Abstract
To examine the role of tumor necrosis factor (TNF)-α in the pathogenesis of degenerative disorders of the central nervous system (CNS), transgenic mice were developed in which expression of murine TNF-α was targeted to astrocytes using a glial fibrillary acidic protein (GFAP)-TNF-α fusion gene. In two independent GFAP-TNFα transgenic lines (termed GT-8 or GT-2) adult (>4 months of age) animals developed a progressive ataxia (GT-8) or total paralysis affecting the lower body (GT-2). Symptomatic mice had prominent meningoencephalitis (GT-8) or encephalomyelitis (GT-2) in which large numbers of B cells and CD4+ and CD8+ T cells accumulated at predominantly perivascular sites. The majority of these lymphocytes displayed a memory cell phenotype (CD44high, CD62Llow, CD25−) and expressed an early activation marker (CD69). Parenchymal lesions contained mostly CD45+ high, MHC class II+, and Mac-1+ cells of the macrophage microglial lineage with lower numbers of neutrophils and few CD4+ and CD8+ T cells. Cerebral expression of the cellular adhesion molecules ICAM-1, VCAM-1, and MAdCAM as well as a number of α- and β-chemokines was induced or up-regulated and preceded the development of inflammation, suggesting an important signaling role for these molecules in the CNS leukocyte migration. Degenerative changes in the CNS of the GFAP-TNFα mice paralleled the development of the inflammatory lesions and included primary and secondary demyelination and neurodegeneration. Disease exacerbation with more extensive inflammatory lesions that contained activated cells of the macrophage/microglial lineage occurred in GFAP-TNFα mice with severe combined immune deficiency. Thus, persistent astrocyte expression of murine TNF-α in the CNS induces a late-onset chronic inflammatory encephalopathy in which macrophage/microglial cells but not lymphocytes play a central role in mediating injury.
Tumor necrosis factor (TNF)-α is a multifunctional pro-inflammatory cytokine pivotal in the regulation of the host response during infection and inflammation and is also implicated in the pathogenesis of many autoimmune diseases. 1-3 A convincing body of evidence implicates TNF-α in the pathogenesis of inflammation in the central nervous system (CNS), including multiple sclerosis (MS), 4,5 stroke, 6 and infectious diseases ranging from cerebral malaria 7 and bacterial meningitis 8 to HIV encephalopathy. 9 The sources for TNF-α production in these pathological states may be quite varied, and in addition to infiltrating leukocytes such as macrophages and T cells, significant local production of this cytokine may also come from astrocytes, 10,11 microglia, 12,13 and possibly neurons. 14
Although it is clear that TNF-α is present in the CNS during various insults and that resident neural cells can express TNF-α providing a local source of production for this cytokine, divergent views (see, for example, Refs. 15 and 16 ) have evolved as to the role of TNF-α in these pathological states with experimental evidence supporting both detrimental and protective functions. Various manipulations that reduce TNF-α levels, including administration of anti-TNF-α neutralizing antibodies or a soluble TNF type I receptor protein or treatment with the TNF-suppressing drug Rolipram, ameliorate or even prevent experimental autoimmune encephalomyelitis (EAE) 17-20 or cerebral malaria 21,22 in mice. Intracisternal injection of TNF-α promotes a vigorous inflammatory response with infiltration of the meninges and ventricles with large numbers of leukocytes. 23 Finally, numerous in vitro studies also support the notion that TNF-α plays a central role in the evolution of neuroinflammation and as well may contribute directly to degenerative CNS disease. In particular, TNF-α is a potent inducer of both cellular adhesion molecule expression by cerebrovascular endothelial cells 24 and astrocytes 25 and chemokine expression by microglia 26,27 and astrocytes 27,28 and promotes demyelination and oligodendrocyte injury. 29,30
Against this body of evidence for pro-inflammatory and harmful effects of TNF-α, recent studies in gene knockout mice deficient for the TNF genes or their corresponding TNF receptors have provided evidence for an alternative, possible anti-inflammatory or protective function of this cytokine in CNS disease. Mice deficient for TNF-α 31 or TNF-α and TNF-β 32 were not only found to be susceptible to the development of EAE but also invariably exhibited a more severe and protracted form of the inflammatory demyelinating disease. In a similar vein, the neuronal injury caused by cerebral ischemia or excitotoxic amino acids was found to be exacerbated in mice deficient for both the p55 and p75 TNF receptors. 33 However, not all studies using knockout mice support a beneficial action of TNF-α in CNS disease; for example, mice deficient for both TNF-α and TNF-β are resistant to cerebral malaria and show a marked reduction in CNS inflammation. 34 It should be noted, as has recently been outlined, 16 that experiments using the current generation of gene knockout mice to understand the role of cytokines such as TNF-α in disease are confounded by a number of issues that warrant caution in interpreting the results.
Transgenic mice with CNS-targeted expression of cytokines offer an alternative approach to gene knockout animals for the study of cytokine functions in the intact CNS. 35 In the case of TNF-α, development of transgenic mice that express either murine TNF-α from its own promoter with expression apparently in neurons 36 or human TNF-α from the GFAP promoter with expression in astrocytes 37 was reported by the same laboratory. In both cases, transgenic animals appear to have had very high TNF-α expression in the brain and exhibited at an early age severe and invariably lethal neurological deficits in association with extensive CNS inflammation and degenerative pathology. In addition to the possible adverse impact of TNF-α on the normal development of the CNS in these transgenic mice, the severe neurological disorder exhibited by these animals necessitated intervening actions to suppress TNF-α levels to be able to breed transgenic mice for further study. In a separate study, Taupin and colleagues reported that transgenic mice with oligodendrocyte-targeted expression of TNF-α driven by the myelin basic protein promoter failed to develop spontaneous disease but developed more severe disease when induced with EAE. 38 Although in this latter report brain levels of transgene encoded TNF-α were shown to be similar to those found in mice with EAE, the targeted expression of this cytokine to oligodendrocytes could be problematic as these cells are not known to produce this cytokine. In addition, oligodendrocytes may be susceptible to nonspecific injury resulting from transgene expression per se. 39
With the aim of addressing the issue of the role of TNF-α in the initiation and perpetuation of disease in the adult CNS, we set out here to generate transgenic mice (termed GFAP-TNFα) with expression of this cytokine targeted to a cell (ie, the astrocyte) that is known to produce TNF-α 10,11 at levels that did not compromise the development and breeding viability of the animal. To this end we describe two independent lines of transgenic mice that exhibit no detectable spontaneous disease until greater than 4 months of age, after which time a progressive and eventually fatal neurological disorder ensues. On further characterization, florid meningoencephalomyelitis and degenerative disease was found in the CNS of symptomatic GFAP-TNFα mice. Interestingly, disease exacerbation with more extensive lesions that contained activated cells of the macrophage/microglial lineage occurred in GFAP-TNFα mice with severe combined immune deficiency. Thus, persistent astrocyte expression of murine TNF-α in the CNS is sufficient to induce a chronic inflammatory encephalopathy in which macrophage/microglial cells but not lymphocytes play a central role in mediating injury.
Materials and Methods
Mice
C57BL/6J × SJL F2 mice used for the development of the transgenic lines and Balb/cByJscid/scid (SCID) mice were obtained from Jackson Laboratories (Bar Harbor, ME). For the breeding of GFAP-TNFαscid/scid mice, transgenic mice of the GT-2 line (see below) were mated with Balb/cByJscid/scid mice. GFAP-TNFα × Balb/cByJscid/+ F1 mice were backcrossed with Balb/cByJscid/scid mice, and SCID offspring were identified by analysis of plasma IgG levels using an immunodiffusion assay kit (ICN, Costa Mesa, CA). All mice were maintained in specific-pathogen-free conditions in the closed breeding colony of the Scripps Research Institute.
Generation of Transgenic Mice
The CNS- and astrocyte-specific expression obtained for fusion gene constructs under the control of the murine GFAP promoter is well documented. 35,40 To obtain astrocyte expression of murine TNF-α, a modified strategy was used to that previously used for the generation of the GFAP-IL6 41 and GFAP-IL3 42 transgenic mice. Briefly, a 2.2-kb Sfi-1/Not-1 fragment containing the murine GFAP promoter and an SV-40 small intron was excised from the GFAP-IL3 plasmid construct 42 and cloned into the vector pGEMEX-1 (Promega, Madison, WI) to generate the construct pGF. Next, a 0.65-kb Sma-1/EcoRV fragment containing the human growth hormone (hGH) polyadenylation signal sequence was cut from the plasmid pIC-hGH (kindly provided by Dr. Jan Allison, Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia) and ligated into the EcoRV site of pGF to generate the construct pGF.GH. Finally, a 1.8-kb EcoN1/EcoRI genomic DNA (a genomic clone containing the entire murine TNF-α and TNF-β gene locus was kindly provided by Dr. Beutler, Southwestern Medical Center, University of Texas) fragment spanning the coding region of murine TNF-α gene was modified by adding Not-1 linkers and was then ligated into the Not-1 site in pGF.GH. After screening for correct orientation, the GFAP-TNFα fusion gene fragment was excised with Sfi-1/EcoRI and purified from plasmid DNA before microinjection into fertilized eggs of C57BL/6J × SJL F2 hybrid mice. Transgenic offspring were identified by slot blot analysis of tail DNA using a 32P-labeled random primer hGH DNA fragment as a probe.
RNA Preparation
Transgenic or control mice were killed, and the organs were then removed and immediately snap frozen in liquid nitrogen. Samples were stored at −70°C pending RNA preparation. Total RNA was extracted with TRIZOL reagent (Gibco-BRL, Grand Island, NY) used according to the manufacturer’s instructions. The concentration of RNA was determined by ultraviolet spectroscopy at 260 nm.
RNase Protection Assay (RPA)
The production and characterization of the multiprobe RPA probe sets used for the detection of cytokine or chemokine gene expression have been described previously. 43,44 To distinguish between transgene-encoded TNF-α (tgTNF) mRNA and the endogenously transcribed TNF-α (eTNF) mRNA, RPA probes were constructed that targeted the hGH sequence (to detect tgTNF) (GenBank accession number M13438; nucleotides (nt) 2453 to 2653) or the TNF-α 3′ untranslated region (UTR; to detect eTNF) (GenBank accession number M11731; nt 1110 to 1357). In addition, to detect total TNF-α mRNA, a previously described 43 RPA probe was used. In all cases, a fragment of the RPL32-4A gene 45 served as an internal loading control. RPAs were performed as described previously. 46
Routine Histology, in Situ Hybridization, and Immunohistochemistry
For routine histology, organs were removed, fixed in 4% buffered paraformaldehyde, and embedded in paraffin for hematoxylin and eosin or luxol fast blue staining, immunohistochemistry, and in situ hybridization. In addition, some fixed brain and spinal cord specimens were transferred to 20% sucrose in PBS and stored overnight at 4°C. After cryoprotection, the tissue was then snap frozen in liquid nitrogen and 10-μm cryostat sections prepared for oil red O staining to visualize neutral fat. Briefly, stock oil red O (Sigma Chemical Co., St. Louis, MO) was prepared at 3 mg/ml in 99% isopropanol. Immediately before use, stock was diluted 1:4 in distilled H2O to obtain a working solution. Sections were stained for 15 minutes in working solution, counterstained for 1 minute in Ehrlichs hematoxylin, rinsed in tap water, coverslipped using an aqueous mounting medium (Aquamount, Lerner Laboratories, Pittsburgh, PA), and immediately inspected by light microscopy.
