Abstract
Accumulating evidence suggests that the early pulmonary inflammation pathogenesis in cystic fibrosis (CF) may be associated with an abnormal increase in the production of pro-inflammatory cytokines in the CF lung, even in the absence of infectious stimuli. We have postulated that if baseline abnormalities in airway epithelial cell production of cytokines occur in CF, they should be manifested in the CF bronchial submucosal glands, which are known to express high levels of CFTR (cystic fibrosis transmembrane conductance regulator) protein, the gene product mutated in CF disease. Immunohistochemical analyses showed that CF bronchial submucosal glands in patients homozygous for the ΔF508 deletion expressed elevated levels of the endogenous chemokine interleukin (IL)-8 but not the pro-inflammatory cytokines IL-1β and IL-6, compared with non-CF bronchial glands. Moreover, basal protein and mRNA expression of IL-8 were constitutively up-regulated in cultured ΔF508 homozygous CF human bronchial gland cells, in an unstimulated state, compared with non-CF bronchial gland cells. Furthermore, the exposure of CF and non-CF bronchial gland cells to an elevated extracellular Cl− concentration markedly increased the release of IL-8, which can be corrected in CF gland cells by reducing the extracellular Cl− concentration. We also found that, in contrast to non-CF gland cells, dexamethasone did not inhibit the release of IL-8 by cultured CF gland cells. The selective up-regulation of bronchial submucosal gland IL-8 could represent a primary event that initiates early airway submucosal inflammation in CF patients. These findings are relevant to the pathogenesis of CF and suggest a novel pathophysiological concept for the early and sustained airway inflammation in CF patients.
Chronic endobronchial inflammation and bacterial infection are thought to be the main cause of mortality and morbidity in cystic fibrosis (CF), a genetic disease characterized by defective Cl− secretion and enhanced Na+ absorption across airway epithelia. 1-3 Although airway inflammation in CF patients is often viewed as a response to infectious stimuli, recent findings indicate that inflammation and infection are early events in CF lung disease and suggest that airway inflammation may arise without concomitant bacterial infection. 4,5 The description of the interleukin (IL)-8 protein as an important tissue-derived chemotactic cytokine for neutrophils 6,7 suggests that polymorphonuclear neutrophil accumulation that takes place during lung inflammation in CF patients may also be mediated, in part, by local tissue-derived factors.
The production of tumor necrosis factor (TNF)-α, IL-1β, IL-6, and IL-8 and other pro-inflammatory cytokines by airway epithelial cells and lung macrophages along with the accumulation and activation of neutrophils in the CF airways may underlie the early pathogenesis of CF lung disease. 4,5,8 Before lung infection, an excessive release of pro-inflammatory cytokines and an increased number of neutrophils have been reported in the bronchoalveolar lavage fluids of CF patients. 4,5 Moreover, Noah et al 9 recently demonstrated that the IL-8 levels in bronchoalveolar lavage fluids, in comparison with other pro-inflammatory cytokine levels, are markedly increased in children with CF compared with non-CF children with a bacterial infection of the lower airways. Thus, it is possible that excessive inflammation in the CF lung may be related to constitutive abnormalities in the regulation of pro-inflammatory cytokine expression by CF airway epithelial cells, independent of infectious stimuli. In this context, we have recently shown in CF mutant mice 10 that airway inflammation may be a direct consequence of mutant CFTR (cystic fibrosis transmembrane conductance regulator) protein expression, the gene product in CF disease, as evidence for an increased number of inflammatory cells was observed in the lamina propria of CF mice in the absence of any sign of infection. All of these data favor the hypothesis that an endogenous pathway for airway inflammation may exist in CF airway cells before the manifestation of a bacteria-related infection. Submucosal gland epithelial cells are of special interest in this regard, given their ability to express the highest level of CFTR protein in comparison with other human airway epithelial cell types 11,12 and also their ability to provide the bulk of the fluid component of airway secretions. Recently, it was shown that CFTR Cl− channel dysfunction in CF tracheal submucosal gland cells leads to abnormal transepithelial salt and fluid secretion. 13 Based on these observations it is reasonable to postulate that intrinsic abnormalities related to the mutant CFTR protein in the bronchial submucosal gland cell type could lead to early inflammation in CF airways. At present, it is not known whether CF human bronchial glands in vivo could represent a major local source of pro-inflammatory cytokines and, in particular, for the IL-8 chemokine. Nor is it known whether the abnormally elevated Cl− concentration described for the airway secretions of CF patients 14,15 can modulate the inflammatory responses in airways. The concept of bronchial gland epithelial cells as an important component of airway inflammation in ΔF508 homozygote CF patients has been applied to understanding the pathogenesis of early pulmonary inflammation in CF patients. 4,5 We therefore decided to address the following questions. 1) Is there evidence of elevated levels of pro-inflammatory cytokines IL-1β, IL-6, and IL-8 expressed by ΔF508 homozygous CF human bronchial submucosal glands compared with non-CF bronchial submucosal glands in vivo? And 2) If so, what are the levels and differential expression of these cytokines, in particular for the IL-8 chemokine, according to the extracellular Cl− concentration by subcultures of ΔF508 homozygous CF human bronchial gland cells compared with levels measured in non-CF bronchial gland cells in vitro?
Materials and Methods
Human Bronchial Tissues
Human CF bronchial tissue was obtained from eight recipients undergoing lung transplant operations (the CF patients were all ΔF508 homozygous; four females and four males; mean age, 17.3 years; age range, 9 to 27 years). Tissue for control experiments was obtained from four non-CF patients (two males with primary pulmonary hypertension, aged 28 and 29 years, respectively, and two males with pulmonary idiopathic fibrosis, aged 40 and 61 years, respectively). For all experiments, bronchial segments were prepared within 5 hours after lung resection and were incubated in a serum-free medium composed of a 1:1 mixture of Dulbecco’s modified Eagles medium (DMEM) and Ham’s F-12 medium supplemented with the antibiotics colistin (200 U/ml), penicillin G (100 U/ml), and streptomycin (100 μg/ml).
Histological Examination of Bronchial Submucosal Tissues
The upper lobar bronchi of six CF patients and three non-CF disease controls (one with primary pulmonary hypertension and two with pulmonary idiopathic fibrosis) were dissected form the pathological lung resected before transplantation, fixed with 10% formalin, and embedded in paraffin for light microscopy. Transverse bronchial sections were stained with a Giemsa solution. Areas of the submucosal connective tissue surrounding glands was selected and analyzed. A minimum of 24 microscopy fields (>450 mm 2 of submucosal tissues) were examined at a magnification of ×400, and the number of inflammatory cells was quantified using a computer-assisted analysis system (CAS-200; Becton-Dickinson, Oxford, UK). The total number of inflammatory cells was counted in each delineated submucosal area. Results were expressed as inflammatory cells per square millimeter. Polymorphonuclear neutrophils could be easily recognized by their lobular nuclei and were quantified in each area examined. The percentage of neutrophils was also calculated.
