Abstract
Immunohistochemistry was used to look for the expression of human herpesvirus-6 (HHV-6) antigens in a well characterized series of benign, atypical, and malignant lymphoid lesions, which tested positive for the presence of HHV-6 DNA. A panel of specific antibodies against HHV-6 antigens, characteristic either of the early (p41) or late (p101K, gp106, and gp116) phases of the viral cycle, was applied to the lymphoid tissues from 15 non-Hodgkin’s lymphomas, 14 Hodgkin’s disease cases, 5 angioimmunoblastic lymphadenopathies with dysproteinemia, 14 reactive lymphadenopathies, and 2 cases of sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease). In lymphomatous tissues, the expression of late antigens was documented only in reactive cells, and mainly in plasma cells. Of interest, the expression of the early p41 antigen was detected in the so-called “mummified” Reed-Sternberg cells, in two Hodgkin’s disease cases. In reactive lymphadenopathies, the HHV-6 late antigen-expressing cells were plasma cells, histiocytes, and rare granulocytes distributed in interfollicular areas. In both cases of Rosai-Dorfman disease, the p101K showed an intense staining in follicular dendritic cells of germinal centers, whereas the gp106 exhibited an intense cytoplasmic reaction in the abnormal histiocytes, which represent the histological hallmark of the disease. The expression of HHV-6 antigens is tightly controlled in lymphoid tissues. The lack of HHV-6 antigen expression in neoplastic cells and the limited expression in degenerating Reed-Sternberg cells argue against a major pathogenetic role of the virus in human lymphomagenesis. The detection of a rather unique pattern of viral late antigen expression in Rosai-Dorfman disease suggests a possible pathogenetic involvement of HHV-6 in some cases of this rare lymphoproliferative disorder.
Human herpesvirus-6 (HHV-6) is ubiquitous in the human adult population throughout the world, with seroconversion occurring early in life. 1 Primary infection with HHV-6 in young children may cause exanthem subitum 2 and acute febrile illness, 3 whereas in adults it may cause, although very rarely, hepatitis, 4 encephalitis, 5 and a mononucleosis-like syndrome. 6 Like all other human herpesviruses, HHV-6 is capable of remaining latent in host cells subsequent to primary infection and then reactivating in an immunocompromised state. Relevant to this, HHV-6 has been recently recognized as an opportunistic pathogen in patients with acquired immune deficiency syndrome 7 and in transplant recipients. 8
Although classified as a β-herpesvirus on the basis of genetic criteria, HHV-6 could be assigned to the γ-herpesvirus subfamily for its biological properties. Indeed, HHV-6 efficiently replicates in vitro and induces a cytopathic effect in CD4+ T lymphocytes, but it has been also shown to be tropic for other hematopoietic cell types, namely CD8+ T lymphocytes, B cells, natural killer cells, monocytes/macrophages, and megakaryocytes. 9-13 HHV-6 infection of leukocyte cultures in vitro induces various immunomodulatory effects, 14,15 as well as the suppression of T-lymphocyte functions. 16
HHV-6 may be well considered an oncogenic virus, given the ability of specific viral genomic fragments to transform animal and human cell lines in vitro, which in turn may induce tumors in animal models. 17-19 The first specific oncoprotein of HHV-6, called ORF-1, has also been recently identified. 20 Furthermore, since its first isolation from the peripheral blood of patients with lymphomas, 21 serological 22,23 and molecular 22,24-27 studies have suggested an association between HHV-6 infection and lymphoproliferative diseases.
At present, it is still largely unknown which cell types harbor HHV-6 in lymphoid tissues in vivo. The limited number of viral genomes in infected cells also hampers the application of DNA in situ hybridization techniques to identify the target cells of HHV-6 infection, either reactive or neoplastic, in pathological lymphoid tissues positive for the presence of HHV-6 DNA, as determined by polymerase chain reaction (PCR). The detection of specific HHV-6 antigens by immunohistochemistry has provided a unique and sensitive tool for the identification of infected cells both in fresh cellular populations and in archival tissue sections. Recently, this technical approach has been revealed to be successful in localizing HHV-6-infected cells in brain tissues from patients with multiple sclerosis, providing the first strong evidence toward an etiological relationship. 28
Thus, we judged it appropriate to use immunohistochemistry with a panel of antibodies for different viral antigens to look for the presence and distribution of HHV-6 antigen-expressing cells in the lymphoid tissues positive for the presence of HHV-6 DNA from a well characterized series of patients with benign, atypical, and malignant lymphoproliferative diseases.
Materials and Methods
Patients
Tissues from 15 non-Hodgkin’s lymphomas (NHL), 14 Hodgkin’s disease (HD) cases, 5 angioimmunoblastic lymphadenopathies with dysproteinemia (AILD), 14 reactive lymphadenopathies, and 2 cases of sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease) were included in the study. In all cases of NHL and HD, the diagnosis was determined on the basis of the histological analysis of a lymph node biopsy, and revised according to the REAL classification. 29 All of the NHL cases were subjected to standard immunophenotyping procedures as well as to Southern blot analysis of immunoglobulin (Ig) heavy and light chain genes and of T-cell receptor β-chain gene, to confirm either the B- or T-cell lineage. 22 The distribution of NHL cases according to lymphoma subtype was as follows: 4 follicular center, 2 mantle cell, 2 marginal zone B cell, 3 diffuse large B cell, 1 Burkitt’s, 2 peripheral T cell, and 1 anaplastic large cell. The distribution of HD cases according to subtype was as follows: 6 mixed cellularity, 5 nodular sclerosis, 2 lymphocyte predominance, and 1 lymphocyte depletion. The series of 14 reactive lymphadenopathies consisted of 5 cases of florid follicular hyperplasia, 4 cases with a predominantly paracortical lesion, 4 cases with sinus histiocytosis, and 1 histiocytic necrotizing lymphadenitis.
