Abstract
Tumor necrosis factor-α is up-regulated in a variety of different human immune-inflammatory and fibrotic pulmonary pathologies. However, its precise role in these pathologies and, in particular, the mechanism(s) by which it may induce fibrogenesis are not yet elucidated. Using a replication-deficient adenovirus to transfer the cDNA of tumor necrosis factor-α to rat lung, we have been able to study the effect of transient but prolonged (7 to 10 days) overexpression of tumor necrosis factor-α in normal adult pulmonary tissue. We have demonstrated that local overexpression resulted in severe pulmonary inflammation with significant increases in neutrophils, macrophages, and lymphocytes and, to a lesser extent, eosinophils, with a peak at day 7. By day 14, the inflammatory cell accumulation had declined, and fibrogenesis became evident, with fibroblast accumulation and deposition of extracellular matrix proteins. Fibrotic changes were patchy but persisted to beyond day 64. To elucidate the mechanism underlying this fibrogenesis, we examined bronchoalveolar fluids for the presence of the fibrogenic cytokine transforming growth factor-β1 and tissues for induction of α-smooth muscle actin-rich myofibroblasts. Transforming growth factor-β1 was transiently elevated from day 7 (peak at day 14) immediately preceding the onset of fibrogenesis. Furthermore, there was a striking accumulation of myofibroblasts from day 7, with the most extensive and intense immunostaining at day 14, ie, coincident with the up-regulation of transforming growth factor-β1 and onset of fibrogenesis. Thus, we have provided a model of tumor necrosis factor-α-mediated pulmonary inflammation and fibrosis in normal adult lung, and we suggest that the fibrogenesis may be mediated by the secondary up-regulation of transforming growth factor-β1 and induction of pulmonary myofibroblasts.
Tumor necrosis factor-α (TNF-α) is a pleiotropic cytokine. It has been detected in a variety of human pulmonary diseases, but its role in these pathologies is not well understood. Based on its in vitro effects on leukocyte activation, adhesion molecule expression, and endothelial cell biology, it is also likely to have proinflammatory activities in vivo. 1 In addition, it may have fibrogenic potential, given that it is both mitogenic and chemotactic for fibroblasts, 2 although its effects on collagen gene expression are inhibitory. 3 Clearly, however, up-regulation of TNF-α in vivo does not always result in the same biological outcome. In particular, TNF-α is detected in many inflammatory and immune diseases that resolve without tissue fibrosis, 1,4 yet in other pathologies TNF-α up-regulation is associated with fibrotic sequelae. 5,6 Whether these outcomes depend directly on TNF-α or indirectly on induction of other bioactive molecules, particularly the cytokines and growth factors, from both immune-inflammatory and structural cells remains unclear.
To investigate the functional activities of local pulmonary overexpression of TNF-α in lung, we have chosen to exploit a transient gene transfer approach using a replication-deficient recombinant adenovirus vector to transfer the cDNA of murine TNF-α to the respiratory epithelium. Using similar cytokine-expressing adenovectors under control of high-efficiency constitutive promoters, we and others have shown that after intratracheal administration, the vectors infect respiratory epithelium and result in local and transient, but sustained, production of their transgene protein in the background of a normally developed adult lung. 7-9 This is likely to mimic the type of cytokine up-regulation seen in human immune and inflammatory pulmonary pathologies.
Using this gene transfer approach we have been able to demonstrate that transient local overexpression of high levels of TNF-α in adult rat lung results in intense pulmonary accumulation of neutrophils, macrophages, and lymphocytes and, to a lesser extent, eosinophils over 2 to 10 days without gross distortion of the lung architecture. This is followed by the development of patchy interstitial fibrogenesis, associated with significant increases in transforming growth factor-β1 (TGF-β1) protein and accumulation of myofibroblasts.
