Abstract
p53-dependent expression of p21WAF-1/CIP1 has been studied in murine intestinal epithelium after exposure to ionizing radiation. In un-irradiated small intestine, neither p53 nor p21WAF-1/CIP1 could be detected by immunohistochemistry. After irradiation (8 Gy), there was a time- and dose-dependent increase in the expression of both proteins. In the small bowel, the positional expression of p53 and p21WAF-1/CIP1 was similar but not coincident. Both proteins could be observed throughout the crypts with greatest frequency of expression over the first 15 cell positions, which includes the stem cell population (approximately positions 3 to 5) and the proliferating, transit cell population (approximately positions 5 to 15). p53-positive cells were primarily distributed toward the base of the crypt relative to p21WAF-1/CIP1. Subdivision of the p53-positive cell population revealed that the cells with strongest p53 immunoreactivity were positioned farther toward the base of the crypt, and their distribution was approximately coincident with the frequency distribution of apoptotic cells. Cells that were either weakly or moderately immunoreactive for p53 were located toward the middle of the crypt and were approximately coincident with the distribution of p21WAF-1/CIP1. The numbers of both p53- and p21WAF-1/CIP1-positive cells declined steadily with time, and by 6 days after irradiation there were very few immunoreactive cells to observe. Radiation-induced increase in p53 and p21WAF-1/CIP1 expression was not detected in mice homozygously null for p53. Expression of p21WAF-1/CIP1 and incorporation of tritiated thymidine were found to be mutually exclusive. In the large bowel, p21WAF-1/CIP1 and p53 expression were observed along the entire length of the colonic crypts after irradiation (8 Gy), and, unlike in the small intestine, this expression was not only maintained but increased over 72 hours. p21WAF-1/CIP1 immunoreactivity was detected in large intestine epithelium up to 6 days after irradiation. The differential expression of p21WAF-1/CIP1, observed between the large and small bowel and within the small intestinal crypts, is discussed.
After DNA damage, cells are observed to undergo cell cycle arrest and/or apoptosis. This process involves a number of factors, including the detection and signaling of DNA damage, which is dependent on the function of proteins such as ATM and Ku1; the ability to process signals from DNA damage recognition proteins, in which the effector protein p53 plays a key role; 2,3 the ability of a cell to repair DNA damage; 4 the cellular threshold for apoptosis, which, among other factors, is determined by the dynamic equilibrium that exists between the different members of the Bcl-2 protein family. 5
We have investigated the response of intestinal epithelium to ionizing (γ) radiation, in vivo. p53 protein levels are up-regulated rapidly in response to DNA damage induced by a number of noxious stimuli, including ionizing radiation, 2 ultraviolet irradiation, 6 cytotoxic drugs, 2,7 and hypoxia. 8 It has now been shown that p53 transcriptionally regulates many genes. This regulation may either be positive 9,10 or negative. 9,11 p53 has been shown to regulate the expression of genes important for both cell cycle arrest (an event that has been proposed to allow the cell time to repair), such as p21WAF-1/CIP1, 12,13 and apoptosis, eg, bax 14-16 and killer/DR5. 17
The expression of p21WAF-1/CIP1 protein mediates p53-dependent cell cycle arrest. 13,18-20 p21WAF-1/CIP1 inhibits cell cycle progression by binding to and inhibiting the function of cyclin-dependent kinases and proliferating cell nuclear antigen. 21,22 In addition, p21WAF-1/CIP1 expression is associated with cell senescence. 23 Loss of p21WAF-1/CIP1 function is associated with the attenuation of cell cycle arrest after DNA damage 18 and with the failure of human fibroblasts to senesce in vitro. 24
It has been shown previously, by ourselves and others, 25-28 that p53 protein expression in the intestinal epithelium is increased in response to γ-irradiation. The acute apoptotic response (3 to 6 hours after irradiation) observed in the intestinal epithelium was shown to be entirely dependent on p53 expression, because it was abrogated in mice homozygously null for p53. In addition, these studies demonstrated that at later times after irradiation (12 to 24 hours), apoptosis could occur independently of p53 expression.
In this paper, we have characterized the in vivo expression of p21WAF-1/CIP1 in response to ionizing radiation, using immunohistochemistry. We have contrasted the response of the different cellular hierarchies within individual intestinal crypts. The different responses observed between the epithelia of the small and large bowel have also been examined.
Materials and Methods
Animals
Male BDF-1 (C57BL × DBA/2) mice and male p53-wild-type (wt) and p53-null mice were bred in house. p53-wt and p53-null mice were originally obtained from Donehower et al. 29 Mice were kept under a 12-hour light:12-hour dark cycle with lights on at 7:00 AM and were allowed free access to food and water. Mice used in the experiments were between 10 and 12 weeks of age.
Exposure of Animals to γ-Radiation
Mice were irradiated with a 137Cs source, with a dose rate of 3.8 Gy/minute. Animals were sacrificed by cervical dislocation at set times after irradiation, and the small and large bowel were removed.
Immunohistochemistry
For immunohistochemistry, tissue was fixed in 4% formaldehyde in phosphate-buffered saline (pH 7.4), before dehydration in alcohols and embedding in wax. Tissue sections were cut using a microtome at a thickness of 3 μm.
Rabbit polyclonal anti-p21WAF-1/CIP1 immunoglobulin G was obtained from Oncogene Research Products (pc55, through Amersham International, Little Chalfont, UK). Rabbit polyclonal anti-p53 immunoglobulin G (cm5) was a kind gift from Prof. D. Lane (Dundee, UK).
