Abstract
The expression of the β1C integrin, an alternatively spliced variant of the β1 subunit, was investigated in human adult and fetal tissues. In the adult, β1C immunoreactivity was found in nonproliferative, differentiated simple, and/or pseudostratified epithelia in prostate glands and liver bile ducts. In contrast, β1C was undetectable in stratified squamous epithelium of the epidermis and/or in hepatocytes. Luminal prostate epithelial cells expressed β1C in vivo and in vitro, but no β1C was seen in basal cells, which are proliferating cells. Fetal prostate expressed β1C in differentiated glands that had a defined lumen, but not in budding glands, indicating that β1C is a marker of prostate epithelium differentiation. The β1C and the common β1A variants are differentially distributed: β1A was found in luminal and basal epithelial as well as in stromal cells in the prostate. In the liver, β1C and β1A were coexpressed in biliary epithelium, whereas vascular cells expressed only β1A. Because we found β1C in nonproliferative and differentiated epithelium, we investigated whether β1C could have a causal role in inhibiting epithelial cell proliferation. The results showed that exogenous expression of a β1C, but not of a β1A, cytoplasmic domain chimeric construct, completely inhibited thymidine incorporation in response to serum by prostate cancer epithelial cells. Consistent with these in vitro results, β1C appeared to be downregulated in prostate glands that exhibit regenerative features in benign hyperplastic epithelium. These data show that the presence of β1C integrins in epithelial cells correlates with a nonproliferative, differentiated phenotype and is growth inhibitory to prostate epithelial cells in vitro. These findings indicate a novel pathophysiological role for this integrin variant in epithelial cell proliferation.
Cell interactions with extracellular matrix proteins control proliferation, differentiation, and survival, 1-3 as well as tumor growth, angiogenesis, and metastasis. 4 These interactions are predominantly mediated by integrins, cell adhesion receptors composed of an α and a β subunit. 5-7 In addition to mediating physical interactions, integrins signal intracellularly through their cytoplasmic domains. 8 The cytoplasmic domains also control integrin affinity for ligands and subcellular localization. 8-10
The cytoplasmic domain of the β1 subunit, in its canonical form (β1A), is highly conserved from fungi and invertebrates to vertebrates. 11 Experimental modifications of the β1 cytoplasmic domain have been shown to affect cell proliferation, 12,13 development, 14 migration, 15,16 integrin localization, 17,18 and mitogen-activated protein kinase activation 19,20 and phosphorylation of focal adhesion kinase or paxillin. 15,21-23 Alternative splicing events, by creating variant cytodomains, generate functionally distinct integrin complexes. 8 Alternatively spliced forms of the β (β1, β3, β4, and β5) and α (α3, α6, and α7) integrin cytoplasmic domains have been identified, 8,24 thus adding further complexity to the regulatory pathways mediated by integrins.
The integrin β1 variants are known as: the canonical β1A, β1B, β1C, β1C-2, and β1D; they differ in the COOH-terminal sequences. 8 The β1C integrin (formerly β1S) 25 is generated by the presence of an unspliced intervening 116-bp sequence (exon C), which causes a frame shift in the 3′ end of the β1 subunit and codes for a unique 48-amino acid COOH-terminal sequence. Unlike β1A, β1C inhibits cell proliferation and is downregulated in cancer cells. 12,13,26
We show here that β1C is expressed in a subset of epithelial cells exhibiting a nonproliferating and differentiated phenotype, and that it is lost in regenerative areas of prostate glands. We also provide evidence suggestive of a causal role for the β1C cytodomain in suppressing epithelial cell proliferation.
Materials and Methods
Cells and Reagents
The PC3 human prostatic carcinoma cell line was obtained from American Type Culture Collection (Manassas, VA). PC3 cells were maintained in RPMI (Life Technologies Inc., Gaithersburg, MD) supplemented with 10% heat-inactivated fetal calf serum (FCS) (Gemini Bioproducts Inc., Calabasas, CA), 2 mmol/L glutamine (Gemini Bioproducts Inc.), 100 μg/ml streptomycin-100 U/ml penicillin (Gemini Bioproducts Inc.), 0.1 mmol/L nonessential amino acids (Life Technologies), and 1 mmol/L sodium pyruvate (Life Technologies). Human prostate luminal epithelial cells, BPH-1, 27 were maintained in RPMI supplemented with 2.5% FCS, 2 mmol/L glutamine, and 100 μg/ml streptomycin-100 U/ml penicillin.
