Abstract
The scientific dogma that multiple sclerosis (MS) is a disease caused by a single pathogenic mechanism has been challenged recently by the heterogeneity observed in MS lesions and the realization that not all patterns of demyelination can be modeled by autoimmune-triggered mechanisms. To evaluate the contribution of local tumor necrosis factor (TNF) ligand/receptor signaling pathways to MS immunopathogenesis we have analyzed disease pathology in central nervous system-expressing TNF transgenic mice, with or without p55 or p75TNF receptors, using combined in situ terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling and cell identification techniques. We demonstrate that local production of TNF by central nervous system glia potently and selectively induces oligodendrocyte apoptosis and myelin vacuolation in the context of an intact blood-brain barrier and absence of immune cell infiltration into the central nervous system parenchyma. Interestingly, primary demyelination then develops in a classical manner in the presence of large numbers of recruited phagocytic macrophages, possibly the result of concomitant pro-inflammatory effects of TNF in the central nervous system, and lesions progress into acute or chronic MS-type plaques with axonal damage, focal blood-brain barrier disruption, and considerable oligodendrocyte loss. Both the cytotoxic and inflammatory effects of TNF were abrogated in mice genetically deficient for the p55TNF receptor demonstrating a dominant role for p55TNF receptor-signaling pathways in TNF-mediated pathology. These results demonstrate that aberrant local TNF/p55TNF receptor signaling in the central nervous system can have a potentially major role in the aetiopathogenesis of MS demyelination, particularly in MS subtypes in which oligodendrocyte death is a primary pathological feature, and provide new models for studying the basic mechanisms underlying oligodendrocyte and myelin loss.
Primary demyelination is a hallmark of multiple sclerosis (MS) 1 and a prominent pathological feature of several other inflammatory diseases of the central nervous system (CNS) that is considered to contribute significantly to functional neurological deficits. It is a complex immune-mediated process that involves the active destruction and phagocytosis of myelin by activated microglia/macrophages 2 and can be accompanied by death of the myelin-forming oligodendrocytes by apoptotic 3-5 or necrotic 3,6 processes. The study of animal models such as experimental autoimmune encephalomyelitis (EAE) 7-9 and Theiler’s virus-induced encephalomyelitis 10,11 has shown that myelin damage can be triggered by myelin- and non-myelin-specific T cells through release of cytokines and recruitment of macrophages or through the binding of antibodies such as those specific for myelin oligodendrocyte glycoprotein (MOG) to the myelin sheath and targeting of complement and antibody-dependent cell cytotoxicity (ADCC) effector mechanisms. However, these mechanisms alone are not sufficient to account for the heterogeneous patterns of myelin destruction observed in MS, particularly in a high percentage of patients where oligodendrocyte death is a primary pathological feature. 3
Considerable evidence has implicated members of the tumor necrosis factor (TNF) ligand/receptor superfamily, particularly TNF and Fas/Fas ligand (FasL), in the pathogenesis of MS. 12,4,6 TNF and its receptors (TNFRs) are up-regulated in active MS lesions 2,13-15 and levels of TNF in the cerebrospinal fluid of MS patients correlate with disease severity. 16 The described effects of TNF on cultured CNS cells such as astrocyte proliferation, 17 microglial proliferation and reactivity, 18 and endothelial cell activation 19 are consistent with an inflammatory role in the CNS and the induction of immune reactivity. 20,21 Most relevant to a role in demyelination is increasing evidence that the TNF ligand/receptor system is involved in triggering oligodendrocyte death. Both the p55TNFR and the p75TNFR are selectively expressed on oligodendrocytes located at the edge of active MS lesions 15 and several studies have shown that TNF can kill cultured oligodendrocytes. 6,22-24 However, although a large body of information supports a potentially major role for TNF during MS immunopathogenesis and TNF blockade can prevent the development of EAE, 25-28 recent studies showing that both CNS inflammation and demyelination develop when EAE is induced in TNF- or TNF/lymphotoxin α-deficient mice have called into question the pathogenic potential of TNF along autoimmune-triggered pathways of inflammation and demyelination. 29-33
To determine whether local TNF/TNFR signaling can play a role in MS aetiopathogenesis, particularly in those subtypes of MS in which demyelinating events precede inflammation 3,34 and are suggestive of a non-autoantigen-induced mechanism, we have analyzed disease pathology in CNS-expressing TNF transgenic mice 35,36 and their backcrosses into p55TNFR-deficient 37 or p75TNFR-deficient 38 backgrounds. We show that local production of TNF by CNS glial cells can selectively induce through the p55TNFR oligodendrocyte apoptosis, primary inflammatory demyelination, and the generation of MS-type plaques which have oligodendrogliopathy as a primary pathological feature.
Materials and Methods
Transgenic and Knockout Mice
TNF transgenic lines Tg6074 and TgK21 express murine TNF-globin and glial fibrillary acid protein (GFAP)-human transmembrane TNF-globin transgenes, respectively, specifically in their CNS. 35,36 Mice deficient in the p55TNFR (p55−/−), p75TNFR (p75−/−) or TNF (TNF−/−) were generated by homologous recombination in embryonic stem cells and have been described elsewhere. 37-39 Animal maintainance and appropriate crosses between these strains were performed under specific pathogen-free conditions in the animal facility of the Hellenic Pasteur Institute.
Histopathological Analyses
Mice were transcardially perfused with ice-cold 4% paraformaldehyde (PFA) in PBS under deep ether anesthesia. The partially dissected brain and whole vertebral column were postfixed by immersion in the same fixative for 3 hours at 4°C and transferred into PBS. Brains and spinal cords were dissected and embedded in paraffin. Sections 3 to 5 μm thick were stained with hematoxylin/eosin, Luxol fast blue/periodic acid-Schiff (LFB/PAS), or Bielschowsky silver stain according to standard procedures.
Immunocytochemistry
Immunohistochemical staining was performed on paraffin sections as described elsewhere. 40 Primary antibodies were as follows: rabbit anti-proteolipid protein (PLP) (Serotec, Oxford, UK) (1/1000), mouse anti-2′ 3′-cyclic nucleotide-3′-phosphodiesterase (CNPase) (Affinity, Nottingham, UK) (1/900), sheep anti-mouse Ig (Amersham, Little Chalfont, UK) (1/200), rabbit anti-cow GFAP (Dakopatts, Glostrup, Denmark) (1/200), rabbit anti-murine TNF (Genzyme, Cambridge, MA) (1/500), rat anti-mouse F4/80 (Serotec) (1/10), rat anti-mouse Mac-1 (Boehringer-Mannheim, Mannheim, Germany) (1/100), rat anti-human CD3 (Serotec) (1/400), rabbit anti-mouse S-100 (Serva, Heidelberg, Germany) (1/100), and anti-microtubule associated protein-2 (MAP-2) (kindly provided by G. Wiche, Institute of Biochemistry and Molecular Cell Biology, Vienna) (1/400). Lectin staining was performed using GSI-B4 (Sigma, Deisenhofen, Germany). For double immunostaining peroxidase substrates 3′,3′-diaminobenzidine (DAB) (Sigma) and 3-amino-9-ethylcarbazole (AEC) (Sigma) were combined with alkaline phosphatase substrate nitroblue tetrazolium (NBT)/brom-chlor-indolyl phosphate (BCIP) (Boehringer Mannheim).