Immunohistochemistry was performed using biotin-conjugated goat anti-rabbit secondary antibody (Pierce and Warriner, Chester, UK), horseradish peroxidase-linked avidin-biotin complex reagents (Vector Laboratories, Peterborough, UK), and 3′,3′-diaminobenzidine as the immunodetection substrate, as previously described. 30
Cell Scoring
Apoptosis
Apoptotic cells, mitotic cells, and cells showing immunoreactivity for p21WAF-1/CIP1 and p53 were scored on a cell-positional basis within the crypts of the small and large bowel according to the method of Ijiri and Potten. 31 A minimum of 1000 cells (50 half-crypts) were counted from each mouse in every group. Apoptosis was assessed on the evidence of morphological characteristics, such as cell shrinkage, chromatin condensation, and margination and cellular fragmentation. 32 Mitotic cells were identified by virtue of chromatin condensation in the absence of cytoplasmic and nuclear shrinkage. In many mitotic cells, discrete chromosomal structure can be observed, and in addition, mitotic cells appear horizontally displaced, away from the other epithelial cells, toward the crypt lumen. To determine the number of cells in S-phase of the cell cycle at given times after the exposure to γ-radiation, animals were injected intraperitoneally with 925 kBq of [3H]thymidine (248 GBq/mmol, in 0.1 ml of physiological saline) 40 minutes before sacrifice. Tissue was then fixed in Carnoy’s fixative before wax embedding and sectioning. Tissue sections were rehydrated and coated in K-5 nuclear track emulsion (Ilford, Cheshire, UK). After the emulsion had dried, sections were boxed and exposed for 3 days at 4°C. Slides were developed using Kodak D-19 developer and fixed with Hypam fixative (Ilford). Sections were counterstained with hematoxylin before dehydration and mounting.
Immunohistochemistry
p53 and p21WAF-1/CIP1 immunoreactivity were classified according to their intensity. Using light microscopy, it could be seen that for both p53 and p21WAF-1/CIP1, certain cells exhibited noticeably stronger immunoreactivity than the rest. These cells were classified as strongly stained. All other cells were classified together as either weakly or moderately stained. This is a fairly subjective approach; however, reproducibility of scoring was observed between mice in the same groups and was also checked using other observers.
Western Blotting
Epithelial cell preparations using a modified Weiser technique and Western blotting were carried out as previously described. 30 Immunodetection was carried out using enhanced chemiluminescence (Amersham, UK). Rabbit anti-actin antibody was obtained from Sigma Chemical Co. (Poole, UK).
Results
The intestinal epithelium from both BDF-1 and p53-wt animals showed time- and dose-related increases in apoptosis and in p53 and p21WAF-1/CIP1 immunoreactivity after exposure to 8 Gy γ-radiation. The frequencies of apoptosis and p21WAF-1/CIP1- and p53-immunoreactivity were scored for each cell position within the intestinal crypts at given times after irradiation.
Small Intestine
Apoptosis, p53, and p21WAF-1/CIP1 Expression after 8 Gy γ-Radiation
Small intestinal crypts show a characteristic peak in apoptotic frequency at cell position 3 to 6, 4 to 48 hours after irradiation (Figure 1 ▶ , left, solid black line). 33 Changes in p53 and p21WAF-1/CIP1 immunoreactivity were observed coincidentally with apoptosis. Neither p53 nor p21WAF-1/CIP1 could be detected in un-irradiated epithelium. p53 immunoreactivity was detectable 1 hour after irradiation and p21WAF-1/CIP1 after 2 hours (data not shown). The distribution of p53-positive cells (Figure 1 ▶ , left, shaded line) was mainly toward the base of the small intestinal crypts relative to the distribution of p21WAF-1/CIP1-positive cells (Figure 1 ▶ , left, dotted line). p53 immunoreactivity was maximal at 4 hours after irradiation, showed a gradual decline at 24 hours and 48 hours, and was almost absent by 72 hours (see Table 1 ▶ ).
Figure 1.
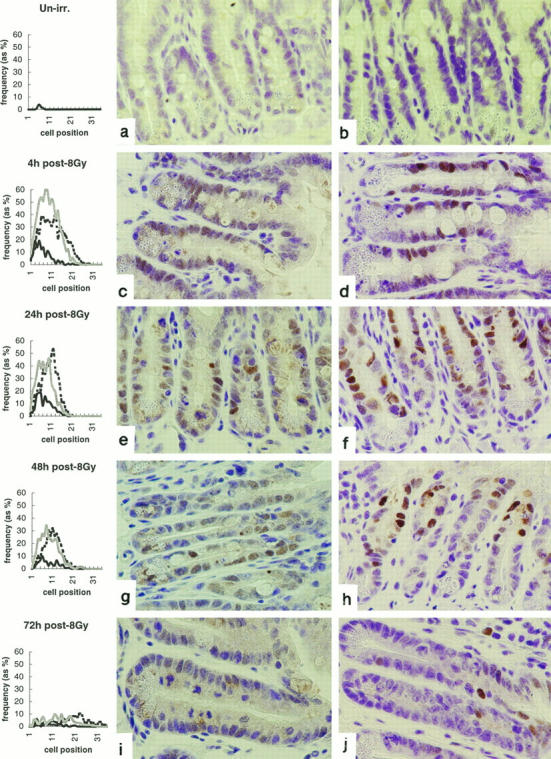
Radiation-induced apoptosis and expression of p53 and p21WAF-1/CIP1 in murine small intestinal epithelium. Line graphs show distributions of apoptotic (bold, solid line), p53-positive (shaded line), and p21WAF-1/CIP1-positive (dashed line) cells in small intestinal crypts at indicated times after exposure to 8 Gy γ-radiation. Cells are scored on a positional basis, as previously described (Ijiri and Potten 1983). 31 a, c, e, g, and i illustrate p53 immunoreactivity; b, d, f, h, and j show P21WAF-1/CIP1 immunoreactivity. Data are mean results from a minimum of three mice at each time point. At least 1000 cells (50 half-crypts) were scored from each mouse. The data are from one representative experiment typical of three.
Table 1.
