Abstract
The stem cells of rapidly renewing tissues give rise to transiently proliferating cells, which in turn give rise to postmitotic terminally differentiated cells. Although the existence of a transiently proliferating compartment has been proposed for the prostate, little molecular anatomical evidence for its presence has been obtained to date. We used down-regulation of the cyclin-dependent kinase inhibitor p27Kip1 to identify cells capable of entering the proliferative phase of the cell cycle and, therefore, competent to fulfill the role of the transiently proliferating compartment. We examined the expression of p27Kip1 in relation to its role in the development of prostatic carcinoma. Formalin-fixed paraffin-embedded specimens from matched samples of normal-appearing prostate tissue, benign prostatic hyperplasia, high-grade prostatic intraepithelial neoplasia, primary adenocarcinomas, and pelvic lymph node metastases were evaluated by comparative immunohistochemistry against p27Kip1. In normal-appearing prostate epithelium, moderate to strong nuclear staining of p27Kip1 was present in greater than 85% of the terminally differentiated secretory cells. The normal basal cell compartment, believed to contain prostatic stem cells, showed distinctive p27Kip1 expression; acini in epithelial benign prostatic hyperplasia tissue contained more p27Kip1-negative basal cells than acini from non-benign prostatic hyperplasia tissue. A third layer of cells was identified that was sandwiched between the basal cells and the luminal cells, and this layer was consistently p27Kip1 negative. This intermediate layer was accentuated in the periurethral region, as well as in prostate tissue that had been subjected to prior combined androgen blockade. We hypothesize that, on appropriate additional mitogenic stimulation, cells in this layer, and other p27Kip1-negative basal cells, are competent for rapid entry into the cell cycle. Consistent with the fact that cancer cells are capable of cell division, all cases of high-grade prostatic intraepithelial neoplasia and invasive carcinoma also showed down-regulation of p27Kip1 as compared with the surrounding normal-appearing secretory cells. In pelvic lymph node metastases, p27Kip1 expression was also reduced. In summary, our results suggest that lack of nuclear p27Kip1 protein may delineate a potential transiently proliferating subcompartment within the basal cell compartment of the human prostate. In addition, these studies support the hypothesis that reduced expression of p27Kip1 removes a block to the cell cycle in human prostate epithelial cells and that dysregulation of p27Kip1 protein levels may be a critical early event in the development of prostatic neoplasia.
In several rapidly renewing tissues, cell types are organized whereby stem cells, transiently proliferating (TP) cells, and mature terminally differentiated cells occupy discrete locations and often form stratified layers. 1-3 A similar stem cell-driven hierarchical arrangement has been postulated for the more slowly renewing adult prostate. 4-6 There is still debate, however, regarding the location and nature of prostatic stem cells, and there has been little molecular anatomical evidence accumulated in support of a TP compartment in the prostate.
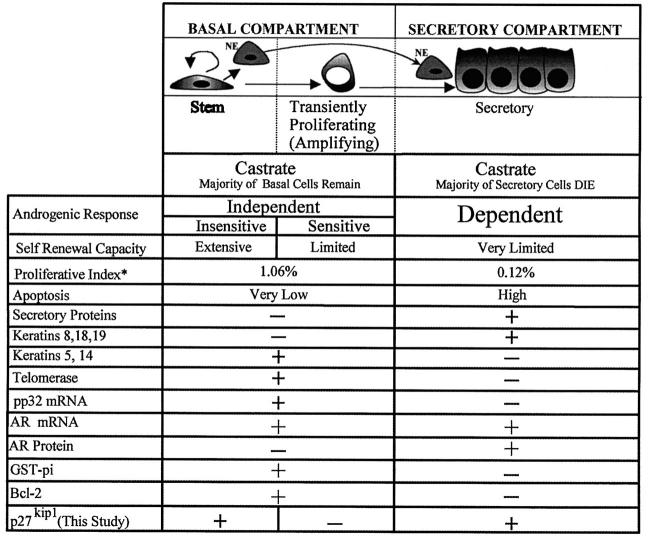
As indicated in the model shown in Figure 1 ▶ , the majority of prostatic epithelial cells in the adult gland are androgen dependent for survival. 7 For example, castration of the male rat leads to programmed cell death in the prostate with loss of up to 90% of the total epithelial cells. 7,8 The remaining epithelial cells do not require androgen for survival and are thus considered androgen independent. At least some of these surviving androgen-independent epithelial cells remain androgen sensitive, because the subsequent administration of exogenous androgens to the castrate animal results in induction of proliferation and the regeneration of the prostate to the normal size and morphology. By experimentally cycling serum androgen levels, this process of involution and subsequent androgen-induced regeneration can be repeated numerous times. Such results led Isaacs and Coffey 4 to postulate a stem cell model of prostate organization whereby slowly proliferating androgen-independent reserve stem cells give rise to a second population of more rapidly cycling androgen-independent but androgen-responsive amplifying cells. (This proposed amplifying population is analogous to the TP cells seen in other organ systems and will be referred to as such in accordance with those systems; see Figure 1 ▶ .) Although these TP cells may be actively cycling, their capacity for self-renewal is greatly diminished as compared with the reserve stem cells (Figure 1) ▶ , which maintain their numbers. Rather, by undergoing a limited number of population doublings, these TP cells are postulated to amplify the number of epithelial secretory cells derived from the stem cell compartment. The TP cells respond to androgens by giving rise to more mature cells (previously referred to as transit cells) with very limited proliferative potential that subsequently undergo terminal differentiation into androgen-dependent secretory cells. 4
Figure 1.
Stem cell model of prostate epithelial cell organization and growth. See text for references. Black nuclei, p27Kip1 positive. White nuclei, p27Kip1 negative. NE, neuroendocrine cells. *Proliferative index, percentage of cells in proliferative phase as measured by PCNA immunostaining from McNeal et al. 25 GST-pi, glutathione S-transferase-π.
Prostatic epithelium consists of two major compartments, basal and secretory. 9-11 In the basal compartment, one or two layers of cells are situated between the basement membrane and the overlying secretory cells. 9,10 Basal cells are distinguished by their morphology, ranging from small flattened cells with condensed chromatin and scant cytoplasm to more cuboidal cells with increased amounts of cytoplasm and more open-appearing chromatin. Basal cells uniquely express cytokeratins 5 and 1412,13, low relative levels of androgen receptor (AR), 14,15 phosphoprotein 32, 16,17 glutathione S-transferase-π, 18 and Bcl-2, 19 and they lack expression of the major prostatic secretory proteins, such as prostate-specific antigen and prostate-specific acid phosphatase in the human 20 and prostatic binding protein in the rat ventral prostate. 21 The secretory compartment generally consists of a luminal layer of columnar cells. The apical aspect of the secretory cells projects into the lumen, and the basal aspect rests on the basal cells. 9-11 Secretory cells express prostate-specific antigen and prostate-specific acid phosphatase, high relative levels of AR, 14,15,22 cytokeratins 8 and 18, and they lack expression of cytokeratins 5 and 14, 13,23 glutathione S-transferase-π 18 (usually), and Bcl-2 19 (see Figure 1 ▶ ).
