated as anti-all huXVIII and anti-long huXVIII antibodies (see Materials and Methods).
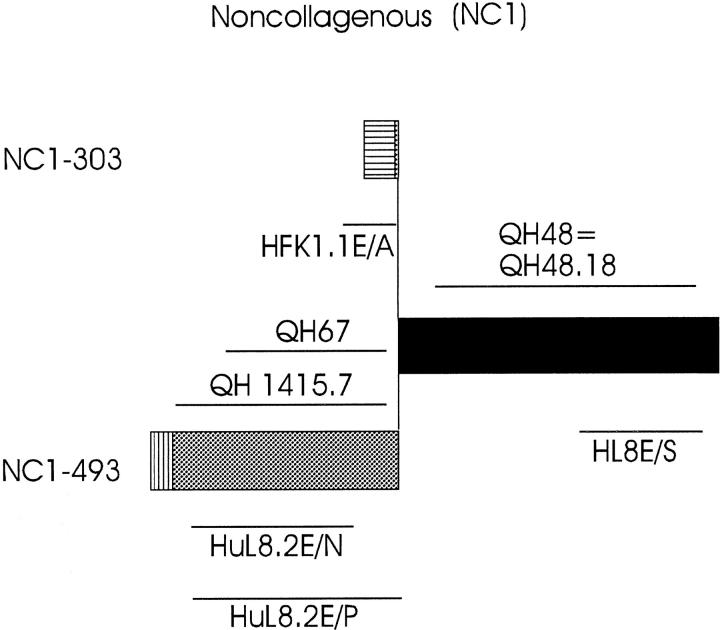
Figure 1.
Probes used for in situ hybridization analysis and the cDNA fragments used for antigen production and for affinity purification of the human type XVIII collagen-specific antibodies. Schematic structures of the variant N-terminal NC1 domains are shown. Noncollagenous sequences common to all variants are shown in black, noncollagenous sequences specific to different variants in gray, and putative signal peptides of the different variants by vertical hatching. The cDNA fragments, named QH48 and QH48.18, used for production and purification of the anti-all huXVIII antibody, and QH67 and QH1415.7, used for production and purification of the anti-long huXVIII, are shown above the schematic structure. HuL8.2E/N and HL8E/S, shown below the schematic structure, represent the RNA probes used for in situ hybridization analysis.
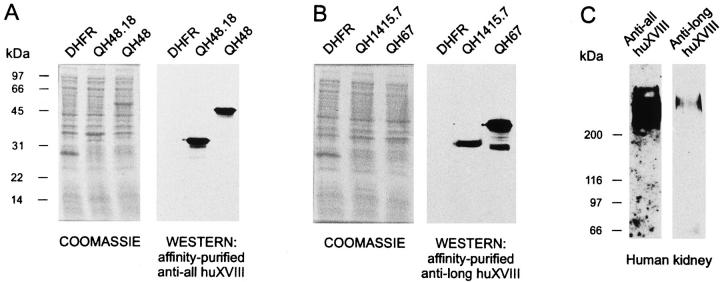
The affinity-purified antibodies were first characterized using crude bacterial cell lysates expressing variant polypeptide sequences. The anti-all huXVIII antibody was tested using the DHFR construct as a negative control and QH48 and QH48.18 constructs (the latter lacking the DHFR sequence) as positive ones. No immunosignal was visible in the DHFR sample, as expected, whereas QH48 and QH48.18 gave specific signals of 52 and 32 kd, respectively, demonstrating that the anti-all huXVIII antibody is capable of detecting type XVIII polypeptides (Figure 2A) ▶ . In the case of the anti-long huXVIII antibody, no immunosignal was visible using the DHFR sample, whereas 44- and 33-kd bands were detected with the QH67 and QH1415.7 constructs, respectively (the latter lacking the DHFR sequence) (Figure 2B) ▶ . Thus, the anti-long huXVIII antibody detected polypeptide sequences derived from the long type XVIII variants. A minor 32-kd band was also detected in the QH67 sample, which was most probably a degradation product from QH67, because it was not detected in the DHFR or QH1415.7 samples.
Figure 2.
Characterization of antibodies to human type XVIII collagen by Western blotting. Bacterial cell lysates expressing various constructs detected by anti-all huXVIII antibody (A) and anti-long huXVIII antibody (B). Coomassie Brilliant Blue stainings demonstrate equal loading in the gels. Western blot analysis demonstrates specific signals only in samples expressing type XVIII-derived polypeptide sequences. DHFR, expression of DHFR; QH48.18, expression of type XVIII-derived sequences from the common region of XVIII without the DHFR leader sequence; QH48, expression of the same fragment of type XVIII but with the DHFR leader sequence; QH1415.7, expression of type XVIII-derived sequences from the long variants of XVIII without the DHFR leader sequence; QH67, expression of the slightly shorter fragment of the long variants of XVIII but with the DHFR leader sequence. See Figure 1 ▶ for the positions of the various expression constructs. C: Western blot analysis of human kidney protein lysate. Thirty micrograms of protein was run in 7% SDS-PAGE and blotted, after which the filter was first stained with the anti-long huXVIII antibody by enhanced chemiluminescence, and then the filter was stripped off the antibodies and stained with the anti-all huXVIII antibody. The exposure time with both antibodies was the same, 15 minutes.
Both antibodies were further characterized using a human kidney protein lysate in Western blotting. Adult human kidney contains marked amounts of mRNAs for the short XVIII variant and much lower amounts of mRNAs for the long variant. 11 The two antibodies gave overlapping specific results, as expected (Figure 2C) ▶ . The anti-all huXVIII antibody resulted in a broad, smear-like band above the highest 200-kd molecular weight standard, as is typical of proteoglycans containing long GAG side chains of different lengths.
