Abstract
Mouse mast cell protease (MMCP) mRNA expression was examined by in situ hybridization histochemistry. Peritoneal mast cells (PMCs) of WBB6F1-+/+ mice expressed MMCP-2, MMCP-4, MMCP-5, and MMCP-6 mRNAs, but did not express MMCP-1 mRNA. When proliferation of PMCs was induced by culturing them in methylcellulose with T cell-derived cytokines, cells in mast cell colonies expressed MMCP-1 mRNA. These mast cells were transferred to a suspension culture to induce further proliferation. The phenotype of the resulting PMC-derived cultured mast cells was similar to that of bone marrow-derived cultured mast cells. When 105 PMC-derived cultured mast cells or 105 bone marrow-derived cultured mast cells were injected into the stomach wall of mast cell-deficient WBB6F1-W/Wv mice, mast cells that appeared in the mucosa and muscularis propria were similar to mast cells in the stomach of intact WBB6F1-+/+ mice, indicating the injected cells adapted to a new tissue environment. In contrast, when 105 PMCs were injected into the stomach wall of WBB6F1-W/Wv mice, the injected PMCs did not adapt to the mucosa. When 20 PMCs were injected, they proliferated and adapted to the mucosal environment. The present results suggest that PMCs adapt to new environments when proliferation occurs before redifferentiation.
Six distinct serine proteases designated mouse mast cell proteases (MMCP) 1, 2, 4, 5, 6, and 7 and mouse mast cell carboxypeptidase A have been identified in the secretory granules of mouse mast cells by protein sequencing and/or by cDNA cloning. 1-7 In situ hybridization histochemistry of mast cell proteases has been previously used for characterization of mast cell phenotypes. 8-10 For example, MMCP-2 mRNA is expressed by mast cells located in the mucosa of the stomach of (WB × C57BL/6)F1-+/+ (hereafter, WBB6F1-+/+) mice but not by mast cells in the muscularis propria. 10 On the other hand, mRNAs of MMCP-4 and MMCP-6 are not expressed by mast cells in the mucosa but are expressed by cells in the muscularis propria of WBB6F1-+/+ mice. 10
We investigated changes in protease expression phenotype of mast cells after transplantation of bone marrow-derived cultured mast cells (BMCMCs) or peritoneal mast cells (PMCs) of WBB6F1-+/+ mice into tissues of mast cell-deficient WBB6F1-W/Wv mice. 10-13 When BMCMCs that expressed MMCP-2 mRNA were transplanted into the stomach wall, mast cells that appeared in the mucosa expressed MMCP-2 mRNA, but mast cells that appeared in the muscularis propria did not, indicating BMCMC adaptation to a new tissue environment. However, PMCs that also expressed MMCP-2 mRNA did not adapt to the muscularis propria. MMCP-2 mRNA continued to be expressed after settlement in the mucosa and muscularis propria of WBB6F1-W/Wv mice. PMCs did not alter their MMCP-4 and MMCP-6 mRNA expression phenotypes after settlement in the mucosa and the muscularis propria of WBB6F1-W/Wv mice. Although mast cells in the stomach mucosa of WBB6F1-+/+ mice did not express MMCP-4 and MMCP-6 mRNAs, approximately one-half of mast cells in the stomach mucosa of WBB6F1-W/Wv recipient mice expressed MMCP-4 and MMCP-6 mRNAs after transplantation of PMCs of WBB6F1-+/+ mice. 10 Although 10 5 BMCMCs were proliferating before transplantation, 12 the injection of 10 5 PMCs in this limited area appeared to inhibit their own proliferation. 13
BMCMCs are undifferentiated and uncommitted cells that can adapt to new environments, whereas PMCs are differentiated and committed cells that can no longer adapt. BMCMCs proliferate in suspension culture containing T cell-derived cytokines, but naive PMCs do not. We examined whether proliferation of mast cells influenced their adaptability. Three approaches were used to induce proliferation: 1) generating cultured mast cells from PMCs (PCMCs) by plating PMCs in methylcellulose containing T cell-derived cytokines and then transferring them to suspension culture, 2) helminth infection, and 3) transplantation of a few (20) instead of many (105) PMCs into the stomach wall of WBB6F1-W/Wv mice. All three procedures induced proliferation and phenotype changes. We used BMCMCs as the undifferentiated and uncommitted control and a large number of PMCs as the differentiated and committed control. In transplantation experiments, we used WBB6F1-+/+ mice as a control of in vivo phenotype. We compared the MMCP-expression phenotype of transplanted mast cells of WBB6F1-+/+ mouse origin with mast cells in tissues of WBB6F1-+/+ mice.