Materials and Methods
Adenoviral Vectors and Administration to Rat Lung
A replication-deficient recombinant adenovirus expressing full-length murine TNF-α cDNA under control of an efficient murine cytomegalovirus promoter and with the interferon-γ-secretory leader sequence was used to locally overexpress TNF-α in the lung. Its construction and demonstration of release of the secreted form of TNF-α have been previously described. 10 This vector or a control vector with identical deletions (AdDL70-3) 11 at a dose of 10 9 plaque-forming units in 300 μl of phosphate-buffered saline was instilled intratracheally to anesthetized Sprague Dawley rats weighing 220 to 280 g. All animal experiments were approved by the McMaster University Animal Ethics Committee. Rats were provided with food and water ad libitum and were examined at 1, 3, 7, 14, 21, 28, or 64 days. Blood was taken from the abdominal aorta, and bronchoalveolar lavage (BAL) was performed as previously described. 8 BAL total cell counts were determined using a hematocytometer. Differential cell counts were performed on cytospins stained with Diff-Quik (Baxter, McGaw Park, IL) by randomly counting 500 cells/cytospin. Three to five animals per group were evaluated. BAL fluid and sera were stored at −20°C until TGF-β1 and TNF-α levels were determined in BAL fluid and serum as described below.
TNF-α and TGF-β Measurements
Concentrations of TNF-α in BAL fluid and serum were determined using a commercial enzyme-linked immunosorbent assay (ELISA) kit specific for murine and rat TNF-α (R&D Systems, Minneapolis, MN) with a sensitivity of 5 pg/ml. TGF-β1 in BAL fluid was measured using a commercial human TGF-β1 ELISA kit that efficiently detects rat TGF-β1 protein due to the high homology of TGF-β1 across species (R&D Systems). The assay detects only the active form of TGF-β1, and samples were activated before measurement according to the manufacturer’s instructions. The sensitivity of this kit is 5 pg/ml. Analysis was performed on samples from three to six animals per group.
Lung Fixation and Histological Examination
Lungs were removed and fixed by perfusion with neutral buffered formaldehyde before routine processing and paraffin embedding. Multiple sections from each lobe were stained with hematoxylin and eosin or Elastic van Gieson, a specific histochemical stain for collagen and elastin. Lungs from three to six rats per time point were examined.
Immunohistochemical Staining for α-Smooth Muscle Actin
Lung tissue sections were deparaffinized, and endogenous peroxidase was blocked. Sections were then treated with blocking goat serum for 30 minutes and were incubated for 16 hours with a monoclonal anti-α-smooth muscle actin (α-SMA) antibody (Sigma Chemical Co., St. Louis, MO) at a dilution of 1:100. Control sections were treated with control mouse immunoglobulin G. Sections were then incubated for 15 minutes with biotinylated goat anti-mouse immunoglobulin (Histostain-SPTM Bulk Kit; Zymed Labororatories, Inc., San Francisco, CA) and treated for 10 minutes in streptavadin-peroxidase conjugate. Finally, they were placed in a substrate chromogen mixture, and color was allowed to develop for 15 minutes before counterstaining with Mayer’s hematoxylin.
Data Analysis
Data are expressed as means ± standard error of the mean (SEM). Statistical analysis was performed using an unpaired t-test. The difference was considered statistically significant when P < 0.05.
Results
TNF-α Protein Expression in Vivo after TNF-α Gene Transfer to the Lung
To assess the effects of local overexpression of TNF-α in lung tissue, AdTNF-α or control virus AdDL70-3 was injected intratracheally into rat lung. TNF-α protein expression in lung was then examined at various time points by assaying BAL fluid by ELISA. In rats infected with AdTNF-α, TNF-α protein expression was high between days 1 and 7, peaked at day 3 (154.9 ± 45.3 ng/ml), and rapidly declined by day 14 (Figure 1) ▶ . In comparison, levels of TNF-α induced by the control virus were very low, with a peak also at day 3 (0.14 ± 0.12 ng/ml) (data not shown). These differences between AdTNF-α- and AdDL70-3-infected rats were highly significant P < 0.0001 at day 3 and P = 0.0062 at day 7. To confirm that the transgene product was largely compartmentalized in the lung, sera were taken at various time points and assayed by ELISA for TNF-α protein. TNF-α was not detected in sera of any rats infected with control virus. In rats infected with AdTNF-α, TNF-α protein was detected in animals only at days 1 and 3, and levels were about 100- to 300-fold lower than in the BAL fluid, ie, 0.21 ± 0.051 ng/ml at day 1 and 0.53 ± 0.06 ng/ml at day 3.
Figure 1.
