Abstract
A two-color immunofluorescent analysis indicated that dendritic cells (DCs) in the human axillar lymph nodes (ie, lymph nodal DCs (LnDCs)) can be classified into three subsets. The first subset consists of CD1a+/CD86− or dim/CD83− or dim nondendriform DCs found mainly in lymph sinuses, the second is of CD1a−/CD86+/CD83+ dendriform DCs scattered in normal T zones, and the third is of large CD1abright/CD86+/CD83+ dendriform DCs occasionally found in hyperplastic T zones. A three-color flow cytometric analysis, immunoperoxidase staining, and electron microscopic observation indicated that the majority of LnDCs corresponded to the first subset, which showed distinctive characteristics of DCs but did not fulfill the ultrastructural criteria for interdigitating reticulum cells (IDCs) and did not contain Birbeck granules. When LnDCs were cultured for 7 days, they became large CD1adim/CD86+/CD83+ dendriform cells, which formed large complexes with many T cells and exhibited distinctive ultrastructural features of interdigitating reticulum cells. LnDCs cultured in the presence of granulocyte/macrophage colony-stimulating factor became markedly larger CD1abright/CD86+/CD83+ dendriform cells forming large complexes with numerous T cells. These findings suggest that cells of the first subset represent immature LnDCs just migrating from epidermis, those of the second subset represent interdigitating reticulum cells, and those of the third subset represent interdigitating reticulum cells probably stimulated with certain immunostimulatory cytokines such as granulocyte/macrophage colony-stimulating factor. It is also suggested that either the second or the third subsets of LnDCs are derived from the first subset.
Dendritic cells (DCs) are potent antigen-presenting cells with the unique ability to activate resting T cells and to prime the immune response. Peripheral DCs can exist at various stages of differentiation. Interdigitating reticulum cells (IDCs) are one of the main classes of DCs in lymphoid tissues, including lymph nodes (LN), spleen, and thymus. 1-4 IDCs are defined exclusively by their unique ultrastructural characteristics, such as numerous interdigitating cytoplasmic projections on their surface, electron-lucent abundant cytoplasm containing well-developed tubulovesicular structures, and a deeply indented eu-chromatin-rich nucleus. 1-4
It is generally accepted that IDCs are derived from Langerhans cells (LCs), which migrate from the epidermis to the draining LN upon picking up antigens. 5,6 However, several important questions remain to be answered as to the differences between immature lymph nodal DCs (LnDCs) just migrating from the epidermis and IDCs, how the immature LnDCs mature into IDCs, and what effects immunoregulatory cytokines have on their maturation into IDCs.
Shinzato et al 7 and Shamoto et al 8 have revealed that LnDCs are heterogeneous in expressing CD1a antigen, and demonstrated that LCs in superficial LN, which are defined by the presence of Birbeck granules, are positive for CD1a, whereas IDCs are negative for this antigen, and suggested that LC and IDC are different in origin. Moreover, although DCs have been considered to be closely related to the myelomonocytic lineage, it was recently revealed that in the thymus there exists a novel subset of DCs expressing some T-cell antigens (CD8 and/or CD4) and Fas ligand, as well as DC-associated antigens, and that they are ontogenetically closely related to T cells. 9-12 Thus, it seems possible that LnDCs consist of plural subsets of different origin. It is, therefore, important to define the morphological and immunophenotypic differences among LnDCs and clarify what causes the heterogeneity of LnDCs.
In the present study, we investigated the morphology and the immunophenotype of LnDCs in human axillar LN obtained from patients with breast cancer by multicolor immunofluorescent staining and flow cytometry. We also investigated the immunophenotype and the behaviors of cultured LnDCs. We paid particular attention to their expression of CD1 and CD86 (B7-2) molecules, because increasing evidence suggests that CD1 molecules are novel antigen-presenting molecules not belonging to major histocompatibility complex class II molecules involved in the presentation of nonpeptide-antigens such as lipids and glycolipids to particular subsets of T cells, 13 and it has been shown that CD86 plays a major role as costimulatory molecules in major histocompatibility complex class II-mediated peptide antigen presentation. 14
We demonstrate here that LnDCs in human axillar LN are heterogeneous and can be classified into three subsets according to their morphology; location; and expression of CD1a, CD86, and CD83. We describe the morphology and the immunophenotype of these three subsets of LnDCs and report evidence that strongly suggests that all three subsets of LnDCs are of the same origin.
Materials and Methods
Antibodies
The expression of leukocyte cell surface markers was assessed using the following fluorescein isothiocyanate (FITC)-conjugated murine monoclonal antibodies (mAbs): anti-CD1a, anti-HLA-DR, anti-CD14, and anti-CD32 (FcγR-II) from DAKO (Glostrup, Denmark), and anti-CD86 (B7-2) from TALK-TEC (San Diego, CA). The phycoerythrin (PE)-conjugated murine mAbs anti-CD1a, anti-HLA-DR, anti-CD14, and anti-CD86 were from TALK-TEC, and anti-CD83 was from Immunotech (Marseille, France). Per-CP-conjugated anti-HLA-DR was purchased from Becton Dickinson Systems (Mountain View, CA). The nonconjugated murine mAbs anti-CD1a, anti-CD3, anti-CD4, anti-CD8, anti-CD86 (B70/B7-2), and HLA-DR were purchased from TALK-TEC, anti-CD1b and anti-CD1c were from Serotec (Oxford, England), and anti-CD83 was from Immunotech.
Affinity-purified rabbit polyclonal antibodies to the bovine S-100β subunit were prepared as described previously. 15,16 As the second antibody for immunoperoxidase staining, horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) and horseradish peroxidase-conjugated goat anti-rabbit IgG were purchased from TAGO (Tokyo, Japan).
Cytokines
Recombinant human (rh) granulocyte/macrophage-colony stimulating factor (GM-CSF) and interleukin-10 (IL-10) were purchased from Genzyme Systems (Cambridge, MA).
Preparation of Frozen Sections, Cell Suspension, and Culture Conditions
Human axillar LNs were surgically obtained from six patients with breast cancer under informed consent. All of these LNs were devoid of cancer metastasis. Three LNs were used for preparing frozen sections, which were fixed in acetone for 10 minutes and examined immunohistochemically.
The other three LNs were cut into 2-mm 3 cubes, and a single-cell suspension was obtained by gentle pressure with a glass homogenizer. After the removal of dead cells and erythrocytes by Ficoll-Hypaque density gradient centrifugation, the cells were suspended in 10% heat-inactivated, low-endotoxin (<6 pg/ml) fetal calf serum (Irvine Scientific, Santa Ana, CA)-supplemented RPMI 1640 (Nissui, Tokyo, Japan). The cells were then cultured at a concentration of 10 6 cells/ml at 37°C in 5% CO2 in humidified air, and the culture media were half-changed every 3 days. The cells were cultured for 7 days and examined.
Removal of CD86+ LnDCs from the Fresh LN Suspension
CD86+ LnDCs were removed on occasion from the fresh LN suspension by complement-mediated lysis using anti-CD86 mAbs (IgG2b; TALK-TEC) and rabbit complement (Cedarlane Laboratories Ltd, Hornby, Ontario, Canada) as described previously. 17 After washing, the LN cells were cultured for 14 days and examined by flow cytometry.
Electron Microscopy
For electron microscopic examination, macrophages were removed from the fresh LN suspension by complement-mediated lysis using anti-CD14 mAb (IgG2a; TALK-TEC) as described above. Fresh or cultured LN cells from which phagocytic macrophages were removed by complement-mediated lysis as described above were fixed with 2% gluteraldehyde solution for 1 hour, postfixed with 2% OsO4, and embedded in Epon 812. Ultrathin sections doubly stained with uranyl acetate and lead citrate were examined by electron microscopy as described previously. 18
Flow Cytometric Analysis
Suspended LN cells were examined by three-color flow cytometry. Cells (n = 106) were incubated with optimal concentrations of fluorescent dye-conjugated mAbs for 45 minutes on ice. The cells were then washed in phosphate-buffered saline containing 2% fetal calf serum and 0.2% sodium azide and fixed in 3% formaldehyde. A FACScan (Becton Dickinson Systems) was used to analyze the cells.