Rabbit antibodies specific for the β1C or the β1A subunit cytoplasmic domains were generated and affinity purified as previously described. 26 The following antibodies were used: fluorescein isothiocyanate-conjugated rat monoclonal antibody to mouse CD4, clone YTS 191.1 (Caltag Laboratories, San Francisco, CA); mouse monoclonal antibody to the human β1 integrin extracellular domain, mAb 13 (Beckton Dickinson, San Jose, CA) and kindly provided by Dr. K. M. Yamada (National Institutes of Health, Bethesda, MD); mouse monoclonal antibodies to basal cytokeratins 1, 5, 10, and 14, clone 34βE12 (Enzo Diagnostic, Farmingdale, NY) or to type I and type II cytokeratins, clone AE1/AE3 (Boehringer Mannheim, Indianapolis, IN); and mouse monoclonal antibody to cytokeratins 8 and 18, clone CAM 5.2 (Becton Dickinson). Fluorescein isothiocyanate-rat immunoglobulin G (IgG) were purchased from Caltag.
Tissue Specimens
Samples (autopsy or surgical specimens) were obtained from the files of the Department of Pathology at Yale New Haven Hospital. The following adult tissues were included in the study: benign prostate (62 samples, including 25 benign prostatic hyperplastic samples), normal liver (12 samples), normal gallbladder (4 samples), normal kidney (5 samples), and normal lung (1 sample). Developing tissues from spontaneous or therapeutic abortions corresponding to different gestational ages were also included: prostate (4 samples, 18 to 22 weeks; 9 samples, 23 weeks or later) and liver (3 samples, 16 to 19 weeks). Hematoxylin and eosin sections were analyzed from all samples to assess the integrity of the tissue selected for the evaluation of β1C, β1A, or cytokeratin immunoreactivity. All tissues were obtained under review board-approved protocols.
Immunohistochemistry
Immunostaining of β1C, β1A, and cytokeratins was performed essentially as described, 26 with minor modifications. For immunostaining of liver specimens, nonspecific binding of biotin/avidin was prevented using the Avidin/Biotin blocking kit from Vector Laboratories (Burlingame, CA), according to the manufacturer’s instructions. For immunostaining of prostate specimens, endogenous peroxidase was quenched with 3% H2O2 for 5 minutes at room temperature, microwave treatment was avoided, blocking was achieved with either 50% goat or horse serum in Tris-buffered saline containing 0.2% bovine serum albumin for 20 minutes at room temperature; each incubation step was followed by three washes with Tris-buffered saline. The specificity of the affinity-purified antibody to the β1C 785 to 808 peptide 12 in immunohistochemical staining was confirmed as follows: before the immunostaining procedure, the affinity-purified antibody to β1C was preincubated for 30 minutes at 4°C with 10 to 30 μg/ml of either β1C 785 to 808 peptide or a control fibrinogen-derived peptide. 28
Immunoblotting and Immunoprecipitation
Frozen prostate tissue, obtained from autopsy specimens, was homogenized using lysis buffer containing 100 mmol/L Tris, pH 7.5, 150 mmol/L NaCl, 0.1% Triton X-100 (Sigma Chemical Co., St. Louis, MO), 5% sodium dodecyl sulfate (SDS; American Bioanalytical, Natick, MA), 1 mmol/L phenylmethylsulfonyl fluoride (Life Technologies), 10 μg/ml leupeptin (Calbiochem, San Diego, CA), 1 mmol/L benzamidine (Sigma), 1 μmol/L d-phenylalanyl-l-prolyl-l-arginine chloromethyl ketone (Boehringer Mannheim), 10 μg/ml soybean trypsin inhibitor (Life Technologies), using an OMNI 2000 homogenizer (OMNI International Inc., Gainesville, VA). Insoluble material was removed by centrifugation at 14,000 × g for 30 minutes at 4°C. The protein content in each lysate was quantitated using the BCA protein assay reagent (Pierce, Rockford, IL) according to the manufacturer’s instructions. To detect β1C integrin, 200 μg of detergent tissue extract was electrophoresed on a 7.5% SDS-polyacrylamide gel under reducing conditions, transferred to nitrocellulose (Schleicher & Schuell, Keene, NH), and immunostained as described, 26 using either 5 μg/ml affinity-purified antibody to β1C; 5 μg/ml nonimmune rabbit IgG; or 2.5 μg/ml mAb 13, monoclonal antibody to β1 integrin.
