Abstract
Matrix metalloproteinase-2 (MMP2) activation is associated with basement membrane remodeling that occurs in injured tissues and during tumor invasion. The newly described membrane-type MMPs (MT-MMPs) form a family of potential MMP2 activators. We investigated the localization and steady-state levels of MT1-MMP and MT2-MMP mRNA, compared with those of MMP2 and tissue inhibitor of MMP-2 in 22 hepatocellular carcinomas, 12 liver metastases from colonic adenocarcinomas, 13 nontumoral samples from livers with metastases, 10 benign tumors, and 6 normal livers. MMP2 activation was analyzed by zymography in the same series. The expression of MT1-MMP mRNA and the activation of MMP-2 were increased in hepatocellular carcinomas, metastases, and cholestatic nontumoral samples. MT2-MMP mRNA was rather stable in the different groups. MT1-MMP mRNA levels, but not MT2-MMP mRNA, correlated with MMP-2 and tissue inhibitor of MMP-2 mRNA levels and with MMP2 activation. In situ hybridization showed that MT1-MMP mRNA was expressed in stromal cells, and MT2-MMP mRNA was principally located in both hepatocytes and biliary epithelial cells. Consistently, freshly isolated hepatocytes expressed only MT2-MMP mRNA, and culture-activated hepatic stellate cells showed high levels of MT1-MMP mRNA. These results indicate that in injured livers, MMP2 activation is related to a coordinated high expression of MMP2, tissue inhibitor of MMP-2, and MT1-MMP. Furthermore, the finding of a preferential expression of MT2-MMP in hepatocytes, together with our previous demonstration that the activation of stellate cell-derived MMP2 in co-culture requires interactions with hepatocytes (Am J Pathol 1997, 150:51–58), suggests that parenchymal cells might play a pivotal role in the MMP2 activation process.
Degradation of the extracellular matrix is associated with most physiological and pathological processes requiring tissue remodeling by the matrix metalloproteinase (MMP) superfamily. 1,2 Five classes of MMP have been identified so far, based on their structure and/or substrate specificity, including collagenases, gelatinases, stromelysins, elastases, and membrane-type metalloproteinases (MT-MMPs). All MMPs display common features, particularly the requirement of a proteolysis step to be activated. 3 Unlike other MMPs, the activation of MMP2 (gelatinase A/72-kd type IV collagenase) takes place at the cell surface, 4,5 which confers to this unique metalloproteinase a pivotal role in migration and growth of tumor cells, a process requiring the remodeling of basement membranes, the thin extracellular matrices that underlie epithelial, endothelial, and nerve cells.
Cytoplasmic membrane components responsible for MMP2 activation are not yet completely identified. A major breakthrough in the knowledge of MMP2 activation process came from the recent discovery of MT-MMPs, the new class of MMPs sharing a transmembrane domain. 6 Thus, it has been shown in vitro that MT1-MMP might be associated to the tissue inhibitor of metalloproteinase 2 (TIMP2), both acting together as a receptor for pro-MMP2 and leading to the cleavage of the zymogen. In support of this, an overexpression of MT1-MMP mRNA was reported in tumoral tissues and correlated to the activation of MMP2 in brain, 7 head and neck, 8 breast, 9 and gastrointestinal cancers. 10 Three additional members of the MT-MMP family were recently identified, ie, MT2-MMP, 11 MT3-MMP, 12 and MT4-MMP. 13 Both MT2-MMP and MT3-MMP were demonstrated to activate MMP2 in vitro. 6,14,15
In the liver, basement membrane remodeling occurs in various physiological and pathological events, including regeneration, fibrogenesis, and the development of tumors. However, the mechanisms whereby MMP2 becomes activated are poorly documented, particularly in human livers. High levels of MMP2 and TIMP2 mRNA were reported in hepatocellular carcinomas (HCCs), cholangiocarcinomas, and liver metastases, compared with their low levels of expression in normal livers. 16-20 Accordingly, MMP2 was revealed by immunohistochemistry in HCCs, its expression being related to tumor aggressiveness 21 and prognosis. 22 Recently, we have shown that MMP2 and TIMP2 mRNA were predominantly expressed by stromal cells in primary and secondary tumors, 19,20 and increased MT1-MMP expression was reported in HCCs. 23 In addition, in vitro studies demonstrated that rat stellate cells, particularly in their activated myofibroblast-like phenotype, can express MMP2, TIMP2, and, interestingly, MT1-MMP mRNA. 24,25 However, a coordinated expression of these components by cultured stellate cells appeared not sufficient to achieve a significant activation of pro-MMP2, as this process requires cell-cell interactions with hepatocytes through an unidentified membrane component. 24 Accordingly, the involvement of an additional component(s) to the ternary complex of activation associating MT1-MMP, MMP2, and TIMP2 was suggested in several model systems. 26-28 Because MT2-MMP was recently identified in the liver, we hypothesized that it could trigger the cleavage of pro-MMP2 in association with MT1-MMP. Indeed, among the four MT-MMPs recently identified in human tissues, only MT1-MMP 29 and MT2-MMP 11 were found in the liver, with MT3-MMP 12 and MT4-MMP 13 being nearly undetectable by Northern blot. Thus, we investigated the localization and the steady-state levels of both MT1-MMP and MT2-MMP mRNA in liver cancers and compared them with those of MMP2 and TIMP2. These data were correlated to the MMP2 gelatinolytic activity revealed by zymography analyses. The results indicate that MMP2 activation is related to a coordinated high expression of MMP2, TIMP2, and MT1-MMP in injured human livers and that hepatocytes might modulate the activation of MMP2 through the expression of MT2-MMP.
Materials and Methods
Tissue Samples
Liver tissue samples were obtained from 63 surgical patients. The samples consisted of 10 benign tumors, 22 HCCs, 12 hepatic metastases from colonic adenocarcinomas, and 13 distant nontumorous samples from livers with metastases. Controls were 6 explanted livers from donors excluded from the transplantation protocol due to extra-abdominal infection unrelated to the cause of death. Access to this material was in agreement with French laws and satisfied the requirements of the local Ethics Committee. After macroscopic examination by a pathologist, representative samples were fixed in buffered formalin and embedded in paraffin for histopathological routine diagnosis; a part of the fresh material was snap frozen in isopentane cooled in liquid nitrogen and stored at −80°C until use. Before mRNA or protein extraction, 5-μm frozen serial sections were obtained from the tissue blocks, stained with methylene blue, and observed under light microscopy. Similarly, formalin-fixed, paraffin-embedded material from the same patient was analyzed independently.