All of these tissue samples harbored HHV-6 DNA sequences, as detected by PCR, which was performed as previously reported. 22 One NHL, 30 two HD, 22 and the five AILD 26 cases have been previously described. In one NHL case and in two HD cases, the viral copy number was so high that it was detectable also by Southern blot analysis. 22,30 Genotype characterization, performed by a PCR assay, 26 showed the HHV-6 variant B genome in all cases, with the exception of three AILD cases, showing HHV-6 variant A genome in two cases and a mixture of HHV-6 variant A and B genomes in one case, as previously reported. 26 Furthermore, tissues from five NHL, four HD, and five reactive lymphadenopathy cases, which tested negative for HHV-6 sequences by PCR, were also included in the study to serve as negative controls in immunohistochemical experiments.
Immunohistochemistry
Sections from formalin-fixed, paraffin-embedded tissues were stained with an avidin-biotin complex immunodetection system in a TechMate instrument (BioTek Solutions, Santa Barbara, CA). Sections (4 μm) mounted on positively charged slides were deparaffinized with xylene and rehydrated, immersed for 10 minutes in 3% hydrogen peroxide/methanol to quench endogenous peroxidase, and microwaved for 10 minutes in citrate buffer. 28 After slow cooling, the sections were incubated successively with primary antibody, biotin-labeled goat anti-mouse or anti-rabbit Ig, streptavidin-biotin peroxidase complex, and 3,3′-diaminobenzidine tetrahydrochloride chromogen. Tissues were counterstained with hematoxylin. Mouse IgG1 monoclonal antibody (mAb) to HHV-6B virion protein p101K (late antigen) was obtained from P. Pellett (Centers for Disease Control and Prevention, Atlanta, GA) and from Chemicon International (Temecula, CA) and used at a dilution of 1:200. 28,31 Mouse IgG2a mAb C5 to DNA binding protein p41 (early antigen) was used at a dilution of 1:50 (Biodesign International, Kennebunkport, ME). 28,32 Mouse IgG2b mAb to gp106 (late antigen) 33 and IgG2b mAb to gp116 (late antigen) (both from Advanced Biotechnologies, Inc., Columbia, MD) were used at a dilution of 1:50. The specificity of these antibodies was confirmed by testing isotype-matched control mouse mAbs against human IgG (IgG1, X931; IgG2a, X943; and IgG2b, X944; Dakopatts, Glostrup, Denmark) in selected HHV-6-positive cases. Control and test antibodies were used at the same IgG concentrations. Antibodies to other herpesviruses included the anti-human cytomegalovirus mAbs DDG9 and CCH2 (Dakopatts) to early and immediate-early antigens, rabbit polyclonal anti-herpes simplex virus 1 antibody B114 (Dakopatts), and anti-Epstein-Barr virus (EBV) mAb to latent membrane protein-1 (LMP-1) (Dakopatts). The antibody panel also included CD3, UCHL-1 (CD45RO), L-26 (CD20), KP1 (CD68), S-100, CD21, Ber-H2 (CD30), and EMA/E29 (all from Dakopatts), and Leu-M1 (CD15; Becton-Dickinson, San Jose, CA). All antibodies were used as recommended by the manufacturers.
Results
Immunohistochemical Localization of HHV-6 Antigens in Lymphoid Tissues
NHL
The expression of HHV-6 antigens was investigated by immunohistochemistry in 15 NHL cases, which tested positive for HHV-6 DNA by PCR. One of these cases was also positive by Southern blot analysis, indicating high copy number latent integration of the HHV-6 genome, as previously reported. 30 Neoplastic cells were consistently negative for HHV-6 antigens in all NHL tissues examined, even in the B-NHL case (follicular center type) with so a high copy number that it tested positive for HHV-6 DNA by Southern blot analysis. Cytoplasmic staining was observed with the antibody p101K in rare plasma cells interspersed among neoplastic cells and in some isolated spindle-shaped stromal cells located in the lymph node capsule and in the surrounding fibroadipose tissue. No reactivity was observed with antibodies gp106, gp116, and p41.
AILD
In five cases of AILD, previously shown to be positive for HHV-6 DNA by PCR, 26 the immunostaining was observed with antibody p101K, gp106, and gp116 in scattered plasma cells. In two cases, focal collections of positively staining plasma cells were present (Figure 1A) ▶ . No reactivity was observed with antibody p41.
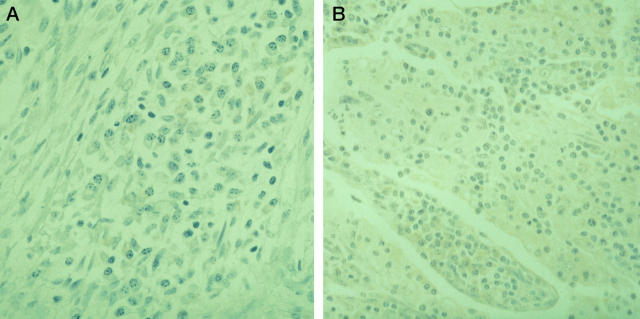
Figure 1.