BPH-1 cells were surface iodinated and proteins immunoprecipitated using rabbit antisera to β1C or to β1A or using normal rabbit serum, as previously described. 12 The immunocomplexes were separated on a 7.5% SDS-polyacrylamide gel under reducing conditions and visualized by autoradiography.
Inducible Expression of CD4-β1C and CD4-β1A Chimeric Constructs
HindIII DNA fragment, encoding the cytoplasmic domain of β1C (nucleotides 2357 to 2613), 25 was isolated from the pBJ1-β1C plasmid. The fragment was inserted into the HindIII site of the Ch2 chimera described by Lukashev et al 22 which consists of the extracellular domain of murine CD4 joined to the transmembrane domain of the β1 integrin. The resulting construct was designated Chβ1C. Correct assembly of the construct was verified by nucleotide sequencing. The Ch1 chimeric construct, designated here Ch1β1A, containing the extracellular domain of murine CD4 and the transmembrane and cytoplasmic domains of the β1A integrin, has been described by Lukashev et al. 22 Each chimeric construct is expressed under the control of the mouse metallothionein I promoter, which can be induced by addition of ZnSO4 to the growth medium. PC3 cells were electroporated using a Genepulser apparatus set at 250 V and 900 μF using either 10 or 30 μg of the Ch1β1A or the Chβ1C chimera, respectively. Hygromycin-resistant cells were selected using growth medium containing 0.2 mg/ml hygromycin B (Boehringer Mannheim). Hygromycin-resistant colonies were pooled, and the resultant population was analyzed for cell surface expression of each chimeric construct by fluorescence-activated cell sorting using rat monoclonal antibody to mouse CD4. As negative control antibody, isotype-matched rat IgG was used. Stable transfectants were maintained in growth medium containing 0.2 mg/ml hygromycin B.
Cell Proliferation Assay
PC3 stable transfectants were starved for 24 hours in serum-free medium. Surface expression of chimeric constructs was induced by treating the cells with 75 μmol/L ZnSO4 (Sigma) in growth medium for 6 hours at 37°C. Cells were then detached with 0.05% trypsin/0.53 mmol/L ethylenediaminetetraacetic acid (Life Technologies), washed three times in serum-free medium, and analyzed by fluorescence-activated cell sorting using rat monoclonal antibody to mouse CD4 or isotype-matched rat IgG. Positive cells expressing comparable levels of the chimeric constructs were sorted using FACStar (Becton Dickinson). After sorting, the cell transfectants (15 × 103) were resuspended in serum-free medium and added to 96-well microtiter plates (ICN Biomedicals, Costa Mesa, CA) coated with 3 μg/ml fibronectin to ensure comparable attachment. After 1 hour and 30 minutes at 37°C, cells were washed twice, incubated either in the absence or in the presence of 10% FCS for 18 hours at 37°C, and pulsed with 1 μCi[3H]thymidine/well (5.0 Ci/mmol; Amersham Life Sciences, Arlington Heights, IL) during the last 3 hours of the 18-hour culture. Thymidine incorporation was evaluated as described. 12 In each experiment, duplicate or triplicate observations were performed, and the values are reported as mean ± standard error (SE). In parallel, in each experiment, the number of attached cells was evaluated after fixing and staining the cells with 0.5% crystal violet (Sigma), as described. 29 The results were evaluated using a 630-nm wavelength filter in a Titertek Multiskan Bichromatic enzyme-linked immunosorbent assay reader (ICN). Group differences were compared using one-way analysis of variance followed by Bonferroni post hoc contrast.