For in situ hybridization, the TNF-α probe used was 35S-labeled, single-stranded antisense or sense RNA to murine TNF-α. For probe synthesis, a cDNA for murine TNF-α (kindly provided by Genentech, South San Francisco, CA) was subcloned into pBluescript-KS (Stratagene, La Jolla, CA) and antisense or sense RNAs synthesized using T7 or T3 RNA polymerase, respectively. In situ hybridization was performed as described by Simmons et al 47 with modifications. Briefly, paraffin sections were deparaffinized and rehydrated in graded alcohols. After post-fixation in 4% formaldehyde, proteinase K treatment (2.4 mg/100 ml of 5X TE buffer at 37°C for 15 minutes) and acetylation (250 μl of acetic anhydride in 100 ml of PBS for 10 minutes), the slides were dehydrated in graded alcohol and dried. The 35S-labeled sense or antisense probes were hybridized to the tissue overnight at 56°C. After digestion with RNAse A (Promega), slides were washed in decreasing concentrations of SSC buffer. After the last SSC step, slides were blocked with serum (Vector Laboratories, Burlingame, CA) and immunohistochemistry was performed where applicable. The primary antibody against GFAP (Dako, Carpinteria, CA) was incubated overnight at 4°C. Antibody-labeled cells were detected using a Vectastain kit (Vector) according to the manufacturer’s instructions. After dehydration in graded alcohol, slides were air dried and exposed for 5 days to Cronex film (DuPont, Wilmington, DE). Slides were then dipped in Kodak NTB-2 emulsion, dried, and stored in the dark for 2 weeks. Subsequently, slides were developed, counterstained with Mayer’s hematoxylin, and examined by bright field microscopy.
Immunohistochemistry for the detection of TNF-α protein was performed on paraformaldehyde-fixed, paraffin-embedded sections. After dewaxing and rehydration, slides were incubated in 1.5% normal goat serum (Vector) in PBS containing 0.1% Saponin (Sigma) for 30 minutes. After blocking, slides were incubated overnight at room temperature with a polyclonal rabbit anti-murine TNF-α antibody (Genzyme, Cambridge, MA) diluted 1:1000 in the blocking buffer. All following steps were performed using the Vectastain kit (Vector) according to the manufacturer’s instructions.
For immunophenotyping and cellular adhesion molecule immunostaining, mice were killed and organs were removed and immediately snap frozen in isopentane and stored at −70°C until sectioning. Sagittal cryomicrotome cut sections of 10 μm were air dried and either stored dehydrated at −70°C or directly processed. Immediately before staining, tissue sections were fixed in cold (−20°C) acetone/methanol (1:1) for 45 seconds and nonspecific binding was blocked by incubating sections for 30 minutes in PBS containing 3% rabbit and 3% goat serum. Sections were then incubated for 2 hours at room temperature in rat monoclonal antibodies to identify leukocytes (CD45 from Pharmingen, San Diego, CA), lymphocytes (CD4, CD8, and B220 from Pharmingen), NK cells (DX5; Pharmingen), neutrophils (MCA771; Serotec, Raleigh, NC), activation markers (MHC class II, clone M5/114 and Mac-1, and clone TIB 126 from American Type Culture Collection, Rockville, MD), and vascular or cellular adhesion molecules (MAdCAM, VCAM-1, and Endoglin from Pharmingen and ICAM-1 clone YN11.1 kindly provided by Dr. F. Takei, Toronto, Canada). All antibodies were used at a final concentration of 5 μg/ml diluted in the blocking buffer. Bound antibody was detected using a biotinylated anti-rat antibody (Southern Biotechnology Associates, Birmingham, AL) followed by avidin-labeled horseradish peroxidase (Sigma). Staining used 3′,3′-diaminobenzidine (Sigma) as substrate. Before mounting, sections were counterstained with Mayer’s hematoxylin and dehydrated in graded ethanols.
Quantitative Neuropathological Assessment
Briefly, as previously described, 41,48 animals were perfused with cold 4% paraformaldehyde in pH 7.4 PBS. The right hemibrain was serially sectioned at 40 μm of thickness with the Vibratome 2000 (Leica) for subsequent immunocytochemical/computer-aided image analysis. The left hemibrain was embedded in paraffin and serially sectioned for studies of DNA fragmentation. To determine neuronal integrity, blind-coded vibratome sections were immunolabeled with antibodies against the dendritic marker MAP-2 (mouse monoclonal antibody, diluted 1:1000; Boehringer Mannheim, Indianapolis, IN) and against the marker for interneurons, parvalbumin (mouse monoclonal, diluted 1:1000; Sigma). For MAP-2, after overnight incubation, sections were incubated with the fluorescein isothiocyanate (FITC)-conjugated horse anti-mouse IgG (Vector). The sections were then transferred to SuperFrost slides (Fisher Scientific, Tustin, CA), mounted under glass coverslips with anti-fading media (Vector), and analyzed with the MRC1024 LSCM (Bio-Rad, Richmond, CA). 49 The percent area of the neuropil covered by MAP-2-immunoreactive dendrites was performed with the IMAGE software, as previously described. 48 Anti-parvalbumin-immunostained cells were counted in 10 consecutive fields (0.1 mm; two each) along the side of the gyrus using a 40× objective and a gridded 10× eyepiece lens.
Cells undergoing DNA fragmentation were identified by using a commercially available kit (Apoptag, Oncor, Gaithersburg, MD) or a modified version of the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-biotin nick-end labeling method of Gavriely. 50 The main modification was the absence of tissue pretreatment with H2O2 (thus preventing nonspecific DNA fragmentation) and a slight difference in the ratio of TdT to biotinylated dUTP to reduce background. For this purpose, paraffin sections were dewaxed and rehydrated, washed in Tris-buffered saline, and treated with proteinase K in 5X TE buffer (at room temperature; 10 μg/ml for 10 minutes), followed by a 1-hour incubation at 37°C in a solution containing TdT (0.3 enzyme units/μl) and biotin-16-dUTP (0.02 nM/μl; Boehringer) in 1X TdT buffer with cobalt chloride. After this incubation, sections were washed in PBS and incubated for 30 minutes at room temperature in avidin-horseradish peroxidase complex (ABC Elite, Vector) followed by diaminobenzidine (0.05%). Paraffin sections from the adult rat mammary gland processed in parallel served as a positive control. Negative control experiments consisted of processing sections either in the absence of TdT or dUTP. Labeled sections were analyzed with an image analysis apparatus to determine the numbers of TdT-positive cells per unit area.
Ultrastructural Assessment
Unless stated otherwise, morphological examination of the brain and spinal cord was performed on symptomatic GFAP-TNFα mice or age-matched normal littermates. Before killing, animals were anesthetized with intraperitoneal injection (2 ml/kg) of a solution consisting of pentobarbital (12.5 mg/ml) and diazepam (12.5 mg/ml) in 0.9% NaCl. The anesthetized animals were perfused intracardially with a solution of 5% phosphate-buffered glutaraldehyde. The brain and spinal cord were then removed, and after overnight fixation at 4°C, tissues were post-fixed in 1% aqueous osmium tetroxide solution for 2 hours, dehydrated using graded alcohols and polyphylene oxide, and infiltrated with resin. Overnight infiltration was followed by embedding in fresh araldite resin. Thick sections (1 μm) were cut with glass knives and stained with paraphenylenediamine (PPD) or methylene blue azure II in preparation for light microscopy. Ultra-thin sections from selected blocks were cut with a diamond knife and stained with uranyl acetate and lead citrate before electron microscopic examination.
Leukocyte Isolation
Leukocytes were isolated as described 51,52 with minor modifications. Briefly, mice were killed by halothane inhalation and perfused with 20 ml of PBS unless stated otherwise. Brains were rapidly removed and mechanically dissociated by sequentially forcing the tissue through 210- and 70-μm Nitex meshes. The cell suspension was enzymatically digested with DNase I (28 U/ml) and collagenase (0.2 mg/ml) for 1 hour at 37°C in a shaking incubator in Hanks’ balanced salt solution without serum. After quenching the digestion with the addition of 10% fetal bovine serum (final concentration), the cell suspension was separated on a discontinuous 1.033/1.088/1.122 Percoll gradient. Leukocytes were collected from the interfaces and the 1.033 Percoll fraction. Myelin and cell debris separated above the gradient.
Flow Cytometry
Biotin-conjugated, FITC-conjugated, and phycoerythrin-conjugated antibodies against mouse CD4, CD8, CD25, CD44, CD45, B220, CD62 (Mel-14), and CD69 (Pharmingen), and MHC class II (M5/114, ATCC) were reacted with the cells isolated from the Percoll gradient or lymph node cell suspensions. The cells were then analyzed with a FACScan by using Cell Quest acquisition software (Becton Dickinson, Mountain View, CA).
Results
Generation and Characterization of GFAP-TNFα Transgenic Mice
From the initial founder generation, five mice were identified as positive for transgene integration. By postnatal day 14, two founder animals appeared runted, developed severe motor impairment, and died between days 21 and 32. Subsequent analysis of the brain from one of these mice revealed high expression of TNF-α in association with extensive and severe encephalomyelitis (not shown). Of the remaining founders, all were bred successfully, and analysis of transgene expression confirmed TNF-α expression in the CNS of offspring derived from two of the three founders. For further study, stable transgene-expressing lines termed GT-2 and GT-8 were then established from the two transgene-expressing founders. Mice from the GT-2 line developed paraparesis from 4 months of age or older and progressed to total hind limb paralysis and premature death around 2 months after the onset of paraparesis. Mice from the GT-8 line had a different physical presentation and showed symptoms of ataxia from 6 months of age or older, the severity of which increased progressively, leading to death of the animal by approximately 12 months of age. After the onset of motor impairment, mice from both lines displayed muscle spasms and became progressively cachectic.
