Abstract
Expression of cyclins A and E and cyclin-dependent kinase 2 (CDK2) was examined immunohistochemically in 190 cases of human lung carcinoma. Cyclin A and CDK2 were expressed in the majority of squamous cell carcinomas, small cell carcinomas, and large cell carcinomas, but in significantly fewer cases of adenocarcinomas. Cyclin E was expressed in a minority of all subtypes. In particular, well differentiated cells in squamous cell carcinoma stained positively for cyclin E; in contrast, cyclin A was expressed in the nonkeratinized proliferating areas of the tumor nests. Immunoblotting revealed that all these proteins were expressed at higher levels in tumor tissues than in adjacent normal tissues. Immunoprecipitation also revealed higher levels of cyclin A and cyclin E associated with CDK2 in tumor tissues. Furthermore, tumor tissues which exhibited higher cyclin A and CDK2 expression also had higher CDK2 kinase activity. However, cyclin E-associated kinase activity was barely detectable even in tumor samples exhibiting higher cyclin E expression. Consistent with these data, elevated expression of cyclin A correlated to shorter survival periods in contrast to expression of cyclin E, which correlated to longer survival periods. These results suggest that in human lung carcinomas, elevated expression of active cyclin A-CDK2 complexes with associated higher CDK2 kinase activity is critical for promoting cell cycle progression and unrestrained proliferation of tumor cells and can be a predictive marker for patients’ prognosis. On the other hand, immunohistochemical detection of cyclin E-CDK2 reflects accumulation of inactive forms of protein complexes, implying differentiation or senescence of the tumor and the better prognosis.
Cell proliferation is ultimately dependent on cell cycle control and the decision to continue to proliferate is made mainly during G1 phase as a result of the activities of G1 cyclins and CDK complexes. 1-8 Cyclin D is expressed initially in the G1 phase and associated kinase activity, manifested mainly by CDK4, oscillates from mid-G1. Cyclin E is expressed periodically, assembling with CDK2 and inducing maximum kinase activity at the G1/S transition. 7,8 Subsequently cyclin A is expressed and is thought to be required, in association with CDK2 and CDC2 (cell-cycle division 2), for progression through the S phase and the G2/M transition, respectively. 9
Among these cyclins only cyclin D1 has been identified as a proto-oncogene, designated PRAD1. It is overexpressed in lung, breast, gastric, and esophageal carcinomas at a frequency ranging from 13 to 60% with or without amplification of the 11q13 region. 10-15 Amplification and/or overexpression of cyclin E has also been reported in colorectal and breast carcinomas. 16-21 Overexpression of cyclin A has been reported in several cases of cultured cell lines from alveolar epithelial cells of the lung. 22 In addition, the cyclin A gene was found to be the unique insertion site of hepatitis B virus (HBV) in one clonal hepatoma. Cyclin A may thus play a role in the continuous proliferation of liver cells and ultimately in the pathogenesis of hepatocellular carcinoma. 23,24 Based on these observations cyclins and CDKs are simply believed to be positive regulators of cell cycles and the pathological mechanisms of tumorigenesis and tumor cell proliferation in human lung carcinoma due to aberrant expression of various cell cycle regulators have not been fully analyzed. The histopathological heterogeneity of human lung carcinomas suggests that they may be caused by diverse cellular mechanisms. In this study we focused on the G1/S and S to G2 transitions in the cell cycle and examined the expression of cyclins A and E as well as their catalytic partner, CDK2, by immunohistochemistry. Furthermore, we performed immunoblotting analysis and in vitro kinase reaction assays to examine the expression of these molecules and their associated kinase activity in matched sets of tumor and normal tissues of the lung and in cultured cell lines of human lung carcinoma. Finally, we analyzed the data in relation to patient survival rates.
Materials and Methods
Cases and Histological Classification
For this study we examined 190 cases of primary lung carcinoma obtained from surgical material including biopsies and from autopsies in the Department of Pathology, Kitasato University Hospital, between 1980 and 1996. According to the World Health Organization (WHO) histological classification, 25 these cases included 55 squamous cell carcinomas (SCC), 58 adenocarcinomas (AC), 36 small cell carcinomas (SmCC), and 41 large cell carcinomas (LCC).
Archival Tissue Samples and Immunohistochemistry
All archival tissue samples were routinely fixed in formalin and embedded in paraffin. Deparaffinized sections were autoclaved (120°C, 2 atm, 20 min) in 20 mmol/L citrate buffer, pH 6.0. 26 Immunostaining was performed with primary antibodies at the following dilutions: anti-cyclin A (monoclonal, Novocastra, Newcastle, United Kingdom), 1:500 dilution; anti-cyclin E (monoclonal, Novocastra), 1:100; anti-CDK2 antibody (polyclonal, SANTA CRUZ, Santa Cruz, CA), 1:2000. The specificity of these antibodies was confirmed by immunoblotting (see Figure 4 ▶ ). The conventional streptavidin-biotinylated horseradish peroxidase complex method (LSAB Kit, DAKO Japan, Kyoto, Japan) was used according to the manufacturer’s instructions. Colorization was performed by the peroxidase-diaminobenzidine (DAB) method.
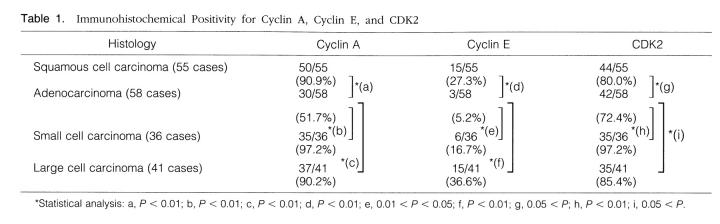
Figure 4.
Immunoblotting analysis of cyclin A, cyclin E, and CDK2 proteins expressed in surgically resected lung tissues and in human lung carcinoma cell lines. Lysates (50 μg of protein) prepared from paired tumor/normal tissues as well as human lung carcinoma cell lines were subjected to immunoblotting analysis. N, normal tissue; T, tumor tissue.
Scoring Immunoreactivity
The number of tumor cells with positive staining varied among the cases. The percentage of positive cells in each case was estimated by counting 500 tumor cells in 10 high-power fields and semiquantatively evaluated into one of the following five immunoreactivity groups: (a) immunoreactivity completely absent (negative, 0%); (b) <5%; (c) <30%; (d) <50%; and (e) up to 100%. In the present study, cases showing >5% of positive tumor cells were defined as “positive.”
Patients’ Follow-Up
Patients’ outcome data were collected from hospital charts. Informative patient charts were available for 41 of SCC, 38 of AC, 17 of SmCC, and 29 of LCC cases. Follow-up period ranged from 2 months to 14 years after the pathological diagnosis.
Statistical Analysis
In immunohistochemical analysis, the ratios of positive cases to total cases among the four histological subtypes were compared and the differences were statistically examined by normal distribution analysis. Patients’ survival curve was calculated using Kaplan-Meier analysis with the data obtained from hospital charts. The correlations between the rates of expression of cyclins or CDK2 and overall survival time were analyzed and the differences between the groups were tested for statistical significance with the Mantel-Cox test.