Laser Scanning Confocal Microscopy
For double staining of TNF and CNPase, essentially the same immunocytochemistry procedure was used as for normal light microscopy. Briefly, sections were incubated with both primary antibodies overnight, then incubated with secondary antibodies (rhodamine-conjugated goat anti-mouse (Jackson ImmunoResearch Laboratories, West Grove, PA) and biotinylated sheep anti-rabbit (Amersham)) for 2 hours at room temperature. In a third step, sections were incubated with avidin-conjugated Cy2 (Amersham) for 1 hour at room temperature. After rinsing with PBS, sections were embedded in PBS/glycerol (1:9) with 3% DABCO (Sigma) and placed in a coverslip. Fluorescent preparations were examined using a Carl Zeiss laser scan microscope equipped with an argon laser (488 and 514 nm excitation) and two HeNe lasers (543 and 633 nm excitation) (Carl Zeiss, Jena, Germany). The rhodamine fluorescence (CNPase) was excited with the 543-nm laser. The emitted light was detected on photomultiplier 2 with a 557–640 bandpass filter. Cy2 (TNF) was excited with the 488-nm laser and was detected on photomultiplier 3 using a 515–565 bandpass filter. Scanning with the 543- and 488-nm lasers was performed sequentially. After this, sections made with 543- and 488-nm lasers lying in the same Z plane were merged to produce a single picture. Overlaid pictures were printed with a Sony digital color printer (Cy2 signal green, rhodamine red, co-localization yellow).
In Situ Detection of Nuclear DNA Fragmentation
Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling (TUNEL) was performed on paraffin sections as described previously. 41 Sections were double-labeled with antibodies to CNPase or GFAP using AEC or DAB for color development. Sections were counterstained with hematoxylin.
In Situ Hybridization of PLP mRNA
Detection of PLP mRNA was performed on brain and spinal cord paraffin sections. Nonisotopic in situ hybridization was performed as described. 42 Briefly, 5-μm paraffin sections were dewaxed, pretreated with 10 μg of proteinase K (Sigma) in Tris-buffered solution , pH 7.2, and incubated with digoxigenin-labeled probes specific for PLP. Sections were incubated for 1 hour with alkaline phosphatase-labeled anti-digoxigenin antibody (Boehringer-Mannheim). Substrate was visualized using NBT/BCIP (Boehringer-Mannheim). All sections were double-stained with anti-PLP antibodies as described above with triple APAAP using Fast Red as substrate. Sections were counterstained with hematoxylin.
Electron Microscopic Analyses
Animals were intracardially perfused under deep ether anesthesia with ice-cold 2% PFA, 0.5% glutaraldehyde in 0.1 mol/L phosphate buffer, pH 7.2, for 1 minute followed by ice-cold 3% glutaraldehyde in 0.1 mol/L phosphate buffer for 5 minutes. Brains and spinal columns were removed, immersion-fixed for 24 hours in phosphate-buffered 3% glutaraldehyde, postfixed in 2% osmium tetroxide solution, and subsequently embedded in epoxy resin. Ultrathin sections were cut and counterstained with uranyl acetate and lead citrate.
Results
Cellular Source of Murine TNF and Human Transmembrane TNF Transgene Expression in Tg6074 and TgK21 Mice
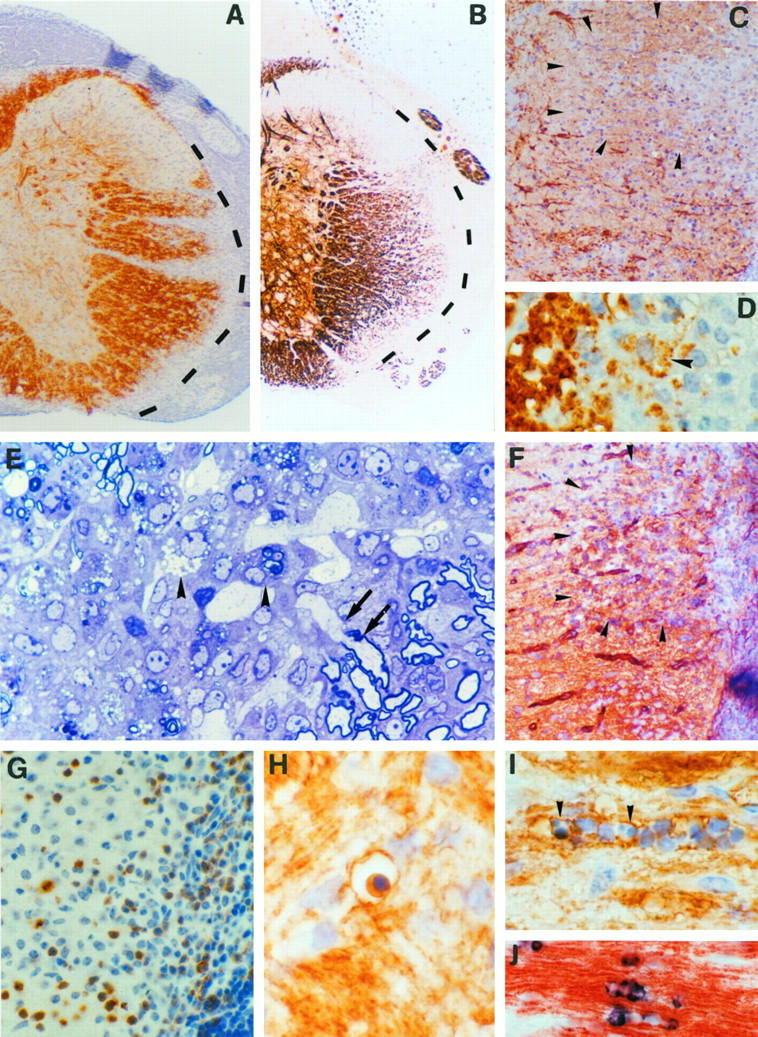
Transgenic mice which show CNS-specific expression of a murine TNF transgene (the Tg6074 line) or astrocyte-targeted expression of an uncleavable transmembrane human TNF mutant (the TgK21 line) have been reported previously. 35,36 TgK21 mice express human transmembrane TNF under the control of the GFAP promoter region, and immunocytochemistry on serial spinal cord sections with antibodies to human TNF and GFAP showed that the TNF transgene is expressed by GFAP-positive astrocytes. 36 To identify the precise cellular source of murine TNF transgene expression in the CNS of Tg6074 mice, we have used immunocytochemical techniques to localize murine TNF immunoreactivity in the CNS. Tissues were taken from Tg6074TNF−/− double transgenic mice to exclude simultaneous expression from the endogenous TNF gene. Transgene-positive cells had a highly ramified appearance and were numerous throughout the brain in both white and gray matter. Double immunostaining for murine TNF and markers for mature CNS cell types showed that the TNF transgene is not expressed in either GFAP-positive astrocytes or F4/80-, Mac-1-, or GSI-B4 lectin-positive microglia/macrophages (not shown). Single immunostaining of serial 0.5- to 1-μm paraffin sections for TNF and S100 (marker for astrocytes and immature glia43) (Figure 1, A and B) ▶ , MAP-2 (neurons) (Figure 1, C and D) ▶ , or CNPase (oligodendrocytes) (Figure 1, E–G) ▶ , demonstrated that TNF immunoreactive cells show low immunoreactivity for all of these markers when compared to mature cell lineages, indicating that they are probably CNS glial progenitor cells. Examination of double-stained sections for TNF and CNPase by confocal microscopy confirmed that the TNF transgene is expressed by CNPase-positive cells (Figure 1H) ▶ .
Figure 1.