Immunohistochemical Staining
For the immunohistochemical analysis of bronchial tissues, frozen bronchial tissue samples were embedded in OCT (Miles Tissue Tek, Elkhart, IN), immersed in liquid nitrogen, and stored at −80°C. Bronchial cryosections (5 μm thick) deposited onto gelatin-coated glass slides were stored at −20°C after air drying and rehydrated in 0.1 mol/L PBS at pH 7.2. Sets of consecutively cryofixed sections were then blocked with PBS/1% bovine serum albumin for 10 minutes and stained for IL-1β, IL-6, IL-8, and lysozyme, a specific protein marker of bronchial gland serous-type cells. 16,17 Monoclonal antibodies against the cytokines IL-1β, IL-6, and IL-8 (dilution, 1:100) were purchased from Biosource International (Camarillo, CA). Rabbit antiserum to human lysozyme (dilution, 1:500) was purchased from Dakopatts (Glostrup, Denmark). In all immunofluorescence experiments, bound antibodies were detected using the streptavidin-fluorescein isothiocyanate (FITC) system (Amersham International, Amersham, UK). Secondary antibodies of goat biotinyled anti-mouse and anti-rabbit IgG fractions (Boehringer Mannheim, Meylan, France) and streptavidin-FITC were used at a dilution of 1:50. Negative controls were performed using either nonimmune mouse or rabbit IgG fractions (Sigma Chemical Co., St Louis, MO).
The co-localization of the IL-8 chemokine and lysozyme was performed by indirect double immunofluorescence in cryofixed CF and non-CF bronchial sections. Sections were incubated for 60 minutes at room temperature in PBS/1% bovine serum albumin containing 1 μg/ml anti-human IL-8 antibody. After staining with the IL-8 monoclonal antibody, sections were incubated for 60 minutes in PBS/1% bovine serum albumin containing 2 μg/ml lysozyme polyclonal antibody, washed in three changes of PBS/1% bovine serum albumin for 5 minutes each time followed by incubation in PBS/1% bovine serum albumin containing 2 μg/ml of both donkey anti-mouse FITC-conjugated antibody and donkey anti-rabbit Texas-red-conjugated antibody for 45 minutes. After rinsing in three changes of PBS/1% bovine serum albumin for 10 minutes each time, all specimens were counterstained with Harris hematoxylin solution for 10 seconds, mounted in citifluor antifading solution (Agar Scientific, Stansted, UK), and observed by using a Zeiss Axiophot microscope (Zeiss, Le Pecq, France) employing epifluorescence and Nomarski differential interference illumination.
Isolation and Culture of Bronchial Submucosal Gland Cells
Human bronchial gland (HBG) cells were isolated from eight ΔF508 homozygous CF patients and from four non-CF patients as described above. Cell isolation and subcultivation procedures were performed as described previously. 17,18 In brief, cells were isolated by enzymatic digestion from bronchial submucosa and grown onto type I collagen-coated 25-cm 2 tissue culture flasks in a DMEM/Ham’s F12 mixture (50/50%, v/v) supplemented with 1% Ultroser G (a serum substitute from Sepracor, Villeneuve-la-Garenne, France), glucose (10 g/L), and sodium pyruvate (0.33 g/L). Penicillin G (100 U/ml) and streptomycin (100 μg/ml) were also added. The culture medium was replaced every 3 days. After 2 weeks in primary culture at 37°C under 5% CO2 in air, cells were treated with 0.25% trypsin, 0.5 mmol/L EDTA in a Ca2+- and Mg2+-free PBS solution. The removal of contaminated fibroblasts from the primary culture was carried out using a selective trypsination procedure as previously described. 17 Cells were then grown in 25-cm 2 tissue culture flasks. After 4 weeks, third-passage HBG cells had proliferated and exhibited characteristics of homogeneous submucosal epithelial and secretory gland cells, including two protein markers specific to the glandular serous-type cell, these being lysozyme and secretory leukocyte proteinase inhibitor (SLPI) also known as antileukoprotease. 17,18
Functional Cl− Channel Activity of CFTR Protein
The phosphorylation-regulated Cl− channel activity of CFTR was assessed using the halide-sensitive fluorescent dye 6-methoxy-N-(3-sulfopropyl)-quinolinium (SPQ) as previously described. 19 The CF and non-CF HBG cells grown on type I collagen-coated glass coverslips were loaded with 3.5 mmol/L SPQ in a hypotonic chloride buffer (1:1 mixture of distilled water and a chloride buffer containing 130 mmol/L NaCl, 2.4 mmol/L K2HPO4, 10 mmol/L d-glucose, 1 mmol/L CaSO4, 1 mmol/L MgSO4, and 10 mmol/L HEPES, pH 7.4) for 10 minutes at 37°C. Cells were then rinsed twice and incubated with the chloride buffer for 15 minutes at 37°C. Cells were placed in a temperature-controlled chamber (37°C) on the stage of an inverted microscope (Zeiss IM35) and incubated in a nitrate buffer in which NaCl was replaced by 103 mmol/L NaNO3. Cyclic AMP (cAMP) stimulation was achieved by exposing the cells to 25 μmol/L forskolin (Sigma Chemical Co.). The chloride secretion of approximately 60 to 80 cells was estimated by measurement of SPQ fluorescence variations obtained using an excitation light wavelength at 365 nm and emission light wavelength at >395 nm through a 32× planachromat objective. A software-driven shutter in the excitation light path was used to automatically illuminate cells for 2 seconds and simultaneously record the fluorescent image every minutes for 15 minutes. Mean variations in SPQ fluorescence after cAMP stimulation were plotted against time over the 15-minute period. The mean number of cells analyzed per non-CF and CF HBG cell monolayer culture was at least 65. Data are presented as the relative fluorescence, this being 100X (Ft/Fo), where Ft is the fluorescence intensity at time t and Fo is the fluorescence intensity at time 0.
RNA Isolation and Northern Blot Analysis
Total cellular RNA was extracted from 25-cm 2 culture flasks of confluent third-passage ΔF508 homozygous CF HBG and non-CF HBG cells using an acid guanidinium/phenol/chloroform method (Trizol, GIBCO BRL, Gaithersburg, MD). For Northern analyses, aliquots of 15 μg of total RNA (determined by spectrophotometry, 260-nm wavelength) were denatured and size fractionated by electrophoresis through a 1.0% agarose/7.0% formaldehyde gel. The integrity of the RNA was confirmed by observing under ultraviolet (UV) light the 28 S and 18 S ribosomal bands after ethidium bromide staining. For Northern blots, the RNA was transferred onto a nylon membrane (Hybond N; Amersham International) by capillary transfer and UV cross-linked to the membrane. Filters were hybridized at 50°C with a 32P end-labeled oligonucleotide probe with the sequence 5′GTT-GGC-GCA-GTG-TGG-TCC-ACT-CTC-AAT-CAC-3′ using a random prime DNA labeling kit (Boehringer Mannheim). Membranes were hybridized for 15 hours at 50°C, washed twice for 10 minutes in 2X SSC, 0.01% SDS, 10 minutes in 1X SSC, 0.1% SDS at room temperature and 15 minutes in 0.1X SSC, 0.1% SDS at 50°C, and finally autoradiographed for 1 week at −70°C. Autoradiogram signal strengths of hybridized mRNA were quantified by scanning densitometry (GS 690 imaging densitometer and Molecular Analyst software; Bio-Rad, Richmond, CA). Adjustments for small differences in loading were made using densitometry of the ethidium bromide fluorescence of the 28 S band of the gel photographed before blotting.