Several lines of evidence suggest that prostate secretory cells arise from maturation of cells derived from the basal cell compartment. First, immunohistochemical and radiolabeling experiments show that the bulk of the proliferating pool in the normal-appearing human prostate is restricted to some of the basal cells. 24,25 Second, cell types with combined phenotypic characteristics intermediate between those of basal cells and secretory cells are present in both the developing and the adult prostate. For example, electron microscopic studies of the human prostate show that there are scattered cells with morphological features of both basal and secretory cells that reach from the basement membrane to the lumen. 26 In the rat neonatal prostate grown in organ culture, the cells that make up the initial solid epithelial buds co-express both the basal cell-specific cytokeratins 5 and 14, as well as the cytokeratins 8 and 18. As the prostate differentiates under androgenic stimulation, the ducts canalize, basal and luminal cells become morphologically distinct, and the cytokeratin pattern changes to reflect that in the adult 27 such that only basal cells express cytokeratins 5 and 14. In addition, double-labeling studies have shown that in androgen-cycling experiments in rats and in the normal human prostate and prostate cancer, individual cells are found that have cytokeratin expression profiles of both basal and secretory cells. 28,29 Indeed, it has been suggested that these latter cells may represent the “amplifying compartment” or TP compartment in the prostate, as outlined in Figure 1 ▶ . 28-30 Finally, Bonkhoff et al 5 have also shown that the human prostate contains cells that express both basal cell-specific keratin and prostate-specific antigen, as well as other cells that express both prostate-specific antigen and chromogranin A (a neuroendocrine cell marker). These results imply the existence of cells that are intermediate in differentiation between basal and secretory cells, and others that are intermediate between basal and neuroendocrine cells. Thus, given that prostate basal cells make up the bulk of proliferating cells and that they appear capable of multiple lines of differentiation, prostatic stem cells are most likely located in the basal compartment. 5,6
High-grade prostatic intraepithelial neoplasia (PIN) is the presumed precursor lesion to many, if not most, prostatic adenocarcinomas. 31,32 As in precursor lesions of cancer of the colon and cervix, there is an overall increase in the proliferative fraction in PIN. Also, the compartmentalization of proliferating cells is altered such that the ratio of proliferating secretory-type cells to proliferating basal cells is greatly increased. 24,25 The mechanisms for such altered proliferation in cancer precursor lesions are not understood. Although cell proliferation and differentiation are controlled in the prostate by circulating androgens, little is known regarding the molecular mechanisms by which these processes are coordinated in normal tissue and altered in prostatic neoplasia.
Progression through the cell cycle is controlled by the activated cyclin:cyclin-dependent kinase complexes. 33 Members of the cip/kip family of cyclin-dependent kinase inhibitors, such as p27Kip1 and p21/waf1/cip1, bind to and inhibit the activity of the cyclin:cyclin-dependent kinase complexes resulting in a block in the progression through the cell cycle. 33 p27Kip1 is widely expressed in mammalian tissues and has been implicated in cell cycle arrest. 34,35 Increased levels of p27Kip1 appear to serve as a barrier for progression to S phase and may signal exit from the cell cycle. 36,37 In vitro experiments have shown that p27Kip1 levels are high in quiescent cells, but that in response to mitogens, the levels fall and they remain low in proliferating cells. Withdrawal of mitogens results in re-expression of p27Kip1 to high levels. 38 Although in the vast majority of tumor types that have been examined, the p27Kip1 gene is altered only very rarely, several different neoplasms, including those from the breast, lung, colon, stomach, and prostate show reduced p27Kip1 protein levels. 37,39-47
In the prostate, down-regulation of p27Kip1 occurs in the vast majority of adenocarcinomas, 39-41 and reduced levels positively correlate with Gleason grade. 39-41 In addition, reduced levels may be useful as a predictor of prognosis independent of grade and stage. 40,41 We have begun to test the hypothesis that p27Kip1 down-regulation renders cells capable of serving as the TP compartment in the prostate. In addition, we sought to determine the potential role of down-regulation of p27Kip1 protein expression in the development of prostatic neoplasia by examining expression in high-grade PIN. We propose that down-regulation of p27Kip1 in human prostate removes a block to cell cycle progression that renders cells capable of rapidly entering into the proliferative phase of the cell cycle on appropriate additional stimulation. In addition, we propose that critical alterations in the normal regulatory control of p27Kip1 levels occur early in prostatic carcinogenesis.
Materials and Methods
Pathological Specimens
Formalin-fixed, paraffin-embedded archival tissues containing primary prostatic carcinomas, high-grade PIN, and pelvic lymph node metastases were obtained from The Johns Hopkins Hospital (Baltimore, MD). All of these specimens were from radical prostatectomies and concomitant pelvic lymph node dissections performed between 1991 and 1998. The mean age of patients was 59.6 years (range, 47 to 71). Specimens were selected to represent a limited spectrum of Gleason scores ranging from 6 to 9, with a wide spectrum of pathological stages (N = 41). Pathological stage ranged from organ confined (pT2) to T(any)N1Mx, using the TNM system of the American Joint Committee on Cancer and the International Union Against Cancer. Control tissues consisting of tonsils, esophagus, colon, epidermis, and urinary bladder were obtained from the surgical pathology archives of The Johns Hopkins Hospital.
Monoclonal Antibodies and Immunohistochemistry
Mouse anti-human monoclonal antibodies were obtained as follows: anti-p27Kip1 (dilution, 1:800; Transduction Laboratories, Lexington, KY); 34βE12 (1:50; Enzo Biochem, Inc., Farmingdale, NY), KI-67 (clone MIB-1, 1:100; Immunotech-Coulter, Miami, FL); proliferating cell nuclear antigen (PCNA, 1:250; Transduction Laboratories); and AR (clone AR-A441), which was generously provided by Dr. Dean P. Edwards (University of Colorado Health Sciences Center, Denver, CO). Immunohistochemistry was performed as described 48 using diaminobenzidine (brown) as the chromagen with a light hematoxylin (blue) counterstain. All primary antibody incubations were carried out for 45 minutes at room temperature. For 34βE12-cytokeratin staining, the sections were pretreated with protease type 27 (Sigma Chemical Co., St. Louis, MO) at 2 mg/ml for 20 minutes at 37°C before incubation with the primary antibodies. Because our pilot studies showed that large specimens that are slowly fixed over several hours retain strong p27Kip1 expression only in the outer aspect of the samples, which are promptly fixed, and because p27Kip1 protein levels are regulated by specific proteolysis, 49,50 we used samples that were obtained immediately after surgical resection that were harvested fresh. All specimens consisted of portions of tumor and normal tissue that were immersed in 10% neutral buffered formalin immediately after surgical resection.