An S100/CNPaselow/MAP-2low CNS glial precursor cell is the cellular source of murine TNF transgene expression in Tg6074 mice. Immunostaining of serial brain sections for TNF (A, arrowheads) and S100 (B, arrowheads) revealed that the Tg6074 transgene is expressed by a subpopulation of S100-positive cells. Immunostaining of serial brain sections for TNF (C) and MAP-2 (D), revealed that strongly MAP-2 positive neurons (D, arrows) are negative for TNF (C, arrows), while the TNF-positive cell (C, arrowhead) expressed lower levels of MAP-2 (D, arrowhead) when compared to the surrounding neurons (D, arrows). Immunostaining of serial brain sections for TNF (E) and CNPase (F) revealed that the TNF-positive cell also stained weakly for CNPase. (G) CNPase immunostaining of Tg6074 hippocampus demonstrated the weak reactivity of these highly branched precursor cells (arrowhead) when compared to the strong staining of adult oligodendrocytes (arrows). (H) Double immunofluorescence for CNPase (red) and TNF (green) examined by confocal microscopy demonstrated the co-localization (yellow) of the murine TNF transgene in CNPase positive cells. Magnifications: A, B ×792; C-F ×990; G ×335; H ×1608.
CNS Expression of Murine TNF in Tg6074 Transgenic Mice Triggers Oligodendrocyte Apoptosis, Primary Demyelination, and Formation of Chronic MS-Type Plaques
To determine the effect of expression of a murine TNF transgene by resident cells of the CNS on oligodendrocytes and myelin in vivo, we carried out a detailed histopathological and ultrastructural analysis of disease in Tg6074 transgenic mice (Figures 2 and 3 ▶ ▶ , Tables 1 and 2 ▶ ▶ ).
Figure 2.
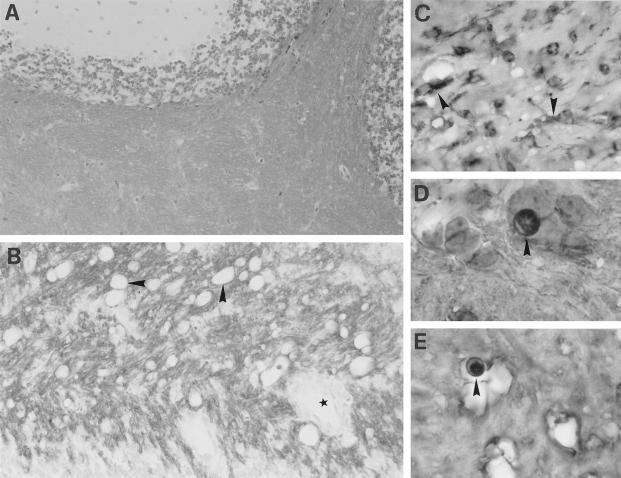
Primary demyelination associated with oligodendrocyte apoptosis leads to chronic plaque formation in Tg6074 transgenic mice. Early primary demyelinating lesions develop by 4 weeks of age and are characterized by myelin vacuolation, as shown by LFB/PAS staining (A, arrow), and in situ hybridization for PLP mRNA (B, black) and immunocytochemistry for PLP protein (B, red); arrowheads show myelin vacuoles. Oligodendrocyte apoptosis revealed by fragmented nucleus as shown by MOG/hematoxylin at 2 weeks of age (C) and by a pyknotic nucleus as shown by CNPase/hematoxylin at 8 weeks of age (D). Confluent demyelinating plaques develop by 10 weeks of age (E-I). LFB/PAS staining of the cerebellum (E) revealed loss of myelin and the presence of numerous PAS positive macrophages which at the lesion edge contained myelin degradation products (E, asterisk; F). In situ hybridization for PLP mRNA (G, black) counterstained with anti-PLP (G, red) showed the loss of oligodendrocytes and myelin with a few remaining oligodendrocytes in the lesion area containing PLP mRNA (G, arrowhead). The borders of the cerebellar dentate nucleus (ND) are indicated. Bielschowsky silver staining revealed moderate axonal damage (H, asterisk) . CD3 immunostaining revealed T lymphocyte infiltration in the cerebellum (I). Magnifications: A, ×68; B, ×388; C, ×990; D, ×1232; E, ×68; F, ×266, G, ×99; H, ×308; I, ×161.
Figure 3.
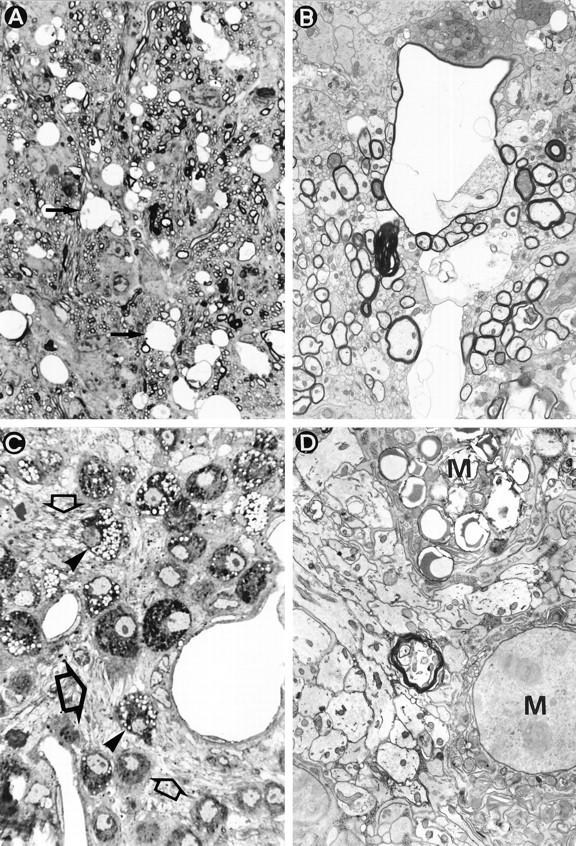
Primary demyelination develops in Tg6074 transgenic mice. At 4 weeks of age, semithin sections revealed myelin vacuolation (A, arrows). Electron microscopy of the same area as Figure 3A ▶ reveals large vacuoles in the myelin sheath (B). At 9 weeks of age, semithin sections revealed active primary demyelination characterized by high numbers of phagocytic macrophages (C, arrowheads), and the presence of naked axons (C, open arrows). (D) Electron microscopy of the same area as Figure 3C ▶ shows a cluster of demyelinated axons in the close vicinity of phagocytic macrophages (M). In the middle a single myelinated axon can still be seen. Magnifications: A, ×740; B, ×4133; C, ×925; D, ×6125.
Table 1.
Time Course of Pathology in Tg6074 Mouse Brain
| Age | Mice (n) | Inflammation | Vacuolation | SFD | Plaques | Oligodendrocyte apoptosis | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Meninges | Parenchyma | CER | CI | CER | CI | CER | CI | |||
| 1 | 2 | − | − | − | − | − | − | − | − | + |
| 2 | 2 | + | +/− | − | − | − | − | − | − | + |
| 4 | 2 | + | + | + | + | + | + | − | − | + |
| 5 | 2 | + | + | + | + | + | + | − | − | + |
| 6.5 | 2 | + | + | + | + | + | + | + | − | + |
| 8 | 4 | + | + | + | + | + | + | + | +* | + |
| 10 | 4 | + | + | + | + | + | + | + | + | + |
Age: weeks after birth. Inflammation: presence of mononuclear cells in meninges and parenchyma. Vacuolation: presence of vacuolation in cerebellum (CER) or capsula interna (CI). SFD, single fiber degeneration as determined by LFB staining or PLP immunostaining; Plaques, presence of confluent demyelinated lesions in cerebellum or capsula interna. Oligodendrocyte apoptosis: presence of CNP-positive apoptotic cells.
−, not present; +, present; +/−, some mononuclear cells present in 1 of 2 animals.
*2 of 4 animals showed confluent lesions in the CI.
Table 2.