Immunoperoxidase Staining
For immunocytochemical staining, cells were smeared on glass slides with a cytospin (Sakura, Tokyo, Japan), fixed in acetone for 10 minutes at room temperature, and air dried. For the detection of cytoplasmic S-100b protein, cells were fixed in 4% paraformaldehyde solution for 15 minutes, washed with phosphate-buffered saline, and smeared on glass slides. Indirect immunoperoxidase staining was performed as described previously. 18 After immunoperoxidase staining, cells were counterstained with methyl green.
Two-Color Immunofluorescent Staining
Frozen sections of axillar LN and smeared LN cells were fixed with acetone for 10 minutes. The preparations were incubated with both FITC-conjugated and PE-conjugated mAbs (20 μg protein/ml) for 30 minutes at room temperature. After washing, the preparations were examined by fluorescent microscopy.
Results
LnDCs in Frozen Sections
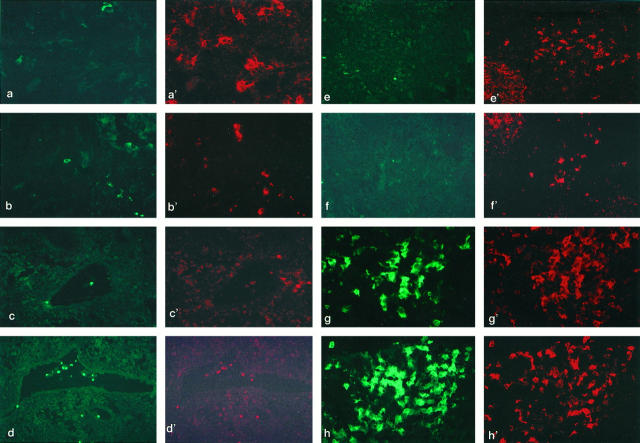
LnDCs in the axillar LN were identified as large HLA-DRbright/CD14− dendriform cells, whereas phagocytic macrophages were HLA-DRdim/CD14+ cells (Figure 1a,a’) ▶ . CD1a, CD86, and CD83 were also expressed on some LnDCs (Figure 1b,b’) ▶ . Although all LnDCs were strongly positive for HLA-DR, they were heterogeneous in expressing CD1a, CD86, CD83, and CD32. Three subsets of LnDCs, CD1a+/CD86− or dim/CD83− or dim LnDCs, CD1a−/CD86+/CD83+ LnDCs, and CD1abright/CD86+/CD83+ LnDCs, were identified. The first subset of CD1a+/CD86− or dim/CD83− or dim LnDCs consisted of round or ovoid cells lacking dendritic morphology, smaller than the other two subsets of LnDCs, and were found mainly in the lymph sinuses and in the parenchyma adjacent to the lymph sinuses (Figure 1c to d’) ▶ . They were uniformly positive for CD1a, and some of them were weakly positive for CD86 (Figure 1c,c’) ▶ , CD83 (Figure 1d,d’) ▶ , and/or CD32 (data not shown).
Figure 1.
Two-color immunofluorescent microscopic analysis on the frozen sections of the axillar lymph nodes obtained from the patients with breast cancer. a and a’: T zone stained with FITC-conjugated anti-CD14 (a) and PE-conjugated anti-HLA-DR (a’). Several large dendriform cells corresponding to IDCs are strongly positive for HLA-DR (a’) but negative for CD14 (a). Only a small number of cells corresponding to macrophages are positive for CD14 and HLA-DR. Magnification, ×270. b and b’: T zone stained with FITC-conjugated anti-CD14 (b) and PE-conjugated anti-CD86 (b’). The LnDCs are strongly positive for CD86 (b’) but negative for CD14 (b). ×50. c and c’: Lymph sinus stained with FITC-conjugated anti-CD1a (c) and PE-conjugated CD86 (c’). The LnDCs in the lymph sinus lacking dendriform morphology are positive for CD1a but not for CD86. Note that dendriform LnDCs found in the T zone are strongly positive for CD86 but almost negative for CD1a. ×40. d and d’: Lymph sinus stained with FITC-conjugated anti-CD1a (d) and PE-conjugated anti-CD83 (d’). The CD1a+ LnDCs found in the lymph sinus are weakly positive for CD83. ×40. e and e’: T zone stained with FITC-conjugated anti-CD1a (e) and PE-conjugated anti-CD86 (e’). The LnDCs in the T zone are strongly positive for CD86 (e) but negative for CD1a (e’). CD86 is also expressed on some germinal center cells (upper left). ×40. f and f’: T zone stained with FITC-conjugated anti-CD1a ( f ) and anti-CD83 ( f’ ). The LnDC in the T zones are strongly positive for CD83 ( f’ ) but negative for CD1a ( f ). CD83 is also expressed on some germinal center cells (upper left). ×40. g and g’: T zone showing reactive hyperplasia stained with FITC-conjugated anti-CD1a (g) and PE-conjugated anti-CD86 (g’). The large dendriform LnDCs found in groups are strongly positive for both CD1a (g) and CD86 (g’). ×80. h and h’: T zone showing reactive hyperplasia stained with FITC-conjugated anti-CD1a ( f ) and PE-conjugated anti-CD83 ( f’ ). A considerable number of the large dendriform LnDCs found in groups are strongly positive for CD1a (h) and CD83 (h’). ×80.
Cells of the second subset of CD1a−/CD86+/CD83+ LnDCs were found to be scattered in normal T zones and exhibited distinctive dendritic morphology. They were positive for both CD86 and CD83 but negative for CD1a (Figure 1e to f’) ▶ .
Cells of the third subset of CD1abright/CD86+/CD83+ LnDCs were found in only one of the three LNs examined. They were found in T zones showing reactive hyperplasia. They were larger than LnDCs in the first and the second subsets, exhibited extensive dendritic morphology, and tended to be found in groups (Figure 1e to f’) ▶ . One of the most remarkable features of them was the strong expression of CD1a. Their staining intensity for CD86 tended to be in proportion to that for CD1a, but their staining intensity for CD83 tended to be in inversely proportion to that for CD1a. Cells of the second and the third subsets of LnDC were negative for CD32 (data not shown).
Suspended Fresh LnDCs
Immunoperoxidase Staining
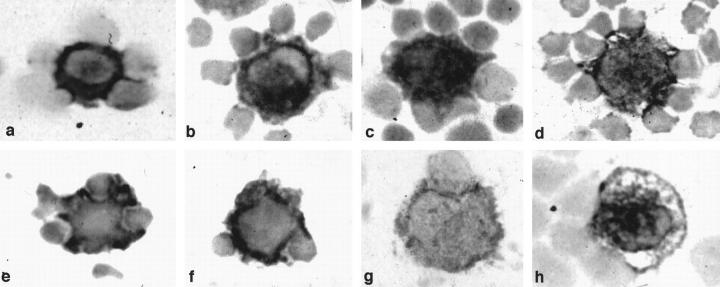
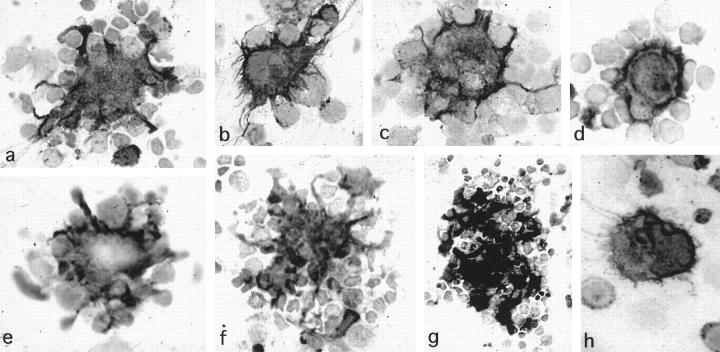
Smeared fresh LN cells were examined by immunoperoxidase methods. LnDCs were identified as large cells with irregular contours strongly positive for HLA-DR (Figure 2a) ▶ ; for CD1a, CD1b, and CD1c (Figure 2, b to d) ▶ ; and for S-100b protein (Figure 2, e and f) ▶ . HLA-DR was also expressed on considerable numbers of lymphocytes and macrophages, whereas CD1 molecules and S-100b protein were specifically expressed in LnDCs. Some LnDCs were also positive for CD83 (Figure 2g) ▶ and CD86 (Figure 2h) ▶ . LnDCs were found to attach to several T cells, which were mostly helper T cells (CD3+/CD4+/CD8−).