Semiquantitative Staining of p27Kip1 in Invasive Prostate Cancer
Semiquantitative grading of the tumors with respect to p27Kip1 immunohistochemical staining was carried out by estimation of the percentage of tumor cells in a representative section of carcinoma that were strongly positive for nuclear p27Kip1 immunoreactivity, as compared with surrounding normal-appearing prostatic acinar secretory cells.
Statistical Analysis
Statistical analysis was carried out on a Dell Pentium II personal computer using Stata 5.0 software for Windows 95.
Results
Expression of p27Kip1 in Nonneoplastic Intact and Androgen-Ablated Human Prostate
In nonneoplastic normal-appearing prostate from all zones, moderate to strong p27Kip1 staining was present in the vast majority (range, 85 to 100%) of nuclei of secretory cells (Figure 2, B, C, E, F, and G ▶ ; Figure 3, A, E, and F ▶ ). Although cytoplasmic staining was present in normal-appearing secretory cells, it was weak or negative in the vast majority of these cells. Scattered collections of small lymphocytes that showed strong positive nuclear p27Kip1 immunoreactivity were invariably present, serving as internal positive controls. In the basal cell compartment, expression of p27Kip1 was much more variable than in the secretory compartment (Figure 2B) ▶ , such that there was a spectrum of acinar profiles. In some acini, all basal cells were negative or markedly reduced for p27Kip1, whereas in others, there was a mixture of both strongly positive and negative/markedly reduced cells, and in others all basal cells were strongly positive. This variability was examined systematically in basal cells in the various zones of the prostate (as defined by McNeal), 9-11 as well as in nodular hyperplasia benign prostatic hyperplasia (BPH) tissue.
Figure 2.
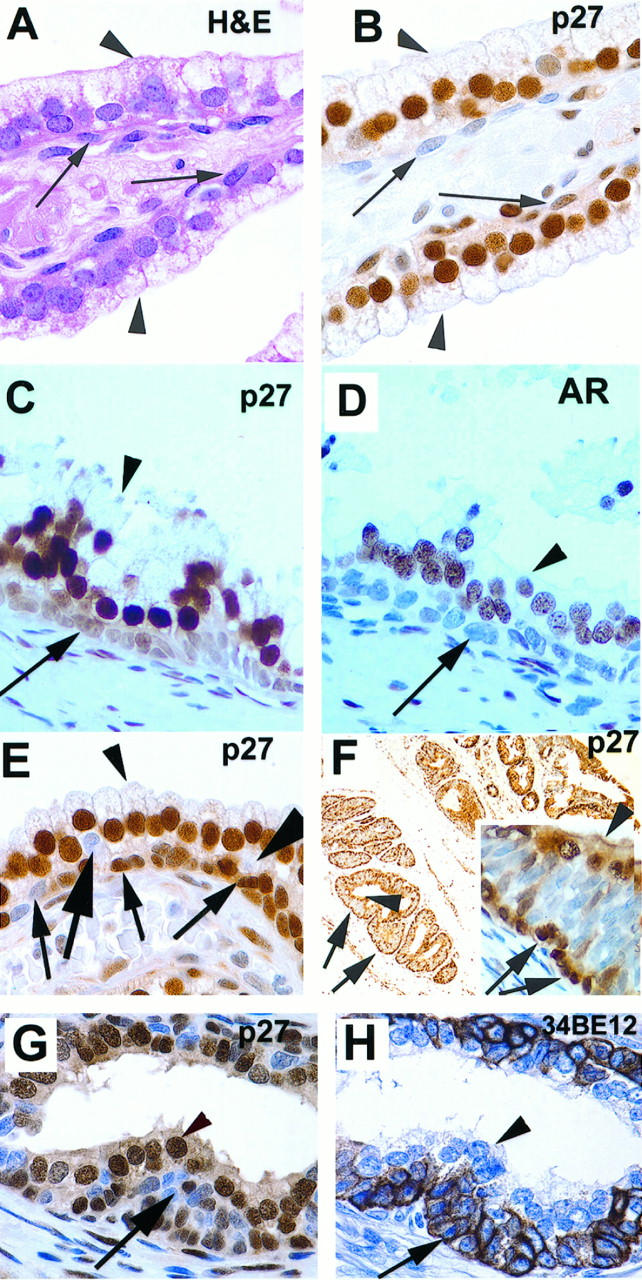
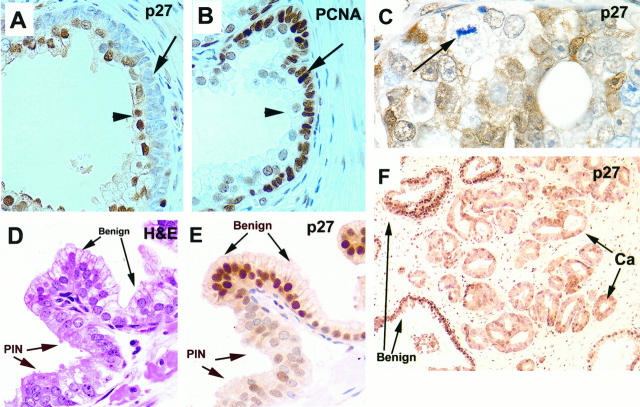
Two populations of prostate basal cells defined by p27Kip1 expression. Arrows: Basal cells. arrowheads: Luminal secretory cells. Stains are indicated. A: Histologically normal prostate (magnification, ×630). B: Section adjacent to that shown in A, showing heterogeneous basal cell expression of p27Kip1 (×630). C: Stong uniform staining of p27Kip1 in epithelial secretory cells in the region of BPH, with markedly less staining in basal cells (×400). D: Section adjacent to that shown in C showing strong nuclear staining of AR in secretory cells (×400). E: An intermediate layer of p27Kip1-negative cells in histologically normal prostate (×630). F: Periurethral glands show accentuated intermediate layer of p27Kip1-negative cells (×50; inset shows higher power view, ×400). G and H: Benign prostate tissue from patient pretreated with androgen ablation therapy shows intermediate cell layer that is p27Kip1 negative and positive for cytokeratin 903 (34βE12) (×630).
Figure 3.
Inverse correlation of p27Kip1 expression with proliferation and down-regulation in high-grade PIN and adenocarcioma. A and B: Epithelial BPH tissue with prominent basal layer that is negative for p27Kip1 and positive for PCNA (×400). D and E: Down-regulation of p27Kip1 in high-grade PIN. High-grade PIN is present together in the same acinus with histologically normal prostate tissue. Note abrupt transition from PIN to normal and corresponding abrupt transition in nuclear expression of p27Kip1 (×400). C and F: Down-regulation of p27Kip in invasive prostatic carcinoma prostate carcinoma. Note that the cell with the mitotic figure and arrow is devoid of p27Kip1 (C, ×630; F, ×100).
