Abstract
The t(2;5)(p23;q35) translocation associated with CD30-positive anaplastic large cell lymphoma results in the production of a NPM-ALK chimeric protein, consisting of the N-terminal portion of the NPM protein joined to the entire cytoplasmic domain of the neural receptor tyrosine kinase ALK. The ALK gene products were identified in paraffin sections by using a new anti-ALK (cytoplasmic portion) monoclonal antibody (ALKc) that tends to react more strongly than a previously described ALK1 antibody with the nuclei of ALK-expressing tumor cells after microwave heating in 1 mmol/L ethylenediaminetetraacetic acid buffer, pH 8.0. The ALKc monoclonal antibody reacted selectively with 60% of anaplastic large cell lymphoma cases (60 of 100), which occurred mainly in the first three decades of life and consistently displayed a T/null phenotype. This group of ALK-positive tumors showed a wide morphological spectrum including cases with features of anaplastic large cell lymphoma “common” type (75%), “lymphohistiocytic” (10%), “small cell” (8.3%), “giant cell” (3.3%), and “Hodgkin’s like” (3.3%). CD30-positive large anaplastic cells expressing the ALK protein both in the cytoplasm and nucleus represented the dominant tumor population in the common, Hodgkin’s-like and giant cell types, but they were present at a smaller percentage (often with a perivascular distribution) also in cases with lymphohistiocytic and small cell features. In this study, the ALKc antibody also allowed us to identify small neoplastic cells (usually CD30 negative) with nucleus-restricted ALK positivity that were, by definition, more evident in the small cell variant but were also found in cases with lymphohistiocytic, common, and “Hodgkin’s-like” features. These findings, which have not been previously emphasized, strongly suggest that the neoplastic lesion (the NPM-ALK gene) must be present both in the large anaplastic and small tumor cells, and that ALK-positive lymphomas lie on a spectrum, their position being defined by the ratio of small to large neoplastic cells. Notably, about 15% of all ALK-positive lymphomas (usually of the common or giant cell variant) showed a cytoplasm-restricted ALK positivity, which suggests that the ALK gene may have fused with a partner(s) other than NPM. From a diagnostic point of view, detection of the ALK protein was useful in distinguishing anaplastic large cell lymphoma cases of lymphohistiocytic and small cell variants from reactive conditions and other peripheral T-cell lymphoma subtypes, as well as for detecting a small number of tumor cells in lymphohemopoietic tissues. In conclusion, ALK positivity appears to define a clinicopathological entity with a T/null phenotype (“ALK lymphomas”), but one that shows a wider spectrum of morphological patterns than has been appreciated in the past.
Anaplastic large cell lymphoma (ALCL) was first described by Stein et al 1 as a tumor with distinctive histological and immunohistological features, eg, preferential paracortical and intrasinusoidal lymph node involvement by sheets of large anaplastic cells. The tumor cells express the Ki-1 antigen, 1 a molecule that was later renamed CD30 and shown to be a receptor for CD30L, a member of the tumor necrosis factor ligand family. 2 The tumor, which represents about 5 to 10% of non-Hodgkin lymphomas in adults and 30 to 40% of large-cell lymphomas in children, is a highly aggressive lymphoma that usually presents as stage III to IV disease frequently associated with systemic symptoms and extranodal involvement, especially skin and bone. 3 However, despite its aggressive features, the disease can be cured in a high percentage of cases. 3,4
ALCL is associated with a t(2;5) chromosomal translocation 5 that fuses the ALK (anaplastic lymphoma kinase) and the NPM (nucleophosmin) genes, 6,7 leading to the formation of a chimeric NPM-ALK protein (p80) 6-8 consisting of the N-terminal portion of NPM 9 linked to the cytoplasmic domain of the neural receptor tyrosine kinase ALK. 10,11 The chimeric NPM-ALK protein is thought to play a key role in lymphomagenesis by aberrant phosphorylation of intracellular substrates. 12,13
These discoveries allowed the development of reverse transcription (RT)-polymerase chain reaction (PCR) assays for the detection of NPM-ALK transcripts 14 and the generation of polyclonal 15-17 and monoclonal antibodies (mAbs) 18 directed against the cytoplasmic portion of the ALK molecule. Extensive studies have demonstrated the presence of NPM-ALK gene and/or its protein product in cases of ALCL. The percentage of NPM-ALK-positive cases in different studies has varied between 30% and 60%, but a picture has emerged of a tumor that consistently presents with primary, systemic disease, shows a T/null phenotype, and usually occurs in the first three decades of life. 16,19-28
A poorly investigated issue in this field concerns the correlation between histological features of ALCL and NPM-ALK protein expression. After the first description of ALCL by Stein et al in 1985, 1 it became evident that neither anaplastic morphology nor CD30 expression could be regarded as absolute defining criteria for ALCL. Several pathologists reported morphological variants (eg, “common type,” “lymphohistiocytic,” “small cell,” “neutrophil-rich,” “sarcomatoid,” or “Hodgkin’s like”), 29-36 which shared the same basic architectural features of ALCL, but which differed in terms of tumor cell cytology and the admixture of inflammatory cells. This raised the question whether the heterogeneous morphological features of the ALCL represent different clinicopathological entities or are just variants of a single disease.
In this paper, we have addressed this point by immunohistological labeling of a series of 100 cases of ALCL for ALK protein expression using a new mAb (ALKc) and also a previously reported anti-ALK antibody ALK1. 18 We also investigated the nature of the small atypical cells that represent the predominant neoplastic population in the so-called lymphohistiocytic 31 and small-cell variants 32 of ALCL, an issue that has not been addressed in previous studies.
Materials and Methods
Generation of Recombinant NPM-ALK Fusion Protein
An NPM-ALK cDNA fragment corresponding to the whole open reading frame of the NPM-ALK protein was generated by PCR using oligonucleotide primers spanning the NPM ATG and the ALK TGA triplets. The PCR product was cloned into the pCRII vector of the TA cloning system (Invitrogen, San Diego, CA), checked by sequencing, and subcloned in PGEX-4T-1 (Pharmacia Biotech, Piscataway, NJ) to produce a glutathione S-transferase NPM-ALK full-length fusion protein. The protein was expressed in the HB101 Escherichia coli strain and purified by affinity chromatography following the manufacturer’s instructions.
Production of the ALKc mAb
A fusion between the spleen cells of BALB/c mice previously immunized intraperitoneally with 150-μg aliquots of recombinant protein and the NS-1 myeloma cell line was carried out, as described previously. 37 Hybridoma supernatants were screened by the immunoalkaline phosphatase (alkaline phosphatase-antialkaline phosphatase, APAAP) technique 38 in cytocentrifuge preparations of a human cell line (Karpas 299) that carries the t(2;5). 39 Selected hybridomas were cloned by limiting dilution. Five hybridoma supernatants out of approximately 1000 tested showed strong immunocytochemical staining of the Karpas 299 cell line but were unreactive on cryostat sections of normal human tonsil. Further testing on paraffin sections of ALCL bearing the (2;5) translocation showed that one of the supernatants reacted strongly with tumor cells but did not stain normal cells. The hybridoma was cloned to produce the ALKc clone used in subsequent studies.