Materials and Methods
Mice
WBB6F1-+/+ and-W/Wv were raised by crossing WB-W/+ females and C57BL/6-Wv/+ males at Japan SLC (Hamamatsu, Japan). The resulting WBB6F1-+/+ and-W/Wv mice were identified by their coat color. WBB6F1-W/Wv mice have white hair and black eyes and are genetically deficient in mast cells. 11,14,15 Mice were used at 2 to 4 months of age and killed by decapitation under ether anesthesia.
Establishment of BMCMCs
Pokeweed mitogen-stimulated spleen cell-conditioned medium (PWM-SCM) was prepared as described by Nakahata et al. 16 To obtain BMCMCs, bone marrow cells were harvested from 2-month-old WBB6F1-+/+ mice. Culture flasks (Nunc, Roskilde, Denmark) containing 1 × 106/ml bone marrow cells in α-minimal essential medium (α-MEM; ICN Biomedicals, Costa Mesa, CA) supplemented with 10−4 mol/L 2-mercaptoethanol, 10% PWM-SCM, and 10% fetal bovine serum (Nippon Bio-Supply Center, Tokyo, Japan) were incubated at 37°C in a humidified atmosphere of 5% CO2 in air. One-half of the medium was replaced every 7 days, and more than 95% of cells were BMCMCs 4 weeks after culture initiation.
Purification of PMCs and Establishment of PCMCs
Purification of PMCs was performed according to the method described by Yurt et al. 17 In brief, peritoneal cells (6 to 10 × 107) were suspended in 1 ml of Tyrode’s buffer, layered on 2 ml of 22.5% (w/v) metrizamide (density, 1.120 g/ml, Sigma Chemical Co., St. Louis, MO), and centrifuged at room temperature for 15 minutes at 400 × g. The cells remaining at the buffer-metrizamide interface were aspirated and discarded; the cells in the pellet were washed and resuspended in 1 ml of Tyrode’s buffer. Mast cells represented 70 to 80% of the nucleated cells in this preparation. To obtain PMC suspensions of ≥99% purity, the procedure just described was repeated using the 70 to 80% pure mast cell suspensions. Cells were counted with a standard hemocytometer. Purified PMCs were identified by phase-contrast microscopy.
Clonal cell culture in methylcellulose (Sigma) was carried out according to the method described by Kanakura et al. 18 One ml of a culture mixture containing 10 3 PMCs, α-MEM, 1% methylcellulose, 30% fetal bovine serum, 1% deionized bovine serum albumin (Sigma), 10−4 mol/L 2-mercaptoethanol, and 10% (v/v) PWM-SCM was plated in 35-mm tissue culture dishes. Dishes were incubated at 37°C in a humidified atmosphere flushed with 5% CO2 in air. Mast cell colonies were counted on day 14, and individual colonies were lifted from methylcellulose medium using a 3-μl Eppendorf pipette under direct microscopic visualization and were collected in Eppendorf microcentrifuge tubes containing 0.5 ml of α-MEM. After washing two times with α-MEM, the cells were used for cytological examination and further amplified in suspension culture. The cells from each mast cell colony were seeded in single wells in 24-well microtiter plates (Corning Costar, Tokyo, Japan) containing 0.4 ml α-MEM supplemented with 10−4 mol/L 2-mercaptoethanol, 20% fetal bovine serum, 0.2% deionized bovine serum albumin, and 10% PWM-SCM. Culture plates were incubated at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Half of the medium was replaced every 7 days. When significant proliferation occurred in a well of the 24-well plate, the cells in the well were transferred to a well in a 6-well microtiter plate (Corning). Eight weeks after initiation of the suspension culture, we selected the wells in which PCMCs proliferated to ≥10 5 cells. Cells contained in two or three wells were pooled and used for cytological examination and transplantation to the stomach wall.
Preparation of Probes for in Situ Hybridization
Total RNA was extracted from BMCMCs of WBB6F1-+/+ mice. Single-strand cDNA was generated with a specific antisense primer for MMCP-2, 8 MMCP-4, 19 MMCP-5, 20 and MMCP-6 8,19 by reverse transcriptase (Takara, Kyoto, Japan) from total RNA. Each cDNA was amplified by a Perkin-Elmer Cetus (Norwalk, CT) DNA thermal cycler using Taq DNA polymerase (Takara). 19 PCR products were subcloned into the EcoRV site of the Bluescript KS(−) plasmid (Stratagene, La Jolla, CA), which contains T3 and T7 promoters to generate riboprobes, and the sequence was confirmed with a model 373A DNA sequencer (Applied Biosystems, Foster City, CA). The MMCP-1 plasmid was kindly donated by Dr. J. Wastling (Department of Veterinary Clinical Studies, University of Edinburgh, Edinburgh, United Kingdom). The plasmid was either linearized with HincII and transcribed with T7 RNA polymerase to generate an antisense probe or linearized with EcoRI and transcribed with T3 RNA polymerase to generate a sense probe.