Figure 2.
Immunoperoxidase staining of the fresh smeared LN cells. Fresh smeared LnDCs are strongly positive for HLA-DR (a), CD1a (b), CD1b (c), CD1c (d), and S-100b protein (e and f ). Some of the LnDCs are positive for CD83 (g) and CD86 (h). They exhibit irregular contours and attach to several T cells. (Magnification, ×1400).
Double Immunofluorescent Staining
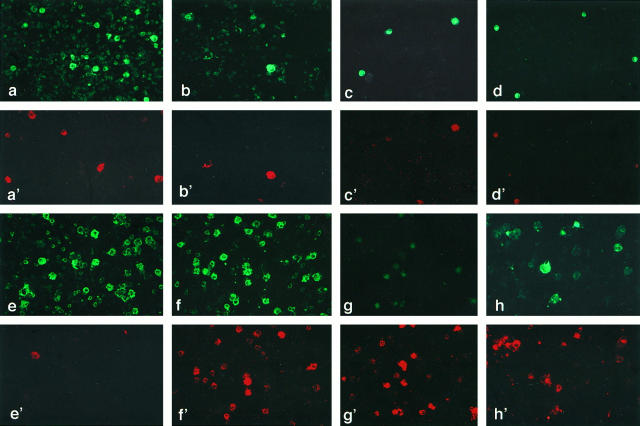
In the fresh LN suspensions, the LnDCs could be easily distinguished from phagocytic macrophages by the strong expression of HLA-DR and the negativity for the monocyte/macrophage-specific marker CD14. As shown in Figure 3a,a’ ▶ , LnDCs were identified as large cells strongly positive for HLA-DR and negative for CD14, whereas macrophages were large cells weakly positive for HLA-DR and strongly positive for CD14. The majority of HLA-DRbright LnDCs were also positive for CD1a (Figure 3b,b’) ▶ . Some LnDCs expressing CD1a were weakly positive for CD86 and CD83 (Figure 3 c–d’) ▶ . None of them expressed T cell-associated antigens, such as CD3, CD4, or CD8 (data not shown).
Figure 3.
Two-color immunofluorescent staining of smeared LN cells. a and a’: Fresh LN cells stained with FITC-conjugated anti-HLA-DR (a) and PE-conjugated anti-CD14 (a’). The LnDCs are strongly positive for HLA-DR but not CD14, whereas phagocytic macrophages are weakly positive for HLA-DR and strongly positive for CD14. Magnification, ×160. b and b’: Fresh LN cells stained with FITC-conjugated anti-HLA-DR (b) and PE-conjugated anti-CD1a (b’). The LnDCs strongly expressing HLA-DR are also strongly positive for CD1a. (×160). c and c’: Fresh LN cells stained with FITC-conjugated anti-CD1a (c) and PE-conjugated anti-CD86 (c’). Some of the LnDCs strongly expressing CD1a are positive for CD86. (×160). d and d’: Fresh LN cells stained with FITC-conjugated anti-CD1a (d) and PE-conjugated anti-CD83 (d’). Some of the LnDCs strongly expressing CD1a are weakly positive for CD83. (×80). e and e’: LN cells cultured for 7 days stained with FITC-conjugated anti-HLA-DR (e) and PE-conjugated anti-CD14 (e’). The large LnDCs with irregular contours are strongly positive for HLA-DR but negative for CD14, whereas only a small number of macrophages are weakly positive for HLA-DR and strongly positive for CD14. ×160. f and f’: LN cells cultured for 7 days stained with FITC-conjugated anti-HLA-DR (f) and PE-conjugated anti-CD86 (f’). The vast majority of the large irregular LnDCs strongly positive for HLA-DR are also positive for CD86. ×160. g and g’: LN cells cultured for 7 days stained with FITC-conjugated anti-CD1a (g) and PE-conjugated anti-CD86 (g’). CD1a expression by the LnDCs is largely down-regulated, but their CD86 expression is significantly up-regulated during the culture. ×160. h and h’: LN cells cultured for 7 days stained with FITC-conjugated anti-HLA-DR (h) and PE-conjugated anti-CD83 (h’). The LnDCs strongly positive for HLA-DR are also positive for CD83. ×160.
Flow Cytometric Analysis
Fluorescent microscopy indicated that LnDCs in the axillar LN were heterogeneous in expressing CD1a, CD86, CD83, and CD32, as described above. Therefore, we precisely analyzed their heterogeneity by three-color flow cytometry. We examined LN cells obtained from the three patients by flow cytometry, and the results were virtually the same. Therefore, we describe herein the results obtained from one representative case.
As shown in Figure 4 ▶ , LnDCs expressing CD1a accounted for approximately 1% of all LN cells. They formed a distinctive population of cells expressing the highest level of HLA-DR. Some CD1a+ LnDCs were weakly positive for CD86 (approximately 20%), CD83 (approximately 10%), and CD32 (approximately 25%).
Figure 4.
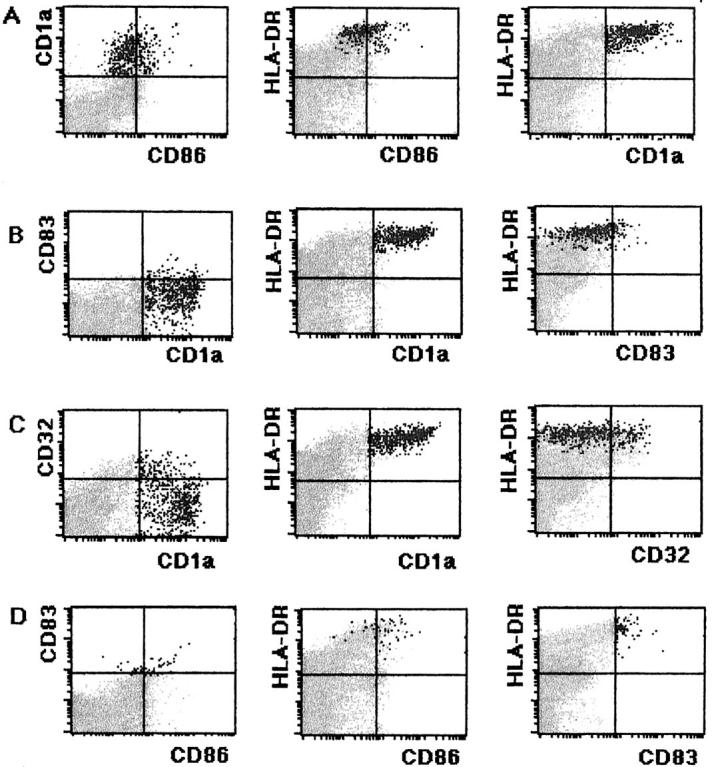
Flow cytometric analysis of the fresh LN cells. A: Fresh LN cells treated with FITC-conjugated anti-CD86, PE-conjugated anti-CD1a, and Per-CP-conjugated anti-HLA-DR. Black dots indicate CD1a+ LnDCs, which express the highest level of HLA-DR and comprise approximately 1% of total cells. Approximately 20% of CD1a+ LnDCs express CD86 weakly. B: Fresh LN cells treated with FITC-conjugated anti-CD1a, PE-conjugated anti-CD83, and Per-CP-conjugated anti-HLA-DR. Black dots indicate CD1a+ LnDCs. Approximately 10% of CD1a+ LnDCs express CD83 weakly. C: Fresh LN cells treated with FITC-conjugated anti-CD1a, PE-conjugated anti-CD32, and Per-CP-conjugated anti-HLA-DR. Black dots indicate CD1a+ LnDCs. Approximately 25% of CD1a+ LnDCs are weakly positive for CD32. D: Fresh LN cells treated with FITC-conjugated CD86, PE-conjugated CD83, and Per-CP-conjugated anti-HLA-DR. Black dots indicate CD83+ LnDCs. The majority of LnDCs expressing CD83 are weakly positive for CD86.