Cell Lines
Cultured cell lines from human lung carcinomas were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum. These included Lu-130, Lu-134B, Lu-135 (small cell carcinomas) kindly provided by the National Cancer Center Research Institute (Tokyo) and PC-13 (poorly differentiated adenocarcinoma) provided by the Tokyo Medical College.
Paired Tumor/Normal Lung Tissue Collection
Fresh fragments of paired tumor and adjacent normal tissues were obtained from surgically resected specimens and were used for immunoblotting analysis, immunoprecipitation (IP), and in vitro kinase reaction assay. These comprised 3 cases of SCC, 3 of AC, 1 of SmCC, and 2 of LCC. (Histopathological and immunohistochemical profiles of those cases are listed in Table 5 ▶ .)
Table 5.
Histological and Immunohistochemical Profiles of Cases used for Immunoblotting, Immunoprecipitation, and In Vitro Kinase Reaction
| Case no. | Histological subtype | % positive cells on immunohistochemistry | ||
|---|---|---|---|---|
| Cyclin A | Cyclin E | CDK2 | ||
| 1 | Squamous, W* | 6 | 25 | 11 |
| 2 | Squamous, M* | 47 | 7 | 29 |
| 3 | Squamous, M | 55 | 3 | 27 |
| 4 | Adenocarcinoma, W | 1 | 2 | 24 |
| 5 | Adenocarcinoma, W | 3 | 1 | 9 |
| 6 | Adenocarcinoma, P* | 28 | 3 | 28 |
| 7 | Small cell carcinoma | 60 | 11 | 41 |
| 8 | Large cell carcinoma | 23 | 38 | 37 |
| 9 | Large cell carcinoma | 35 | 20 | 35 |
*Subtypes: W, well differentiated; M, moderately differentiated; P, poorly differentiated.
Preparation of Cell Lysates and Tissue Extracts
For protein extraction, cells and tissues were lysed in high-salt lysis buffer (0.5% Nonidet P-40 (NP-40), 0.1% SDS, 50 mmol/L Tris-HCl, pH 8.0, 0.25 mol/L NaCl, 5 mmol/L EDTA, 50 mmol/L NaF, 0.5 mmol/L phenylmethylsulfonyl fluoride (PMSF), 5 μg/ml aprotinin, 5 μg/ml leupeptin). 27 Fresh tissues were homogenized in high-salt lysis buffer on ice and the resultant lysates were sonicated on ice 4 times for 10 seconds each time. 28 Lysates were clarified by centrifugation at 10,000 × g for 5 minutes.
Immunoblotting Analysis
Equal amounts (50 μg) of protein were used for immunoblotting. The same specific antibodies used in the immunohistochemical staining were used in the following dilutions: anti-cyclin A, 1:300; anti-cyclin E, 1:100; and anti-CDK2 antibody, 1:150. Each protein was detected by the sequential application of a specific primary antibody followed by an alkaline phosphatase-conjugated secondary antibody (Promega, Madison, WI, 1:6000 dilution). Colorization was performed with nitroblue tetrazolium (NBT) and 5-bromo-4-chloro-3-indol-phosphate (BCIP) (Bio-Rad, Gathersburg, MD) in 100 mmol/L Tris buffer, pH 9.6. For immunoprecipitation followed by immunoblotting, cells were lysed in NP-40 lysis buffer (50 mmol/L Tris-HCl, pH 7.4, 0.5% NP-40, 0.15 mol/L NaCl, 50 mmol/L NaF, 1 mmol/L dithiothreitol (DTT), 1 mmol/L PMSF, 5 μg/ml of aprotinin, 5 μg/ml of leupeptin). 29 Lysates (200 μg of protein) were incubated with p13suc1-Sepharose for 4 hours at 4°C. The precipitates were used for further immunoblotting analysis for cyclin A or cyclin E. Alternatively, lysates were incubated with anti-cyclin A (diluted 1:300) or anti-cyclin E (diluted 1:100) antibodies for 1 hour at 4°C followed by an additional 1 hour incubation with protein G-Sepharose beads at 4°C. 29 The immunoprecipitates were used for immunoblotting analysis for CDK2.
In Vitro Kinase Reaction
For immunocomplex kinase reactions for CDK2, cells were lysed in NP-40 lysis buffer with addition of 1 mmol/L Na3VO4. 30 Lysates (250 μg of protein) were incubated with anti-CDK2 antibody (diluted 1:150) for 1 hour followed by an additional 1 hour of incubation with protein A-Sepharose beads at 4°C. For cyclin A- or cyclin E-associated kinase reactions, lysates (250 μg of protein) were incubated with anti-cyclin A (diluted 1:300) or anti-cyclin E antibody (1:100) for 1 hour followed by an additional 1 hour of incubation with protein G-Sepharose beads at 4°C. A bacterially-expressed fragment of the retinoblastoma protein (pRB, amino acids 385–928) fused to glutathione S-transferase (GST) was used as a substrate (0.5 μg of protein) in 50 μl of kinase reaction buffer (50 mmol/L Tris-HCl [pH 7.2], 10 mmol/L MgCl2, 1 mmol/L DTT, 20 mmol/L [γ-32P]ATP(5 μCi;1 μCi = 37 kBq, ICN, Irvine, CA). 27,30 After incubation for 10 minutes at room temperature, the sample was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by autoradiography.
Results
Immunohistochemistry
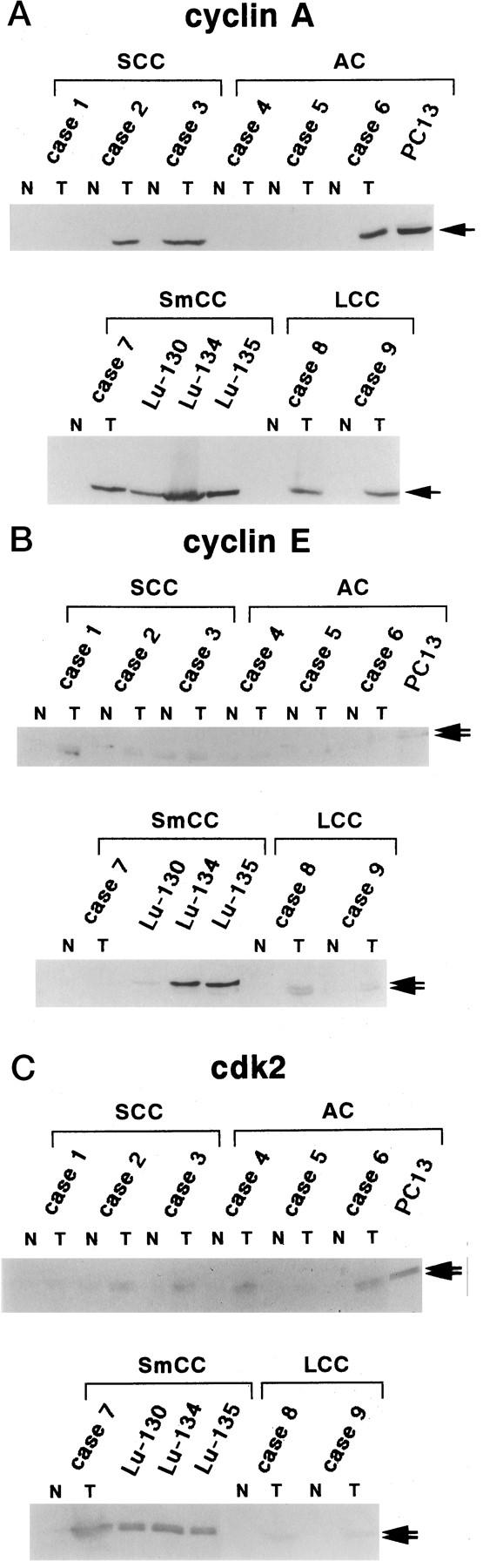
Positive immunohistochemical staining for cyclin A, cyclin E, and CDK2 was confined almost exclusively to the nuclei of the tumor cells (Figure 1) ▶ . The overall results of the immunohistochemical analysis are summarized in Table 1 ▶ .