To determine the expression characteristics of the transgene-encoded product versus the endogenous TNF-α gene, an RPA was developed that permitted distinction between the TNF-α transcript, the TNF-α 3′ UTR encoded by the endogenous TNF gene, and the 3′ hGH region encoded by the transgene. A representative assay is shown in Figure 1A ▶ . There was little detectable expression of the TNF-α gene in the CNS tissue of wild-type controls. However, TNF-α mRNA was found to be expressed at elevated levels in the forebrain, cerebellum, spinal cord, and eye from mice of both transgenic lines. In mice of the GT-2 line, the highest level of TNF-α mRNA expression was found in the spinal cord (Figure 1A) ▶ , whereas in the GT-8 line, the expression of TNF-α mRNA was highest in the cerebellum (data not shown). Consistent with the production of transgene-encoded TNF-α, in mice from both transgenic lines, a similar pattern of expression was observed in these CNS regions for the hGH transcript with the highest expression of the hGH mRNA detectable in the spinal cord of symptomatic GT-2 animals (Figure 1A) ▶ and cerebellum of GT-8 mice (not shown). Although not as high as the expression for the hGH mRNA, elevated expression of the TNF-α 3′ UTR transcript was clearly detectable in these same CNS regions, indicating there was also activation of the endogenous TNF-α gene. A survey of a number of peripheral organs revealed that in wild-type mice TNF-α and 3′ UTR but not hGH transcript was constitutively expressed in spleen, lung, heart, and thymus (Figure 1A) ▶ . In the transgenic mice, increased expression of the TNF-α and 3′ UTR transcript was detectable in the muscle, spleen, kidney, heart, lung, and thymus. A somewhat lower level of hGH mRNA was observed in the lung, heart, and thymus, particularly in symptomatic mice. Thus, there was some leakage of the transgene GFAP promoter expression in the periphery of symptomatic mice, however, and in contrast to the CNS, the elevated expression of TNF-α mRNA seen in these peripheral organs was due mostly to increased activation of the endogenous gene.
Figure 1.
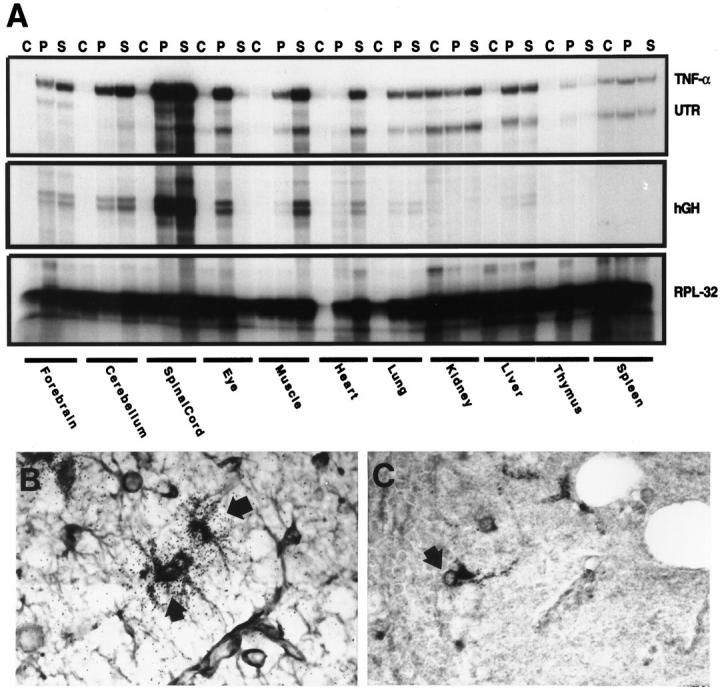
Analysis of TNF-α expression. A: Transgene versus endogenous TNF-α expression was distinguished using target-specific probes generated as described in Materials and Methods. Organs were removed from control mice (C) or presymptomatic (P) and symptomatic (S) GT-2 mice. Total RNA (10 μg) was then used for analysis by RPA. B: In situ hybridization and immunohistochemistry was performed as described in Materials and Methods. In situ hybridization was used to detect TNF-α and combined with immunohistochemical staining for GFAP. Co-localization of TNF-α RNA and GFAP protein is shown for astrocytes (arrows) in the cerebellum of a GT-8 transgenic mouse. Original magnification, ×500. C: Immunohistochemical stain for TNF-α demonstrates expression of this protein in cells (arrow) with astrocyte morphology in the cerebellum of a GT-8 transgenic mouse. Original magnification, ×500.
In the presymptomatic GT-2 and GT-8 animals, the astrocyte-specific expression of TNF-α in the brain was demonstrated using a combination of immunohistochemistry for GFAP and in situ hybridization for TNF-α RNA. Cells strongly positive for GFAP as well as for TNF-α RNA were scattered predominantly throughout the spinal cord and to a lesser extent the cerebellum and thalamus of GT-2 mice or the cerebellum, hippocampus, and cerebral cortex of GT-8 animals (Figure 1B) ▶ . TNF-α mRNA expression was not detectable by this method in CNS tissue of wild-type mice. By immunohistochemical staining, cells immunopositive for TNF-α with the morphological appearance of astrocytes were observed with a similar distribution as astrocytes expressing TNF-α RNA (as detected by in situ hybridization above) in the CNS of mice from both the GT-2 and GT-8 lines (Figure 1C) ▶ . No cells immunopositive for TNF-α were observed in the CNS tissue of wild-type controls (not shown).
Pathological Alterations in the CNS of Symptomatic but Not Asymptomatic GFAP-TNFα Mice
In mice of both the GT-2 (1 to 3 months of age) and GT-8 (1 to 6 months of age) lines, before the onset of physical signs, light microscopic examination of the brain and spinal cord failed to reveal any overt pathological alterations compared with wild-type controls. However, in symptomatic transgenic mice, a most striking finding was the presence of large areas of dense mononuclear cell infiltration at meningeal, perivascular, and parenchymal locations (Figure 2) ▶ . These infiltrates varied in intensity throughout the brain, being minimal in cortical regions to particularly florid in the spinal cord (Figure 2E) ▶ or the cerebellum (Figure 2B) ▶ of mice from the GT-2 or GT-8 lines, respectively. The density of mononuclear cell infiltration therefore coincided with the levels of TNF-α expressed in the various brain regions. Infiltrating cells located at perivascular sites were, on the whole, quite homogeneous with the morphological characteristics of lymphocytes and were often seen to form well organized, follicular-like structures (Figure 2C) ▶ whereas parenchymal lesions were less well organized and appeared to contain a more heterogeneous cell population (Figure 2F) ▶ . In more advanced-stage lesions, areas of hemorrhage were present and indicated vascular damage.
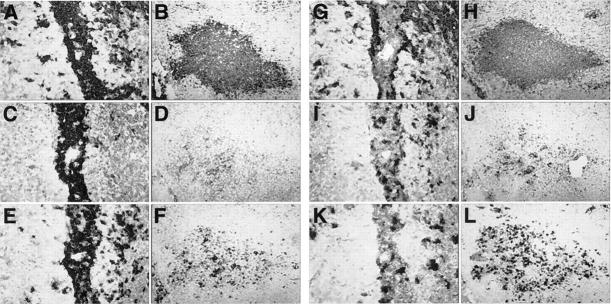
Figure 2.
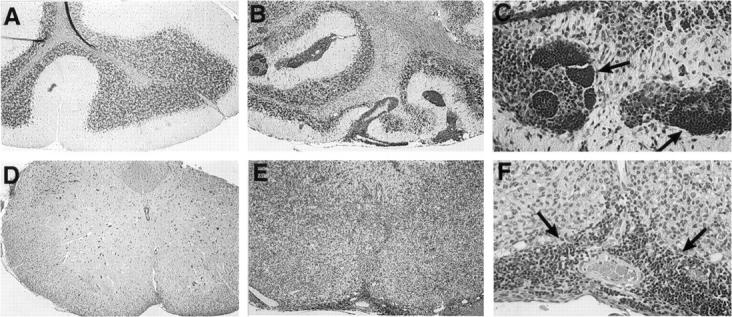
Routine histological analysis of the CNS. Luxol fast blue stains showing normal morphology of cerebellum and spinal cord is shown in A and D, respectively. Original magnification, ×125. In contrast, large areas of perivascular and parenchymal infiltration as seen in cerebellum (B) or spinal cord (E) from symptomatic GT-8 or GT-2 transgenic mice, respectively. Original magnification, ×125. Higher magnification (×500) view of same sections in B and E, showing dense well organized follicular-like structures (C, arrows) and a perivascular infiltrate (F, arrows).
The extent of degenerative CNS disease in the GFAP-TNFα mice was next assessed. In specimens from symptomatic but not presymptomatic mice, luxol fast blue staining revealed areas of marked white matter pallor suggestive of demyelination overlapping with the inflammatory lesions in the spinal cord of GT-2 mice or cerebellum of GT-8 mice (not shown). Compared with wild type (Figure 3, A and C) ▶ , active destruction of myelin was clearly evident in the GFAP-TNFα mice with an overt loss of CNPase immunostaining (Figure 3B) ▶ and the presence of numerous oil-red-O-positive (Figure 3D) ▶ cells in association with the white matter lesions. This was confirmed in plastic sections from tissue prepared for electron microscopy (EM). Both primary demyelination and Wallerian degeneration were identified in areas of active disease (Figure 4) ▶ . In these regions, the normal compact appearance of the white matter (Figure 4A) ▶ was effaced by masses of infiltrating cells (Figure 4, B and C) ▶ . Small groups of demyelinated axons and solitary axonal profiles lacking their myelin sheaths appeared within the inflammatory infiltrate (Figure 4, A–C) ▶ . These groups of contiguous demyelinated axons were more readily identified in electron micrographs from the affected region (Figure 4, D and E) ▶ . Clusters of macrophages were scattered through the spinal cord parenchyma. Many of them were foamy (Figure 4B) ▶ , and ultra-thin sections from such clusters showed lipid droplet accumulation in the vicinity of demyelinated axons (Figure 4E) ▶ . Mast cells were also observed in the inflamed meninges. Greatly swollen axons undergoing Wallerian degeneration were found in regions of the spinal cord compressed by infiltrating cells (Figure 4C) ▶ and distal to the site of inflammation. Inflammation and fibrosis of the spinal meninges and Wallerian degeneration in nerve roots and peripheral nerves was also identified. Axons undergoing Wallerian degeneration were very swollen (Figure 4, B and F) ▶ , and electron microscopy revealed masses of darkly staining organelles, characteristic of that process (Figure 4F) ▶ .
Figure 3.
