Abstract
It has long been believed that trypsin is normally synthesized only in the pancreas. In the present study, expression of trypsin in human and mouse nonpancreatic tissues was examined. Northern blot analysis of normal human tissues indicated that the trypsin gene is expressed at high levels in the pancreas and spleen and considerably in the small intestine. However, in situ hybridization and immunohistochemistry demonstrated that trypsin is widely expressed in epithelial cells of the skin, esophagus, stomach, small intestine, lung, kidney, liver, and extrahepatic bile duct, as well as splenic and neuronal cells. In the spleen, trypsin message was detected in macrophages, monocytes, and lymphocytes in the white pulp. In the brain, it was detected in the nerve cells of the hippocampus and cerebral cortex. Analysis by gelatin zymography confirmed the presence of a latent or an active form of trypsin in various normal mouse tissues. Reverse transcription-polymerase chain reaction analysis also confirmed the expression of trypsin genes in the spleen, liver, kidney, and brain of normal mice. Such a broad distribution of trypsin suggests its general roles in the maintenance of normal epithelial cell functions, the immune defense system, and the central nervous system.
Secretory proteinases play essential roles in many physiological processes, such as food digestion, blood coagulation, fibrinolysis, and control of blood pressure. 1 They are also involved in various pathological processes, including abnormal blood coagulation, inflammation, tumor invasion, and atherosclerosis. Metalloproteinases 2 and serine proteinases 3 are the major groups of secretory proteinases.
Trypsin is one of the best characterized serine proteinases. It has long been known that trypsin is produced as a zymogen (trypsinogen) in the acinar cells of the pancreas, is secreted into the duodenum, is activated into the mature form of trypsin by enterokinase, and functions as an essential food-digestive enzyme. 4 However, little is known about the distribution and function of trypsin in other normal tissues. To date, four trypsin (or trypsinogen) genes have been characterized in humans: trypsin 1, 5 trypsin 2, 5 trypsin 3, 6 and trypsin 4. 7 Trypsins 1, 2, and 3 have been demonstrated as the zymogens in human pancreatic juice. 8 In mice, only a single type of trypsin has been identified. 9
Past studies have shown that trypsins or trypsin-like enzymes are produced by human cancer cells of the stomach, 10,11 ovary, 12 lung, 13 colon, 14 and others. 14 The tumor-derived trypsin is likely to contribute to tumor invasion and metastasis by degrading extracellular matrix proteins and by activating the latent forms of matrix metalloproteinases (MMPs). 10-12 Recently, we found that trypsin 2 is expressed by vascular endothelial cells around gastric tumors and in patients with disseminated intravascular coagulation. 15 Trypsin-like enzymes have been observed in some normal tissues. 13,16,17 The trypsin 4 gene is expressed in the human brain. 7 These facts suggest broad distribution and function of trypsin in normal tissues. In the present study, we surveyed the expression of trypsin mRNA and protein in various human and mouse tissues.
Materials and Methods
Tissue Specimens and Sample Preparation
All human tissues were obtained by autopsy performed less than 3 hours postmortem and were immediately fixed in 10% formalin. The paraffin-embedded sections were mounted on aminoacyl silane-coated glass slides and used for in situ hybridization and immunohistochemical analysis. Normal mouse tissues were obtained from 8-week-old female ICR mice, washed with cold phosphate-buffered saline (PBS), and stored at −40°C until use. Digestive tract tissues were washed with PBS containing 0.1% (w/v) sodium dodecyl sulfate to avoid contamination of pancreatic trypsin and then washed further with PBS alone. The frozen tissues were thawed, homogenized in 5 volumes (w/v) of 50 mmol/L Tris-HCl (pH 7.5) containing 0.1% (w/v) Triton X-100, and cleared by centrifugation. For activation of trypsinogen, 10 μl of the tissue extract (5 mg protein/ml) was mixed with 2 μl of 50 μg/ml enterokinase (Biozyme; South Wales, UK) and 3 μl of 50 mmol/L CaCl2 and incubated at 37 °C for 1 hour, followed by mixing with an equal volume of 2× sodium dodecyl sulfate sample buffer without 2-mercaptoethanol.