A: AILD; positive staining with p101K antibody in a focal collection of plasma cells. Immunoperoxidase method; magnification, ×170. B and C: HD, nodular sclerosis type; positive staining with p41 antibody in R-S cells with shrunken cytoplasm and pyknotic nuclei (mummified cells). Immunoperoxidase method; ×330. D: HD, nodular sclerosis type; positive staining with p41 antibody in mummified R-S cells compared with negative staining in a viable R-S cell (right side). Immunoperoxidase method; ×330.
HD
The expression of HHV-6 antigens was investigated by immunohistochemistry in 14 lymph nodes affected by HD that were positive for HHV-6 DNA by PCR. Two of these cases were positive also by Southern blot analysis, as previously reported. 22 In all cases, complete effacement of the lymph node architecture was observed, and no remnants of previous normal structure could be detected. Only rare cells were found to exhibit cytoplasmic reaction for HHV-6 antigens, as evidenced by the p101K and gp116 antibodies. No staining was observed with the gp106 antibody. The morphology of the positive cells was that of reactive histiocytes and plasma cells. Isolated granulocytes also showed a positive reaction. Reed-Sternberg (R-S) and Hodgkin cells were consistently negative for HHV-6 antigens.
The p41 antibody stained rare plasma cells and isolated granulocytes in all cases. Of interest, in the two cases with a high enough copy number of HHV-6 sequences to be detectable by Southern blot analysis, this antibody also showed positive immunohistochemical staining in R-S cells with shrunken cytoplasm and pyknotic nuclei. These were considered to represent degenerating “mummified” cells 34 (Figure 1, B and C) ▶ . No staining was observed in viable R-S and Hodgkin cells with antibody p41 (Figure 1D) ▶ . In the same cases, there was clear evidence of staining of rare plasma cells and granulocytes in the reactive component, in addition to the positive mummified cells.
The HD cases were also examined with anti-EBV mAb to LMP-1. Positive reactivity was observed in seven cases and restricted only to R-S and Hodgkin cells, whereas reactive lymphoid cells were consistently negative (not shown). Of note, in the two HD cases with mummified cells positive for HHV-6 p41, immunostaining for EBV LMP-1 was negative both in viable R-S and Hodgkin cells and in mummified cells. Moreover, using immunohistochemistry for EBV LMP-1 and for four HHV-6 antigens, we could not demonstrate the co-infection of the two viruses in lymphoid cells, as the only lymphoid cells expressing HHV-6 antigens were rare plasma cells, which invariably stained negative for LMP-1.
Nonneoplastic Lymph Nodes
Fourteen nonneoplastic lymph nodes, including five cases of florid follicular hyperplasia, four cases with a predominantly paracortical lesion, four cases with sinus histiocytosis, and one histiocytic necrotizing lymphadenitis as well as two cases of Rosai-Dorfman disease, were studied for the expression of HHV-6 antigens. All of the examined cases were positive for HHV-6 DNA sequences by PCR.
In most cases, only isolated cells stained positive with the HHV-6-specific antibodies p101K, gp106 and gp116. Antibody p41 showed no reaction. The number of immunopositive cells was always less than 1% of the total lymph node cell burden, being usually lower than 0.5%. No staining was observed in the single case of histiocytic necrotizing lymphadenitis. Positive cells were found to correspond to plasma cells and histiocytes, whereas endothelial cells, follicular dendritic cells, and different types of lymphoid cells (lymphocytes, centrocytes, centroblasts, and immunoblasts) were consistently immunonegative. Isolated granulocytes, present in dilated sinuses in two cases of reactive lymphadenopathy with sinus histiocytoses, showed a positive reaction. Positive cells were not homogeneously distributed in the nodal tissue but appeared to be scattered in interfollicular regions (Figure 2A) ▶ . Hyperplastic germinal centers were usually negative, showing a bland, doubtful staining only in one case.
Figure 2.
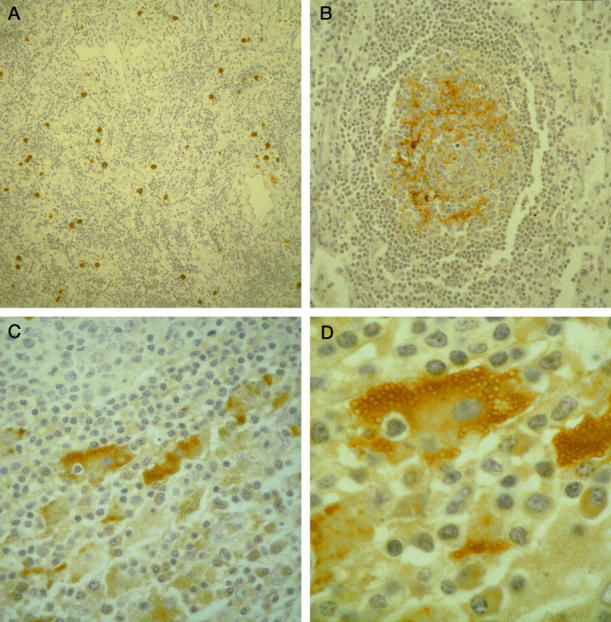
A: Reactive lymphadenopathy with a predominantly paracortical lesion; positive staining with p101K antibody in scattered interfollicular cells. Immunoperoxidase method; magnification, ×125. B: Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease); positive staining with p101K antibody in follicular dendritic cells of a reactive germinal center. Immunoperoxidase method; ×170. C: Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease); intense cytoplasmic reaction with gp106 antibody in large, abnormal histiocytes located within distended sinuses. Isolated plasma cells are also positive. Immunoperoxidase method; ×250. D: Higher magnification of an abnormal histiocyte with emperipolesis in Rosai-Dorfman disease; intense cytoplasmic reaction with gp106 antibody. Immunoperoxidase method; ×400.