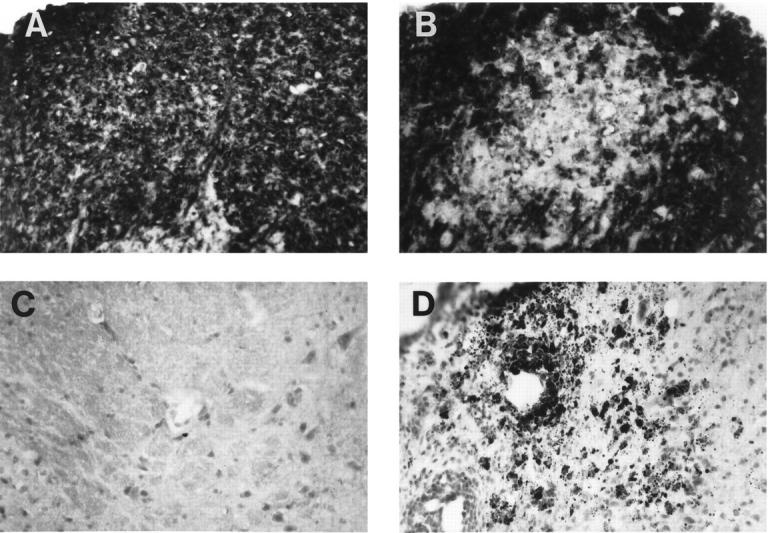
Active demyelination in the spinal cord. A and B: CNPase immunohistochemical stain on control (A) and GT-2 (B) spinal cord sections. Original magnification, ×312. C and D: Oil red O stain and immunohistochemistry was performed as described in Materials and Methods. Oil red O stain of neutral fat in wild-type control (C) and GT-2 (D) spinal cord. Original magnification, ×312.
Figure 4.
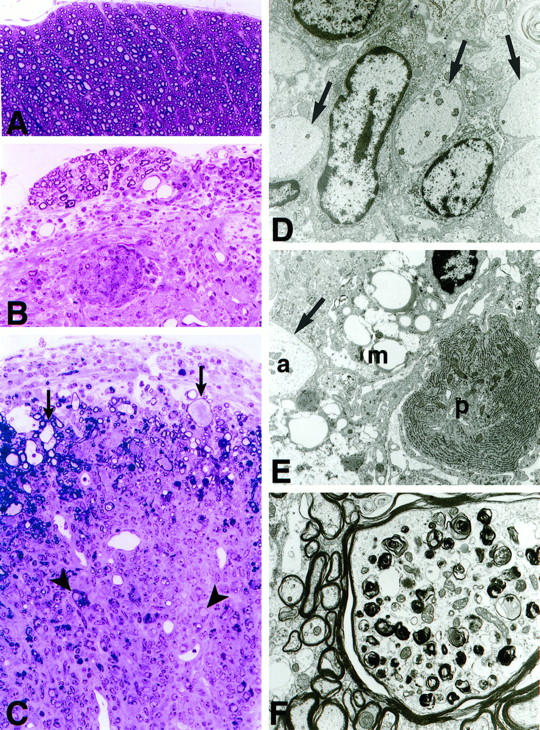
A: Transverse section from the midthoracic spinal cord of a wild-type (control) mouse showing normal white matter. Note the compact appearance of the myelinated fibers and the absence of inflammatory cells from meninges and parenchyma (araldite-embedded 1-μm-thick section stained with methylene blue. Original magnification, ×200. B: Transverse section of spinal cord from a symptomatic GT-2 mouse with inflammatory changes in the meninges and cord parenchyma. A cluster of foamy macrophages is present at the center of the picture. C: Section from a severely affected region in which the meninges are thickened and infiltrated by mononuclear cells. Within the densely infiltrated spinal cord tissue normal structures are effaced and individual demyelinated axons (arrowheads) as well as small groups of demyelinated axons are present. Original magnification, ×200. Note the greatly swollen axonal profiles (arrows) indicating Wallerian degeneration in groups of axons beneath the inflamed meninges at the top of the picture. D: Electron micrograph illustrating primary demyelination in a group of contiguous axons (arrows) from the severely inflamed region. The axoplasm appears quite normal, but the myelin has been completely removed. The nucleated cells may represent activated microglia having an elongated cytoplasm and scanty cytoplasm. Original magnification, ×5000. E: A foamy macrophage (m) packed with lipid droplets appears between a demyelinated axon (a, arrow) and a plasma cell (p) in a severely inflamed region. Original magnification, ×6000. F: A massively swollen axon is present and is surrounded by normal-appearing myelinated fibers. Note the accumulation of darkly staining organelles, including lysozomal profiles, mitochondria, and membranous debris within the affected axon. Original magnification, ×5500.
The extent and natural history of neurodegenerative change in the CNS of the GFAP-TNFα mice was further assessed using quantitative morphometric analysis. The overall integrity of the dendritic complexity was examined by labeling sections with antibodies against MAP-2. Confocal analysis of the sections showed that in presymptomatic transgenic mice there was a little or no detectable alterations in the neocortex, hippocampus, or cerebellum. In contrast, in symptomatic GFAP-TNFα mice moderate to severe loss of dendritic structures was evident both in pyramidal neurons in the hippocampus and neocortex as well as in Purkinje cells in the cerebellum (Figure 5A) ▶ . These neurodegenerative changes were accompanied by significant astrogliosis as revealed by the GFAP antibody (not shown). As neurons in these areas express high levels of calcium-binding proteins and have been shown previously to be lost in human 49 and murine 41 neuroinflammatory disease states, we analyzed the integrity of these neuronal populations with antibodies against parvalbumin. This analysis showed similar numbers of parvalbumin-immunoreactive interneurons in the presymptomatic mice as compared with wild-type controls (Figure 5B ▶ , top panels). In contrast, in the symptomatic mice there was widespread and severe loss of parvalbumin-immunoreactive cells in the neocortex, hippocampus, and cerebellum.
Figure 5.
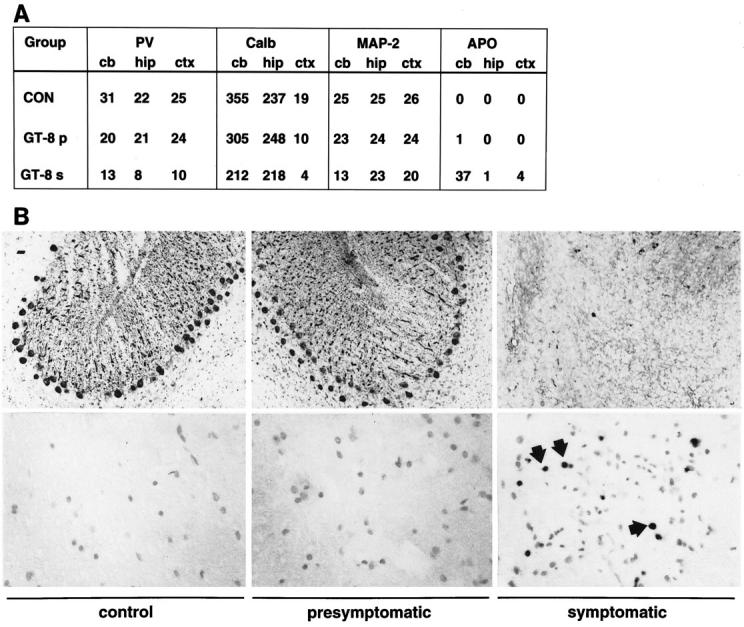
Neurodegenerative changes in the CNS of symptomatic GT-8 transgenic mice. Immunohistochemical stains and their quantification were performed as described in Materials and Methods. A: Quantitative analysis of changes in parvalbumin (PV), calbindin (Calb), MAP-2 levels, and in DNA fragmentation (APO). B, upper panel: Parvalbumin-immunostained sections showing cerebellum from wild-type mice and presymptomatic or symptomatic GT-8 mice Original magnification, ×650. B, lower panel: Analysis of DNA fragmentation by TUNEL stain in the spinal cord from control mice and presymptomatic or symptomatic GT-2 mice. Original magnification, ×640.
TNF-α is known to be a potent inducer of apoptosis, and based on in vitro studies this pathway has been implicated in TNF-α-mediated toxicity to oligodendrocytes. 29,30 To obtain evidence as to whether the astrocyte-targeted expression of TNF-α in vivo was associated with increased apoptosis we analyzed for DNA fragmentation in situ, using TUNEL staining methods. Little or no nuclear staining was evident in any brain region analyzed from wild-type or presymptomatic GFAP-TNFα mice (Figure 5, A and B ▶ , lower panel). In contrast, in symptomatic GFAP-TNFα mice, numerous nuclei positive for TUNEL staining were observed in the CNS inflammatory lesions of GT-8 mice (Figure 5B ▶ , lower panel) or GT-2 mice (not shown), suggesting these were leukocytes undergoing apoptosis.
Immunophenotypic and Spatial Heterogeneity of the CNS-Infiltrating Cells
The phenotypic composition of the inflammatory infiltrates observed in symptomatic mice from both transgenic lines was examined in detail by immunohistochemical staining and revealed differences between the perivascular and parenchymal compartments (Figure 6) ▶ . Thus, the perivascular infiltrates contained mostly lymphocytes of which B220-positive B lymphocytes were abundant and formed dense accumulations that were likely responsible for the follicular-like structure of these infiltrates noted above (Figure 2) ▶ . Both CD4- and CD8-positive T lymphocytes were also prevalent and were scattered throughout the perivascular infiltrates (Figure 6, E and I ▶ ▶ , respectively). Cells strongly positive for Mac-1, and presumed to be activated macrophage/microglia, circumscribed the perivascular lymphocytic infiltrates (Figure 6G) ▶ . In contrast to the perivascular infiltrates, infiltrates in the parenchyma contained mostly CD45-high, Mac-1-positive cells of the macrophage lineage with fewer numbers of both CD4- and CD8-positive T lymphocytes and few B lymphocytes (Figure 6, B, H, F, J, and D) ▶ . Numerous neutrophils were also identified scattered throughout the parenchymal lesions (Figure 6L) ▶ . In addition to the perivascular and parenchymal infiltrates, strong staining for the macrophage marker Mac-1 was observed on ramified cells adjacent to lesion areas and were assumed to represent activated microglia (Figure 6, G and H) ▶ . A similar assessment of CNS tissue from presymptomatic mice of each line failed to detect the presence of increased numbers of leukocytes when compared with wild-type controls (not shown).
Figure 6.
Immunophenotypic characterization of inflammatory lesions in the cerebellum of a symptomatic GT-8 mouse. Immunohistochemistry was performed as described in Materials and Methods. Comparison of a perivascular (A, C, E, G, I, and K) with a parenchymal lesion (B, D, F, H, J, and L) immunostained for CD45 (A and B), B220 (C and D), CD4 (E and F), Mac-1 (G and H), CD8 (I and J), and neutrophils (K and L).