Exposure of CF and Non-CF HBG Cell Monolayers to Low and High Cl− Concentration
Before the exposure of cells to either low (85 mmol/L), intermediate (135 mmol/L), or high (170 mmol/L) Cl− concentration, third-passage confluent monolayers of ΔF508 homozygous CF and non-CF HBG cells were incubated for 16 hours in an Ultroser G-free RPMI 1640 medium in 95% air/5% CO2 to ensure that cells were in a quiescent state. At the end of the 16-hour period, the culture supernatants from an additional 6-hour period were collected and stored at −80°C until tested for the presence of the cytokines IL-1β, IL-6, IL-8, and IL-10, as described below. In this way, individual monolayers of CF and non-CF HBG cells were exposed for an additional 6-hour period to Cl− solutions containing either 85 mmol/L, 135 mmol/L, or 170 mmol/L Cl−, respectively. Immediately after each period of cell exposure, supernatants were collected and stored at −80°C until tested for the presence of cytokines. The three chloride-containing solutions used in this study (85 mmol/L Cl−, 135 mmol/L Cl−, and 170 mmol/L Cl−) contained 1 mmol/L CaCl2, 20 mmol/L KCl, and either 60 mmol/L NaCl, 105 mmol/L NaCl, or 148 mmol/L NaCl, pH 7.4, respectively, as previously reported. 15
In another set of experiments, at the end of the first 16-hour period in the Ultroser G-free RPMI 1640 medium, the production of IL-8 by third-passage confluent cultures of CF and non-CF HBG cells was analyzed in response to exposure to glucocorticoids previously solubilized in Ultroser G-free RPMI 1640 medium (1, 5, and 10 μmol/L dexamethasone; Sigma Chemical Co.). From individual monolayers of ΔF508 homozygous CF and non-CF HBG cells, IL-8 secretion was measured over the subsequent 6-hour culture period in the absence or the presence of the different concentrations of dexamethasone.
ELISA for Cytokines
Cytokine concentrations in the culture supernatants of third-passage confluent ΔF508 homozygous CF and non-CF HBG cells were determined by following the manufacturer’s instructions in commercially available ELISA kits (Biosource International). The ELISAs for IL-1β, IL-6, IL-8, and IL-10 were sensitive to a level of 5 pg/ml. In experiments where defined amounts of particular recombinant human cytokines (used as standards) were added to supernatants, the total recovery of each of the cytokines was always close to 100%. Analysis of TNF-α was not performed, however, due to the lack of supernatant samples after analysis of the cytokines described above. Cell viability was confirmed by trypan blue exclusion after all experimental interventions. The uniformity of the cell monolayer was determined by quantifying the cell number per well. Total cellular protein concentrations were measured using the Bradford method (Bio-Rad Laboratories). All results are expressed as pg/ml/10 6 cells.
Statistical Analysis
Results are expressed as means ± SD. Each data point was performed in triplicate at least, and each cell culture experiment was performed at least three times. Differences in cytokine levels were analyzed by the Student’s t-test for paired and unpaired samples.
Results
Histological Findings
The mean number of the inflammatory cells and, in particular, the number of polymorphonuclear neutrophils found in six ΔF 508 homozygous CF patients and in three non-CF disease controls are reported in Table 1 ▶ . In CF patients, the mean number of inflammatory cells was higher (1856 ± 721 cells/mm2; n = 6) as compared with non-CF disease controls (910 ± 352 cells/mm2; n = 3), but the difference was not significant between the two groups. In the submucosal periglandular areas examined, a predominance of mononucleated cells was observed in the two groups. Although the mean number of neutrophils in the CF patient group (116 ± 65 cells/mm2) was higher than that obtained from non-CF disease control group (65 ± 16 cells/mm2), the difference was not significant. Interestingly, when expressed in percentage of total inflammatory cells, the percentage of neutrophils was low and similar in the two groups of subjects analyzed (6 ± 2% in CF and 8 ± 4% in non-CF disease controls).
Table 1.
Individual Counts for Inflammatory Cells and Polymorphonuclear Neutrophils in Bronchial Submucosal Connective Tissues Surrounding Secretory Glands in CF Patients and Non-CF Disease Controls
| Patients | Age (years) | Sex | Total inflammatory cells | Neutrophils | |
|---|---|---|---|---|---|
| ΔF508 | |||||
| Homozygous CF | |||||
| CF1 | 21 | M | 1283 ± 1254 | 59 ± 56 | |
| CF2 | 26 | F | 2051 ± 1441 | 202 ± 171 | |
| CF3 | 23 | F | 754 ± 999 | 41 ± 28 | |
| CF4 | 9 | F | 2104 ± 1314 | 90 ± 114 | |
| CF5 | 27 | M | 2794 ± 2154 | 138 ± 100 | |
| CF6 | 16 | M | 2141 ± 1232 | 141 ± 98 | |
| Non-CF controls | |||||
| Hypertension | 28 | M | 793 ± 486 | 52 ± 39 | |
| Idiopathic fibrosis | 61 | M | 1301 ± 562 | 59 ± 39 | |
| Idiopathic fibrosis | 40 | F | 631 ± 349 | 84 ± 65 |
The values represent the number of cells per square millimeter of tissue examined and are expressed as mean ± SD of at least 24 microscopic fields (magnification, ×400) of transverse bronchial sections analyzed for each patient in both the two CF and non-CF groups. M, male; F, female.
Expression of the Chemokine IL-8 Is Elevated in CF Bronchial Submucosal Gland Structures
To evaluate the possibility that the endogenous expression of pro-inflammatory cytokines is up-regulated in CF bronchial submucosal gland epithelial cells, we monitored the immunoreactivity of cytokines IL-1β, IL-6, and IL-8 in consecutive serial bronchial tissue sections obtained from eight ΔF508 homozygous CF patients and from four non-CF disease patients. Figure 1 ▶ provides a representative set of the eight CF and four non-CF cryofixed bronchial sections and shows the expression and localization of IL-8 protein by immunofluorescence. The surface epithelium of CF bronchial tissues (Figure 1, a and b) ▶ and of non-CF bronchial tissues (data not shown) demonstrated a notable absence of immunoreactivity to the IL-8 antibody.
Figure 1.