Northern Blotting and in Situ Hybridization
Nylon membranes blotted with polyadenylated RNAs, Multiple Tissue Northern Blots (Clontech Laboratories, Palo Alto, CA), were used to analyze expression of trypsin mRNAs in various human tissues except for the skin. Polyadenylated RNA from human skin was purchased from Nippon Gene (Tokyo, Japan) and used for Northern blotting analysis of trypsin mRNAs. Hybridization of trypsin mRNAs with a 32P-labeled trypsin 1 cDNA (nucleotides 1 to 482 in Ref. 5 ) and subsequent autoradiography were carried out as described before. 12,15 For in situ hybridization analysis of trypsin messages in various human tissues, sense and antisense RNA probes were prepared from a cDNA fragment (nucleotides 131 to 482 in Ref. 5 ) of human trypsin 1.15 In situ hybridization was carried out as reported previously. 15 The hybridized signals were visualized by the alkaline phosphatase reaction. In this analysis, the RNA probe was expected to hybridize to trypsin 1, 2, 3, and 4 mRNAs under the experimental conditions used. The tissue sections were counterstained with methyl green.
Reverse Transcription-Polymerase Chain Reaction and Nucleotide Sequences
For reverse transcription-polymerase chain reaction (RT-PCR) analysis, the following two primers were designed: sense, 5′-CTACAAATACCGCATCCAAGT-3′, and antisense, 5′-ACCTCGTCCAGACCCAACAA-3′, which corresponded to nucleotides 208 to 228 and 473 to 492, respectively, of mouse trypsin (or trypsinogen) cDNA. 9 For nucleotide sequencing, RT-PCR products of approximately 280 bp were electrophoretically separated and cloned into a pGEM-T Easy vector (Promega, Madison, WI). The nucleotide sequences of the cloned DNA fragments were determined by the dideoxynucleotide method, using cycle sequencing with a LI-COR automatic sequencer (Lincoln, NE).
Immunohistochemistry
Immunohistochemical staining of human tissues for trypsin was carried out with a mouse monoclonal antibody raised against human pancreatic trypsin (Chemicon; Temecula, CA), as described before. 12 Briefly, 4-μm-thick paraffin sections were dewaxed, rehydrated, and immersed in 0.3% hydrogen peroxide-containing methanol for inactivation of intrinsic peroxidase. Then the sections were incubated with the anti-trypsin monoclonal antibody at 37°C for 1 hour. The labeled antigen was visualized by the 3,3′-diaminobenzidine reaction with a HistoFine kit (Nichirei, Tokyo, Japan). The trypsin antibody was reactive to pancreatic trypsins/trypsinogens 1 and 2 as tested by immunoblotting.
Gelatin Zymography
Unless otherwise noted, gelatin zymography was carried out on 14% polyacrylamide slab gels (90 mm long, 90 mm wide, and 0.75 mm thick) under nonreducing conditions, as described before. 10,15 Molecular weight markers were purchased from Bio-Rad (Richmond, CA). To distinguish metalloproteinases and serine proteinases, enterokinase-treated tissue extracts were incubated with 5 mmol/L ethylenediaminetetraacetic acid or with 10 mmol/L diisopropyl fluorophosphate.
Determination of Protein Concentration
Protein concentration was determined by the dye method with a Bio-Rad protein assay kit (Bio-Rad), using bovine immunoglobulin G as the standard.
Results
Expression of Trypsin in Normal Human Tissues
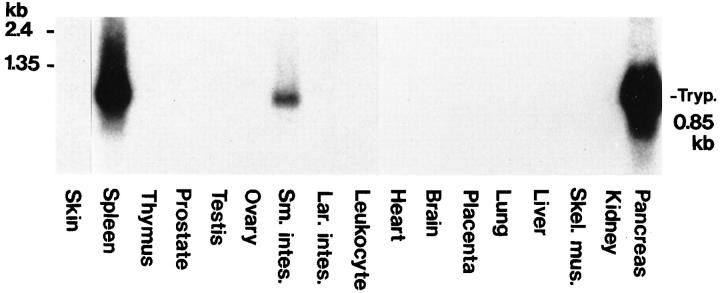
Expression of trypsin in various human normal tissues was analyzed by Northern blotting with trypsin 1 cDNA as a probe, which presumably hybridizes to trypsin 2, 3, and 4 mRNAs, as well as trypsin 1 mRNA. As shown in Figure 1 ▶ , trypsin mRNA of approximately 0.85 kb was detected strongly in the pancreas and spleen and clearly in the small intestine. Other tissues, including the T lymphocyte-producing tissue thymus, did not show the hybridization signal for the transcript.
Figure 1.
Expression of trypsin mRNA in various human tissues. Nylon membranes blotted with 2 μg of polyadenylated RNA from the indicated human tissues were hybridized with a 32P-labeled human trypsin 1 cDNA probe as described in Materials and Methods. Poly(A)+ RNA from the skin was run on a separate gel. Sm. intes., small intestine; Lar. intes., large intestine; Skel. mus., skeletal muscle; Tryp., trypsin mRNA (850 bases).