An interesting reactivity with p101K and gp106 antibodies was observed in both cases of Rosai-Dorfman disease. The p101K antibody exhibited an intense staining in follicular dendritic cells of germinal centers (Figure 2B) ▶ , both hyperplastic and burned out, present in areas in which the normal nodal architecture was still retained. In the two Rosai-Dorfman disease cases, adjacent tissue sections were stained either with anti-HHV-6 p101K or with CD21 antibodies, respectively. The same distribution of positive cells in the follicles was observed with both antibodies, providing further evidence that the HHV-6-positive cells in the follicles corresponded to follicular dendritic cells. With antibody gp106, an intense granular positive reaction was observed in the cytoplasm of abnormal histiocytes located within distended sinuses (Figure 2, C and D) ▶ . Emperipolesis was present inside some positive cells (Figure 2, C and D) ▶ . The distribution of the staining was not uniform throughout the lymph nodes but tended to be localized in discrete regions. A weak positive reaction was detected in isolated plasma cells with both p101K and gp106 antibodies (Figure 2C) ▶ .
Specificity of Immunohistochemical Localization of HHV-6 Antigens
The authenticity and specificity of the immunohistochemical results was supported by the following series of findings. First, we could document a good concordance between the results obtained by PCR/Southern blot analysis and the immunohistochemical assay. In fact, all of the specimens harboring HHV-6 DNA sequences, as detected by molecular methods, were also positive at least with one anti-HHV-6 antibody. Conversely, we observed the complete unreactivity of the anti-HHV-6 antibodies (p41, p101K, gp 106, and gp 116) on the lymph node sections from control specimens that tested negative for HHV-6 DNA by PCR. Second, the specificity of the staining reaction was confirmed by testing isotype-matched control mouse mAbs against human IgG (IgG1, X931; IgG2a, X943; and IgG2b, X944; Dakopatts) in selected HHV-6-positive cases (Figure 3, A and B) ▶ . Control and test antibodies were used at the same IgG concentrations. Furthermore, selected HHV-6-positive specimens that stained positively with antibodies to HHV-6 did not show a positive immunohistochemical reaction when reacted with an mAb to human cytomegalovirus, with a polyclonal antibody to herpes simplex virus 1, and with mAb to EBV LMP-1. On the other hand, it should be noted that controlled studies have ascertained that these antibodies do not display cross-reactivity to other herpes or common human viruses. 28,31-33 Finally, the specificity and suitability of p101K and p41 antibodies for in situ detection of HHV-6 antigens in human tissues have been already successfully reported by one of us (RG). 28
Figure 3.
A: AILD; negative staining with an isotype-matched control mouse mAb against human IgG1. Immunoperoxidase method; magnification, ×300. B: Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease); negative staining with isotype-matched control mouse mAb against human IgG2b. Immunoperoxidase method; ×200.
Discussion
A pathogenetic association between HHV-6 infection and NHLs has been previously suggested on the basis of the following findings: 1) the first isolation of the virus from the peripheral blood of patients with NHL, related to acquired immune deficiency syndrome or not; 21 2) the detection of HHV-6 sequences in the lymphomatous tissues by PCR 27 and, although rarely, also by Southern blot analysis, 24,25,30 which reflected an unusually high amount of viral DNA; and 3) the very recent establishment of an HHV-6 latently infected cell line from the pathological tissue of one case of HHV-6-positive and EBV-negative Burkitt’s lymphoma. 35 Identification of the viral genome in the neoplastic cells certainly represents one of the most important and classical parameters to suggest an etiological relationship between an oncogenic DNA virus and a human tumor. Because DNA in situ hybridization studies have invariably failed to document the presence of the viral genome in the lymphomatous cells, we judged it appropriate to apply a sensitive immunohistochemical technique, looking for the possible expression of viral antigens, occurring during the early or late phase of the viral cycle, in a series of HHV-6-infected lymphoma tissues. The expression of viral antigens is restricted to reactive cells and is invariably absent in neoplastic cells in all cases examined. A hit-and-run mechanism of HHV-6, in which HHV-6 might have contributed to the initial transformation of lymphoid cells and then is released by the cells themselves once they become fully malignant, is always possible but cannot be proven. Similarly, indirect effects of herpesvirus-infected reactive cells on the neoplastic clone, within the lymphomatous lesion, is suggestive but still speculative, in the case of HHV-6 infection. The negative results of our immunohistochemical study, performed with the largest panel of antibodies to HHV-6 antigens now available, argues against a major “orthodox” role for HHV-6 infection in the occurrence of human NHLs.
We were the first to document an unusually high frequency of HHV-6 sequences in the pathological tissues of AILD, by PCR. 26 Using immunohistochemistry in the same series of cases, we showed that the expression of viral antigens is absent in T lymphocytes, which are considered the proliferating elements in such a disease, 36 arguing against a direct role for HHV-6 in the development of this atypical lymphoproliferation. The frequent presence of HHV-6 DNA may be also related to a reactivation or to a primary infection of this herpesvirus, favored by the immunesuppression of AILD patients, who, indeed, often die from severe opportunistic infections. 36 However, the fact that the expression of HHV-6 antigens is limited to a small proportion of cells and restricted to plasma cells, suggests that an active disseminated infection with HHV-6 is uncommon, even under conditions of severe immune impairment, which are typical of AILD.