Other Antibodies
The mAb ALK1 raised against a fragment (amino acids 419 to 520) of the cytoplasmic portion of ALK protein has been described previously. 18 Immunophenotyping of ALCL in paraffin sections was performed with antibodies directed against the following antigens: CD45, CD45RO, CD3, and CD20 (all obtained from DAKO A/S, Glostrup, Denmark); CD30/Ber-H2 (kindly provided by Prof. H Stein, Free University of Berlin, Berlin, Germany); and CD45RA, CD68, CD79a, and PML proteins (generated in the investigators’ laboratories).
Expression of the NPM-ALK Protein in HeLa Cells
NPM-ALK cDNA corresponding to the whole open reading frame of the protein was subcloned in the pcDNA3 expression vector (Invitrogen) and used for transient transfection of HeLa cells by the calcium chloride-HEPES-buffered-saline (HBS) method. 40 As negative control, HeLa cells were transfected in parallel with the plasmid vector containing no insert.
Western Blotting
Western blotting of cell lysates of the human cell lines U937, Karpas 299, Daudi, and Rh30 rhabdomyosarcoma 6 was performed as previously described 18 using mAbs ALKc and ALK1 (diluted 1:5).
Enzyme-Linked Immunosorbent Assay
The reactivity of the antibodies ALKc and ALK1 or the negative control reagent MR12 (mouse anti-rabbit MR12, prepared in the laboratory of Mason et al) 18 was tested against DHFR-ALK (a recombinant protein containing amino acids 1359 to 1460 of the full-length ALK receptor protein used to raise the antibody ALK1), by using a previously described enzyme-linked immunosorbent assay technique. 18
Cell Lines
A variety of human cell lines of different origin, MOLT-4, Jurkat, and Peer (T cell); Daudi, Nalm 12, and Cess (B cell); L-428 and L540 (Hodgkin’s); Karpas 299, JB6, Su-DHL1 (ALCL bearing t(2;5)); K-562 (erythroid); U937, HL60, KG1, and NB4 (myeloid); and HeLa (carcinoma), were maintained in culture in RPMI 1640 containing 10% fetal calf serum (Life Technologies, Inc., Grand Island, NY). Cytospins were prepared from exponentially growing cells, fixed in acetone for 10 minutes at room temperature, and then used for immunocytochemical studies.
Tissue Processing for Immunohistochemistry
Paraffin-embedded tissue samples had been fixed either in 10% buffered formalin for 24 hours to 1 week (most cases) or in Brasil-Dubosq or B5 for 2 hours (a minority of cases). Paraffin sections on silane-coated slides were rehydrated and subjected to microwaving (750 W for three cycles of 5 minutes each) using either 0.01 mol/L citrate buffer, pH 6.0, 41 or 1-mmol/L ethylenediaminetetraacetic acid buffer, pH 8.0, 42 as antigen retrieval solution. After microwave heating, sections were allowed to cool at room temperature for approximately 20 minutes, washed with Tris-buffered saline, and immunostained.
Frozen sections from snap frozen samples (when available) were air dried overnight and fixed in acetone for 10 minutes.
Normal Human Tissues
Normal lymphohemopoietic tissues comprised tonsil (n = 10), spleen (n = 5), bone marrow (n = 5), and thymus (n = 3). Samples representative of all extrahemopoietic tissues were also investigated. All tissues were diagnostic biopsies or were obtained at the time of autopsy.
Reactive and Neoplastic Lymphoid Samples
The following nonneoplastic conditions were studied: follicular hyperplasia (n = 10), toxoplasmic lymphadenitis (n = 5), tubercular lymphadenitis (n = 2), Kikuchi’s lymphadenitis (n = 5), sarcoidosis (n = 3), and reactive T-immunoblastic proliferations (n = 2).
A total of 510 cases of lymphoid neoplasms that included 100 cases of ALCL and 40 cases of acute and chronic myeloid disorders were retrieved from the authors’ institutions. Lymphomas and leukemias were categorized according to the REAL 43 and FAB 44 classifications. In all cases, diagnosis was based on morphological examination of conventionally stained tissue sections supplemented by immunophenotyping. Diagnostic immunomorphological criteria for ALCL were those originally established by Stein et al, 1 and an attempt was made to assign each case to one of the following morphological subtypes of ALCL: common, lymphohistiocytic, small cell, giant cell, or Hodgkin’s-like, according to previously defined criteria. 29,31,32,36,43 The assessment was made independently by two investigators (BF and SAP). All cases were reviewed for the second time after immunostaining for the ALK protein, and controversial cases were discussed to reach a consensus.
Nonhemopoietic Tumors
The following nonhemopoietic tumors were investigated: 50 carcinomas from various sites, 10 melanomas, 50 soft tissue tumors of different types (including 35 cases of rhabdomyosarcomas), and 25 tumors of the central nervous system.
Immunoenzymatic Labeling
All sections were stained by the immunoalkaline phosphatase (APAAP) technique, as previously described. 38 Briefly, paraffin sections were incubated with the primary mAbs, followed by rabbit anti-mouse immunoglobulin (Dako, Glostrup, Denmark) and APAAP complexes. To maximize the sensitivity of the method, steps 2 and 3 were repeated once each. All antibody steps were for 30 minutes with intervening 5-minute washes in 0.05 mol/L Tris-buffered saline, pH 7.6. Endogenous alkaline phosphatase was blocked with 1 mmol/L levamisole. 45 Slides were then counterstained for 5 minutes in Gill’s hematoxylin and mounted in Kaiser’s glycerol gelatin (Merck, Darmstadt, Germany).
RT-PCR Analysis
RT-PCR studies were performed as previously described. 24
Results
ALKc Reacts with the Intracytoplasmic Region of ALK
Western blotting of the t(2;5)-positive Karpas 299 cell line using antibody ALKc revealed an 80-kd immunoreactive polypeptide that corresponded in size to the NPM-ALK fusion protein (Figure 1) ▶ . ALKc also detected the full-length 200 kd ALK protein present in the rhabdomyosarcoma cell line Rh30 (Figure 1) ▶ . Identical bands, although at lower intensity, were detected by the ALK1 antibody (not shown). No bands were detected by the ALKc mAb in lysates of the U937 and Daudi cell lines that served as negative controls (Figure 1) ▶ .
Figure 1.
Western blotting. In lysates of the Karpas 299 cell line, the ALKc mAb identifies a band of 80 kd, corresponding in size to the full-length NPM-ALK fusion protein. ALKc also detects a band of 200 kd, (corresponding to the full-length ALK protein) in lysates of the rhabdomyosarcoma cell line Rh30. No bands are detected in the negative controls (lysates of the U937 and Daudi cell lines).
Antibody ALKc gave strong diffuse cytoplasmic positivity and also labeling of nucleoli (Figure 2, A and B) ▶ in ALCL cell lines bearing t(2;5) (Karpas 299, JB6 and SuDHL1). No other human cell lines were labeled by the ALKc mAb. As expected (due to expression of full-length ALK), ALKc gave strong diffuse cytoplasmic positivity in the absence of nuclear labeling (Figure 2C) ▶ in a rhabdomyosarcoma sample that had been previously shown to react with the ALK1 antibody. Staining of the same biopsy with the ALK1 and ALKc antibodies at high dilution showed expression of the ALK protein even at the level of cell membrane (Figure 2D) ▶ .