Interestingly, the Western analysis with the anti-long huXVIII resulted in a similar smear-like signal, but with less intensity (Figure 2C) ▶ . Moreover, the signal with the anti-long huXVIII antibody overlapped the one obtained with the anti-all huXVIII antibody, but consisted only of the upper part of the broad smear detected with the latter, a finding consistent with the expected polypeptide lengths of the long and short variants. Also, the intensities of the bands were in agreement with the results obtained by immunohistochemistry (see below, Figure 6 ▶ ), in which the anti-long huXVIII gave far weaker immunosignals than the anti-all huXVIII. The specificity of the immunostainings was further demonstrated by incubating the affinity-purified type XVIII antibodies with molar excess solution of the corresponding human type XVIII collagen-derived polypeptides and using these mixtures for additional stainings. Immunostaining of tissue sections with these mixtures did not show any immunoreactivity (data not shown). These results suggest that the two antibodies can be used to detect human type XVIII collagen in a specific manner.
Figure 6.

In situ hybridization and immunofluorescence stainings for localization of type XVIII collagen mRNAs and protein in human fetal and adult kidney. A and B: The cells of the glomerular structures (Gl), those of Bowman’s capsule (arrows), and the epithelial cells of the lower parts of the nephron (Tu) are labeled for type XVIII mRNAs. The probe in A is HL8E/S antisense RNA, and that in B is the corresponding sense RNA (probes shown in Figure 1 ▶ ) B: Hybridization with the sense probe shows the extent of the background labeling. C: Immunostaining of fetal kidney with the anti-all huXVIII antibody shows clear immunostaining in the peripheral BM of the glomerular capillaries and in the mesangial area of the glomeruli. The BM zones of Bowman’s capsule (arrow) and some tubules (Tu) are also strongly stained. D: Immunostaining of the fetal kidney with anti-long huXVIII antibody shows immunoreactivity in the peripheral BM zones of the glomerular capillaries and in the mesangial area, but there is no staining in the BM zones of Bowman’s capsule or the tubules. E: Immunostaining of adult kidney with the anti-all huXVIII antibody shows strong staining in the BM zones of the tubules (Tu). The BM zone of Bowman’s capsule (arrow) and the glomerular mesangium give clear staining, whereas that in the glomerular BM zone is very weak. F: Immunostaining of the adult kidney with anti-long huXVIII antibody shows only faint fluorescence in the BM zones and mesangium of the glomeruli, whereas the tubules are negative for this antibody. Magnification: A to D and F, ×400; E, ×250.
Because type XVIII collagen chains contain several potential sites for the attachment of GAG side chains, we wanted to study whether type XVIII is in a proteoglycan form, at least in kidney. As the kidney sample was a commercial one already homogenized in a high concentration of sodium dodecyl sulfate, we were not able to perform digestions of sugar chains with that sample. Therefore, we isolated crude proteoglycan fractions from a normal placenta and a kidney from a patient with congenital nephrosis, Finnish type, and treated those samples with heparin lyase II and III (Figure 3) ▶ . In contrast to the commercial normal kidney sample, we were not able to detect type XVIII in a nondigested proteoglycan fraction isolated from a patient (Figure 3 ▶ , lanes 3 and 5), probably because of the different nature of the samples. However, after heparin lyase II and III digestions, strong signals of 180 kd were detected (Figure 3 ▶ , lanes 4 and 9). The normal placenta sample gave identical results but with very weak signal intensities (data not shown). Thus, type XVIII contains heparan sulfate side chains at least in some tissues. Chondroitinase ABC did not have any effect compared with the nondigested samples (data not shown). The collagenous nature of the 180-kd band was verified by demonstration that after heparin lyase III digestion, the band was sensitive to bacterial collagenase (Figure 3 ▶ , lane 10). The anti-long huXVIII antibody was negative in all samples studied (data not shown), whereas in the commercial sample it gave a weak smear (Figure 2C) ▶ . This difference was likely due to the fact that the tissue section from the same patient sample almost totally lacked glomeruli, which are virtually the only structures in kidney positive for the anti-long huXVIII antibody in immunofluorescence stainings.
Figure 3.
Characterization of the glycosaminoglycan side chains attached to type XVIII in kidney. A crude proteoglycan fraction was isolated from a kidney from a patient with congenital nephrosis, Finnish type. Proteoglycans were digested with heparin lyase II (lanes 1 to 4) or heparin lyase III (lanes 5 to 10), and the latter samples were further treated with bacterial collagenase. The samples were fractionated by reduced 7% SDS-PAGE followed by Coomassie staining (lanes 1 , 2, and 5 to 7) or to Western blotting using the anti-all huXVIII antibody (lanes 3, 4, and 8 to 10). Both heparin lyase II and III resulted in appearance of a 180-kd band (lanes 4 and 9), which was also sensitive to bacterial collagenase (lane 10). A 120-kd band in lane 7 represents bacterial collagenase.
Expression of Type XVIII Collagen mRNAs and Protein in Human Tissues
In situ hybridization was performed on paraffin and cryosections from five normal placentas and on sections representing eight types of tissue from two fetuses with Down’s syndrome and a fetus with adenoid malformation of the lung (Table 1) ▶ . The sections included samples from the heart, liver, kidney, pancreas, skin, lung, thymus, and bone. Adult human skin and liver sections were also analyzed by in situ hybridization (Table 1) ▶ . Two antisense RNA probes, HL8E/S, covering some of the sequences encoding the NC1 domain region common to all type XVIII collagen chains, and HuL8.2E/P, covering sequences specific to the long cDNA variant were used for the hybridizations (Figure 1) ▶ . The same probes in the reverse orientation (sense RNA) served as negative controls for nonspecific hybridization. Only an evenly distributed background of autoradiography grains was seen with the sense probes. The results with respect to specific hybridization were generally the same in the cryosections and paraffin sections.
Table 1.