A pathogenetic association between HHV-6 infection and the development of HD is apparently more solid and based on the following epidemiological evidence, obtained by our group and others: 1) higher frequency and higher titers of anti-HHV-6 antibodies in HD patients than in NHL patients or in blood donors, 22 2) correlation between anti-HHV-6 antibody titers and the clinical course and prognosis of HD, 23 and 3) higher frequency of HHV-6 sequences by PCR and Southern blot analysis in HD than in NHL cases. 22,27 Again, so far, in situ DNA hybridization studies have failed to identify HHV-6 in the putative neoplastic cells of HD, namely in Hodgkin and R-S cells and have simply documented the presence of viral genomes in normal, reactive lymphocytes. 37 We provide here the first evidence that, although rarely, HHV-6 may infect R-S cells. HHV-6 infection is latent, as only p41, an early antigen, is expressed. Infected R-S cells represent the so-called mummified cells, ie, R-S cells that have undergone apoptosis. 34 It is not possible to assess whether viral infection of R-S cells has been favored by the apoptotic process of the cells or the virus has triggered the programmed cell death of the cells themselves. Relevant to this, HHV-6 has been shown to induce apoptosis in infected T cells in vitro. 38 If an apoptotic effect of HHV-6 on R-S cells is confirmed, the virus might have a protective rather than a pathogenetic role in such a disease. Of interest, HHV-6-expressing R-S cells could not be identified in HD cases with a low HHV-6 copy number (ie, PCR positive), but only in the two cases of HD harboring an extraordinarily high amount of viral DNA, sufficient to test positive also by Southern blot analysis. We are well aware of the fact that mummified cells may be prone to nonspecific staining. However, it should be noted that mummified cells stained positive only with p41, but stained negative with each of three anti-HHV-6 antibodies for late antigens (p101K, gp106, and gp116), and with antibodies to three herpes viruses (cytomegalovirus, herpes simplex virus, and EBV). Moreover, in two HHV-6 DNA-negative HD cases examined as controls, mummified cells were present but did not react with p41. These data definitely argue against a nonspecific staining of mummified cells at least for a early HHV-6 antigen, recognized by p41 antibody, in the two HD cases with high viral load.
In conclusion, as R-S cells in the vast majority of HD cases resulted negative for the expression of viral antigens, a causative role for HHV-6 infection in the development of HD cannot be assessed. However, as we show, for the first time, that HHV-6 may have a tropism for R-S cells in vivo, the possibility exists that other as-yet-uncharacterized proteins may be expressed in HD tissues. Relevant to this, we recently identified the first HHV-6 protein, called ORF-1, which has a transactivating and transforming activity mediated through the binding to p53. 20 As we already detected the sequences encoding for ORF-1 in HD tissues in vivo, 20 we are currently looking for the expression of this oncoprotein in R-S and Hodgkin cells. Furthermore, similarly to what occurs in AILD, HHV-6 infection of HD tissues is mainly a latent infection, and the expression of viral antigens indicative of a replicative cycle was limited to a small number of cells and restricted to few cell types, including plasma cells. Similarly, in cases of EBV-associated HD, the expression of lytic genes of EBV is a very rare phenomenon in R-S cells. 39 Moreover, the expression of HHV-6 antigens in plasma cells, which seems a constant finding in HHV-6-associated lymphoproliferative diseases, again reminds us of the behavior of EBV. For example, in the EBV-induced lymphomas arising in the severe combined immunodeficiency mouse model, EBV-infected tumor cells show a plasma cell phenotype and a reduced expression of viral latent genes. 40
Although HHV-6 primary infection is a well-recognized cause of mononucleosis-like illnesses, 6 there is not a histological pattern of the lymph node that has been considered characteristic of HHV-6 infection, as paracortical expansion is generally typical of EBV-induced infectious mononucleosis, 41 or giant germinal center hyperplasia with increased vascularity seems to be often related to human herpesvirus-8 infection of the lymph nodes. 42 That is why we judged it appropriate to investigate a highly heterogeneous series of reactive lympadenopathies, with different histological features, selected simply on the basis of their positivity for HHV-6 DNA by PCR. EBV infection may have different patterns of infection in nonneoplastic lymph nodes. Indeed, in typical EBV-induced infectious mononucleosis, large numbers of EBV-positive B lymphoid blasts are detectable in extrafollicular areas, whereas germinal centers are free of EBV-positive cells. 41 In other reactive lymph nodes harboring EBV sequences, EBV-positive cells are small lymphocytes generally present in extrafollicular areas but, sometimes, also in germinal centers. 41 In our extensive immunohistochemical study, we did not observe different patterns of HHV-6 antigen expression in the various types of lymphadenopathies examined. The localization of HHV-6-expressing cells in interfollicular areas and their absence in germinal centers were a constant feature, whereas the infected cell types were the same, ie, plasma cells, histiocytes, and granulocytes in all cases examined. It should also be noted that lymphocytes, which tested invariably negative for HHV-6 antigens, seem to be not permissive for viral replication, in contrast to what occurs in vitro. 12 The possibility still exists that as-yet-uncharacterized viral proteins may be expressed in infected lymphocytes, in vivo.