Figure 1.
a: Squamous cell carcinoma of the lung showing ubiquitous positive staining for cyclin A and CDK2, but only scattered positive staining for cyclin E. b: Adenocarcinoma showing focal positive staining for cyclin A and CDK2. Small cell carcinoma (c) and large cell carcinoma (d) also showed almost diffuse positive staining for cyclin A and CDK2, but focal positive staining for cyclin E. All immunohistochemical staining was confined to the nuclei. Streptavidin-biotin peroxidase method with hematoxylin counterstain; magnification, ×500.
Table 1.
Immunohistochemical Positivity for Cyclin A, Cyclin E, and CDK2
Cyclin A staining revealed that the vast majority of SCC (50/55 cases), SmCC (35/36), and LCC (37/41) cases exhibited positive staining irrespective of the percentage of positive cells per case. Positive staining in non-neoplastic cells was also occasionally identified, for instance, in cells within the germinal center of the lymphoid follicle. Although positive cells in SmCC (Figure 1c) ▶ and LCC (Figure 1d) ▶ were almost ubiquitously identified within the tumor nests, those in SCC were identified predominantly in the basal areas of tumor nests and well differentiated, keratinized areas, often localized in the central portion of the nests, remained unstained (Figure 2a) ▶ . In AC, 52% (30/58) stained positively, and 70% of these (21/30) contained fewer than 30% total positive cells (Table 2) ▶ . Furthermore, the proportion of positive tumor cells was inversely correlated with the degree of morphological differentiation. In LCC, positive staining was identified in all histological subtypes, ie, ordinary, spindle, and giant cell types, and there was no predilection for a certain subtype. The ratio of positive cases to total cases was lower to a statistically significant degree in AC than in the other three subtypes (Table 1) ▶ .
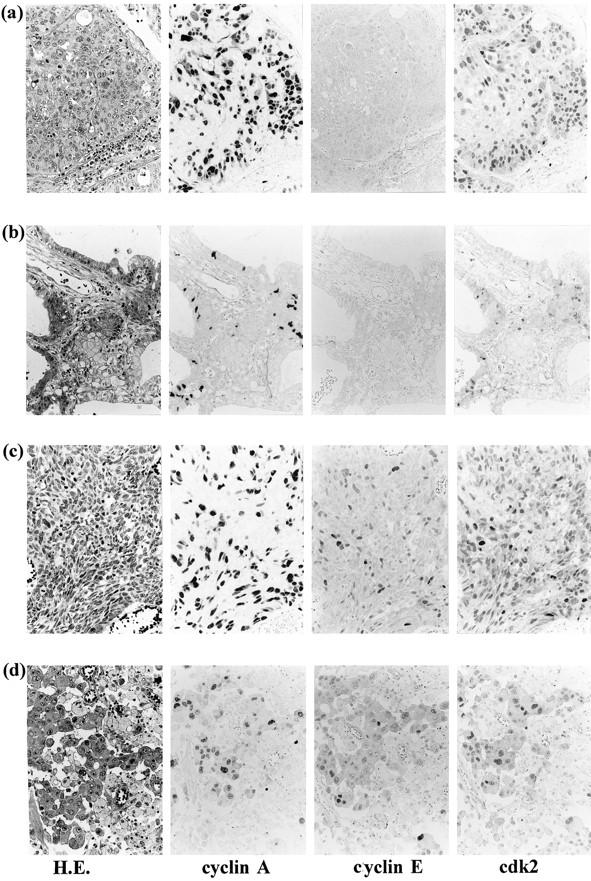
Figure 2.
Immunohistochemical staining for cyclin A, cyclin E, and CDK2 on serial sections. Co-expression of cyclin E and CDK2 in well differentiated cells of squamous cell carcinoma (a) and squamoid cells in large cell carcinoma (b). Coexpression of cyclin A and CDK2 around the basal area of tumor nest in moderately differentiated squamous cell carcinoma (c). Streptavidin-biotin peroxidase method with hematoxylin counterstain; magnification, ×1,000.
Table 2.
Semiquantative Evaluation of Immunohistochemical Positivity for Cyclin A
| Histology | % positive tumor cells | Total no. of cases | ||||
|---|---|---|---|---|---|---|
| 0 | <5% | <30% | <50% | <100% | ||
| Squamous cell carcinoma (55 cases) | ||||||
| W* | 1 | 3 | 6 | 5 | 1 | 16 |
| M* | 0 | 1 | 7 | 13 | 6 | 27 |
| P* | 0 | 0 | 0 | 3 | 9 | 12 |
| Adenocarcinoma (58 cases) | ||||||
| W | 5 | 16 | 4 | 0 | 0 | 25 |
| M | 2 | 5 | 12 | 3 | 0 | 22 |
| P | 0 | 0 | 5 | 5 | 1 | 11 |
| Small cell carcinoma (36 cases) | 0 | 1 | 9 | 12 | 14 | 36 |
| Large cell carcinoma (41 cases) | 0 | 4 | 9 | 19 | 9 | 41 |
*Subtypes: W, well differentiated; M, moderately differentiated; P, poorly differentiated.
Cyclin E staining was found to be less frequent and less intense than cyclin A in all types of carcinomas (Tables 1 and 3 ▶ ▶ and Figure 1 ▶ ). In the SCC cases, 27% (15/55) stained positively, particularly tumor cells in differentiated areas that were unstained by cyclin A staining (Figure 2a) ▶ . In AC, only 5% (3/58) cases of moderately and poorly differentiated subtypes exhibited positive staining (Figure 1c ▶ and Table 3 ▶ ). In SmCC, 17% (6/36) stained positively and none of these 6 cases contained more than 30% total positive cells (Figure 1c ▶ and Table 3 ▶ ). In LCC, 37% (15/41) stained positively (Figure 1d ▶ and Table 3 ▶ ). In several of these LCC cases, positive tumor cells were found to be squamoid or large multinucleated giant cells with rich cytoplasm which were unstained by cyclin A staining (Figure 1d and 2b) ▶ ▶ .