To detect relatively weak expression of trypsin genes by specific cells, trypsin mRNAs in various human tissues were analyzed by in situ hybridization with a digoxigenin-labeled trypsin 1 antisense RNA probe. This antisense RNA probe was expected to recognize the messages for trypsin 1, 2, 3, and 4 genes. When the pancreas was subjected to the in situ hybridization analysis, acinar cells showed a strong hybridization signal with the antisense probe, but they showed little signal with the sense probe (data not shown).
When the spleen was subjected to in situ hybridization, the cells in the white pulp showed a strong signal for trypsin mRNA (Figure 2A) ▶ . The hybridization signal was especially strong in larger cells, which appeared to be macrophages and monocytes. Many lymphocytes in the white pulp also showed a positive signal for trypsin mRNA. In contrast, the trypsin message was faint, if present at all, in spleen cells of the red pulp (data not shown).
Figure 2.
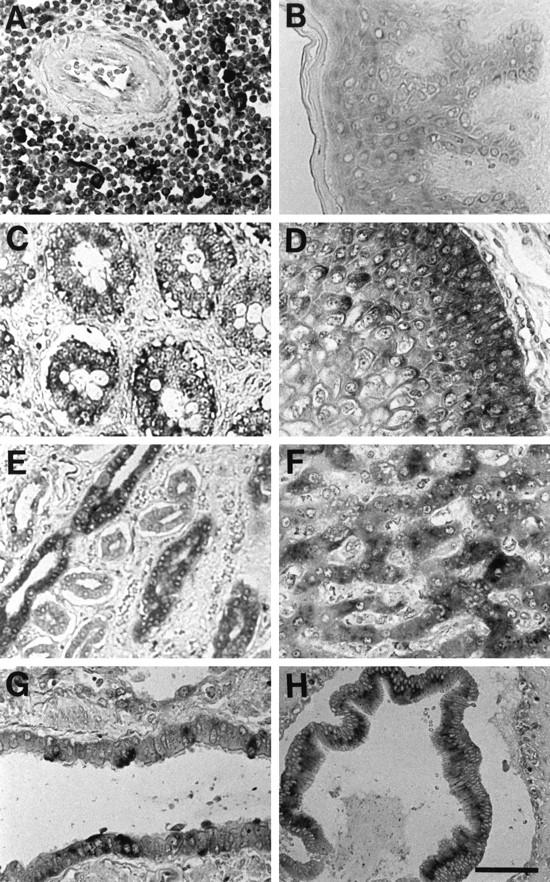
In situ hybridization analysis for localization of trypsin mRNA in various human tissues. Lymphoid nodule in the white pulp of the spleen (A), skin (B), small intestine (C), esophagus (D), collecting ducts of the kidney (E), liver (F), bronchiole of the lung (G), and extrahepatic bile duct (H). The dark staining indicates the presence of trypsin message. Experimental conditions are described in Materials and Methods. Bar, 60 μm.
Trypsin mRNA was also detected in epithelial cells of the skin, small intestine, esophagus, kidney, liver, lung, and extrahepatic bile duct (Figure 2, B to H) ▶ . Stratified squamous epithelial cells expressed trypsin message in the esophagus and skin. In the esophagus, the signal for trypsin mRNA was more intense in the basal and parabasal cells (Figure 2D) ▶ , whereas in the skin, the signal was evenly distributed from the basal cells to the stratum spinous cells (Figure 2B) ▶ . In the kidney, the epithelial cells of the collecting ducts were densely stained with the antisense probe, and those of other segments of nephron were weakly stained (Figure 2E) ▶ . In the lung, the bronchial and bronchiolar epithelial cells were focally stained for trypsin transcript (Figure 2G) ▶ . In the liver, hepatocytes clearly showed the positive signal for trypsin mRNA (Figure 2F) ▶ . Gastric epithelial cells were also positive for trypsin message, but the thyroid, testis, and cardiac muscle did not show specific reactivity to the trypsin antisense probe (data not shown).
To verify the synthesis of trypsin in the human tissues, immunohistochemical analysis with the anti-trypsin antibody was carried out (Figure 3) ▶ . The immunoreactivity to trypsin showed essentially the same distribution as that of the trypsin message detected by in situ hybridization in all of the tissues except the skin. In the skin, the trypsin antigen was more abundantly detected in the basal keratinocytes than in the stratum spinosum (Figure 3A) ▶ .