Figure 2.
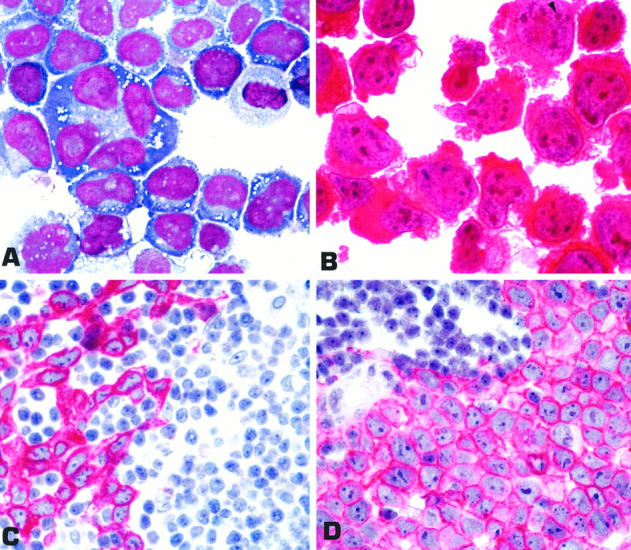
A: Karpas 299 ALCL cell line bearing the t (2;5) translocation (cytospin; May-Grunwald-Giemsa; magnification, ×1000). B: Strong diffuse cytoplasmic positivity and nucleolar labeling (arrowhead) for the ALK protein (immunostaining with ALKc; APAAP technique; hematoxylin counterstain; ×1000). C: Lymph node metastatic involvement by rhabdomyosarcoma (paraffin section stained with ALKc). Tumor cells show strong diffuse cytoplasmic positivity, whereas nuclei are negative. No labeling for ALK is observed in the residual lymphoid tissue. D: At a higher dilution of the antibody, cell membrane expression of the protein becomes more evident. C and D: APAAP technique; hematoxylin counterstain; ×800).
ALK Protein Expression in Normal Lymphohemopoietic Tissues
ALK protein was not detected with antibody ALKc in any of the normal and reactive lymphohemopoietic tissues tested on cryostat and paraffin sections. The only reactivity in normal tissues was observed in the brain (weak positivity of a few neural cells).
ALK Protein Expression in Human Lymphomas
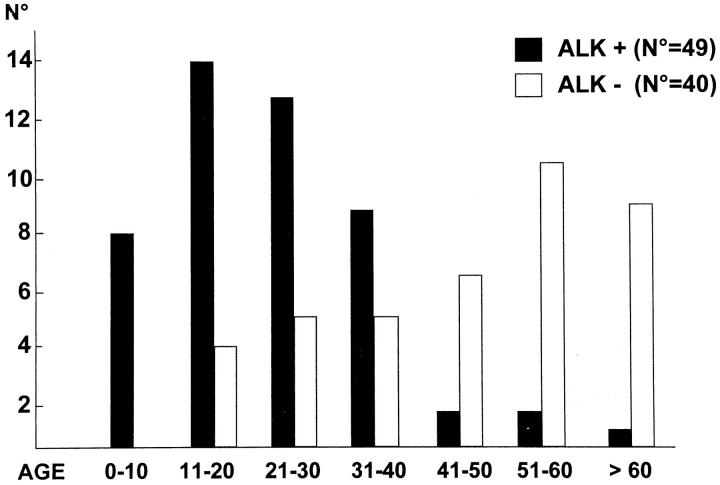
Paraffin sections from 510 cases of lymphomas representative of the different categories in the REAL classification, 43 were analyzed for expression of ALK protein(s) (Table 1) ▶ . Reactivity was restricted to approximately 60% of ALCL (all of T or null phenotype) (Table 1) ▶ . These ALK-positive lymphoma cases usually presented in the first three decades of life, whereas ALK-negative ALCL cases were most frequent in older patients with a plateau in the sixth decade (Figure 3) ▶ . The lower incidence of ALK-positive cases in the first decade as compared to the second and third ones (Figure 3) ▶ is a bias due to the limited number of pediatric patients (less than 14 years old) who are usually referred to the institutions participating to this study.
Table 1.
Expression of ALK Protein(s) in Human Lymphohemopoietic Neoplasms
| Lymphoma*/Leukemia | Number of cases (N = 550) | ALK-positive tumor cells |
|---|---|---|
| Non-Hodgkin’s lymphomas | ||
| B cell derived | ||
| Pre-B-cell acute lymphoblastic leukemia | 20 | 0 /20 |
| Small lymphocytic/B-cell chronic lymphocytic leukemia | 45 | 0 /45 |
| Hairy cell leukemia | 15 | 0 /15 |
| Mantle cell lymphoma | 22 | 0 /22 |
| Marginal zone lymphoma | 15 | 0 /15 |
| Follicle center lymphoma | 56 | 0 /56 |
| Diffuse large cell lymphoma | 100 | 0 /100 |
| Burkitt’s lymphoma | 13 | 0 /13 |
| T cell derived | ||
| Acute lymphoblastic leukemia | 8 | 0 /8 |
| Mycosis fungoides | 10 | 0 /10 |
| Peripheral T-cell lymphoma | 30 | 0 /30 |
| Anaplastic large cell lymphoma | 100 | 60 /100 |
| Hodgkin’s disease | ||
| Nodular, lymphocyte predominance | 10 | 0 /10 |
| Nodular sclerosis | 45 | 0 /45 |
| Mixed cellularity | 21 | 0 /21 |
| Chronic and acute myeloid disorders | ||
| Chronic myeloid leukemia | 10 | 0 /10 |
| Idiopathic myelofibrosis | 5 | 0 /5 |
| Polycythemia rubra vera | 5 | 0 /5 |
| Acute myeloid leukemias† | 20 | 0 /20 |
All staining results were obtained with the ALKc mAb. ALCLs were stained in parallel with the ALKc and ALK1 antibodies.
*Categorized according to the REAL classification. 43
†M1 (n = 2), M2 (n = 6), M3 (n = 5), M4 (n = 5), M5 (n = 2).
Figure 3.
Age distribution (years) of ALK-positive and ALK-negative anaplastic large cell lymphomas.
The ALK protein was usually detectable in all neoplastic cells, but in a few cases occasional large clearly neoplastic cells failed to express ALK. A correlation between ALK expression pattern and cell tumor size was observed, in that nuclear plus cytoplasmic (the most frequent pattern) or cytoplasm-restricted ALK positivity (15% of cases) was usually observed in the large anaplastic cells, whereas small neoplastic cells mostly showed purely nuclear labeling. ALK expression in the nucleus was sometimes associated with nucleoli, but, more frequently, was of diffuse type. This is likely to be an artifact, eg, diffusion of the antigen from nucleoli to nucleoplasm related to fixation and/or embedding procedures. In fact, this finding can be observed also with other nuclear-located proteins, eg, PML, which changes artifactually its nuclear speckled pattern (due to localization of PML to nuclear bodies) into a nuclear diffuse pattern in routinely fixed paraffin-embedded samples. 46 ALK nuclear labeling was significantly stronger when microwave antigen retrieval was performed with ethylenediaminetetraacetic acid buffer than with citrate buffer.