Table 3.
Semiquantitative Evaluation of Immunohistochemical Positivity for Cyclin E
| Histology | % positive tumor cells | Total no. of cases | ||||
|---|---|---|---|---|---|---|
| 0 | <5% | <30% | <50% | <100% | ||
| Squamous cell carcinoma (55 cases) | ||||||
| W* | 5 | 6 | 3 | 1 | 1 | 16 |
| M* | 15 | 4 | 6 | 1 | 1 | 27 |
| P* | 7 | 3 | 1 | 1 | 0 | 12 |
| Adenocarcinoma (58 cases) | ||||||
| W | 22 | 3 | 0 | 0 | 0 | 25 |
| M | 19 | 2 | 1 | 0 | 0 | 22 |
| P | 7 | 2 | 2 | 0 | 0 | 11 |
| Small cell carcinoma (36 cases) | 28 | 2 | 6 | 0 | 0 | 36 |
| Large cell carcinoma (41 cases) | 22 | 4 | 9 | 4 | 2 | 41 |
*Subtypes: W, well differentiated; M, moderately differentiated; P, poorly differentiated.
The number of cases positive for CDK2 staining and the proportion of positive cells in each case were similar to those obtained for cyclin A staining (Tables 1 and 4) ▶ ▶ . In addition, there was no obvious correlation between the degree of differentiation and positive CDK2 staining. In the immunohistochemical investigation on serial sections of all types of carcinomas, tumor cells expressing cyclin A also displayed positive staining for CDK2 protein at a high frequency (Figures 1 and 2c) ▶ ▶ . However, well differentiated cells in SCC and squamoid cells in LCC, both of which stained positively for cyclin E, also revealed positive staining for CDK2 (Figure 2a and 2b) ▶ .
Table 4.
Semiquantitative Evaluation of Immunohistochemical Positivity for CDK2
| Histology | % positive tumor cells | Total no. of cases | ||||
|---|---|---|---|---|---|---|
| 0 | <5% | <30% | <50% | <100% | ||
| Squamous cell carcinoma (55 cases) | ||||||
| W* | 1 | 5 | 5 | 3 | 2 | 16 |
| M* | 0 | 3 | 7 | 11 | 6 | 27 |
| P* | 0 | 2 | 2 | 5 | 3 | 12 |
| Adenocarcinoma (58 cases) | ||||||
| W | 2 | 5 | 14 | 4 | 0 | 25 |
| M | 4 | 3 | 8 | 7 | 0 | 22 |
| P | 0 | 2 | 4 | 4 | 1 | 11 |
| Small cell carcinoma (36 cases) | 0 | 1 | 13 | 15 | 7 | 36 |
| Large cell carcinoma (41 cases) | 0 | 6 | 14 | 15 | 6 | 41 |
*Subtypes: W, well differentiated; M, moderately differentiated; P, poorly differentiated.
Correlation of Immunohistochemical Positivity with Patients’ Survival
Staining results of cyclins A and E and CDK2 were evaluated for their correlation with patients’ survival rate. The cyclin A-positive group revealed lower survival rate compared with negative group in non-SmCC at a statistically significant level (P < 0.02, Figure 3a ▶ ). In SmCC cases, correlation could not be analyzed due to the paucity of cyclin A-negative cases. Conversely, the cyclin E-positive group showed a significantly higher survival rate compared with the cyclin E-negative group in both SmCC and non-SmCC groups (Figure 3b) ▶ . For CDK2 staining, no specific correlation was found with patients’ survival (data not shown).
Figure 3.
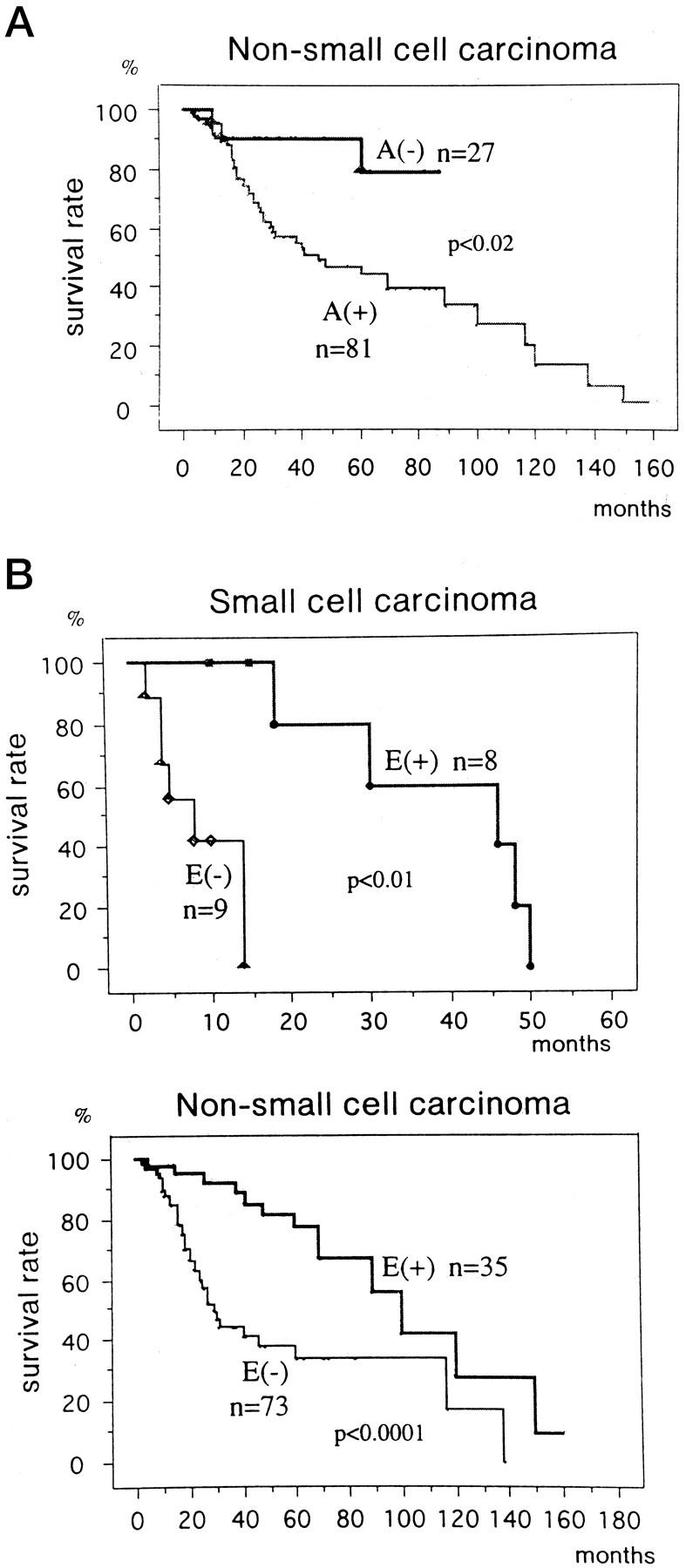
Overall survival curves of the 125 patients (17 small cell carcinoma and 108 non-small cell carcinoma cases) stratified according to the immunohistochemical expression of cyclin A (A) or cyclin E (B). A, cyclin A; E, cyclin E; +, positive; −, negative.