Figure 3.
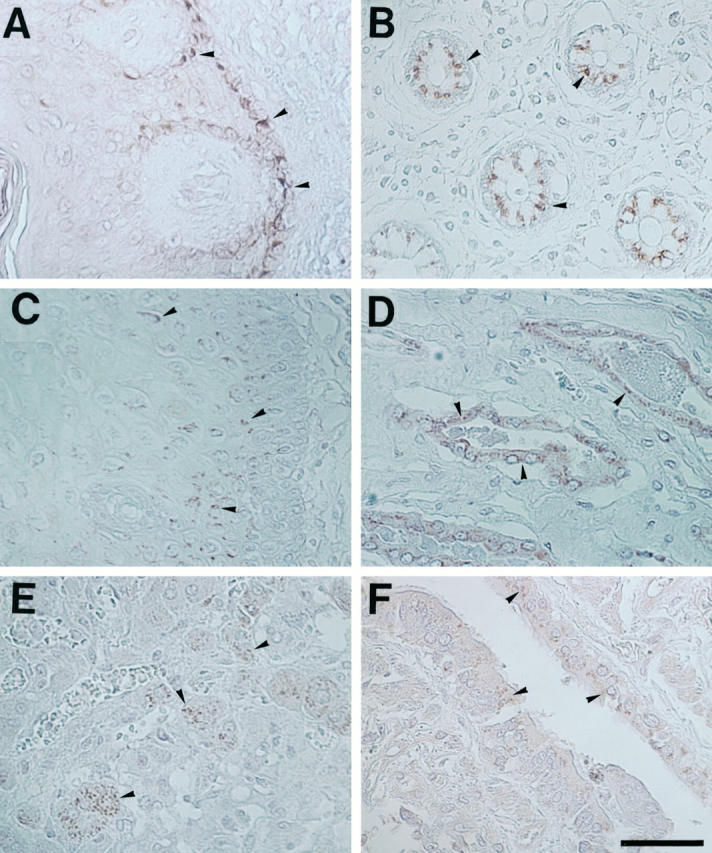
Immunohistochemical analysis for localization of trypsin protein in various human tissues. A, skin; B, small intestine; C, esophagus; D, collecting ducts of the kidney; E, liver; F, bronchiole of the lung. Arrowheads: Positive staining for trypsin protein. Experimental conditions are described in Materials and Methods. Bar, 60 μm.
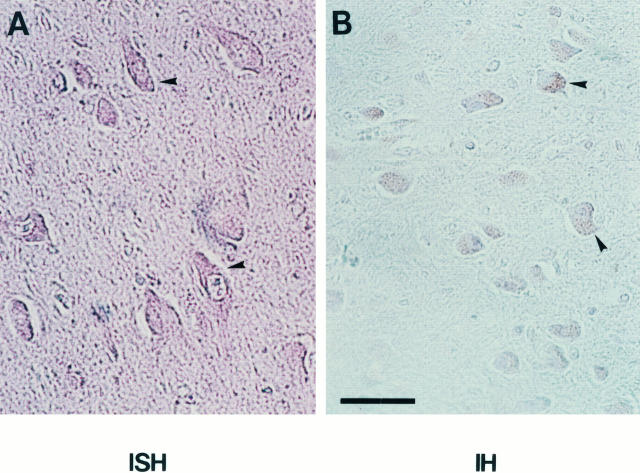
We also examined the expression of the trypsin gene and its protein product in the brain by in situ hybridization and immunohistochemistry (Figure 4) ▶ . Both trypsin message and protein were detected in nerve cells of the hippocampus and the cerebral cortex.
Figure 4.
In situ hybridization and immunohistochemistry for trypsin expression in the hippocampus. A: In situ hybridization with an antisense RNA probe for trypsin mRNA. B: Immunohistochemistry with an anti-human-trypsin monoclonal antibody. Arrowheads: Positive staining for trypsin message (A) or trypsin protein (B). Bar, 60 μm.