About 75% of the ALK-positive cases showed the morphology of the classic or common type (Table 2) ▶ characterized by a proliferation of large, bizarre cells (Figure 4A) ▶ , which tended to invade lymph node sinuses and infiltrate the paracortex, sometimes in a pseudocohesive pattern. In most cases, the large cells expressed the ALK protein strongly both in the cytoplasm and in the nucleus (Figure 4B) ▶ , a small proportion of large cells showing nucleus-restricted ALK positivity being rarely observed (Figure 4C) ▶ . In about one-third of these cases, a percentage of small cells (range, 5 to 30%) that showed nucleus-restricted reactivity for the ALK protein (Figure 4D) ▶ was also present. This population of cells was usually not recognized on conventionally stained paraffin sections. About 15% of all ALK-positive cases showed restricted expression of the ALK protein in the cytoplasm of large neoplastic cells (Figure 4E) ▶ . With the exception of one case of giant cell type (see below), all samples showed a “common” type morphology and were devoid of ALK-positive small cells.
Table 2.
Morphological Features of 60 ALK-Positive Lymphomas
| Morphological appearance | Number ALK+/tested | Tumor cells; Pattern of ALK positivity |
|---|---|---|
| Common | 45 /68 | 23/45; all c+ n* |
| 15/45; many c+ n,† few n‡ | ||
| 7/45; all c* | ||
| Lymphohistiocytic | 6 /6 | 6/6; few c+ n,† many n‡ |
| Small cell | 5 /5 | 5/5; few c+ n,† many n‡ |
| Giant cell | 2 /6 | 1/2; all c+ n* |
| 1/2; all c* | ||
| Hodgkin’s like | 2 /15 | 1/2; all c+ n* |
| 1/2; many c+ n,† many n‡ | ||
| Total | 60 /100 |
c, cytoplasmic; n, nuclear.
*Only large anaplastic cells (no small tumor cells were observed in these cases).
†Mostly large anaplastic cells.
‡Mostly small tumor cells.
Figure 4.

A: ALCL, common type (lymph node paraffin section; Giemsa; magnification, ×800). B to D: Various expression patterns of the ALK protein in ALCL of the common type are shown. B: Large anaplastic cells with strong cytoplasmic and nucleolar ALK positivity in the absence of ALK-positive small cells. C: Large anaplastic cells showing ALK expression in the nucleus and cytoplasm (large arrow) or only in the nucleus (small arrow) admixed with a few small cells with nucleus-restricted ALK positivity (arrowhead), * Mitotic figure. D: Mixture of large anaplastic cells (ALK positive in the cytoplasm and nucleus) (large arrows) and small cells with irregular nuclei showing nucleus-restricted ALK expression (small arrows). E and F: Two ALCL cases, one of common type E: and the other with giant cell morphology F: showing strong diffuse ALK positivity confined to the cytoplasm. A part of the cytoplasm, probably corresponding to the Golgi area, appears unlabeled. The arrow in F points to a giant multinucleated cell. B to F: lymph node paraffin sections immunostained with ALKc antibody; APAAP technique; B to D, ×800; E and F, ×1000).
Two of the 60 ALK-positive cases were of giant cell morphology; in one of them, ALK labeling was both nuclear and cytoplasmic, and in the other was restricted to the cytoplasm (Figure 4F) ▶ .
Six of the 60 ALK-positive cases displayed the histological features of the so-called lymphohistiocytic variant of ALCL, as first described by Pileri et al 31 (Figure 5A) ▶ . In the majority of these cases, clustered or isolated CD30-positive large anaplastic cells displayed both cytoplasmic and nuclear ALK positivity and were accompanied by a variable percentage of small cells showing nucleus-restricted ALK positivity (Figure 5B) ▶ . These small cells showed weak or absent CD30 staining (not shown). In some samples, ALK-positive small cells were morphologically indistinguishable from reactive lymphocytes, having scant cytoplasm and round nuclei (Figure 5, A and B) ▶ , and ALK labeling was the only way they could be recognized, especially when present at a low percentage in the paracortex of the lymph node.
Figure 5.

A: ALCL, lymphohistiocytic. Small lymphoid cells with round nuclei (small arrows) are admixed with reactive histiocytes (arrowheads) and scattered large anaplastic cells (large arrow) (lymph node paraffin section; hematoxylin and eosin; magnification, ×800). B: The large cells are ALK positive in the cytoplasm and nucleus (large arrow), whereas the small round cells show nucleus-restricted ALK positivity (small arrow) (×800). C: ALCL, small-cell variant. Tumor cells show irregular nuclei and “water-clear” cytoplasm (arrow) (lymph node paraffin section; H&E; ×800). D: Same case as in C showing small tumor cells with irregular nuclei and nucleus-restricted ALK positivity (arrows) (×800). E: Another case of ALCL with small-cell morphology. Many small tumor cells are admixed with a few large anaplastic cells arranged around vessels (perivascular pattern). * Lumen of the vessel (lymph node paraffin section; H&E; ×300). F: Same case as in E. The small tumor cells show strong nuclear ALK positivity and faint ALK expression in the cytoplasm. The large anaplastic cells around the vessels are strongly ALK positive both in the cytoplasm and nucleus. *, Lumen of the vessel (×600). B, D, and F: Lymph node paraffin sections immunostained with ALKc antibody; APAAP technique; hematoxylin counterstain.
Five of the 60 ALK-positive lymphomas in this series showed features of the small cell variant of ALCL. 32 Four of five had concomitant lymph node and skin involvement. Small to medium-sized tumor cells with irregular nuclei (Figure 5C) ▶ were admixed with small numbers of large anaplastic tumor cells, which tended to localize around blood vessels (Figure 5E) ▶ . The small neoplastic cells usually showed a nucleus-restricted ALK positivity (Figure 5D) ▶ , occasionally associated with a faint cytoplasmic labeling (Figure 5F) ▶ , which never attained the intensity of the cytoplasmic positivity observed in the scattered large cells located around the blood vessels (Figure 5F) ▶ .
Two ALK-positive lymphomas showed Hodgkin’s-like features, eg, bands of sclerosis dividing the lymph node parenchyma into nodules that contained tumor cells with a “lacuna-like” appearance. One of the cases was characterized by common type cytology, whereas the other showed the presence of two tumor cell populations segregated in different areas of the lymph node (Figure 6, A and B) ▶ , ie, large lacunar-like cells (ALK positive both in the cytoplasm and nucleus) within the nodules and small-sized elements with nucleus-restricted ALK positivity encased within the fibrous bands surrounding the nodules, which were regarded as reactive lymphocytes at conventional morphology. Minimal bone marrow involvement in this case could be documented only by ALK staining (Figure 6, C and D) ▶ .
Figure 6.

