Abstract
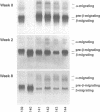
Apo E, a key regulator of cholesterol-rich lipoprotein metabolism, is synthesized by numerous extrahepatic tissues. Although its synthesis in macrophages is documented, the contribution of macrophage-derived apo E to hepatic clearance of serum cholesterol is unknown. To address this issue bone marrow transplantation was performed on hypercholesterolemic apo E-deficient mice with either syngeneic apo E-deficient mouse bone marrow cells (E0-control) or wild-type mouse bone marrow cells expressing apo E (E0-treated). E0-control and E0-treated mice were fed either a regular chow diet or an atherogenic diet (designated E0-control-HF and E0-treated-HF). Serum cholesterol levels dropped dramatically in the E0-treated mice largely due to a reduction in their VLDL cholesterol. No changes were seen in the E0-control mice. After 4 wk serum cholesterol in E0-treated-HF mice was about four-fold lower compared to E0-control-HF animals. Moreover, the extent of atherosclerosis in the E0-treated-HF mice after 14-16 wk was greatly reduced. Wild-type apo E mRNA was detected in the liver, spleen, and brain of the E0-treated mice indicating that apo E gene transfer was successfully achieved through bone marrow transplantation. More importantly, the level of apo E expression was sufficient to reduce the severe hypercholesterolemia of the apo E-deficient mice fed either chow or atherogenic diets.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basu S. K., Brown M. S., Ho Y. K., Havel R. J., Goldstein J. L. Mouse macrophages synthesize and secrete a protein resembling apolipoprotein E. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7545–7549. doi: 10.1073/pnas.78.12.7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S. K., Ho Y. K., Brown M. S., Bilheimer D. W., Anderson R. G., Goldstein J. L. Biochemical and genetic studies of the apoprotein E secreted by mouse macrophages and human monocytes. J Biol Chem. 1982 Aug 25;257(16):9788–9795. [PubMed] [Google Scholar]
- Beisiegel U., Weber W., Ihrke G., Herz J., Stanley K. K. The LDL-receptor-related protein, LRP, is an apolipoprotein E-binding protein. Nature. 1989 Sep 14;341(6238):162–164. doi: 10.1038/341162a0. [DOI] [PubMed] [Google Scholar]
- Boak J. L., Christie G. H., Ford W. L., Howard J. G. Pathways in the development of liver macrophages: alternative precursors contained in populations of lymphocytes and bone-marrow cells. Proc R Soc Lond B Biol Sci. 1968 Feb 27;169(1016):307–327. doi: 10.1098/rspb.1968.0013. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dawson P. A., Lukaszewski L. M., Ells P. F., Malbon C. C., Williams D. L. Quantification and regulation of apolipoprotein E expression in rat Kupffer cells. J Lipid Res. 1989 Mar;30(3):403–413. [PubMed] [Google Scholar]
- Driscoll D. M., Getz G. S. Extrahepatic synthesis of apolipoprotein E. J Lipid Res. 1984 Dec 1;25(12):1368–1379. [PubMed] [Google Scholar]
- Gale R. P., Sparkes R. S., Golde D. W. Bone marrow origin of hepatic macrophages (Kupffer cells) in humans. Science. 1978 Sep 8;201(4359):937–938. doi: 10.1126/science.356266. [DOI] [PubMed] [Google Scholar]
- Godleski J. J., Brain J. D. The origin of alveolar macrophages in mouse radiation chimeras. J Exp Med. 1972 Sep 1;136(3):630–643. doi: 10.1084/jem.136.3.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon V., Innerarity T. L., Mahley R. W. Formation of cholesterol- and apoprotein E-enriched high density lipoproteins in vitro. J Biol Chem. 1983 May 25;258(10):6202–6212. [PubMed] [Google Scholar]
- Koo C., Innerarity T. L., Mahley R. W. Obligatory role of cholesterol and apolipoprotein E in the formation of large cholesterol-enriched and receptor-active high density lipoproteins. J Biol Chem. 1985 Oct 5;260(22):11934–11943. [PubMed] [Google Scholar]