Small Bowel
| Time (hours) | % apoptosis | % p21WAF-1/CIP1 positive | % p53 positive | % [3H]thymidine | |||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Weak | Strong | Total | Weak | Strong | Total | Dual* | ||
| 4 | 6 | 24.7 | 19.3 | 5.4 | 34.3 | 27.9 | 6.4 | 3.9 | 0.8 |
| 24 | 8.2 | 25.9 | 19.9 | 6 | 23.3 | 20.4 | 2.7 | 15.1 | 2.9 |
| 48 | 3.8 | 16.5 | 12.8 | 3.7 | 18.5 | 16.6 | 1.9 | 22.8 | 2.1 |
| 72 | 1.1 | 4.8 | 4.4 | 0.4 | 4.7 | 4.4 | 0.3 | 27.7 | 0.2 |
*Cells showing both [3H]thymidine incorporation and p21WAF-1/CIP1 immunoreactivity.
p21WAF-1/CIP1 expression peaked at 24 hours. There was a slow drift in the distribution of p21WAF-1/CIP1-positive cells toward the top of the crypts, and by 72 hours, these cells were exiting the crypts and could be observed on the lower portion of the villi. p21WAF-1/CIP1 expression could still be observed 96 hours after irradiation; however, by 6 days only very rare, positively staining cells could be observed on the villi (see Figure 7, b and d ▶ ).
Figure 7.

Comparison of the longevity of radiation-induced p21WAF-1/CIP1 expression in the small and large bowel. Shown are large bowel (a and c) and small bowel (b and d) at either 96 hours (a and b) or 6 days (c and d) after irradiation. Data are the mean results from a minimum of four mice at each time point. At least 1000 cells (50 half-crypts) were scored from each mouse. The data are from one experiment typical of two.
Subdivision of p53-Positive Cell Populations and the Distribution of p21WAF-1/CIP1
The expression of both p53 and p21WAF-1/CIP1 was subclassified as either weak/moderate or strong (Table 1) ▶ . Data from crypts 4 hours after exposure to ionizing radiation are shown in Figure 2 ▶ and reveal that cells with strongest p53 immunoreactivity (Figure 2 ▶ , bold solid line) were positioned farther toward the crypt base and that their distribution was approximately coincident with the positional distribution of apoptotic cells (Figure 2 ▶ , fine solid line), as previously shown by Merritt et al. 25 The majority of apoptotic cells, however, showed no p53 immunoreactivity: this may reflect the loss or masking of the epitope recognized by the anti-p53 antibody during apoptosis.
Figure 2.
Relationship between weak and strong p53 and p21WAF-1/CIP1 expression and apoptosis. Shown is the subdivision of the p53-positive cell population into weakly/moderately immunoreactive (shaded line) and strongly immunoreactive (solid, bold line) groups. Also shown are the distribution of apoptotic cells (solid, fine line) and p21WAF-1/CIP1-positive cells (dashed line). Data are for the 4-hour time point after exposure of BDF-1 mice to 8 Gy γ-radiation. Data are mean results from a minimum of three mice at each time point. At least 1000 cells (50 half-crypts) were scored from each mouse. The data are from one representative experiment typical of three.
The distribution of weak/moderate p53 immunoreactivity (Figure 2 ▶ , shaded line) was centered toward the top of the crypt and was approximately coincident with the distribution of p21WAF-1/CIP1-positive cells (Figure 2 ▶ , fine dashed line). There was no difference between the distribution of cells that were either weakly or moderately stained for p21WAF-1/CIP1 and that of those cells that were strongly stained for p21WAF-1/CIP1 (data not shown).
Effect of Radiation Dose on p21WAF-1/CIP1 Expression
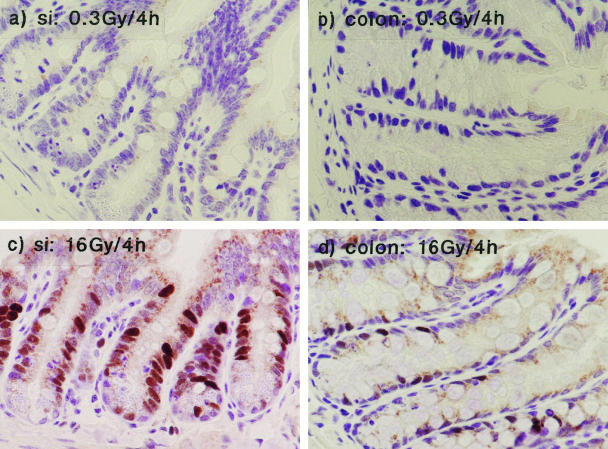
In the small bowel, the radiation-induced increase in p21WAF-1/CIP1 showed dose dependency. Figure 3 ▶ contrasts the response to 0.3 and 16 Gy 4 hours after exposure to γ-radiation. Exposure to 0.3 Gy resulted in minimal p21WAF-1/CIP1 expression (Figure 3, a and b ▶ , and Table 2 ▶ ). Considerable expression of p21WAF-1/CIP1 was induced after exposure to 16 Gy, with a greater percentage of p21WAF-1/CIP1-positive cells than was observed after exposure to 8 Gy (39%; compare 25%: see Table 2 ▶ ).
Figure 3.
The effect of radiation dose on p21WAF-1/CIP1 expression. a and b: p21WAF-1/CIP1 expression in small and large bowel, respectively, after 0.3 Gy. c and d: p21WAF-1/CIP1 expression after 16 Gy.
Table 2.
Comparison of p21WAF-1/CIP1 Expression, 4 Hours after Exposure to Different Doses of γ-Radiation
| Dose | % p21WAF-1/CIP1-positive cells | ||
|---|---|---|---|
| Total | Weak | Strong | |
| SI | |||
| 0.3 Gy/4 hours | 4.3 | 4.3 | 0 |
| 8 Gy/4 hours | 24.7 | 19.3 | 5.4 |
| 16 Gy/4 hours | 38.6 | 31.2 | 7.4 |
| LI | |||
| 0.3 Gy/4 hours* | |||
| 8 Gy/4 hours | 24.5 | 18.5 | 6 |
| 16 Gy/4 hours | 13.6 | 11.9 | 2.7 |
*Not detected.