BPH tissue was defined strictly as tissue derived from the transition zone that showed classic distinct nodules composed of mixtures of epithelium and stroma. Furthermore, the glands consisted of tall columnar secretory cells with pale staining cytoplasm (by hematoxylin and eosin staining) and occasional papillary infoldings. The intervening and immediately surrounding stroma was invariably mildly hypercellular. In these nodular hyperplastic lesions, the basal cells were often prominent, appearing slightly hyperchromatic and more rounded than those in nonhyperplastic tissue (Figure 3A) ▶ . Areas of BPH that were highly inflamed were excluded from these analyses. For these comparisons, we assigned a given acinus to one of two groups. Acini in the high-staining group contained strong staining in greater than 50% of the identifiable basal cells, as compared with overlying secretory cells, whereas acini in the low-staining group contained strong staining in ≤50% of basal cells. The results of these analyses (analysis of variance using Bartlett’s test of equal variances) indicated significant differences among the groups of tissues analyzed (Table 1 ▶ ; F3,28 = 8.47; P = 0.0004). Pairwise comparisons (Table 2) ▶ showed that BPH tissue contained a significantly higher percentage of low-staining acinar profiles than normal-appearing peripheral zone and normal-appearing transition zone. Although the central zone had a somewhat higher mean score of low staining acinar profiles, the results as compared with the other zones were not statistically significant.
Table 1.
Basal Cell Immunoreactivity in Different Zones of the Human Prostate and BPH
| n | Mean* | SD | |
|---|---|---|---|
| Peripheral zone | 9 | 6.8 | 3.3 |
| Transition zone | 6 | 9.2 | 4.0 |
| Central zone | 6 | 10.5 | 2.6 |
| BPH | 11 | 13.7 | 2.7 |
*Mean numbers represent the mean number of acinar profiles out of a total of 20 for each specimen that showed >50% of the basal cells to be negative or markedly reduced in staining for p27Kip1 as compared with overlying secretory cells within the same acinar profile.
Table 2.
Adjusted Significance (Bonferroni’s Correction) of Differences of p27Kip1 Immunoreactivity in Different Prostate Zone and BPH*
| BPH | Central | Peripheral | |
|---|---|---|---|
| Central | 0.31 | ||
| Peripheral | <0.001 | 0.19 | |
| Transition | 0.05 | 1.0 | 0.95 |
*Numbers represent adjusted P values for the matrix comparisons.
We next examined the relation between expression of p27Kip1 and ARs. ARs have been reported to be present in the majority of secretory cells but are either absent or markedly reduced in the vast majority of cells within the basal compartment. 14,51,52 We analyzed seven specimens of nonneoplastic prostate tissue from five patients using adjacent sections that were stained with p27Kip1 or ARs. In each case, the staining pattern in the secretory epithelium of normal-appearing prostate showed a strict concordance between AR nuclear staining and p27Kip1 nuclear staining (Figure 2, C and D) ▶ . As a result of the very weak staining for AR in the basal cells in general, it was difficult to determine with certainty whether there was a strict concordance between AR and p27Kip1 in the basal compartment overall, although there was clear concordance in many acini, in which both markers were markedly decreased or negative as compared with adjacent overlying secretory epithelium.
Prostatic ducts/acini are generally assumed to contain two cell layers, although as previously noted, electron microscopic studies have shown occasional layering of apparent basal cells in normal prostate. We also noticed a third, intermediate, zone of cells that was present variably in all zones of the normal prostate, was localized between the basal-most cells and the luminal secretory cells, and was consistently negative for p27Kip1 (Figure 2E ▶ , large arrows). This third layer of p27Kip1-negative cells was most easily recognized in the periurethral gland region of the prostate, where it was often accentuated. Figure 2F ▶ shows the periurethral gland region with three cellular zones where the basal-most cells and the luminal cells stain positively, yet a distinct multilayered middle zone of cells negative for p27Kip1 (Figure 2F ▶ , inset) can be identified. To rule out the possibility that the periurethral gland region does not simply reflect benign bladder mucosa extending into prostatic acini, we examined the expression of p27Kip1 in normal bladder mucosa. By contrast to the periurethral region, benign urothelial mucosa from the urinary bladder revealed an absence of a distinct zone of cells that were negative for p27Kip1. Rather, approximately 90% of the total nuclei in the bladder mucosa were strongly positive for nuclear p27Kip1 staining, and although negative cells predominated near the basal-most aspect, they were also scattered throughout all layers of the mucosa.
The appearance of a potential third middle layer of cells was further investigated in androgen-deprived human prostates, because these typically show an increase in the number of layers that make up the basal compartment. 53,54 Figure 2, G and H ▶ , is from a patient who was treated with androgen blockade therapy before prostatectomy; the basal compartment appears prominent, hyperchromatic, and multilayered. Staining of an adjacent section with 34βE12 for basal cell-specific cytokeratins demonstrates that these cells are part of the basal cell compartment (Figure 2H) ▶ . We examined five prostates from patients who were treated with androgen deprivation therapy before radical prostatectomy, and all have shown this pattern of increased numbers of p27Kip1-negative basal cells that often appear as a third layer in the prostate epithelium. These cells may be proliferation competent and poised for rapid growth after androgen stimulation. Our results support the hypothesis that there is a variable third layer of prostatic acinar cells that are defined by p27Kip1 negativity, basal cell specific cytokeratin expression, and prominence in periurethral and androgen-deprived prostate tissue.
Because p27Kip1 levels are negatively correlated with proliferation, 36,55 we examined p27Kip1 expression levels in mitotic cells and in cells in the proliferative phase of the cycle using the proliferation markers Ki-67 and PCNA. In all cells with mitotic figures, generally only seen in PIN and invasive cancer, p27Kip1 expression was negative (Figure 3C) ▶ . As noted previously in normal-appearing prostate epithelium, the majority of the nonmitotic but proliferating cells were in the basal compartment. In these proliferative cells, p27Kip1 was generally low or absent, as determined by staining of adjacent sections. There were no clear examples of cells that were strongly p27Kip1 positive and positive for either of the proliferation markers. In acini in which the majority of the basal cells were negative for p27Kip1, the overall proliferative fraction was elevated over those acini in which the basal cells were uniformly positive for p27Kip1. At times, entire layers of p27Kip1-negative basal cells were positive for PCNA (Figure 3, A and B) ▶ . However, because not all p27Kip1-negative cells were in the proliferative phase of the cell cycle, we conclude that absence of p27Kip1 in prostate epithelial cells may be necessary yet not sufficient for entrance into the proliferative phase of the cell cycle.