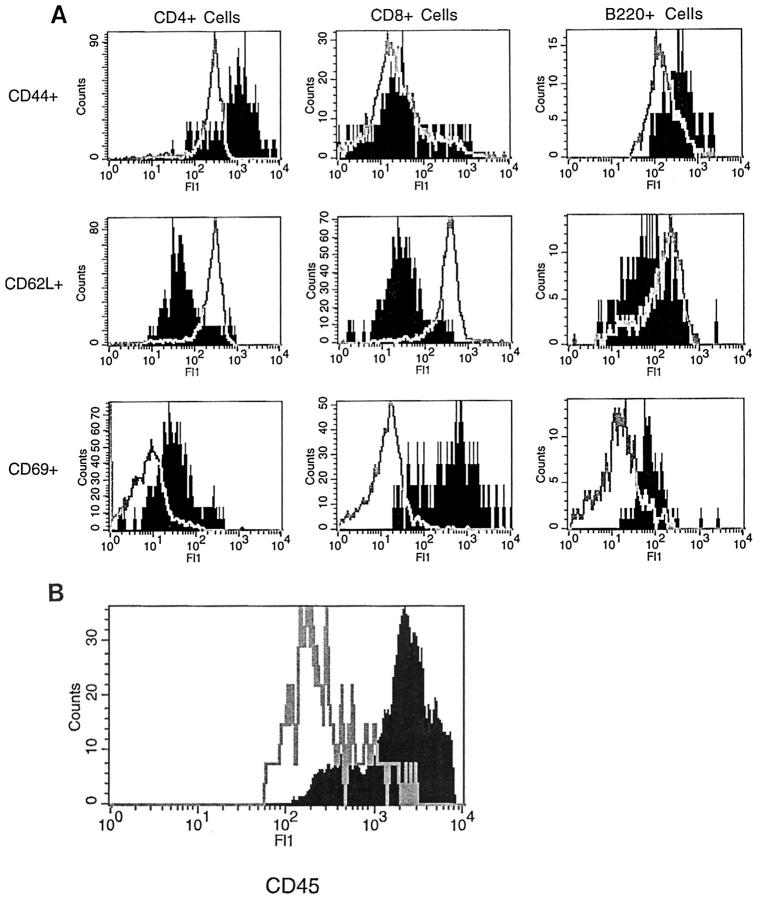
The activation status of the CNS-infiltrating cells in symptomatic mice of the GT-8 line was next examined by flow cytofluorimetric analysis. Both CD4+ and CD8+ T cells in the infiltrating leukocyte population displayed a memory phenotype, expressing high levels of CD44 and low levels of CD62L (MEL-14), as examined by flow cytometry (Figure 7A) ▶ . Lymph node T and B cells were used as markers of CD44low, CD62Lhigh cells. Although all B cells, naive and activated, express relatively high levels of CD44 as compared with T cells, CD44 levels were still increased on B cells from the TNFα mice. Examination of two commonly used markers of acute activation showed an unusual dissociation of CD25 (IL-2 receptor) and CD69 (very early activation antigen) expression. CD25 was virtually absent in the infiltrating leukocyte population (not shown), whereas CD69 expression was elevated in both T and B cells, being the highest in CD8+ cells (Figure 7A) ▶ . Microglia activation was monitored by analysis of levels of CD45 expression. In contrast to all other CD45-expressing cells in adult mice, microglia express low levels in their unactivated state and intermediate levels in their activated state. All other nucleated cells of hemopoietic origin express high levels of CD45. 52,53 In all symptomatic mice (GT-2 and GT-8), there is reduction or loss of the CD45low population and the appearance of CD45intermediate and CD45high populations, indicating both microglial activation and leukocyte infiltration (Figure 7B) ▶ . In early symptomatic mice, this shift in CD45 levels was seen even in the absence of detectable T cell infiltration, suggesting that large scale leukoctye infiltration was secondary to microglia/macrophage activation (data not shown).
Figure 7.
Flow cytofluorimetric analysis of mononuclear cells isolated from the lymph node of wild-type control or spinal cord of symptomatic GT-2 mice. A: Characterization of lymphocyte phenotypes. Expression of CD44, CD62L, and CD69 by CD4+, CD8+, or B220 cells in control (open histogram) or transgenic (solid histogram ) mice. Similar patterns of expression were seen in the cerebellum of symptomatic mice from the GT-8 line. Infiltrating T and B cells were not detected in the CNS of control mice. B: CD45 levels of FcR-positive cells isolated from control (solid histogram) or from transgenic (open histogram) mice. Note that the x axis is in log scale for both A and B.
Cellular Adhesion Molecule and Chemokine Expression Precede Disease Onset
To begin to elucidate the mechanisms by which TNF-α may promote the recruitment of leukocytes to the brain, we examined the expression of a number of cellular adhesion molecules (CAMs), cytokines, and chemokines in CNS specimens from wild-type control and presymptomatic and symptomatic GFAP-TNFα mice from each line.
CAM expression was analyzed by immunohistochemical staining and revealed low levels of ICAM-1 and VCAM-1 expression but no MAdCAM expression by cerebrovascular endothelium in control mice (Figure 8) ▶ . In contrast, in presymptomatic GFAP-TNFα mice, significant up-regulation of ICAM-1 and VCAM-1 expression was observed on large and small cerebrovascular endothelium whereas induction of MAdCAM molecule expression was found to be prominent on small vessel endothelium (Figure 8) ▶ . Expression of these CAMs was further increased in symptomatic GFAP-TNFα mice with ICAM-1 and VCAM-1 also being expressed by some infiltrating leukocytes. Endoglin expression was the same in wild-type and GFAP-TNFα mice.
Figure 8.
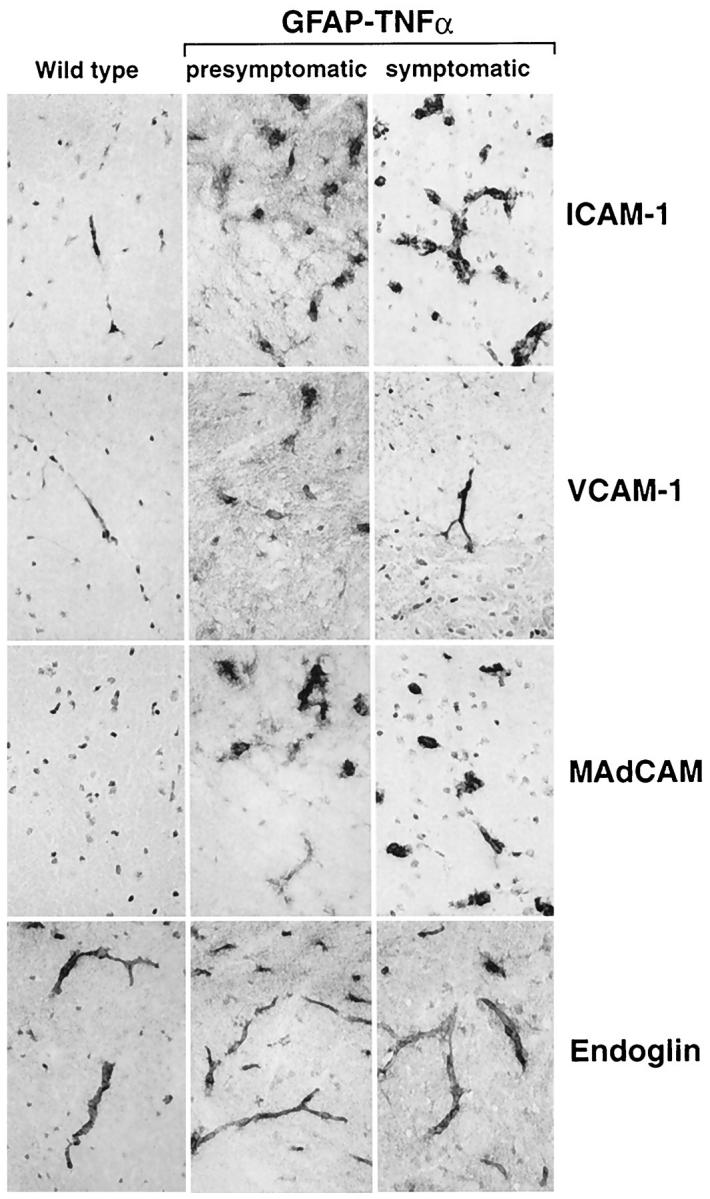
Expression of cellular adhesion molecules in the spinal cord. Frozen sections (10 μm) were prepared and immunostained with specific monoclonal antibodies against murine ICAM-1, VCAM-1, MAdCAM, or endoglin as described in Materials and Methods. All panels are the same original magnification (×500).
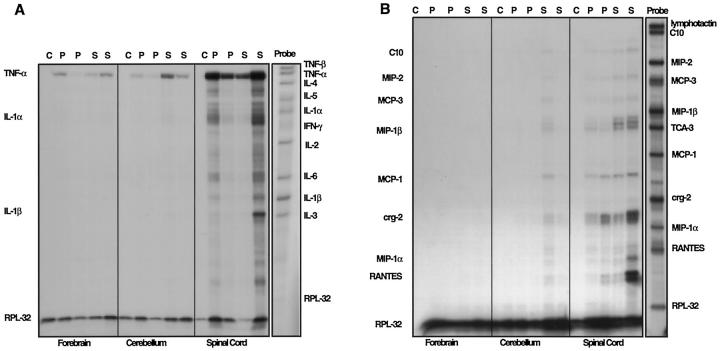
Cytokines and, more particularly, chemokines may also contribute to the recruitment of inflammatory cells into the CNS. In forebrain, cerebellum, and spinal cord of control mice little or no cytokine mRNA expression was detectable (Figure 9A) ▶ . Consistent with our findings above, in presymptomatic and symptomatic mice of each transgenic line, TNF-α mRNA was readily detectable in these brain regions. With the exception of the IL-1α and IL-1β mRNAs, which exhibited a modest increase in spinal cord or cerebellum of symptomatic mice of the GT-2 or GT-8 mice, respectively, expression of transcripts for other cytokines, including TNF-β, IL-2, -3, -4, -5, and -6, and IFN-γ, were not detectable. Similar to the cytokines, in control CNS tissue, no chemokine gene expression was detectable (Figure 9B) ▶ . In contrast to the cytokine genes, however, expression of a number of α and β chemokine transcripts was induced in tissue from presymptomatic transgenic mice and increased further in CNS tissue from symptomatic GFAP-TNFα mice and included C10, MIP-2, MCP-3, MIP-1β, MCP-1, crg-2, and RANTES (Figure 9B) ▶ . The level of chemokine mRNA expression in the different brain regions paralleled that for the TNF-α mRNA and was highest in the spinal cord or cerebellum from GT-2 or GT-8 mice, respectively.
Figure 9.
Cytokine and chemokine expression in the CNS of GFAP-TNFα mice (GT-2). Total RNA from CNS tissue from control (c), presymptomatic GT-2 (p), or symptomatic (s) GT-2 was analyzed for cytokine (A) and chemokine (B) expression as described in Materials and Methods. Principal areas with altered cytokine and chemokine expression co-localized with transgene expression.