Pathological Features
All specimens were routinely processed for histology, ie, hematoxylin-eosin-saffran and Sirius red staining. HCCs were graded according to Edmondson and Steiner. 30 Other pathological features studied in HCCs included tumor size, histological and cytological features, fibrosis in the nontumorous part of the liver, tumor encapsulation, tumor necrosis, and presence of portal tumor emboli. Myofibroblasts and endothelial cells were characterized by immunohistochemical staining with anti-α-SM-1, a monoclonal IgG2a recognizing α-smooth muscle actin (α-SMA) (Sigma, St. Louis, MO) and with monoclonal mouse IgG1κ anti-human von Willebrand Factor (Dako, Glostrup, Denmark), respectively, using standard immunoperoxidase techniques. 20
Cell Isolation and Culture
Hepatic stellate cells and hepatocytes were isolated from liver fragments of patients undergoing hepatectomy for secondary tumors. Stellate cells were purified by a single-step density gradient centrifugation with Nycodenz (Nyegaard and Co., Oslo, Norway) after tissue dissociation by perfusion with a pronase and collagenase solution. 31 They were subsequently routinely cultured until passage 6. Human hepatocytes were obtained using the two-step collagenase perfusion method. 32
Nucleic Acid Probes
The cDNA probes for MMP2 and TIMP2 were previously obtained from human liver and human placenta, respectively. 19 For the preparation of MT1-MMP cDNA probe, reverse transcription-polymerase chain reaction products from a human liver were cloned into the pTag plasmid vector (R&D Systems, UK) as previously described. 24 Sequencing with the Sequenase Version 2.0 DNA sequencing kit (United States Biochemical, Cleveland, OH) confirmed the identity of the constructs with previously published sequences. The probe for MT2-MMP was a 1268-bp EcoRI fragment from the plasmid pBlue/M2/1-1268 (a gift from Dr. H. Will, In Vitek GMbH, Berlin, Germany). For 18S ribosomal RNA, a 25-mer oligoprobe was used. 33
Northern and Dot Blots
After histological analysis, frozen blocks making up a volume of 1.5 to 3 cm 3 were used for RNA extraction by the guanidinium thiocyanate/cesium chloride method. 34 Histological analysis of the frozen blocks before RNA extraction from large tissue samples ensured that sampling was both macroscopically and microscopically consistent. From each sample, 15 μg RNA were resolved by electrophoresis through a 1.2% denaturing agarose slab gel and transferred onto a Hybond N+ nylon membrane (Amersham, Buckinghamshire, UK). The efficacy of transfer was assessed by methylene blue staining after UV cross-linking. cDNA probes were 32P labeled (1 × 10 8 to 1 × 10 9 cpm/μg cDNA) using the Rediprime kit (Amersham) and [α-32P]dCTP, 3000 Ci/mmol (Amersham). Hybridizations with cDNA probes were performed in 0.5 mol/L Na2H PO4, 12 H2O; 20 mmol/L H3 PO4, pH 7.2 containing 7% sodium dodecyl sulfate (SDS), 1 mmol/L ethylenediaminetetraacetic acid and 1% bovine serum albumin for 16 hours at 65°C. Filters were washed in 3× standard saline citrate/0.1%SDS three times for 1 minute each and two times for 15 minutes each at 65°C followed by 0.1× standard saline citrate/0.1%SDS two times for 15 minutes each at 65°C. Amersham Hyperfilm-MP films were exposed with enhancing screens at −80°C.
Dot blots were performed as described. 35 Briefly, each RNA sample was blotted in triplicate at 1, 2.5, and 5 μg/μl using a filtration manifold. Hybridizations with cDNA probes were performed as indicated above. Blots were autoradiographed for 72 hours for MT1-MMP, MT2-MMP, and MMP2 each; 24 hours for TIMP2; and 12 hours for 18S rRNA. Densitometry scanning of the autoradiograms was performed with the Densylab program (Bioprobe Systems, Les Ulis, France). Hybridizations were performed under conditions, probe concentrations, and film exposure times that gave a linear relationship between densitometry signal and amount of RNA loaded (range tested, 1 to 5 μg RNA; r = 0.97 to 0.99). Relative mRNA amounts were corrected for minor differences in RNA loading by normalization with the signal obtained for 18S rRNA. Values were thus expressed as MT1-MMP/18S, MT2-MMP/18S, MMP2/18S, and TIMP2/18S RNA ratios.
In Situ Hybridization
In situ hybridization with sense and antisense cRNA probes was performed as previously described by Musso et al. 20 Tissues were hybridized with 8 × 10 3 cpm/μl of probe and exposed to ILFORD G5 emulsion (Ilford Anitec, Lyon, France) at 4°C for 2 weeks, developed in Kodak D19, fixed with Kodak Unifix (Kodak-Pathé, Chalons-sur-Saone, France), and stained with hematoxylin and eosin.
Zymography Analysis
The proenzyme and activated forms of MMP2 were detected by zymography using SDS-7% polyacrylamide gels copolymerized with 1 mg/ml gelatin. Sections of 5 μm/1 cm 2 were scraped off the slides. 19 Protein content was measured, and equal amounts of samples were homogenized in sample buffer and directly electrophoresed. The gels were washed twice at room temperature for 10 minutes in 2.5% Triton X-100 and for 20 minutes in water and incubated overnight at 37°C in 50 mmol/L Tris-HCl, pH 8, containing 5 mmol/L CaCl2 and 1 μmol/L ZnCl2. 36 Gels were stained in 30% methanol/10% acetic acid containing 0.5% Coomassie Brilliant Blue G250. Both proenzyme and active proteinase forms were detected as clear bands and analyzed by densitometric scanning using a computer-assisted analysis. 37 Five levels of intensity were defined ranging from no signal to maximal densitometric value, and 66 kd and 62 kd bands were classified as follows: −, null; +, mild; ++, moderate; +++, strong; and ++++, intense. No variation in the classification was observed among three separate analyses for the same samples.
Statistical Analysis
Values were expressed as mean ± SD. The Mann-Whitney U test was used to test the significance of the differences between means. Spearman rank order correlation coefficients were used to express the association between the different variables within the study groups. Data were analyzed using the Statistica release 4.3B software package (StatSoft, Inc., Tulsa, OK).
Results
Pathological Features
Patients with HCC comprised 21 males and 1 female; the mean age ± SD was 64 ± 8 years. Edmonson’s score was II in 17 cases, III in 4 cases, and IV in one case. Eleven patients had portal embolization, and 9 HCCs were encapsulated. All 12 liver metastases were from colonic cancers (8 males and 4 females; the mean age ± SD was 61 ± 9 years). Nontumoral samples from metastatic livers included nontumoral fragments with minimal histopathological changes (n = 7) and those with cholestasis (n = 6), as described. 20 Nontumor samples with minimal histopathological changes showed edematous portal tracts with leukocyte infiltrates, Kupffer cell hyperplasia, and sinusoidal dilatation. Nontumoral samples with cholestasis showed portal fibrosis without septa or intracellular cholestasis and a type I ductular reaction characterized by the proliferation of biliary epithelial cells with tortuosity and elongation of pre-existing ducts. Benign tumors comprised seven focal nodular hyperplasias and three adenomas (one male and nine females; the mean age ± SD was 37 ± 12 years). Control livers were histologically normal.