To examine further the relationship between p27Kip1 negativity and proliferative status, we stained sections of oral mucosa, epidermis, esophagus, and colon. In each tissue, many of the cells in the basal zone or crypts were devoid of staining for p27Kip1. As the cells matured toward the surface of these epithelia, nuclear p27Kip1 expression increased and cell proliferation decreased. However, not all of the p27Kip1-negative cells were in the proliferative phase of the cycle as determined by PCNA and Ki-67 staining (data not shown). These results suggest that down-regulation of p27Kip1 may be necessary but not sufficient for progression to the proliferative phase of the cell cycle in human epithelial cells in vivo. In lymphoid tissue of the tonsils and lymph nodes, by contrast, we found an essentially 1:1 inverse relation between the staining for the proliferative marker Ki-67 and p27Kip1, as previously noted. 56
Expression of p27Kip1 in High-Grade PIN and Invasive Prostate Carcinoma
In high-grade PIN there was a consistent down-regulation (11 of 11 cases) of p27Kip1 as compared with adjacent benign prostatic epithelium. This down-regulation consisted of an increased number of cells that were devoid of nuclear expression and a more pronounced generalized decrease in overall nuclear p27Kip1 intensity. In each case, between 50 and 75% of high-grade PIN cells showed markedly reduced or negative nuclear staining as compared with adjacent and surrounding secretory cells. In most cases, there was an increased amount of cytoplasmic staining in PIN versus the normal-appearing epithelial secretory cells. Figure 3, D and E ▶ , demonstrates down-regulation of p27Kip1 in high-grade PIN in a prostatic acinus that contains both benign epithelium and high-grade PIN within the same structure.
In invasive carcinomas, there was down-regulation of p27Kip1 as compared with adjacent normal-appearing epithelium (Figure 3, C and F) ▶ . Again, this consisted of both an increase in the total number of negative nuclei and a generalized decrease in intensity in the majority of tumor cells. As in high-grade PIN, invasive tumor cells at times contained p27Kip1 cytoplasmic immunoreactivity (Figure 3C) ▶ .
To determine whether there was a relation between p27Kip1 expression and tumor grade or pathological stage, specimens from 41 patients were analyzed. All specimens contained an internal positive control of benign, noninflamed, nonatrophic prostatic acinar secretory cells, and >85% of these cells were strongly positive for nuclear p27Kip1. No cases were completely devoid of tumor cell staining, and staining for p27Kip1 in invasive prostatic carcinoma was heterogeneous. Semiquantitative grading was performed by estimating the percentage of tumor cells in the specimen that were strongly positive for nuclear p27Kip1 expression. This method was used as opposed to previous methods that counted the negative tumor cells at high power, because there was extensive heterogeneity of tumor cell staining for p27Kip1, which creates a bias depending on which areas are selected for counting. In addition, it was relatively simple to identify strongly positive tumor cells, and this facilitated counting. The scoring ranged from 0 to 70% strongly positive tumor cells. Specimens were divided into two groups in relation to staining for p27Kip1 (Table 3) ▶ ; group 1 (n = 20) had ≥10% of the tumor cells strongly positive for nuclear p27Kip1, and group 2 (n = 21) had <10% of tumor cells strongly positive. For pathological grade, specimens were also divided into two groups, intermediate (Gleason score 6; n = 11) and high (Gleason score ≥7; n = 30). Among this cohort of patients (Table 3) ▶ , p27Kip1 staining proved to be a significant predictor of high Gleason score (odds ratio, 7.7; SE = 6.75; z = 2.36; P = 0.018; 95% confidence interval, 1.42–42.7) but not of pathological stage at radical prostatectomy (odds ratio, 2.8; SE = 2.03; z = 1.48; P = 0.15; 95% confidence interval, 0.62–11.59). This data set is limited in number (n = 41) and, therefore, may not reveal a potential weak association between p27Kip1 expression and pathological stage. As a control for this data set, however, linear regression analysis showed that Gleason grade was highly predictive of pathological stage at radical prostatectomy (n = 41; P < 0.0001; r 2 = 0.40; slope = 0.85; SE = 0.17; 95% confidence interval, 0.51–1.19). Thus, we do expect that if a strong relation between p27Kip1 and pathological stage at radical prostatectomy were present, we would likely have uncovered it. Lymph node metastases (n = 11), which were all detected as microscopic lesions identified after radical prostatectomy and concomitant pelvic lymph node dissection, all showed decreased levels of p27Kip1 immunoreactivity as compared with surrounding benign lymphoid cells (no normal prostate is present in the lymph nodes for comparison) and as compared with staining of secretory cells from a separate section from the patient’s prostate.
Table 3.
Associations Between Reduced or Absent p27Kip1 Nuclear Staining and Gleason Grade and Pathological Stage at Radical Prostatectomy
| Tumor cell staining | |||
|---|---|---|---|
| ≥10% strong | <10% strong | Total | |
| Gleason grade | |||
| 6 | 9 | 2 | 11 |
| 7 | 10 | 15 | 25 |
| 8 | 0 | 3 | 3 |
| 9 | 1 | 1 | 2 |
| Total | 20 | 21 | 41 |
| Pathological stage | |||
| T2b | 8 | 4 | 12 |
| T3a,b | 7 | 12 | 19 |
| T3c | 2 | 2 | 4 |
| T(any), N1 | 3 | 3 | 6 |
| Total | 20 | 21 | 41 |
The integers in the table represent the number of cases in each category. The percentage indicates an estimate of the percentage of tumor cells in the specimen that showed strong nuclear p27Kip1 staining as compared with adjacent nonneoplastic secretory epithelium. Overall N = 41.
Discussion
In tissues that undergo rapid physiological renewal, such as squamous epithelium, the gastrointestinal tract, and bone marrow, cell turnover is governed by a small number of stem cells. The stem cells are characterized by a generalized undifferentiated state and a long cell-cycle time, thus limiting their total number of cycles. The stem cells achieve tissue replenishment by giving rise to a TP compartment 1-3 that serves to amplify the divisions of the stem cell compartment. TP cells are characterized by rapid proliferation but limited proliferative capacity. The progeny of TP cells give rise to fully mature postmitotic cells. In these tissues, there is a topographic hierarchy of stem cells, TP cells, and maturing/mature terminally differentiated cells. We used the expression levels of the cyclin-dependent kinase inhibitor p27Kip1 as a marker of competent cells capable of becoming the TP compartment in the prostate.
In the current study, we focused to a large extent on characterizing expression of p27Kip1 in various zones of the normal-appearing prostate, as well as epithelial BPH tissue, analyses that have not been reported on in a systematic fashion thus far. We found that in normal-appearing prostate tissues, p27Kip1 was expressed in >85% of nuclei of secretory cells, which have been shown previously to have a very low proliferative rate (<0.1%). 24,25 By contrast, the basal cell compartment, which contains the bulk of any of the proliferating cells in the normal-appearing human prostate, was much more heterogeneous and contained a significant number of p27Kip1-negative cells. This implies the existence of at least two populations of basal cells, one that is p27Kip1 negative and another that is p27Kip1 positive. In addition, there was a distinct third layer of cells that was present variably, which was localized between the basal-most cells and the luminal cells. This layer of cells was characterized by basal cell-specific cytokeratin expression and an absence of p27Kip1 immunoreactivity. This layer was accentuated in the periurethral regions of normal-appearing prostate and in prostatic tissue treated with androgen deprivation therapy. Based on these results, we hypothesize that the prostate contains a variable middle compartment. This compartment is p27Kip1 negative (Figure 4) ▶ . It functions as a potential TP compartment with cells that are intermediate in phenotype between the predominantly resting stem cells (p27Kip1 positive) in the basal compartment and the resting mature cells in the secretory compartment. These cells are capable of a limited number of proliferation events and are also capable of differentiating into mature postmitotic cells. We are currently extending these studies to include double-labeling experiments with p27Kip1 and various cytokeratins to determine whether the cells that we have identified as p27Kip1 negative, and the presumed TP compartment, co-localize with cells previously found to have intermediate patterns of cytokeratin expression. 29 We are also extending these studies to examination of the rat prostate, in which our preliminary experiments (De Marzo et al, unpublished results) indicate strong expression of p27Kip1 in the vast majority of secretory epithelium of all lobes of the adult prostate and seminal vesicle, except in actively proliferating cells, and in the majority of the remaining resting epithelial cells of the ventral prostate after castration-induced involution.