TNF-α protein concentration in BAL fluid. BAL fluid samples were collected from rats receiving AdTNF-α or control virus at various time points and were assayed for TNF-α by ELISA. Control virus resulted in production of 0.14 ± 0.12 ng/ml TNF-α (data not graphed). Results shown are for AdTNF-α-infected rats and are expressed as means ± SEM from three to six animals/time point. *P < 0.0001 and **P = 0.0062, respectively, between AdTNF-α and control virus.
Cellular Changes in the BAL Fluid after TNF-α Gene Transfer to the Lung
To assess the cellular responses after overexpression of TNF-α in the lung, total and differential cell counts in BAL fluid were quantitated at a variety of time points. Infection with the control vector resulted in low levels of neutrophilia at day 1 and, subsequently, a small increase in the number of macrophages and lymphocytes at day 7 (Figure 2A ▶ /B). In contrast, infection with AdTNF-α resulted in a dramatic accumulation of neutrophils, macrophages (Figure 2A) ▶ , and lymphocytes in the BAL fluid (Figure 2B) ▶ . Cell accumulation was evident from day 3 but was most significant at day 7, when, compared with control vector-treated rats, AdTNF-α-treated rats demonstrated a 39-fold increase in neutrophils, a 6-fold increase in macrophages, and a 17-fold increase in lymphocytes. There was also a small increase in eosinophils. The cellular accumulation resolved thereafter. Neutrophil and macrophage numbers returned toward those observed in rats infected with control virus by day 14, and lymphocytes numbers returned to control levels by day 21.
Figure 2.

Effects of TNF-α overexpression on macrophage and neutrophil accumulation (A) and lymphocyte and eosinophil accumulation (B) in BAL fluid. Rats were infected intratracheally with AdTNF-α or control virus, and BAL fluid were recovered as described in text. Differential cell counts were then determined on Diff-Quik-stained cytospins. Results are expressed as means ± SEM from three to five animals/time point.
Pulmonary Tissue Responses after TNF-α Gene Transfer to the Lung
Rats infected with control virus appeared healthy, and microscopic examination showed some minor inflammatory changes with a few neutrophils and mononuclear cells in the perivascular and peribronchial areas at days 1, 3, and 7. These inflammatory changes did not extend into the alveolar spaces or septae beyond, and they rapidly cleared with no evidence of tissue remodeling or fibrosis, similar to the minimal changes seen with a second control vector expressing the marker gene β-galactosidase. 8 In contrast, rats receiving AdTNF-α lost weight and appeared lethargic. Macroscopically, areas of consolidation were evident at early time points. Microscopic examination of their lungs revealed widespread and severe inflammatory changes beginning 24 hours after infection, when there was accumulation of neutrophils in the alveolar spaces and of neutrophils and mononuclear cells in the interstitium, particularly in perivascular and peribronchial areas (data not shown). These changes were much more severe than in control vector-treated rats. By day 3, accumulation of inflammatory cells was more pronounced (Figure 3A) ▶ , and by day 7, in keeping with the kinetics of cell accumulation in the BAL fluid, there was intense tissue inflammation with dramatic accumulation of neutrophils and mononuclear cells, particularly in perivascular and peribronchial areas, but extending widely throughout the parenchyma right up to the pleural surface (Figure 3B) ▶ . The alveolar septae were expanded by infiltrated inflammatory cells, but the general tissue architecture was maintained. Only a few eosinophils were observed. By day 14, the tissue neutrophilia had resolved while there was still accumulation of perivascular and peribronchial mononuclear cells, although this was much less severe than at day 7. However, at this time point, there was thickening of some alveolar walls and emergence of areas of interstitial fibrosis with accumulation of fibroblasts and destruction of normal tissue architecture (Figure 3C) ▶ . Deposition of disorganized collagen and elastic fibers was evident on Elastic van Gieson staining (Figure 3D) ▶ . Inflammation had greatly declined by day 21, but some patchy fibrotic changes persisted, with areas of fibrosis scattered throughout the parenchyma, sometimes reaching the pleural surface. These fibrotic changes persisted up to day 64 (Figure 3E) ▶ , when there was deposition of more organized collagen and elastin fibers. Inflammatory changes had largely resolved by this point.
Figure 3.