A very peculiar pattern of expression of HHV-6 antigens was documented in both cases of Rosai-Dorfman disease. The expression of p101K antigen in follicular dendritic cells seems to be a rather specific phenomenon, as it tested absent in all of the other lymphadenopathy cases examined. It has been previously reported that EBV is able to infect and transform follicular dendritic cells in vitro. 43 Infection of follicular dendritic cells with herpesviruses may be an underestimated phenomenon that merits further investigation. Clearly, the most interesting finding is represented by the demonstration of a HHV-6 protein in the late phase of the viral cycle in a significant proportion of the abnormal histiocytes, which represent the hallmark of the Rosai-Dorfman disease. Our study extends the results by Levine and colleagues, 44 who documented the presence of HHV-6 DNA in such cells by in situ DNA hybridization. The novelty and the interest of our finding is that we provide the first evidence that HHV-6 not only may infect the abnormal histiocytes of Rosai-Dorfman disease but, more importantly, it is functionally active in such cells. Although the diagnostic cells of Rosai-Dorfman disease have many histiocyte-associated morphological features, they also have an uncommon phenotype, as they express monocyte/macrophage-associated markers and the dendritic cell-associated marker S-100. 45 Of interest, the expression of HHV-6 genes in lymphoid cells/tissues, either normal or pathological, as detected by Northern blot analysis, is a very rare phenomenon, documented so far only in the neoplastic cells of two cases of the rare S-100-positive T-cell chronic lymphoproliferative disease, 46 suggesting that S-100-positive cells are in some way more permissive for HHV-6 replication. Rosai-Dorfman disease has been proposed to represent an exaggerated immunological response to an infectious agent, and a pathogenetic association with EBV has been previously reported. 44,45 Thus, we suggest that HHV-6 should also be investigated as another possible trigger of the uncontrolled proliferation of abnormal histiocytes in such disease.
Acknowledgments
The authors thank Dr. Christine Morris from the Cytogenetic and Molecular Oncology Unit, Department of Pathology, Christchurch School of Medicine (Christchurch, New Zealand), for helpful discussion and enlightened suggestions.
Footnotes
Address reprint requests to Dr. Giuseppe Torelli, Department of Medical Sciences, Section of Hematology, Policlinico, Via del Pozzo 71, 41100 Modena, Italy. E-mail: mluppi@unimo.it.
Supported by the Associazione Italiana per la Ricerca sul Cancro (Milan, Italy). ML is a recipient of a fellowship for AIDS research from the Istituto Superiore di Sanità (Rome, Italy).
References
- 1.Okuno T, Takahashi K, Balachandran K, Shiraki K, Yamanishi K, Takahashi M, Baba K: Seroepidemiology of human herpesvirus 6 infection in normal children and adults. J Clin Microbiol 1989, 27:651-653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamanishi K, Okuno T, Shiraki T, Takahashi M, Kondo T, Asano Y, Kurata T: Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet 1988, i:1065-1067 [DOI] [PubMed] [Google Scholar]
- 3.Pruksananonda P, Breese-Hall C, Insel RA, McIntyre K, Pellett PE, Long CE, Schnabel KC, Pincus PH, Stamey FR, Dambaugh TR, Stewart JA: Primary human herpesvirus 6 infection in young children. N Engl J Med 1992, 326:1445-1450 [DOI] [PubMed] [Google Scholar]
- 4.Irving WL, Cunningham AL: Serological diagnosis of infection with human herpesvirus 6. Br Med J 1990, 300:156-159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merelli E, Sola P, Barozzi P, Torelli G: An encephalitic episode in a multiple sclerosis patient with human herpesvirus 6 latent infection. J Neurol Sci 1996, 137:42-46 [DOI] [PubMed] [Google Scholar]
- 6.Steeper TA, Horwitz CA, Ablashi DV, Salahuddin SZ, Saxinger C, Saltzman R, Schwartz B: The spectrum of clinical and laboratory findings resulting from human herpesvirus 6 (HHV-6) in patients with mononucleosis-like illness not resulting from Epstein-Barr virus or cytomegalovirus. Am J Clin Pathol 1990, 93:776-783 [DOI] [PubMed] [Google Scholar]
- 7.Lusso P, Gallo RC: Human herpesvirus 6 in AIDS. Immunol Today 1995, 16:67-71 [DOI] [PubMed] [Google Scholar]
- 8.Singh N, Carrigan DR: Human herpesvirus-6 in transplantation: an emerging pathogen. Ann Intern Med 1996, 124:1065-1071 [DOI] [PubMed] [Google Scholar]
- 9.Ablashi DV, Lusso P, Lung CL, Salahuddin SZ, Josephs SF, Llana T, Kramarsky B, Biberfeld P, Markham PD, Gallo RC: Utilization of human hematopoietic cell lines for the propagation and characterization of HBLV (human herpesvirus 6). Int J Cancer 1988, 42:787-791 [DOI] [PubMed] [Google Scholar]