Disease Exacerbation in GFAP-TNFαSCID/SCID Mice
To determine the relative contribution of the lymphocytes and therefore the adaptive immune response to the development of the neurological disorder observed in the GFAP-TNFα mice, animals of the GT-2 line were crossed with SCID mice that lack T and B lymphocytes. 54 Compared with GFAP-TNFα+/+ or GFAP-TNFαSCID/+ animals, GFAP-TNFαSCID/SCID mice exhibited earlier physical deterioration by 2 months of age characterized initially by the onset of severe ataxia, which then progressed to hind limb paralysis and was followed soon after by the death of the animals. Histological examination of the brain and spinal cord revealed the presence of more widely disseminated lesions in the spinal cord, cerebellum, and striatum of the GFAP-TNFαSCID/SCID mice. Luxol fast blue staining showed that considerable demyelination was evident in these lesion areas (not shown). Immunophenotypic analysis revealed the presence of large numbers of CD45high (Figure 10A) ▶ , Mac-1 (Figure 10B) ▶ , and MHC class II (Figure 10F) ▶ positive macrophage/microglial cells within and surrounding the lesions (Figure 10) ▶ . However, and in contrast to the GFAP-TNFα+/+ or GFAP-TNFαSCID/+ animals, the inflammatory lesions in the GFAP-TNFαSCID/SCID mice did not contain T and B lymphocytes (Figure 10, C–E) ▶ .
Figure 10.
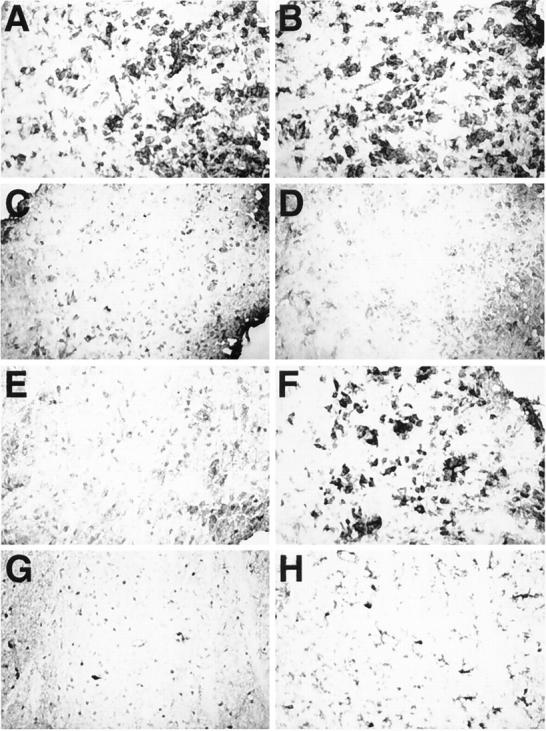
Immunophenotypic characterization of inflammatory lesions in GFAP-TNFα SCID/SCID mice. Frozen sections (10 μm) of brain from transgenic (A–F) or wild-type (G and H) mice were immunostained with monoclonal antibodies to identify leukocytes (CD45; A and G), macrophage/microglia (Mac-1; B and H), T lymphocytes (CD4 and CD8; C and D, respectively), B lymphocytes (E), and MHC class II (F).
Discussion
TNF-α is assumed to play a critical role in the pathogenesis of many neuroinflammatory and neurodegenerative disorders of the CNS. However, recent experimental results 31-33 derived particularly from studies in gene knockout mice deficient for either TNF-α itself or the TNF p55 and/or p75 receptors have brought into question the previously widely held view that this cytokine was pro-inflammatory and contributed to CNS injury. In the present study, chronic expression of TNF-α was targeted to astrocytes in the intact CNS of transgenic mice to better understand the CNS pathobiology of this cytokine. The results indicated that under these circumstances and in the transgenic lines studied, TNF-α expression was clearly deleterious, resulting in the development of degenerative disease affecting both white and gray matter. Significantly, this disorder occurred with a late onset and was closely linked to the development of a robust and rapidly progressing inflammatory process in the CNS. Similar features exist in neurological disorders in adult humans where dysregulated TNF-α production is found, including MS, 55,56 HIV encephalopathy, 57,58 and the infectious meningoencephalitides 8 or in the experimental animal models of EAE 59,60 and cerebral malaria. 61 Therefore, the GFAP-TNFα mice represent a relevant in vivo model to evaluate the contribution of TNF-α to the initiation and perpetuation of neuroinflammatory disease in the adult CNS.
It was notable that in the GFAP-TNFα mice described here, there was a long preclinical phase in which little neurodegeneration or apoptosis was discernible. Direct toxicity of TNF-α to different neural cells and structures has been documented using in vitro culture systems. Thus, TNF-α is cytotoxic to primary oligodendrocytes and also mediates myelin injury 29,30 whereas in primary cultures of human neurons TNF-α potentiates glutamate neurotoxicity. 62 In contrast to these findings, in vitro 63 and in vivo 33 studies provide evidence of a neuroprotective function of TNF-α. In addition, chronic intracerebral infusion of TNF-α was found not to be associated with significant neuronal or oligodendrocyte toxicity. 64 Clearly, there are major differences between the different model systems; nevertheless, our findings suggest that despite its chronic production in the CNS, at levels that eventually provoke significant inflammatory disease, TNF-α alone does not induce apoptosis in neural cells such as oligodendrocytes and does not directly cause CNS injury.
The preclinical phase in the GFAP-TNFα mice was also marked by the absence of detectable infiltrating leukocytes in the CNS. However, this phase was later replaced by the sudden onset of an inflammatory response with recruitment and infiltration of the CNS with large numbers of leukocytes. Therefore, this transgenic model provides an opportunity to study the role of TNF-α in the initiation of inflammation in the CNS and raises questions as to why there was such a long delay before the activation of the inflammatory response and as to what the nature of initiating signals may have been that were driving this process. Evidence derived from the study of acute inflammatory responses highlight an intrinsic resistance of the CNS to the recruitment and activation of leukocytes. 65 Such resistance may emanate from structural elements such as the blood-brain barrier 66 as well as from suppressive factors produced constitutively in the CNS, including cytokines such as TGF-β 67 and certain classes of gangliosides. 68 Clearly, all of these may contribute to the delayed initiation of the inflammatory response in the CNS of the GFAP-TNFα mice. In any case, the chronic production of TNF-α by astrocytes in the GFAP-TNFα mice did eventually overcome these barriers to leukocyte recruitment. Leukocyte trafficking is known to be complex, involving multiple factors in which key participants include the endothelial expressed cellular adhesion molecules (CAMs), chemokines, and cytokines. 69,70 A survey of the expression of these molecules in the CNS of the GFAP-TNFα mice documented marked increases or induction in the expression of a number of endothelial CAMs and of a variety of members of both the α and β chemokine gene families. These alterations likely represent a direct action of TNF-α, which is known to be a potent regulator of CAM expression by cerebrovascular endothelium, 71 and of chemokine expression by various neural cells types. 70 The CNS expression of the CAMs and the chemokine genes was found to be coordinately induced in the presymptomatic stages in the GFAP-TNFα mice and may therefore indicate a central role of these factors in the subsequent initiation of the inflammatory cascade.
The CNS inflammatory lesions in the GFAP-TNFα mice displayed some degree of heterogeneity between perivascular and parenchymal locations with the former being composed of predominantly lymphocytes whereas the latter contained predominantly macrophage/microglia, with neutrophils and T lymphocytes and few B lymphocytes. The highly organized appearance of the perivascular lesions was due mainly to the accumulation of large numbers of B lymphocytes and was reminiscent of follicular lymphoid tissue. The similarities of these lesions to peripheral lymphoid tissue was further underscored by the phenotypic properties of the cerebrovascular endothelium, which had the morphology of high endothelial venules and expressed MAdCAM, a molecule usually found on vessels in mesenteric lymph nodes and the expression of which is known to be induced by TNF-α. 69 Despite the robust nature and organization of the lymphocytic response, only a few of the T lymphocytes (as judged by the IL-2R expression) and B lymphocytes (as judged by the presence of plasma cells) were activated, with the vast majority being memory-type cells. In addition, expression of a number of cytokine genes associated with T lymphocyte activation, including IFN-γ and IL-2, were not detectable in the CNS of the symptomatic GFAP-TNFα mice. In all, the cytoarchitectural characteristics and the general lack of functional activation of the inflammatory response that we have observed in the CNS of the GFAP-TNFα mice are similar to those reported for TNF-α when expressed by pancreatic β cells in the islets of Langerhans 72,73 and by pulmonary cells in the lung 74 of transgenic mice. Recent studies in mice show that persistent exposure to TNF-α in vivo or in vitro suppresses Th1 and Th2 lymphocyte responses markedly. 75 Therefore, these and the transgenic studies highlight a possible down-regulatory role at the level of the T lymphocyte for TNF-α in chronic inflammatory states.
The development of severe CNS degenerative features were observed to parallel the onset and evolution of the inflammatory disease in the GFAP-TNFα mice. As noted above, in view of the absence of such features in the presymptomatic mice, we concluded that the inflammatory response is central to the pathogenesis of the CNS injury. The white matter lesions, which were particularly prominent, shared many features found in human inflammatory demyelinating disorders such as MS and included primary demyelination, remyelination, and the presence of foamy macrophages. However, unlike MS, extensive neurodegeneration affecting hippocampus and frontal cortex as well as the cerebellum and spinal cord was also seen and presumably reflected the more disseminated nature of the inflammatory lesions found in the transgenic mice. We suggest some of these neurodegenerative changes may have arisen from ischemic injury and edema resulting from vascular damage due to the massive and rapid accumulation of leukocytes within the cerebrovascular compartment. In support of this, hemorrhaging was evident in advanced lesions and resulted from injury to the vessel. In addition, ischemic injury may help to explain the pronounced axonal damage observed at sites distal to the inflammatory focus. It should be noted that vascular damage consequent to TNF-α-mediated inflammation appears to be a central mechanism in the pathogenesis of neurodegeneration in a number of experimental disorders, including cerebral malaria 24 and bacterial meningitis. 8 It is known from studies in EAE that although activated CD4-positive lymphocytes constitute a small fraction of the lymphocyte population that enters the CNS, these few cells are able to coordinate a significant destructive autoimmune process that is targeted to the white matter. 76 It was therefore conceivable that although there was little lymphocyte activation in the CNS of the symptomatic GFAP-TNFα mice, a possible autoimmune mechanism may still be involved in contributing to the CNS injury. This notion was also supported by the observation of induction of MHC class II expression in the inflammatory lesions coupled with enhanced macrophage/microglial activation and function. However, our data in the GFAP-TNFα SCID/SCID mice clearly suggest that if there is involvement of a classical T-lymphocyte-regulated autoimmune response in these transgenic mice it is not a significant direct determining factor in the development of degenerative disease. On the contrary, the fact that the clinical presentation was of earlier onset and the CNS inflammatory lesions were more widespread in GFAP-TNFα SCID/SCID mice suggested that T and possibly B lymphocytes may have a role in counteracting the recruitment and/or destructive potential of the macrophage/microglia that formed the inflammatory lesions.