Myelin Pathology in the Cerebellum of Tg6074 Transgenic Mice
| Age (wk) | Mice (n) | Myelin | ||
|---|---|---|---|---|
| Normal (%) | Vacuolation (%) | Demyelination (%) | ||
| 4–6 | 4 | 60 ± 5.2 | 40 ± 5.2 | 0 |
| 6–8 | 6 | 23 ± 5.1 | 27 ± 10.2 | 50 ± 9.9 |
| 10 | 4 | 18 ± 3.3 | 30 ± 17.0 | 53 ± 19.0 |
The total area of cerebellar white matter (average, 0.5 mm2) and the relative proportions (% with SEM) of demyelinated (confluent) and vacuolated areas were determined using an ocular morphometric grid.
The first histopathological changes were observed from 1 week of age and involved single-scattered oligodendrocytes which stained positively for TUNEL and showed nuclear changes characteristic of apoptosis in the corpus callosum (Figure 2C) ▶ , the initial site of transgene expression, together with widespread microglial activation. Oligodendrocyte apoptosis developed in the absence of immune infiltration and blood-brain barrier (BBB) damage in the CNS parenchyma (not shown), although minimal BBB damage and immune infiltration could be observed at the meninges.
By 4 weeks of age, early demyelinating events consisting of myelin swelling and the formation of vacuoles with single fiber degeneration were observed (Tables 1 and 2) ▶ ▶ . This was localized by luxol fast blue staining or PLP in situ hybridization and immunocytochemistry for PLP protein in the cerebellar white matter, the capsula interna (Figure 2, A and B) ▶ , and, to a minor degree, the optic tracts. Examination of semithin (Figure 3A) ▶ and thin (Figure 3B) ▶ sections by electron microscopy showed myelin swelling within the periaxonal space of the myelin sheath. Axons within the demyelinating area appeared intact (Figure 3B) ▶ , showing that TNF-mediated CNS damage is selective for oligodendrocytes and myelin. Although there was pronounced inflammation at the brain meninges at this stage, immune cell infiltration in the brain parenchyma and BBB leakage were minor. The myelin vacuolation observed in transgenic mice closely resembled that seen in chronic MS lesions 2 and other demyelinating diseases such as HIV vacuolar myelopathy, which is associated with massive vacuolation of myelin, axonal preservation, and in part with infiltration of the periaxonal space by macrophages/microglia. 44
In later lesions (8 to 10 weeks of age), confluent symmetrical plaques of primary demyelination had developed in all animals tested (Tables 1 and 2) ▶ ▶ . Plaques were characterized by loss of both myelin and oligodendrocytes as shown by double PLP in situ hybridization and PLP immunocytochemistry (Figure 2G) ▶ , and oligodendrocyte apoptosis (Figure 2D) ▶ but remyelinating events were not observed. Ultrastructural analysis showed the presence of numerous demyelinated axons within the lesions (Figure 3D) ▶ . In the plaque there were also abundant activated microglia/macrophages (Figures 2E, 3C, and 3D) ▶ ▶ and at the plaque edge several of these cells contained myelin degradation products (Figure 2F) ▶ demonstrating active myelin degradation. Moderate axonal damage was evident within the plaques (Figure 2H) ▶ . Such lesions are accompanied by BBB leakage, some perivascular lymphocyte infiltration (Figure 2I) ▶ , and astroglial scarring. All histopathological changes described were restricted to brain samples where the transgene is abundantly expressed. Such demyelinating plaques remarkably resemble those of chronic MS lesions. 3,45
Astrocytic Expression of Transmembrane Human TNF Triggers Oligodendrocyte Apoptosis, Primary Demyelination with Axonal Loss, and the Development of Acute MS-Type Lesions
To investigate whether a human transmembrane TNF protein expressed by astrocytes could also trigger oligodendrocyte and myelin damage in vivo and thereby assess the importance of p55TNFR signaling via a contact-dependent manner, we also analyzed CNS tissues from TgK21 transgenic mice (Figure 4 ▶ > and
Figure 4.
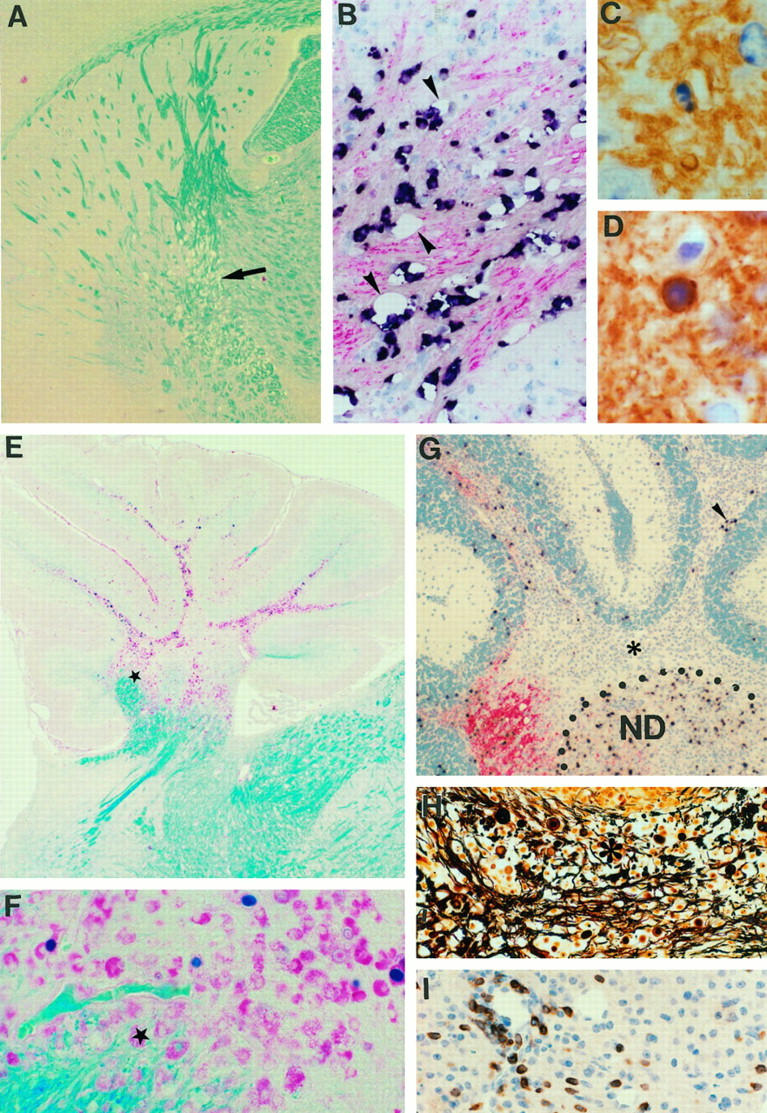
Acute lesions with primary demyelination and axonal damage develop in TgK21 transgenic mice. Demyelination was observed in the spinal cord of TgK21 mice by 4 weeks of age, together with massive meningeal and parenchymal inflammation as shown by immunocytochemistry for PLP protein counterstained with hematoxylin (A). The outer border of the CNS parenchyma is indicated by a dotted line. Axonal loss was observed by Bielschowsky silver staining (B). GFAP immunostaining revealed astrocytic loss in the acute plaque (C) (arrowheads outline plaque). In the lesion several phagocytic macrophages were detected (D and E, arrowheads) that contain PLP positive degradation products (D, arrowhead). Toluidine blue-stained semithin resin sections of the spinal cord revealed that primary demyelination was specific for the CNS myelin leaving PNS myelin completely unaffected (E, arrows). Severe BBB disruption was shown by immunostaining with anti-Ig (F). Arrowheads indicate Ig deposition in the acute plaque. CD3 immunostaining revealed T cell infiltration in the lesions (G). CNPase/hematoxylin shows a CNPase positive oligodendrocyte with a pyknotic nucleus characteristic of apoptotic cell death (H). CNPase immunostaining counterstained with hematoxylin revealed rows of oligodendrocytes with pyknotic and fragmented nuclei characteristic of apoptosis (I, arrowheads). TUNEL (J, black) counterstained with anti-CNPase (J, red) confirmed that such oligodendrocytes were undergoing apoptosis. Magnifications: A and B ×99; C and F ×388; D and J, ×990; E, ×925; G, ×484; H, ×1895; I, ×1232.