Figure 4.
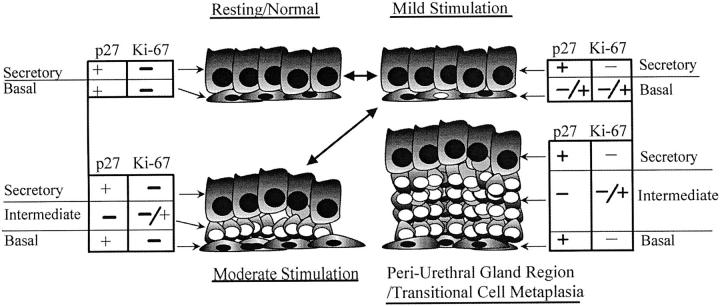
Model of stem cell hierarchy in human prostate tissue in relation to nonproliferating/non-proliferation-competent cells (p27Kip1+/Ki-67−), proliferation-competent cells (p27Kip1−/Ki-67−), and actively proliferating cells (p27Kip1−/Ki-67+). Black nuclei, p27Kip1 positive. White nuclei, p27Kip1 negative.
In our current working model, the presence of a third cell layer relates to the potential proliferative activity state of the cells in a given acinus or duct. An acinus or duct will temporarily expand the pool of p27Kip1 negative basal cells by down-regulation of the protein product to prime itself for cell proliferation if the appropriate stimulus is presented; in other words, the p27Kip1-negative cells are competent for subsequent proliferation. Since we postulate that the absence of p27Kip1 removes a critical block to cell cycle progression in prostate tissue but does not appear to be sufficient for proliferation by itself, we are not suggesting that the mere absence of p27Kip1, as in the periurethral gland region, renders cells highly susceptible to neoplastic transformation. In fact, BPH tissue does not progress to carcinoma in the vast majority of cases, yet we found an increase in the percentage of acini that contained a high percentage of basal cells staining negative for p27Kip1. This increase may help explain, however, why epithelial BPH tissue does have an elevated proliferation rate as compared with non-BPH prostatic tissue. 57 Whether down-regulation of p27Kip1 in the basal cells of BPH relates to stroma-derived growth factors remains to be determined.
By contrast to normal-appearing secretory epithelium, in all high-grade PIN lesions, primary invasive carcinomas and regional lymph node metastases, the expression of p27Kip1 was decreased as compared with adjacent nonneoplastic epithelial secretory cells and/or lymphoid cells. These findings are concordant with other recent studies, 39-41 and they implicate a decrease in nuclear p27Kip1 expression as an essential early event in the development of prostatic neoplasia. Additionally, our studies showing increased numbers of secretory cells in PIN that are p27Kip1 negative support the hypothesis that central to the neoplastic process is the disruption of the tightly controlled spatial organization of proliferation and differentiation. This was suggested previously for the colon by el-Deiry et al 58 and Polyak et al, 59 who have shown that the compartmentalization of expression of the proliferative marker Ki-67 and the differentiation marker p21/waf1/cip1 is altered in precancerous colon lesions, the aberrant crypt focus, and tubulovillous adenoma, but not in nonneoplastic hyperplastic polyps. In cancer precursor lesions, neoplastic cells appear to bypass the normal topological signals that restrict proliferation to the crypts, such that the proliferating neoplastic cells are found as high up as the luminal surface. Similar alterations occur in preinvasive neoplastic lesions of the uterine cervix, where a long-standing criterion for the diagnosis of cervical squamous intraepithelial neoplasia has been abnormal localization of mitotic cells, away from the parabasal region toward the surface. We postulate that abnormal down-regulation of p27Kip1 in secretory cells of high-grade PIN may be responsible, at least in part, for the markedly increased proliferation rate of these secretory cells.
In summary, we report that lack of expression of p27Kip1 in prostate epithelium may be a specific indicator for TP cells of the normal human prostate. We postulate that p27Kip1 levels play a major role in regulating the cell cycle in normal, hyperplastic, and neoplastic prostate epithelial cells and that down-regulation of p27Kip1 renders these cells competent for proliferation. We also report that down-regulation of p27Kip1 occurs in all examined cases of high-grade PIN and in invasive primary and metastatic prostate carcinomas. These findings suggest that p27Kip1 down-regulation is an essential step in the development and maintenance of the malignant prostatic epithelial cell phenotype.
Acknowledgments
The authors thank Josephine Geh for performing the immunohistochemical staining for this work.
Footnotes
Address reprint requests to Dr. Angelo M. De Marzo, The James Buchanan Brady Urological Institute, 145 Marburg Building, 600 North Wolfe Street, Baltimore, MD 21287-2101. E-mail: ademarz@welchlink.welch.jhu.edu.
Supported in part by Public Health Services Grants DK22000-25 and CA-58236-04.