In Situ Hybridization
BMCMCs, mast cells in PMC-derived colonies, PCMCs, and PMCs were each collected, washed with phosphate-buffered saline, and mixed with 2% agarose (FMC BioProducts, Rockland, ME). The mixture was fixed with freshly prepared 4% paraformaldehyde in 0.1 mol/L phosphate buffer (pH 7.4) overnight and then dehydrated and embedded in paraffin. The stomachs of WBB6F1-W/Wv mice were removed 5 weeks after injection of mast cells, opened, and flattened onto a rubber plate. Fixation and embedding of the tissues was performed by the procedure used for the suspended cells. Serial sections (4 μm thick) were cut, and odd-number sections were stained with alcian blue and nuclear fast red to identify mast cells and even-number sections were used for in situ hybridization. Hybridization was carried out as described previously with minor modifications. 21 Digoxigenin-labeled single-strand RNA probes were prepared using a DIG RNA labeling kit (Boehringer Mannheim GmbH Biochemica, Mannheim, Germany) according to the manufacturer’s instructions. Controls included 1) hybridization with the sense probe, 2) RNase A treatment (20 μg/ml) before hybridization, and 3) withholding of the antisense RNA probe and the anti-digoxigenin antibody. 21 None of the three controls showed any positive signals.
Proportion of Protease mRNA-Expressing Mast Cells
The number of alcian blue-positive mast cells and that of MMCP-1, MMCP-2, MMCP-4, MMCP-5, or MMCP-6 mRNA-expressing cells were counted in adjacent sections. In cases in which the number of mast cells in adjacent sections were few, data from many pairs of adjacent sections were pooled.
Infection of Helminth
The strain of Strongyloides venezuelensis used in this study was originally isolated from a wild brown rat in Okinawa, Japan, established as a laboratory strain, 22 and is now maintained in the Miyazaki Medical College with serial passages in Wistar rats. 23 Third-stage infective larvae (L3) were obtained from fecal culture by the filter paper method. 23 The degree of infection was monitored daily by egg excretion in feces from five animals. WBB6F1-+/+ mice were infected by subcutaneous injections of 1000 L3. Mice were killed on day 12, and the stomach was removed and used for in situ hybridization histochemistry.
Transplantation into the Stomach Wall
Recipient WBB6F1-W/Wv mice were anesthetized with Nembutal, the peritoneal cavity was opened, and the stomach was exposed. BMCMCs, PCMCs or PMCs from WBB6F1-+/+ mice were counted with a standard hemocytometer and injected into the wall of the glandular stomach of WBB6F1-W/Wv mice. 10,24 Cells (10 5 or 20) suspended in 0.1 ml α-MEM were injected with a tuberculin syringe. Each mouse received two injections recorded by tattooing with India ink. WBB6F1-W/Wv mice were killed 5 weeks after injections. Injection sites identified by the presence of India ink were removed. Serial sections were made, and one section was stained with alcian blue and nuclear fast red, and the next section was used for in situ hybridization. Numbers of mast cells were counted with a microscope. When we injected 20 PMCs, the section whose adjacent section contained ≥50 alcian blue-positive mast cells was used for in situ hybridization.
Results
PMCs expressed MMCP-2, MMCP-4, MMCP-5, and MMCP-6 mRNAs but did not express MMCP-1 mRNA. We plated 10 3 PMCs in methylcellulose containing PWM-SCM to induce proliferation, and approximately 25% of PMCs formed colonies. Half of the mast cells in large mast cell colonies (≥64 cells) expressed MMCP-1 mRNA (Table 1 ▶ and Figure 1 ▶ ). Large colonies were transferred to suspension culture for expansion. Approximately 40% of large colonies generated ≥10 5 mast cells after 8 weeks of growth. The phenotype of the resulting PCMCs was similar to that of mast cells in PMC-derived colonies and BMCMCs (Table 1) ▶ .
Table 1.
Proportion of Mast Cells That Expressed MMCP of Each Type in Various Mast Cell Populations of WBB6F1-+/+ Mice
| Mast cells | Proportion of protease mRNA-expressing cells to alcian-blue positive cells (%) | ||||
|---|---|---|---|---|---|
| MMCP-1 | MMCP-2 | MMCP-4 | MMCP-5 | MMCP-6 | |
| PMCs | 0 | 59 | 80 | 81 | 82 |
| Mast cells in PMC-derived colonies* | 52† | 75‡ | 74 | 73 | 79 |
| PCMCs | 68† | 83† | 68 | 69 | 78 |
| BMCMCs | 73† | 92† | 67 | 72 | 80 |
Ninety to 100 mast cells of each type were scored.
*One hundred mast cell colonies were pooled.
†P < 0.01, when compared with the value of PMCs by χ2 test.
‡P < 0.05, when compared with the value of PMCs by χ2 test.