Histopathological changes were detected from the first week of age in all mice tested as a progressive accumulation of inflammatory cells with associated BBB damage at the meninges of the spinal cord and widespread astrocytic gliosis (not shown). In situ TUNEL counterstained for CNPase revealed the selective apoptosis of oligodendrocytes from the second week of age (not shown). By the fourth week of age, demyelinating lesions covering 30% of the original myelin mass (Figure 4A) ▶ had developed in the spinal cord at sites of extreme inflammation (Figure 4G) ▶ and extensive BBB breakdown (Figure 4F) ▶ . Myelin loss was accompanied by axonal damage (Figure 4B) ▶ and astrocyte loss (Figure 4C) ▶ . The presence of numerous phagocytic macrophages containing PLP positive myelin degradation products in the affected areas (Figure 4, D and E) ▶ showed that myelin was being phagocytosed by these cells. As in chronic lesions mentioned above, in situ hybridization for PLP mRNA showed that PLP-expressing oligodendrocytes were progressively depleted from demyelinating lesions (not shown). Combined TUNEL/CNPase immunocytochemistry showed that this loss could be accounted for at least in part by oligodendrocyte apoptosis (Figure 4, H–J) ▶ . Interestingly, TNF triggered demyelination selectively in CNS myelin, leaving myelin of the peripheral nervous system intact (Figure 4E) ▶ . The histopathological and ultrastructural characteristics of spinal cord lesions in TgK21 mice are consistent with those observed in typical acute MS, where destructive lesions include loss of myelin, oligodendrocytes, axons, and astrocytes. 3,45
The p55TNFR Is Necessary for the Development of TNF-Triggered CNS Inflammation and Primary Demyelination in Transgenic Mice
The present findings that both murine and human TNF can trigger the development of CNS inflammation, oligodendrocyte apoptosis, and demyelination in transgenic mice indicate that the p55TNFR plays a dominant role in the initiation of this phenotype, as human TNF is unable to signal through the murine p75TNFR. 46 To investigate further the specificity of TNFR signaling in TNF-triggered CNS pathology, we generated Tg6074 and TgK21 transgenic mice which were deficient in the p55TNFR and Tg6074 mice which were deficient for the p75TNFR. All Tg6074p55−/− and TgK21p55−/− mice generated remained entirely free of clinical symptoms for the study period of 8 months, while Tg6074p55+/+ and TgK21p55+/+ littermates developed disease as expected by 4 and 3 weeks of age, respectively. Histopathological analysis of CNS tissues from Tg6074p55−/−- (n = 4) and TgK21p55−/− (n = 5) mice aged up to 6 months found no evidence of inflammation or demyelination (Figure 5A) ▶ , showing that the presence of the p55TNFR is necessary for TNF to trigger these effects. Interestingly, TgK21p55+/− mice developed much delayed neurological symptoms by the sixth month of age. At the histological level the CNS from such mice (n = 2) showed symmetrical, focal demyelinated plaques at the capsula interna and in the spinal cord (not shown). In contrast, Tg6074p55+/− mice remained symptom-free during a study period of 12 months, and such mice (n = 2) showed no sign of pathology at the histological level. These results show that the levels of expression of the p55TNFR play a critical role in determining the pattern and extent of TNF-triggered CNS inflammation and primary demyelination in vivo. In contrast to Tg6074p55−/− mice, CNS pathology developed in Tg6074p75−/− (n = 2) mice with myelin vacuolation, inflammation and oligodendrocyte apoptosis as in Tg6074p75+/+ controls (Figure 5, B–E) ▶ , showing that when TNF is overexpressed it can trigger both inflammation and demyelination through the p55TNFR, even in the absence of the p75TNFR.
Figure 5.
TNF-triggered CNS demyelination is dependent on the presence of the p55TNF receptor. Ten-week-old Tg6074p55−/− mice show normal myelin with a normal distribution of PLP protein (A). In contrast, the cerebellum from a 4-week-old Tg6074p75−/− mouse immunostained for PLP showed myelin vacuolation (B, arrowheads) and inflammation (B, asterisk) similar to that observed in normal Tg6074 mice of the same age. Staining for the lectin GSI-B4 in Tg6074p75−/− mice revealed microglial activation in the cerebellar white matter (C, arrowheads). CNPase immunostaining in Tg6074p75−/− mice revealed early nuclear changes characteristic of apoptosis (D) and a CNPase-positive oligodendrocyte with a pyknotic nucleus (E). Magnifications: A, ×161; B, ×388; C, ×792; D, ×1232; E, ×990.
Discussion
Through the application of modern immunopathology techniques such as those which allow the precise histopathological identification of cell death and cell proliferation processes to the study of MS, it has become clear that MS lesions and patterns of demyelination are heterogeneous among patients and may therefore be mediated by different pathological mechanisms. In some patients myelin damage proceeds with preservation of oligodendrocytes, whereas in others oligodendrocytes are the primary target of the destructive process. 3,47,48 To explain the relative selectivity of degeneration of myelin in MS, emphasis has been given to autoimmune-mediated mechanisms of demyelination. 49,50 The study of EAE has provided important information concerning mechanisms of T cell-mediated and antibody-mediated demyelination where macrophages are important effector cells. 7,8,10 However, oligodendrocyte death does not seem to be a feature of EAE 51,52 and remyelination capacity is retained. It is clear that certain subtypes of MS, particularly those showing primary oligodendrogliopathy and absence of remyelinating events, cannot be accounted for by such antigen-triggered processes alone. TNF is a potent inflammatory mediator which is involved at multiple levels of immune regulation 53-55,39 and has also been strongly implicated in the pathogenesis of neuroinflammatory and demyelinating diseases. 12,56-58 In this study we demonstrate that TNF produced by resident CNS glia in two transgenic mouse lines can selectively induce oligodendrocyte apoptosis and primary demyelination in vivo as primary pathogenic effects. Myelin vacuolation observed in early transgenic lesions closely resembled that observed in MS 2 and HIV-induced vacuolar myelopathy 44 and depending on the context of TNF transgene expression in the CNS the demyelinating process progressed to the development of classical chronic or destructive acute MS-type lesions.