Results
β1C Expression in Epithelial Cells in Adult and Fetal Tissues
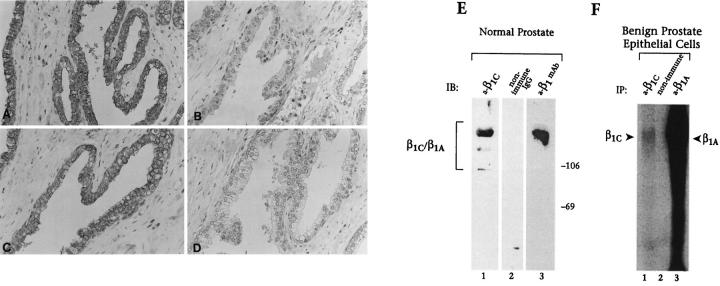
Immunohistochemical analysis of adult and fetal human tissue specimens was performed using an affinity-purified antibody to β1C; β1C was detected in a subset of epithelial cells. Specifically, simple or pseudostratified epithelia in prostate, liver, gallbladder, lung, and kidney were positive for β1C (see Figures 1 through 6 and 8 ▶ ▶ ▶ , and data not shown) in all the analyzed cases, whereas stratified squamous epithelium of the epidermis was negative (Figure 1E) ▶ . In the prostate, β1C was preferentially expressed in the luminal glandular epithelium (Figure 1A) ▶ . In contrast, the basal epithelium, composed of proliferating cells 30 identified by an antibody to basal cell-specific cytokeratins 1, 5, 10, and 14 (Figure 1D) ▶ , did not reveal detectable levels of β1C (Figure 1A) ▶ . Similarly, β1C immunoreactivity was undetectable in stromal and endothelial cells in all the analyzed tissues (see Figures 1 through 6 ▶ ▶ ▶ ). The specificity of the immunoreactivity of the antibody to β1C (Figure 2A) ▶ was confirmed by 1) inhibition by the β1C peptide, 785 to 808, used as the antigen (Figure 2B) ▶ , 2) immunoblotting of detergent lysate of prostate tissue (Figure 2E) ▶ , and 3) immunoprecipitation of surface-iodinated BPH-1 luminal epithelial cells (Figure 2F) ▶ . The immunoblotting and immunoprecipitation both revealed bands with an electrophoretic mobility similar to that of β1A (Figure 2, E and F) ▶ . 12
Figure 1.
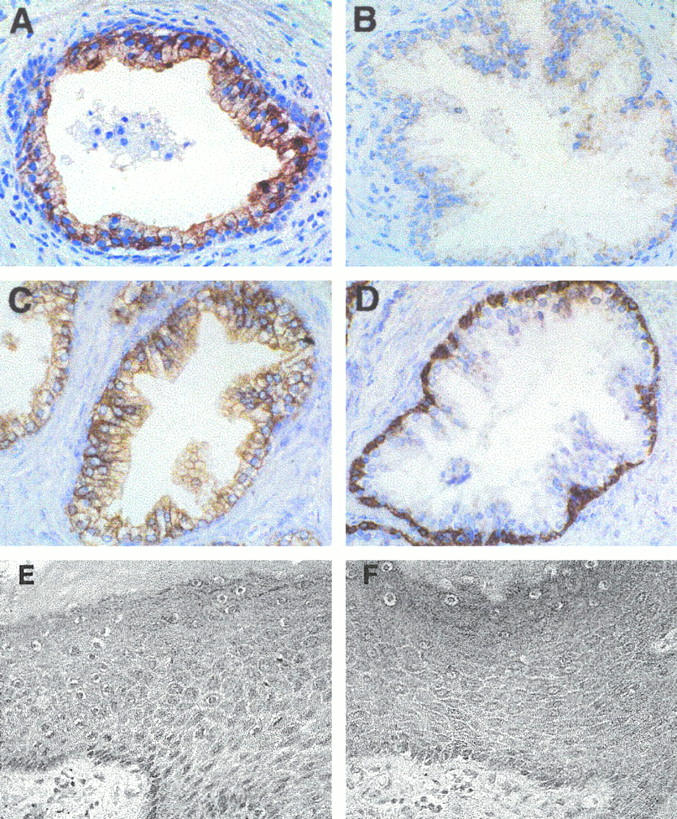
The β1C integrin is expressed in luminal epithelium of adult prostate but not in the epidermis. The immunohistochemical analysis was performed on adult prostate (A through D) and on adult skin tissue sections (E and F) using 1.85 μg/ml affinity-purified antibody to β1C (A and E); 1.85 μg/ml of nonimmune rabbit IgG (B and F); 5 μg/ml AE1/AE3, monoclonal antibody to type I and type II cytokeratins (C); or 0.2 μg/ml 34βE12, monoclonal antibody to cytokeratins 1, 5, 10, and 14 (D). Magnification, ×200. Note that β1C is undetectable in the basal layer of the adult prostate gland (A).