Location of Type XVIII Collagen mRNAs in Human Tissues by in situ Hybridization
| Tissue* | mRNA location† |
|---|---|
| Placenta and gestational endometrium | Epithelial cells of the villi, myofibroblasts in the villous stroma, unorganized cytotrophoblasts of the trophoblastic columns, endothelial cells of the capillaries, pericystic cells of spiral arteries, epithelial cells of the gestational endometrium, and large decidual cells of the decidual membrane |
| Adult and fetal liver | Fetal hepatocytes, hepatocytes of the adult liver, endothelial cells, and epithelial cells of the bile ducts |
| Adult and fetal skin | Keratinocytes of the epidermis, endothelial cells, epithelial cells of the sweat glands, and cells of the hair follicle |
| Fetal heart | Heart muscle cells and endothelial cells |
| Fetal kidney | Epithelial cells of the lower part of the nephron, endothelial cells, cells of Bowman’s capsule, unorganized cells of the metanephric blastema, and cells of the glomeruli |
| Fetal pancreas | Cells of the acinar epithelium and endothelial cells |
| Fetal thymus | Endothelial cells |
| Fetal bone | Periostic cells, perichondrial cells, and immature mesenchymal cells surrounding the developing bone |
*The tissues used were 10-gestational week placentas, 19- and 20-week fetuses, and normal adult tissues.
†The probes used were HL8E/S, detecting all mRNA variants, and HuL8.2E/P, specific to the long variant (see Figure 1 ▶ ). Adult and fetal liver hepatocytes were the only convincingly labeled cells in the hybridizations using the probe HuL8.2E/P (data not shown).
Cryosections from two placentas; a fetus; and normal adult liver, kidney, skin, and skeletal neck muscle were analyzed by immunofluorescence staining using the affinity-purified anti-all huXVIII and anti-long huXVIII antibodies (Table 2) ▶ . Double-immunofluorescence staining and stainings of adjacent sections using an antibody to type IV collagen were used to verify the location of the type XVIII collagen signal in relation to the BMs in the tissue samples. The same approach was also adopted with CD34, α-smooth muscle actin, and FLT4 antibodies to aid in the identification of endothelial cells, smooth muscle structures, and small lymphatic vessels, respectively.
Table 2.
Immunofluorescence Staining of Human Tissues*
| Tissue | Protein location | |
|---|---|---|
| NC1-303 variant | NC1-493 variant | |
| Placenta and gestational endometrium | BM zone of the villous epithelium, around individual cytotrophoblasts, BMs of capillaries and larger blood vessels, fibrovascular stroma of the fibrotic villi, and BMs under the epithelium in the endometrial glands | Around some of the myofibroblasts of the villi and in larger blood vessel walls of the gestational endometrium |
| Adult liver | Clear staining of BMs of capillaries and bile ducts | Sinusoidal staining |
| Fetal liver† | BM zones of blood vessels and developing bile ducts and staining around developing portal areas | Interrupted sinusoidal staining |
| Skin | Dermoepidermal BM, BMs of dermal capillaries, sweat glands, and hair follicles, and around smooth muscle cells and peripheral nerves | Dermoepidermal BM and BM zone of hair follicles |
| Fetal heart† | Around individual heart muscle cells, BM of capillaries, and walls of larger vessels | Walls of larger vessels |
| Adult striated neck muscle | Thin, interrupted staining around muscle cells, BM zones of capillaries, larger blood vessels, peripheral nerve fibers and the musculotendinal junction, and walls of larger vessels | Walls of larger vessels and very weak staining in the musculotendinal junction |
| Adult kidney | BM of the tubules, Bowman’s capsule, BMs of capillaries and larger vessels, glomerular mesangium, and walls of larger vessels | Glomerular mesangium and glomerular BM and walls of larger vessels |
| Fetal kidney† | BM zone and the mesangium of the glomeruli, and BM of tubules, Bowman’s capsule, BMs of capillaries and larger vessels, and walls of larger vessels | BM and the mesangium of the developing glomeruli and walls of larger vessels |
| Fetal pancreas† | BM zones of developing pancreatic acini and ducts, and capillaries and larger vessels | Walls of larger vessels |
| Fetal lung† | BM zones under the epithelium of developing alveolar structures and bronchi, around smooth muscle cells of bronchial walls, and vascular structures | Walls of larger vessels |
*Immunofluorescence staining with affinity-purified polyclonal antibodies detecting both type XVIII chain variants and specifically the long variant were used to locate the corresponding protein. Immunofluorescence staining seen with anti-all huXVIII antibodies but not with anti-long huXVIII antibodies was considered to represent occurrence of the short variant.
†The tissues used were from a 17-gestational week fetus.
Placenta
The human placenta gave an intense signal for collagen XVIII mRNAs in the cells of the double-layered trophoblastic epithelium of the villi and in the unorganized cytotrophoblasts of the trophoblastic columns, including multinucleated syncytial giant cells, with the probe HL8E/S, encoding sequences common to all α1(XVIII) chains (Figure 3 ▶ , A and B). The signals in the endothelial cells of the villi were consistent, although not as strong as in the epithelial cells, whereas labeling of the villous myofibroblasts was weak (Figure 3 ▶ , A and B). The stromal cells of the gestational endometrium seemed to express the mRNAs faintly, whereas the signal in the epithelial cells of the endometrial glands and in the large decidual cells of the decidual membrane was considerably stronger (not shown). The pericystic cells and the endothelial cells of the placental spiral arteries were also clearly positive with the probe HL8E/S (not shown).
The BM zone under the double-layered trophoblastic epithelium and the blood vessels of the stromal villi showed positive staining with the anti-all huXVIII antibody (Figure 4 ▶ , C and E), and the fibrovascular stroma of the villi was also positive, particularly in the case of fibrotic villi. There was also staining for type XVIII collagen around individual cytotrophoblasts of the trophoblastic columns and around some stromal myofibroblasts of the villi (Figure 4C) ▶ . Immunofluorescence analysis of the decidual membrane of the placenta using the anti-all huXVIII antibody showed a strong positive staining in the maternal blood vessels and a faint staining lining the epithelial cells in the endometrial glands (not shown).
Figure 4.