CD45 expression can be used to discriminate infiltrating macrophages from resident microglia in the adult mouse CNS. 51 In view of their high expression of this marker, it is likely that the CNS lesion-associated cells in the GFAP-TNFα SCID/SCID mice were derived from peripheral macrophages recruited to the CNS, although it cannot be ruled out that these cells were also microglia with up-regulated CD45 expression. In any case, the studies in the GFAP-TNFα SCID/SCID mice highlight a dramatic direct response by cells of the macrophage microglial lineage to chronic TNF-α production in the CNS. This response, which included recruitment, accumulation, and activation concomitant with significant tissue destruction, therefore highlights a central role of the macrophage/microglial cell as a mediator of inflammatory disease in the CNS of the GFAP-TNFα model. The CNS white matter appears to be a major target in this regard in which macrophage/microglia were involved in causing significant demyelination. We have previously documented the spontaneous induction of macrophage/microglial-mediated demyelinating disease after chronic astrocyte-targeted expression of the cytokine IL-3. 42 More recently, persistent disease with increased macrophage/microglial reactivity was seen after induction of EAE in MBP-TNFα transgenic mice that do not develop spontaneous disease. 38 There is now accumulating evidence implicating cells of the macrophage/microglial lineage as primary effectors in the pathogenesis of inflammatory demyelinating disorders 77,78 and neurodegenerative disorders 79 in humans. Our findings here link directly TNF-α, a cytokine for which expression is increased in the CNS in these same disorders, to the function of macrophage/microglia and suggest a possible mechanism whereby the reactivity of these cells could be perpetuated by both paracrine and autocrine pathways. Therefore, approaches directed at breaking this link, for example, suppressing macrophage/microglial reactivity, may provide potential therapeutic strategies for CNS inflammatory disorders the efficacy of which could be tested in the GFAP-TNFα model.
Footnotes
Address reprint requests to Dr. Iain L. Campbell, Department of Neuropharmacology, CVN9, The Scripps Research Institute, 10550 N. Torrey Pines Road, La Jolla, CA, 92037. E-mail: icamp@scripps.edu.
Supported by USPHS grant MH 50426 (I.L. Campbell). A.K. Stalder is a postdoctoral fellow of the National Multiple Sclerosis Society. A. Pagenstecher was a postdoctoral fellow of the Deutsche Forschungsgemeinschaft.
A. Pagenstecher’s current address: Department of Neuropathology, University of Freiburg, Freiburg, Germany.
References
- 1.Fiers W: Tumor necrosis factor: characterization at the molecular, cellular and in vivo level. FEBS Lett 1991, 285:199-212 [DOI] [PubMed] [Google Scholar]
- 2.Grunfeld C, Palladino MA, Jr: Tumor necrosis factor: immunologic, antitumor, metabolic and cardiovascular activities. Adv Intern Med 1990, 35:45-72 [PubMed] [Google Scholar]
- 3.Beutler B, Grau GE: Tumor necrosis factor in the pathogenesis of infectious diseases. Crit Care Med 1993, 21:S423-S435 [PubMed] [Google Scholar]
- 4.Raine CS: Multiple sclerosis: TNF revisited with promise. Nature Med 1995, 1:211-214 [DOI] [PubMed] [Google Scholar]
- 5.Merrill JE: Proinflammatory and antiinflammatory cytokines in multiple sclerosis and central nervous system acquired immunodeficiency syndrome. J Immunol 1992, 12:167-170 [DOI] [PubMed] [Google Scholar]
- 6.Feuerstein GZ, Liu T, Barone FC: Cytokines, inflammation, and brain injury: role of tumor necrosis factor-α. Cerebrovasc Brain Metab Rev 1994, 6:341-360 [PubMed] [Google Scholar]
- 7.Grau GE, Modlin RL: Immune mechanisms in bacterial and parasitic diseases: protective immunity versus pathology. Curr Opin Immunol 1991, 3:480-485 [DOI] [PubMed] [Google Scholar]
- 8.Quagliarello V, Scheld WM: Bacterial meningitis: pathogenesis, pathophysiology, and progress. N Engl J Med 1992, 327:864-872 [DOI] [PubMed] [Google Scholar]
- 9.Griffin DE: Cytokines in the brain during viral infection: clues from HIV-associated dementia. J Clin Invest 1997, 100:2948-2951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung IY, Benveniste EN: Tumor necrosis factor-α production by astrocytes. J Immunol 1990, 144:2999-3007 [PubMed] [Google Scholar]
- 11.Lieberman AP, Pitha PM, Shin HS, Shin ML: Production of tumor necrosis factor and other cytokines by astrocytes stimulated with lipopolysaccharide or a neurotropic virus. Proc Natl Acad Sci USA 1989, 86:6348-6352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SC, Liu W, Dickson DW, Brosnan CF, Berman JW: Cytokine production by human fetal microglia and astrocytes: differential induction by lipopolysaccharide and IL-1β. J Immunol 1993, 150:2659-2667 [PubMed] [Google Scholar]
- 13.Chao CC, Hu S, Sheng WS, Peterson PK: Tumor necrosis factor-α production by human fetal microglial cells: regulation by other cytokines. Dev Neurosci 1995, 17:97-105 [DOI] [PubMed] [Google Scholar]
- 14.Breder CD, Tsujimoto M, Terano Y, Scott DW, Saper CB: Distribution and characterization of tumor necrosis factor-α-like immunoreactivity in the murine central nervous system. J Comp Neurol 1993, 337:543-567 [DOI] [PubMed] [Google Scholar]
- 15.Rothwell NJ, Luheshi GN: Brain TNF: damage limitation or damaged reputation? Nature Med 1996, 2:746-747 [DOI] [PubMed] [Google Scholar]
- 16.Steinman L: Some misconceptions about understanding autoimmunity through experiments with knockouts. J Exp Med 1997, 185:2039-2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruddle NH, Bergman CM, McGrath KM, Lingenheld EG, Grunnet ML, Padula SJ, Clark RB: An antibody to lymphotoxin and tumor necrosis factor prevents transfer of experimental allergic encephalomyelitis. J Exp Med 1990, 172:1193-1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selmaj K, Raine CS, Cross AH: Anti-tumor necrosis factor therapy abrogates autoimmune demyelination. Ann Neurol 1991, 1991:694-700 [DOI] [PubMed] [Google Scholar]
- 19.Klinkert WEF, Kojima K, Lesslauer W, Rinner W, Lassmann H, Wekerle H: TNF-α receptor fusion protein prevents experimental autoimmune encephalomyelitis and demyelination in Lewis rats: an overview. J Neuroimmunol 1994, 72:163-168 [DOI] [PubMed] [Google Scholar]
- 20.Sommer N, Loschmann PA, Northoff GH, Weller M, Steinbecher A, Steinbach JP, Lichtenfels R, Meyermann R, Reitmuller A, Fontana A, Dichgans J, Martin R: The antidepressant Rolipram suppresses cytokine production and prevents autoimmune encephalomyelitis. Nature Med 1995, 1:244-248 [DOI] [PubMed] [Google Scholar]
- 21.Garcia I, Miyazaki Y, Araki K, Araki M, Lucas R, Grau GE, Milon G, Belkaid Y, Montixi C, Lesslauer W, Vassalli P: Transgenic mice expressing high levels of soluble TNF-R1 fusion protein are protected from lethal septic shock and cerebral malaria, and are highly sensitive to Listeria monocytogenes and Leishmania major infections. Eur J Immunol 1995, 25:2401-2407 [DOI] [PubMed] [Google Scholar]
- 22.Lucas R, Juillard P, Decoster E, Redard M, Burger D, Donati Y, Giroud C, Monso-Hinard C, Kesel TD, Buurman WA, Moore MW, Dayer J-M, Fiers W, Bluethmann H, Grau GE: Crucial role of tumor necrosis factor (TNF) receptor 2 and membrane-bound TNF in experimental cerebral malaria. Eur J Immunol 1997, 27:1719-1725 [DOI] [PubMed] [Google Scholar]
- 23.Saukkonen K, Sande S, Cioffe C, Wolpe S, Sherry B, Cerami A, Tuomanen E: The role of cytokines in the generation of inflammation and tissue damage in experimental gram-positive meningitis. J Exp Med 1990, 171:439-448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grau GE, Lou J: TNF in vascular pathology: the importance of platelet-endothelial interactions. Res Immunol 1993, 144:355-363 [DOI] [PubMed] [Google Scholar]
- 25.Satoh J-I, Kastrukoff LF, Kim SU: Cytokine-induced expression of intercellular adhesion molecule-1 (ICAM-1) in cultured human oligodendrocytes and astrocytes. J Neuropathol Exp Neurol 1991, 50:215-226 [DOI] [PubMed] [Google Scholar]
- 26.McManus CM, Brosnan CF, Berman JW: Cytokine induction of MIP-1α and MIP-1β in human fetal microglia. J Immunol 1998, 160:1449-1455 [PubMed] [Google Scholar]
- 27.Hayashi M, Luo Y, Laning J, Strieter RM, Dorf ME: Production and function of monocyte chemoattractant protein-1 and other β-chemokines in murine glial cells. J Neuroimmunol 1995, 60:143-150 [DOI] [PubMed] [Google Scholar]
- 28.Hurwitz AA, Lyman WD, Berman JW: Tumor necrosis factor α and transforming growth factor β upregulate astrocyte expression of monocyte chemoattractant protein-1. J Neuroimmunol 1995, 57:193-198 [DOI] [PubMed] [Google Scholar]
- 29.Louis J-C, Magal E, Takayama S, Varon S: CNTF protection of oligodendrocytes against natural and tumor necrosis factor-induced death. Science 1993, 259:689-692 [DOI] [PubMed] [Google Scholar]
- 30.Selmaj K, Raine CS, Farooq M, Norton WT, Brosnan CF: Cytokine cytotoxicity against oligodendrocytes: apoptosis induced by lymphotoxin. J Immunol 1991, 147:1522-1529 [PubMed] [Google Scholar]
- 31.Liu J, Marino MW, Wong G, Grail D, Dunn A, Bettadapura J, Slavin AJ, Old L, Bernard CCA: TNF is a potent anti-inflammatory cytokine in autoimmune-mediated demyelination. Nature Med 1998, 4:78-83 [DOI] [PubMed] [Google Scholar]
- 32.Frei K, Eugster H-P, Bopst M, Constantinescu CS, Lavi E, Fontana A: Tumor necrosis factor α and lymphotoxin α are not required for the induction of acute experimental autoimmune encephalomyelitis. J Exp Med 1997, 185:2177-2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruce AJ, Bolinf W, Kindy MS, Peschon J, Kraemer PJ, Carpenter MK, Holtsberg FW, Mattson MP: Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nature Med 1996, 2:788-794 [DOI] [PubMed] [Google Scholar]
- 34.Rudin W, Eugster H-P, Bordmann G, Bonato J, Muller M, Yamage M, Ryffel B: Resistance to cerebral malaria in tumor necrosis factor-α/β-deficient mice is associated with a reduction of intercellular adhesion molecule-1 up-regulation and T helper type 1 response. Am J Pathol 1997, 150:257-266 [PMC free article] [PubMed] [Google Scholar]