Expression of MT1-MMP and MT2-MMP mRNA
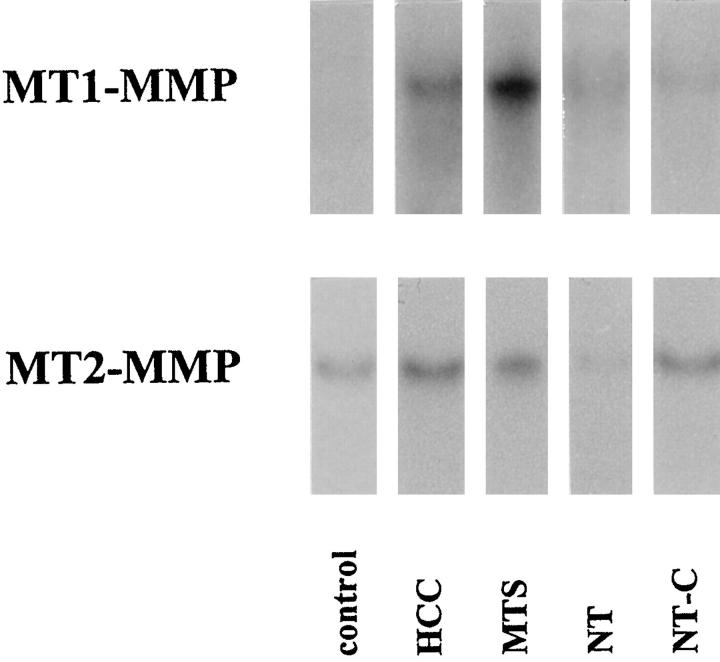
Total RNA was extracted from human biopsies, and specific mRNA levels were compared between normal livers, benign tumors, HCCs, metastases from colonic adenocarcinomas, and nontumoral areas from livers with metastases. Northern blots of MT1-MMP and MT2-MMP mRNA of one representative sample of each group are illustrated in Figure 1 ▶ . In normal livers, MT1-MMP mRNA species were barely detectable, and MT2-MMP mRNA species were conspicuous. Staining intensity for both MT1-MMP and MT2-MMP mRNA increased in HCCs, metastases, and nontumoral liver with cholestasis. Semiquantitative analysis of the steady-state MT1-MMP and MT2-MMP mRNA levels was performed by dot blot (Figure 2) ▶ . MT1-MMP mRNA levels were increased in HCCs (4 ± 2.7) and in metastases (11.7 ± 3.8) compared with normal liver (1 ± 0.4) (P < 0.001 and P < 0.001, respectively) and compared with benign tumors (1.15 ± 0.82) (P < 0.001 and P < 0.0001, respectively). In nontumoral areas with cholestasis, MT1-MMP mRNA level was increased fourfold versus controls (P < 0.01), whereas in nontumoral areas without cholestasis, a 2.2-fold increase was found (P < 0.01). The steady-state MT2-MMP mRNA levels were not different in HCCs from those in normal livers and benign tumors. MT2-MMP mRNA levels were increased in metastases versus controls (3.5-fold, P < 0.001). In nontumoral areas of metastatic livers, MT2-MMP mRNA levels were slightly higher than in controls (1.8-fold, P < 0.05), and in nontumoral areas with cholestasis, MT2-MMP mRNA levels were 3.4-fold increased versus controls (P < 0.001).
Figure 1.
Northern blot analysis of MT1-MMP and MT2-MMP mRNA in human livers. Total RNAs were extracted from liver biopsies, resolved by electrophoresis onto agarose gels, and blotted onto Hybond N nylon. MTS, hepatic metastasis from colonic adenocarcinomas; NT, nontumorous samples from livers with metastases (with either minimal histopathological changes (NT) or cholestasis (NT-C).
Figure 2.
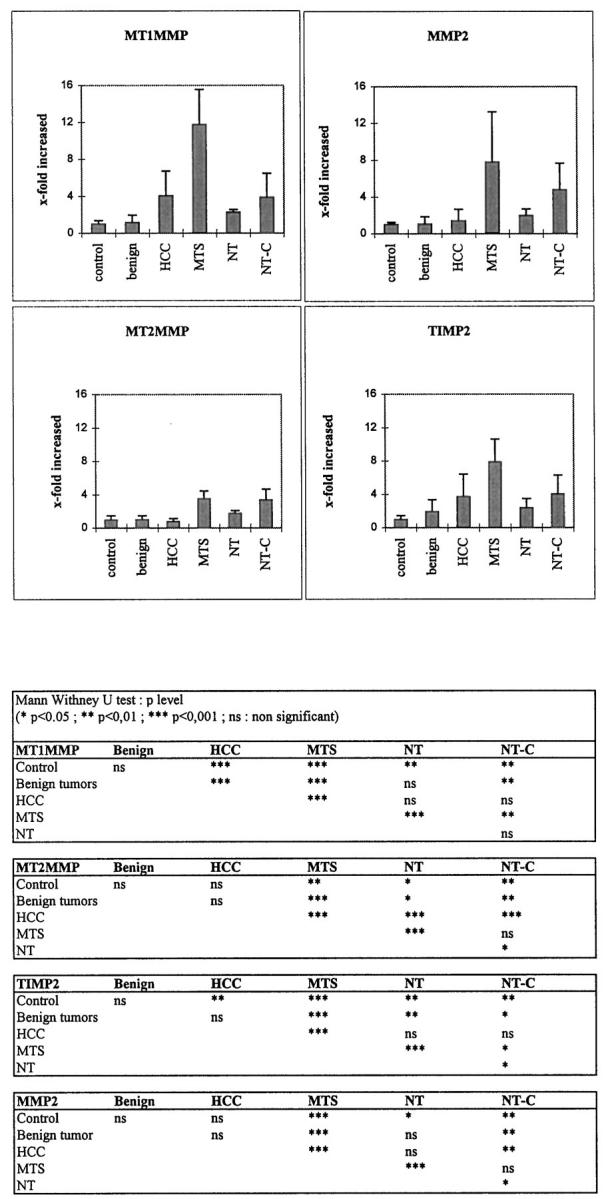
Dot blot analysis of the steady-state MT1-MMP, MT2-MMP, MMP2, and TIMP2 mRNA levels in control livers, benign tumors, HCCs, hepatic metastases from colonic adenocarcinomas (MTS), and nontumorous samples from livers with metastases with either minimal histopathological changes (NT) or cholestasis (NT-C). Top: Densitometric values from each probe within the linear range were normalized with the 18S oligoprobe and plotted. Columns, mean; bars, SD. Bottom: The Mann-Whitney U test was used to test the significance of the differences between means.
Correlation between MT1-MMP and MT2-MMP Expression and the Activation of MMP2
To analyze whether the expression levels of MT1-MMP and MT2-MMP mRNAs were associated with MMP2 activation, we further studied both the expression of MMP2 and TIMP2 mRNA by semiquantitative dot-blot analysis and the relative amounts of both inactive and active forms of MMP2 by zymography.
MMP2 and TIMP2 mRNA Levels (Figure 2) ▶
In metastases, both MMP2 and TIMP2 mRNA levels were similarly increased (7.8- and 8-fold, respectively, P < 0.001) compared with normal livers and benign tumors. In HCCs, unlike TIMP2 mRNA levels (3.7-fold increase, P < 0.01), MMP2 mRNA levels were not statistically different (1.4-fold ± 1.2) versus controls. In nontumoral areas with cholestasis, both MMP2 and TIMP2 mRNA were increased compared with normal livers (4.8- and 4-fold, respectively), whereas in the absence of cholestasis (nontumoral samples), the values were lower (1.9- and 2.4-fold). There was no significant difference between benign tumors and controls for both MMP2 and TIMP2 mRNA levels.