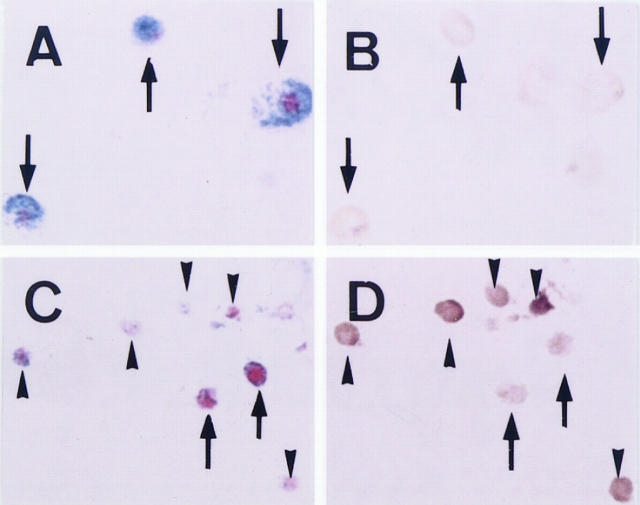
Figure 1.
Expression of MMCP-1 mRNA demonstrated by in situ hybridization. A: PMCs of WBB6F1-+/+ mice stained with alcian blue and nuclear fast red. B: Section adjacent to that shown in A. Arrows in A and B show the same cells that do not express MMCP-1 mRNA. C: Mast cells in PMC-derived colonies of WBB6F1-+/+ mouse origin stained with alcian blue and nuclear fast red. Mast cells in PMC-derived colonies are significantly smaller than PMCs. Moreover, alcian blue-positive granules are significantly fewer in the former than in the latter. D: Section adjacent to that shown in C. Arrows and arrowheads in C and D show the same cells. Arrows show the cells that do not express MMCP-1 mRNA, and arrowheads show the cells that express MMCP-1 mRNA. Magnification, ×500.
MMCP-1 mRNA was not expressed by mast cells in the stomach mucosa of intact mice. Because MMCP-1 mRNA appeared to be expressed after stimulation by T-cell-derived cytokines, we investigated the effect of helminth infection on the expression of MMCP-1 mRNA. When WBB6F1-+/+ mice were infected with S. venezuelensis, mast cells in the stomach mucosa remarkably increased in number, and more than half of the mast cells expressed MMCP-1 mRNA (Figure 2) ▶ .
Figure 2.
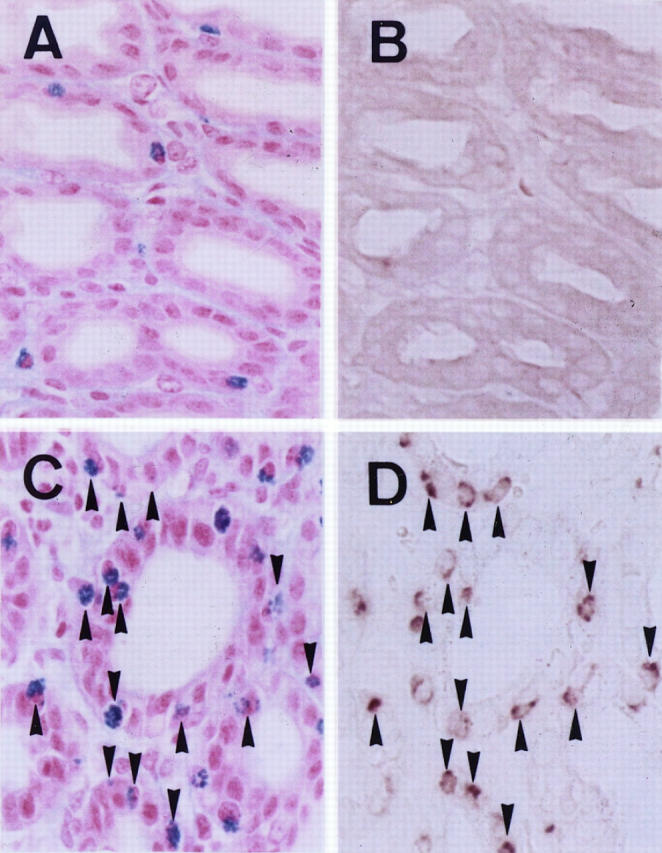
Effect of S. venezuelensis infection on the MMCP-1 mRNA expression in the stomach mucosa of WBB6F1-+/+ mice, demonstrated by in situ hybridization. A: Stomach mucosa of nontreated mouse stained with alcian blue and nuclear fast red. B: Section adjacent to that shown in A; no MMCP-1 mRNA-expressing cells were observed. C: Stomach mucosa of the infected mouse stained with alcian blue and nuclear fast red. D: Section adjacent to that shown in C. Many mast cells expressing MMCP-1 mRNA were observed. Arrowheads indicate the same mast cells in the paired photographs. Magnification, ×375.