In situ hybridization and immunofluorescence stainings for localization of type XVIII collagen mRNAs and protein in human placenta. A: Light-field image. B: Corresponding dark-field image. There is a strong in situ hybridization signal in the cells of the double-layered trophoblastic epithelium of the villi (curved arrows) and the cytotrophoblastic cells of the trophoblastic columns (Tr), whereas the signals in the endothelial cells (arrowheads) and the fibroblastic stromal cells are consistent, but clearly weaker. Inserts in A and B show higher magnification. The section in A and B was hybridized with the antisense RNA probe HL8E/S (probes shown in Figure 1 ▶ ). C: Immunostaining with the anti-all huXVIII antibody shows a strong immunofluorescence staining in the BM zone of the epithelium (curved arrows) and the capillaries (arrowheads) of the villi, around the fibroblastic stromal cells (thin arrows), and around the individual cylotrophoblastic cells of the trophoblastic columns (Tr). Because of the curving of the epithelium in some parts of the placental section, the staining of the epithelium in C is not identical with that of typical staining of the BM, but the staining with the anti-all huXVIII antibody showed linear immunofluorescence reaction more strictly under the epithelium of the villi in most parts of the section, as can be seen in E. D: Immunostaining with the anti-long huXVIII antibody shows immunoreactivity only around myofibroblastic stromal cells (arrows), whereas the capillaries are negative (arrowheads). E: and E′: Stainings of the placental villi with the anti-all huXVIII antibody (E) and with an antibody to human type IV collagen (E′) show linear staining under the epithelial cells (arrows), typical for BMs as well as around capillaries (arrowheads). Magnification: A and B, ×100; inserts, ×250; C to E′, ×250.
Liver
Interestingly, the hepatocytes of both the fetal and adult liver expressed type XVIII collagen mRNAs. In situ hybridization studies showed clear hybridization signals in hepatocytes (Figure 5 ▶ , A and B). The endothelial cells and the epithelial cells of the bile ducts in the portal areas were also positive, whereas the erythropoietic cells in the fetal liver were negative.
Figure 5.

In situ hybridization and immunofluorescence stainings for localization of type XVIII collagen mRNAs and protein in human fetal and adult liver. A: Light-field image. B: Corresponding dark-field image. There is a clear hybridization signal in the fetal hepatocytes open arrows, whereas the erythropoietic cells are negative (A, short arrows) with the antisense RNA probe HL8E/S. A, insert: Higher magnification. B, insert: Higher magnification, light-field image of the hybridization with the control RNA probe for comparison (probes shown in Figure 1 ▶ ). C: Immunostaining of fetal liver with the anti-all huXVIII antibody shows interrupted linear staining along the developing sinusoids. There is strong immunoreactivity around the developing portal areas (Po) and in the BM zone of the blood vessel (arrow). D: Immunostaining of the fetal liver with anti-long huXVIII antibody shows immunoreactivity only along the developing sinusoidal structures, whereas the structures in the portal area (Po) are negative, as is the BM zone of the blood vessel (arrow). E: Immunostaining of adult liver with the anti-all huXVIII antibody shows linear staining along the sinusoids. The walls of the larger vessels (straight arrows) and the BM zones of the bile ducts (bd, curved arrows ) and capillaries (arrowheads) are also strongly stained. F: Immunostaining of adult liver with the anti-long huXVIII antibody reveals the same linear staining along the sinusoids. The staining in the walls of the larger vessels is markedly weaker (straight arrow), whereas the BM zones of the capillaries and bile ducts remain negative with this antibody. Magnification: A to D, ×250; inserts, ×400; E and F, ×100.
Immunofluorescence stainings of fetal liver using the anti-all huXVIII antibody revealed an interrupted staining along the developing sinusoidal structures between the hepatocytes, as well as staining around the developing portal areas and in the BM zone under the endothelium in the blood vessels (Figure 5C) ▶ . In the adult human liver the anti-all huXVIII antibody gave a clear, linear staining along the sinusoids (Figure 5E) ▶ . The walls of the arteries of the portal tracts were very strongly stained, as was the BM zone under the epithelium of the bile duct (Figure 5E) ▶ . The capillaries in the portal areas also showed clear immunoreactivity (Figure 5E) ▶ .
Kidney
The epithelial cells of the lower part of the nephron in the developing fetal kidney, especially the collecting ducts, were clearly labeled for type XVIII collagen mRNAs using probe HL8E/S (Figure 6 ▶ , A and B). A positive hybridization signal was seen in the unorganized cells of the metanephric blastema and the cells of the primitive glomeruli (not shown) and also in the cells of the more mature glomeruli (Figure 6A) ▶ . The cells of Bowman’s capsule were likewise positive (Figure 6A) ▶ , whereas the epithelial cells of the proximal tubules were negative (not shown). The vascular structures of the kidney were also strongly labeled for type XVIII collagen mRNA (not shown).
Immunofluorescence staining of the fetal kidney with the anti-all huXVIII antibody showed clear staining in the glomerular BMs and in the BM zones of most of the tubules and of Bowman’s capsule (Figure 6C) ▶ . The metanephric blastemal cells of the subcapsular fetal cortex were negative to immunofluorescence staining, whereas there was linear staining in the developing Bowman’s capsule around them (not shown). Some immature glomeruli and the more mature glomeruli toward the juxtamedullar region demonstrated clear positivity in the mesangium and in the BMs of the capillaries once the latter had formed (not shown). The BM zones of Bowman’s capsules were always stained. The anti-all huXVIII antibody showed strong positive staining in the BM zones of the tubules in the adult human kidney (Figure 6E) ▶ . Bowman’s capsule and the glomerular mesangium were clearly stained, whereas the glomerular BM staining was considerably weaker than that in the fetal glomeruli (Figure 6E) ▶ . All of the vascular structures in the fetal and adult kidney samples were linearly stained with the anti-all huXVIII antibody (not shown). Treating the kidney tissue with heparin lyase II or III before incubation with the primary antibody improved the glomerular staining, whereas treating the tissue samples with several other enzymes or buffers (see Materials and Methods) did not have any effect (not shown). This coincides well with the susceptibility of the kidney extracts for treatment with heparin lyase II and III (Figure 3) ▶ .