- 10.Kondo K, Kondo T, Okuno T, Takahashi M, Yamanishi K: Latent human herpesvirus 6 infection of human monocytes: macrophages. J Gen Virol 1991, 72:1401-1408 [DOI] [PubMed] [Google Scholar]
- 11.Levy JA, Ferro F, Lennette ET, Oshiro L, Poulin L: Characterization of a new strain of HHV-6 (HHV-6 SF) recovered from the saliva of an HIV infected individual. Virology 1990, 178:113-121 [DOI] [PubMed] [Google Scholar]
- 12.Lusso P, Markham PD, Tschachler ET, di Marzo Veronese F, Salahuddin SZ, Ablashi DV, Pahwa S, Korhn K, Gallo RC: In vitro cellular tropism of human B-lymphotropic virus (human herpesvirus 6). J Exp Med 1988, 167:1659-1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lusso P, Malnati MS, Garzino-Demo A, Crowley RW, Long EO, Gallo RC: Infection of natural killer cells by human herpesvirus 6. Nature 1993, 362:458-462 [DOI] [PubMed] [Google Scholar]
- 14.Flamand L, Gosselin J, D’Addario M, Hiscott J, Ablashi DV, Gallo RC, Menezes J: Human herpesvirus 6 induces interleukin-1β and tumor necrosis factor α, but not interleukin-6, in peripheral blood mononuclear cell cultures. J Virol 1991, 65:5105-5110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kikuta H, Nakane A, Lu H, Tagushi Y, Minagawa T, Matsumoto S: Interferon induction by human herpesvirus 6 in human mononuclear cells. J Infect Dis 1990, 162:35-38 [DOI] [PubMed] [Google Scholar]
- 16.Flamand L, Gosselin J, Stefanescu I, Ablashi D, Menezes J: Immunosuppressive effect of human herpesvirus 6 on T-cell functions: suppression of interleukin-2 synthesis and cell proliferation. Blood 1995, 85:1263-1271 [PubMed] [Google Scholar]
- 17.Razzaque A: Oncogenic potential of human herpesvirus-6 DNA. Oncogene 1990, 5:1365-1370 [PubMed] [Google Scholar]
- 18.Razzaque A, Williams O, Wang J, Rhim JS: Neoplastic transformation of immortalized human epidermal keratinocytes by two HHV-6 DNA clones. Virology 1993, 195:113-120 [DOI] [PubMed] [Google Scholar]
- 19.Thompson J, Choudhury S, Kashanchi F, Doniger J, Bernemann Z, Frenkel N, Rosenthal LJ: A transforming fragment within the direct repeat region of human herpesvirus type 6 that transactivates HIV-1. Oncogene 1994, 9:1167-1175 [PubMed] [Google Scholar]
- 20.Kashanchi F, Araujo J, Doniger J, Muralidhar S, Hoch R, Khleif S, Mendelson E, Thompson J, Azumi N, Brady J, Luppi M, Torelli G, Rosenthal LJ: Human herpesvirus (HHV-6) ORF-1 transactivating gene exhibits malignant transforming activity and its protein binds to p53. Oncogene 1997, 14:359-367 [DOI] [PubMed] [Google Scholar]
- 21.Salahuddin SZ, Ablashi DV, Markham PD, Josephs SF, Sturzenegger S, Kaplan M, Halligan M, Biberfeld P, Wong-Staal F, Kramarsky B, Gallo RC: Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science 1986, 234:596-600 [DOI] [PubMed] [Google Scholar]
- 22.Torelli G, Marasca R, Luppi M, Selleri L, Ferrari S, Narni F, Mariano MT, Federico M, Ceccherini-Nelli L, Bendinelli M, Montagnani G, Montorsi M, Artusi A: Human herpesvirus-6 in human lymphomas: identification of specific sequences in Hodgkin’s lymphomas by polymerase chain reaction. Blood 1991, 77:2251-2258 [PubMed] [Google Scholar]
- 23.Levine PH, Ebbesen P, Ablashi DV, Saxinger WC, Nordentoft A, Connelly RR: Antibodies to human herpesvirus-6 and clinical course in patients with Hodgkin’s disease. Int J Cancer 1992, 51:53-57 [DOI] [PubMed] [Google Scholar]
- 24.Jarrett RF, Gledhill S, Qureshi F, Crae SH, Madhock R, Brown I, Evans I, Krajewski A, O’Brien CJ, Cartwright RA, Venables P, Onions DE: Identification of human herpesvirus-6 specific DNA sequences in two patients with non-Hodgkin’s lymphoma. Leukemia 1988, 2:496-502 [PubMed] [Google Scholar]
- 25.Josephs SF, Buchbinder A, Streicher HZ, Ablashi DV, Salahuddin SZ, Guo H-G, Wong-Staal F, Cossman J, Raffeld M, Sundeen J, Levine P, Biggar R, Krueger GRF, Fox RI, Gallo RC: Detection of human B lymphotropic virus (human herpesvirus 6) sequences in B cell lymphoma tissues of three patients. Leukemia 1988, 2:132-135 [PubMed] [Google Scholar]
- 26.Luppi M, Marasca R, Barozzi P, Artusi T, Torelli G: Frequent detection of human herpesvirus-6 sequences by polymerase chain reaction in paraffin-embedded lymph nodes from patients with angioimmunoblastic lymphadenopathy and angioimmunoblastic lymphadenopathy-like lymphoma. Leuk Res 1993, 17:1003-1011 [DOI] [PubMed] [Google Scholar]
- 27.Di Luca D, Dolcetti R, Mirandola P, de Re V, Secchiero P, Carbone A, Boiocchi M, Cassai E: Human herpesvirus 6: a survey of presence and variant distribution in normal peripheral lymphocytes and lymphoproliferative disorders. J Infect Dis 1994, 99:533-535 [DOI] [PubMed] [Google Scholar]
