Abstract
We determined that mitochondrial respiration reduced cytosolic oxidant stress in vivo and scavenged extramitochondrial superoxide anion (O2-.) in vitro. First, Saccharomyces cerevisiae deficient in both the cytosolic antioxidant cupro-zinc superoxide dismutase (Cu,Zn-SOD) and electron transport (Rho0 state) grew poorly (P < 0.05) in 21% O2 compared with parent yeast and yeast deficient only in electron transport or Cu,Zn-SOD, whereas anaerobic growth was the same (P > 0.05) in all yeast. Second, isolated yeast and mammalian mitochondria scavenged extramitochondrial O2-. generated by xanthine/xanthine oxidase. Yeast mitochondria scavenged 42% more (P < 0.05) extramitochondrial O2-. during pyruvate/malate-induced respiration than in the resting state. Addition of either antimycin (respiratory chain inhibitor) or FCCP (respiratory chain uncoupler) prevented increased O2-. scavenging. Mitochondria isolated from yeast deficient in the mitochondrial manganous superoxide dismutase (Mn-SOD) increased (P < 0.05) O2-. scavenging 56% during respiration. This apparent SOD activity, expressed in units of SOD activity per milligram of mitochondrial protein, was the same (9 +/- 0.6 vs. 10 +/- 1.0; P = 0.43) as the O2-. scavenging of mitochondria with Mn-SOD, suggesting that respiration-dependent mitochondrial O2-. scavenging was nonenzymatic. Finally, isolated rat liver and lung mitochondria also increased (P < 0.05) O2-. scavenging during respiration. We speculate that respiring mitochondria, via the protonmotive pump, present a polarized, proton-rich surface that enhances nonenzymatic dismutation of extramitochondrial O2-. and that this is a previously unrecognized function of mitochondrial respiration with potential physiological ramifications.
Full text
PDF
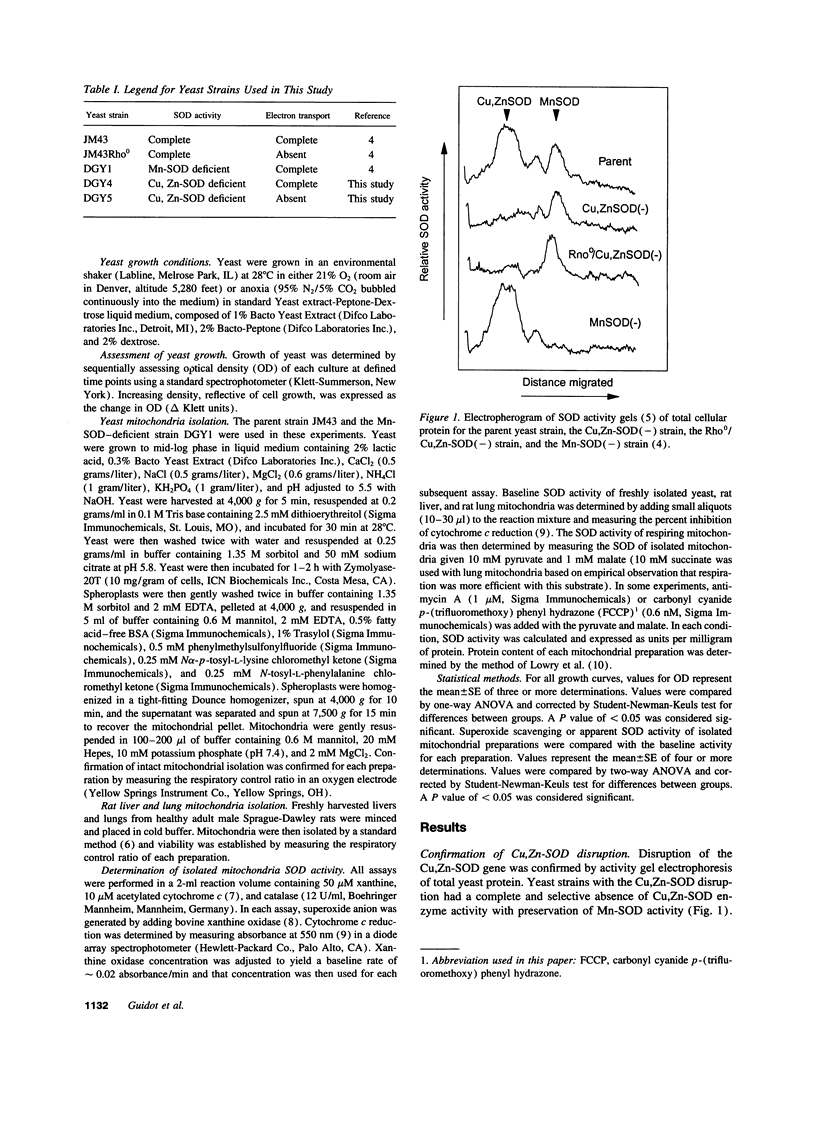