Muscular tissues
In situ hybridizations of fetal heart revealed clear signals both in the endothelial cells and in the cardiac muscle cells with the probe detecting both type XVIII mRNA variants (Figure 7 ▶ , A and B). Immunofluorescence staining with the anti-all huXVIII antibody showed staining around individual heart muscle cells and around numerous small capillaries (Figure 7C) ▶ . This reaction was very similar to that for type IV collagen, except that the staining in the capillaries was not as intense (not shown). The larger blood vessels were more strongly stained for type XVIII than for type IV (not shown).
Figure 7.
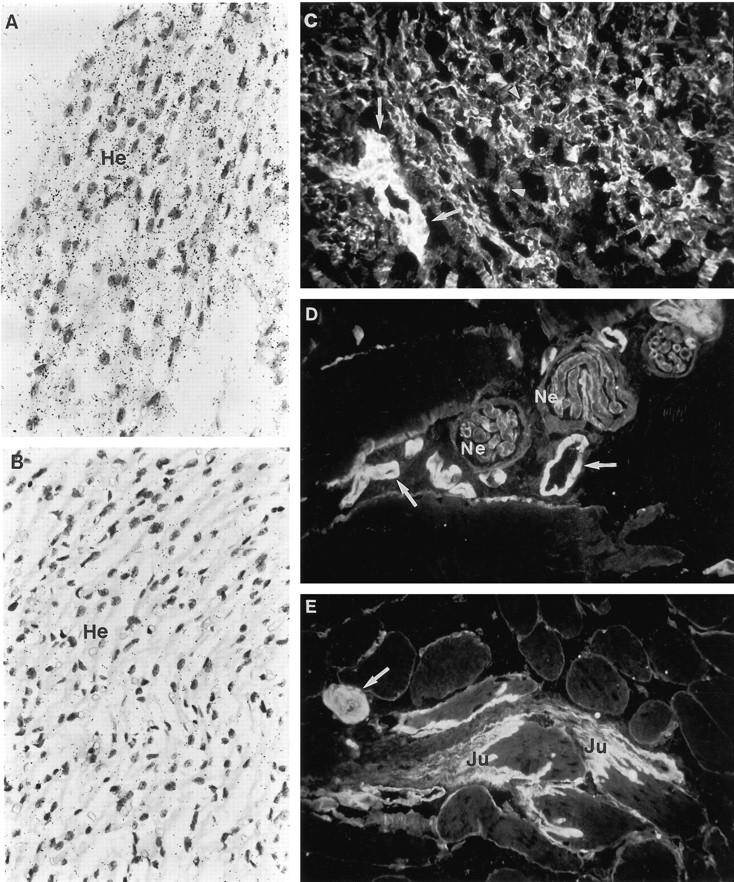
In situ hybridization and immunofluorescence stainings for localization of type XVIII collagen mRNAs and protein in human muscle tissues. A: Fetal heart muscle cells (He) clearly express type XVIII collagen mRNAs. B: Hybridization with the control probe. The probe in A is antisense RNA HL8E/S and that in B is the corresponding sense RNA (probes shown in Figure 1 ▶ ). C: Immunostaining of fetal heart with the anti-all huXVIII antibody shows immunoreactivity around the heart muscle cells and small capillaries (arrowheads) and in a vessel wall (arrow). D and E: Immunostaining of adult skeletal muscle with the anti-all huXVIII antibody shows strong immunofluorescence in the BM zones of the vessels (arrows) between the muscle bundles. There is also clear linear staining in the BM zones of the peripheral nerves (Ne), whereas the staining in the BMs of the endomycium is thin and interrupted. Strong immunoreactivity is seen at the musculotendinal junction (Ju). Magnification: A to C and E ×250; D ×400.
The anti-all huXVIII antibody revealed thin, interrupted staining in the BM region around individual adult skeletal muscle fibers, whereas the staining in the musculotendinal junction was considerably stronger, extending further into the fibers of the tendon (Figure 7 ▶ , D and E). There was also strong staining in the blood vessels between the muscle bundles (Figure 7 ▶ , D and E). Furthermore, strong staining was seen around the individual Schwann cells and encircling the whole peripheral nerves located between the muscle cells (Figure 7D) ▶ . The reaction in some of the small endomycial vessels between individual muscle fibers was either very weak or nonexistent. Some of these vessels were stained with a lymphatic vessel-specific antibody, suggesting that the lymphatic vessels had only low amounts of type XVIII collagen or lacked it altogether (not shown).
Skin
The keratinocytes of the epidermis in adult and fetal skin were found to express type XVIII collagen mRNAs (not shown). The signal was mainly seen in the basal cells, although some positivity could be seen in all cell layers of the epidermis. The epithelial cells of the sweat glands, cells of the hair follicles, and endothelial cells also contained type XVIII mRNAs (not shown).
Strong positive staining with the anti-all huXVIII antibodies was seen in the dermal-epidermal BM zone of the adult skin, at capillaries, and around individual smooth muscle cells (Figure 8A) ▶ . The BM region around the sweat glands and hair follicles (Figure 8A) ▶ and the BM zones of the nerve fibers showed linear immunofluorescence staining reactions (not shown), markedly resembling the staining seen with a monoclonal antibody for type IV collagen (not shown).
Figure 8.