- Lee S. H., Starkey P. M., Gordon S. Quantitative analysis of total macrophage content in adult mouse tissues. Immunochemical studies with monoclonal antibody F4/80. J Exp Med. 1985 Mar 1;161(3):475–489. doi: 10.1084/jem.161.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. T., Xu Y. F., Wu J. Y., Chan L. Immunoreactive apolipoprotein E is a widely distributed cellular protein. Immunohistochemical localization of apolipoprotein E in baboon tissues. J Clin Invest. 1986 Oct;78(4):947–958. doi: 10.1172/JCI112685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton M. F., Gish R., Hubl S. T., Bütler E., Esquivel C., Bry W. I., Boyles J. K., Wardell M. R., Young S. G. Phenotypes of apolipoprotein B and apolipoprotein E after liver transplantation. J Clin Invest. 1991 Jul;88(1):270–281. doi: 10.1172/JCI115288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley R. W., Ji Z. S., Brecht W. J., Miranda R. D., He D. Role of heparan sulfate proteoglycans and the LDL receptor-related protein in remnant lipoprotein metabolism. Ann N Y Acad Sci. 1994 Sep 10;737:39–52. doi: 10.1111/j.1749-6632.1994.tb44300.x. [DOI] [PubMed] [Google Scholar]
- Palinski W., Ord V. A., Plump A. S., Breslow J. L., Steinberg D., Witztum J. L. ApoE-deficient mice are a model of lipoprotein oxidation in atherogenesis. Demonstration of oxidation-specific epitopes in lesions and high titers of autoantibodies to malondialdehyde-lysine in serum. Arterioscler Thromb. 1994 Apr;14(4):605–616. doi: 10.1161/01.atv.14.4.605. [DOI] [PubMed] [Google Scholar]
- Piedrahita J. A., Zhang S. H., Hagaman J. R., Oliver P. M., Maeda N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4471–4475. doi: 10.1073/pnas.89.10.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plump A. S., Smith J. D., Hayek T., Aalto-Setälä K., Walsh A., Verstuyft J. G., Rubin E. M., Breslow J. L. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992 Oct 16;71(2):343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- Shand F. L., Bell E. B. Studies on the distribution of macrophages derived from rat bone marrow cells in xenogeneic radiation chimaeras. Immunology. 1972 Apr;22(4):549–556. [PMC free article] [PubMed] [Google Scholar]
- Thomas E. D., Ramberg R. E., Sale G. E., Sparkes R. S., Golde D. W. Direct evidence for a bone marrow origin of the alveolar macrophage in man. Science. 1976 Jun 4;192(4243):1016–1018. doi: 10.1126/science.775638. [DOI] [PubMed] [Google Scholar]
- Tsoi M. S., Dobbs S., Brkic S., Ramberg R., Thomas E. D., Storb R. Cellular interactions in marrow-grafted patients. II. Normal monocyte antigen-presenting and defective T-cell-proliferative functions early after grafting and during chronic graft-versus-host disease. Transplantation. 1984 Jun;37(6):556–561. [PubMed] [Google Scholar]
- Virolainen M. Hematopoietic origin of macrophages as studied by chromosome markers in mice. J Exp Med. 1968 May 1;127(5):943–952. doi: 10.1084/jem.127.5.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. L., Dawson P. A., Newman T. C., Rudel L. L. Apolipoprotein E synthesis in peripheral tissues of nonhuman primates. J Biol Chem. 1985 Feb 25;260(4):2444–2451. [PubMed] [Google Scholar]
- Zannis V. I., Cole F. S., Jackson C. L., Kurnit D. M., Karathanasis S. K. Distribution of apolipoprotein A-I, C-II, C-III, and E mRNA in fetal human tissues. Time-dependent induction of apolipoprotein E mRNA by cultures of human monocyte-macrophages. Biochemistry. 1985 Jul 30;24(16):4450–4455. doi: 10.1021/bi00337a028. [DOI] [PubMed] [Google Scholar]
- Zhang S. H., Reddick R. L., Piedrahita J. A., Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992 Oct 16;258(5081):468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- van Furth R. Origin and kinetics of monocytes and macrophages. Semin Hematol. 1970 Apr;7(2):125–141. [PubMed] [Google Scholar]
- van Furth R. Origin and turnover of monocytes and macrophages. Curr Top Pathol. 1989;79:125–150. [PubMed] [Google Scholar]