Effect of p53 Status on p21WAF-1/CIP1 Expression
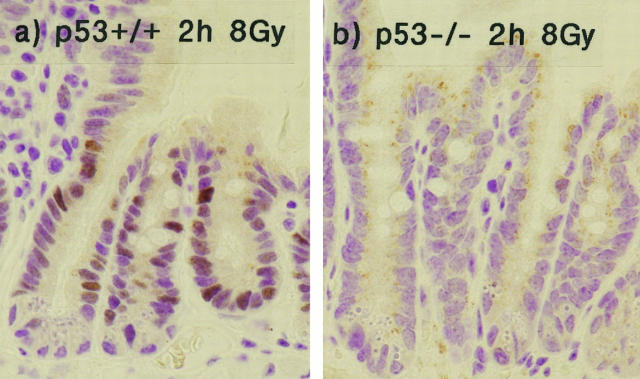
The expression of p21WAF-1/CIP1 was confirmed to be dependent on p53 function, as mice homozygously null for p53 showed no radiation-induced increase in p21WAF-1/CIP1, as detected by immunohistochemistry (Figure 4) ▶ . The radiation-induced increase in p21WAF-1/CIP1 was confirmed by Western blotting, which also confirmed the absence of p21WAF-1/CIP1 up-regulation in p53-null mice (Figure 5) ▶ . Control Westerns blots for actin showed comparable levels of expression in samples from both p53-wt and p53-null mice (data not shown). Even transfer of protein to nitrocellulose membrane was confirmed by staining with Ponceau-S (data not shown).
Figure 4.
Radiation-induced p21WAF-1/CIP1 expression is dependent on p53 function. This figure shows p21WAF-1/CIP1 immunoreactivity in wild-type mice (a) or mice homozygously null for p53 (b) 2 hours after exposure to 8 Gy γ-radiation.
Figure 5.
Western blot demonstrating radiation-induced increase in p21WAF-1/CIP1 expression in the small intestine of mice that are either wt or homozygously null for p53. Figures along the top indicate time (hours) after irradiation (8 Gy). Western samples were prepared by pooling epithelial cell preparations from at least three mice.
Large Intestine
p53 and p21WAF-1/CIP1 Expression after 8 Gy γ-Radiation
In the large intestinal epithelium, radiation-induced p53 and p21WAF-1/CIP1 expression showed both spatial and temporal differences from the small bowel (Figure 6) ▶ . The p53 response was attenuated (proportion of p53-positive cells per half-crypt) relative to that observed in the small intestinal epithelium (see Tables 1 and 3 ▶ ▶ ). In contrast to the small bowel, however, the number of p53-positive cells also declined more slowly up to 72 hours after irradiation. The p21WAF-1/CIP1 response was of a similar magnitude to that in the small bowel up to 24 hours; however, the frequency of p21WAF-1/CIP1 expression gradually increased up to 72 hours and was still present up to 6 days after irradiation. This longevity of p21WAF-1/CIP1 expression in the large bowel, relative to that observed in the small bowel, is shown in Figure 7 ▶ .
Figure 6.

Radiation-induced apoptosis and expression of p53 and p21WAF-1/CIP1 in murine large intestinal epithelium. Line graphs show distributions of apoptotic cells (bold, solid line), p53-positive cells (shaded line), and p21WAF-1/CIP1 in large intestinal crypts, at indicated times, after exposure to 8 Gy γ-radiation. Cells are scored on a positional basis, as previously described (Ijiri and Potten 1983). 31 a, c, e, g, and i illustrate p53-immunoreactivity; b, d, f, h, and j show p21WAF-1/CIP1 immunoreactivity. Data are mean results from a minimum of three mice at each time point. At least 1000 cells (50 half-crypts) were scored from each mouse. The data are from one representative experiment typical of three.
Table 3.
Large Bowel
| Time (hours) | % apoptosis | % p21WAF-1/CIP1 positive | % p53 positive | % [3H]thymidine | |||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Weak | Strong | Total | Weak | Strong | Total | Dual* | ||
| 4 | 6.3 | 24.5 | 18.5 | 6 | 17.9 | 17.7 | 0.2 | 0.2 | 0.1 |
| 24 | 3.1 | 25.3 | 18 | 7.3 | 15.4 | 14.5 | 0.9 | 1.7 | 0.4 |
| 48 | 0.8 | 32.2 | 23.2 | 9 | 12.8 | 12.4 | 0.4 | 5.8 | 2.8 |
| 72 | 1.1 | 38.3 | 27.5 | 10.8 | 11.4 | 10.8 | 0.6 | 10.2 | 2.8 |
*Cells showing both [3H]thymidine incorporation and p21WAF-1/CIP1 immunoreactivity.
Effect of Radiation Dose on p21WAF-1/CIP1 Expression
As in the small intestine, no p21WAF-1/CIP1 was detected in colonic epithelial crypts 4 hours after exposure to 0.3 Gy γ-radiation. Exposure to 16 Gy γ-radiation resulted in a large increase in p21WAF-1/CIP1 immunoreactivity; however, in contrast to the small bowel, exposure to 16 Gy resulted in a smaller increase in p21WAF-1/CIP1 expression than an 8-Gy exposure (Figure 3) ▶ . This was an unexpected observation; however, it remains to be determined whether this effect is demonstrated at other time points.
p21WAF-1/CIP1 Expression and [3H]Thymidine Incorporation
Incorporation of [3H]thymidine was measured to estimate the number of cells undergoing DNA synthesis. In both the small and large intestinal crypts exposure to γ-radiation severely depressed thymidine incorporation (Figures 8 and 9) ▶ ▶ . There was a gradual recovery in thymidine incorporation, and by the 72-hour time point, it had returned to normal. Cells re-entering the cell cycle (thymidine labeled) were observed at a lower position in the crypts than the p21WAF-1/CIP1-positive cells. Positional analysis of p21WAF-1/CIP1 immunoreactivity and [3H]thymidine incorporation revealed that they were almost mutually exclusive: fewer than 10% of p21WAF-1/CIP1-positive cells at any one time demonstrated [3H]thymidine incorporation, although the frequency of dual-labeling cells did show a gradual time-dependent increase (Figure 8 ▶ and Tables 1 and 3 ▶ ▶ ).