In situ hybridization and immunofluorescence stainings for localization of type XVIII collagen mRNAs and protein in human adult skin, and fetal pancreas and lung. A: Immunostaining of adult skin with the anti-all huXVIII antibody shows strong immunoreactivity in the dermal-epidermal BM zone and in the BM zones of the capillaries (arrowheads) and hair follicles in the dermis (De). Ep, epidermis. B: Immunostaining of the adult skin with the anti-long huXVIII antibody shows immunoreactivity in the dermal-epidermal (De and Ep) BM zone, whereas there is no convincing staining in other dermal structures. C: A section of fetal pancreas hybridized with the HL8E/S antisense RNA probe is shown. There is a clear hybridization signal in the epithelial cells of the pancreatis acini (C and D, curved arrows). D: Hybridization with the sense probe shows the amount of the background labeling (probes shown in Figure 1 ▶ ). E: Immunostaining of fetal pancreas with the anti-all huXVIII antibody shows linear staining in the BM zones of the acini. The BM zone of the larger blood vessel is also stained (arrows), whereas the immunoreactivity in the small capillaries is very weak or unconvincing (arrowheads). F and G: Immunostaining of fetal lung with the anti-all huXVIII antibody shows staining in the BM zones of the developing alveolar structures (curved arrow) and in the small capillaries (arrowheads). In the center there is a bronchus with a linear double contour, signifying staining of the BM region under the bronchial epithelium and around the smooth muscle cells in the bronchial wall (thin arrows). H: Immunostaining of the fetal lung with the anti-long huXVIII antibody shows immunoreactivity only in the walls of the larger vessels (arrows). Magnification: A and B, ×100; C and D, ×400; E to H, ×250.
Pancreas
In situ hybridization analysis of the fetal pancreas revealed a clear signal in the epithelial cells of the developing acini (Figure 8 ▶ , C and D), whereas the ductal epithelial cells were not convincingly positive (not shown). The endothelial cells of the capillaries also expressed the mRNAs. Strong linear immunofluorescence was seen in the BM zone under the epithelium of the pancreatic acini (Figure 8E) ▶ and in the BM region of the pancreatic ducts. The signal around the small capillaries situated in the fibrous stroma and between the developing acinar structures was faint or nonexistent, whereas the staining around larger vessels was considerably stronger with the anti-all XVIII antibody (Figure 8E) ▶ .
Lung
Immunostaining of fetal lung tissue with anti-all huXVIII antibodies showed linearly stained BM zones around the developing alveolar structures (Figure 8F) ▶ . The BM regions of the bronchi in the fibrotic areas were also linearly stained, often with a double contour representing the BM zone under the bronchial epithelium and staining around the smooth muscle cells of the bronchial wall (Figure 8G) ▶ . The staining of the numerous small capillaries between the alveoles varied from very weak to strong in some capillaries and larger vessels (Figure 8 ▶ , F and G). Despite this clear immunofluorescence signal and detection of the mRNA in the fetal lung by Northern analysis, repeated in situ hybridization experiments did not reveal a convincing signal. This may be due to the low amount of the mRNA in the tissue.
Differential Expression of Short and Long Variants of α1(XVIII) Collagen Chains
Marked differences were seen in the occurrence of the NC1-303 and NC1-493 variants of the α1(XVIII) chain. The NC1-303 variant is clearly the main one in most locations, whereas the NC1-493 variant has a more restricted distribution, the strongest stainings for it being found in the liver sinusoids (Figure 5 ▶ , D and F). The immunostaining together with the Northern and in situ data suggest that the sinusoidal staining is largely derived from the NC1-493 variant. In skin, the BM zones of the epidermal-dermal junction and the hair follicles were stained for the anti-long huXVIII antibody (Figure 8B) ▶ , but not the other structures within the dermis, including the BM zones around the smooth muscle cells, indicating that they contain only the short variant. Interestingly, the anti-long huXVIII antibody gave a clear immunofluorescence reaction in placenta only around the fibroblasts of the villi (Figure 4D) ▶ , whereas the other stained structures of the placental villi contained the short variant of the collagen.
One notable finding was the lack of staining for the NC1-493 variant in capillaries and only weak staining in the walls of large arteries (Figure 4D ▶ , 5F ▶ , and 8H ▶ ), compared with strong signals with the anti-all huXVIII antibody (Figure 4C ▶ ; 5 ▶ , A, C, and E; 7 ▶ , C to E; and 8 ▶ , A and E to G). Only the vascular smooth muscle structures contain the long variant, given that there is staining in the walls of the larger vessels in all tissues studied but not in other smooth muscle structures such as the smooth muscle cells in the dermis (Figure 8B) ▶ or in the bronchial walls (not shown). The strong staining seen with the anti-all huXVIII antibody in the epithelial BM zones of the bile ducts, kidney tubules, pancreatic acini and ducts, lung alveoli, and bronchial structures and in the BM zones of Bowman’s capsule and peripheral nerves, was lacking with the anti-long huXVIII antibody. The anti-long huXVIII antibody stained the glomerular BM of the fetal kidney, whereas the glomerular mesangium of the adult kidney was stained but the glomerular BM only very weakly so (Figure 6 ▶ , D and F). No staining for the long variant could be found around the cardiac muscle cells of the heart, but faint staining could be seen at the musculotendinal junction in the skeletal muscle (not shown).
Discussion
Antibodies were produced against a protein fragment corresponding to NC1 sequences common to the human N-terminal variants and another corresponding to sequences specific to the NC1-493 variant, and these were used to visualize the type XVIII collagen variants in a number of human tissues. They were also used for Western blotting, which resulted in detection of overlapping high-molecular weight bands above the 200-kd standard in a kidney extract. This size is in agreement with the previous finding of a 200-kd band thought to represent α1(XVIII) chains in cultured mouse embryonic stem cells. 9 The human type XVIII collagen contains several putative sites for N-linked glycosylation and GAG side chain attachment, but it has not been known whether any of these sites are functional. The Western analysis of human kidney extracts showed a broad band typical of proteoglycans containing multiple side chains of various sizes, suggesting that some of these putative sites may be in use. Further analyses by heparin lyase III digestion of the kidney and placenta extracts indicated that at least in these tissues, type XVIII collagen contains heparin sulfate glycosaminoglycan side chains. The size difference between the 200-kd type XVIII collagen in embryonic stem cells 9 and the 180-kd type XVIII in the kidney analyzed here can be explained with species differences in other posttranslational modifications or with some differences in the SDS-PAGE running conditions.