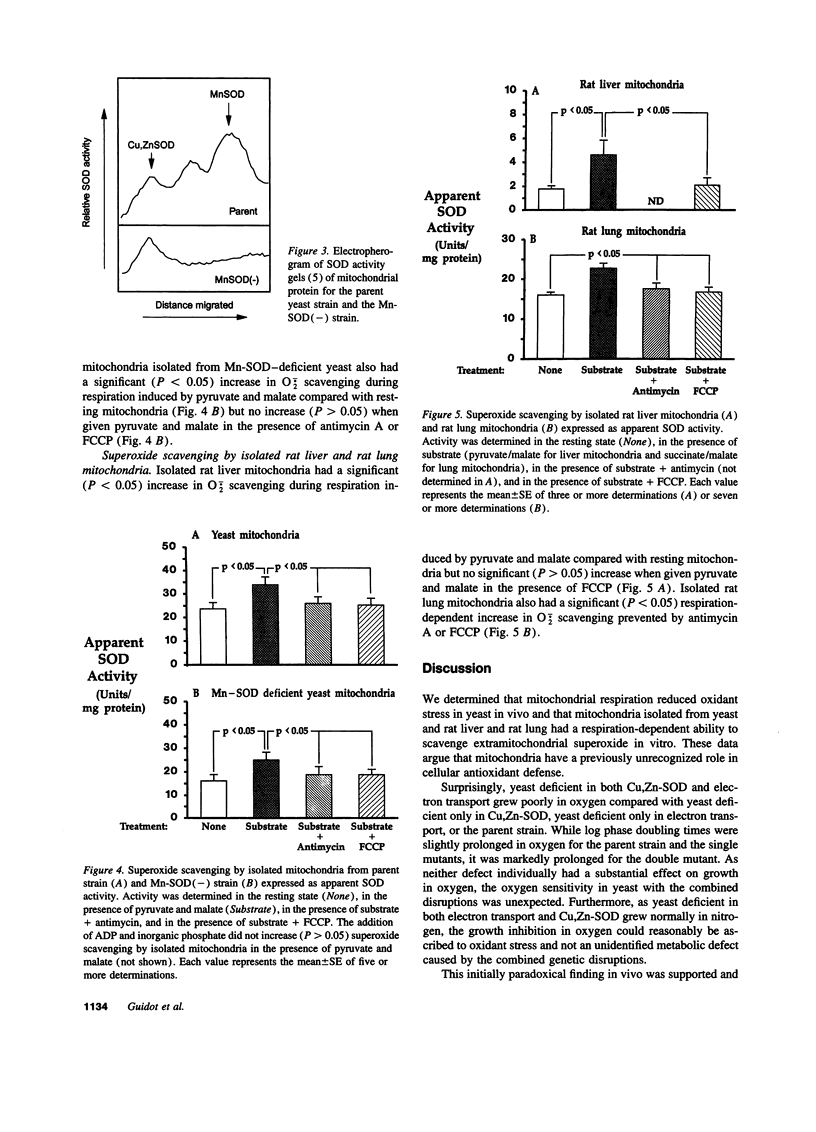
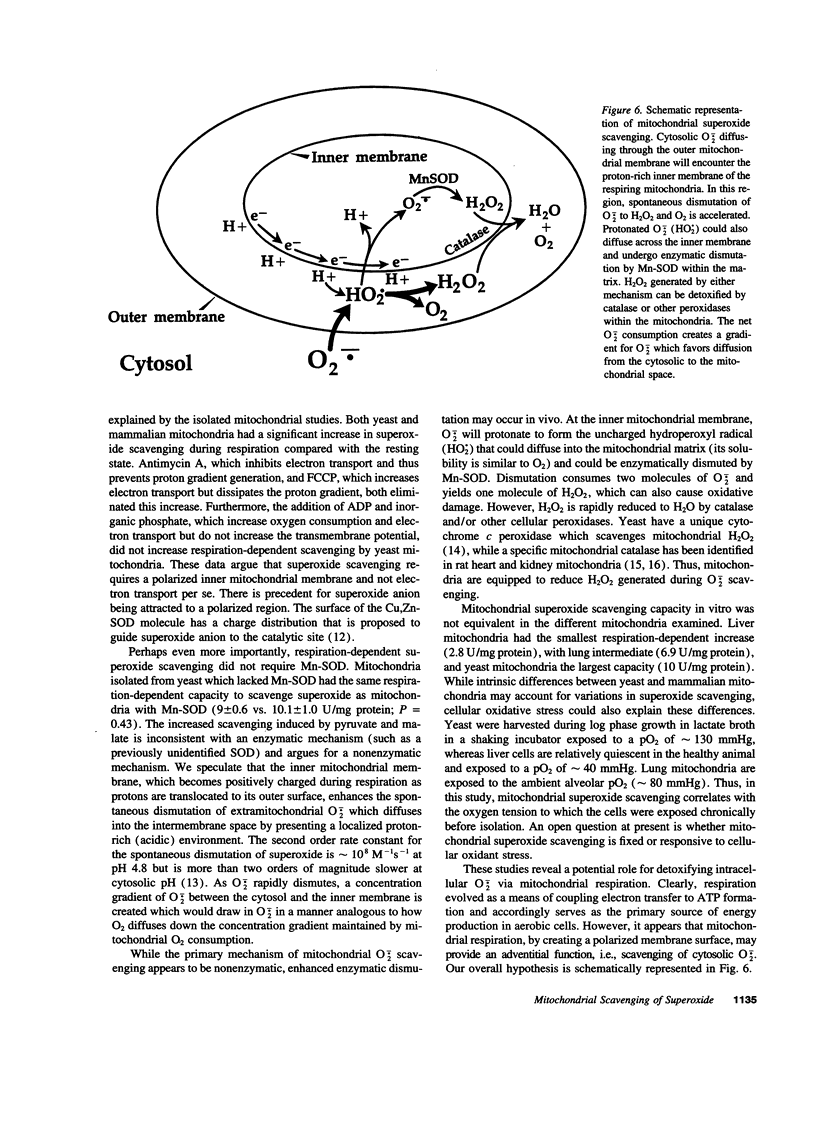

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzi A., Montecucco C., Richter C. The use of acetylated ferricytochrome c for the detection of superoxide radicals produced in biological membranes. Biochem Biophys Res Commun. 1975 Jul 22;65(2):597–603. doi: 10.1016/s0006-291x(75)80188-4. [DOI] [PubMed] [Google Scholar]
- Cadenas E. Biochemistry of oxygen toxicity. Annu Rev Biochem. 1989;58:79–110. doi: 10.1146/annurev.bi.58.070189.000455. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., McCord J. M., Fridovich I. Preparation and assay of superoxide dismutases. Methods Enzymol. 1978;53:382–393. doi: 10.1016/s0076-6879(78)53044-9. [DOI] [PubMed] [Google Scholar]
- Cross A. R., Jones O. T. Enzymic mechanisms of superoxide production. Biochim Biophys Acta. 1991 May 6;1057(3):281–298. doi: 10.1016/s0005-2728(05)80140-9. [DOI] [PubMed] [Google Scholar]
- Getzoff E. D., Tainer J. A., Weiner P. K., Kollman P. A., Richardson J. S., Richardson D. C. Electrostatic recognition between superoxide and copper, zinc superoxide dismutase. Nature. 1983 Nov 17;306(5940):287–290. doi: 10.1038/306287a0. [DOI] [PubMed] [Google Scholar]
- Guidot D. M., McCord J. M., Wright R. M., Repine J. E. Absence of electron transport (Rho 0 state) restores growth of a manganese-superoxide dismutase-deficient Saccharomyces cerevisiae in hyperoxia. Evidence for electron transport as a major source of superoxide generation in vivo. J Biol Chem. 1993 Dec 15;268(35):26699–26703. [PubMed] [Google Scholar]