Figure 8.
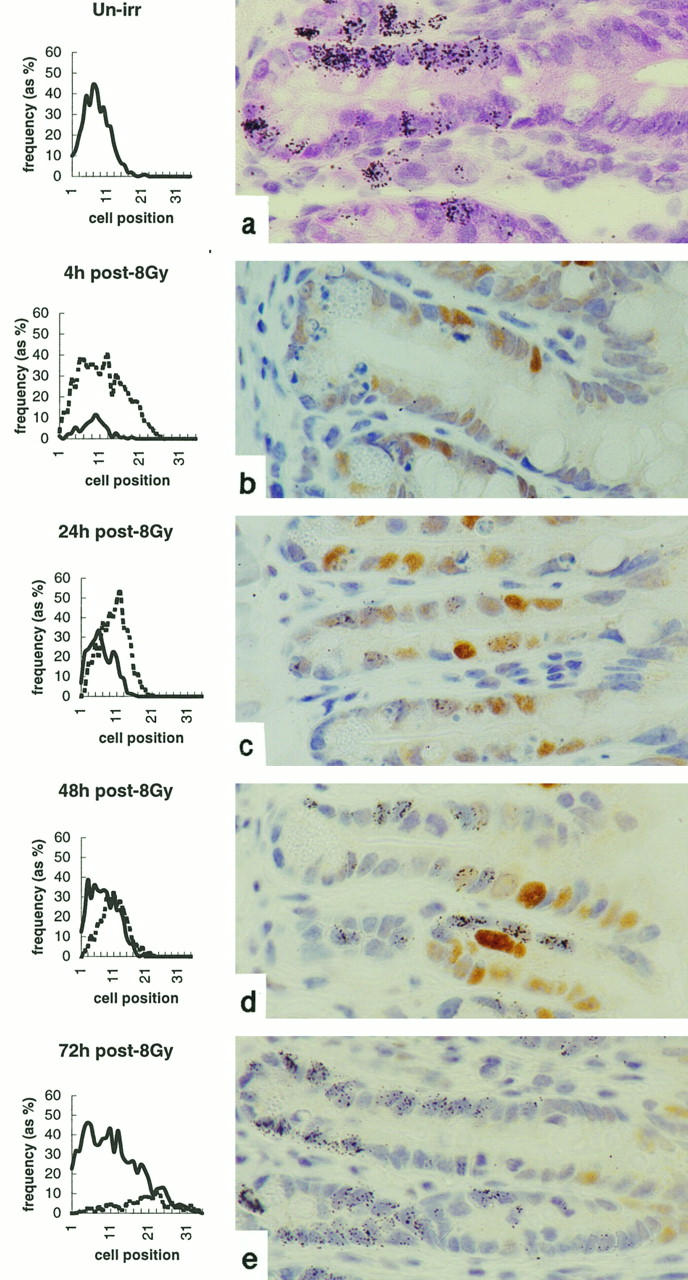
Effect of radiation on [3H]thymidine incorporation and its relationship to p21WAF-1/CIP1 expression in the small bowel. Line graphs show the distribution of thymidine-labeled (solid line) and p21WAF-1/CIP1-positive (dashed line) cells at indicated times after exposure to 8 Gy γ-radiation. Accompanying plates demonstrate more clearly the distribution of thymidine incorporation (as black silver grains) and p21WAF-1/CIP1 immunoreactivity. Data are mean results from a minimum of three mice at each time point. At least 1000 cells (50 half-crypts) were scored from each mouse. The data are from one experiment typical of two.
Figure 9.

Effect of radiation on [3H]thymidine incorporation and its relationship to p21WAF-1/CIP1 expression in the large bowel. Line graphs show the distribution of thymidine-labeled (solid line) and p21WAF-1/CIP1-positive (dashed line) cells at indicated times after exposure to 8 Gy γ-radiation. Accompanying plates demonstrate more clearly the distribution of thymidine incorporation (as black silver grains) and p21WAF-1/CIP1 immunoreactivity. Data are mean results from a minimum of three mice at each time point. At least 1000 cells (50 half-crypts) were scored from each mouse. The data are from one experiment typical of two.
Discussion
The data presented here clearly demonstrate the heterogeneity in the response of different cell populations within the intestinal epithelium to γ-radiation, with respect to expression of p53 and p21WAF-1/CIP1, apoptosis, and inhibition of DNA synthesis. If it is presumed that the potential for cellular damage induced by exposure to γ-radiation is equivalent for all of the cells of a given crypt, then the heterogeneous response must represent phenotypic variations defined by the topological position of each epithelial cell within the crypt.
Within the small intestinal epithelium, the distribution of γ-radiation-induced p21WAF-1/CIP1 expression was centered toward the top of the crypts relative to the distribution of p53 and apoptotic cells. When the population of p53-positive cells was subclassified as either weakly/moderately stained or strongly stained, it was revealed that the distribution of cells strongly positive for p53 was coincident with that for apoptosis (4 hours after exposure to 8 Gy γ-radiation). Previous work from this laboratory had also identified coincidence of p53-positive cells and apoptotic cells after irradiation at 8 Gy. 25 The greater number of p53-positive cells observed after irradiation in the current study may be explained by a different immunostaining protocol and a different batch of cm5 antibody. Arai et al 34 have also reported the positional association of p53 expression and apoptosis. The distribution of cells that were weakly/moderately positive for p53 was positionally coincident with p21WAF-1/CIP1-positive cells, 4 hours after exposure to 8 Gy γ-radiation. This cell population showed little frequency of apoptosis.
The apoptotic response in the large intestinal epithelium was attenuated relative to that in the small intestine. The acute response (at 4 hours) was very similar. However, at the later time points, apoptotic events were much less frequent in the large bowel. This was associated with a greater percentage of cells within the large bowel crypts showing radiation-induced p21WAF-1/CIP1 expression and a lower frequency of p53-positive cells, especially those demonstrating strong immunoreactivity.