The protease mRNA expression phenotype of mast cells differed between the mucosa and muscularis propria of the stomach of WBB6F1-+/+ mice. 10 Mast cells in the mucosa expressed MMCP-2 mRNA but did not express MMCP-4, MMCP-5, or MMCP-6 mRNAs. On the contrary, mast cells in the muscularis propria did not express MMCP-2 mRNA but did express MMCP-4, MMCP-5, and MMCP-6 mRNAs. In previous experiments, we injected 10 5 PMCs into the stomach wall of mast cell-deficient WBB6F1-W/Wv mice 10 and found that the protease expression phenotype of PMCs did not adapt to the new environment. MMCP-2 mRNA continued to be expressed in the muscularis propria, and MMCP-4 and MMCP-6 mRNA continued to be expressed in the mucosa. In the present experiment, we injected 20 PMCs into the stomach wall of WBB6F1-W/Wv mice. Mast cells that appeared in the muscularis propria continued to express MMCP-2 mRNA, but the proportion of MMCP-2 mRNA-expressing cells was significantly decreased (Table 2) ▶ . The expression of MMCP-4, MMCP-5, and MMCP-6 mRNAs was abolished in the mucosa (Table 2) ▶ .
Table 2.
Proportion of Mast Cells that Expressed MMCP of Each Type in the Stomach of WBB6F1-W/Wv Mice after Transplantation of Various Mast Cell Populations of WBB6F1-+/+ Mouse Origin
| Mast cells | No. of injected cells | Proportion of protease mRNA-expressing cells to alcian-blue positive cells (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MMCP-1 | MMCP-2 | MMCP-4 | MMCP-5 | MMCP-6 | |||||||
| Mucosa* | Muscularis | Mucosa* | Muscularis | Mucosa* | Muscularis | Mucosa* | Muscularis | Mucosa* | Muscularis | ||
| Mast cells in+/+ mice | 0 | 0 | 0 | 89 | 0† | 0† | 42 | 0† | 50 | 0† | 51 |
| BMCMCs‡ | 105 | 10†§ | 0 | 93 | 3† | 0† | 46 | 0† | 58 | 0† | 59 |
| PCMCs‡ | 105 | 6¶∥ | 0 | 87 | 10†§ | 0† | 41 | 0† | 53 | 0† | 52 |
| PMCs‡ | 20 | 0 | 0 | 78 | 32†§ | 0† | 43 | 0† | 49 | 0† | 52 |
| PMCs‡ | 105 | 0 | 0 | 83 | 74§ | 45§ | 43 | 53§ | 54 | 56§ | 54 |
*Pooled data of two or three injection sites when BMCMCs, PCMCs, 20 PMCs, or 105 PMCs were injected. Eighty-five to 104 mast cells were scored.
†P < 0.01, when compared with the value of mast cells that appeared in the stomach of WBB6F1-W/Wv mice after injection of 105 PMCs of WBB6F1-+/+ mice.
‡Results obtained after injection of mast cells of each type into the stomach wall of WBB6F1-W/Wv mice.
§P < 0.01, when compared with the value of mast cells in the stomach of intact WBB6F1-+/+ mice.
¶P < 0.05, when compared with the value of mast cells that appeared in the stomach of WBB6F1-W/Wv mice after injection of 105 PMCs of WBB6F1-+/+ mice.
∥P < 0.05, when compared with the value of mast cells in the stomach of intact WBB6F1-+/+ mice.
We then transplanted 10 5 PCMCs or 10 5 BMCMCs derived from WBB6F1-+/+ mice into the stomach wall of WBB6F1-W/Wv mice. MMCP-1 mRNA continued to be expressed in mast cells in the mucosa but not in mast cells in the muscularis propria (Table 2) ▶ . The proportion of MMCP-1 mRNA-expressing mast cells in the mucosa was lower than that of PCMCs and BMCMCs (Tables 1 and 2) ▶ ▶ . Expression of MMCP-2 mRNA continued in mast cells in the mucosa and muscularis propria, but the proportion of MMCP-2 mRNA-expressing cells in the muscularis propria was significantly lower when 10 5 PCMCs were injected than when 10 5 PMCs were injected (Table 2) ▶ . The expression of MMCP-4, MMCP-5, and MMCP-6 mRNAs was abolished in mast cells in the mucosa of WBB6F1-W/Wv mice after the injection of 10 5 PCMCs. Injection of 10 5 PCMCs had results similar to those of injection of 10 5 BMCMCs.
We counted the number of mast cells in the mucosa and the muscularis propria of the stomach of WBB6F1-W/Wv mice 5 weeks after injection of 20 PMCs. The results shown in Table 3 ▶ indicate the proliferation of injected PMCs after settlement.