References
- 1.Potten CS, Morris RJ: Epithelial stem cells in vivo. J Cell Science 1988, 10(Suppl):45-62 [DOI] [PubMed] [Google Scholar]
- 2.Potten CS, Loeffler M: Stem cells: attributes, cycles, spirals, pitfalls and uncertainties: lessons for and from the crypt. Development 1990, 110:1001-1020 [DOI] [PubMed] [Google Scholar]
- 3.Potten CS: Stem Cells. ed 1. London, Academic Press, 1997
- 4.Isaacs JT, Coffey DS: Etiology and disease process of benign prostatic hyperplasia. Prostate 1989, 2(Suppl):33-50 [DOI] [PubMed] [Google Scholar]
- 5.Bonkhoff H, Stein U, Remberger K: Multidirectional differentiation in the normal, hyperplastic, and neoplastic human prostate: simultaneous demonstration of cell-specific epithelial markers. Hum Pathol 1994, 25:42-46 [DOI] [PubMed] [Google Scholar]
- 6.Bonkhoff H, Remberger K: Differentiation pathways and histogenetic aspects of normal and abnormal prostatic growth: a stem cell model. Prostate 1996, 28:98-106 [DOI] [PubMed] [Google Scholar]
- 7.Kyprianou N, Isaacs JT: Activation of programmed cell death in the rat ventral prostate after castration. Endocrinology 1988, 122:552-562 [DOI] [PubMed] [Google Scholar]
- 8.DeKlerk DP, Coffey DS: Quantitative determination of prostatic epithelial and stromal hyperplasia by a new technique: biomorphometrics. Invest Urol 1978, 16:240-245 [PubMed] [Google Scholar]
- 9.McNeal JE: Regional morphology and pathology of the prostate. Am J Clin Pathol 1968, 49:347-357 [DOI] [PubMed] [Google Scholar]
- 10.McNeal JE: The zonal anatomy of the prostate. Prostate 1981, 2:35-49 [DOI] [PubMed] [Google Scholar]
- 11.McNeal JE: Normal histology of the prostate. Am J Surg Pathol 1988, 12:619-633 [DOI] [PubMed] [Google Scholar]
- 12.Brawer MK, Peehl DM, Stamey TA, Bostwick DG: Keratin immunoreactivity in the benign and neoplastic human prostate. Cancer Res 1985, 45:3663-3667 [PubMed] [Google Scholar]
- 13.Sherwood ER, Berg LA, Mitchell NJ, McNeal JE, Kozlowski JM, Lee C: Differential cytokeratin expression in normal, hyperplastic and malignant epithelial cells from human prostate. J Urol 1990, 143:167-171 [DOI] [PubMed] [Google Scholar]
- 14.Bonkhoff H, Remberger K: Widespread distribution of nuclear androgen receptors in the basal cell layer of the normal and hyperplastic human prostate. Virchows Arch A Pathol Anat Histopathol 1993, 422:35-38 [DOI] [PubMed] [Google Scholar]
- 15.Leav I, McNeal JE, Kwan PW, Komminoth P, Merk FB: Androgen receptor expression in prostatic dysplasia (prostatic intraepithelial neoplasia) in the human prostate: an immunohistochemical and in situ hybridization study. Prostate 1996, 29:137-145 [DOI] [PubMed] [Google Scholar]
- 16.Walensky LD, Coffey DS, Chen TH, Wu TC, Pasternack GR: A novel Mr 32,000 nuclear phosphoprotein is selectively expressed in cells competent for self-renewal. Cancer Res 1993, 53:4720-4726 [PubMed] [Google Scholar]
- 17.Kadkol SS, Brody JR, Epstein JI, Kuhajda FP, Pasternack GR: The novel nuclear phosphoprotein pp32 is highly expressed in intermediate and high grade prostate cancer. Prostate 1998, 34:231-237 [DOI] [PubMed] [Google Scholar]
- 18.Lee WH, Morton RA, Epstein JI, Brooks JD, Campbell PA, Bova GS, Hsieh WS, Isaacs WB, Nelson WG: Cytidine methylation of regulatory sequences near the pi-class glutathione S-transferase gene accompanies human prostatic carcinogenesis. Proc Natl Acad Sci USA 1994, 91:11733-11737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonnell TJ, Troncoso P, Brisbay SM, Logothetis C, Chung LW, Hsieh JT, Tu SM, Campbell ML: Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res 1992, 52:6940-6944 [PubMed] [Google Scholar]
- 20.Epstein JI: PSA and PAP as immunohistochemical markers in prostate cancer. Urol Clin North Am 1993, 20:757-770 [PubMed] [Google Scholar]
- 21.Sensibar JA, Alger B, Tseng A, Berg L, Lee C: Proteins of the rat prostate. III. Effect of testosterone on protein synthesis by the ventral prostate of castrated rats. J Urol 1990, 143:161-166 [DOI] [PubMed] [Google Scholar]
- 22.Sar M, Lubahn DB, French FS, Wilson EM: Immunohistochemical localization of the androgen receptor in rat and human tissues. Endocrinology 1990, 127:3180-3186 [DOI] [PubMed] [Google Scholar]
- 23.Yang Y, Hao J, Liu X, Dalkin B, Nagle RB: Differential expression of cytokeratin mRNA and protein in normal prostate, prostatic intraepithelial neoplasia, and invasive carcinoma. Am J Pathol 1997, 150:693-704 [PMC free article] [PubMed] [Google Scholar]
- 24.Bonkhoff H, Stein U, Remberger K: The proliferative function of basal cells in the normal and hyperplastic human prostate. Prostate 1994, 24:114-118 [DOI] [PubMed] [Google Scholar]
- 25.McNeal JE, Haillot O, Yemoto C: Cell proliferation in dysplasia of the prostate: analysis by PCNA immunostaining. Prostate 1995, 27:258-268 [DOI] [PubMed] [Google Scholar]
- 26.Mao P, Angrist A: The fine structure of the basal cell of human prostate. Lab Invest 1966, 15:1768-1782 [PubMed] [Google Scholar]
- 27.Lipschutz JH, Foster BA, Cunha GR: Differentiation of rat neonatal ventral prostates grown in a serum-free organ culture system. Prostate 1997, 32:35-42 [DOI] [PubMed] [Google Scholar]
- 28.Verhagen AP, Aalders TW, Ramaekers FC, Debruyne FM, Schalken JA: Differential expression of keratins in the basal and luminal compartments of rat prostatic epithelium during degeneration and regeneration. Prostate 1988, 13:25-38 [DOI] [PubMed] [Google Scholar]
- 29.Verhagen AP, Ramaekers FC, Aalders TW, Schaafsma HE, Debruyne FM, Schalken JA: Colocalization of basal and luminal cell-type cytokeratins in human prostate cancer. Cancer Res 1992, 52:6182-6187 [PubMed] [Google Scholar]
- 30.Xue Y, Smedts F, Debruyne FM, de la Rosette JJ, Schalken JA: Identification of intermediate cell types by keratin expression in the developing human prostate. Prostate 1998, 34:292-301 [DOI] [PubMed] [Google Scholar]
- 31.McNeal JE, Bostwick DG: Intraductal dysplasia: a premalignant lesion of the prostate. Hum Pathol 1986, 17:64-71 [DOI] [PubMed] [Google Scholar]
- 32.Epstein JI: Pathology of prostatic intraepithelial neoplasia and adenocarcinoma of the prostate: prognostic influences of stage, tumor volume, grade, and margins of resection. Semin Oncol 1994, 21:527-541 [PubMed] [Google Scholar]