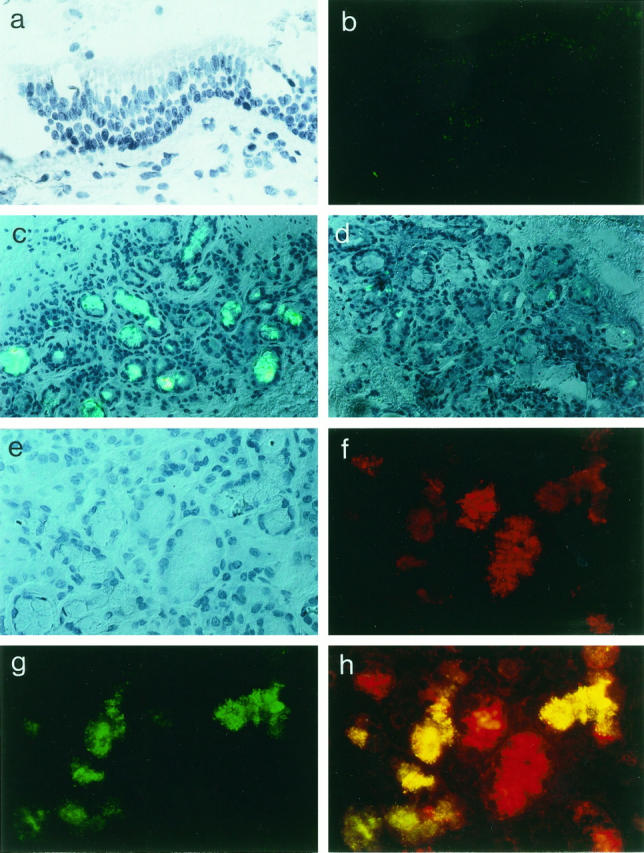
Expression and localization of chemokine IL-8 in human ΔF508 homozygous CF and non-CF bronchial tissues. Analysis of frozen tissue sections (5 μm thick) in CF bronchial surface epithelium (a and b) and submucosal gland structures from ΔF508 homozygous CF patients (c and e–h) and non-CF (control) patients is presented (d). Shown are Nomarski photomicrographs (a and e) and immunofluorescence micrographs for detection of IL-8 with FITC (green; c, d, and g) and lysozyme with Texas red (red; f and h). Note the absence of immunostaining for IL-8 at the level of ΔF508 homozygous CF bronchial surface epithelium (b) as in non-CF bronchial surface epithelium (not shown). In contrast, dense IL-8 staining is specifically observed in most of the submucosal gland cells from CF bronchial tissues (c) but is not identified in the submucosal glad cells from non-CF bronchial tissues (d). At higher magnification (×400), ΔF508 homozygous CF bronchial submucosal glands (e–h) are photographed using fluorescence filters for simultaneous red (lysozyme; f)/green (IL-8; g) visualization. The yellow color (h) indicates significant co-staining of IL-8 and lysozyme in ΔF508 homozygous CF bronchial submucosal gland serous-type cells. Results are representative of the findings in eight ΔF508 homozygous CF patients and four non-CF (control) subjects. All sections are counterstained with hematoxylin.
An examination of bronchial submucosal cryosections at low magnification revealed an intense immunoreactivity for IL-8 inside the submucosal glandular structures lying in the connective tissue of CF patients (Figure 1c) ▶ compared with non-CF bronchial tissues (Figure 1d) ▶ . Immunostaining for IL-8 was observed exclusively in the submucosal glands, with gland cells displaying strong immunoreactivity interspersed with unstained gland cells. The distal portion of bronchial submucosal glands contains a branching network of tubules that contain serous cells. At higher magnification (Figure 1, e–h) ▶ , the expression of IL-8 protein in CF bronchial gland structures was analyzed by double immunofluorescence using antibodies to both lysozyme, a specific marker for the gland serous-type cell (Figure 1f) ▶ , and IL-8 (Figure 1, g and h) ▶ . In CF bronchial submucosal glands, both the lysozyme-positive gland cells (serous-type cells) and lysozyme-negative gland cells (mucous-type cells) exhibited a high-level reaction to the IL-8 antibody.
In a second set of experiments, we examined whether pro-inflammatory cytokines other than the IL-8 chemokine were concomitantly up-regulated in the same ΔF508 CF bronchial submucosal tissue under investigation. Immunohistochemical analyses were performed on serial consecutive sections of the eight CF and four non-CF cryofixed bronchial tissues with specific antibodies to IL-1β and IL-6, respectively. Both of these cytokines are known to be markedly increased in cultured airway epithelial cells after exposure to viral 20,21 and bacterial products. 22-24 Sections showed a very low and similar level of staining for IL-1β and IL-6 in both CF and non-CF bronchial submucosal gland structures, respectively (data not shown).
Functional Activity of CFTR Protein in CF and Non-CF Bronchial Gland Cells in Culture
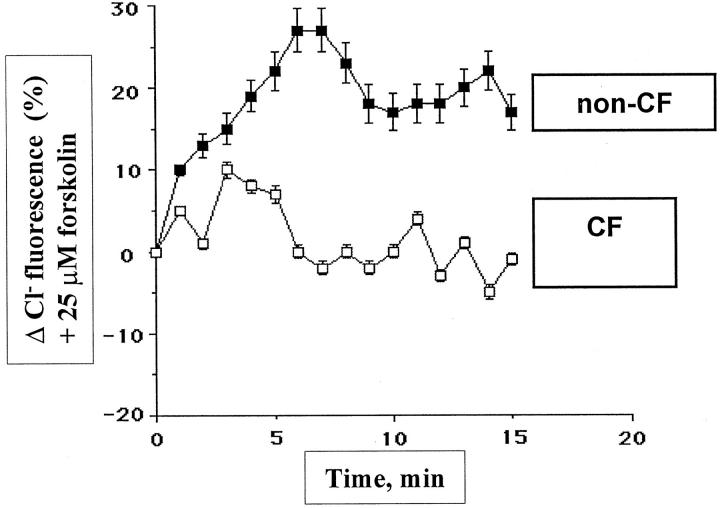
The CFTR defect in CF epithelial cells manifests itself as a defective chloride secretion in response to stimulation to cAMP agonists. To investigate the functional activity of the CFTR protein in both cultured CF and non-CF HBG cells, we made measurements of Cl− efflux in tight confluent third-passage HBG cell monolayers. We examined the Cl− secretion of non-CF and CF HBG cells after their exposure to 25 μmol/L forskolin. Results shown in Figure 2 ▶ are typical of subcultures of HBG cells isolated from all of the eight CF patients and all four non-CF control patients. Cultured HBG cells from non-CF patients demonstrated significant (P < 0.001) increases in Cl− efflux in response to exposure to forskolin compared with cultured HBG cells from ΔF508 homozygous CF patients. SPQ-loaded non-CF HBG cells exhibited a significant response to cAMP stimulation (ie, a CFTR functionality), as demonstrated by a significant increase (27%) in the fluorescence signal 6 minutes after the onset of the stimulation. In contrast, no significant Cl− efflux in response to forskolin stimulation was detected after the same period of time in CF HBG cell cultures from eight CF patients.
Figure 2.
Functional CFTR protein is demonstrated in cultured non-CF HBG cells but not in ΔF508 homozygous CF HBG cells. Changes in SPQ halide efflux for non-CF and ΔF508 homozygous CF HBG cell cultures were evaluated as described in Materials and Methods. Cells were stimulated with 25 μmol/L forskolin (time 0), and the Cl− secretion was estimated by measurements of SPQ fluorescence over a 15-minute time period. Data are presented as the relative fluorescence, this being 100X (Ft/Fo), where Ft is the fluorescence intensity at time t and Fo is the fluorescence intensity at time 0. A significant increase in Cl− efflux in response to cAMP stimulation is observed in monolayers of non-CF HBG cells. In contrast, no significant change in cAMP-stimulated Cl− efflux is observed in monolayers of ΔF508 homozygous CF HBG cells. Data shown are representative of responses obtained from four experiments for each condition.