Type XVIII collagen was found in virtually all of the BM zones of the fetal and adult human tissues studied here. A conspicuous finding in all tissues was immunostaining of the capillaries as well as the larger blood vessels, which also showed strong staining in the vessel wall. Previous studies of type XVIII collagen expression in the mouse detected this collagen most notably in association with blood vessels, but also in the BM zone of the skin and faintly in Bowman’s capsule in the kidney. 9 We found this collagen to be expressed very clearly in the human fetal and adult kidney in the Bowman’s capsule, the glomeruli, and the tubules and have recently observed it in this same location in the mouse kidney using antibodies against mouse type XVIII collagen-derived fragments (M Rehn et al, unpublished results). In addition, type XVIII collagen was found adjacent to the trophoblastic epithelium of the placental villi and in the endometrial glands of the gestational endometrium; adjacent to the skeletal muscle, smooth muscle, and heart muscle cells; surrounding the peripheral nerves; at the epidermal-dermal junction and in the dermal appendices in the skin; under the epithelium of the pancreatic acini and ducts; and around the alveoli and bronchi in the lung. In the liver it appeared in a continuous deposit along the sinusoids and surrounding the biliary ducts. In situ hybridizations demonstrated that hepatocytes contain marked amounts of α1(XVIII) collagen chain mRNAs, suggesting that the strong liver expression is mainly due to synthesis of type XVIII collagen by hepatocytes. This finding is in striking contrast with other collagens known to be synthesized in the liver, as they are produced by nonparenchymal cells such as myofibroblasts and endothelial cells. 17,18
In its prominent occurrence at vascular and epithelial BM zones throughout the human fetus and in the adult tissues studied here, type XVIII collagen resembles another widely expressed BM component, type IV collagen, consisting of α1 and α2 chains. 19,20 Thus, type XVIII collagen appears to be a ubiquitous BM component. A new BM collagen chain suggested as possibly identical to type XVIII collagen has recently been identified with a monoclonal antibody, JK-132, originally produced against human type IV collagen. 21,22 Immunohistochemically, this antibody reacted exclusively with the mesangial matrix of the glomeruli in the normal kidney, and it also reacted strongly with the expanded glomerular matrix found in diabetes. 23 In view of the extensive expression of type XVIII collagen in the kidney and the fact that the amino acid sequences of the peptide fragments recognized by JK-132 21 were not found in the collagen sequences, 11 JK-132 appears to recognize a protein that is distinct from type XVIII collagen.
Comparison of the expression of the NC1-493 and NC1-303 variant chains revealed marked differences. The long variant appeared to be virtually the only one present in liver sinusoids and probably around some myofibroblasts in the placental villi, a clearly detectable variant in the epidermal BM and in the glomerular mesangium and glomerular BM during glomerulogenesis, and a minor variant in vessel walls and skeletal muscle. It could not be detected at all in other locations, although several enzymatic pretreatments were performed to minimize the possibility of the epitopes for the human type XVIII-specific antibodies being masked. Thus, the short variant is the one found in most conventional BMs, including those of blood vessels and the various epithelial structures, and around muscular structures. The 192 N-terminal amino acid residues specific to the long variant apparently confer some functional property needed above all in the liver sinusoids, but also at certain other locations. Interestingly, the finding of the long variant around myofibroblasts in the placental villi could signify that the extensive N terminus has some cell-associated functions. The possible importance of the noncollagenous portions of type XVIII collagen is not restricted to the variant N terminus; however, because since the 20 kd C-terminal fragment of the NC11 domain specifically inhibits endothelial proliferation and potently inhibits angiogenesis and tumor growth. 12 This finding fits well with the prominent capillary location of type XVIII collagen, suggesting that the C-terminal fragment may represent a source of regulatory activity released by proteolytic cleavage of the intact molecule under conditions of induced angiogenesis. Nevertheless, the prominent synthesis of type XVIII collagen by hepatocytes also suggests the possibility that it may be secreted into the blood and recruited and activated at sites undergoing angiogenesis as needed.
The homologous collagen types XVIII and XV show both similarities and differences in their tissue distribution. Both are found conspicuously around blood vessels and at the epidermal BM. Type XV collagen is not found as widely in subepithelial BMs as type XVIII, 7,24 however, and it is virtually lacking in the fetal liver and has a more restricted distribution in the fetal kidney and lung. On the other hand, type XVIII collagen occurs less prominently around skeletal muscle cells and, in contrast to type XV, is virtually absent from the fibrillar collagen matrix. Thus, even though the tissue distributions of the two homologous collagens are partially overlapping, their roles in tissues may be characterized by certain distinctive features.
Acknowledgments
We thank Jaana Väisänen, Annikki Huhtela, and Riitta Karvonen for their expert technical assistance; Pasi Hägg and Timo Väisänen for the generous gift of the fetal tissues used in immunofluorescence analysis; and Prof. Kari Alitalo (University of Helsinki, Finland) for the gift of antibody to FLT4.
Footnotes
Address reprint requests to Dr. Taina Pihlajaniemi, Department of Medical Biochemistry, University of Oulu, Kajaanintie 52 A, 90220 Finland. E-mail: taina.pihlajaniemi@oulu.fi.
Supported by grants from the Health Sciences Council of the Academy of Finland, the Sigrid Juselius Foundation, the Finnish Medical Society Duodecim, and FibroGen Inc. (South San Francisco, CA).