Figure 2.
In vivo and in vitro expression of β1C in prostate cells. A through D, a representative case of adult normal prostate tissue is shown. β1C immunostaining (A) was specifically inhibited by antibody absorption on the β1C 785 to 808 peptide (10 μg/ml) used as antigen (B) but not on an irrelevant peptide (C). The affinity-purified antibody to β1C (A through C) or nonimmune rabbit IgG (D) were used at a final concentration of 5 μg/ml. E: newborn (1-month-old) prostate tissue detergent extract was electrophoresed on 7.5% SDS-polyacrylamide gel under reducing conditions, transferred to nitrocellulose membranes, and immunostained using 5 μg/ml affinity-purified antibody to β1C (a-β1C, lane 1); 5 μg/ml nonimmune rabbit IgG (nonimmune IgG, lane 2); or 2.5 μg/ml mAb 13, monoclonal antibody to β1 (a-β1 mAb, lane 3). Proteins were visualized by enhanced chemiluminescence. Prestained marker proteins in kd are shown. The bracket (E) indicates the expected electrophoretic mobility of β1C and β1A. F: BPH-1 cells were surface iodinated and proteins immunoprecipitated using rabbit antisera either to β1C (a-β1C, lane 1) or β1A (a-β1A, lane 3), or normal rabbit serum (non immune, lane 2). The immunocomplexes were separated on a 7.5% SDS-polyacrylamide gel under reducing conditions and visualized by autoradiography. Arrowheads indicate β1C and β1A. Magnification, ×200 (A through D).
Figure 3.
Expression of β1C in luminal epithelium of fetal prostate. The immunohistochemical analysis was performed on a 20-week-old fetus using 1.85 μg/ml affinity-purified antibody to β1C (A and C); 1.85 μg/ml nonimmune rabbit IgG (B); or 0.2 μg/ml 34βE12, monoclonal antibody to cytokeratins 1, 5, 10, and 14 (D); or 5 μg/ml AE1/AE3, monoclonal antibody to type I and type II cytokeratins (E). The β1C immunostaining was specifically inhibited by antibody absorption on the β1C 785 to 808 peptide (30 μg/ml) used as antigen (C). Magnification, ×100. Note that β1C expression is undetectable in the basal layer of the fetal prostate gland (A).