Activation of MMP2
Gelatinolytic activity of 53 biopsies was monitored by gel substrate analysis in 5 controls, 7 benign tumors, 19 HCCs, 12 metastases, and 10 nontumoral areas of metastatic livers without (n = 4) or with (n = 6) cholestasis in three independent experiments (Figure 3) ▶ . Both Mr 62,000 active and Mr 66,000 latent forms of MMP2 were increased in metastases, HCCs, and nontumoral liver samples with cholestasis compared with control and benign tumor groups in which no gelatinolytic activity was detected. In nontumoral areas of metastatic liver without cholestasis, the latent form of MMP2 was detected, but not the active 62-kd form. The percentage of the active form (62 kd) expressed as the ratio 62/(62 + 66) was increased in metastases (42% ± 11%) compared with that of HCCs (22% ± 23%) and nontumoral areas of livers with cholestases (29% ± 10%).
Figure 3.
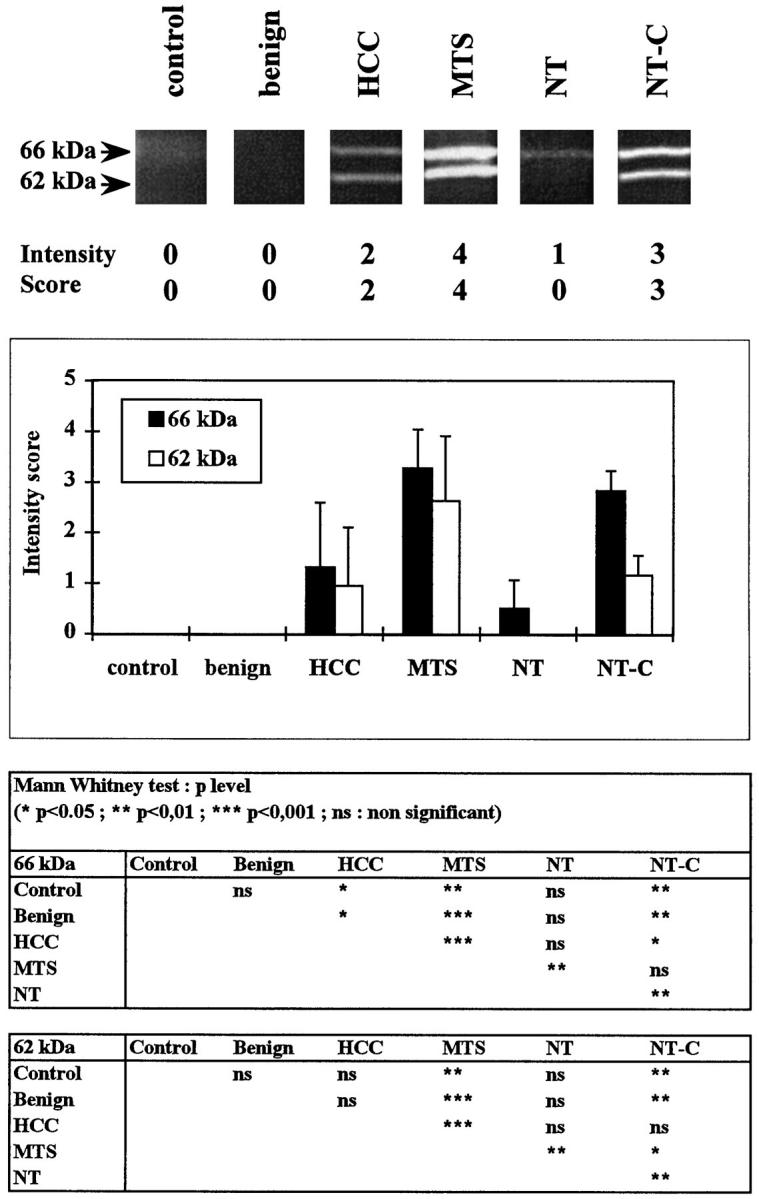
Zymography analyses. Sections (5 μm/1 cm2) from 53 biopsy samples (control livers, benign tumors, HCCs, hepatic metastases from colonic adenocarcinomas (MTS), and nontumorous samples from livers with metastases with either minimal histopathological changes (NT) or cholestasis (NT-C) were homogenized in sample buffer and electrophoresed on an SDS-7% polyacrylamide gel copolymerized with 1 mg/ml gelatin. Five intensity levels based on densitometric analyses were determined for the Mr 66 and Mr 62 kd bands. Top: Representative zymogram with densitometric range. Middle: Semiquantitative analyses of gelatinolytic activity. Bottom: Results are expressed as means ± SD. The Mann-Whitney U test was used to test the significance of the differences between means.
Correlation Study
The relationships between MT1-MMP, MT2-MMP, MMP2, and TIMP2 expression levels were further analyzed (Table 1) ▶ . In HCCs, metastases, and cholestases, the steady-state MT1-MMP mRNA levels correlated both with MMP2 (r = 0.62, 0.77, and 0.88, respectively) and TIMP2 mRNA levels (r = 0.90, 0.77, and 0.88, respectively). In addition, MMP2 mRNA levels correlated with TIMP2 mRNA levels (r = 0.7, 0.67, and 0.83, respectively). No correlation was found between MT2-MMP mRNA levels and those of MT1-MMP, MMP2, and TIMP2 in all of the groups.
Table 1.
Correlation of MT1-MMP, MT2-MMP, MMP2, and TIMP2 mRNA Levels in Hepatocellular Carcinomas, Metastases, and Cholestasis
| HCC | MTS | NT-C | |
|---|---|---|---|
| MT1 vs TIMP2 | 0.9*** | 0.77** | 0.88* |
| MT1 vs MT2 | 0.14 | 0.1 | 0.2 |
| MT1 vs MMP2 | 0.62** | 0.77** | 0.88* |
| TIMP2 vs MT2 | 0.184 | 0.35 | 0.54 |
| TIMP2 vs MMP2 | 0.70*** | 0.67* | 0.83* |
| MT2 vs MMP2 | 0.28 | 0.03 | 0.48 |
Spearman rank order correlation (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
HCC, hepatocellular carcinomas; MTS, metastases; NT-C, cholestasis
Because HCCs showed a high variability in specific mRNA expression levels and MMP2 activity, correlation analyses between MMP2 activity and MT1-MMP, MT2-MMP, MMP2, and TIMP2 mRNA levels were performed (Table 2) ▶ . MMP2 mRNA expression was correlated with the proform of MMP2 (r = 0.68) and the active form of MMP2 (r = 0.56). The steady-state MT1-MMP and TIMP2 mRNA levels were highly correlated with the presence of the 66-kd proform (r = 0.77 and 0.90, respectively) and the 62-kd active form MMP2 (r = 0.76 and 0.82, respectively). By contrast, MT2-MMP mRNA levels were not associated with any level of gelatinolytic activity. No significant correlation was found between the clinical features studied and the enhanced secretion of MMP2.
Table 2.