Zymographic Analysis of Mouse Tissue Extracts
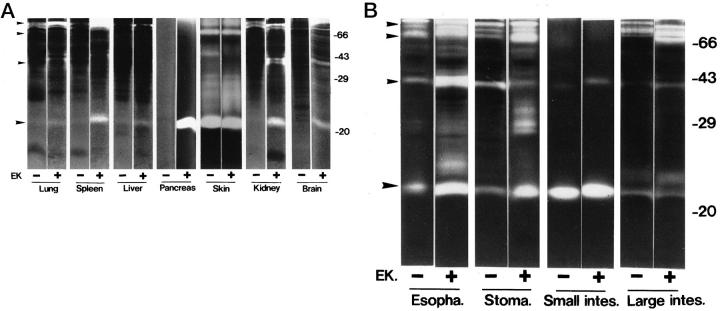
To confirm the presence of tryptic activity in nonpancreatic tissues, various tissues of normal mice were extracted and analyzed by gelatin zymography in the absence of Ca2+ before and after activation by enterokinase (Figure 5) ▶ . When pretreated with enterokinase, the pancreas showed a high gelatinolytic activity at 23 kd, which appeared to correspond to the active form of trypsin (Figure 5A) ▶ . The 23-kd gelatinolytic activity was strongly inhibited when the extract was treated with the serine proteinase inhibitor diisopropyl fluorophosphate (data not shown). Furthermore, immunoblotting analysis with a polyclonal antibody against human pancreatic trypsin confirmed that the 23-kd gelatinolytic activity was due to mouse trypsin (data not shown).
Figure 5.
Gelatinolytic activities of extracts from 11 kinds of normal mouse tissues. Extracts were prepared from the indicated tissues, incubated without (−) or with (+) enterokinase (EK), and then applied to gelatin zymography in the absence of Ca2+, as described in Materials and Methods. The protein amount loaded on the gel was approximately 20 ng for the pancreas, 200 ng for the small intestine, and 20 μg for the other tissues. B: Esopha., esophagus; Stoma., stomach; Small intes., small intestine; Large intes., large intestine. A and B: Ordinate, molecular weight in kd. Large arrowheads (bottom), gelatinolytic activity that appears to correspond to trypsin (23 kd). Small arrowheads (top three), positions of 100-, 70-, and 40-kd gelatinolytic activities.
The 23-kd trypsin activity was detected in all of the enterokinase-treated tissue extracts tested, ie, the lung, spleen, liver, skin, kidney, brain, esophagus, stomach, small intestine, and large intestine (Figure 5, A and B) ▶ . The trypsin activity was high in the pancreas, skin, small intestine, esophagus, and stomach and moderate in the brain, spleen, and kidney. Even in the absence of enterokinase, almost the same level of trypsin activity was detected in the skin and small intestine. In the esophagus, stomach, and large intestine, the trypsin activity was detected more weakly in the absence of enterokinase than in its presence. These results indicated that these tissues contained an endogenous activator of trypsinogen, most likely enterokinase. The remaining tissues showed little activity at 23 kd in the absence of enterokinase treatment, suggesting that they contained a very low level, if any, of enterokinase.
The gelatin zymography also exhibited gelatinolytic activities with approximate molecular sizes of 100, 70, and 40 kd, in addition to the 23-kd activity (Figure 5) ▶ . These activities were especially high in the enterokinase-treated extracts of the spleen, brain, esophagus, stomach, and large intestine. In other experiments with trypsin-secreting human cancer cell lines, we have found that similar gelatinolytic activities are produced by sodium dodecyl sulfate-stable complexes of active trypsin with secreted forms of Alzheimer amyloid protein precursor (or protease nexin II) and their partially degraded products (unpublished data). Alzheimer amyloid protein precursors, which contain the Kuniz-type serine proteinase inhibitor domain, are known to be secreted from various types of cultured cells. 18 Therefore, it is most likely that the gelatinolytic activities at 100, 70, and 40 kd in the tissue extracts are derived from the trypsin-Alzheimer amyloid protein precursor complexes.
PCR Analysis of Trypsin Genes Expressed in Normal Mouse Tissues
To determine trypsin species expressed in some normal tissues of mice, we carried out RT-PCR analysis for trypsin mRNAs and subsequent sequencing of the amplified DNA fragments. The RT-PCR amplified a 280-bp product from the RNAs isolated from the brain, kidney, spleen, and liver. Each DNA product was isolated, cloned into a plasmid vector, and sequenced. The sequence analyses showed that the nucleotide sequences of the amplified 280-bp products from the brain, kidney, and spleen were completely identical to that of mouse trypsin cDNA (nucleotides 208 to 492), indicating that at least these tissues express the pancreatic trypsin gene (data not shown). The sequence of the PCR product from the liver RNA was very similar but not identical to that of the trypsin cDNA.