Histopathological effects induced by overexpression of AdTNF-α at various time points. A, B, C, and E are stained with hematoxylin and eosin; D is stained with Elastic van Gieson (collagen stained red and elastin black). Cells are shown on day 3 (A), day 7 (B), day 14 (C and D), and day 64 (E) after infection. Magnifications: ×160 (A, B, and E) and ×250 (C and D). Arrows show fibrogenic response in the interstitium. Arrowhead shows patchy scarring and distorted architecture in the interstitium. b, bronchial structure; v, vascular structure.
Examination of TGF-β1 Production after TNF-α Gene Transfer to the Lung
To help address the mechanism of TNF-α-induced interstitial fibrosis, we examined the content of TGF-β1 in BAL fluid by ELISA. We and others have shown TGF-β1 to be a key fibrogenic cytokine in the lung. 7,12 Increased levels of TGF-β1 were evident in AdTNF-α-treated rats from day 3 with a peak at day 14 (P = 0.0057, AdTNF-α compared with control) and a decrease back toward control levels by day 64 (Figure 4) ▶ . No such increase in TGF-β1 was observed in control vector-infected rats.
Figure 4.
TGF-β1 concentration in BAL fluid. BAL samples were collected from rats receiving AdTNF-α or control virus (AdDL70-3) at various time points and were assayed for TGF-β1 by ELISA. Results are expressed as means ± SEM from three to six animals/time point. *P = 0.0057 between AdTNF-α and control virus.
Immunolocalization of Myofibroblasts after TNF-α Gene Transfer to the Lung
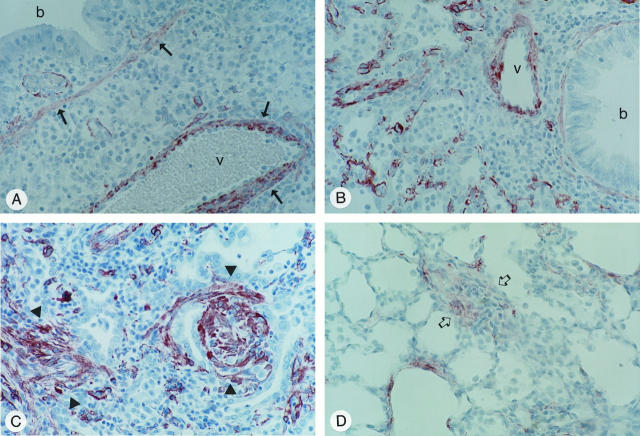
The myofibroblast is a fibroblast-like cell characterized by the expression of α-SMA 13 and is thought to be important in fibrogenesis and wound healing. 14 To assess whether TNF-α overexpression resulted in the generation of myofibroblasts during the fibrogenic process, we examined lung tissues for induction of α-SMA-expressing myofibroblasts using a monoclonal antibody directed against α-SMA. In control vector lungs and in lungs of AdTNF-α-infected rats, immunostaining of bronchial wall and vascular smooth muscle was observed as expected. There was no other immunostaining in control virus-treated animals at any time points. In AdTNF-α-treated rats, there was no increase in α-SMA immunostaining above control virus-infected animals at day 3 (Figure 5A) ▶ ; however, by day 7, immunostaining of fibroblastic cells was evident widely throughout the parenchyma (Figure 5B) ▶ . By day 14, when fibrogenesis was evident, there was intense and extensive immunostaining of fibroblastic cells in fibrotic foci within the interstitium (Figure 5C) ▶ . Immunostaining within these fibrotic areas persisted up to day 64, although it was not as extensive or intense as it had been on day 14 (Figure 5D) ▶ . The fibroblast-like cells staining for α-SMA also immunostained with an antibody directed against vimentin. This confirmed that these cells were myofibroblasts and not smooth muscle cells.
Figure 5.
α-SMA-positive cells in lung tissue from rats infected with AdTNF-α. Lungs were fixed and processed for immunohistochemical staining using a monoclonal antibody directed against α-SMA. A: Cells on day 3 after infection. Immunoreactivity is primarily localized to the smooth muscle layer (arrow) around a bronchial structure (b) and a vessel (v). B and C: Cells on days 7 and 14 after infection, respectively. Arrowheads in C indicate intense immunostaining of fibroblastic cells in fibrotic foci. D: Cells on day 64. Arrows indicate less intense immunostaining in a fibrotic area. Magnification: ×250 (A to D).