Correlation between MMP2 Activity and MT1-MMP, MT2-MMP, MMP2 and TIMP2 mRNA Levels in Hepatocellular Carcinoma
| MT1 | MT2 | MMP2 | TIMP2 | |
|---|---|---|---|---|
| 66 kd | 0.77*** | 0.1 | 0.68** | 0.90*** |
| 62 kd | 0.76*** | 0.1 | 0.56* | 0.82*** |
| 62/(62+ 66) ratio | 0.71*** | 0.09 | 0.38 | 0.72*** |
Spearman rank order correlation, n = 19 (***, p < 0.001; **, p < 0.01; *, p < 0.05)
Cells Involved in MT1-MMP and MT2-MMP Expression
To determine the cellular distribution of MT1-MMP and MT2-MMP mRNA, both in situ hybridization on liver slices and Northern blot analysis with freshly isolated and cultured hepatocytes and stellate cells were performed.
In Situ Hybridization
In normal livers, MT1-MMP mRNA was barely detectable in bile duct epithelial cells. Hepatocytes were negative (Figure 4A) ▶ . In cholestatic livers, both bile duct epithelial cells and underlying myofibroblasts were heavily stained (Figure 4B) ▶ . In HCCs, MT1-MMP transcripts were located preferentially in stromal cells (Figure 4D) ▶ , tumor cells being negative. MT1-MMP-positive stromal cells included endothelial cells and myofibroblasts, as shown by immunohistochemical detection of von Willebrand factor in contiguous sections (Figure 4E) ▶ and α-smooth muscle actin within myofibroblasts (not shown). In metastases from colonic adenocarcinomas, MT1-MMP mRNA was detected in myofibroblasts within the tumor stroma, and in α-smooth muscle actin-positive sinusoidal cells at the tumor-host interface (Figure 4, F and G) ▶ . Endothelial cells of tumor vessels were also positive (not shown).
Figure 4.
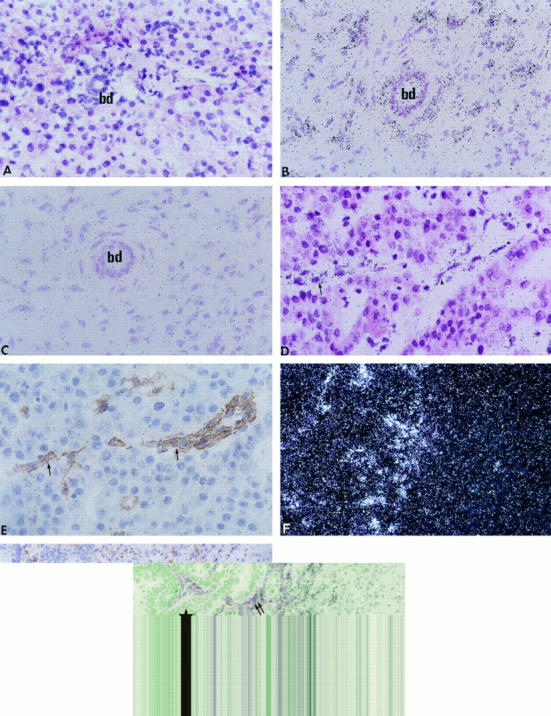
In situ hybridization of MT1-MMP mRNA in normal liver (A), cholestatic liver (B), HCC (D), and metastasis from a colonic adenocarcinoma (F). Controls include an immunohistochemical detection of von Willebrand factor in HCC (E, section contiguous to D) and α-smooth muscle actin in metastasis (G, section contiguous to F) and in situ hybridization with the sense probe (C, section contiguous to B). bd, bile duct; star, tumor; arrows, endothelial cells; double arrows, myofibroblasts. Bright-field (A to D) and dark-field (F) photomicrographs of autoradiographs stained with hematoxylin and eosin. Magnification: A to E, ×400; F and G, ×200.
MT2-MMP transcripts were detected in hepatocytes in normal livers. Portal fibroblasts showed background signal (Figure 5A) ▶ . Biliary epithelial and vascular endothelial cells were positive (not shown). In cholestatic livers, bile ducts and periportal hepatocytes were strongly labeled (Figure 5, B and C) ▶ . Myofibroblasts within the edematous stroma surrounding proliferating and distended bile ducts were negative, and vascular endothelial cells were positive (not shown). No significant signal was detected in controls with the MT2-MMP sense probe (Figure 5D) ▶ . In HCCs, MT2-MMP mRNA was detected in tumor cells, particularly in cells at the invasion front, adjacent to the tumor stroma (Figure 5, E and F) ▶ . In metastases, tumor cells and hepatocytes at the host-tumor interface were homogeneously labeled (Figure 5G) ▶ .
Figure 5.
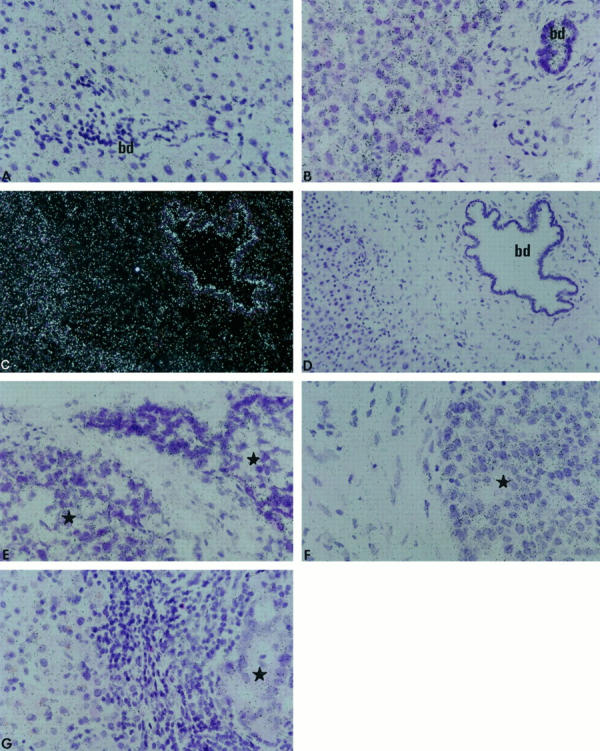
In situ hybridization of MT2-MMP mRNA in normal liver (A), cholestatic liver (B and C), HCCs (E and F), and metastasis from a colonic adenocarcinoma (G). Control with sense probe in situ hybridization is shown (D, section contiguous to C). bd, bile duct; star, tumor. Bright-field (A, B, and D to G) and dark-field (C) photomicrographs of autoradiographs stained with hematoxylin and eosin. Magnification: A, B, and E to G, ×400; C and D, ×200.
Isolated and Cultured Cells
The expression of MT1-MMP and MT2-MMP was investigated in human freshly isolated hepatocytes and culture-activated stellate cells. Northern blot analyses revealed a high steady-state MT1-MMP mRNA level in culture-activated stellate cells (Figure 6) ▶ ; MT1-MMP mRNA was not detectable in freshly isolated hepatocytes (Figure 6) ▶ and in short-term primary cultures (not shown). MT2-MMP mRNA was expressed at high levels in freshly isolated hepatocytes, which contrasted with a low expression level in culture-activated stellate cells.