CF HBG Cells Produce More Constitutive IL-8 Chemokine Compared with Non-CF HBG Cells
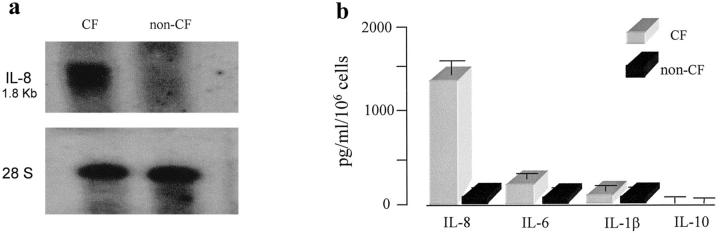
Consistent with our results of immunofluorescence detection of IL-8 protein in CF bronchial submucosal gland cells in situ, Northern blot analyses revealed that resting ΔF508 homozygous CF HBG cells constitutively produce high levels of IL-8 mRNA transcripts. Under similar culture conditions (ie, in an unstimulated resting state), there was no evidence of endogenous IL-8 mRNA transcripts in non-CF HBG cells (Figure 3a) ▶ . Moreover, the spontaneous production of IL-8 by subcultures of CF HBG cells was 13-fold higher compared with non-CF HBG cells (Figure 3b) ▶ .
Figure 3.
Comparison of the endogenous IL-8 mRNA expression and spontaneous levels of indicated cytokines released by cultured ΔF508 homozygous CF and non-CF HBG cells in the unstimulated (basal) state. Northern blot analysis (a) shows the presence of endogenous IL-8 mRNA transcripts in CF HBG cells. No evidence of endogenous IL-8 mRNA transcripts is found in non-CF HBG cells, under the same conditions of culture. Shown are Northern blots of total cellular RNA (15 μg/lane) from CF and non-CF HBG cells hybridized with a 32P-labeled IL-8 cDNA probe (top) and normalized to 28 S expression (bottom). The 1.8-kb IL-8 mRNA transcript is indicated. ELISAs (b) of the spontaneous production of the indicated cytokines in 6-hour supernatants show that the level of IL-8 release is 13-fold higher in CF HBG cells compared with non-CF HBG cells. Other cytokine levels are lower, and no significant difference between CF and non-CF HBG cell cultures is found. Values represent means ± SD of eight ΔF508 homozygous CF HBG and four non-CF HBG cell cultures, respectively, each assayed in triplicate.
The possibility that the up-regulation of IL-8 observed in CF HBG cells could be related to a spontaneous release of IL-1β and/or IL-6 by cultured CF HBG cells was investigated because these pro-inflammatory cytokines have previously been shown to be present in high amounts in CF airway secretions and known to be potent stimuli for the IL-8 secretion. 20,21 As shown in Figure 3b ▶ , similar and low levels of cytokines IL-1β and IL-6 were found in the supernatants from both CF and non-CF HBG cell cultures. As normal human bronchial surface epithelial cells were recently reported to constitutively produce the anti-inflammatory cytokine IL-10, which appeared to be down-regulated in CF human bronchial surface epithelial cells, 25 we also compared cultured CF and non-CF HBG cells for their ability to produce this cytokine. Negligible levels of IL-10 were found in the supernatants of both the CF and non-CF HBG cell cultures (Figure 3b) ▶ .
Reduction of the Extracellular Cl− Concentration Allows CF HBG Cells to Markedly Decrease Their IL-8 Secretion Level
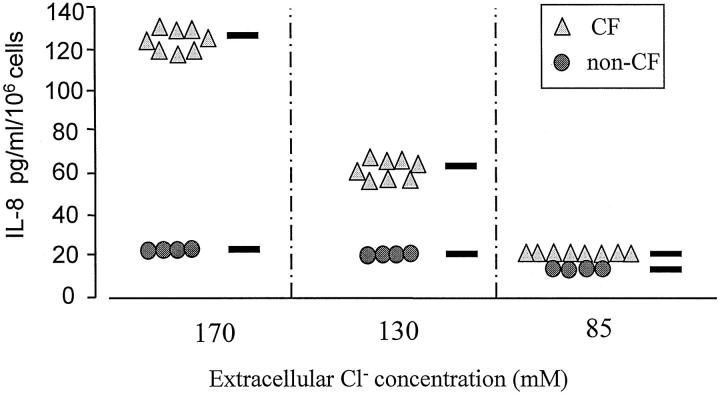
To further mimic the in vivo situation, in which a change in NaCl concentration occurs in CF bronchial secretions 14,15 as a result of CFTR deficiency (ie, 85 mmol/L in non-CF versus 120 to 170 mmol/L in CF), we examined whether both CF and non-CF HBG cells display differential expression in their release of IL-8 according to the electrolyte concentration in the extracellular medium. As shown in Figure 4 ▶ , the exposure of cell cultures to a high Cl− concentration (170 mmol/L) for 6 hours resulted in a 6.5-fold increase in IL-8 production in CF HBG cells compared with similarly treated non-CF HBG cells. More importantly, in the presence of low (85 mmol/L) and intermediate (135 mmol/L) Cl− concentration, ΔF508 homozygous CF HBG cells behaved similarly to non-CF HBG cells. Lowering the extracellular Cl− concentration from 170 mmol/L to 85 mmol/L resulted in a significant decrease (P < 0.001) in IL-8 production by CF HBG cells down to the levels of IL-8 produced by non-CF HBG cells exposed to the same low electrolyte content. These data indicate that the extracellular Cl− concentration markedly regulates the IL-8 release by CF HBG cells.
Figure 4.
Effect of the extracellular concentration of Cl− on the IL-8 production by cultured ΔF508 homozygous CF and non-CF HBG cells. Confluent monolayers of CF and non-CF HBG cells were covered by 2 ml of a solution containing 1 mol/L CaCl2, 20 mol/L KCl, and either 60 mol/L NaCl or 105 mol/L NaCl or 148 mol/L NaCl (total Cl− concentration indicated for each data set), pH 7.4. IL-8 levels were assayed in 6-hour supernatants collected from CF and non-CF HBG cell cultures exposed to a low (85 mol/L), intermediate (135 mol/L), or high (170 mol/L) Cl− concentration, respectively, as indicated. Lowering the extracellular Cl− concentration to 85 mol/L decreased the IL-8 release of CF HBG cells to a level very similar to that observed in non-CF HBG cells. Each data point represents the average of three determinations for eight ΔF508 homozygous CF HBG cell cultures and four non-CF HBG cell cultures.
Release of IL-8 Is Modestly Reduced in CF Gland Cells after Dexamethasone Treatment
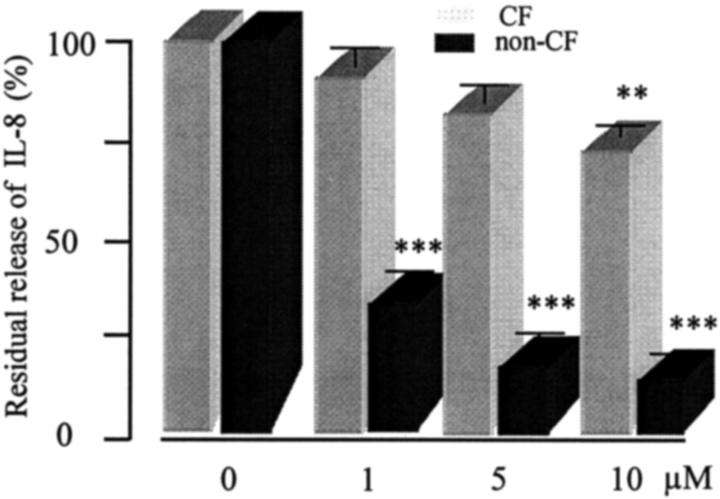
In a final set of experiments, we tested the capacity of different concentrations of glucocorticoid (1, 5, and 10 μmol/L dexamethasone) to affect the release of the IL-8 chemokine by ΔF508 homozygous CF and non-CF HBG cell cultures. Over a 6-hour culture period, the spontaneous IL-8 production of untreated CF and non-CF HBG cells was 1485 pg/ml/10 6 cells and 114 pg/ml/10 6 cells, respectively. Figure 5 ▶ shows that CF HBG cells were refractory to glucocorticoid treatment at lower concentrations (1 and 5 μmol/L) whereas the high concentration (10 μmol/L) of dexamethasone decreased by 25% the basal level of IL-8 production by CF HBG cells. In contrast to CF HBG cells, dexamethasone (1 and 5 μmol/L) significantly (P < 0.001) reduced the spontaneous release of IL-8 in a dose-dependent fashion in non-CF HBG cells.