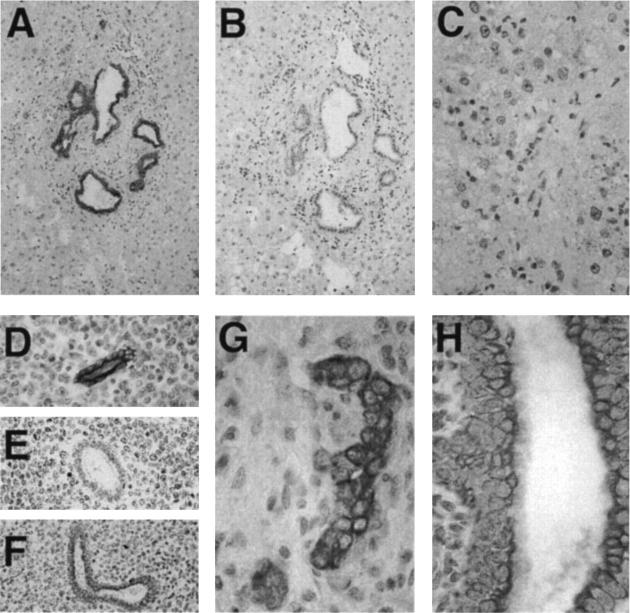
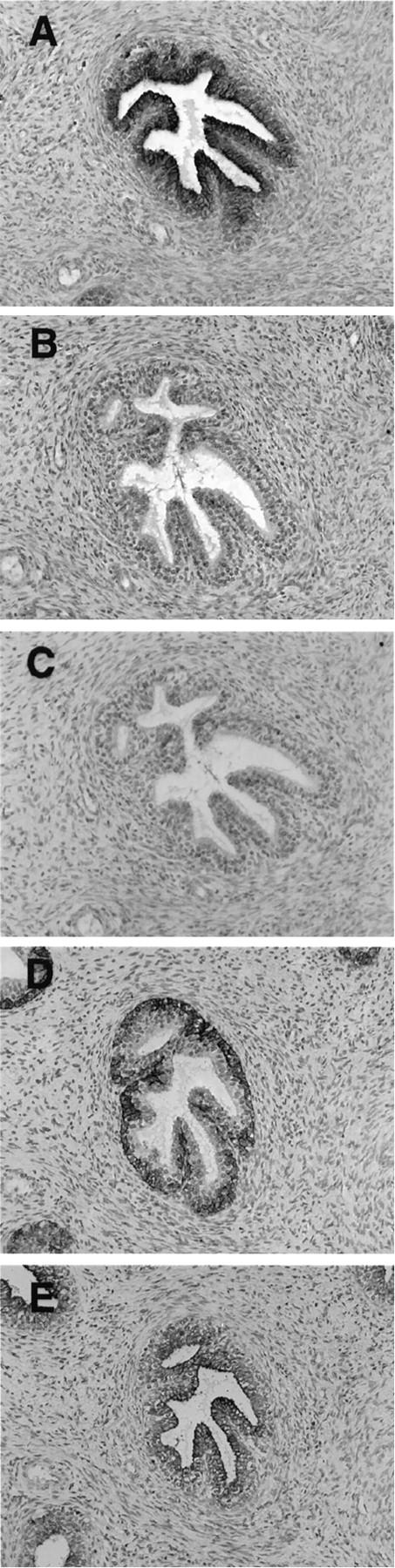
Immunohistochemical analysis performed on second-trimester fetal tissues showed that, as in adult tissue, β1C was confined to glandular epithelium in completely differentiated glands with a defined lumen (Figures 3A and 4A ▶ ▶ and not shown), whereas it was absent in budding glands (Figure 4A) ▶ . Cytokeratin 8 and 18 staining was used for the identification of prostate glands 31 that had not differentiated completely and lacked a lumen (Figure 4C) ▶ . The basal epithelium, identified using an antibody to basal cell-specific cytokeratins 1, 5, 10, and 14 (Figure 3D) ▶ , did not contain detectable β1C (Figure 3A) ▶ . The specificity of β1C immunoreactivity (Figure 3A) ▶ was confirmed by inhibitions with the β1C peptide used as the antigen (Figure 3C) ▶ . These staining results suggest that β1C is a marker of prostate epithelial cell differentiation.
Figure 4.
Expression of β1C in prostate budding glands. The immunohistochemical analysis was performed on a 20-week-old fetus using 1.85 μg/ml affinity-purified antibody to β1C (A); 1.85 μg/ml of nonimmune rabbit IgG (B); or 1.25 μg/ml CAM 5.2, monoclonal antibody to cytokeratins 8 and 18 (C). Magnification, ×200. The expression of β1C coincides with a differentiated phenotype of the prostate gland: note that β1C is detectable only when a lumen is well defined in the gland.