Figure 6.
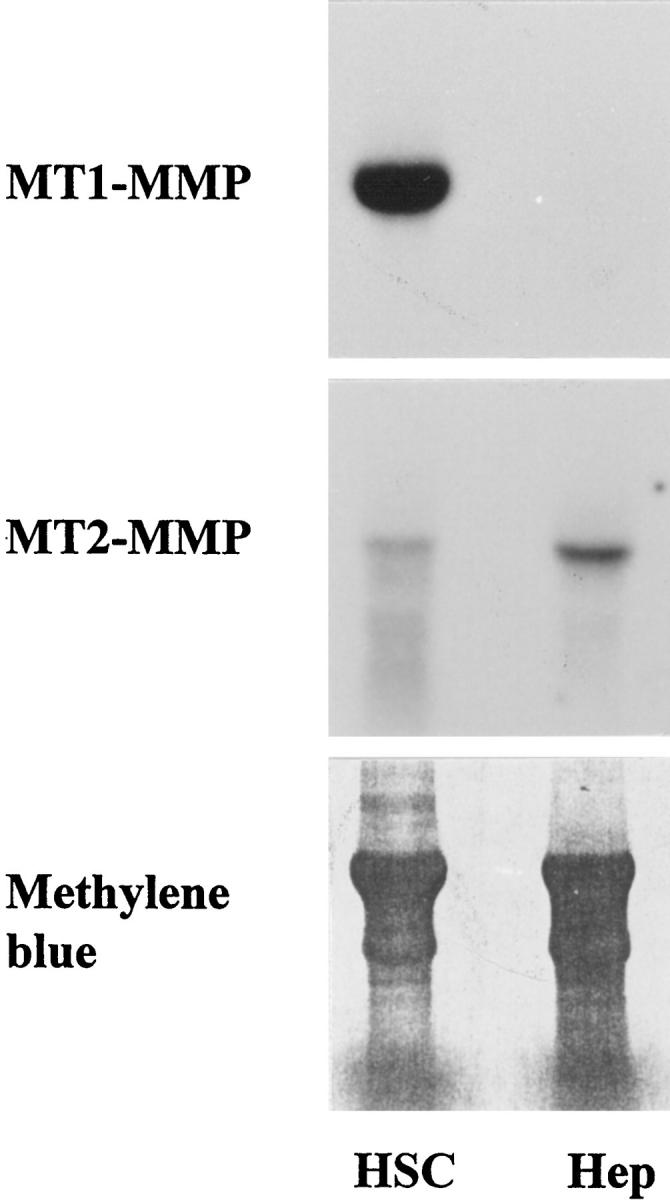
Northern blots of MT1-MMP and MT2-MMP mRNA in culture-activated stellate cells at passage 6 (HSC) and in freshly isolated hepatocytes (Hep). Note the strong expression of MT1-MMP mRNA in stellate cells, which contrasts with the low levels of MT2-MMP mRNA. Hepatocytes are positive for MT2-MMP mRNA only. Bottom: Transferred RNA stained with methylene blue.
Discussion
Activation of MMP2 is tightly related to basement membrane remodeling in tumor invasion and in tissue injury. In this study, we show that two potential activators of MMP2, ie, MT1-MMP and MT2-MMP, have distinct patterns of expression and distribution in normal and pathological human livers. MT1-MMP mRNA showed increased levels in primary and secondary liver cancers and in cholestases, was of stromal origin, and correlated with MMP2 activation, whereas MT2-MMP mRNA did not show significant changes and originated mainly in hepatocytes.
The increased levels of MT1-MMP mRNA in human liver cancers, compared with control livers or benign tumors, are in agreement with previous demonstrations of an overexpression of MT1-MMP mRNA in tumors from other tissues. 38-41 In nontumoral areas from metastatic livers, MT1-MMP mRNA levels were associated with the intensity of histological changes, probably because important basement membrane remodeling occurs during cholestasis and the ductular reaction. 42,43 This is in contrast with the absence of increased MT1-MMP mRNA levels in benign tumors, which could be related to a limited potential for extracellular matrix remodeling. Conversely, the expression pattern of MT2-MMP was strikingly different from that of MT1-MMP. The increase in MT2-MMP mRNA level in metastases versus control was moderate (3.5-fold) compared with those of MT1-MMP (11.7-fold), MMP2 (8-fold), and TIMP2 (8-fold). These observations are probably related to a higher MT2-MMP mRNA basal level in normal livers, whereas MT1-MMP mRNA was nearly undetectable.
A current hypothesis about the mechanism(s) of MMP2 activation involves interplays between pro-MMP2, MT1-MMP, and TIMP2. 4 Accordingly, we show that MMP2, TIMP2, and MT1-MMP mRNA overexpression are coordinated in primary and secondary cancers and in cholestases. In addition, they correlated with MMP2 activation in HCCs. Such a simultaneous increase in MMP2, MT1-MMP, and TIMP2 mRNA levels was previously reported in tissue remodeling during embryogenesis, 44 as well as a simultaneous increase in MT1-MMP and MMP2 mRNA levels in a number of tumors. Our study extends these findings and emphasizes the importance of the coordinated expression of MMP2, TIMP2, and MT1-MMP, which may lead to the formation of a ternary complex in the MMP2 activation process.
However, recent reports have suggested that a mere expression of the three components of the ternary complex could not account for an activation of MMP2 in several model systems 26-28 and that an additional factor(s) might be involved in this process. Based on this hypothesis, we have investigated the expression of MT2-MMP, another potential activator of MMP2 that was previously shown to be expressed in the liver. Surprisingly, no correlation between MT2-MMP mRNA levels and other parameters, including MMP2, TIMP2, MT1-MMP, and the active form of MMP2 were found, regardless of the liver pathology. Such a lack of correlation between MMP2 activation and the expression of MT2-MMP was reported in human invasive breast carcinomas. 9 Interestingly, in situ hybridization demonstrated a contrasted distribution between MT1-MMP and MT2-MMP mRNA. Thus, MT1-MMP mRNA was associated principally to stromal cells, which were previously shown to express both MMP2 and TIMP2 mRNA, 19,20 whereas MT2-MMP mRNA was found mostly in hepatocytes and in bile duct epithelial cells. These observations were confirmed in vitro, because culture-activated stellate cells were found to express MT1-MMP mRNA at high levels, but not MT2-MMP mRNA, whereas hepatocytes contained high amounts of MT2-MMP mRNA. These apparent discrepancies raised the interesting hypothesis that hepatocytes may contribute to the membrane-mediated MMP2 activation by expressing MT2-MMP at the level of intimate hepatocyte-stellate cell contacts, as it was previously suggested from our co-culture experiments. 24 Although MT2-MMP mRNA expression was not directly correlated to MMP2 activation in HCCs, changes in MT2-MMP expression might be located to tumoral hepatocytes adjacent to surrounding stromal cells and/or involve posttranslational changes of the protein. Thus, dot-blot analyses could not take into account local changes in MT2-MMP mRNA levels as a consequence of its high basal expression in all of the samples, including normal livers. Therefore, it cannot be ruled out that the contribution of cancer cells and/or proliferative bile duct cells to MT2-MMP expression might be involved in MMP2 activation.