- 35.Campbell IL, Stalder AK, Chiang C-S, Bellinger R, Heyser CJ, Steffensen S, Masliah E, Powell HC, Gold LH, Henriksen SJ, Siggins GR: Transgenic models to assess the neuropathogenic actions of cytokines in the central nervous system. Mol Psychiatry 1997, 2:125-129 [DOI] [PubMed] [Google Scholar]
- 36.Probert L, Akassoglou K, Pasparakis M, Kontogeorgos G, Kollias G: Spontaneous inflammatory demyelinating disease in transgenic mice showing central nervous system-specific expression of tumor necrosis factor α. Proc Natl Acad Sci USA 1995, 92:11294-11298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akassoglou K, Probert L, Kontogeorgos G, Kollias G: Astrocyte-specific but not neuron-specific transmembrane TNF triggers inflammation and degeneration in the central nervous system of transgenic mice. J Immunol 1997, 158:438-445 [PubMed] [Google Scholar]
- 38.Taupin V, Renno T, Bourbonniere L, Peterson AC, Rodriguez M, Owens T: Increased severity of experimental autoimmune encephalomyelitis, chronic macrophage/microglial reactivity, demyelination in transgenic mice producing tumor necrosis factor-α in the central nervous system. Eur J Immunol 1997, 27:905-913 [DOI] [PubMed] [Google Scholar]
- 39.Turnley AM, Morahan G, Okano H, Bernard O, Mikoshiba K, Allison J, Bartlett PF, Miller JFAP: Dysmyelination in transgenic mice resulting from expression of class I histocompatibility molecules in oligodendrocytes. Nature 1991, 353:566-569 [DOI] [PubMed] [Google Scholar]
- 40.Campbell IL, Powell HC: Role of cytokines in demyelinating disease studied in transgenic mice. Methods 1996, 10:462-477 [DOI] [PubMed] [Google Scholar]
- 41.Campbell IL, Abraham CR, Masliah E, Kemper P, Inglis JD, Oldstone MBA, Mucke L: Neurologic disease induced in transgenic mice by the cerebral overexpression of interleukin 6. Proc Natl Acad Sci USA 1993, 90:10061-10065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiang C-S, Powell HC, Gold L, Samimi A, Campbell IL: Macrophage/microglial-mediated primary demyelination and motor disease induced by the central nervous system production of interleukin-3 in transgenic mice. J Clin Invest 1996, 97:1512-1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hobbs MV, Weigle WO, Noonan DJ, Torbett BE, McEvilly RJ, Koch RJ, Cardenas GJ, Ernst DN: Patterns of cytokine gene expression by CD4+ T cells from young and old mice. J Immunol 1992, 150:3602-3068 [PubMed] [Google Scholar]
- 44.Asensio VC, Campbell IL: Chemokine gene expression in the brain of mice with lymphocytic choriomeningitis. J Virol 1997, 71:7832-7840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dudov KP, Perry RP: The gene family encoding the mouse ribosomal protein L32 contains a uniquely expressed intron-containing gene and an unmutated processed gene. Cell 1984, 37:457-468 [DOI] [PubMed] [Google Scholar]
- 46.Stalder AK, Campbell IL: Simultaneous analysis of multiple cytokine receptor mRNAs by RNAse protection assay in LPS-induced endotoxemia. Lymphokine Cytokine Res 1994, 13:107-112 [PubMed] [Google Scholar]
- 47.Simmons DM, Arriza JL, Swanson LW: A complete protocol for in situ hybridization of messenger RNAs in brain and other tissues with radiolabeled single-stranded RNA probes. J Histotechnol 1989, 12:169-181 [Google Scholar]
- 48.Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L: Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature 1994, 367:188-193 [DOI] [PubMed] [Google Scholar]
- 49.Masliah E, Ge N, Achim CL, Hansen LA, Wiley CA: Selective neuronal vulnerability in HIV encephalitis. J Neuropathol Exp Neurol 1992, 51:585-593 [DOI] [PubMed] [Google Scholar]
- 50.Gavrieli Y, Sherman Y, Ben-Sasson SA: Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 1992, 119:493-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ford AL, Goodsall AL, Hickey WF, Sedgwick JD: Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. J Immunol 1995, 154:4309-4321 [PubMed] [Google Scholar]
- 52.Carson MJ, Reilly CR, Sutcliffe JG, Lo D: Mature microglia resemble immature antigen-presenting cells. Glia 1998, 22:72-85 [DOI] [PubMed] [Google Scholar]
- 53.Sedgewick JD, Schwender S, Imrich H, Dorres R, Butcher GW, ter Meulen V: Indirect and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci USA 1991, 88:7438-7442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bosma GC, Custer RP, Bosma MJ: A severe combined immunodeficiency syndrome in the mouse. Nature 1983, 301:527-530 [DOI] [PubMed] [Google Scholar]
- 55.Hofman FM, Hinton DR, Johnson K, Merrill JE: Tumor necrosis factor identified in multiple-sclerosis brain. J Exp Med 1989, 170:607-612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Selmaj K, Raine CS, Cannella B, Brosnan CF: Identification of lymphotoxin and tumor necrosis factor in multiple sclerosis lesions. J Clin Invest 1991, 87:949-954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wesselingh SL, Power C, Glass JD, Tyor WR, McArthur JC, Farber JM, Griffin JW, Griffin DE: Intracerebral cytokine messenger RNA expression in acquired immunodeficiency syndrome dementia. Ann Neurol 1993, 33:576-582 [DOI] [PubMed] [Google Scholar]
- 58.Wesselingh SL, Takahashi K, Glass JD, McArthur JC, Griffin JW, Griffin DE: Cellular localization of tumor necrosis factor mRNA in neurological tissue from HIV-infected patients by combined reverse transcriptase/polymerase chain reaction in situ hybridization and immunohistochemistry. J Neuroimmunol 1997, 74:1-8 [DOI] [PubMed] [Google Scholar]
- 59.Kennedy MK, Torrance DS, Picha KS, Mohler KM: Analysis of cytokine mRNA expression in the central nervous system of mice with experimental autoimmune encephalomyelitis reveals that IL-10 mRNA expression correlates with recovery. J Immunol 1992, 149:2496-2505 [PubMed] [Google Scholar]
- 60.Renno T, Krakowski M, Piccirillo C, Lin J-y, Owens T: TNF-α expression by resident microglia and infiltrating leukocytes in the central nervous system of mice with experimental allergic encephalomyelitis. J Immunol 1995, 154:944-953 [PubMed] [Google Scholar]
- 61.Medana IM, Hunt NH, Chaudhri G: Tumor necrosis factor-α expression in the brain during fatal murine cerebral malaria. Am J Pathol 1997, 150:1473-1486 [PMC free article] [PubMed] [Google Scholar]
- 62.Chao CC, Hu S: Tumor necrosis factor-α potentiates glutamate neurotoxicity in human fetal brain cultures. Dev Neurosci 1994, 16:172-179 [DOI] [PubMed] [Google Scholar]
- 63.Cheng B, Christakos S, Mattson MP: Tumor necrosis factors protect neurons against metabolic-excitotoxic insults and promote maintenance of calcium homeostasis. Neuron 1994, 12:139-153 [DOI] [PubMed] [Google Scholar]
- 64.Yamasaki T, Kikuchi H, Moritake K, Nagao S, Iwasaki K, Paine JT, Kagawa T, Namba Y: A morphological and ultrastructural investigation of normal mouse tissue after intracerebral injection of tumor necrosis factor. J Neurosurg 1992, 77:279-287 [DOI] [PubMed] [Google Scholar]
- 65.Andersson P-B, Perry VH, Gordon S: Intracerebral injection of proinflammatory cytokines or leukocyte chemotaxins induces minimal myelomonocytic cell recruitment to the parenchyma of the central nervous system. J Exp Med 1992, 176:255-259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perry VH, Anthony DC, Bolton SJ, Brown HC: The blood-brain barrier and the inflammatory response. Mol Med Today 1997, 335–341 [DOI] [PubMed]
- 67.Finch CE, Laping NJ, Morgan TE, Nichols NR, Pasinetti GM: TGF-β1 is an organizer of responses to neurodegeneration. J Cell Biochem 1993, 53:314-322 [DOI] [PubMed] [Google Scholar]
- 68.Irani DN, Lin K-I, Griffin DE: Regulation of brain-derived T cells during acute central nervous system inflammation. J Immunol 1997, 158:2318-2326 [PubMed] [Google Scholar]
- 69.Butcher EC: Cellular and molecular mechanisms that direct leukocyte traffic. Am J Pathol 1990, 136:3-11 [PMC free article] [PubMed] [Google Scholar]
- 70.Ransohoff RM, Glabinski A, Tani M: Chemokines in immune-mediated inflammation of the central nervous system. Cytokine Growth Factor Rev 1996, 7:35-46 [DOI] [PubMed] [Google Scholar]
- 71.Lassmann H, Rossier K, Zimprich F, Vass K: Expression of adhesion molecules and histocompatibility antigens at the blood-brain barrier. Brain Pathol 1991, 1:115-123 [DOI] [PubMed] [Google Scholar]
- 72.Higuchi Y, Herrera P, Muniesa P, Huarte J, Belin D, Ohashi P, Aichele P, Orci L, Vassalli J-D, Vassalli P: Expression of a tumor necrosis factor α transgene in murine pancreatic β cells results in severe and permanent insulitis without evolution towards diabetes. J Exp Med 1992, 176:1719-1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Picarella DE, Kratz A, Li C-B, Ruddle NH, Flavell RA: Transgenic tumor necrosis factor (TNF)-α production in pancreatic islets leads to insulitis, not diabetes. J Immunol 1993, 150:4136-4150 [PubMed] [Google Scholar]
- 74.Miyazaki Y, Araki K, Vesin C, Garcia I, Kapanci Y, Whitsett JA, Piguet P-F, Vassalli P: Expression of a tumor necrosis factor-α transgene in murine lung causes lymphocytic and fibrosing alveolitis. J Clin Invest 1995, 96:250-259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cope AP, Liblau RS, Yank X-D, Congia M, Laudanna C, Schreiber RD, Probert L, Kollias G, McDevitt HO: Chronic tumor necrosis factor alters T cell responses by attenuating T cell receptor signaling. J Exp Med 1997, 185:1573-1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Steinman L: A few autoreactive cells in an autoimmune infiltrate control a vast population of nonspecific cells: a tale of smart bombs and the infantry. Proc Natl Acad Sci USA 1996, 93:2253-2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sriram S, Rodriguez M: Indictment of the microglia as the villain in multiple sclerosis. Neurology 1997, 48:464-470 [DOI] [PubMed] [Google Scholar]
- 78.Benveniste EN: Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J Mol Med 1997, 75:165-173 [DOI] [PubMed] [Google Scholar]
- 79.Dickson DW, Lee SC, Mattiace LA, Yen S-HC: Microglia and cytokines in neurological disease, with special reference to AIDS and Alzheimer’s disease. Glia 1993, 7:75-83 [DOI] [PubMed] [Google Scholar]