Thus, LnDCs in the axillar LN were heterogeneous, and CD1a+/CD86− or dim/CD83− or dim LnDCs predominated. Although CD1a−/CD86+/CD83+ dendriform LnDCs were clearly detected in T zones of the axillar LN by immunofluorescent microscopy, they were scarcely detected as a distinctive population by flow cytometry, indicating that their percentage in total LN cells was very low.
Ultrastructure
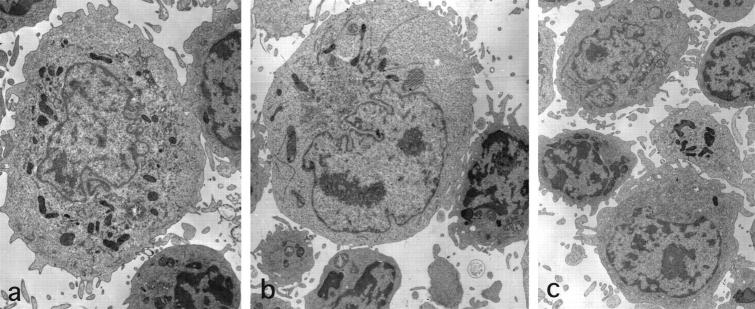
To avoid confusing immature LnDCs with macrophages by electron microscopy, macrophages were removed from the LN suspension by complement-mediated lysis using an anti-CD14 mAb. All of the LnDCs (20 of 20) identified by electron microscopy exhibited immature features of LnDCs, which did not fulfill the ultrastructural criteria of IDCs (Figure 5, a to c) ▶ . Namely, they were larger than lymphocytes and had abundant cytoplasm containing irregularly shaped nuclei and extended numbers of short finger-like cytoplasmic projections on their surface, with which they attached to a few T cells. They possessed abundant and relatively electron-dense cytoplasm with irregular nuclei and well developed cellular organelles including mitochondria, dense bodies, and tubulovesicular structures, and they attached to neighboring T cells through their projections. Birbeck granules were not observed.
Figure 5.
Ultrastructure of fresh LnDCs obtained from the axillar LN. The LnDCs are large cells with villous projections, through which they attach to neighboring T cells. They possess irregular nuclei and relatively electron-dense cytoplasm containing tubulovesicular structures and dense bodies. They do not fulfill the ultrastructural criteria for IDC and do not contain Birbeck granules. Magnification: ×4285 (a), ×5000 (b), and ×3750 (c).
Cultured LnDCs
Immunoperoxidase Staining
LN cells decreased in number nonselectively during the culture in vitro, and this was due to nonspecific cell death during the culture. The morphology and the immunophenotype of LnDCs changed significantly during the culture for 7 days. They were found to be completely nonadherent cells, whereas phagocytic macrophages mostly adhered to the plastic bottom of the culture dishes. The LnDCs became larger and extended numerous fine cytoplasmic projections and formed large complexes with many T cells. They were strongly positive for HLA-DR, CD86, CD83, and S-100b protein (Figure 6, a to e) ▶ .
Figure 6.
Immunoperoxidase staining of LnDCs cultured for 7 days (a to e). a: A large dendriform LnDC strongly positive for HLA-DR form complexes with many T cells. Magnification, ×800. b: An HLA-DRbright LnDC extending numerous fine cytoplasmic projections, with which it attaches to T cells. ×1200. c: An LnDC strongly positive for CD86. ×600. d: An LnDC strongly positive for CD83. ×800. e: An LnDC strongly positive for S-100b protein. ×800. f and g: Immunoperoxidase staining for HLA-DR of LnDCs cultured for 7 days in the presence of 10 ng/ml of rhGM-CSF. f: LnDCs strongly positive for HLA-DR form large complexes with numerous T cells. ×400. g: Several LnDCs strongly positive for HLA-DR aggregate and form large complexes with numerous T cells. ×500. h: Immunoperoxidase staining for HLA-DR of an LnDC cultured for 7 days in the presence of 10 ng/ml of rhIL-10. Note an HLA-DR+ LnDC that does not attach to T cells. ×800.
Two-Color Immunofluorescent Staining
The cultured LnDCs were large HLA-DRbright/CD14− dendriform cells, whereas phagocytic macrophages, which were very few in the nonadherent fraction, were HLA-DRdim/CD14+ cells (Figure 3e,e’) ▶ . They became larger in size and exhibited irregular contours. Most of the LnDCs became strongly positive for CD86 and CD83, but their CD1a expression was significantly down-regulated (Figure 3, f to h’) ▶ . Thus, most of the LnDCs became large CD1adim/CD86+/CD83+ dendriform cells during the culture.
Flow Cytometry
The flow cytometric analysis indicated that the CD1a expression by DCs was significantly down-regulated (Figure 7A) ▶ and that the CD1a+ LnDCs decreased in number (approximately 0.25% in cultured LN cells). In contrast, the CD83 and CD86 expressions of the LnDCs were up-regulated (Figure 7, B and D) ▶ . LnDCs became negative for CD32 (Figure 7C) ▶ .
Figure 7.