Figure 5.
Effect of dexamethasone treatment on the IL-8 production by cultured ΔF508 homozygous CF and non-CF HBG cells. IL-8 levels were measured in 6-hour supernatants from CF and non-CF HBG cell cultures after addition of dexamethasone at final concentrations of 1, 5, and 10 μmol/L, respectively. Data are expressed as a percentage of IL-8 recovered 6-hour after dexamethasone addition by comparison to values obtained from untreated CF and non-CF HBG cell cultures (control, 100%), respectively. Interestingly, dexamethasone treatment significantly (P < 0.001) blocks the IL-8 release of non-CF HBG cells but, in contrast, failed to block the IL-8 release of CF HBG cells, even when levels were increased to 5 μmol/L. Data represent means ± SD of eight CF HBG cell cultures and four non-CF HBG cell cultures, each assayed in triplicate. **P < 0.01; ***P < 0.001.
Discussion
The early appearance of airway inflammation, distinct from that of airway infection, 4,5 prompts the proposal of a more endogenous pathway for the origin of airway inflammation in CF patients. The findings presented here suggest that the early endobronchial inflammation in CF is associated with an up-regulated constitutive expression of chemokine IL-8 at the bronchial submucosal level in CF gland secretory epithelial cells, which represent the predominant site of CFTR expression in human airways. 11,12 As shown here, the abnormally high IL-8 production by ΔF508 homozygous CF HBG cells appeared selective. In this way, secretion levels of other cytokines, such as IL-1β, IL-6, and the anti-inflammatory cytokine IL-10, by CF HBG cells in culture were low and similar to non-CF HBG cells in the unstimulated resting state, indicating no generalized disturbance of the ΔF508 homozygous genotype of CF HBG cells.
Our findings of a nonsignificant increase of neutrophils surrounding submucosal secretory glands in CF bronchial tissues as compared with non-CF periglandular tissues may appear surprising. A possible explanation for the relatively low number of neutrophils in CF bronchial submucosa and high number of neutrophils found in bronchoalveolar lavage in CF patients 4,5,9 could be their low resident time in the bronchial submucosal connective tissue and their rapid migration across the tissues into the airway lumen. To date, the primary cause initiating recruitment of neutrophils in CF lungs remains unknown. Studies with human recombinant IL-8 in SCID mice 6 have shown that IL-8 is a strong chemoattractant for neutrophils and activates neutrophils to release prestored chemotactic products mediating T lymphocyte and monocyte accumulation at sites of inflammation. In airway epithelial cells, the CFTR protein can be detected in subcellular components, such as the endoplasmic reticulum (ER) and vesicles, 26-29 and in the membrane of secretory granules of submucosal glands. 12 The different locations of CFTR protein suggest the existence of intracellular mechanisms whereby a mutated CFTR in CF HBG cells could lead to abnormalities in IL-8 secretion, as recently demonstrated by Moss et al 30 who showed a reduced IL-10 secretion by human CD4+ T lymphocytes expressing mutant CFTR. Recently, it has been suggested that the CFTR protein, depending on its level of expression, is a multifaceted molecule with multiple roles in epithelial cells. 28 Combined with our previous observation 10 showing airway inflammation in mutant CF mice raised in pathogen-free conditions, the present study reinforces our idea that CF submucosal gland secretory cells may be the first and predominant source of IL-8, which initiates the early airway mucosal inflammation in CF patients. Although it is tempting to speculate that the selective up-regulated expression of chemokine IL-8 by CF HBG cells could be related to a CFTR-dependent mechanism, the relationships between the mislocalization of mutated CFTR protein, the regulation of secretory processes, and the up-regulated IL-8 production in CF HBG cells remain open to debate. Irrespective of the mechanisms involved in the selective up-regulation of IL-8 expression by CF HBG cells, our observations support the notion that abnormal constitutive secretion of IL-8 by CF HBG cells is primarily due to a dysregulated endogenous pathway rather than a consequence of a general response to airway inflammation. Consequently, neutrophils activated by locally secreted IL-8 release elastase and oxidants that stimulate airway surface epithelial cells to produce other chemotactic factors, such as TNF-α, IL-1β, IL-6, and IL-8, 22,31 which may generate and perpetuate an inflammatory vicious cycle in CF airways. This inflammation can be thereafter greatly amplified after Pseudomonas aeruginosa infection, as recently demonstrated by Heeckeren et al 32 in CF mice. In CF, it is known that the airway epithelium is exposed to P. aeruginosa products that have previously been shown to actively increase the production of most of the pro-inflammatory cytokines by airway epithelial cells. 31,33 It has been suggested that a feedback mechanism exists, involving activated neutrophils, phagocytosis of bacteria, and epithelial IL-8, resulting in a persistent inflammation and infection in CF airways. 34
One of the most striking results that we obtained in the present study is the NaCl-dependent production of IL-8 by cultured CF HBG cells. The finding that the level of IL-8 release from CF bronchial submucosal gland cells is significantly increased during exposure to high (170 mmol/L) and intermediate (135 mmol/L) Cl− concentration is of particular significance in CF where the NaCl concentration in bronchial secretion liquids has been shown to be higher than that found in normal subjects. 14,15 Recent data have shown that high NaCl concentrations in CF airway surface fluid contribute to the diseased state by impairing the neutrophil killing of P. aeruginosa 35 and by inhibiting the bactericidal properties of CF airway fluid associated with a decreased activity of β-defensin-1 peptides produced by airway cells. 36 Our study shows, in addition, that high NaCl concentrations may contribute to and sustain an exaggerated and prolonged inflammatory state by releasing high amounts of IL-8 from CF airway submucosal glands. This aberrant secretion of IL-8 by CF gland cells can be fully corrected by reducing the extracellular NaCl concentration. Therefore, it will be of importance to define the ionic composition of fluid secreted by CF and non-CF submucosal gland cells more precisely and to characterize the contribution of these ions to surface airway fluids. Although a recent work revealed no differences in the ionic composition of airway surface liquids from bronchial regions of CF and non-CF patients, 37 the ionic composition of liquids secreted by bronchial submucosal gland cells from CF and non-CF patients is still unknown.