A: ALCL, Hodgkin’s-like variant (lymph node paraffin section; H&E; magnification, ×250). Notice a large neoplastic nodule (*) composed of anaplastic cells with lacunar appearance (inset, top right; ×800) that is surrounded by strands of fibrous tissue. Encased in the fibrous tissue are many small cells with irregular nuclei (inset, bottom left; ×500). B: APAAP immunostaining with ALKc (hematoxylin counterstain; ×250). Large anaplastic cells in the nodule (*) express the ALK protein in both the cytoplasm and the nucleus (inset, top right; ×800), whereas the small elements show nucleus-restricted ALK expression (inset, bottom left ; ×800). C and D: Same case as in A shows minimal bone marrow involvement by small cells with nucleus-restricted ALK positivity (C, arrow) and large cells (a tumor cell in mitosis strongly positive in the cytoplasm is pointed in D). C and D: Bone marrow trephine biopsy stained with ALKc; hematoxylin counterstain; ×800.
Forty percent of all ALCL cases were ALK-negative. A wide morphological spectrum was also seen in these tumors, the common type being the most frequent. However, a higher percentage of cases with giant cell and Hodgkin’s like morphology were observed in this group. None of the ALK-negative ALCL cases showed lymphohistiocytic and small cell features.
ALK Protein Expression in Nonhemopoietic Tumors
One hundred biopsies representative of a large variety of nonhemopoietic tumors were all ALK-negative, with the exception of a single case of rhabdomyosarcoma (1 of 35 tested), which showed strong cytoplasmic and cell membrane-associated labeling for the ALK protein (Figure 2, C and D) ▶ .
Comparison of ALKc and ALK1 Antibodies
Antibody ALK1 but not ALKc or the negative control antibody MR12 reacted with the recombinant protein ALK-DHFR in the enzyme-linked immunosorbent assay. No cross-blocking of ALK1 and ALKc antibodies was observed at Western blotting, where ALKc gave stronger bands than ALK1. At immunohistochemistry, the two antibodies gave essentially identical reactions in most cases, but ALKc reacted more strongly than ALK1 with the nuclei of tumor cells (especially those of the small size) in at least 15 to 20% of specimens. One of the 60 ALK-positive lymphomas reacted with ALKc but not with ALK1. Conversely, two cases (one fixed in formalin and the other in B5) were ALKc negative/ALK1 positive.
All together, these findings demonstrate that the mAb ALKc is directed against a different ALK epitope from that recognized by antibody ALK1 and suggest that the highest yield of information is obtained when both the antibodies are employed for the study of ALCL.
RT-PCR Studies
As shown in Table 3 ▶ , the finding of ALK expression at the nuclear level correlated with a positive RT-PCR test for NPM-ALK. However, in the two cases in which labeling was restricted to the cytoplasm, RT-PCR was negative.
Table 3.
Correlation between ALK Expression and RT-PCR for NPM-ALK in 11 ALK-Positive Lymphomas
| ALK-positive lymphoma | Morphology | ALK expression pattern (ALKc) | NPM-ALK |
|---|---|---|---|
| 1 | Common type | Nuclear and cytoplasmic (large cells) | + |
| 2 | Common type | Nuclear and cytoplasmic (large cells) | + |
| 3 | Common type | Nuclear and cytoplasmic (mostly large cells)* | + |
| 4 | Common type | Nuclear and cytoplasmic (large cells) | + |
| 5 | Common type | Nuclear and cytoplasmic (large cells) | +† |
| 6 | Common type | Nuclear and cytoplasmic (mostly large cells)* | + |
| 7 | Common type | Cytoplasm restricted (large cells) | − |
| 8 | Giant cell type | Cytoplasm restricted (large cells) | − |
| 9 | Lymphohistiocytic | Nucleus restricted (mostly small cells)‡ | + |
| 10 | Lymphohistiocytic | Nucleus restricted (mostly small cells)‡ | + |
| 11 | Small cell type | Nucleus restricted (mostly small cells)‡ | + |
*A percentage (5 to 30%) of small cells with nucleus-restricted ALK positivity was also present.
†RT-PCR was not performed in this case but conventional cytogenetic examination showed typical t(2;5).
‡A small number of large anaplastic cells with nuclear plus cytoplasmic ALK expression was also detected.
Discussion
Initial studies of the t(2;5) chromosomal translocation showed that this anomaly is associated with CD30-positive large cell lymphomas. 5 It subsequently became apparent that a small cell variant, in which only a minority of the neoplastic cells were of large size, could sometimes be found among t(2;5)-positive lymphomas. 32 Cases were also reported of a lymphohistiocytic lymphoma, 31 in which reactive macrophages outnumber, and often obscure, the underlying large cell lymphoma. Other distinctive histological pictures were also occasionally observed. 36
The production of polyclonal antibodies and mAbs to ALK protein 15-18 made it possible to detect, by immunohistological techniques, the NPM-ALK fusion protein generated by the t(2;5) translocation. This has allowed the histological features of large numbers of ALK-positive lymphomas to be reviewed. 16,18,28,47,48 In the present paper, we document by using a new anti-ALK mAb, ALKc, the range of morphological appearances found among these neoplasms.
The ALK-positive lymphomas in this series included five cases that showed features of the small cell variant. These cases were of interest for several reasons. First, ALK reactivity was seen not only in the large neoplastic cells but also in the numerous smaller neoplastic cells. This implies that the genetic lesion (the NPM-ALK gene), which is presumed to play a direct causal role in the genesis of ALK-positive lymphomas, 6,7 must be present in all of the neoplastic cells. The large cells cannot, therefore, represent a subclone that has arisen in a low-grade (small cell) lymphoma after the acquisition of the (2;5) anomaly. In this context, it may be noted that a recent study has addressed this possibility directly by studying a series of transformed T-cell lymphomas and has confirmed that histological progression is not accompanied by the appearance of the (2;5) translocation. 49 Immunocytochemistry also identified a typical perivascular pattern of large cells. Similar findings have been recently reported by Benharroch et al. 50
Small neoplastic cells expressing ALK protein were, by definition, most evident in the small cell variant but were also found in cases showing other histological patterns. In the lymphohistiocytic lymphomas, they were particularly obvious in immunostained sections, and it is possible that most cases of this neoplasm should be considered as examples of the small variant of ALCL in which large numbers of reactive histiocytes have accumulated. Approximately one-third of ALK-positive lymphomas cases showing the common histological pattern also contained a minor population of small cells showing nucleus-restricted ALK reactivity.
We suggest that many ALK-positive lymphomas lie on a spectrum, their position being defined by the ratio of small to large neoplastic cells (Figure 7) ▶ . The tissue distribution of tumor cells (eg, “perivascular pattern”), the occurrence of sclerosis (occasionally imparting to the lesion a Hodgkin’s-like appearance), and the presence of reactive histiocytes or other cell types, probably induced by the release of cytokines by tumor cells (especially those of small size) may add to the heterogeneous morphological pattern of ALK-positive lymphomas. The recognition of the small neoplastic cells in this study was facilitated by the ALKc mAb, which tends to label them more strongly than does ALK1, 51 the first anti-ALK mAb to be described, 18 presumably because it detects a different epitope. Antigen retrieval in ethylenediaminetetraacetic acid buffer, which acts by chelating calcium ions (possibly responsible for masking of several nucleus-located antigens), 42 may have contributed further to these findings.
Figure 7.
ALK-positive lymphomas show a broad morphological spectrum, their position being defined by the ratio of small to large neoplastic cells and the presence of accompanying inflammatory cells.