The molecular and cellular mechanisms by which TNF induces CNS inflammation and demyelination in transgenic mice appear to be very specific. The p55TNFR is known to mediate cell death responses (apoptosis) and proliferative effects by differential signaling through intracellular pathways. 59-62 Our observation that oligodendrocyte apoptosis is one of the first pathological effects of TNF transgene expression in the CNS suggests that TNF may exert a direct cytotoxic effect on oligodendrocytes. This is substantiated by evidence showing that oligodendrocytes can express TNFRs both in vitro 63,64 and in MS lesions 15 and by ample evidence that TNF can trigger oligodendrocyte death in vitro. 23,6 Furthermore, the recent finding that inhibitors of ICE/CED-3 proteases prevent TNF-mediated oligodendrocyte apoptosis 24 show that at least in vitro TNF can trigger intracellular death-signaling pathways in these cells. The observed concomitant inflammatory effects of TNF in the CNS of transgenic mice, including astrocytosis, microgliosis, and endothelial cell activation 36 are likely to contribute significantly to progression of the demyelinating process and plaque formation through additional inflammatory mechanisms of myelin and axonal damage. The development of conditional mutant mice in which the p55TNFR can be activated or inactivated in specific CNS cell lineages will allow the individual contributions of different TNF-mediated effects to CNS inflammation and demyelination to be evaluated. Further to an essential role for the p55TNFR in the initiation of TNF-mediated inflammation and demyelination, our studies in transgenic mice have demonstrated that the cellular source of TNF expression within the CNS also plays a critical role in determining whether inflammation and demyelination will develop. 36 Our finding that transmembrane human TNF can trigger demyelination when produced by astrocytes but not neurons 36 and the observation by others that transmembrane TNF is more effective than soluble TNF in killing oligodendrocytes in vitro 65 strongly suggest that TNF-mediated demyelination depends on appropriate cellular contacts between TNF-producing cells and oligodendrocytes or intermediate cells such as microglia/macrophages, and implicate transmembrane TNF as an important effector of oligodendrocyte death in vivo.
Although primary demyelination is the major hallmark of MS, axonal loss correlates with inflammatory activity and is observed in lesions as they age. 45,66 Similarly, in TNF transgenic mice axonal damage is not observed as a primary pathogenic effect of TNF expression suggesting that neurons, in contrast to oligodendrocytes, may not be direct targets of TNF cytotoxicity in vivo but are damaged following immune infiltration at sites of oligodendrocyte/myelin damage. This conclusion is consistent with several lines of evidence which show that TNF can be neuroprotective, 67-69 probably through the induction of NF-κB and NF-κB-regulated genes in neurons. 68 Our observation that the context of TNF expression within the CNS determines whether the resulting lesion will be chronic or acute may therefore relate to the differential capacity of TNF to trigger parenchymal inflammation and BBB leakage from different cell sources. TNF induces the development of acute demyelinating lesions when expressed by astrocytes in TgK21 mice. Astrocytes form intimate associations with the BBB through their foot processes and induce BBB properties in CNS endothelial cells in vitro 70 and astrocyte-specific expression of TNF seems to be crucial for endothelial cell activation and BBB damage in transgenic mice 36 (Akassoglou, Bauer, Lassmann, Kollias, and Probert, unpublished observations), suggesting a vigorous inflammatory component characteristic of acute demyelinating lesions with axonal loss. Taken together, these results indicate that whereas oligodendrocyte apoptosis is primary and TNF-dependent, axonal damage is secondary to BBB damage and immune cell infiltration of the CNS and points to the interesting possibility that axonal damage in MS, which is largely responsible for permanent disability, may be limited by treatments that restrict leukocyte trafficking at the BBB irrespective of disease etiology.
Our results demonstrate that TNF is a potent and selective effector of oligodendrocyte death and primary demyelination in vivo and implicate TNF/p55TNFR signaling as a potentially important mechanism of non-antigen-driven demyelination in MS. In addition, the characterization of the p55TNFR as the dominant receptor in mediating TNF-induced oligodendrocyte cytotoxicity and inflammation in the CNS identifies p55TNF signaling pathways as potentially important targets for cell-specific interventions. The finding that TNF-induced lesions in mice bear remarkable histopathological resemblance to MS lesions, particularly those characterized by primary oligodendrogliopathy, establish TNF transgenic mice as new animal models for MS.
Table 3.
Time Course and Extent of Pathology in TgK21 Mouse Spinal Cord
| Age | Mice (n) | Inflammation | Oligodendrocyte apoptosis | Myelin | ||
|---|---|---|---|---|---|---|
| Meninges | Parenchyma | Normal (%) | Demyelination (%) | |||
| 1 | 2 | + | − | − | 100 | 0 |
| 2 | 2 | + | − | − | 100 | 0 |
| 3 | 3 | + | +/− | + | 100 | 0 |
| 4 | 3 | + | + | + | 67± 8 | 33± 15 |
Age: weeks after birth. Inflammation: presence of mononuclear cells in meninges and parenchyma. Oligodendrocyte apoptosis: presence of CNP-positive apoptotic cells.
−, not present; +, present. The total area of spinal cord white matter (average, 0.5 mm2) and the relative proportions (% with SEM) of demyelinated (confluent) areas were determined using an ocular morphometric grid.
Acknowledgments
We thank Dr. Horst Bluethmann for providing the p55TNFR knockout mice, Dr. Mark Moore for providing the p75TNFR knockout mice and Dr. Roly Foulkes (Celltech, Berkshire, UK) for the TN3 neutralizing anti-murine TNF antibodies used for maintainance of the Tg6074 line. We also thank Helene Breitschopf, Angela Kury, Elisabeth Gurnhofer, Anna Kefalaki, Marianne Leisser, and Spiridoula Papandreou for expert technical assistance.
Footnotes
Address reprint requests to Lesley Probert, PhD., Department of Molecular Genetics, Hellenic Pasteur Institute, 127 Vas. Sofias Ave., 115 21, Athens, Greece. E-mail: lesley_probert@hol.gr.
Supported by European Commission Grants BIO4-CT96–0174 and BIO4-CT96–0077, Hellenic General Secretariat for Research and Technology EPETII/PENED 1995 Program Grant 1629, and European Molecular Biology Organisation Short Term Fellowship ASTF 8543 to KA.