Discussion
TNF-α is an “early-wave” alarm-type cytokine. It appears to be up-regulated soon after injury in a number of tissues including lung, and increased TNF-α expression has certainly been documented in a wide variety of different human pulmonary diseases and animal models. However, our understanding of its precise role, particularly in the process of pulmonary fibrogenesis, is poorly understood. An effective strategy of assessing the role of any cytokine in vivo is to locally overexpress it in a tissue-specific manner, and there are several ways to achieve this. Administration of recombinant cytokines is one possibility. Using this approach, Ulich et al 15 administered recombinant TNF-α to rat lung and demonstrated induction of moderate neutrophilia, which resolved within 48 hours, leaving no evidence of permanent tissue damage. An alternative strategy is to develop a tissue-specific transgenic animal model, and using this approach, Miyazaki et al 16 reported their findings in a transgenic model in which TNF-α gene expression was driven from the lung-specific SP-C promoter. In contrast to the recombinant protein experiments, these mice developed an early lymphocytic alveolitis, followed later by a fibrogenic response. Although both of these approaches provide useful information on the in vivo biological effects of TNF-α, they may not accurately reflect the type of overexpression seen in adult human disease, in which TNF-α up-regulation is likely to be more prolonged than in experiments using recombinant proteins, but shorter than the lifelong overexpression of genetic transgenics. In addition, unlike the permanent transgenic models in which abnormal lung development can occur as a consequence of transgene overexpression in utero, most adult lung disease occurs in the background of a normally developed lung. 16,17 To overcome some of these problems and to examine the role of TNF-α overexpression in normal adult lung, we chose a transient transgenic approach using a replication-deficient adenovirus. 10 We demonstrated that delivery of the adenoviral gene transfer vector resulted in high levels of transgene overexpression with a peak at day 3 and a rapid decline after 7 days. This induced rapid and intense accumulation of neutrophils, macrophages, and lymphocytes from 24 hours. Inflammation in both BAL fluid and tissue was maximal at day 7, ie, shortly after the peak of transgene protein expression. Inflammation thereafter started to resolve, although inflammatory changes were still evident at days 21 and 28, long after transgenic protein was no longer detectable. The inflammatory changes were most severe in the peribronchial areas, likely reflecting the high concentrations of transgene protein in these areas. However, these changes also spread throughout the parenchyma up to the pleural surface, either as a result of diffusion of the cytokine itself, or more likely by TNF-α-mediated induction of other inflammatory cytokines such as interleukin-8, granulocyte-macrophage colony-stimulating factor, or macrophage chemotactic protein-1 from adjacent cells in a paracrine fashion. 18,19 The neutrophilia in this model was more severe than in either the recombinant protein or transgenic models, perhaps because of higher local tissue concentrations of TNF-α. Lymphocytic accumulation was a notable feature in the transgenic model, as in the present study, but increases in the number of macrophages seem specific to our model. The mechanisms of this TNF-α-mediated accumulation of neutrophils, lymphocytes, macrophages, and eosinophils are likely to involve both direct effects of TNF-α itself on regulation of adhesion molecule expression and induction of other cytokines and growth factors capable of mediating leukocyte chemotaxis and survival. 1 After the intense inflammation in the tissue, there was evidence of a fibrogenic response. This was evident from day 14, when there was accumulation of fibroblasts and deposition of both collagen and elastin. The destruction of normal lung architecture and the persistence of these changes to day 64 likely indicate that this fibrogenesis is, at least in part, irreversible. Certainly, TNF-α has been shown to be up-regulated in a number of fibrotic human and animal pulmonary pathologies, 5,6,20,21 and there is evidence that inhibiting early TNF-α expression with either anti-TNF-α antibodies or TNF-α antagonists inhibits fibrogenesis. 