Immunoblotting Analysis
To further confirm the results observed by immunohistochemical staining, immunoblotting analysis was performed using lysates obtained from paired tumor and normal tissue samples of the lung as well as cultured human lung carcinoma cell lines. Profiles of these cases in immunohistochemical stainings are summarized in Table 5 ▶ .
Cyclin A blotting revealed one major band of 58 kd, suggesting the absence of any rearrangement or chimeric protein formation (Figure 4a) ▶ . In cell lines and tumor tissues that exhibited higher numbers of positively stained cells on immunohistochemistry (cases 2, 3, 6–9), significantly higher levels of cyclin A expression were detected compared with normal lung tissue from the same cases. The level of protein expression correlates well with the number of positive cells revealed by immunohistochemistry (Figure 4 ▶ and Table 5 ▶ ). All cell lines, 1 AC and 3 SmCC, also exhibited higher level of expression.
Cyclin E blotting revealed two major bands of 49 and 43 kd, corresponding to the two alternatively spliced forms of human cyclin E (Figure 4b) ▶ . 31 Although expression of cyclin E was elevated in SCC (cases 1–3) and LCC (cases 8 and 9), this was not observed in the cases of AC (cases 4–6) and SmCC (case 7), consistent with the lower numbers of positive cells detected by immunohistochemistry. Although two cell lines of SmCC, Lu-134 and Lu-135, exhibited noticeably higher levels of expression, other cell lines, one AC (PC13) and one SmCC (Lu130), revealed levels of expression similar to that in the tumor tissue.
Anti-CDK2 blotting revealed two major bands of 34 and 33 kd corresponding to the hyper- and unphosphorylated forms of human CDK2 (Figure 4c) ▶ . 27 The levels of CDK2 expression almost paralleled those revealed by anti-cyclin A blotting (Figure 4c) ▶ .
Immunoblotting Analysis of CDK-Associated Cyclin A and Cyclin E
To evaluate the levels of cyclin A and cyclin E associated with CDK in the cases which showed significantly higher level of expression by immunoblotting analysis, we subjected p13suc1 precipitates prepared from the selected tissue samples and cell lines to sequential immunoblotting analysis. As shown in Figure 5a ▶ , the amount of cyclin A and cyclin E associated with CDK which bound to p13suc1-Sepharose was generally higher in cell lines and in tumor samples compared with matched adjacent normal lung tissues. To further confirm the association of CDK2 with cyclin A or cyclin E, we subjected anti-cyclin A or anti-cyclin E immunoprecipitates to sequential immunoblotting for CDK2. As shown in Figure 5b ▶ , we observed higher levels of CDK2-cyclin complexes in these tumor tissues and in cell lines.
Figure 5.
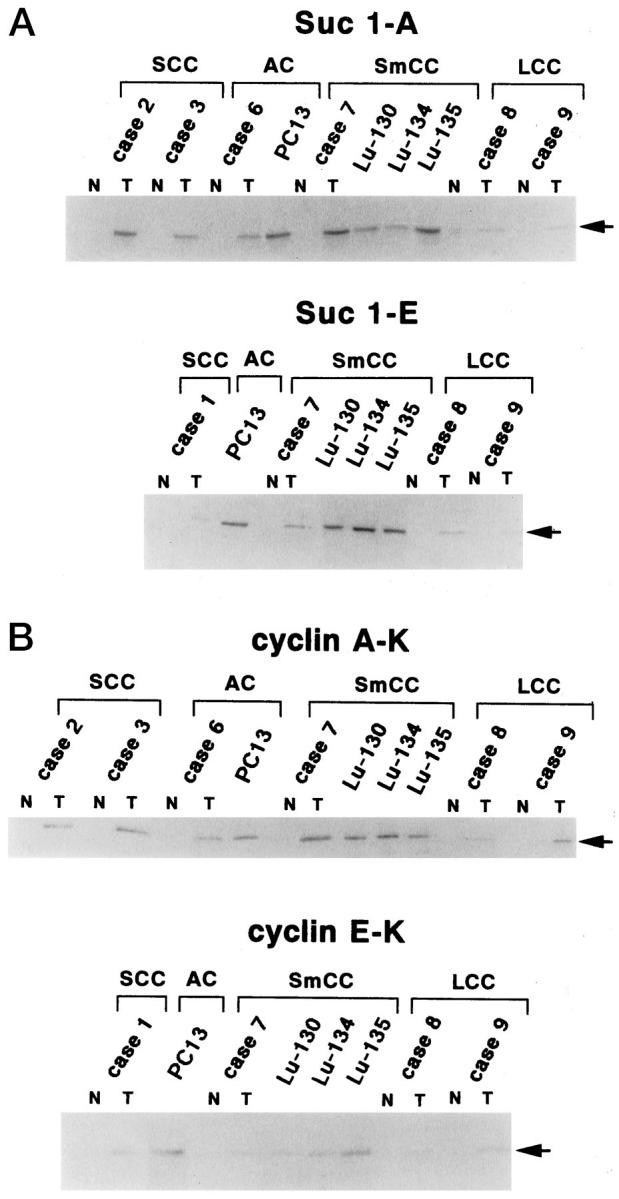
Levels of CDK-associated cyclin A and cyclin E and levels of CDK2 associated with cyclin A and cyclin E. Lysates prepared from surgically resected lung tissues and from cell lines (200 μg) were precipitated with p13suc1-Sepharose (for cyclin A and cyclin E blotting) or immunoprecipitated with anti-cyclin A or anti-cyclin E antibodies (for CDK2 blotting). The precipitates were subjected to further immunoblotting analysis with the respective antibodies. Only those cases in which high-level expression of the respective proteins was observed by immunoblotting analysis were subjected to precipitation by anti-cyclin A, cyclin E antibodies, or p13suc1. Suc 1-A, Suc 1-E: Precipitation by p13suc1-Sepharose followed by cyclin A or cyclin E blotting, respectively, Cyclin A-K, Cyclin E-K: IP by anti-cyclin A or anti-cyclin E antibodies followed by CDK2 blotting, respectively. N, normal tissue; T, tumor tissue.