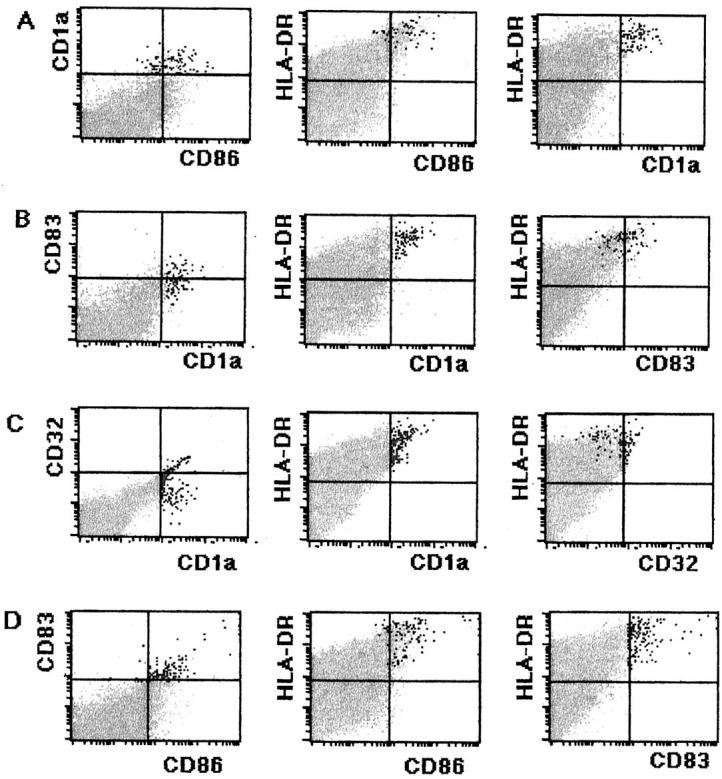
Flow cytometric analysis of LN cells cultured for 7 days. A: Cultured LN cells treated with FITC-conjugated anti-CD86, PE-conjugated anti-CD1a, and Per-CP-conjugated anti-HLA-DR. Black dots indicate CD1a+ LnDCs, which comprise approximately 0.25% of total cells. CD1a expression by LnDCs is significantly down-regulated, and their CD86 expression is up-regulated. B: Cultured LN cells treated with FITC-conjugated anti-CD1a, PE-conjugated anti-CD83, and Per-CP-conjugated anti-HLA-DR. Black dots indicate CD1a+ LnDCs. CD83 expression by LnDCs is slightly up-regulated. C: Cultured LN cells treated with FITC-conjugated anti-CD1a, PE-conjugated anti-CD32, and Per-CP-conjugated anti-HLA-DR. Black dots indicate CD1a+ LnDCs. CD1a+ LnDCs are mostly converted to be negative for CD32. D: Cultured LN cells treated with FITC-conjugated CD86, PE-conjugated CD83, and Per-CP-conjugated anti-HLA-DR. Black dots indicate CD83+ LnDCs. The expressions of CD86 and CD83 by LnDC are up-regulated during the culture.
Electron Microscopy
The LnDCs cultured for 7 days tended to show the typical ultrastructural features of IDCs. Of the 20 LnDCs identified by electron microscopy, 12 fulfilled the ultrastructural criteria of IDCs; ie, they were large cells extending numerous interdigitating cytoplasmic projections on their surfaces; they possessed electron-lucent, abundant cytoplasm containing a deeply indented eu-chromatin-rich nucleus and well-developed cytoplasmic organelles including tubulovesicular structures; and they formed attachments with neighboring T cells through their numerous interdigitating cytoplasmic projections (Figure 8, a and b) ▶ . The other 8 LnDCs exhibited some of these ultrastructural characteristics incompletely (data not shown).
Figure 8.
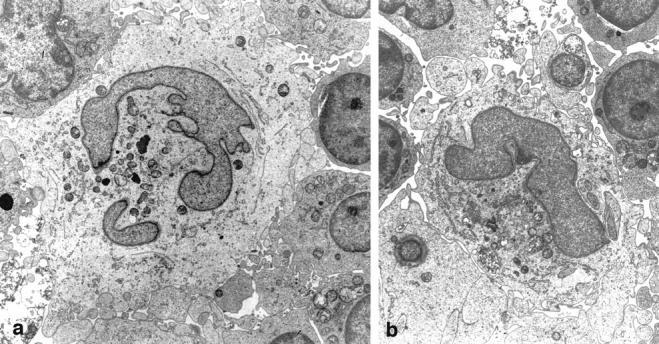
An LnDC cultured for 7 days exhibits the distinctive ultrastructural features of IDC. Magnification: ×5200 (a) and ×4400 (b).
Effects of Cytokines
To clarify the effects of immunoregulatory cytokines including GM-CSF and IL-10, LN cells were cultured for 7 days in the presence of 10 ng/ml of rhGM-CSF or rhIL-10 and then examined. GM-CSF improved the viability of LnDC and up-regulated their CD1a, CD86, and CD83 expressions (Figure 9, A and B) ▶ . GM-CSF promoted the formation of complexes with T cells by LnDCs. LnDCs treated with GM-CSF tended to aggregate and formed larger complexes with numerous T cells. The flow cytometric analysis indicated that GM-CSF significantly up-regulated the expressions of CD1a and CD86 (Figure 7, f and g) ▶ .
Figure 9.
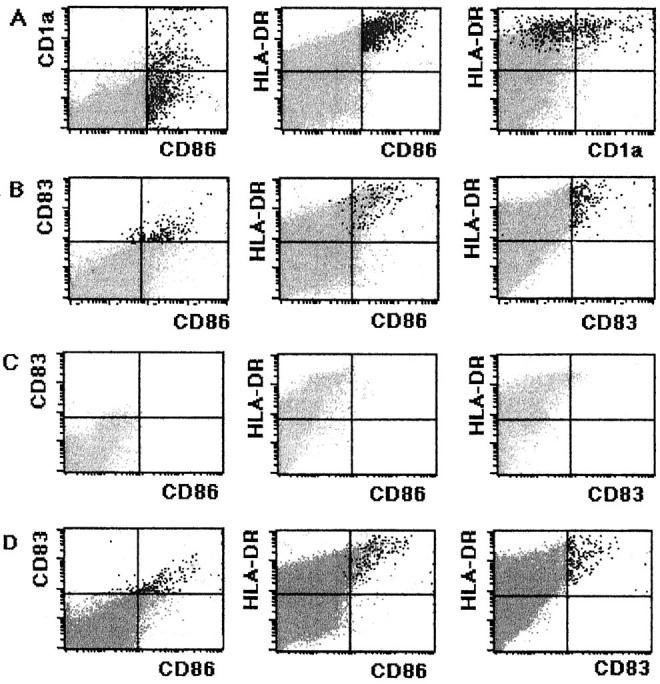
A and B: Flow cytometric analysis of LN cells cultured for 7 days in the presence of 10 ng/ml of rhGM-CSF. A: GM-CSF-treated LN cells incubated with FITC-conjugated anti-CD86, PE-conjugated anti-CD1a, and Per-CP-conjugated HLA-DR. Black dots indicate CD86+ cells. CD86 and CD1a expressions by the LnDCs are significantly up-regulated. B: GM-CSF-treated LN cells incubated with FITC-conjugated anti-CD86, PE-conjugated anti-CD83, and Per-CP-conjugated HLA-DR. Black dots indicate CD83+ LnDCs. GM-CSF up-regulates CD86 and CD83 expressions by LnDCs. C and D: Flow cytometric analysis of the LN cell fraction from which CD86+ cells were removed at the start of the culture. C: Noncultured CD86+ cell-deleted LN cell fraction treated with FITC-conjugated anti-CD86, PE-conjugated anti-CD83, and Per-CP-conjugated anti-HLA-DR. No CD86+ LnDCs are detected by flow cytometry. D: The 14-day-cultured CD86+ cell-deleted LN cell fraction treated with FITC-conjugated anti-CD86, PE-conjugated anti-CD83, and Per-CP-conjugated anti-HLA-DR. Note a considerable number of CD86+/CD83+ LnDCs.
Conversely, IL-10 worsened the viability of LnDCs (0.1% in nonadherent LN cells) and strongly inhibited their formation of complexes with T cells (Figure 7h) ▶ . The flow cytometric analysis indicated that IL-10 down-regulated their CD1a and CD86 expression (data not shown).
Generation of CD86+/CD83+ LnDCs from the CD86+ Cell-Deleted LN Culture in Vitro
The flow cytometry indicated that although CD1a+/CD86−/CD83− or dim LnDCs predominated in the LN suspension at the start of the culture, CD1adim/CD86+/CD83+ LnDCs became predominant at the end of the culture. To exclude the possibility that CD1a+/CD86−/CD83− or dim LnDCs selectively died during the culture and that, as a result, CD1adim/CD86+/CD83+ LnDCs, which were present in the culture in only a small number at the start of the culture, became predominant at the end of culture, we deleted CD86+ cells from the LN suspension by complement-mediated lysis at the start of culture. The LN cells were then cultured for 14 days and examined by flow cytometry. As shown in Figure 8, C and D ▶ , there were virtually no CD86+ LnDCs at the start of the culture, but a considerable number of CD86+/CD83+ LnDCs were found at the end of the culture. These findings strongly suggested that CD1a+/CD86− or dim/CD83− or dim LnDCs themselves matured into CD1adim/CD86+/CD83+ LnDCs during the culture.