22,23 Equally, however, TNF-α up-regulation has been documented in other inflammatory and immune lung pathologies in which normal lung repair processes ensue without evidence of fibrotic reactions. 1 This raises the question of the precise role of TNF-α in vivo during pulmonary fibrogenesis and whether TNF-α may be acting indirectly through up-regulation of other fibrogenic molecules during pathological processes with a fibrotic sequela. In particular, it appears that TGF-β1 is up-regulated in many of the pathologies in which TNF-α is associated with fibrosis but not in those where TNF-α expression results in immune-inflammatory damage without a fibrotic outcome. 5,24,25 To address this hypothesis, we examined the AdTNF-α-infected animals for up-regulation of TGF-β1 protein and found increased protein synthesis from day 3, with a peak around day 7 to 14, immediately preceding the onset of fibrogenesis. To further investigate the mechanism of the fibrogenesis in this model, we then examined tissue for induction of α-SMA-expressing myofibroblasts. Myofibroblasts have been demonstrated in a variety of fibrotic and repairing tissues 14,26 and have been shown to be the major cells producing procollagen mRNA in the bleomycin model of fibrosis. 27 In the AdTNF-α-treated rats, aside from the expected immunopositive bronchial and vascular smooth muscle, we found α-SMA and vimentin-immunopositive fibroblast-like cells from day 7 scattered widely throughout the parenchyma. By day 14, there was intense immunostaining within fibroblast-like cells in the fibrotic foci, and staining persisted to day 64 although, its intensity and extent were lessened. Interestingly, in the studies of Rubbia-Brandt et al, 28 in which TNF-α was administered subcutaneously by osmotic minipump, fibroblast accumulation occurred, but there was no evidence of induction of myofibroblasts and minimal collagen deposition, suggesting that TNF-α overexpression alone is insufficient to generate myofibroblasts. 28 In contrast, overexpression of TGF-β1 in lung or skin results in induction of many α-SMA-rich myofibroblasts and extensive collagen deposition. 7,29 These data suggest that in the present model, induction of myofibroblasts may be secondary to the increased TGF-β1 protein rather than to a direct effect of TNF-α. Certainly, the temporal association of increased TGF-β1 and induction of myofibroblasts at days 7 to 14 would support this concept. Interestingly, in the transgenic study of Miyazaki et al, 16 no evidence of TGF-β gene activation was found, and there was no induction of myofibroblasts. However, only late time points, after fibrogenesis was established, were examined. The cellular origin of the TGF-β1 in this current model is under investigation. In other fibrogenic models, macrophages, 30,31 eosinophils, 32 and tissue structural cells 1 have all been shown to be important sources, and it is therefore noteworthy that macrophage and eosinophil numbers are increased early in our model. It is interesting to note that based on our own studies of adenovector-mediated overexpression of other fibrogenic cytokines, including TGF-β1, 7 granulocyte-macrophage colony-stimulating factor, 33 and now TNF-α, there appears to be a correlation between the levels of TGF-β1, the extent of myofibroblast induction, and fibrosis. Specifically, in the TGF-β1 model, TGF-β1 levels are very high, myofibroblasts are very numerous, and fibrosis is severe, but in the TNF-α model, levels of TGF-β1 are lower; myofibroblast induction is less than in the TGF-β1 model, especially at later time points; and fibrosis is correspondingly less severe. The granulocyte-macrophage colony-stimulating factor model lies intermediately between the other models in all three parameters: TGF-β1 induction, myofibroblast presence, and fibrosis. 34
In conclusion, our study has provided evidence that in normal adult lung, transient overexpression of TNF-α results in intense but transient inflammation and patchy interstitial fibrosis associated with induction of TGF-β1 and myofibroblasts.
Acknowledgments
We thank Xueya Feng, Duncan Chong, and Susanna Goncharova for their invaluable technical assistance.
Footnotes
Address reprint requests to Dr. Jack Gauldie, Room 2N16, Health Sciences Center, Department of Pathology, McMaster University, Hamilton, Ontario, Canada L8N 3Z5. E-mail: gauldie@fhs.csu.mcmaster.ca.
Supported by the Medical Research Council (Canada) and the Norman Salvesen Emphysema Research Trust (United Kingdom). PJS is a Parker B. Francis Fellow.