These data support the hypothesis that the fate of an individual cell to undergo either p53-mediated growth arrest or apoptosis in response to γ-radiation is dependent on the concentration of active p53 protein, with high p53 expression resulting in apoptosis and low p53 expression resulting in growth arrest. Similar proposals regarding p53 action have been put forward by others using in vitro models. 35-37 At levels of p53 expression below that capable of inducing neither growth arrest nor apoptosis, p53 has been proposed to suppress apoptosis 36 and to promote differentiation. 37 Such a hypothesis suggests that p53-binding domains, within promoter sequences of p53-regulated genes, display different affinities for binding p53. It could be, therefore, that the p53-binding domain of the p21WAF-1/CIP1 has a higher affinity for p53 than that of the p53-regulatory sequences in genes that regulate the apoptotic process within the cell, as originally suggested by Chen et al. 35 This is certainly true for mutant forms of p53. 38,39 Studies by Gottlieb et al 40 suggest that p53 function may show cell- and tissue-specific regulation in vivo.
What determines the degree of p53 expression within the different cell populations in the small intestinal crypts and the intestinal epithelium as a whole? Factors that could affect the degree of p53 protein expression include the ability of the cell to detect DNA damage and the efficiency of signal transduction pathways between DNA damage-recognition proteins and effector proteins such as p53. The failure of such pathways is clearly illustrated in individuals with ataxia telangiectasia. 3 Transcriptional regulation of the p53 gene itself through signal transduction pathways may also be of some importance. Studies by Komarova et al 41 suggest that the absolute level of p53 mRNA transcript within a cell directly determines the ability of the cell to up-regulate and maintain p53 protein levels. Finally, regulation may occur by the targeting of p53 protein for inactivation/degradation via the binding of mdm-2. 42-45
Cell- and tissue-specific variation in the efficacy of p53 to execute either cell cycle arrest or apoptosis could be determined by the expression of other proteins that directly interact with p53 to affect its transcriptional activity. Examples of such proteins are IRF-1 46 and p33ING1, 47 both of which have been shown to be essential for the transcriptional activity of p53 in vitro.
It has been demonstrated that there is an association between the expression of p21WAF-1/CIP1 and attenuation of apoptosis. Waldman et al 48,49 have shown that after treatment with agents such as Adriamycin and γ-radiation, the human colorectal tumor cell line HCT116, which has transcriptionally functional p53, underwent cell cycle arrest. However, cell clones with nonfunctional p21WAF-1/CIP1 underwent apoptosis.48. Waldman et al 49 also demonstrated this effect of p21WAF-1/CIP1 expression in vivo, using HCT116 xenografts in nude mice. Similar results using the same colorectal cell line were obtained by Wouters et al. 50 Studies by Polyak et al 51 and Chen et al 35 suggest that enforced expression of wt p53 in tumor cell lines can induce apoptosis irrespective of p21WAF-1/CIP1 status. These studies suggest, therefore, that p21WAF-1/CIP1 does not provide a dominant signal for the suppression of apoptosis and fit well with observations of cell- and tissue-dependent efficacy of p53-mediated transcriptional activation. 40
One of the major differences between the response of the large and small intestinal epithelial cells to γ-radiation was the relative longevity of p53 and p21WAF-1/CIP1 expression observed in the large bowel. One possible reason is that cell proliferation within the crypts of the large intestine is much slower compared with the crypts of the small intestine (cell cycle times are 12 hours and 35 hours for crypt epithelium from murine small and large intestine, respectively: also, the number of cells undergoing DNA synthesis at anyone time is two to three times greater in the small intestine). 52 Therefore, labeled cells migrate more slowly up the crypts in the large intestine. The large intestinal crypts are also longer than those in the small intestine (large intestinal crypt length is approximately 45 cells, compared with small intestinal crypt length, which is approximately 25 cells). Immunoreactivity is, therefore, observed for a longer period in the large intestinal crypt epithelium.
In summary, γ-radiation exposure resulted in apoptosis and a reduction in the fraction of proliferating cells, as indicated by a decrease in [3H]thymidine incorporation. These cellular responses were associated with a time- and dose-dependent increase in the expression of p53 and p21WAF-1/CIP1. Heterogeneity in the response of the small intestinal epithelium to γ-radiation was observed. Cells at the base of the small intestinal crypts showed strong p53 expression and a higher frequency of apoptosis relative to the cells toward the top of the crypt. The latter showed primarily weak p53 expression that was correlated with an increased frequency of p21WAF-1/CIP1 expression and cell survival. Regional variation was also noted in the response of the intestinal epithelium to ionizing radiation. The small bowel showed a greater increase in radiation-induced p53 expression, relative to the large bowel, with greater numbers of p53 positive cells at all time points up to 48 hours. A larger proportion of cells that were strongly positive for p53 was also observed in the small bowel. In contrast, a greater number of p21WAF-1/CIP1-positive cells were found in the large intestinal epithelium and also in the top half of the small intestinal crypts; this was associated with a lower frequency of radiation-induced apoptosis in these regions. Coincidence between p21WAF-1/CIP1 expression and resistance to γ-radiation-induced apoptosis has been observed by others. 48-51 However, it has been shown that p21WAF-1/CIP1 does not act as a dominant suppressor of apoptosis. 35,51 It would appear, therefore, that resistance to γ-radiation-induced apoptosis is related to a reduced ability to increase functional p53 to a level sufficient to initiate apoptosis.
Footnotes
Address reprint requests to Dr. James W. Wilson, CRC Epithelial Biology Laboratory, Cell & Tumor Biology Section, Paterson Institute for Cancer Research, Manchester M20 4BX, United Kingdom. E-mail: jwilson@picr.man.ac.uk.
Supported by the Cancer Research Campaign. DMP is funded by the British Digestive Foundation and the Medical Research Council.