Table 3.
Number of Mast Cells Observed in the Stomach of WBB6F1-W/Wv Mice after the Transplantation of 20 PMCs of WBB6F1-+/+ Mice
| Injection sites | No. of mast cells per injection site | |
|---|---|---|
| Mucosa | Muscularis | |
| 1* | 310 | 940 |
| 2* | 980 | 1020 |
| 3 | 0 | 0 |
| 4 | 7 | 3 |
| 5 | 119 | 13 |
| 6 | 140 | 20 |
| 7 | 0 | 13 |
| 8 | 210 | 650 |
| 9* | 760 | 1440 |
| 10 | 0 | 20 |
| 11 | 3 | 2 |
| 12 | 24 | 0 |
Twenty peritoneal mast cells were injected into the stomach wall of 6 WBB6F1-W/Wvmice. Each mouse received two injections.
*Used to obtain results shown in Table 2 ▶ .
Discussion
Expression of MMCP-1 mRNA was observed in mast cells proliferating in the gastric mucosa of S. venezuelensis-infected mice. In contrast, mast cells in the mucosa of intact mice did not express MMCP-1 mRNA. This is consistent with the results of Scudamore et al, 25 which show that MMCP-1 expression is induced in mast cells in the gastric and intestinal mucosa by infection with Nippostrongyloides brasiliensis. Because the gastric mucosa is not infested with S. venezuelensis, MMCP-1 mRNA expression appeared to be induced by diffusible factors. In fact, Finkelman et al 26 reported that the expression of interleukin (IL)-3, IL-4, IL-5, IL-9, and IL-10 mRNAs increases in mesenteric lymph nodes and Peyer’s patches after infection with N. brasiliensis. Addition of IL-9 and IL-10 to culture medium has been shown to increase the expression of MMCP-1 mRNA in BMCMCs. 27,28 IL-9 and IL-10 may play a role in MMCP-1 mRNA induction in both mucosal mast cells of parasite-infected mice and BMCMCs.
PMCs did not express MMCP-1 mRNA, but one-half of PCMCs did express it. Approximately 10% of PMCs formed large mast cell colonies in methylcellulose, and ≥10 5 PCMCs developed from 40% of the large mast cell colonies. Roughly 4% of PMCs augmented up to 10 5 PCMCs and appeared to change the MMCP expression phenotype after proliferation. It is possible that mast cells with extensive proliferation potential may change the MMCP expression phenotype.
PCMCs were derived from PMCs, whereas BMCMCs originated from mast cell precursors in bone marrow. Despite differences of origin, MMCP expression phenotype of PCMCs and BMCMCs were similar. The MMCP expression phenotype appeared to be dependent on the microenvironment rather than on mast cell origin. It is believed that cytokines contained in the medium are the most important environmental factor. When 10 5 PCMCs, BMCMCs, or PMCs were transplanted into the stomach wall of WBB6F1-W/Wv mice, PCMCs behaved similarly to BMCMCs, not to PMCs. The proportion of MMCP-2 mRNA+ cells markedly decreased in the muscularis propria, and MMCP-4 mRNA+, MMCP-5 mRNA+ and MMCP-6 mRNA+ cells disappeared in the mucosa. After proliferation in culture, PMCs appeared to adapt to the new tissue environments.
When PMCs were injected into the stomach wall of mast cell-deficient WBB6F1-W/Wv mice, the MMCP expression phenotype of mast cells appeared to be influenced by the number of PMCs injected. The proportion of MMCP-2 mRNA+ cells in the muscularis propria was significantly lower after the injection of 20 PMCs than after the injection of 10 5 PMCs. Although MMCP-4 mRNA+, MMCP-5 mRNA+, or MMCP-6 mRNA+ mast cells remained in the mucosa of WBB6F1-W/Wv mice after injection of 10 5 PMCs, such mast cell types were not detectable after injection of 20 PMCs. Given that MMCP-2 mRNA+ mast cells were not detectable in the muscularis propria of WBB6F1-+/+ mice, and that MMCP-4 mRNA+, MMCP-5 mRNA+, or MMCP-6 mRNA+ mast cells were not detectable in the mucosa of WBB6F1-+/+ mice, PMCs appeared to adapt to the new tissue environment after transplantation of 20 but not after transplantation of 10 5 cells. Because at least a part of the 20 PMCs proliferated after injection into the stomach wall of WBB6F1-W/Wv mice, PMCs appeared to change the MMCP expression phenotype after proliferation. This is consistent with the results of Sonoda et al, 24 which demonstrate that chondroitin sulfate-containing mucosal mast cells develop after the injection of heparin-containing PMCs in the mucosa of WBB6F1-W/Wv mice. Glycosaminoglycan content is not a direct result of a single gene action. Moreover, the presence of heparin by individual mast cells was indirectly shown by staining with berberine sulfate. In situ hybridization histochemistry of MMCPs is a better method to clarify the change of mast cell phenotypes after the introduction into new tissue environment.