In conclusion, we demonstrate that MMP2 activation in liver injury is related to a coordinated overexpression of MMP2, TIMP2, and MT1-MMP in stromal cells. In addition, our data would suggest that both hepatocytes and bile duct epithelial cells might play a major role in the activation process of MMP2, through the expression of MT2-MMP.
Acknowledgments
We are indebted to Dr. H. Will (In Vitek, Berlin, Germany) for providing the Plasmid pBlue/M2/1-1268 and for helpful discussions. We thank Dr. B. Turlin (Laboratoire d’Anatomie et Pathologie, Hôpital Pontchaillou, Rennes, France) for histological examination of the samples, Dr. D. Lotrian for analyzing the follow-up of patients, and Profs. A. Guillouzo and V. Lagente for critical reading of the manuscript.
Footnotes
Address reprint requests to Bruno Clément, INSERM U456, Facultés de Médecine et de Pharmacie, Université de Rennes I, 2 avenue Léon Bernard, 35043 Rennes, France. E-mail: bruno.clement@univ-rennes1.fr.
Supported by the Institut National de la Santé et de la Recherche Médicale, the Secrétariat d’Etat à la Santé (programme PHRC 1997, DRRC Bretagne), and the Association pour la Recherche contre le Cancer.
References
- 1.Matrisian LM: Metalloproteinases and their inhibitors in matrix remodelling. Trends Genet 1990, 6:121-125 [DOI] [PubMed] [Google Scholar]
- 2.Stetler-Stevenson WG, Aznavoorian S, Liotta LA: Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol 1993, 9:541-573 [DOI] [PubMed] [Google Scholar]
- 3.Birkedal-Hansen H: Proteolytic remodeling of extracellular matrix. Curr Opin Cell Biol 1995, 7:728-735 [DOI] [PubMed] [Google Scholar]
- 4.Corcoran ML, Hewitt RE, Kleiner DE, Jr, Stetler-Stevenson WG: MMP-2: expression, activation and inhibition. Enzyme Protein 1996, 49:7-19 [DOI] [PubMed] [Google Scholar]
- 5.Yu AE, Hewitt RE, Kleiner DE, Stetler-Stevenson WG: Molecular regulation of cellular invasion: role of gelatinase A and TIMP-2. Biochem Cell Biol 1996, 74:823-831 [DOI] [PubMed] [Google Scholar]
- 6.Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M: A matrix metalloproteinase expressed on the surface of invasive tumor cells. Nature (Lond) 1994, 370:61-65 [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto M, Mohanam S, Sawaya R, Fuller GN, Seiki M, Sato H, Gokaslan ZL, Liotta LA, Nicolson GL, Rao JS: Differential expression of membrane-type matrix metalloproteinase and its correlation with gelatinase activation in human malignant brain tumors in vivo and in vitro. Cancer Res 1996, 56:384-392 [PubMed] [Google Scholar]
- 8.Yoshizaki T, Sato H, Maruyama Y, Murono S, Furukawa M, Park C, Seiki M: Increased expression of membrane type 1-matrix metalloproteinase in head and neck carcinoma. Cancer 1997, 79:139-144 [DOI] [PubMed] [Google Scholar]
- 9.Ueno H, Nakamura H, Inoue M, Imai K, Noguchi M, Sato H, Seiki M, Okada Y: Expression and tissue localization of membrane-types 1, 2, and 3 matrix metalloproteinases in human invasive breast carcinomas. Cancer Res 1997, 57:2055-2060 [PubMed] [Google Scholar]
- 10.Mori M, Mimori K, Shiraishi T, Fujie T, Baba K, Kusumoto H, Haraguchi M, Ueo H, Akiyoshi T: Analysis of MT1-MMP and MMP2 expression in human gastric cancer. Int J Cancer 1997, 74:316-321 [DOI] [PubMed] [Google Scholar]
- 11.Will H, Hinzmann B: cDNA sequence and mRNA tissues distribution of a novel human matrix metalloproteinase with a potential transmembrane segment. Eur J Biochem 1995, 231:602-608 [DOI] [PubMed] [Google Scholar]
- 12.Takino T, Sato H, Shinagawa A, Seiki M: Identification of the second membrane-type matrix metalloproteinase (MT-MMp-2) gene from a human placenta cDNA library. J Biol Chem 1995, 270:23013-23020 [DOI] [PubMed] [Google Scholar]
- 13.Puente XS, Pendas AM, Llano E, Velasco G, Lopez-Otin C: Molecular cloning of a novel membrane-type matrix metalloproteinase from a human breast carcinoma. Cancer Res 1996, 56:944-949 [PubMed] [Google Scholar]
- 14.Kolkenbrock H, Hecker-Kia A, Orgel D, Ulbrich N, Will H: Activation of progelatinase A and progelatinase A/TIMP-2 complex by membrane-type matrix metalloproteinase. J Biol Chem 1997, 378:77-76 [DOI] [PubMed] [Google Scholar]
- 15.Tanaka M, Sato H, Takino T, Iwata K, Inoue M, Seiki M: Isolation of a mouse MT2-MMP gene from a lung cDNA library and identification of its product. FEBS Lett 1997, 402:219-222 [DOI] [PubMed] [Google Scholar]
- 16.Milani S, Herbst H, Schuppan D, Grappone C, Pelligrini G, Pinzani M, Casini A, Calabro A, Ciancio G, Stefanini F, Burroughs AK, Surrenti C: Differential expression of matrix metalloproteinase-1 and -2 genes in normal and fibrotic human liver. Am J Pathol 1994, 144:528-537 [PMC free article] [PubMed] [Google Scholar]
- 17.Nakatsukasa H, Ashida K, Higsahi T, Ohgughi S, Tsuboi S, Hino N, Nouso K, Urabe Y, Kinugasa N, Yoshida K, Uematsu S, Ishizaki M, Kobayashi Y, Tsusi T: Cellular distribution of transcripts for tissue inhibitor of metalloproteinases 1 and 2 in human hepatocellular carcinomas. Hepatology 1996, 24:82-88 [DOI] [PubMed] [Google Scholar]
- 18.Osaki I, Yamamoto K, Mizuta T, Setoguchi Y, Morito F, Sakai T: Detection of matrix metalloproteinase gene expression by reverse transcription polymerase chain reaction in human hepatocellular carcinomas. Int Hepatol Commun 1996, 6:36-42 [Google Scholar]
- 19.Théret N, Musso O, Campion JP, Turlin B, Loréal O, L’Helgoualc’h A, Clément B: Overexpression of matrix metalloproteinase-2 and tissue inhibitor of matrix metalloproteinase-2 in liver from patients with gastrointestinal adenocarcinoma and no detectable metastasis. Int J Cancer 1997, 74:1-7 [DOI] [PubMed] [Google Scholar]
- 20.Musso O, Théret N, Campion JP, Turlin B, Milani S, Grappone C, Clément B: In situ detection of matrix metalloproteinase-2 (MMP2), and the metalloproteinase inhibitor TIMP2 transcripts in human primary hepatocellular carcinoma, and in liver metastasis. J Hepatol 1997, 26:593-605 [DOI] [PubMed] [Google Scholar]