- Klug D., Rabani J., Fridovich I. A direct demonstration of the catalytic action of superoxide dismutase through the use of pulse radiolysis. J Biol Chem. 1972 Aug 10;247(15):4839–4842. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Mertens-Strijthagen J., De Schrijver C., Wattiaux-De Coninck S., Wattiaux R. A centrifugation study of rat-liver mitochondria, lysosomes and peroxisomes during the perinatal period. Eur J Biochem. 1979 Aug 1;98(2):339–352. doi: 10.1111/j.1432-1033.1979.tb13193.x. [DOI] [PubMed] [Google Scholar]
- Pedersen P. L., Greenawalt J. W., Reynafarje B., Hullihen J., Decker G. L., Soper J. W., Bustamente E. Preparation and characterization of mitochondria and submitochondrial particles of rat liver and liver-derived tissues. Methods Cell Biol. 1978;20:411–481. doi: 10.1016/s0091-679x(08)62030-0. [DOI] [PubMed] [Google Scholar]
- Radi R., Sims S., Cassina A., Turrens J. F. Roles of catalase and cytochrome c in hydroperoxide-dependent lipid peroxidation and chemiluminescence in rat heart and kidney mitochondria. Free Radic Biol Med. 1993 Dec;15(6):653–659. doi: 10.1016/0891-5849(93)90169-u. [DOI] [PubMed] [Google Scholar]
- Radi R., Turrens J. F., Chang L. Y., Bush K. M., Crapo J. D., Freeman B. A. Detection of catalase in rat heart mitochondria. J Biol Chem. 1991 Nov 15;266(32):22028–22034. [PubMed] [Google Scholar]
- Salin M. L., McCord J. M. Superoxide dismutases in polymorphonuclear leukocytes. J Clin Invest. 1974 Oct;54(4):1005–1009. doi: 10.1172/JCI107816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waud W. R., Brady F. O., Wiley R. D., Rajagopalan K. V. A new purification procedure for bovine milk xanthine oxidase: effect of proteolysis on the subunit structure. Arch Biochem Biophys. 1975 Aug;169(2):695–701. doi: 10.1016/0003-9861(75)90214-3. [DOI] [PubMed] [Google Scholar]
- Yonetani T. Studies on cytochrome c peroxidase. II. Stoichiometry between enzyme, H2O2, and ferrocytochrome c and enzymic determination of extinction coefficients of cytochrome c. J Biol Chem. 1965 Nov;240(11):4509–4514. [PubMed] [Google Scholar]