- 28.Challoner PB, Smith KT, Parker JD, MacLeod DL, Coulter SN, Rose TM, Schultz E, Bennett JL, Garber RL, Chang M, Schad PA, Stewart PM, Nowinski, Brown JP, Burmer GC: Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc Natl Acad Sci USA 1995, 92:7440-7444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JKC, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC, Grogan TM, Isaacson PG, Knowles DM, Mason DY, Muller-Hermelink H-K, Pileri S, Piris MA, Ralfkiaer E, Warnke RA: A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 1994, 84:1361-1392 [PubMed] [Google Scholar]
- 30.Torelli G, Barozzi P, Marasca R, Cocconcelli P, Merelli E, Ceccherini-Nelli L, Ferrari S, Luppi M: Targeted integration of human herpesvirus 6 in the p arm of chromosome 17 of human peripheral blood mononuclear cells in vivo. J Med Virol 1995, 46:178-188 [DOI] [PubMed] [Google Scholar]
- 31.Pellett PE, Sánchez-Martínez D, Dominguez G, Black JB, Anton E, Greenamoyer C, Dambaugh TR: A strongly immunoreactive virion protein of human herpesvirus 6 variant B strain Z29: identification and characterization of the gene and mapping of a variant-specific monoclonal antibody reactive epitope. Virology 1993, 196:521-531 [DOI] [PubMed] [Google Scholar]
- 32.Agulnick AD, Thompson JR, Iyengar S, Pearson G, Ablashi DV, Ricciardi RP: Identification of a DNA-binding protein of human herpesvirus 6, a putative DNA polymerase stimulatory factor. J Gen Virol 1993, 74:1003-1009 [DOI] [PubMed] [Google Scholar]
- 33.Okuno T, Shao H, Asada H, Shiraki K, Takahashi M, Yamanishi K: Analysis of human herpesvirus 6 glycoproteins recognized by monoclonal antibody OHV1. J Gen Virol 1992, 73:443-447 [DOI] [PubMed] [Google Scholar]
- 34.Henry K: Neoplastic disorders of lymphoreticular tissue: thymus, lymph nodes, spleen and lymphatics. Symmers WSC eds. Systemic Pathology. 1992, vol 7.:pp 868-879 Churchill Livingstone, London [Google Scholar]
- 35.Bandobashi K, Daibata M, Kamioka M, Tanaka Y, Kubonishi I, Taguchi H, Ohtsuki Y, Miyoshi I: Human herpesvirus 6 (HHV-6)-positive Burkitt’s lymphoma: establishment of a novel cell line infected with HHV-6. Blood 1997, 90:1200-1207 [PubMed] [Google Scholar]
- 36.Freter CE, Cossman J: Angioimmunoblastic lymphadenopathy with dysproteinemia. Semin Oncol 1993, 20:627-635 [PubMed] [Google Scholar]
- 37.Valente G, Secchiero P, Lusso P, Abele MC, Jemma C, Reato G, Kerim S, Gallo RC, Palestro G: Human herpesvirus 6 and Epstein-Barr virus in Hodgkin’s disease: a controlled study by polymerase chain reaction and in situ hybridization. Am J Pathol 1996, 149:1501-1510 [PMC free article] [PubMed] [Google Scholar]
- 38.Inoue Y, Yasukawa M, Fujita S: Induction of T-cell apoptosis by human herpesvirus 6. J Virol 1997, 71:3751-3759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pallesen G, Sandvej K, Hamilton-Dutoit SJ, Rowe M, Young LS: Activation of Epstein-Barr virus replication in Hodgkin and Reed-Sternberg cells. Blood 1991, 78:1162-1165 [PubMed] [Google Scholar]
- 40.Rochford R, Hobbs MV, Garnier J-L, Cooper NR, Cannon MJ: Plasmacytoid differentiation of Epstein-Barr virus-transformed B cells in vivo is associated with reduced expression of viral latent genes. Proc Natl Acad Sci USA 1993, 90:352-356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niedobitek G, Herbst H, Young LS, Brooks L, Masucci MG, Crocker J, Rickinson AB, Stein H: Patterns of Epstein-Barr virus infection in non-neoplastic lymphoid tissue. Blood 1992, 79:2520-2526 [PubMed] [Google Scholar]
- 42.Luppi M, Barozzi P, Maiorana A, Artusi T, Trovato R, Marasca R, Savarino M, Ceccherini-Nelli L, Torelli G: Human herpesvirus-8 DNA sequences in human immunodeficiency virus-negative angioimmunoblastic lymphadenopathy and benign lymphadenopathy with giant germinal center hyperplasia and increased vascularity. Blood 1996, 87:3903-3909 [PubMed] [Google Scholar]
- 43.Lindhout E, Lakeman A, Mevissen MLCM, de Groot C: Functionally active Epstein-Barr virus-transformed follicular dendritic cell-like cell lines. J Exp Med 1994, 179:1173-1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levine PH, Jahan N, Murari P, Manak M, Jaffe ES: Detection of human herpesvirus 6 in tissue involved by sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease). J Infect Dis 1992, 166:291-295 [DOI] [PubMed] [Google Scholar]
- 45.Woda BA, Sullivan JL: Reactive histiocytic disorders. Am J Clin Pathol 1993, 99:459-463 [DOI] [PubMed] [Google Scholar]
- 46.Braun DK, Pellett PE, Hanson CA: Presence and expression of human herpesvirus 6 in peripheral blood mononuclear cells of S100-positive, T-cell chronic lymphoproliferative disease. J Infect Dis 1995, 171:1351-1355 [DOI] [PubMed] [Google Scholar]