References
- 1.Martin R, McFarland HF, McFarlin DE: Immunological aspects of demyelinating diseases. Annu Rev Immunol 1992, 10:153-187 [DOI] [PubMed] [Google Scholar]
- 2.Brosnan CF, Raine CS: Mechanisms of immune injury in multiple sclerosis. Brain Pathol 1996, 6:243-257 [DOI] [PubMed] [Google Scholar]
- 3.Lucchinetti CF, Bruck W, Rodriguez M, Lassmann H: Distinct patterns of multiple sclerosis pathology indicates heterogeneity in pathogenesis. Brain Pathol 1996, 6:259-274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dowling P, Shang G, Raval S, Menonna J, Cook S, Husar W: Involvement of the CD95 (APO-1/Fas) receptor/ligand system in multiple sclerosis brain. J Exp Med 1996, 184:1513-1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vartanian T, Li Y, Zhao M, Stefansson K: Interferon-γ induced oligodendrocytes cell death: implications for the pathogenesis of multiple sclerosis. Mol Med 1995, 1:732-743 [PMC free article] [PubMed] [Google Scholar]
- 6.D’Souza SD, Bonetti B, Balasingam V, Cashman NR, Barker PA, Troutt AB, Raine CS, Antel JP: Multiple sclerosis: Fas signaling in oligodendrocyte cell death. J Exp Med 1996, 184:2361-2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zamvil SS, Steinman L: The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol 1990, 8:579-621 [DOI] [PubMed] [Google Scholar]
- 8.Wekerle H, Kojima K, Lannes-Vieira J, Lassmann H, Linington C: Animal models. Ann Neurol 1994, 36:S47-S53 [DOI] [PubMed] [Google Scholar]
- 9.Genain CP, Nguyen MH, Letvin NL, Pearl R, Davis RL, Adelman M, Lees MB, Linington C, Hauser SL: Antibody facilitation of multiple sclerosis-like lesions in a nonhuman primate. J Clin Invest 1995, 96:2966-2974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dal Canto MC, Melvold RW, Kim BS, Miller SD: Two models of multiple sclerosis: experimental allergic encephalomyelitis (EAE) and Theiler’s murine encephalomyelitis virus (TMEV) infection; a pathological and immunological comparison. Microsc Res Tech 1995, 32:215-229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez M, Pavelko KD, Njjenga MK, Logan WC, Wettstein PJ: The balance between persistent virus infection and immune cells determines demyelination. J Immunol 1996, 157:5699-5709 [PubMed] [Google Scholar]
- 12.Raine CS: Multiple sclerosis: TNF revisited, with promise. Nat Med 1995, 1:211-214 [DOI] [PubMed] [Google Scholar]
- 13.Hofman FM, Hinton DR, Johnson K, Merrill JE: Tumor necrosis factor identified in multiple sclerosis brain. J Exp Med 1989, 170:607-612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selmaj KW, Raine CS, Cannella B, Brosnan CF: Identification of lymphotoxin and tumor necrosis factor in multiple sclerosis lesions. J Clin Invest 1991, 87:949-954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonetti B, Raine CS: Multiple sclerosis: oligodendrocytes display cell death-related molecules in situ but do not undergo apoptosis. Ann Neurol 1997, 42:74-84 [DOI] [PubMed] [Google Scholar]
- 16.Sharief MK, Hentges R: Association between tumor necrosis factor-α and disease progression in patients with multiple sclerosis. N Engl J Med 1991, 325:467-472 [DOI] [PubMed] [Google Scholar]
- 17.Selmaj KW, Farooq M, Norton WT, Raine CS, Brosnan CF: Proliferation of astrocytes in vitro in response to cytokines: a primary role for tumor necrosis factor. J Immunol 1990, 144:129-135 [PubMed] [Google Scholar]
- 18.Merrill J: Effects of interleukin-1 and tumor necrosis factor-α on astrocytes, microglia, oligodendrocytes, and glial precursors in vitro. Dev Neurosci 1991, 13:130-137 [DOI] [PubMed] [Google Scholar]
- 19.Karmann K, Min W, Fanslow WC, Pober JS: Activation and homologous desensitization of human endothelial cells by CD40 ligand, tumor necrosis factor, and interleukin 1. J Exp Med 1996, 184:173-182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shrikant P, Benveniste EN: The central nervous system as an immunocompetent organ: role of glial cells in antigen presentation. J Immunol 1996, 157:1819-1822 [PubMed] [Google Scholar]
- 21.Merrill JE, Benveniste EN: Cytokines in inflammatory brain lesions: helpful and harmful. Trends Neurosci 1996, 19:331-338 [DOI] [PubMed] [Google Scholar]
- 22.Selmaj KW, Raine CS: Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann Neurol 1988, 23:339-346 [DOI] [PubMed] [Google Scholar]
- 23.Selmaj K, Raine CS, Farooq M, Norton WT, Brosnan CF: Cytokine cytotoxicity against oligodendrocytes: apoptosis induced by lymphotoxin. J Immunol 1991, 147:1522-1529 [PubMed] [Google Scholar]
- 24.Hisahara S, Shoji S, Okano H, Miura M: ICE/CED-3 family executes oligodendrocyte apoptosis by tumor necrosis factor. J Neurochem 1997, 69:10-20 [DOI] [PubMed] [Google Scholar]
- 25.Ruddle NH, Bergman CM, McGrath KM, Lingenheld EG, Grunnet ML, Padula SJ, Clark RB: An antibody to lymphotoxin and tumor necrosis factor prevents transfer of experimental allergic encephalomyelitis. J Exp Med 1990, 172:1193-1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker D, Butler D, Scallon BJ, O’Neill JK, Turk JL, Feldmann M: Control of established experimental allergic encephalomyelitis by inhibition of tumor necrosis factor (TNF) activity within the central nervous system using monoclonal antibodies and TNF receptor-immunoglobulin fusion proteins. Eur J Immunol 1994, 24:2040-2048 [DOI] [PubMed] [Google Scholar]
- 27.Selmaj K, Papierz W, Glabinski A, Kohno T: Prevention of chronic relapsing experimental autoimmune encephalomyelitis by soluble tumor necrosis factor receptor I. J Neuroimmunol 1995, 56:135-141 [DOI] [PubMed] [Google Scholar]
- 28.Sommer N, Loschmann P-A, Northoff GH, Weller M, Steinbrecher A, Steinbach JP, Lichtenfels R, Meyermann R, Riethmuller A, Fontana A, Dichgans J, Martin R: The antidepressant rolipram suppresses cytokine production and prevents autoimmune encephalomyelitis. Nat Med 1995, 1:244-248 [DOI] [PubMed] [Google Scholar]
- 29.Frei K, Eugster H-P, Bopst M, Constantinescu CS, Lavi E, Fontana A: Tumor necrosis factor α and lymphotoxin α are not required for induction of acute experimental autoimmune encephalomyelitis. J Exp Med 1997, 185:2177-2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suen WE, Bergman CM, Hjelmstrom P, Ruddle NH: A critical role for lymphotoxin in experimental allergic encephalomyelitis. J Exp Med 1997, 186:1233-1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korner H, Rimington DS, Strickland DH, Lemckert FA, Pollard JD, Sedgwick JD: Critical points of tumor necrosis factor action in central nervous system autoimmune inflammation defined by gene targeting. J Exp Med 1997, 186:1585-1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, Marino MW, Wong G, Grail D, Dunn A, Bettadapura J, Slavin AJ, Old L, Bernard CCA: TNF is a potent anti-inflammatory cytokine in autoimmune-mediated demyelination. Nat Med 1998, 4:78-83 [DOI] [PubMed] [Google Scholar]
- 33.Steinman L: Some misconceptions about understanding autoimmunity through experiments with knockouts. J Exp Med 1997, 185:2039-2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez M, Scheithauer B: Ultrastructure of multiple sclerosis. Ultrastruct Pathol 1994, 18:3-13 [DOI] [PubMed] [Google Scholar]
- 35.Probert L, Akassoglou K, Pasparakis M, Kontogeorgos G, Kollias G: Spontaneous inflammatory demyelinating disease in transgenic mice showing central nervous system-specific expression of tumor necrosis factor α. Proc Natl Acad Sci USA 1995, 92:11294-11298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akassoglou K, Probert L, Kontogeorgos G, Kollias G: Astrocyte-specific but not neuron-specific transmembrane TNF triggers inflammation and degeneration in the central nervous system of transgenic mice. J Immunol 1997, 158:438-445 [PubMed] [Google Scholar]