CDK2 Kinase Activity
CDK2-associated kinase activity was examined in selected tissue samples and cell lines which showed significantly higher level of protein expression by immunoblotting analysis after IP from the lysates using anti-cyclin A, anti-cyclin E, and anti-CDK2 antibodies. Tumor tissues and cell lines exhibited higher levels of cyclin A- or CDK2-associated kinase activity compared to those obtained from matched normal lung tissues (Figure 6a, b) ▶ . However, there was no significant cyclin E-associated kinase activity in lysates even from tumor tissues expressing higher levels of cyclin E-CDK2 complexes compared with that in normal tissue, except one case of tumor tissue (case 7) and a cell line from SmCC, Lu135 (Figures 5 and 6a) ▶ ▶ .
Figure 6.
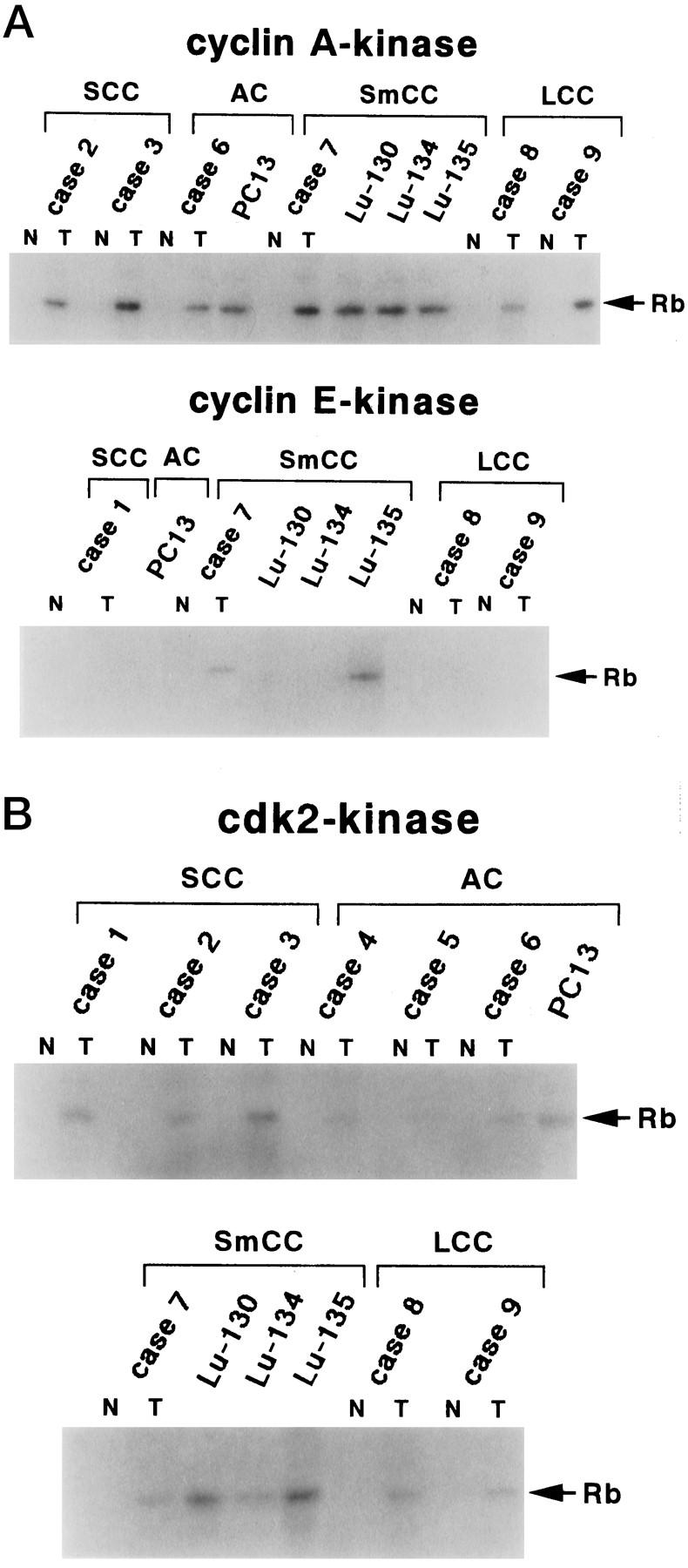
Kinase activities associated with cyclin A, cyclin E, or CDK2. Cyclin A-, cyclin E-, or CDK2-associated kinase activity in surgically resected lung tissues and in cell lines are shown. Lysates (250 μg) were immunoprecipitated with anti-cyclin A, anti-cyclin E, or anti-CDK2 antibodies. The immunocomplex was assayed for kinase activity using GST-RB fusion protein as a substrate. Only those cases in which high-level expression of the respective proteins was observed by immunoblotting analysis were subjected to immunoprecipitation by anti-cyclin A, anti-cyclin E, or anti-CDK2 antibodies. N: normal tissue, T: tumor tissue.
Discussion
Although involvement of cyclin D1 in carcinogenesis has been suggested and strengthened by molecular analysis in human lung carcinoma, its clinicopathological implication is still controversial. 32,33 This and the marked heterogeneity of lung carcinoma led us to consider the possibility of aberrations in other cell cycle regulators.
In this study, we clearly demonstrated diverse implications regarding the expression of cyclin A-CDK2 and cyclin E-CDK2 complexes, both of which have been simply believed to be positive regulators of cell cycle.
Positive immunohistochemical staining for cyclin A was detected in more than 80% of the cases of lung carcinoma. One possibility is that this positive cyclin A staining simply reflects an increase in the proportion of the cell populations in the S through G2/M phases. However, this is unlikely; non-neoplastic lung tissue barely exhibited positive staining for cyclin A, even though fluorescence activated-cell-sorting analysis of non-neoplastic lung tissues revealed that approximately 7% of these cells were in the S through G2/M phases (data not shown). From these observations, we speculate the positive cyclin A staining in immunohistochemistry represents overexpression.
Cyclin A was found to be overexpressed in most SCC, SmCC, and LCC cases. Furthermore, in SCC and AC, poorly differentiated subtypes tended to express cyclin A at a higher frequency and with a higher proportion of positive tumor cells. Analysis of serial sections revealed that many of the cells positive for cyclin A co-expressed CDK2, suggesting that these cells maintain their transformed and aggressive phenotype due to high cyclin A-associated CDK2 kinase activity. The distribution of cyclin A-positive cells in the basal areas of tumor nests of SCC which are interpreted as the proliferating zone, supports this notion. The lower frequency of positive cyclin A staining in AC suggests a lower cell proliferation rate, consistent with a previous report describing lower labeling index for BrdU and proliferating cell nuclear antigen (PCNA) in AC. 34-36
In contrast, expression of cyclin E seemed inversely correlated with the proliferative activity of tumor cells, since well differentiated cells in SCC, which are assumed to have low proliferative activity, stained positively for both cyclin E and CDK2. Possibly, cyclin E plays a role in cellular differentiation, as described for the process of neuronal or osteoblastic cell line differentiation. 27,37 Alternatively, as demonstrated in the present study, CDK2-cyclin E complexes accumulate in these well differentiated cells without catalyzing significant kinase activity, suggested in a previous report using senescent human diploid fibroblasts. 38 If this is the case, the lack of kinase activity could be due to the action of CDK-cyclin inhibitor proteins p21, p27, p57, or others, and/or to changes in the phosphorylation state of CDK2 that modulate its activity. 5,38-41 Although these hypotheses seemed applicable to most of the cases in the present study, we do not have reasonable explanation for a case of SmCC (case 7) and a cell line Lu135 both of which manifested higher cyclin E-associated kinase activity. We speculate that there was alteration in cyclin E gene resulting in stable and functional activation of cyclin E-CDK2 complex or functional inactivation of CDK2 inhibitors, and cyclin E may play a role for the tumor cells to get growth advantage in those particular cases.