References
- 1.Abe N, Muragaki Y, Yoshioka H, Inohue H, Ninomiya Y: Identification of a novel collagen chain represented by extensive interruptions in the triple-helical region. Biochem Biophys Res Commun 1993, 196:576-582 [DOI] [PubMed] [Google Scholar]
- 2.Oh SP, Kamagata Y, Muragaki Y, Timmons S, Ooshima A, Olsen BR: Isolation and sequencing of cDNAs for proteins with multiple domains of Gly-Xaa-Yaa repeats identify a distinct family of collagenous proteins. Proc Natl Acad Sci USA 1994, 91:4229-4233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh SP, Warman ML, Seldin NE, Cheng SD, Knoll JH, Timmons S, Olsen BR: Cloning of cDNA and genomic DNA encoding human type XVIII collagen and localization of the α1(XVIII) collagen gene to mouse chromosome 10 and human chromosome 21. Genomics 1994, 19:494-499 [DOI] [PubMed] [Google Scholar]
- 4.Rehn M, Pihlajaniemi T: α1(XVIII), a collagen chain with frequent interruptions in the collagenous sequence, a distinct tissue distribution, and homology with type XV collagen. Proc Natl Acad Sci USA 1994, 91:4234-4238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rehn M, Hintikka E, Pihlajaniemi T: Primary structure of the α1 chain of mouse type XVIII collagen, partial structure of the corresponding gene, and comparison of the α1(XVIII) chain with its homologue α1(XV) collagen chain. J Biol Chem 1994, 269:13929-13935 [PubMed] [Google Scholar]
- 6.Myers JC, Kivirikko S, Gordon MK, Dion AS, Pihlajaniemi T: Identification of a previously unknown human collagen chain, α1(XV), characterized by extensive interruptions in the triple-helical region. Proc Natl Acad Sci USA 1992, 89:10144-10148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hägg PM, Hägg PO, Peltonen S, Autio-Harmainen H, Pihlajaniemi T: Location of type XV collagen in human tissues and its accumulation in the interstitial matrix of the fibrotic kidney. Am J Pathol 1997, 150:2075-2086 [PMC free article] [PubMed] [Google Scholar]
- 8.Rehn M, Pihlajaniemi T: Identification of three N-terminal ends of type XVIII collagen and tissue-specific differences in the expression of the corresponding transcripts. J Biol Chem 1995, 270:4705-4711 [DOI] [PubMed] [Google Scholar]
- 9.Muragaki Y, Timmons S, Griffith CM, Oh SP, Fadel B, Quertermous T, Olsen BR: Mouse Col18a1 is expressed in a tissue-specific manner as three alternative variants and is localized in basement membrane zones. Proc Natl Acad Sci USA 1995, 92:8763-8767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R: A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature 1996, 382:225-230 [DOI] [PubMed] [Google Scholar]
- 11.Saarela J, Ylikärppä R, Rehn M, Purmonen S, Pihlajaniemi T: Complete primary structure of two variant forms of human type XVIII collagen and tissue-specific differences in the expression of the corresponding transcripts. Matrix Biol 1997, 16:319-328 [DOI] [PubMed] [Google Scholar]
- 12.O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J: Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 1997, 88:277-285 [DOI] [PubMed] [Google Scholar]
- 13.Hogan B, Constantini F, Lacy E: Manipulating the Mouse Embryo: A Laboratory Manual. 1986. Cold Spring Laboratory Press, New York
- 14.Hoeffler H, Childers H, Montminy MR, Lechan RM, Goodman RH, Wolfe HJ: In situ hybridization methods for detection of somatostatin mRNA in tissue sections using antisense RNA probes. Histochem J 1986, 18:597-604 [DOI] [PubMed] [Google Scholar]
- 15.Kivirikko S, Saarela J, Myers JC, Autio-Harmainen H, Pihlajaniemi T: Distribution of type XV collagen transcripts in human tissues and their production by muscle cells and fibroblasts. Am J Pathol 1995, 147:1500-1509 [PMC free article] [PubMed] [Google Scholar]
- 16.Hascall VC, Yanagishita M, Calabro A, Midura R, Rada JA, Chakravarti S, Hassell JR: Isolation and characterization of proteoglycan core protein. Haralson MA Hassell JR eds. Extracellular Matrix: A Practical Approach. 1995, :pp 221-239 IRL Press, Oxford, [Google Scholar]
- 17.Clement B, Grimaud JA, Campion JP, Deugnier Y, Guillouzo A: Cell types involved in collagen and fibronectin production in normal and fibrotic human liver. Hepatology 1986, 6:225-234 [DOI] [PubMed] [Google Scholar]
- 18.Maher JJ, McGuire RF: Extracellular matrix gene expression increases preferentially in rat lipocytes and sinusoidal endothelial cells during hepatic fibrosis in vivo. J Clin Invest 1990, 86:1641-1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez-Hernandez A, Amenta PS: The basement membrane in pathology. Lab Invest 1983, 48:656-677 [PubMed] [Google Scholar]
- 20.Tryggvason K: Molecular Pathology and Genetics of Alport Syndrome: Contributions to Nephrology. 1996. Karger, Basel
- 21.Kino J, Adachi E, Yoshida T, Asamatsu C, Nakajima K, Yamamoto K, Hayashi T: A novel chain of basement membrane-associated collagen as revealed by biochemical and immunohistochemical characterizations of the epitope recognized by a monoclonal antibody against human placenta basement membrane collagen. Am J Pathol 1991, 138:911-920 [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi S, Sasaki T, Takeda Y, Imamura Y, Sado Y, Hayashi T: Possible production of type XVIII collagen by fetal lung fibroblast. Matrix Biol 1996, 15:158-159 [Google Scholar]
- 23.Makino H, Shikata K, Hayashi T, Wieslander J, Haramoto T, Hirata K, Wada J, Yoshida T, Yoshioka K, Ota Z: Immunoreactivity of the JK-132 monoclonal antibody directed against basement membrane collagen in normal and diabetic glomeruli. Virchows Arch 1994, 424:235-241 [DOI] [PubMed] [Google Scholar]
- 24.Myers JC, Dion AS, Abraham V, Amenta PS: Type XV collagen exhibits a widespread distribution in human tissues but a distinct localization in basement membrane zones. Cell Tissue Res 1996, 286:493-505 [DOI] [PubMed] [Google Scholar]