References
- 1.Ulich TR: Tumor necrosis factor. Cytokines of the Lung. 1993, :pp 307-332 Marcel Dekker, Edited by J. Kelleys. New York [Google Scholar]
- 2.Leibovich SJ, Polverini PJ, Shepard MH, Wiseman DM, Shivley V, Nusewir N: Macrophage-induced angiogenesis is mediated by TNF-α. Nature 1987, 329:630-632 [DOI] [PubMed] [Google Scholar]
- 3.Mauviel A, Daireaux F, Redini F, Galera P, Loyau G, Pujol J-P: Tumor necrosis factor inhibits collagen and fibronectin synthesis in human dermal fibroblasts. FEBS Lett 1988, 236:47-52 [DOI] [PubMed] [Google Scholar]
- 4.Nelson S, Bagby GJ, Bainton BG, Wilson LA, Thompson JJ, Summer WR: Compartmentalization of intraalveolar and systemic LPS-induced TNF and the pulmonary inflammatory response. J Infect Dis 1989, 159:189-194 [DOI] [PubMed] [Google Scholar]
- 5.Phan SH, Kunkel SL: Lung cytokine production in bleomycin-induced pulmonary fibrosis. Exp Lung Res 1992, 18:29-43 [DOI] [PubMed] [Google Scholar]
- 6.Piguet PF, Collart MA, Grau GE, Sappino AP, Vassalli P: Requirement of TNF for development of silica-induced pulmonary fibrosis. Nature 1990, 344:245-247 [DOI] [PubMed] [Google Scholar]
- 7.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J: Adenovirus-mediated gene transfer of active transforming growth factor-β 1 induces prolonged severe fibrosis in rat lung. J Clin Invest 1997, 100:768-776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xing Z, Braciak T, Jordana M, Croitoru K, Graham FL, Gauldie J: Adenovirus-mediated cytokine gene transfer at tissue sites: overexpression of IL-6 induces lymphocytic hyperplasia in the lung. J Immunol 1994, 153:4059-4069 [PubMed] [Google Scholar]
- 9.Sime PJ, Xing Z, Foley R, Graham FL, Gauldie J: Transient gene transfer and expression in the lung. Chest 1997, 111:89S-94S [DOI] [PubMed] [Google Scholar]
- 10.Marr RA, Addison CL, Snider D, Muller WJ, Gauldie J, Graham FL: Tumour immunotherapy using an adenoviral vector expressing a membrane mutant of murine TNF-α. Gene Ther 1997, 4:1181-1188 [DOI] [PubMed] [Google Scholar]
- 11.Bett AJ, Haddara W, Prevec L, Graham FL: An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 2. Proc Natl Acad Sci USA 1994, 91:8802-8806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelley J: Transforming growth factor-β. Kelleys J eds. Cytokines of the Lung. 1993, :pp 101-137 Marcel Dekker, New York [Google Scholar]
- 13.Darby I, Skalli O, Gabbiani G: α-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest 1990, 63:21-29 [PubMed] [Google Scholar]
- 14.Desmouliere A: Factors influencing myofibroblast differentiation during wound healing and fibrosis. Cell Biol Int 1995, 19:471-476 [DOI] [PubMed] [Google Scholar]
- 15.Ulich TR, Watson LR, Yin S, Guo K, Wang P, Thang H, del Castillo J: The intratracheal administration of endotoxin and cytokines: characterization of lipopolysaccharide (LPS)-induced interleukin (IL)-1 and tumor necrosis factor (TNF) mRNA expression and the LPS-, IL-1-, and TNF-induced inflammatory infiltrate. Am J Pathol 1991, 138:1485-1496 [PMC free article] [PubMed] [Google Scholar]
- 16.Miyazaki M, Araki Y, Vesin C, Garcia I, Kapanci Y, Whitsett JA, Piguet PF, Vassalli P: Expression of a tumor necrosis factor-α transgene in murine lung causes lymphocytic and fibrosing alveolitis: a mouse model of progressive pulmonary fibrosis. J Clin Invest 1995, 96:250-259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou L, Dey CR, Wert SE, Whitsett JA: Arrested lung morphogenesis in transgenic mice bearing an SP-C-TGF-β1 chimeric gene. Dev Biol 1996, 175:227-238 [DOI] [PubMed] [Google Scholar]
- 18.Strieter RM, Kunkel SL, Showell HJ, Remick DG, Phan SH, Ward PA, Marks RM: Endothelial cell gene expression of a neutrophil chemotactic factor by TNF-α, LPS, and IL-1β. Science 1989, 243:1467-1469 [DOI] [PubMed] [Google Scholar]