Immunoblotting analysis detected cyclins A and E and CDK2 of an apparent wild-type molecular weight. Thus, the possibility of rearrangement, truncation, or chimeric protein formation as previously described for cyclins A and E, 18,21,23,24 is less likely in the case of lung carcinoma.
The clinicopathological implications of amplification/overexpression of cyclins D1 and E in human tumors have been variously described in the literature as positive or negative prognostic factors. 20,21,42,43 G1/S cyclins that are overexpressed but not genetically altered are presumably catalytically inactive and found predominantly in differentiated tumor cells, as are inactive cyclin E-CDK2 complexes detected in our present study, and eventually associated with better prognosis. Indeed, the immunohistochemical positivity of cyclin A was demonstrated to be related with shorter survival and cyclin E with longer survival compared with respective negative groups.
Altogether, our results show that expression of cyclin A-CDK2 complex and its associated kinase activity serve as novel markers that predict proliferative activity and eventually, patients’ prognosis in lung carcinoma. Apparently, these complexes cause an increase in the population of S phase cells and drive the tumorous phenotype, particularly in SCC, SmCC, and LCC. Overexpression of cyclin E-CDK2 complexes, which normally play a crucial role in the G1/S transition, does not seem to be most critical in the cell cycle control in lung carcinoma, and is rather associated with better prognosis. However, based on the data that cyclin E expression was exclusively identified in the tumor cells, cyclin E-CDK2 complex appear to be expressed and accumulated in an inactive form in pararell with down-regulation of cyclin A-CDK2 expression when the tumor cells show differentiation or senescence after the phase of active proliferation.
Acknowledgments
We thank Ms. Tomoko Tsuruta for excellent technical assistance.
Footnotes
Address reprint requests to Toru Kameya, MD, Department of Pathology, Kitasato University School of Medicine, 1-15-1, Sagamihara, Kanagawa 228-8555, Japan. E-mail: ydobashi@med.kitasato-u.ac.jp.
Supported in part by Grants-in-aid for Scientific Research 09770130 and 07670219 from the Ministry of Education, Science and Culture in Japan and by Kitasato University Research Grant for Young Researchers.
References
- 1.Pardee AB: G1 events and regulation of cell proliferation. Science 1989, 246:603-608 [DOI] [PubMed] [Google Scholar]
- 2.Lees E, Faha B, Dulic V, Reed SI, Harlow E: Cyclin E/CDK2 and cyclin A/CDK2 kinase associated with p107, and E2F in a temporary distinct manner. Genes Dev 1992, 6:1874-1885 [DOI] [PubMed] [Google Scholar]
- 3.Xiong Y, Connolly T, Futcher B, Beach D: Human D-type cyclin. Cell 1991, 65:691-699 [DOI] [PubMed] [Google Scholar]
- 4.Sherr CJ: G1 phase progression: cycling on cue. Cell 1994, 79:551-555 [DOI] [PubMed] [Google Scholar]
- 5.Hunter T, Pines J: Cyclins and cancer. II: Cylin D and CDK inhibitors come of age. Cell 1994, 79:573-582 [DOI] [PubMed] [Google Scholar]
- 6.Sherr CJ: Cancer cell cycle. Science 1996, 274:1672-1678 [DOI] [PubMed] [Google Scholar]
- 7.Dulic V, Lees E, Reed SI: Association of human cyclin E with a periodic G1-S phase protein kinase. Science 1992, 257:1958-1961 [DOI] [PubMed] [Google Scholar]
- 8.Koff A, Giordanao A, Desai D, Yamashita K, Harper JW, Elledge S, Nishimoto T, Morgan DO, Franza BR, Roberts JM: Formation and activation of cyclin E-CDK2 complex during the G1-phase of the human cell cycle. Science 1992, 257:1689-1694 [DOI] [PubMed] [Google Scholar]
- 9.Pagano M, Pepperlol R, Verde F, Ansorge W, Draetta G: Cyclin A is required at two points in the human cell cycle. EMBO J 1992, 11:961-971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motokura T, Bloom T, Kim HG, Juppner H, Ruderman JV, Kronenberg HM, Arnold A: A novel cyclin encoded by a bcl-linked candidate oncogene. Nature 1991, 350:512-515 [DOI] [PubMed] [Google Scholar]
- 11.Withers DA, Harvey RC, Faust JB, Melnyk, Carey K, Meeker TC: Characterization of a candidate bcl-1 gene. Mol Cell Biol 1991, 11:4846-4853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lammie GA, Fantl V, Smith R, Schuuring E, Brookes S, Michalides R, Dickson C, Arnold A, Peters G: D11S128, a putative oncogene on chromosome 11q13 is amplified and expressed in squamous cell and mammary carcinomas and linked to BCL-1. Oncogene 1991, 6:439-444 [PubMed] [Google Scholar]
- 13.Jiang W, Kahn SM, Tomita N, Zhang YJ, Lu SH, Weinstein IB: Amplification and expression of the human cyclin D gene in esophageal cancer. Cancer Res 1992, 52:2980-2983 [PubMed] [Google Scholar]
- 14.Buckley MF, Sweeney KJ, Hamilton JA, Sini RL, Manning DL, Nicholson RI, de Fazio A, Watts CK, Musgrove EA, Sutherland RL: Expression and amplification of cyclin genes in human breast cancer. Oncogene 1993, 8:2127-2133 [PubMed] [Google Scholar]
- 15.Diest PJ, Michalides RJ, Jannink I, Valk P, Peterse H, Jong JS, Meijer CJL, Baak PA: Cyclin D1 expression in invasive breast cancer: correlation and prognostic value. Am J Pathol 1995, 150:705-711 [PMC free article] [PubMed] [Google Scholar]
- 16.Resnitzky D, Gossen M, Bujard H, Reed SI: Acceleration of the G1/S phase transition by expression of cyclin D1 and E with an inducible system. Mol Cell Biol 1994, 14:1669-1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohtsubo M, Roberts JM: Cyclin-dependent regulation of G1 in mammlian fibroblasts. Science 1993, 259:1908-1912 [DOI] [PubMed] [Google Scholar]
- 18.Tahara E: Genetic alteration in human gastrointestinal cancers. The application to molecular diagnosis. Cancer 1995, 75:1410-1417 [DOI] [PubMed] [Google Scholar]