References
- 1.Jackson SP: The recognition of DNA damage. Curr Opin Genet Dev 1996, 6:19-25 [DOI] [PubMed] [Google Scholar]
- 2.Kastan MB, Onyckwere O, Sidransky D, Vogelstein B, Craig RW: Participation of p53 protein in the cellular response to DNA damage. Cancer Res 1991, 51:6304-6311 [PubMed] [Google Scholar]
- 3.Kastan MB, Zhan Q, El-Deiry WS, Carrier F, Jacks T, Walsh WV, Plunkett BS, Vogelstein B, Fornace AJ, Jr: A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia telangiectasia. Cell 1992, 71:587-597 [DOI] [PubMed] [Google Scholar]
- 4.Wood RD: DNA repair in eukaryotes. Annu Rev Biochem 1996, 65:135-167 [DOI] [PubMed] [Google Scholar]
- 5.Reed JC: Double identity for proteins of the Bcl-2 family. Nature 1997, 387:773-776 [DOI] [PubMed] [Google Scholar]
- 6.Maltzman W, Czyzyk L: UV irradiation stimulates levels of p53 cellular tumor antigen in non-transformed mouse cells. Mol Cell Biol 1984, 4:1689-1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tishler RB, Calderwood SK, Coleman CN, Price BD: Increases in sequence-specific DNA binding by p53 following treatment with chemotherapeutic and DNA-damaging agents. Cancer Res 1993, 53 (Suppl 10):2212-2216 [PubMed] [Google Scholar]
- 8.Graeber TG, Peterson JF, Tsai M, Monica K, Fornace AJ, Jr, Giaccia AJ: Hypoxia induces accumulation of p53 protein, but activation of a G1-phase checkpoint by low-oxygen conditions is independent of p53 status. Mol Cell Biol 1994, 14:6264-6277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amson RB, Nemani M, Roperch J-P, Israeli D, Bougueleret L, LeGall I, Medhioub M, Linares-Cruz G, Lethrosne F, Pasturaud P, Piouffre L, et al: Isolation of 10 differentially expressed cDNAs in p53-induced apoptosis: activation of the vertebrate homologue of the Drosophila seven in absentia gene. Proc Natl Acad Sci USA 1996, 93:3953-3957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B: A model for p53-induced apoptosis. Nature 1997, 389:300-305 [DOI] [PubMed] [Google Scholar]
- 11.Murphy M, Hinman A, Levine AJ: Wild type p53 negatively regulates the expression of a microtubule-associated protein. Genes Dev 1996, 10:2971-2980 [DOI] [PubMed] [Google Scholar]
- 12.El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B: WAF1, a potential mediator of p53 tumor suppression. Cell 1993, 75:817-825 [DOI] [PubMed] [Google Scholar]
- 13.Dulic V, Kauffman WK, Wilson SJ, Tisty TD, Lees E, Harper JW, Elledge SJ, Reed SI: p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell 1994, 76:1013-1023 [DOI] [PubMed] [Google Scholar]
- 14.Selvakumaran M, Lin H-K, Miyashita T, Wang HG, Krajewski S, Reed JC, Hoffman B, Liebermann D: Immediate early up-regulation of bax expression by p53 but not TGFβ1: a paradigm for distinct apoptotic pathways. Oncogene 1994, 9:1791-1798 [PubMed] [Google Scholar]
- 15.Zhan Q, Fan S, Bae I, Guillouf C, Liebermann DA, O’Connor PM, Fornace AJ: Induction of bax by genotoxic stress in human cells correlates with normal p53 status and apoptosis. Oncogene 1994, 9:3743-3751 [PubMed] [Google Scholar]
- 16.Miyashita T, Reed JC: Tumour suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 1995, 80:293-299 [DOI] [PubMed] [Google Scholar]
- 17.Wu G-S, Burns TF, McDonald ER, III, Jiang W, Meng R, Krantz ID, Kao G, Gan D-D, Zhou J-Y, Muschel R, Hamilton SR, Spinner NB, Markowitz S, Wu G, El-Deiry WS: KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nat Genet 1997, 17:141-143 [DOI] [PubMed] [Google Scholar]
- 18.Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ: Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature 1995, 377:552-557 [DOI] [PubMed] [Google Scholar]
- 19.Waldman T, Kinzler KW, Vogelstein B: p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res 1995, 55:5187-5190 [PubMed] [Google Scholar]
- 20.Del Sal G, Murphy M, Ruaro EM, Lazarevic D, Levine AJ, Schneider C: Cyclin D1 and p21/waf1 are both involved in p53 growth suppression. Oncogene 1996, 12:177-185 [PubMed] [Google Scholar]
- 21.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D: p21 is a universal inhibitor of cyclin kinases. Nature 1993, 366:701-704 [DOI] [PubMed] [Google Scholar]
- 22.Waga S, Hannon GJ, Beach D, Stillman B: The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature 1994, 369:574-578 [DOI] [PubMed] [Google Scholar]
- 23.Noda A, Ning Y, Venable SF, Pereira-Smith OM, Smith JR: Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res 1994, 211:90-98 [DOI] [PubMed] [Google Scholar]
- 24.Brown JP, Wei W, Sedivy J: Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts. Science 1997, 277:831-834 [DOI] [PubMed] [Google Scholar]
- 25.Merritt AJ, Potten CS, Kemp CJ, Hickman JA, Balmain A, Lane DP, Hall PA: The role of p53 in spontaneous and radiation-induced apoptosis in the gastrointestinal tract of normal and p53-deficient mice. Cancer Res 1994, 54:614-617 [PubMed] [Google Scholar]
- 26.Merritt AJ, Allen TD, Potten CS, Hickman JA: Apoptosis in small intestinal epithelia from p53-null mice: evidence for a delayed, p53-independent G2/M-associated cell death after γ-irradiation. Oncogene 1997, 14:2759-2766 [DOI] [PubMed] [Google Scholar]
- 27.Clarke AR, Gledhill S, Hooper ML, Bird CC, Wyllie AH: p53 dependence of early apoptotic and proliferation responses within the mouse intestinal epithelium following γ-irradiation. Oncogene 1994, 9:1767-1773 [PubMed] [Google Scholar]