Discussion
The results of the present study indicate that LnDCs in human superficial LNs are heterogeneous in morphology and in surface immunophenotype. By tissue immunohistochemistry, LnDCs were classified into three subsets; the first subset was of CD1a+/CD86− or dim/CD83− or dim nondendriform LnDCs found mainly in the lymph sinus, the second subset was of CD1a−/CD86+/CD83+ dendriform LnDCs scattered in normal T zones, and the third subset was of large CD1abright/CD86+/CD83+ dendriform LnDCs occasionally found in T zones showing reactive hyperplasia.
It is generally accepted that LnDCs in the superficial LN are derived from epidermal LC; ie, epidermal LCs migrate to the T zones of the draining LN via afferent lymph upon picking up antigens, where they mature into IDCs and present the antigens to T cells. 19 According to this view, it is reasonable to consider that the first subset represents immature LnDCs migrating from the skin, and that the second subset represents IDCs.
The first subset of LnDCs were found mainly in the lymph sinuses and the parenchyma adjacent to them; these LnDCs were smaller than those of the other subsets of LnDCs and were round or ovoid in shape, lacking a dendriform appearance. They were strongly positive for CD1a. Some of them were weakly positive for CD83 and CD86. The flow cytometric analysis indicated that the vast majority of LnDCs in the axillar LN examined could be categorized into the first subset. The immunoperoxidase staining indicated that they were strongly positive for S-100b protein, a characteristic cytoplasmic marker for human DCs, including LCs and IDCs, 16,18,20 and these LnDCs exhibited an ability to form complexes with several T cells, which is known to be one of the most important characteristics of DCs. 21,22 Ultrastructurally, however, they did not contain Birbeck granules and did not fulfill the ultrastructural criteria of IDCs. As shown in this study, some of them expressed CD32, which is a low-affinity Fcγ receptor expressed on macrophages and epidermal LCs, but not on IDCs, and is thought to be important for the capture of opsonized pathogens. 23-26 It seems likely that the expression of CD32 on some of the first subset of LnDCs was a vestige of their earlier form, ie, epidermal LCs.
The first subset of LnDCs also expressed CD1b and CD1c as well as CD1a. CD1a, CD1b, and CD1c are the three isoforms of the human CD1 family. 13 It has been shown that the expressions of CD1a and CD1c are features shared with epidermal LCs and dermal DCs, whereas CD1b is expressed on dermal DCs but not on epidermal LCs. 27 It has been suggested that in human skin, CD1a+/CD1b−/CD1c+ DCs represent nonmigrating forms of DCs, whereas CD1a+/CD1b+/CD1c+ DCs represent active migrating forms of DCs, and that when CD1a+/CD1b−/CD1c+ LCs pick up antigen in the epidermis, they become CD1a+/CD1b+/CD1c+ and move to the dermis, from which they migrate to the draining LN. 28 Thus, it seems likely that the first subset of LnDCs were more closely related to dermal DCs, ie, the migrating forms of DCs, than to epidermal LCs. Based on these findings, it is reasonable to consider that the first subset of LnDCs represent neither LCs nor IDCs, but the immature form of LnDC, ie, the converting form from LCs into IDCs.
The second subset of LnDC, ie, CD1a−/CD86+/CD83+ dendriform LnDCs, were found to be scattered in the normal T zones and exhibited a conspicuous dendritic appearance. This subset of LnDCs was hardly identified by flow cytometry as a distinctive population in the LN suspension, indicating that their percentage in the total LN cells was very low. In the frozen sections, however, they were easily and clearly identified by fluorescent microscopy as dendriform cells strongly expressing HLA-DR, CD86, and CD83. Judging from their immunophenotype, morphology, and location, it is clear that they corresponded to IDCs.
CD83 is a 43 to 45-kd surface glycoprotein with unknown function and is specifically expressed on DCs, including peripheral blood DCs, epidermal LCs, and IDCs. 29-31 It is curious that only some of LnDCs of the first subset were found to be weakly positive for CD83, because it has been reported that epidermal LCs express CD83 strongly. The reason for this discrepancy remains unclear. One possible explanation is that CD83 expression by DCs is temporarily down-regulated during their migration from the skin to the draining LN.
As shown in this study, cells in the second subset of LnDCs were strongly positive for CD86 (B7-2), which is the member of the B7 family of co-stimulatory molecules expressed on professional accessory cells including DCs, macrophages, and B cells, and on activated, but not resting, T cells. 14 It has been shown that CD86 and another member of the B7 family, CD80, are the ligands for CD28 and CTLA-4 expressed on T cells. 14,32-35 It has also been shown that CD86 expressed by accessory cells plays a major role as a co-stimulatory molecule in T-cell activation, and that when T cells are presented antigens by cells lacking CD86 expression, they fall into anergy. 14,34,35 It therefore seems probable that the second subset of LnDCs consists of more functionally mature and important accessory cells compared to the first subset. Judging from their location, morphology, and immunophenotype, it is clear that they correspond to IDCs.
It is of interest that the LnDCs cultured for 7 days, which were large CD1dim/CD86+/CD83+ dendriform cells forming large complexes with many T cells, were strikingly similar to the second subset of LnDC in situ. The electron microscopic observation also indicated that they mostly fulfilled the ultrastructural criteria for IDCs. The present finding that a considerable number of CD86+ LnDCs could be generated from the LN culture from which CD86+ cells had been removed at the start of the culture strongly suggests that these changes were not due to the selective death of CD1+/CD86−/CD83− or dim LnDCs but to their morphological and phenotypic maturation during the culture. Similarly, it has been demonstrated that epidermal LCs with a poor accessory activity mature into IDC-like dendriform cells with a strong accessory activity. 36 We also found that epidermal LCs, which were initially CD1abright/CD86−/CD83dim small cells with an inconspicuous dendriform morphology, matured into IDC-like large CD1adim/CD86+/CD83+ dendriform cells upon culture in vitro (unpublished data). Taken together, it is probable that the second subset of LnDCs is derived from the first subset of LnDCs.
It is unexpected that in the fresh LN suspension the vast majority of LnDCs detected by flow cytometry corresponded to the first subset of immature LnDCs. We examined axillar LN obtained from patients with breast cancer by flow cytometry but had no opportunity to examine those from normal individuals. Therefore, it is unclear whether the present findings are normal or abnormal. The present findings that there were many CD1a+/CD86− or dim/CD83− or dim immature LnDCs in the axillar LN of patients with breast cancer (approximately 1% in total LN cells), whereas CD1a−/CD86+/CD83+ IDCs were scarce, and that the former were able to mature into the latter upon culture, imply that the maturation of immature LnDCs into IDCs was inhibited in these patients. The patients examined in this study underwent a probe biopsy at the tumor site 2 or 3 weeks before the surgical operation. Therefore, it is also possible that the probe biopsy and/or inflammation resulting from the biopsy caused a temporary unusual migration of large numbers of LCs to the draining axillar LN.