- 19.Kunkel SL, Strieter RM, Chensue SW, Basha M, Standford T, Ham J, Remick DG: TNF, IL-8 and chemotactic cytokines. Melli M Parentes L eds. Cytokines and Lipocortins in Inflammation and Differentiation. 1990, :pp 433-444 Wiley-Liss, New York [PubMed] [Google Scholar]
- 20.Piguet PF, Ribaux C, Karpuz V, Grau GE, Kapanci Y: Expression and localization of tumor necrosis factor-α and its mRNA in idiopathic pulmonary fibrosis. Am J Pathol 1993, 143:651-655 [PMC free article] [PubMed] [Google Scholar]
- 21.Vanhée D, Gosset P, Marquette CH, Wallaert B, Lafitte JJ, Gosselin B, Voisin C, Tonnel AB: Secretion and mRNA expression of TNF-α and IL-6 in the lungs of pneumoconiosis patients. Am J Respir Crit Care Med 1995, 152:298-306 [DOI] [PubMed] [Google Scholar]
- 22.Piguet PF, Collart MA, Grau GF, Kapanci Y, Vassalli P: Tumor necrosis factor α cachectin plays a key role in bleomycin-induced pneumopathy and fibrosis. J Exp Med 1989, 170:655-663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piguet PF, Vesin C: Treatment by human recombinant soluble TNF receptor of pulmonary fibrosis induced by bleomycin or silica in mice. Eur Respir J 1994, 7:515-518 [DOI] [PubMed] [Google Scholar]
- 24.Kapanci Y, Desmouliere A, Pache J-C, Redard M, Gabbiani G: Cytoskeletal protein modulation in pulmonary alveolar myofibroblasts during idiopathic pulmonary fibrosis: possible role of TGF-β and TNF-α. Am J Respir Crit Care Med 1995, 152:2163-2169 [DOI] [PubMed] [Google Scholar]
- 25.Fiers W: Tumor necrosis factor. FEBS Lett 1991, 285:199-212 [DOI] [PubMed] [Google Scholar]
- 26.Xing Z, Tremblay GM, Sime PJ, Gauldie J: Overexpression of granulocyte/macrophage colony-stimulating factor induces pulmonary granulation tissue formation and fibrosis by induction of transforming growth factor-β1 and myofibroblast accumulation. Am J Pathol 1997, 150:59-66 [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang K, Rekhter MD, Gordon D, Phan SH: Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis. Am J Pathol 1994, 145:114-125 [PMC free article] [PubMed] [Google Scholar]
- 28.Rubbia-Brandt L, Sappino A-P, Gabbiani G: Locally applied GM-CSF induces the accumulation of α-smooth muscle actin containing myofibroblasts. Virchows Arch B Cell Pathol 1991, 60:73-82 [DOI] [PubMed] [Google Scholar]
- 29.Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G: Transforming growth factor-β1 induces α-smooth actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 1993, 122:103-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khalil N, Whitman C, Zuo L, Danielpour D, Greenberg A: Regulation of alveolar macrophage transforming growth factor-β secretion by corticosteroids in bleomycin-induced pulmonary inflammation in the rat. J Clin Invest 1993, 92:1812-1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khalil N, Corne S, Whitman C, Yacyshyn H: Plasmin regulates the activation of cell-associated latent TGF-β1 secreted by rat alveolar macrophages after in vivo bleomycin injury. Am J Respir Cell Mol Biol 1996, 15:252-259 [DOI] [PubMed] [Google Scholar]
- 32.Zhang K, Flanders KC, Phan SH: Cellular localization of transforming growth factor-β expression in bleomycin-induced pulmonary fibrosis. Am J Pathol 1995, 147:352-361 [PMC free article] [PubMed] [Google Scholar]
- 33.Xing Z, Ohkawara Y, Jordana M, Graham FL, Gauldie J: Transfer of granulocyte-macrophage colony-stimulating factor gene to rat lung induces eosinophilia, monocytosis, and fibrotic reactions. J Clin Invest 1996, 97:1102-1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gauldie J. Sime PJ, Xing Z, Marr R, Tremblay G: TGFβ gene transfer to the lung induces myofibroblast presence and pulmonary fibrosis. Pathol Update 1998, (in press) [DOI] [PubMed]