A possible explanation for our findings of the NaCl dose-dependent release of IL-8 by CF bronchial glands could involve abnormal dynamic changes in intracellular Ca2+ mobilization in response to the external ionic composition, as it has recently been demonstrated that a reduction in the extracellular Na+ concentration causes a rapid release of Ca2+ from internal stores in normal human airway epithelial cells. 38 Furthermore, an abnormal CFTR protein accumulation in the ER of CF gland secretory cells might result in a reduced intracellular Ca2+ handling associated with a defect in the process of exocytosis. 39 The amplitude and the duration of intracellular Ca2+ signals were recently shown to be directly responsible for the differential activation of several Ca2+-sensitive transcriptional regulators, including the transcription nuclear factor-κB (NF-κB), c-JunN-terminal kinase (JNK), and NFAT, which are required for the transcriptional activation of diverse pro-inflammatory cytokines, including IL-8. 40 In CF gland cells, a possible mechanism of action could involve the effect of the accumulation of mutant CFTR protein in the ER 41,42 on the synthesis and secretion of cytokines, as overload of the ER by resident proteins activates NF-κB by causing a rapid Ca2+ release from the ER. 43
In the present study, we have also shown that dexamethasone treatment of CF HBG cells failed to block the IL-8 production and release but, in contrast, significantly blocked the IL-8 release from non-CF HBG cells. In this way, a defective inhibition by dexamethasone in B lymphocytes in CF patients was reported. 44 The action of glucocorticoids is very complex and appears to be via the inhibition of NF-κB complex activation. 45 Whether or not the differential IL-8 response to dexamethasone treatment between ΔF508 homozygous CF and non-CF HBG cells is due to variability in the specific NF-κB complexes or to direct protein-protein interactions with mutated CFTR protein involved in regulating IL-8 gene expression requires further investigation.
In summary, we have demonstrated that CF bronchial submucosal glands constitutively produce high levels of IL-8 in the resting (unstimulated) state, which may represent the primary signal that initiates the early and sustained mucosal inflammation observed in CF lungs. 4,5 We suggest that the basic genetic defect in CF patients may be a contributory factor to the selective up-regulated IL-8 expression observed. Even so, the molecular events related to the up-regulation of the IL-8 gene in CF airway gland epithelial cells together with variations of the extracellular ion concentrations in airway fluids remain to be elucidated.
Acknowledgments
We thank the team of Service du Département de Chirurgie Cardio-Vasculaire (Prof. A. Carpentier), Hôpital Broussais, Paris, France, for their cooperation in providing lung transplant tissues.
Footnotes
Address reprint requests to Dr. Jacky Jacquot, INSERM Unité 314, IFR 53, CHU Maison Blanche, 45, rue Cognacq-Jay, 51092 Reims Cédex, France. E-mail: jacky.jacquot@univ-reims.fr.
Supported by a grant from the Association Française de Lutte contre la Mucoviscidose. The GOEMAR Laboratories (St. Malo, France) fund O. Tabary.
References
- 1.Riordan JR, Rommens JM, Kertem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, Drumm ML, Iannuzzi MC, Collins FS, Tsui LC: Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 1989, 245:1066-1073 [DOI] [PubMed] [Google Scholar]
- 2.Rich DP, Anderson MP, Gregory RJ, Cheng SH, Paul S, Jefferson DM, McCann JD, Klinger KW, Smith AE, Welsh MJ: Expression of cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells. Cell 1990, 62:1227-1233 [DOI] [PubMed] [Google Scholar]
- 3.Boucher RC: Human airway ion transport (part 1). Am J Respir Crit Care Med 1994, 150:271-2814 [DOI] [PubMed] [Google Scholar]
- 4.Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, Riches DWH: Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med 1995, 151:1075-1082 [DOI] [PubMed] [Google Scholar]
- 5.Balough KR, McCubbin M, Weinberger M, Smits W, Ahrens R, Fich R: The relationship between infection and inflammation in the early stages of lung disease from cystic fibrosis. Pediatr Pulmonol 1995, 20:63-70 [DOI] [PubMed] [Google Scholar]
- 6.Taub DT, Anver M, Oppenheim JJ, Longo L, Murphy WJ: T lymphocyte recruitment by interleukin-8. Il-8-induced degranulation of neutrophils releases potent chemoattractants for human T lymphocytes both in vitro and in vivo. J Clin Invest 1996, 97:1931-1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richman-Eisenstat JB, Jorens PG, Hebert CA, Ieki I, Nadel JA: Interleukin-8: an important chemoattractant in sputum of patients with chronic inflammatory airway diseases. Am J Physiol 1993, 26:L413-L418 [DOI] [PubMed] [Google Scholar]
- 8.Dean TP, Dai Y, Shute JK, Church MK, Warner JO: Interleukin-8 concentrations are elevated in bronchoalveolar lavage, sputum, and sera of children with cystic fibrosis. Pediatr Res 1993, 34:159-161 [DOI] [PubMed] [Google Scholar]
- 9.Noah TL, Blach HR, Cheng PW, Wood RE, Leigh MW: Nasal and bronchoalveolar lavage fluid cytokines in early cystic fibrosis. J Infect Dis 1997, 175:638-647 [DOI] [PubMed] [Google Scholar]
- 10.Zahm JM, Gaillard D, Dupuit F, Hinnrasky J, Porteous D, Dorin JR, Puchelle E: Early alterations in airway mucociliary clearance and inflammation of the lamina propria in CF mice. Am J Physiol 1997, 272:C853-C859 [DOI] [PubMed] [Google Scholar]
- 11.Engelhart JF, Yankaskas JR, Ernst SA, Yang Y, Marino CR, Boucher RC, Cohn JA, Wilson JM: Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nature Genet 1992, 2:240-248 [DOI] [PubMed] [Google Scholar]
- 12.Jacquot J, Puchelle E, Hinnrasky J, Fuchey C, Bettinger C, Spilmont C, Bonnet N, Dieterle D, Dreyer D, Pavirani A, Dalemans W: Localization of the cystic fibrosis transmembrane conductance regulator in airway secretory glands. Eur Respir J 1993, 6:169-176 [PubMed] [Google Scholar]
- 13.Jiang C, Finkbeiner WE, Widdicombe JH, Miller SS: Fluid transport across cultures of human tracheal glands is altered in cystic fibrosis. J Physiol 1997, 501:3:637-647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joris L, Dab I, Quinton P: Elemental composition of human airway surface fluid in healthy and diseased airways. Am Rev Respir Dis 1993, 148:1633-1637 [DOI] [PubMed] [Google Scholar]
- 15.Smith JJ, Travis SM, Greenberg EP, Welsh MJ: Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell 1996, 85:229-236 [DOI] [PubMed] [Google Scholar]
- 16.Franken C, Meijer CJLM, Dijkman JH: Tissue distribution of antileucoprotease and lysozyme in humans. J Histochem Cytochem 1989, 37:493-498 [DOI] [PubMed] [Google Scholar]