Taken together, PMCs may change the MMCP expression phenotype after proliferation either in culture or in tissues.
Acknowledgments
The authors thank Dr. J. Wastling of the Department of Veterinary Clinical Studies, University of Edinburgh, for MMCP-1 plasmid.
Footnotes
Address reprint requests to Dr. Yukihiko Kitamura, Department of Pathology, Osaka University Medical School, Yamada-oka, 2-2, Suita 565-0871, Osaka, Japan. Fax 81-6–879-3729. E-mail: kitamura@patho.med.osaka-u.ac.jp.
Supported by grants from the Ministry of Education, Science and Culture of Japan, the Ryoich Naito Foundation for Medical Research, and the Joint Research Project under the Japan-Korea Basic Scientific Promotion Program. Y-ML is a postdoctoral fellow supported by the Japan Society for the Promotion of Science.
References
- 1.Reynolds DS, Stevens RL, Gurley DS, Lane WS, Austen KF, Serafin WE: Isolation and molecular cloning of mast cell carboxypeptidase A: a novel member of the carboxypeptidase gene family. J Biol Chem 1989, 264:20094-20099 [PubMed] [Google Scholar]
- 2.Serafin WE, Reynolds DS, Rogelj S, Lane WS, Conder GA, Johnson SS, Austen KF, Stevens RL: Identification and molecular cloning of a novel mouse mucosal mast cell serine protease. J Biol Chem 1990, 265:423-429 [PubMed] [Google Scholar]
- 3.Serafin WE, Sullivan TP, Conder GA, Ebrahimi A, Marcham P, Johnson SS, Austen KF, Reynolds DS: Cloning of the cDNA and gene for mouse mast cell protease 4: demonstration of its late transcription in mast cell subclasses and analysis of its homology to subclass-specific neutral proteases of the mouse and rat. J Biol Chem 1991, 266:1934-1941 [PubMed] [Google Scholar]
- 4.Reynolds DS, Gurley DS, Austen KF, Serafin WE: Cloning of the cDNA and gene of mouse mast cell protease-6: transcription by progenitor mast cells and mast cells of the connective tissue subclass. J Biol Chem 1991, 266:3847-3853 [PubMed] [Google Scholar]
- 5.McNeil HP, Austen KF, Somerville LL, Gurish MF, Stevens RL: Molecular cloning of the mouse mast cell protease-5 gene: a novel secretory granule protease expressed early in the differentiation of serosal mast cells. J Biol Chem 1991, 266:20316-20322 [PubMed] [Google Scholar]
- 6.Huang R, Blom T, Hellman L: Cloning and structural analysis of MMCP-1, MMCP-4 and MMCP-5, three mouse mast cell-specific serine proteases. Eur J Immunol 1991, 21:1611-1621 [DOI] [PubMed] [Google Scholar]
- 7.McNeil HP, Reynolds DS, Schiller V, Ghildyal N, Gurley DS, Austen KF, Stevens RL: Isolation, characterization, and transcription of the gene encoding mouse mast cell protease 7. Proc Natl Acad Sci USA 1992, 89:11174-11178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kasugai T, Oguri K, Jippo-Kanemoto T, Morimoto M, Yamatodani A, Yoshida K, Ebi Y, Isozaki K, Tei H, Tsujimura T, Nomura S, Okayama M, Kitamura Y: Deficient differentiation of mast cells in the skin of mi/mi mice: usefulness of in situ hybridization for evaluation of mast cell phenotype. Am J Pathol 1993, 143:1337-1347 [PMC free article] [PubMed] [Google Scholar]
- 9.Isozaki K, Tsujimura T, Nomura S, Morii E, Koshimizu U, Nishimune Y, Kitamura Y: Cell type-specific deficiency of c-kit gene expression in mutant mice of mi/mi genotype. Am J Pathol 1994, 145:827-836 [PMC free article] [PubMed] [Google Scholar]
- 10.Jippo T, Tsujino K, Kim HM, Kim DK, Lee YM, Nawa Y, Kitamura Y: Expression of mast-cell-specific proteases in tissues of mice studied by in situ hybridization. Am J Pathol 1997, 150:1373-1382 [PMC free article] [PubMed] [Google Scholar]
- 11.Kitamura Y, Go S, Hatanaka K: Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood 1978, 52:447-452 [PubMed] [Google Scholar]
- 12.Nakano T, Kanakura Y, Asai H, Kitamura Y: Changing processes from bone marrow-derived cultured mast cells to connective tissue-type mast cells in the peritoneal cavity of mast cell-deficient W/Wv mice: association of proliferation arrest and differentiation. J Immunol 1987, 138:544-549 [PubMed] [Google Scholar]