- 37.Rothe J, Lesslauer W, Lotscher H, Lang Y, Koebel P, Kontgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H: Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature 1993, 364:798-802 [DOI] [PubMed] [Google Scholar]
- 38.Erickson SL, de Sauvage FJ, Kikly K, Carver-Moore K, Pitts-Meek S, Gillet N, Sheehan KF, Schreiber RD, Goeddel DV, Moore MW: Decreased sensitivity to tumour-necrosis factor but normal T-cell development in TNF receptor-2-deficient mice. Nature 1994, 372:560-563 [DOI] [PubMed] [Google Scholar]
- 39.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G: Immune and inflammatory responses in TNF α-deficient mice: a critical requirement for TNF α in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med 1996, 184:1397-1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rossler K, Neuchrist C, Kitz K, Scheiner O, Kraft D, Lassmann H: Expression of leucocyte adhesion molecules at the human blood-brain barrier. J Neurosci Res 1992, 31:365-374 [DOI] [PubMed] [Google Scholar]
- 41.Gold R, Schmied M, Giegerich G, Breitschopf H, Hartung HP, Toyka KV, Lassmann H: Differentiation between cellular apoptosis and necrosis by the combined use of in situ tailing and nick translation techniques. Lab Invest 1994, 71:219-225 [PubMed] [Google Scholar]
- 42.Breitschopf H, Suchanek G, Gould RM, Coleman DR, Lassmann H: In situ hybridization with digoxigenin-labeled probes: sensitive and reliable detection method applied to myelinating rat brain. Acta Neuropathol 1992, 84:581-587 [DOI] [PubMed] [Google Scholar]
- 43.Stagaard Janas M, Nowakowski RS, Terkelsen OB, Mollgard K: Glial cell differentiation in neuron-free and neuron-rich regions. I. Selective appearance of S-100 protein in radial glial cells of the hippocampal fimbria in human fetuses. Anat Embryol (Berl) 1991, 184:549-558 [DOI] [PubMed] [Google Scholar]
- 44.Maier H, Budka H, Lassmann H, Pohl P: Vacuolar myelopathy with multinucleated giant cells in the aquired immune deficiency syndrome (AIDS): light and electron microscopic distribution of human immunodeficiency virus (HIV) antigens. Acta Neuropathol 1989, 78:497-503 [DOI] [PubMed] [Google Scholar]
- 45.Raine CS: Demyelinating diseases. Davies RL Robertson DM eds. Textbook of Neuropathology. 1997, :pp 627-714 Williams and Wilkins, Baltimore [Google Scholar]
- 46.Lewis M, Tartaglia LA, Lee A, Bennett GL, Rice GC, Wong GHW, Chen EY, Goeddel DV: Cloning and expression of cDNAs for two distinct murine tumor necrosis factor receptors demonstrate one receptor is species specific. Proc Natl Acad Sci USA 1991, 88:2830-2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ozawa K, Suchanek G, Breitschopf H, Bruck W, Budka H, Jellinger K, Lassmann H: Patterns of oligodendroglia pathology in multiple sclerosis. Brain 1994, 117:1311-1322 [DOI] [PubMed] [Google Scholar]
- 48.Bruck W, Schmied M, Suchanek G, Bruck Y, Breitschopf H, Poser S, Piddlesden S, Lassmann H: Oligodendrocytes in the early course of multiple sclerosis. Ann Neurol 1994, 35:65-73 [DOI] [PubMed] [Google Scholar]
- 49.Raine CS: Multiple sclerosis: a pivotal role for the T cell in lesion development. Neuropathol Appl Neurobiol 1991, 17:265-274 [DOI] [PubMed] [Google Scholar]
- 50.Link H, Baig S, Olsson O, Yu-Ping J, Hojeberg B, Olsson T: Persistent anti-myelin basic protein IgG antibody response in multiple sclerosis cerebrospinal fluid. J Neuroimmunol 1990, 28:237-248 [DOI] [PubMed] [Google Scholar]
- 51.Genain CP, Abel K, Belmar N, Villinger F, Rosenberg DP, Linington C, Raine CS, Hauser SL: Late complications of immune deviation therapy in a nonhuman primate. Science 1996, 274:2054-2057 [DOI] [PubMed] [Google Scholar]
- 52.Bonetti B, Pohl J, Gao Y-L, Raine CS: Cell death during autoimmune demyelination: effector but not target cells are eliminated by apoptosis. J Immunol 1997, 159:5733-5741 [PubMed] [Google Scholar]
- 53.Vassalli P: The pathophysiology of tumor necrosis factors. Annu Rev Immunol 1992, 10:411-452 [DOI] [PubMed] [Google Scholar]
- 54.Sarvetnick N: Mechanisms of cytokine-mediated localized immunoprotection. J Exp Med 1996, 184:1597-1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cope A, Liblau RS, Yang X-D, Congia M, Laudanna C, Schreiber RD, Probert L, Kollias G, McDevitt HO: Chronic tumor necrosis factor alters T cell responses by attenuating T cell receptor signaling. J Exp Med 1997, 185:1573-1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tyor WR, Glass JD, Baumrind N, McArthur JC, Griffin JW, Becker PS, Griffin DE: Cytokine expression of macrophages in HIV-1-associated vacuolar myelopathy. Neurology 1993, 43:1002-1009 [DOI] [PubMed] [Google Scholar]
- 57.Wilt SG, Milward E, Zhou JM, Nagasato K, Patton H, Rusten R, Griffin DE, O’Connor M, Dubois-Dalcq M: In vitro evidence for a dual role of tumor necrosis factor-α in human immunodeficiency virus type 1 encephalopathy. Ann Neurol 1995, 37:381-394 [DOI] [PubMed] [Google Scholar]
- 58.Kordek R, Nerurkar VR, Liberski PP, Isaacson S, Yanagihara R, Gajdusek DC: Heightened expression of tumor necrosis factor α, interleukin 1α, and glial fibrillary acidic protein in experimental Creutzfeldt-Jakob disease in mice. Proc Natl Acad Sci USA 1996, 93:9754-9758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Z-G, Hsu H, Goeddel DV, Karin M: Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-κB activation prevents cell death. Cell 1996, 87:565-576 [DOI] [PubMed] [Google Scholar]
- 60.Wallach D, Boldin M, Varfolomeev E, Beyaert R, Vandenbeele P, Fiers W: Cell death induction by receptors of the TNF family: towards a molecular understanding. FEBS Lett 1997, 410:96-106 [DOI] [PubMed] [Google Scholar]
- 61.Beg AA, Baltimore D: An essential role for NF-κB in preventing TNF-α-induced cell death. Science 1996, 274:782-784 [DOI] [PubMed] [Google Scholar]
- 62.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM: Suppression of TNF-α-induced apoptosis by NF-κB. Science 1996, 274:787-789 [DOI] [PubMed] [Google Scholar]
- 63.Dopp JM, Mackenzie-Graham A, Otero GC, Merrill JE: Differential expression, cytokine modulation, and specific functions of type-1 and type-2 tumor necrosis factor receptors in rat glia. J Neuroimmunol 1997, 75:104-112 [DOI] [PubMed] [Google Scholar]
- 64.Tchelingerian JL, Monge M, Le Saux F, Zalc B, Jacque C: Differential oligodendroglial expression of the tumor necrosis factor receptors in vivo and in vitro. J Neurochem 1995, 65:2377-2380 [DOI] [PubMed] [Google Scholar]
- 65.Zajicek JP, Wing M, Scolding NJ, Compston DAS: Interactions between oligodendrocytes and microglia: a major role for complement and tumour necrosis factor in oligodendrocyte adherence and killing. Brain 1992, 115:1611-1631 [PubMed] [Google Scholar]
- 66.Barnes D, Munro PM, Youl BD, Prineas JW, McDonald WI: The longstanding MS lesion: a quantitative MRI and electron microscopic study. Brain 1991, 114:1271-1280 [DOI] [PubMed] [Google Scholar]
- 67.Cheng B, Christakos S, Mattson MP: Tumor necrosis factors protect neurons against metabolic-excitotoxic insults and promote maintenance of calcium homeostasis. Neuron 1994, 12:139-153 [DOI] [PubMed] [Google Scholar]
- 68.Barger SW, Horster D, Furukawa K, Goodman Y, Krieglstein J, Mattson MP: Tumor necrosis factors α and β protect neurons against amyloid β-peptide toxicity: evidence for involvment of a κB-binding factor and attenuation of peroxide and Ca2+ accummulation. Proc Natl Acad Sci USA 1995, 92:9328-9332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bruce AJ, Boling W, Kindy MS, Peschon J, Kraemer PJ, Carpenter MK, Holtsberg FW, Mattson MP: Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat Med 1996, 2:788-794 [DOI] [PubMed] [Google Scholar]
- 70.Janzer RC, Raff MC: Astrocytes induce blood-brain barrier properties in endothelial cells. Nature 1987, 325:253-257 [DOI] [PubMed] [Google Scholar]