- 33.Roberts JM, Koff A, Polyak K, Firpo E, Collins S, Ohtsubo M, Massague J: Cyclins, Cdks, and cyclin kinase inhibitors. Cold Spring Harb Symp Quant Biol 1994, 59:31-38 [DOI] [PubMed] [Google Scholar]
- 34.Polyak K, Lee MH, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, Massague J: Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 1994, 78:59-66 [DOI] [PubMed] [Google Scholar]
- 35.Toyoshima H, Hunter T: p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell 1994, 78:67-74 [DOI] [PubMed] [Google Scholar]
- 36.Coats S, Flanagan WM, Nourse J, Roberts JM: Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science 1996, 272:877-880 [DOI] [PubMed] [Google Scholar]
- 37.Steeg PS, Abrams JS: Cancer prognostics: past, present and p27 [News: Comment]. Nat Med 1997, 3:152-154 [DOI] [PubMed] [Google Scholar]
- 38.Sherr CJ: Cancer cell cycles. Science 1996, 274:1672-1677 [DOI] [PubMed] [Google Scholar]
- 39.Guo YP, Sklar GN, Borkowski A, Kyprianou N: Loss of the cyclin-dependent kinase inhibitor p27Kip1 protein in human prostate cancer correlates with tumor grade. Clin Cancer Res 1997, 3:2269-2274 [PubMed] [Google Scholar]
- 40.Yang RM, Naitoh J, Murphy M, Wang HJ, Phillipson J, Dekernion JB, Loda M, Reiter RE: Low p27 expression predicts poor disease-free survival in patients with prostate cancer. J Urol 1998, 159:941-945 [PubMed] [Google Scholar]
- 41.Tsihlias J, Kapusta LR, Deboer G, Moravaprotzner I, Zbieranowski I, Bhattacharya N, Catzavelos GC, Klotz LH, Slingerland JM: Loss of cyclin-dependent kinase inhibitor p27Kip1 is a novel prognostic factor in localized human prostate adenocarcinoma. Cancer Res 1998, 58:542-548 [PubMed] [Google Scholar]
- 42.Yasui W, Kudo Y, Semba S, Yokozaki H, Tahara E: Reduced expression of cyclin-dependent kinase inhibitor p27Kip1 is associated with advanced stage and invasiveness of gastric carcinomas. Jpn J Cancer Res 1997, 88:625-629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mori M, Mimori K, Shiraishi T, Tanaka S, Ueo H, Sugimachi K, Akiyoshi T: p27 expression and gastric carcinoma. Nat Med 1997, 3:593. [DOI] [PubMed] [Google Scholar]
- 44.Tan P, Cady B, Wanner M, Worland P, Cukor B, Magi-Galluzzi C, Lavin P, Draetta G, Pagano M, Loda M: The cell cycle inhibitor p27 is an independent prognostic marker in small (T1a,b) invasive breast carcinomas. Cancer Res 1997, 57:1259-1263 [PubMed] [Google Scholar]
- 45.Catzavelos C, Bhattacharya N, Ung YC, Wilson JA, Roncari L, Sandhu C, Shaw P, Yeger H, Morava-Protzner I, Kapusta L, Franssen E, Pritchard KI, Slingerland JM: Decreased levels of the cell-cycle inhibitor p27Kip1 protein: prognostic implications in primary breast cancer. Nat Med 1997, 3:227-230 [DOI] [PubMed] [Google Scholar]
- 46.Loda M, Cukor B, Tam SW, Lavin P, Fiorentino M, Draetta GF, Jessup JM, Pagano M: Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med 1997, 3:231-234 [DOI] [PubMed] [Google Scholar]
- 47.Esposito V, Baldi A, De Luca A, Groger AM, Loda M, Giordano GG, Caputi M, Baldi F, Pagano M, Giordano A: Prognostic role of the cyclin-dependent kinase inhibitor p27 in non-small cell lung cancer. Cancer Res 1997, 57:3381-3385 [PubMed] [Google Scholar]
- 48.Silberman MA, Partin AW, Veltri RW, Epstein JI: Tumor angiogenesis correlates with progression after radical prostatectomy but not with pathologic stage in Gleason sum 5 to 7 adenocarcinoma of the prostate. Cancer 1996, 79:772-779 [DOI] [PubMed] [Google Scholar]
- 49.Alessandrini A, Chiaur DS, Pagano M: Regulation of the cyclin-dependent kinase inhibitor p27 by degradation and phosphorylation. Leukemia 1997, 11:342-345 [DOI] [PubMed] [Google Scholar]
- 50.Loda M, Cukor B, Tam SW, Lavin P, Fiorentino M, Draetta GF, Jessup JM, Pagano M: Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med 1997, 3:231-234 [DOI] [PubMed] [Google Scholar]
- 51.van der Kwast TH, Tetu B: Androgen receptors in untreated and treated prostatic intraepithelial neoplasia. Eur Urol 1996, 30:265-268 [DOI] [PubMed] [Google Scholar]
- 52.Bonkhoff H, Fixemer T, Remberger K: Relation between Bcl-2, cell proliferation, and the androgen receptor status in prostate tissue and precursors of prostate cancer. Prostate 1998, 34:251-258 [DOI] [PubMed] [Google Scholar]
- 53.Vailancourt L, Ttu B, Fradet Y, Dupont A, Gomez J, Cusan L, Suburu ER, Diamond P, Candas B, Labrie F: Effect of neoadjuvant endocrine therapy (combined androgen blockade) on normal prostate and prostatic carcinoma: a randomized study. Am J Surg Pathol 1996, 20:86-93 [DOI] [PubMed] [Google Scholar]
- 54.Gleave ME, Goldenberg SL, Jones EC, Bruchovsky N, Sullivan LD: Biochemical and pathological effects of 8 months of neoadjuvant androgen withdrawal therapy before radical prostatectomy in patients with clinically confined prostate cancer. J Urol 1996, 155:213-219 [PubMed] [Google Scholar]
- 55.Kato JY, Matsuoka M, Polyak K, Massague J, Sherr CJ: Cyclic AMP-induced G1 phase arrest mediated by an inhibitor (p27Kip1) of cyclin-dependent kinase 4 activation. Cell 1994, 79:487-496 [DOI] [PubMed] [Google Scholar]
- 56.Sanchez-Beato M, Saez AI, Martinez-Montero JC, Sol Mateo M, Sanchez-Verde L, Villuendas R, Troncone G, Piris MA: Cyclin-dependent kinase inhibitor p27KIP1 in lymphoid tissue: p27KIP1 expression is inversely proportional to the proliferative index. Am J Pathol 1997, 151:151-160 [PMC free article] [PubMed] [Google Scholar]
- 57.Claus S, Wrenger M, Senge T, Schulze H: Immunohistochemical determination of age related proliferation rates in normal and benign hyperplastic human prostates. Urol Res 1993, 21:305-308 [DOI] [PubMed] [Google Scholar]
- 58.el-Deiry WS, Tokino T, Waldman T, Oliner JD, Velculescu VE, Burrell M, Hill DE, Healy E, Rees JL, Hamilton SR, Kinzler KW, Vogelstein B: Topological control of p21WAF1/CIP1 expression in normal and neoplastic tissues. Cancer Res 1995, 55:2910-2919 [PubMed] [Google Scholar]
- 59.Polyak K, Hamilton SR, Vogelstein B, Kinzler KW: Early alteration of cell-cycle-regulated gene expression in colorectal neoplasia. Am J Pathol 1996, 149:381-387 [PMC free article] [PubMed] [Google Scholar]