- 13.Matsuda H, Kitamura Y, Sonoda T, Imori T: Precursor of mast cells fixed in the skin of mice. J Cell Physiol 1981, 108:409-415 [DOI] [PubMed] [Google Scholar]
- 14.Russell ES, Fondal EL: Quantitative analysis of the normal and four alternative degrees of an inherited macrocytic anemia in the house mouse. I. Number and size of erythrocytes. Blood 1951, 6:892-905 [PubMed] [Google Scholar]
- 15.Russell ES, Snow CM, Murray LM, Cormier JP: The bone marrow in inherited macrocytic anemia in the house mouse. Acta Haematol 1953, 10:247-259 [DOI] [PubMed] [Google Scholar]
- 16.Nakahata T, Spicer SS, Cantey JR, Ogawa M: Clonal assay of mouse mast cell colonies in methylcellulose culture. Blood 1982, 60:352-361 [PubMed] [Google Scholar]
- 17.Yurt RW, Leid RW, Austen KF, Silbert JE: Native heparin from rat peritoneal mast cells. J Biol Chem 1977, 252:518-521 [PubMed] [Google Scholar]
- 18.Kanakura Y, Thompson H, Nakano T, Yamamura T, Asai H, Kitamura Y, Metcalfe DD, Galli SJ: Multiple biodirectional alterations of phenotype and changes in proliferative potential during the in vitro and in vivo passage of clonal mast cell populations derived from mouse peritoneal mast cells. Blood 1988, 72:877-885 [PubMed] [Google Scholar]
- 19.Jippo T, Ushio H, Hirota S, Mizuno H, Yamatodani A, Nomura S, Matsuda H, Kitamura Y: Poor response of cultured mast cells derived from mi/mi mutant mice to nerve growth factor. Blood 1994, 84:2977-2983 [PubMed] [Google Scholar]
- 20.Morii E, Jippo T, Tsujimura T, Hashimoto K, Kim DK, Lee YM, Ogihara H, Tsujino K, Kim HM, Kitamura Y: Abnormal expression of mouse mast cell protease 5 gene in cultured mast cells derived from mutant mi/mi mice. Blood 1997, 90:3057-3066 [PubMed] [Google Scholar]
- 21.Nomura S, Wills AJ, Edwards DR, Heath JK, Hogan BLM: Developmental expression of 2ar (osteopontin) and SPARC (osteonectin) RNA as revealed by in situ hybridization. J Cell Biol 1988, 106:441-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato Y, Toma H: Strongyloides venezuelensis infections in mice. Int J Parasitol 1990, 20:57-62 [DOI] [PubMed] [Google Scholar]
- 23.Khan AI, Horii Y, Tiuria R, Sato Y, Nawa Y: Mucosal mast cells and the expulsive mechanisms of mice against Strongyloides venezuelensis. Int J Parasitol 1993, 23:551-555 [DOI] [PubMed] [Google Scholar]
- 24.Sonoda S, Sonoda T, Nakano T, Kanayama Y, Kanakura Y, Asai H, Yonezawa T, Kitamura Y: Development of mucosal mast cells after injection of a single connective tissue-type mast cells in the stomach mucosa of genetically mast cell-deficient W/Wv mice. J Immunol 1986, 137:1319-1322 [PubMed] [Google Scholar]
- 25.Scudamore CL, McMillan L, Thornton EM, Wright SH, Newlands GFJ, Miller HRP: Mast cell heterogeneity in the gastrointestinal tract: variable expression of mouse mast cell protease-1 (mMCP-1) in intraepithelial mucosal mast cells in nematode-infected and normal BALB/c mice. Am J Pathol 1997, 150:1661-1672 [PMC free article] [PubMed] [Google Scholar]
- 26.Finkelman FD, Madden KB, Cheever AW, Katona IM, Morris SC, Gately MK, Hubbard BR, Gause WC, Urban JF, Jr: Effects of interleukin 12 on immune responses and host protection in mice infected with intestinal nematode parasites. J Exp Med 1994, 179:1563-1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghildyal N, McNeil HP, Stechschulte S, Austen KF, Silberstein D, Gurish MF, Somerville LL, Stevens RL: IL-10 induces transcription of the gene for mouse mast cell protease-1, a serine protease preferentially expressed in mucosal mast cells of Trichinella spiralis-infected mice. J Immunol 1992, 149:2123-2129 [PubMed] [Google Scholar]
- 28.Eklund KK, Ghildyal N, Austen KF, Stevens RL: Induction by IL-9 and suppression by IL-3 and IL-4 of the levels of chromosome 14-derived transcripts that encode late-expressed mouse mast cell proteases. J Immunol 1993, 151:4266-4273 [PubMed] [Google Scholar]