Discussion
The present study demonstrates that trypsin is widely expressed in epithelial cells of the skin, esophagus, stomach, small intestine, large intestine, lung, kidney, liver, and extrahepatic bile duct, as well as leukocytes in the spleen and nerve cells in the brain. Trypsin potently hydrolyzes a wide variety of proteins, including food proteins and extracellular matrix proteins at the carboxyl end of the arginine and lysine residues. In addition, it effectively activates the latent forms of various serine proteinases and metalloproteinases that are involved in blood coagulation, fibrinolysis, or extracellular matrix degradation. For example, trypsin is more potent than plasmin and other serine proteinases in activating the latent forms of many MMPs, including stromelysin 19 and matrilysin. 20 Trypsin is also able to process membrane receptors such as thrombin receptor and proteinase-activated receptor 2. 21
Unexpectedly, the spleen was the highest in trypsin expression of all of the tissues except for the pancreas. Monocytes and macrophages in the white pulp of spleen showed a strong signal for trypsin transcript, whereas lymphocytes showed a weak signal. It is known that many types of leukocytes constitutively secrete gelatinase B (MMP-9) and neutrophil collagenase (MMP-8), which are effectively activated by trypsin. 22,23 Activated human B cells and B-cell lines produce a trypsin-like proteinase, 24 whereas T lymphocytes produce several types of granzymes 25 and tryptase TL2. 26 Very recently, Fukusen and Aoki 17 purified two forms of novel serine proteinases with high similarity to trypsin from mouse spleen. Our PCR analysis showed that mouse spleen expressed the pancreatic trypsin gene at a high level. It is speculated that the splenic trypsin plays some roles in the immune defense.
The small intestine and stomach expressed trypsin at a high level. Jeohn et al 16 purified a trypsin from the intracellular microsomal fraction of porcine gastric mucosa. We have previously found that gastric adenocarcinoma cells secrete trypsins 1 and 2 in latent and active forms. 11,27 Therefore, we assume that in the stomach and intestine, trypsins are secreted and function as digestive enzymes together with the pancreas-derived trypsins. Similarly, trypsin produced by various types of epithelial cells, especially of exocrine glands such as the bile duct and the nephron of the kidney, may function in the digestion of unnecessary excreted proteins as a “pipe cleaner.”
The stratified squamous epithelia in the esophagus and skin expressed trypsin at relatively high levels. In mice, the extracts from these epithelia, as well as the stomach and intestine, contained the active form of trypsin. This implies that in these tissues the secreted latent trypsin (trypsinogen) is continuously activated to the active trypsin by an endogenous activator, probably enterokinase. Recently Yahagi et al 28 have reported that enterokinase gene is expressed in the digestive tract. Proteolytic degradation of intercellular cohesive structures in the stratum corneum is required for desquamation, ie, the cell shedding at the skin surface. 29 Recently, a skin-specific novel serine proteinase called stratum corneum chymotryptic enzyme has been characterized and is expected to be involved in desquamation. 30 Stratum corneum chymotryptic enzyme has homology to trypsin, chymotrypsin, and kallikrein, and its proenzyme is effectively activated by trypsin. It seems likely that the enterokinase-activated trypsin is involved in the desquamation both by directly degrading the intercellular cohesive structures and by activating the latent forms of stratum corneum chymotryptic enzyme and MMPs. As another possibility, trypsin may be involved in the growth and terminal differentiation of epidermal keratinocytes.
The present study also demonstrated the expression of trypsin gene in human and mouse brains. Trypsin 4 cDNA, the protein product of which does not have a signal sequence, has been cloned from human brain cDNA library. 7 In addition, cDNAs for two trypsin-like enzymes expressed in the human brain, neuropsin 31 and neurosin, 32 have been cloned. Neuropsin is specifically expressed in pyramidal neurons of the hippocampus. The physiological roles of these trypsins and trypsin-related enzymes in the central nervous system seem to be among the most important subjects to be studied.
Ubiquitus expression of trypsin genes suggests their essential roles in the maintenance of various cellular functions. Furthermore, aberrant expression of tissue trypsin is likely to be involved in various pathological processes, including tumor invasion and inflammation. Further studies are required for clarifying the detailed functions of trypsin in each type of tissue and cell.
Footnotes
Address reprint requests to Dr. Kaoru Miyazaki, Division of Cell Biology, Kihara Institute for Biological Research, Yokohama City University, 641-12 Maioka-cho, Totsuka-ku, Yokohama 244, Japan. E-mail: miyazaki@yokohama-cu.ac.jp.
Supported by a Research Fellowship of the Japan Society for the Promotion of Science for Young Scientists (to NK) and Grants-in-Aid from the Uehara Memorial Foundation (to KMiy), from the Ministry of Health and Welfare (to KMiy), and from the Ministry of Education, Culture, Sports and Science of Japan (to YN and KMiy).