The restriction of ALK reactivity to the nucleus of these small neoplastic cells is also of interest. Because the NPM-ALK fusion protein lacks NPM nuclear localization signals, 6 transportation within the nuclei of tumor cells most likely occurs via association with the wild-type 38-kd NPM shuttling protein that is able to form heterodimers with NPM-ALK (through a motif, yet to be characterized, localized within its amino-terminal portion). 7,52 There is good evidence that the NPM-ALK kinase accumulates within cell nuclei (and particularly within nucleoli), but there are also good reasons to believe that this is not the site at which it exerts its oncogenic effect. 52 It is possible that NPM-ALK is present in the cytoplasm of the small cells at levels too low to be detected by immunohistochemical labeling (especially after tissue fixation and embedding), but which are sufficient to allow oncogenic phosphorylation of intracytoplasmic substrates. However, the finding that NPM-ALK is expressed at the highest concentration within these cells at a site that is not its site of action is a paradox that requires further study.
The ability to detect by immunocytochemistry a population of ALK-positive small cells also has important diagnostic implications in the following settings: 1) distinction of the lymphohistiocytic variant from benign reactive lymphadenopathies or infection-associated hemophagocytic syndromes, 53 2) differential diagnosis between the small cell variant and peripheral T cell lymphomas or inflammatory infiltrates (especially in skin), 36 and 3) detection of very small number of tumor cells in bone marrow and/or lymph nodes either at the time of initial diagnosis or after therapy.
A final observation of interest is that, in approximately 15% of the ALK-positive lymphomas in this study, labeling appeared to be confined to the cytoplasm. A similar observation has been recently reported in an independent study by Benharroch et al. 50 Our hypothesis is that these cells may carry variant translocations, in which the ALK gene on chromosome 2 is linked to a gene other than NPM. We also assume that the resultant fusion protein(s) activates the ALK kinase by cross-linking (as occurs in the case of NPM-ALK), but that they do not contain any motifs that direct the protein to the nucleus. There is at least one precedent for this in a reported case of ALK-positive ALCL that carried the (1;2) translocation and in which immunolabeling was also confined to the cytoplasm. 18,24 Furthermore, an engineered TPR-ALK construct has been reported that can transform cells but that remains confined to the cytoplasm. 12 It is of interest that the cases in the present study that showed only cytoplasmic labeling for ALK seemed to lack the degree of variation in neoplastic cell size seen in the majority of ALK-positive lymphomas. This hints at the possibility that they may represent a subtype of ALK-positive lymphoma with distinctive histological and possibly clinical features. Further biochemical and molecular biological studies of neoplasms in which ALK positivity is confined to the cytoplasm are therefore clearly likely to be of interest.
In conclusion, this study emphasizes the importance of immunohistological labeling for ALK protein(s) in the evaluation of ALCL. All cases in this series were of T cell or null phenotype, and the age of these patients tended to be lower than that of ALK-negative ALCL (confirming other data). 16 In consequence, ALK positivity appears to define a clinicopathological entity (ALK lymphomas), but one that shows a much wider spectrum of morphological patterns than has been appreciated in the past.
Acknowledgments
We thank Prof. David Y. Mason for reading the manuscript and for the helpful suggestions. We would like also to thank Gisberto Loreti, Cristina Alunni, and Laura Natali-Tanci for the excellent technical assistance and Claudia Tibidò for the excellent secretarial assistance.
Footnotes
Address reprint requests to Brunangelo Falini, Istituto di Ematologia, Policlinico, Monteluce, 06100 Perugia, Italy.
Supported by A.I.R.C. (Associazione Italiana Ricerca Cancro).
References
- 1.Stein H, Mason DY, Gerdes J, O’Connor N, Wainscoat J, Pallesen G, Gatter K, Falini B, Delsol G, Lemke H, Schwarting R, Lennert K: The expression of Hodgkin’s disease associated Ki-1 antigen in reactive and neoplastic lymphoid tissues: evidence that Sternberg-Reed cells and histiocytic malignancies are derived from activated lymphoid cells. Blood 1985, 66:848-858 [PubMed] [Google Scholar]
- 2.Falini B, Pileri S, Pizzolo G, Durkop H, Flenghi L, Stirpe F, Martelli MF, Stein H: CD30 (Ki-1) molecule: a new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood 1995, 85:1-14 [PubMed] [Google Scholar]
- 3.Kadin ME: Primary Ki-1 positive anaplastic large cell lymphoma: a distinct clinicopathologic entity. Ann Oncol 1994, 5:25-30 [DOI] [PubMed] [Google Scholar]
- 4.Zinzani PL, Bendandi M, Martelli M, Falini B, Sabattini E, Amadori S, Gherlinzoni F, Martelli MF, Mandelli F, Tura S, Pileri S: Anaplastic large cell lymphoma: clinical and prognostic evaluation of 90 adult patients. J Clin Oncol 1996, 14:955-962 [DOI] [PubMed] [Google Scholar]
- 5.Mason DY, Bastard C, Rimokh R, Dastugue N, Huret J-L, Kristoffersson U, Magaud J-P, Nezelof C, Tilly H, Vannier J-P, Hemet J, Warnke R: CD30-positive large cell lymphomas (Ki-1 lymphoma) are associated with a chromosomal translocation involving 5q35. Br J Haematol 1990, 74:161-168 [DOI] [PubMed] [Google Scholar]
- 6.Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, Look AT: Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science 1994, 263:1281-1284 [DOI] [PubMed] [Google Scholar]
- 7.Ladany M: The NPM/ALK gene fusion in the pathogenesis of anaplastic large cell lymphoma. Cancer Surv 1997, 30:59-75 [PubMed] [Google Scholar]