The third subset of LnDCs identified in vivo consisted of larger CD1abright/CD86+/CD83+ extremely dendriform LnDCs occasionally found in T zones showing hyperplasia. They were found in groups and seemed to be similar to the increased IDCs seen in dermatopathic lymphadenopathy. The strong CD1a expression by the increased IDCs in dermatopathic lymphadenopathy has been reported. 7,8 We suspect that they represent an activated form of IDCs because of their larger size, extremely dendriform morphology, and strong expression of CD1a. It has been shown that GM-CSF up-regulates the accessory function of monocytes/macrophages and that it induces them to express CD1a. 37,38 GM-CSF has been reported to stimulate the accessory function of DCs. 39 Therefore, we investigated the effects of GM-CSF and also IL-10 on cultured LnDCs. We found that GM-CSF up-regulated the CD1a and CD86 expression by LnDCs and that it induced them to aggregate with each other and to form large complexes with themselves and numerous T cells. Thus, cells of the third subset of LnDCs in situ are similar to GM-CSF-treated LnDCs in vitro. We also found that IL-1β had similar but inconspicuous effects on LnDCs (unpublished data). It seems likely that cells of the third subset of LnDCs represent activated forms of IDCs, which are probably stimulated by certain immunostimulatory cytokines such as GM-CSF and IL-1β secreted by neighboring activated T cells.
One of the most prominent features of the third subset of LnDCs is the strong expression of CD1 molecules. In contrast, the second subset of LnDC (ie, normal IDC) is negative for CD1 molecules. The human CD1 molecules including CD1a, CD1b, and CD1c are nonpolymorphic proteins showing a modest but significant level of homology to both major histocompatibility complex class I and II proteins. 40 They are expressed on the majority of DCs 41 and on GM-CSF-stimulated monocytes. 38 Although the functional role of CD1 molecules remains unclear, increasing evidence suggests that the CD1 system is a novel family of antigen-presenting molecules, separate from those encoded by the major histocompatibility complex, which are engaged in the presentation of nonpeptide antigens such as lipid (mycolic acid) and glycolipid (lipoarabinomannan) to specialized populations of T cells. 42-47 Taken together with the finding that the second subset of LnDCs also strongly expressed CD86, which is the strong co-stimulatory molecule for peptide antigen presentation, it seems probable that LnDCs of the second subset are antigen-presenting cells for exclusively peptide antigens, and that LnDCs of the third subset are antigen-presenting cells for both peptide and nonpeptide antigens. The functional roles of the third subset of LnDCs should be clarified by further investigations.
The present findings indicated that GM-CSF improved the viability of LnDC in vitro. Conversely, IL-10, which is known to be an immunosuppressive cytokine that inhibits the accessory function of LCs, 48 worsened the viability of LnDCs, inhibited their morphological maturation, and significantly down-regulated their formation of complexes with T cells. It seems likely that immunosuppression by IL-10 is partly due to the inhibition of the formation of LnDC-T complexes.
In this study, we demonstrated that LnDC were different from phagocytic macrophages in the extremely strong expression of HLA-DR, the negativity for CD14, and the ability to form complexes with T cells. Moreover, LnDCs were completely nonadherent cells upon culture, whereas phagocytic macrophages were mostly adherent. These findings suggest that LnDCs are fundamentally different from phagocytic macrophages. It is also noteworthy that cultured LnDCs retained the morphological and immunophenotypic characteristics of IDCs and formed large complexes with T cells for a long term (at least for 14 days). In contrast, cultured follicular DCs, which are DCs of a different lineage located in the germinal center of LNs, have been reported to dedifferentiate into fibroblastic cells, losing their morphological and phenotypic characteristics soon after forming large complexes with numerous B cells in vitro. 49 These findings indicate that LnDCs are more readily investigated in vitro than follicular DCs. Further studies should be performed to clarify the functional roles of DCs in the immune system and their significance in various inflammatory and neoplastic diseases.
Footnotes
Address reprint requests to Dr. Kiyoshi Takahashi, Department of Pathology, Okayama University Medical School, Okayama City, 700-8558, Japan.
References
- 1.Veldman AJP: Histophysiology and Electron Microscopy of the Immune Response. Akad Profschrift. Groningen, The Netherlands, NV Boekdrukkerij Dijkstra Niemeyer, 1970
- 2.Kaiserling E, Stein H, Muller-Hermelink HK: Interdigitating reticulum cells in the human thymus. Cell Tissue Res 1974, 155:47-55 [DOI] [PubMed] [Google Scholar]
- 3.Rausch E, Kaiserling E, Goos M: Langerhans cells and interdigitating reticulum cells in the thymus-dependent region in human dermatopathic lymphadenitis. Virchows Arch B Cell Pathol 1977, 25:327-343 [DOI] [PubMed] [Google Scholar]
- 4.Friess A: Interdigitating reticulum cells in the popliteal lymph node of the rat. Cell Tissue Res 1976, 170:43-60 [DOI] [PubMed] [Google Scholar]
- 5.Hoefsmit EC, Duijvestijn AM, Kamperdijk EW: Relation between Langerhans cells, veiled cells, and interdigitating cells. Immunobiology 1982, 161:255-265 [DOI] [PubMed] [Google Scholar]
- 6.Bos JD, Kapsenberg ML: The skin immune system: progress in cutaneous biology. Immunol Today 1993, 14:75-78 [DOI] [PubMed] [Google Scholar]
- 7.Shinzato M, Shamoto M, Hosokawa S, Kaneko C, Osada A, Shimizu M, Yoshida A: Differentiation of Langerhans cells from interdigitating cells using CD1a and S-100 protein antibodies. Biotech Histochem 1995, 70:114-118 [DOI] [PubMed] [Google Scholar]
- 8.Shamoto M, Osada A, Shinzato M, Kaneko C, Shimizu M: A comparative study on Langerhans cells in lymph nodes with dermatopathic lymphadenopathy and histiocytosis X cells. Adv Exp Med Biol 1995, 378:139-141 [DOI] [PubMed] [Google Scholar]
- 9.Li Wu, Chung-Leung Li, Shortman K: Thymic dendritic cell precursor: Relationship to the T lymphocyte lineage and phenotype of the dendritic cell progeny. J Exp Med 1996, 184:903-911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saunders D, Lucas K, Ismaili J, Li Wu, Marraskovsky E: Dendritic cell development in culture from thymic precursor cells in the absence of granulocyte/macrophage colony-stimulating factor. J Exp Med 1996, 184:2185-2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vremec D, Zorbas M, Scollay R, Saunders DJ, Ardavin CF, Wu, Shortman K: The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J Exp Med 1992, 176:47-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ardavin C: Thymic dendritic cells. Immunol Today 1997, 18:350-361 [DOI] [PubMed] [Google Scholar]
- 13.Beckman EM, Brenner MB: MHC class I-like, class II-like and CD1 molecules: distinct roles in immunity. Immunol Today 1995, 16:349-352 [DOI] [PubMed] [Google Scholar]
- 14.Robey E, Allison JP: T-cell activation: integration of signals from the antigen receptor and costimulatory molecules. Immunol Today 1995, 16:306-309 [DOI] [PubMed] [Google Scholar]