References
- 1.Neurath H: Evolution of proteolytic enzymes. Science 1984, 224:350-357 [DOI] [PubMed] [Google Scholar]
- 2.Stetler-Stevenson WG, Aznavoorian S, Liotta LA: Tumor cell interactions with the extracellular matrix during invasion metastasis. Annu Rev Cell Biol 1993, 9:541-573 [DOI] [PubMed] [Google Scholar]
- 3.Saksela O: Plasminogen activation and regulation of pericellular proteolysis. Biochim Biophys Acta 1985, 823:35-65 [DOI] [PubMed] [Google Scholar]
- 4.Dunn BM: Determination of protease mechanism. Beynon RJ Bond JS eds. Proteolytic Enzymes. 1989, :pp 57-81 IRL Press, Oxford, UK, [Google Scholar]
- 5.Emi M, Nakamura Y, Ogawa M, Yamamoto T, Nishide T, Mori T, Matsubara K: Cloning, characterization and nucleotide sequences of two cDNAs encoding human pancreatic trypsinogen. Gene 1986, 41:305-310 [DOI] [PubMed] [Google Scholar]
- 6.Tani T, Kawashima I, Mita K, Takiguchi Y: Nucleotide sequence of the human pancreatic trypsinogen III cDNA. Nucleic Acids Res 1990, 18:1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiegand U, Corbach S, Minn A, Kang J, Muller-Hill B: Cloning of the cDNA encoding human brain trypsinogen and characterization of its product. Gene 1993, 136:167-175 [DOI] [PubMed] [Google Scholar]
- 8.Sheele G, Bieger W: Characterization of human exocrine pancreatic proteins by two-dimensional isoelectric focusing/sodium dodecyl sulfate gel electrophoresis. Gastroenterology 1981, 80:461-473 [PubMed] [Google Scholar]
- 9.Stevenson BJ, Hagenbuechle O, Wellauer PK: Sequence organization and transcriptional regulation of the mouse elastase II and trypsin genes. Nucleic Acids Res 1986, 14:8307-8330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koshikawa N, Yasumitsu H, Umeda M, Miyazaki K: Multiple secretion of matrix serine proteinases by human gastric carcinoma cell lines. Cancer Res 1992, 52:5046-5053 [PubMed] [Google Scholar]
- 11.Kato Y, Koshikawa N, Saito S, Nagashima Y, Miyagi Y, Yasumitsu H, Miyazaki K: Production of trypsins by human gastric cancer cells correlates with their malignant phenotype. Eur J Cancer 1998, 34:1117-1123 [DOI] [PubMed] [Google Scholar]
- 12.Hirahara F, Miyagi Y, Miyagi E, Yasumitsu H, Koshikawa N, Nagashima Y, Kitamura H, Minaguchi H, Umeda M, Miyazaki K: Trypsinogen expression in human ovarian carcinomas. Int J Cancer 1995, 63:176-181 [DOI] [PubMed] [Google Scholar]
- 13.Kawano N, Osawa H, Ito T, Nagashima Y, Hirahara F, Inayama Y, Nakatani Y, Kimura S, Kitajima H, Koshikawa N, Miyazaki K, Kitamura H: Expression of gelatinase A, tissue inhibitor of metalloproteinases-2 (TIMP-2), matrilysin, and trypsin(ogen) in lung neoplasms: an immunohistochemistry study. Hum Pathol 1997, 28:613-622 [DOI] [PubMed] [Google Scholar]
- 14.Koivunen E, Saksela O, Itkonen O, Osman S, Huhtala M-L, Stenman U-H: Human colon carcinoma, fibrosarcoma and leukemia cell lines produce tumor-associated trypsinogen. Int J Cancer 1991, 47:592-596 [DOI] [PubMed] [Google Scholar]
- 15.Koshikawa N, Nagashima Y, Miyagi Y, Yanoma S, Yasumitsu H, Miyazaki K: Expression of trypsin in vascular endothelial cells. FEBS Lett 1997, 409:442-448 [DOI] [PubMed] [Google Scholar]
- 16.Jeohn G-H, Serizawa S, Iwamatsu A, Takahashi K: Isolation and characterization of gastric trypsin from the microsomal fraction of porcine gastric antral mucosa. J Biol Chem 1995, 270:14748-14755 [DOI] [PubMed] [Google Scholar]
- 17.Fukusen N, Aoki Y: Purification and characterization of novel trypsin-like serine proteases from mouse spleen. J Biochem 1996, 119:633-638 [DOI] [PubMed] [Google Scholar]