- 21.Grigioni WF, Garbisa S, D’Errico A, Baccarini P, Stetler-Stevenson WG, Liotta LA, Mancini AM: Evaluation of hepatocellular carcinoma aggressiveness by a panel of extracellular matrix antigens. Am J Pathol 1991, 138:647-654 [PMC free article] [PubMed] [Google Scholar]
- 22.Arii S, Tanaka J, Yamazoe Y, Minematsu S, Morino T, Fujita K, Maetani S, Tobe T: Predictive factors for intrahepatic recurrence of hepatocellular carcinoma after partial hepatectomy. Cancer 1992, 69:913-919 [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto H, Itoh F, Adachi Y, Sakamoto H, Adachi M, Hinoda Y, Imai K: Relation of enhanced secretion of active matrix metalloproteinases with tumor spread in human hepatocellular carcinoma. Gastroenterology 1997, 112:1290-1296 [DOI] [PubMed] [Google Scholar]
- 24.Théret N, Musso O, L’Helgoualc’h A, Clément B: Activation of matrix metalloproteinase-2 from hepatic stellate cells requires interactions with hepatocytes. Am J Pathol 1997, 150:51-58 [PMC free article] [PubMed] [Google Scholar]
- 25.Arthur MJP, Friedman SL, Roll FJ, Bissell DM: Lipocytes from normal rat liver release a neutral metalloproteinase that degrades basement membrane (type IV) collagen. J Clin Invest 1989, 84:1076-1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emonard H, Remacle AG, Noël AC, Grimaud JA, Stetler-Stevenson WG, Foidart JM: Tumor cell surface-associated binding site for the Mr 72,000 type IV collagenase. Cancer Res 1992, 52:5845-5848 [PubMed] [Google Scholar]
- 27.Young TN, Pizzo SV, Stack MS: A plasma membrane-associated component of ovarian adenocarcinoma cells enhances the catalytic efficiency of matrix metalloproteinase-2. J Biol Chem 1995, 270:999-1002 [DOI] [PubMed] [Google Scholar]
- 28.Yu M, Sato H, Seiki M, Thompson EW: Complex regulation of membrane-type matrix metalloproteinase expression and matrix metalloproteinase-2 activation by concanavalin A in MDA-MB-231 human breast cancer cells. Cancer Res 1995, 55:3272-3277 [PubMed] [Google Scholar]
- 29.Takino T, Sato H, Yamamoto E, Seiki M: Cloning of a human gene potentially encoding a novel matrix metalloproteinase having a C-terminal transmembrane domain. Gene 1995, 155:293-298 [DOI] [PubMed] [Google Scholar]
- 30.Edmondson H, Steiner P: Primary carcinoma of the liver: a study of 100 cases among 48900 necropsies. Cancer 1954, 7:462-503 [DOI] [PubMed] [Google Scholar]
- 31.Loréal O, Levavasseur F, Fromaget C, Gros D, Guillouzo A, Clément B: Cooperation of Ito cells and hepatocytes in the deposition of an extracellular matrix in vitro. Am J Pathol 1993, 143:538-544 [PMC free article] [PubMed] [Google Scholar]
- 32.Guguen-Guillouzo C, Campion JP, Brissot P, Glaise D, Bourel M, Guillouzo A: High-yield preparation of isolated human adult hepatocytes by enzymatic perfusion of the liver. Cell Biol Int Rep 1982, 6:625-628 [DOI] [PubMed] [Google Scholar]
- 33.Chan YL, Gutell R, Noller HF, Wool IG: The nucleotide sequence of a rat 18S ribosomal ribonucleic acid gene and a proposal for the secondary structure of 18S ribosomal ribonucleic acid. J Biol Chem 1984, 259:224-230 [PubMed] [Google Scholar]
- 34.Chomczynski P, Sacchi N: Single-step method of MRNA isolation by guanidinium thiocyanate phenol chloroform extraction. Anal Biochem 1987, 162:156-169 [DOI] [PubMed] [Google Scholar]
- 35.Liétard J, Musso O, Théret N, L’Helgoualc’h A, Campion JP, Yamada Y, Clément B: SP1-mediated transactivation of LamC1 promoter and coordinated expression of laminin γ1 and SP1 mRNA in human hepatocarcinomas. Am J Pathol 1997, 151:1663-1672 [PMC free article] [PubMed] [Google Scholar]
- 36.Welgus HG, Campbell EJ, Curry JD, Eisen AZ, Senior RM, Wilhelm SM, Goldberg GI: Neutral metalloproteinases produced by human mononuclear phagocytes. J Clin Invest 1990, 86:1496-1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davies B, Miles DW, Happerfield LC, Naylor MS, Bobrow LG, Rubens RD, Balkwill FR: Activity of type IV collagenases in benign and malignant breast disease. Br J Cancer 1993, 67:1126-1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tokuraku M, Sato H, Murakami S, Okada Y, Watanabe Y, Seiki M: Activation of the precursor of gelatinase A/72 kd type IV collagenase/MMP2 in lung carcinomas correlates with the expression of membrane-type matrix metalloproteinase (MT-MMP) and with lymph node metastasis. Int J Cancer 1995, 64:355-359 [DOI] [PubMed] [Google Scholar]
- 39.Nomura H, Sato H, Seiki M, Mai M, Okada Y: Expression of membrane-type matrix metalloproteinase in human gastric carcinomas. Cancer Res 1995, 55:3263-3266 [PubMed] [Google Scholar]
- 40.Yamamoto M, Mohanam S, Sawaya R, Fuller GN, Seiki M, Sato H, Gokaslan ZL, Liotta LA, Nicolson GL, Rao JS: Differential expression of membrane-type matrix metalloproteinase and its correlation with gelatinase activation in human malignant brain tumors in vivo and in vitro. Cancer Res 1996, 56:384-392 [PubMed] [Google Scholar]
- 41.Yoshizaki T, Sato H, Maruyama Y, Murono S, Furukawa M, Park C, Seiki M: Increased expression of membrane type 1-matrix metalloproteinase in head and neck carcinoma. Cancer 1997, 79:139-144 [DOI] [PubMed] [Google Scholar]
- 42.Takahara T, Furui K, Funaki J, Nakayama Y, Itoh H, Miyabayashi C, Sato H, Seiki M, Ooshima A, Watanabe A: Increased expression of matrix metalloproteinase-II in experimental liver fibrosis in rats. Hepatology 1995, 21:787-795 [PubMed] [Google Scholar]
- 43.Demetris AJ, Seaberg EC, Wennerberg A, Ionellie J, Michalopoulos G: Ductular reaction after submassive necrosis in human: special emphasis on analysis of ductular hepatocytes. Am J Pathol 1996, 149:439-448 [PMC free article] [PubMed] [Google Scholar]
- 44.Kinoh H, Sato H, Tsunezuka Y, Takino T, Kawashima A, Okada Y, Seiki M: MT-MMP, the cell surface activator of proMMP-2 (pro-gelatinase A) is expressed with its substrate in mouse tissue during embryogenesis. J Cell Sci 1996, 109:953-958 [DOI] [PubMed] [Google Scholar]