- 8.Fujimoto J, Shiota M, Iwahara T, Seki N, Satoh H, Mori S, Yamamoto T: Characterization of the transforming activity of p80, a hyperphosphorylated protein in a Ki-1 lymphoma cell line with chromosomal translocation t(2;5). Proc Natl Acad Sci USA 1996, 93:4181-4186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan PK, Chan FY, Morris SW, Liu QR: Isolation and characterization of the human nucleophosmin/B23 (NPM) gene: identification of the YY1 binding site at the 5′ enhancer region. Nucleic Acids Res 1996, 25:1225-1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris SW, Naeve C, Mathew P, James PL, Kirstein MN, Cui X, Witte DP: ALK, the chromosome 2 gene locus altered by the t(2;5) in non-Hodgkin’s lymphoma, encodes a novel neural tyrosine kinase that is highly related to leukocyte tyrosine kinase (LTK). Oncogene 1997, 14:2175-2188 [DOI] [PubMed] [Google Scholar]
- 11.Iwahara T, Fujimoto J, Wen D, Cupples R, Bucay N, Arakawa T, Mori S, Ratzkin B, Yamamoto T: Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene 1997, 14:439-449 [DOI] [PubMed] [Google Scholar]
- 12.Bischof D, Pulford K, Mason DY, Morris SW: Role of nucleophosmin (NPM) portion of the non-Hodgkin’s lymphoma-associated NPM-anaplastic kinase fusion protein in oncogenesis. Mol Cell Biol 1997, 17:2312-2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuefer MU, Look AT, Pulford K, Behm FG, Pattengale PK, Mason DY, Morris SW: Retrovirus-mediated gene transfer of NPM-ALK causes lymphoid malignancy in mice. Blood 1997, 90:2901-2910 [PubMed] [Google Scholar]
- 14.Downing J, Shurtleff S, Zielenska M, Curcio-Brint AM, Behm FG, Head DR, Sandlund JT, Weinburger D, Kossakowska AE, Thorner P, Lorenzana A, Ladanyi M, Morris S: Molecular detection of the t(2;5) translocation of non-Hodgkin’s lymphoma by reverse transcriptase-polymerase chain reaction. Blood 1995, 85:3416-3422 [PubMed] [Google Scholar]
- 15.Shiota M, Fujimoto J, Takenaga M, Satoh H, Ichinohasama R, Abe M, Nakano M, Yamamoto T, Mori S: Diagnosis of t(2;5)(p23;q35)-associated Ki-1 lymphoma with immunohistochemistry. Blood 1994, 84:3648-3652 [PubMed] [Google Scholar]
- 16.Shiota M, Nakamura S, Ichinohasama R, Abe M, Akagi T, Takeshita M, Mori N, Fujimoto J, Miyauchi J, Mikata A, Nanba K, Takami T, Yamabe H, Takano Y, Izumo T, Nagatani T, Mohri N, Nasu K, Satoh H, Katano H, Fujimoto J, Yamamoto T, Mori S: Anaplastic large cell lymphomas expressing the novel chimeric protein p80NPM/ALK: a distinct clinicopathologic entity. Blood 1995, 86:1954-1960 [PubMed] [Google Scholar]
- 17.Hutchinson RE, Banki K, Shuster JJ, Barrett D, Dieck C, Berard CW, Murphy SB, Link MP, Pick TE, Laver J, Schwenn M, Mathew P, Morris SW: Use of an anti-ALK antibody in the characterization of anaplastic large-cell lymphoma of childhood. Ann Oncol 1997, 8:37-42 [PubMed] [Google Scholar]
- 18.Pulford K, Lamant L, Morris S, Butler LH, Wood KM, Stroud D, Delsol G, Mason DY: Detection of ALK and NPM-ALK protein in normal and neoplastic cells with the monoclonal antibody ALK1. Blood 1997, 89:1394-1404 [PubMed] [Google Scholar]
- 19.Herbst H, Anagnostopoulos J, Heinze B, Durkop H, Hummel M, Stein H: ALK gene products in anaplastic large cell lymphomas and Hodgkin’s disease. Blood 1995, 86:1694-1700 [PubMed] [Google Scholar]
- 20.Elmberger PG, Lozano MD, Weisenburger DD, Sanger W, Chan WC: Transcripts of the npm-alk fusion gene in anaplastic large cell lymphoma, Hodgkin’s disease, and reactive lymphoid lesions. Blood 1995, 86:3517-3521 [PubMed] [Google Scholar]
- 21.Wellmann A, Otsuki T, Vogelbruch M, Clark HM, Jaffe ES, Raffeld M: Analysis of the t(2;5)(p23;q35) translocation by reverse transcription polymerase chain reaction in CD30+ anaplastic large cell lymphomas, in other non-Hodgkin’s lymphomas of T-cell phenotype, and in Hodgkin’s disease. Blood 1995, 86:2321-2328 [PubMed] [Google Scholar]
- 22.Lopategui JR, Sun LH, Chan JK, Gaffey MJ, Frierson HF, Jr, Glackin C, Weiss LM: Low frequency association of the t(2;5)(p23;q35) chromosomal translocation with CD30+ lymphomas from American and Asian patients: a reverse transcriptase-polymerase chain reaction study. Am J Pathol 1995, 146:323-328 [PMC free article] [PubMed] [Google Scholar]
- 23.Weisenburger DD, Gordon BG, Vose JM, Bast MA, Chan WC, Greiner TC, Anderson JR, Sanger WG: Occurrence of the t(2;5)(p23;q35) in non-Hodgkin’s lymphoma. Blood 1996, 87:3860-3868 [PubMed] [Google Scholar]
- 24.Lamant L, Meggetto F, Al Saati T, Bruggieres L, Bressac de Paillerets B, Dastague N, Bernheim A, Rubie H, Terrier-Lacombe MJ, Robert A, Brousset P, Rigal F, Schlaifer D, Shiota M, Mori S, Delsol G: High incidence of the t(2;5)(p23;q35) translocation in anaplastic large cell lymphoma and its lack of detection in Hodgkin’s disease: comparison of cytogenetic analysis, reverse transcriptase-polymerase chain reaction, and P-80 immunostaining. Blood 1996, 87:284-291 [PubMed] [Google Scholar]
- 25.Sarris AH, Rajyalakshmi L, Papadimitracopoulou V, Waasdorp M, Dimopoulos M, McBride JA, Cabanillas F, Duvic M, Deisseroth A, Morris S, Pugh WC: Amplification of genomic DNA demonstrates the presence of the t(2;5)(p23;q35) in anaplastic large cell lymphoma, but not in other non-Hodgkin’s lymphomas, Hodgkin’s disease, or lymphomatoid papulosis. Blood 1996, 88:1771-1779 [PubMed] [Google Scholar]
- 26.DeCoteau JF, Butmarc JR, Kinney MC, Kadin ME: The t(2;5) chromosomal translocation is not a common feature of primary cutaneous CD30+ lymphoproliferative disorders: comparison with anaplastic large-cell lymphoma of nodal origin. Blood 1996, 87:3437-3441 [PubMed] [Google Scholar]
- 27.Shiota M, Mori S: Anaplastic large cell lymphomas expressing the novel chimeric protein p80NPM/ALK: a distinct clinicopathologic entity. Leukemia 1997, 11:538-540 [PubMed] [Google Scholar]
- 28.Nakamura S, Shiota M, Nakagawa A, Yatabe Y, Kojima M, Motoori T, Suzuki R, Kagami Y, Ogura M, Morishima Y, Mizoguchi Y, Okamoto M, Seto M, Koshikawa T, Mori S, Suchi T: Anaplastic large cell lymphoma: a distinct molecular pathologic entity: a reappraisal with special reference to p80 (NPM/ALK) expression. Am J Surg Pathol 1997, 21:1420-1432 [DOI] [PubMed] [Google Scholar]
- 29.Stein H: Ki-1 anaplastic large cell lymphoma: is it a discrete entity? Leuk Lymphoma 1993, 10:81-84 [DOI] [PubMed] [Google Scholar]
- 30.Chan JKC, Ng CS, Hui PK, Leungs TW, Lau WH, McGuire LJ: Anaplastic large cell Ki-1 lymphoma: delineation of two morphological types. Histopathology 1989, 15:11-34 [DOI] [PubMed] [Google Scholar]