- 18.Koshikawa N, Nakamura T, Tsuchiya N, Isaji M, Yasumitsu H, Umeda M, Miyazaki K: Purification and identification of a novel and four known serine proteinase inhibitors secreted by human glioblastoma cells. J Biochem 1996, 119:334-339 [DOI] [PubMed] [Google Scholar]
- 19.Umenishi F, Yasumitsu H, Ashida Y, Yamauti J, Umeda M, Miyazaki K: Purification and properties of extracellular matrix-degrading metallo-proteinase overproduced by Rous sarcoma virus-transformed rat liver cell line, and its identification as transin. J Biochem 1990, 108:537-543 [DOI] [PubMed] [Google Scholar]
- 20.Imai K, Yokohama Y, Nakanishi I, Ohuchi E, Fujii Y, Nakai N, Okada Y: Matrix metalloproteinase 7 (matrilysin) from human rectal carcinoma cells: activation of the precursor, interaction with other matrix metalloproteinases and enzymic properties. J Biol Chem 1995, 270:6691-6697 [DOI] [PubMed] [Google Scholar]
- 21.Nystedt S, Emilsson K, Wahlestedt C, Sundelin J: Molecular cloning of a potential proteinase activated receptor. Proc Natl Acad Sci USA 1994, 91:9208-9212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devarajan P, Johnston JJ, Ginsberg SS, van-Wart HE, Berliner N: Structure and expression of neutrophil gelatinase cDNA: identity with type IV collagenase from HT 1080 cells. J Biol Chem 1992, 267:25228-25232 [PubMed] [Google Scholar]
- 23.Hasty KA, Pourmotabbed TF, Goldberg GI, Thompson JP, Spinella DG, Stevens RM, Mainardi CL: Human neutrophil collagenase. J Biol Chem 1990, 265:11421-11424 [PubMed] [Google Scholar]
- 24.Biro A, Sarmay G, Rozsnyay Z, Klein E, Gergely J: A trypsin-like serine proteinase activity on activated human B cells, and various B cell lines. Eur J Immunol 1992, 22:2547-2553 [DOI] [PubMed] [Google Scholar]
- 25.Masson D, Tschopp J: A family of serine esterases in lytic granules of cytolytic T lymphocytes. Cell 1987, 49:679-685 [DOI] [PubMed] [Google Scholar]
- 26.Matsushita K, Taguchi M, Kovacs EJ, Young HA, Oppenheim JJ: Intracellular localization of human monocyte associated interleukin 1 (IL 1) activity and release of biologically active IL 1 from monocytes by trypsin and plasmin. J Immunol 1986, 136:2883-2891 [PubMed] [Google Scholar]
- 27.Koshikawa K, Nagashima Y, Yasumitsu H, Umeda M, Miyazaki K: Identification of single-chain and two-chain forms of trypsinogen 1 produced by a human gastric adenocarcinoma cell line. Biochem J 1994, 303:187-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yahagi Y, Ichinose M, Matsushima M, Matubara Y, Miki K, Kurokawa K, Fukamachi H, Tashiro K, Shiokawa K, Kageyama T, Takahashi T, Inoue H, Takahashi K: Complementary DNA cloning, and sequencing of rat enteropeptidase, and tissue distribution of its mRNA. Biochem Biophys Res Commun 1996, 219:806-812 [DOI] [PubMed] [Google Scholar]
- 29.Lundstrom A, Egelrud T: Cell shedding from human plantar skin in vitro: evidence of its dependence on endogenous proteolysis. J Invest Dermatol 1988, 81:340-343 [DOI] [PubMed] [Google Scholar]
- 30.Hansson L, Stromqvist M, Backman A, Wallbrandt P, Carlstein A, Egelrud T: Cloning, expression and characterization of stratum corneum chymotryptic enzyme. J Biol Chem 1994, 269:19420-19426 [PubMed] [Google Scholar]
- 31.Chen Z-L, Yoshida S, Kato K, Momota Y, Suzuki J, Tanaka T, Ito J, Nishino H, Aimoto S, Kiyama H, Shiosaki S: Expression and activity-dependent changes of a novel limbic-serine protease gene in the hippocampus. J Neurosci 1995, 15:5088-5097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamashiro K, Tsuruoka N, Kodama S, Tsujimoto M, Yamamura Y, Tanaka T, Nakazato H, Yamaguchi: Molecular cloning of a novel trypsin-like serine proteinase (neurosin) preferentially expressed in brain. Biochim Biophys Acta 1997, 1350:11-14 [DOI] [PubMed] [Google Scholar]