- 19.Dutta A, Chandra R, Leiter LM, Lester S: Cyclins as marker of tumor proliferation: immunocytochemical studies in breast cancer. Proc Natl Acad Sci USA 1995, 92:5386-5390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keyomarsi K, O’Leary N, Molnar G, Lees E, Fingert HJ, Pardee AB: Cyclin E, a potential prognostic marker for breast carcinoma. Cancer Res 1994, 54:380-385 [PubMed] [Google Scholar]
- 21.Keyomarsi K, Conte D, Toyofuku W, Fox MP: Deregulation of cyclin E in breast cancer. Oncogene 1995, 11:941-50 [PubMed] [Google Scholar]
- 22.Bui KC, Wu F, Buckley S, Wu L, Williams R, Carbonaro HD, Hall FL, Warburton D: Cyclin A expression in normal, and transformed alveolar epithelial cells. Am J Respir Cell Mol Biol 1993, 9:115-125 [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Chenivesse X, Henglein B, Brechot C: Hepatitis B virus integration in a cyclin A gene in a hepatocellular carcinoma. Nature 1990, 343:555-557 [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Zindy F, Chenivesse X, Lamas E, Henglein B, Brechot C: Modification of cyclin A expression by hepatitis B virus DNA integration in a hepatocellular carcinoma. Oncogene 1992, 7:1653-1656 [PubMed] [Google Scholar]
- 25.: WHO: The World Health Organization histological typing of lung tumours. Second edition. Am J Clin Pathol 1982, 77:123-136 [DOI] [PubMed] [Google Scholar]
- 26.Jiang SX, Kameya T, Sato Y, Yanase N, Yoshimura H, Kodama T: Bcl-2 protein expression in lung cancer and close correlation with neuroendocrine differentiation. Am J Pathol 1996, 148:837-846 [PMC free article] [PubMed] [Google Scholar]
- 27.Dobashi Y, Kudoh T, Matsumine A, Toyoshima K, Akiyama T: Constitutive overexpression of CDK2 inhibits neuronal differentiation of rat pheochromocytoma PC12 cells. J Biol Chem 1995, 270:23031-23037 [DOI] [PubMed] [Google Scholar]
- 28.Matsushime H, Quelle DE, Shurtleff SA, Shibuya M, Sherr CJ, Kato J: D-type cyclin dependent kinase activity in mammalian cells. Mol Cell Biol 1994, 14:2066-2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiong Y, Zhang H, Beach D: D-type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell 1992, 71:505-514 [DOI] [PubMed] [Google Scholar]
- 30.Akiyama T, Ohuchi T, Sumida S, Matsumoto K, Toyoshima K: Phosphorylation of the retinoblastoma protein by CDK2. Proc Natl Acad Sci USA 1992, 89:7900-7904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sewing A, Roenicke V, Burger C, Funk M, Muller R: Alternative splicing of human cyclin E. J Cell Science 1994, 107:581-588 [DOI] [PubMed] [Google Scholar]
- 32.Shapiro GI, Edwards CD, Kobzik L, Godleski J, Richards W, Sugarbaker DJ, Rollins BJ: Reciprocal Rb inactivation and p16INK4 expression in primary lung cancers and cell lines. Cancer Res 1995, 55:505-509 [PubMed] [Google Scholar]
- 33.Betticher DC, Heighway J, Hasleton PS, Altermat HJ, Ryder WDJ, Cerney T, Thatcher N: Prognostic significance of CCND1 (cyclin D1) overexpression in primary resected non-small-cell lung cancer. Br J Cancer 1996, 73:294-300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshida K, Morinaga S, Shimosato Y, Hayata Y: A cell kinetic study of pulmonar adenocarcinoma by an immunoperoxidase procedure after bromodeoxyuridine labeling. Cancer 1989, 64:2284-2291 [DOI] [PubMed] [Google Scholar]
- 35.Kawai T, Suzuki M, Kono S, Shinomiya N, Rokutanda M, Takagi K, Ogata T, Tamai S: Proliferating cell nuclear antigen and Ki-67 in lung carcinoma. Correlation with DNA flow cytometric analysis. Cancer 1994, 74:2468-2475 [DOI] [PubMed] [Google Scholar]
- 36.Esposito V, Baldi A, De Luca A, Micheli P, Mazzarella G, Baldi F, Caputi M, Giordano A: Prognostic value of p53 in non-small cell lung cancer: relationship with proliferating cell nuclear antigen and cigarette smoking. Hum Pathol 1997, 28:233-237 [DOI] [PubMed] [Google Scholar]
- 37.Smith E, Schlegel R, Giordano A, Lian JB, Stein JL, Stein GS: Expression of cell cycle regulatory factors in differentiating osteoblasts: postproliferative up-regulation of cyclin B and E. Cancer Res 1995, 55:5019-5024 [PubMed] [Google Scholar]
- 38.Dulic V, Drullinger LF, Lees E, Reed SI, Stein GH: Altered regulation of G1 cyclins in senescent human diploid fibroblasts: accumulation of inactive cyclin E-CDK2 and cyclin D1-CDK2 complexes. Proc Natl Acad Sci USA 1993, 90:11034-11038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shiekhatter R, Mernelstein F, Fisher RP, Drapkin R, Dynlacht B, Wessling HC, Morgan DO, Reinberg D: Cdk-activating kinase complex is a component of human transcription factor TFIIH. Nature 1995, 374:283-287 [DOI] [PubMed] [Google Scholar]
- 40.Wu L, Yee A, Liu L, Carbonaro HD, Venkatesan N, Tolo VT, Hall FL: Molecular cloning of the human CAK1 gene encoding a cyclin-dependent kinase-activating kinases. Oncogene 1994, 9:2089-2096 [PubMed] [Google Scholar]
- 41.Higashi H, Suzuki I, Saitoh S, Segawa K, Taya Y, Okuyama A, Nishimura S, Kitagawa M: Cyclin-dependent kinase-2 (Cdk2) forms an inactive complex with cyclin D1 since Cdk2 associated with cyclin D1 is not phosphorylated by Cdk7-cyclin-H. Eur J Biochem 1996, 237:460-467 [DOI] [PubMed] [Google Scholar]
- 42.Naggar AK, Steck K, Batsakis JG: Heterogeneity of the proliferative fraction and cyclin D1/CCND1 gene amplification in head and neck squamous cell carcinoma. Cytometry 1995, 21:47-51 [DOI] [PubMed] [Google Scholar]
- 43.Callender T, Naggar AK, Lee MS, Frankenthaler R, Luna MA, Batsakis JG: PRAD-1 (CCND1)/cyclin D1 oncogene amplification in primary head and neck squamous cell carcinoma. Cancer 1994, 74:152-158 [DOI] [PubMed] [Google Scholar]