- 15.Takahashi K, Isobe T, Ohtsuki Y, Akagi T, Sonobe H, Okuyama T: Immunohistochemical study on the distribution of α and β subunits of S-100 protein in human normal and neoplastic tissues. Virchows Arch B Cell Pathol 1984, 45:385-396 [DOI] [PubMed] [Google Scholar]
- 16.Takahashi K, Isobe T, Ohtsuki Y, Sonobe H, Takeda I, Akagi T: Immunohistochemical localization and distribution of S-100 proteins in human lymphoreticular system. Am J Pathol 1984, 497–503 [PMC free article] [PubMed]
- 17.Takahashi K, Isobe T, Ohtsuki Y, Sonobe H, Yamaguchi H, Akagi T: S-100 protein positive T-lymphocyte. Am J Clin Pathol 1985, 83:69-72 [DOI] [PubMed] [Google Scholar]
- 18.Takahashi K, Yamaguchi H, Ishizeki J, Nakajima T, Nakazato Y: Immunohistochemical and immunoelectron microscopic localization of S-100 protein in interdigitating reticulum cells of the human lymph node. Virchows Arch B Cell Pathol 1981, 45:385-396 [DOI] [PubMed] [Google Scholar]
- 19.Steinman RM, Pack M, Inaba K: Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev 1997, 156:25-37 [DOI] [PubMed] [Google Scholar]
- 20.Coccia D, Michetti F, Donato R: Immunochemical and immunocytochemical localization of S-100 protein in normal human skin. Nature 1981, 294:85-87 [DOI] [PubMed] [Google Scholar]
- 21.Flechner ER, Freudenthal PS, Kaplan G, Steinman RM: Antigen-specific T lymphocytes efficiently cluster with dendritic cells in the human primary mixed-leukocyte reaction. Cell Immunol 1988, 111:183-195 [DOI] [PubMed] [Google Scholar]
- 22.Inaba K, Romani N, Steinman RM: An antigen-independent contact mechanism as an early step in T cell-proliferative responses to dendritic cells. J Exp Med 1989, 170:527-542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King PD, Katz DR: Human tonsillal dendritic cells-induced T cell responses: analysis of molecular mechanisms using monoclonal antibodies. Eur J Immunol 1989, 19:581-587 [DOI] [PubMed] [Google Scholar]
- 24.Romani N, Lanz A, Glassel H, Stossel H, Stanzl U, Majdic O, Fritsch P: Cultured human Langerhans cells resemble lymphoid dendritic cells in phenotypes and function. J Invest Dermatol 1989, 93:600-609 [DOI] [PubMed] [Google Scholar]
- 25.Hart DJN, McKenzie JH: Isolation and characterization of human tonsil dendritic cells. J Exp Med 1988, 168:157-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shibaki A, Meunier L, Ra C, Shimada S, Ohkawara A, Cooper KD: Differential responsiveness of Langerhans cell subsets of varying phenotypic states in normal human epidermis. J Invest Dermatol 1995, 104:42-46 [DOI] [PubMed] [Google Scholar]
- 27.Wollenberg A, Kraft S, Hanau D, Bieber T: Immunomorphological and ultrastructural characterization of Langerhans cells and a novel inflammatory dendritic epidermal cell (IDEC) population in lesional skin of atopic eczema. J Invest Dermatol 1996, 106:446-453 [DOI] [PubMed] [Google Scholar]
- 28.Richters CD, Hoekstra MJ, Van Baare J, Du Pont JS, Hoefsmit EC, Kamperdijk EW: Migratory properties and functional capacities of human skin dendritic cells. Br J Dermatol 1995, 133:721-727 [DOI] [PubMed] [Google Scholar]
- 29.Zhou LJ, Schwarting R, Smith HM, Tedder TF: A novel cell-surface molecule expressed by human interdigitating reticulum cells, Langerhans cells, and activated lymphocytes is a new member of the Ig superfamily. J Immunol 1992, 142:735-742 [PubMed] [Google Scholar]
- 30.Zhou LJ, Tedder TF: Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J Immunol 1995, 154:3821-3835 [PubMed] [Google Scholar]
- 31.Bjorck P, Flores-Romo L, Liu YJ: Human interdigitating dendritic cells directly stimulate CD40-activated naive B cells. Eur J Immunol 1997, 27:1266-1274 [DOI] [PubMed] [Google Scholar]
- 32.June CH, Bluestone JA, Nadler LM, Thompson CB: The B7 and CD28 receptor families. Immunol Today 1994, 15:321-331 [DOI] [PubMed] [Google Scholar]
- 33.Freeman GJ, Freedman AS, Segil JM, Lee G, Whitman JF, Nadler LM: B7, a new member of the Ig superfamily with unique expression on activated and neoplastic B cells. J Immunol 1989, 143:2714-2722 [PubMed] [Google Scholar]
- 34.Azuma M, Ito D, Yagita H: B70 antigen is a second ligand for CTLA-4 and CD28. Nature 1993, 366:76-79 [DOI] [PubMed] [Google Scholar]
- 35.Freeman GJ, Gribben JG, Boussioitis VA, Ng JW, Restivo VA, Lomdard LA, Gray GS, Nadler LM: Cloning of B7–2: a CTLA-4 counter-receptor that costimulates human T cell proliferation. Science 1993, 262:909-911 [DOI] [PubMed] [Google Scholar]
- 36.Schuler G, Steinman RM: Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med 1985, 161:526-546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith PD, Lamerson CL, Wong HL, Wahl LM, Wahl SM: Granulocyte-macrophage colony-stimulating factor stimulates human monocyte accessory cell function. J Immunol 1990, 144:3829-3834 [PubMed] [Google Scholar]
- 38.Kasinrerk W, Baumruker T, Majdic O, Knapp W, Stocinger H: CD1 molecule expression on human monocytes induced by granulocyte-macrophage colony-stimulating factor. J Immunol 1993, 150:579-584 [PubMed] [Google Scholar]
- 39.Bowers WE, Ruhoff MS, Goodell EM: Conditioned medium from activated rat macrophages and the recombinant factors, IL-1β and GM-CSF, enhance the accessory activity of dendritic cells. Immunobiology 1990, 180:362-384 [DOI] [PubMed] [Google Scholar]
- 40.Martin LH, Calabi F, Milstein C: Isolation of CD1 genes, a family of major histocompatibility complex-related differentiation antigens. Proc Natl Acad Sci USA 1986, 83:9154-9158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy GF, Bhan AK, Sato S, Mihm MC, Harrist TJ: A new immunologic marker for human Langerhans cells. New Engl J Med 1981, 304:791-792 [DOI] [PubMed] [Google Scholar]
- 42.Blumberg RS, Gerdes D, Ghott A, Porcelli SA, Balk SP: Structure and function of the CD1 family of MHC-like cell surface proteins. Immunol Rev 1995, 147:5-29 [DOI] [PubMed] [Google Scholar]
- 43.Thomssen H, Ivanyi J, Espitia C, Arya A, Londei M: Human CD4−CD8− T-cells and αβ+ T-cell receptor T cells recognize different mycobacteria strains in the context of CD1b. Immunology 1995, 85:33-40 [PMC free article] [PubMed] [Google Scholar]
- 44.Sieling PA, Chatterjee D, Porcelli SA, Prigozy TI, Mazzaccaro JR, Soriano T, Bloom BR, Brenner MB, Kronenberg M, Brennan PJ, Modlin RL: CD1-restricted T cell-recognition of microbial lipoglycan antigens. Science 1995, 269:227-230 [DOI] [PubMed] [Google Scholar]
- 45.Beckman EM, Porcelli SA, Morita CT, Behar SM, Furlong ST, Brenner MB: Recognition of a lipid antigen by CD1-restricted αβ+ T cells. Nature 1994, 372:691-694 [DOI] [PubMed] [Google Scholar]
- 46.Beckman EM, Brenner MB: MHC class I-like, class II-like and CD1 molecules: distinct roles in immunity. Immunol Today 1995, 16:349-352 [DOI] [PubMed] [Google Scholar]
- 47.Behar SM, Porcell SA: A pathway of costimulation that prevent anergy in CD28-T cells: B7-independent costimulation of CD1-restricted T cells. J Exp Med 1995, 182:2007-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ozawa H, Aiba S, Nakagawa, Tagami H: Interferon-gamma and interleukin-10 inhibit antigen presentation by Langerhans cells for T helper type 1 cells by suppressing their CD80 (B7–1) expression. Eur J Immunol 1996, 26:648-652 [DOI] [PubMed] [Google Scholar]
- 49.Tsunoda R, Nakayama M, Onozaki K, Heinen E, Corman N, Kinet DC, Kojima M: Isolation and long-term cultivation of human tonsil follicular dendritic cells. Virchows Arch Abteil B Cell Pathol 1990, 59:95-105 [DOI] [PubMed] [Google Scholar]