- 28.Clarke AR, Howard LA, Harrison DJ, Winton DJ: p53, mutation frequency and apoptosis in the murine small intestine. Oncogene 1997, 14:2015-2018 [DOI] [PubMed] [Google Scholar]
- 29.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A: Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 1992, 356:215-221 [DOI] [PubMed] [Google Scholar]
- 30.Wilson JW, Potten CS: Immunohistochemical localization of BAX and BAD in the normal and BCL-2 null gastrointestinal tract. Apoptosis 1996, 1:183-190 [Google Scholar]
- 31.Ijiri K, Potten CS: Response of intestinal cells of differing topographical and hierarchical status to ten cytotoxic drugs and five sources of radiation. Br J Cancer 1983, 47:175-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kerr JFR, Wyllie AH, Currie AR: Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 1972, 26:239-257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Potten CS: Effects of radiation on murine gastrointestinal cell proliferation. Potten CS Hendry JH eds. Radiation and Gut. 1995, :pp 61-84 Elsevier Science BV, Amsterdam [Google Scholar]
- 34.Arai T, Kida Y, Harmon BV, Gobe GC: Comparative alterations in p53 expression and apoptosis in the irradiated rat small and large intestine. Br J Cancer 1996, 74:406-412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen X, Ko LJ, Jayaraman L, Prives C: p53 levels, functional domains and DNA damage determine the extent of the apoptotic response of tumor cells. Gene Dev 1996, 10:2438-2451 [DOI] [PubMed] [Google Scholar]
- 36.Lassus P, Ferlin M, Piette J, Hibner U: Anti-apoptotic activity of low levels of wild-type p53. EMBO J 1996, 15:4566-4573 [PMC free article] [PubMed] [Google Scholar]
- 37.Ronen D, Schwartz D, Teitz Y, Goldfinger N, Rotter V: Induction of HL-60 cells to undergo apoptosis is determined by high levels of wild-type p53 protein, whereas differentiation of the cells is mediated by lower p53 levels. Cell Growth Differ 1996, 7:21-30 [PubMed] [Google Scholar]
- 38.Friedlander P, Haupt Y, Prives C, Oren M: A mutant p53 that discriminates between p53-responsive genes cannot induce apoptosis. Mol Cell Biol 1996, 16:4961-4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ludwig RL, Bates S, Vousden KH: Differential activation of target cellular promoters by p53 mutants with impaired apoptotic function. Mol Cell Biol 1996, 16:4952-4960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gottlieb E, Haffner R, King A, Asher G, Gruss P, Lonai P, Oren M: Transgenic mouse model for studying the transcriptional activity of the p53 protein: age- and tissue-dependent changes in radiation-induced activation during embryogenesis. EMBO J 1997, 16:1381-1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Komarova EA, Chernov MV, Franks R, Wang K, Armin G, Zelnick CR, Chin DM, Bacus SS, Strark GR, Gudkov AV: Transgenic mice with p53-responsive lacZ: p53 activity varies dramatically during normal development and determines radiation and drug sensitivity in vivo. EMBO J. 1997, 16:1391-1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen J, Wu X, Lin J, Levine AJ: mdm-2 inhibits the G1 arrest and apoptosis functions of the p53 tumor suppressor protein. Mol Cell Biol 1996, 16:2445-2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haupt Y, Barak Y, Oren M: Mdm2 promotes the rapid degradation of p53. Nature 1997, 387:296-299 [DOI] [PubMed] [Google Scholar]
- 44.Midgley CA, Lane DP: p53 protein stability in tumour cells is not directly determined by mutation but is dependent on Mdm2 binding. Oncogene 1997, 15:1179-1189 [DOI] [PubMed] [Google Scholar]
- 45.Shieh S-Y, Ikeda M, Taya Y, Prives C: DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell 1997, 91:325-334 [DOI] [PubMed] [Google Scholar]
- 46.Tanaka N, Ishihara M, Lamphier MS, Nozawa H, Matsuyama T, Mak TW, Aizawa S, Tokino T, Oren M, Taniguchi T: Cooperation of the tumour suppressors IRF-1 and p53 in response to DNA damage. Nature 1996, 382:816-818 [DOI] [PubMed] [Google Scholar]
- 47.Garkavtsev I, Grigorian IA, Ossovskaya VS, Chernov MV, Chumakov PM, Gudkov AV: The candidate tumour suppressor p33ING1 cooperates with p53 in cell growth control. Nature 1998, 391:295-298 [DOI] [PubMed] [Google Scholar]
- 48.Waldman T, Lengauer C, Kinzler KW, Vogelstein B: Uncoupling of S phase and mitosis induced by anticancer agents in cells lacking p21. Nature 1996, 381:713-716 [DOI] [PubMed] [Google Scholar]
- 49.Waldman T, Zhang Y, Dillehay L, Yu J, Kinzler K, Vogelstein B, Williams J: Cell-cycle arrest versus cell death in cancer therapy. Nat Med 1997, 3:1034-1036 [DOI] [PubMed] [Google Scholar]
- 50.Wouters BG, Giaccia AJ, Denko NC, Brown JM: Loss of p21WAF-1/CIP1 sensitizes tumors to radiation by an apoptosis-independent mechanism. Cancer Res 1997, 57:4703-4706 [PubMed] [Google Scholar]
- 51.Polyak K, Waldman T, He T-C, Kinzler KW, Vogelstein B: Genetic determinants of p53-induced apoptosis and growth arrest. Genes Dev 1996, 10:1945-1952 [DOI] [PubMed] [Google Scholar]
- 52.Potten CS: Structure, function and proliferative organisation of mammalian gut. Potten CS Hendry JH eds. Radiation and Gut. 1995, :pp 1-31 Elsevier Science BV, Amsterdam [Google Scholar]