- 31.Pileri S, Falini B, Delsol G, Stein H, Baglioni P, Poggi S, Martelli MF, Rivano MT, Mason DY, Stansfeld AG: Lymphohistiocytic T-cell lymphoma (anaplastic large cell lymphoma CD30+/Ki-1+ with a high content of reactive histiocytes). Histopathology 1990, 16:383-391 [DOI] [PubMed] [Google Scholar]
- 32.Kinney MC, Collins RD, Greer JP, Whitlock JA, Sioutos N, Kadin ME: A small cell-predominant variant of primary Ki-1 (CD30)+ T-cell lymphoma. Am J Surg Pathol 1993, 17:859-868 [DOI] [PubMed] [Google Scholar]
- 33.Mann KP, Hall B, Kamino J, Borowitz MJ, Ratech H: Neutrophil-rich, Ki-1-positive anaplastic large cell malignant lymphoma. Am J Surg Pathol 1995, 19:407-416 [DOI] [PubMed] [Google Scholar]
- 34.Chan JKC, Buchanan R, Fletcher CDM: Sarcomatoid variant of anaplastic large-cell lymphoma. Am J Surg Pathol 1990, 14:983-988 [DOI] [PubMed] [Google Scholar]
- 35.Falini B, Liso A, Pasqualucci L, Flenghi L, Ascani S, Pileri S, Bucciarelli E: CD30+ anaplastic large cell lymphoma, null type, with signet-ring appearance. Histopathology 1997, 30:90-92 [DOI] [PubMed] [Google Scholar]
- 36.Kadin ME: Anaplastic large cell lymphoma and its morphological variants. Cancer Surv 1997, 30:77-86 [PubMed] [Google Scholar]
- 37.Flenghi L, Bigerna B, Fizzotti M, Venturi S, Pasqualucci L, Pileri S, Ye BH, Gambacorta M, Pacini R, Baroni C, Pescarmona E, Anagnostopoulos I, Stein H, Asdrubali G, Martelli MF, Pelicci PG, Dalla Favera R, Falini B: Monoclonal antibodies PG-B6a and PG-B6p recognize, respectively, a highly conserved and a formol-resistant epitope on the human BCL-6 protein amino-terminal region. Am J Pathol 1996, 148:1543-1555 [PMC free article] [PubMed] [Google Scholar]
- 38.Cordell JL, Falini B, Erber WN, Ghosh AK, Abdulaziz Z, MacDonald S, Pulford KAF, Stein H, Mason DY: Immunoenzymatic labelling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem 1984, 32:219-229 [DOI] [PubMed] [Google Scholar]
- 39.Fischer P, Nacheva E, Mason DY, Sherrington PD, Hoyle C, Hayhoe FG, Karpas A: A Ki-1 (CD30)-positive human cell line (Karpas 299) established from a high-grade non-Hodgkin’s lymphoma, showing a 2;5 translocation and rearrangement of the T-cell receptor β-chain gene. Blood 1988, 72:234-240 [PubMed] [Google Scholar]
- 40.Pear WS, Nolan GP, Scott ML, Baltimore D: Production of high-titer helper-free retrovirus by transient transfection. Mol Cell Biol 1993, 90:8392-8396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cattoretti G, Suurmeijer AJH: Antigen unmasking on formalin-fixed paraffin-embedded tissues using microwaves: a review. Adv Anat Pathol 1995, 2:2-9 [Google Scholar]
- 42.Pileri S, Roncador G, Ceccarelli C, Piccioli M, Briskomatis A, Sabattini E, Ascani S, Santini D, Piccaluga PP, Leone O, Damiani S, Ercolessi C, Sandri F, Pieri F, Leoncini L, Falini B: Antigen retrieval techniques in immunohistochemistry: comparison of different methods. J Pathol 1997, 183:116-123 [DOI] [PubMed] [Google Scholar]
- 43.Harris NL, Jaffe ES, Stein H, Banks P, Chan JKC, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC, Grogan TM, Isaacson PG, Knowles DM, Mason DY, Muller-Hermelink HK, Pileri SA, Ralfkier E, Warnke RA: A Revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 1994, 84:1361-1392 [PubMed] [Google Scholar]
- 44.Bennet JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, Sultan C: Proposed revised criteria for the classification of acute myeloid leukemia: a report of the French-American-British Cooperative Group. Ann Int Med 1985, 103:620-625 [DOI] [PubMed] [Google Scholar]
- 45.Ponder BA, Wilkinson WN: Inhibition of endogenous tissue alkaline phosphatase with the use of alkaline phosphatase conjugates in immunohistochemistry. J Histochem Cytochem 1981, 29:981-986 [DOI] [PubMed] [Google Scholar]
- 46.Gambacorta M, Flenghi L, Fagioli M, Pileri S, Leoncini L, Bigerna B, Pacini R, Natali Tanci L, Pasqualucci L, Ascani S, Mencarelli A, Liso A, Pelicci PG, Falini B: Heterogeneous nuclear expression of the promyelocytic (PML) protein in normal and neoplastic human tissues. Am J Pathol 1996, 149:2023-2035 [PMC free article] [PubMed] [Google Scholar]
- 47.Pittaluga S, Wiodarska I, Pulford K, Campo E, Morris SW, Van den Berghe H, De Wolf-Peeters C: The monoclonal antibody ALK1 identifies a distinct morphological subtype of anaplastic large cell lymphoma associated with 2p23/ALK rearrangements. Am J Pathol 1997, 151:343-351 [PMC free article] [PubMed] [Google Scholar]
- 48.Delsol G, Lamant L, Mariamé B, Pulford K, Dastugue N, Brousset P, Rigal-Huguet F, Al Saati T, Cerretti DP, Morris SW, Mason DY: A new subtype of large B-cell lymphoma expressing the ALK kinase and lacking the 2;5 translocation. Blood 1997, 89:1483-1490 [PubMed] [Google Scholar]
- 49.Li G, Salhany KE, Rook AH, Lessin SR: The pathogenesis of large cell transformation in cutaneous T-cell lymphoma is not associated with t(2;5)(p23;q35) chromosomal translocation. J Cutan Pathol 1997, 24:403-408 [DOI] [PubMed] [Google Scholar]
- 50.Benharroch D, Meguerian-Bedoyan Z, Lamant L, Amin C, Brugieres L, Terrier-Lacombe MJ, Haralambieva E, Pulford K, Pileri S, Morris S, Mason DY, Delsol G: ALK-positive lymphoma: a single disease with a broad spectrum of morphology. Blood 1998, 91:2076-2084 [PubMed] [Google Scholar]
- 51.Pileri SA, Mason DY, Mori S, Pulford K, Sabattini E, Roncador G, Piccaluga PP, Stein H, Falini B: Frequent expression of the p80 NPM-ALK chimeric fusion protein in anaplastic large cell lymphoma, lympho-histiocytic type. Am J Pathol 1997, 150:1207-1211 [PMC free article] [PubMed] [Google Scholar]
- 52.Mason DY, Pulford K, Bischof D, Kuefer MU, Butler LH, Lamant L, Delsol G, Morris S: Nucleolar localization of the nucleophosmin-anaplastic lymphoma kinase tyrosine kinase is not required for malignant transformation. Cancer Res 1998, 58:1057-1062 [PubMed] [Google Scholar]
- 53.Pileri S, Sabattini E, Falini B: Lymphohistiocytic T-cell lymphoma and peripheral T-cell lymphoma associated with haemophagocytic syndrome: two recently recognized entities which mimic malignant histiocytosis. Leuk Lymphoma 1992, 6:317-324 [Google Scholar]