- 17.Jacquot J, Spilmont C, Burlet H, Fuchey C, Buisson AC, Tournier JM, Gaillard D, Puchelle E: Glandular-like morphogenesis and secretory activity of human tracheal gland cells in a three-dimensionnal collagen gel matrix. J Cell Physiol 1994, 161:407-418 [DOI] [PubMed] [Google Scholar]
- 18.Maizieres M, Kaplan H, Millot JM, Bonnet N, Manfait M, Puchelle E, Jacquot J: Neutrophil elastase promotes rapid exocytosis in human airway gland cells by producing cytosolic Ca2+ oscillations. Am J Respir Cell Mol Biol 1998, 18:32-42 [DOI] [PubMed] [Google Scholar]
- 19.Brezillon S, Zahm JM, Pierrot D, Gaillard D, Hinnrasky J, Millard H, Klossek JM, Tümmler B, Puchelle E: ATP depletion induces a loss of epithelium functional integrity and down-regulates CFTR (cystic fibrosis transmembrane conductance regulator) expression. J Biol Chem 1997, 272:27830-27838 [DOI] [PubMed] [Google Scholar]
- 20.Noah TL, Becker S: Respiratory syncytial virus-induced cytokine production by a human bronchial epithelial cell line. Am J Physiol 1993, 265:L472-L478 [DOI] [PubMed] [Google Scholar]
- 21.Terajima M, Yamaha M, Sekizawa K, Okinaga S, Suzuki T, Yamada N, Nakayama K, Ohrui T, Oshima T, Numazaki Y, Sasaki H: Rhinovirus infection of primary cultures of human tracheal epithelium: role of ICAM-1 and IL-1b. Am J Physiol 1997, 273:L749-759 [DOI] [PubMed] [Google Scholar]
- 22.Massion PP, Inoue H, Richman-Eisenstat J, Grunberger D, Jorens PG, Housset B, Pittet JF, Wiener-Kronish JP, Nadel JA: Novel Pseudomonas product stimulates interleukin-8 production in airway epithelial cells in vitro. J Clin Invest 1994, 93:26-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoue H, Massion PP, Ueki IF, Grattan KM, Hara M, Dohrman AF, Chan B, Lausier JA, Golden JA, Nadel JA: Pseudomonas stimulates interleukin-8 mRNA expression selectively in airway epithelium, in gland ducts, and in recruited neutrophils. Am J Respir Cell Mol Biol 1994, 11:651-663 [DOI] [PubMed] [Google Scholar]
- 24.DiMango E, Zar HJ, Bryan R, Prince A: Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J Clin Invest 1995, 96:2204-2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonfield TL, Konstan MW, Burfeind P, Panuska JR, Hilliard JB, Berger M: Inflammatory cytokines in cystic fibrosis lungs. Am J Respir Crit Care Med 1995, 152:2111-2118 [DOI] [PubMed] [Google Scholar]
- 26.Barasch J, Kiss B, Prince A, Salman L, Gruenert D, Al-Awqati Q: Defective acidification of intracellular organelles in cystic fibrosis. Nature 1991, 352:70-73 [DOI] [PubMed] [Google Scholar]
- 27.Bradbury NA, Jilling T, Berta G, Sorscher EJ, Bridges R, Kirk KL: Regulation of plasma membrane recycling by CFTR. Science 1992, 256:530-532 [DOI] [PubMed] [Google Scholar]
- 28.Tümmler B, Puchelle E: CFTR: a multifaceted molecule. Trends Cell Biol 1997, 7:250-251 [DOI] [PubMed] [Google Scholar]
- 29.Cheng SH, Gregory JG, Marshall J, Paul S, Souza DW, White GA, O’Riordan CR, Smith AE: Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell 1990, 63:827-83 [DOI] [PubMed] [Google Scholar]
- 30.Moss RB, Bocian RC, Hsu YP, Dong YJ, Kemma M, Wei T, Gardner P: Reduced IL-10 secretion by CD4+ T lymphocytes expressing mutant cystic fibrosis transmembrane conductance regulator (CFTR). Clin Exp Immunol 1996, 106:374-388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruef C, Jefferson DM, Schlegel-Haueter SE, Suter S: Regulation of cytokine secretion by cystic fibrosis airway epithelial cells. Eur Respir J 1993, 6:1429-1436 [PubMed] [Google Scholar]
- 32.Heeckeren AV, Walenga R, Konstan MW, Bonfield T, Davis PB, Ferkol T: Excessive inflammatory response of cystic fibrosis mice to bronchopulmonary infection with Pseudomonas aeruginosa. J Clin Invest 1997, 100:2840-2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kammouni W, Figarella C, Marchand S, Merten M: Altered cytokine production by cystic fibrosis tracheal gland serous cells. Infect Immun 1997, 65:5176-5183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Döring G: The role of neutrophil elastase in chronic inflammation. Am J Respir Crit Care Med 1995, 152:163-168 [DOI] [PubMed] [Google Scholar]
- 35.Tager AM, Wu J, Wermulen MW: High extracellular chloride concentration impairs neutrophil killing of Pseudomonas aeruginosa. Am J Respir Crit Care Med 1998, 157:A259 [Google Scholar]
- 36.Goldmann MJ, Anderson GM, Stolzenberg ED, Kari UP, Zasloff M, Wilson JM: Human β-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell 1997, 88:553-560 [DOI] [PubMed] [Google Scholar]
- 37.Knowles MR, Robinson JM, Wood RE, Pue CA, Mentz WM, Wager GC, Gatzy JT, Boucher RC: Ion composition of airway surface liquid of patients with cystic fibrosis as compared with normal and disease-control subjects. J Clin Invest 1997, 100:2588-2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boitano S, Woodruff ML, Dirksen ER: Reduction of extracellular Na+ causes a release of Ca2+ from internal stores in airway epithelial cells. Am J Physiol 1997, 272:L1189-L1197 [DOI] [PubMed] [Google Scholar]
- 39.Jacquot J, Maizieres M, Spilmont C, Millot JM, Sebille S, Merten M, Kammouni W, Manfait M: Intracellular free Ca2+ dynamic changes to histamine are reduced in cystic fibrosis human tracheal cells. FEBS Lett 1996, 386:123-127 [DOI] [PubMed] [Google Scholar]
- 40.Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI: Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature 1997, 386:855-858 [DOI] [PubMed] [Google Scholar]
- 41.Yang Y, Janich S, Cohn JA, Wilson JM: The common variant of cystic fibrosis transmembrane conductance regulator is recognized by hsp 70 and degraded in a pre-Golgi nonlysosomal compartment. Proc Natl Acad Sci USA 1993, 90:9480-9484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pind S, Riordan JR, Williams DB: Participation of the endoplasmic reticulum chaperone calnexin (p88, IP90) in the biogenesis of the cystic fibrosis transmembrane conductance regulator. J Biol Chem 1994, 269:12784-12788 [PubMed] [Google Scholar]
- 43.Pahl HL, Sester M, Burgert HG, Baeuerle PA: Activation of transcription factor NF-kB by the adenovirus E3/19K protein requires its ER retention. J Cell Biol 1996, 132:511-522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Emilie D, Crevon MC, Chicheportiche R, Auffredou MT, Baro-Ciorbaru R, Lenoir G, Dayer JM, Galanaud P: Cystic fibrosis patient’s B lymphocyte response is resistant to the in vitro enhancing effect of corticoids. Eur J Clin Invest 1990, 20:620-626 [DOI] [PubMed] [Google Scholar]
- 45.Blackwell TS, Christman JW: The role of nuclear factor-kB in cytokine gene regulation. Am J Respir Cell Mol Biol 1997, 17:3-9 [DOI] [PubMed] [Google Scholar]