In the adult liver, β1C immunoreactivity was restricted to biliary epithelium (Figure 5, A, D, and F) ▶ , which stained at the apical, basal, and lateral cell surfaces; no β1C was found in hepatocytes (Figure 5C) ▶ . A similar staining pattern showing expression in bile ducts, but not in hepatocytes, was observed in 16, 17, and 19 week-gestation fetal livers (Figure 5, G and H ▶ , and not shown). The specificity of the staining was confirmed by inhibition with the β1C peptide (Figure 5E) ▶ . The control of β1C expression appears to be independent of β1A, as the two variants are differentially regulated in a cell type-specific manner. In contrast to β1C, which has a distribution restricted to biliary epithelium (Figure 6, A and B) ▶ , the β1A variant was found in bile duct epithelium (Figure 6D) ▶ ; hepatic vasculature (Figure 6C ▶ and not shown); and, weakly, in hepatocytes, as shown previously. 32
Figure 5.
The β1C integrin is expressed in adult and fetal liver bile ducts but not in hepatocytes. Immunoperoxidase staining of β1C was performed on adult (A through F) and 19-week-old fetal (G and H) liver using 5 μg/ml affinity-purified antibody to β1C (A, C through E, G, and H) or 5 μg/ml nonimmune rabbit IgG (B). The β1C immunostaining (D) was specifically inhibited by antibody absorption on the β1C 785 to 808 peptide (10 μg/ml) used as antigen (E), not on an irrelevant peptide (F). Magnification, ×100 (A and B); ×200 (C through F), and ×400 (G and H). Note strong β1C immunoreactivity in the bile ducts (A, D, F, G, and H) and not in hepatocytes (C and G).
Figure 6.
Differential expression of β1C and β1A in adult liver. Immunoperoxidase staining of β1C and β1A in liver with mild parenchyma alterations (steatohepatitis) was performed using 5 μg/ml affinity-purified antibodies to β1C (A and B) or to β1A (C and D). Nonimmune rabbit IgG (5 μg/ml) was used as a negative control (E). Magnification, ×200. Note, β1C is selectively expressed in biliary epithelium (B), whereas β1A is found in bile ducts (D), as well as in sinusoids (C). Arrows (A and C) point to sinusoids.
β1C Inhibits Epithelial Cell Proliferation
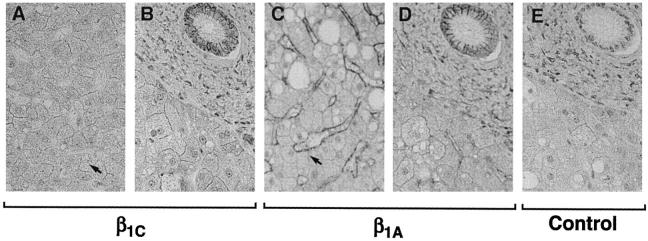
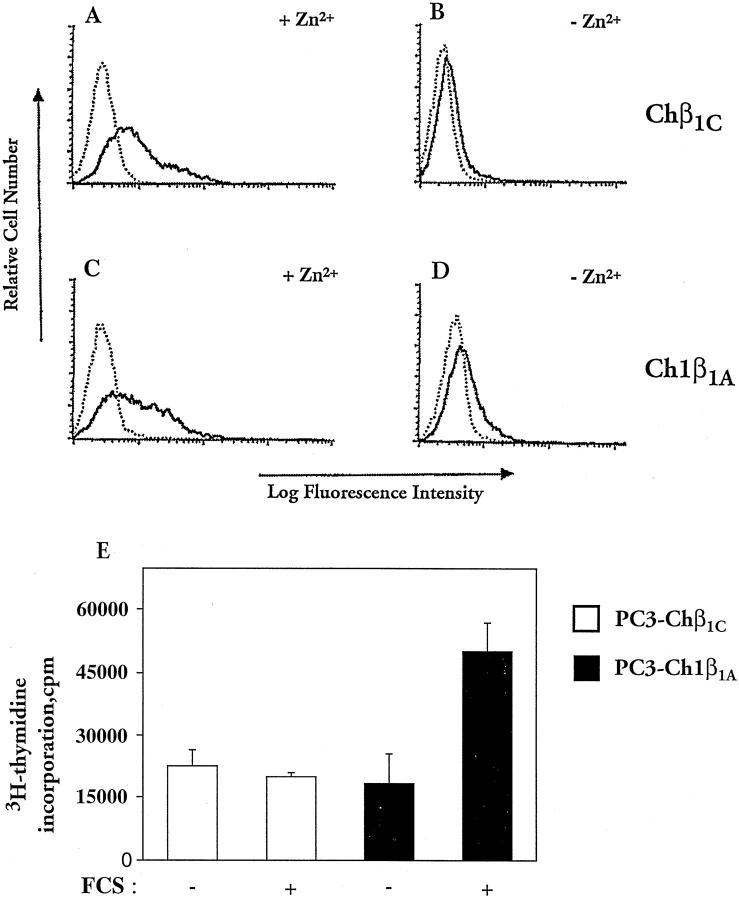
Because β1C has a powerful growth-inhibitory activity in fibroblasts 12,13 and because, as shown above, it is predominantly found in nonproliferative epithelium, we explored the possibility that β1C would have a causal role in inhibiting epithelial cell proliferation. Chimeric constructs containing either β1C (Chβ1C; Figure 7, A and B ▶ ) or β1A (Ch1β1A; Figure 7, C and D ▶ ) cytoplasmic tails under the control of an inducible promoter were stably expressed in PC3 prostate cancer cells. Serum-starved stable transfectants were incubated with ZnSO4 (Figure 7, A and C) ▶ to induce surface expression of the chimeric proteins and sorted by fluorescence-activated cell sorting to isolate positive cells expressing comparable levels of Chβ1C or Ch1β1A. The sorted cells were compared in proliferation assays on fibronectin (Figure 7E) ▶ . Under the experimental conditions used, adhesion to fibronectin was not affected by the expression of either Chβ1C or Ch1β1A (not shown). However, the expression of β1C completely inhibited thymidine incorporation in response to serum in the cells, whereas β1A had no effect.
Figure 7.
Inhibition of prostate cell growth in response to β1C expression. PC3 cells were stably transfected using chimeric constructs containing the cytoplasmic domain of either β1C (Chβ1C, A and B) of β1A (Ch1β1A, C and D). Serum-starved stable transfectants were incubated either in the absence (C and D) or in the presence of 75 μmol/L ZnSO4 (A and C) for 6 hours at 37°C in growth medium to induce surface expression of the chimeric proteins (A and C). Stable cell transfectants were then stained with fluorescein isothiocyanate-conjugated rat monoclonal antibody to mouse CD4 (solid line) or isotype-matched fluorescein isothiocyanate-conjugated rat IgG (dotted line) and sorted by fluorescence-activated cell sorting to isolate positive cells expressing comparable levels of Chβ1C or Ch1β1A. The Chβ1C and the Ch1β1A sorted cells were compared in proliferation assays (E) on 96-well plates coated with 3 μg/ml fibronectin. Attached cells were incubated for 15 hours at 37°C either in the absence or in the presence of 10% FCS and then for an additional 3 hours at 37°C with 1 μCi/well [3H]thymidine to measure their proliferative response to FCS. Results are mean ± SE values of duplicate determinations. Group differences were compared using one-way analysis of variance followed by Bonferroni post hoc contrast. The differences in proliferation between Chβ1C and Ch1β1A transfectants in the presence of 10% FCS and between nonstimulated and 10% FCS-stimulated Ch1β1A transfectants are statistically significant (P < 0.05). A representative experiment of three is shown.
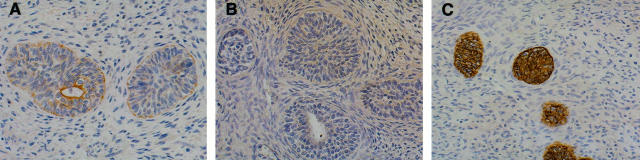
The in vitro data showing strong inhibition of prostate epithelial cell proliferation by β1C were further supported by immunohistochemical analysis of regenerative areas of hyperplastic prostate tissues. Luminal expression of β1C (Figure 8A) ▶ , which was observed in normal and hyperplastic prostatic glandular epithelia of all the analyzed cases, was selectively downregulated in regenerative areas displaying hyperchromatic nuclei and cellular crowding (Figure 8, C and D) ▶ . β1A was always detected in both the basal and luminal layers (Figure 8B ▶ , and not shown). These results suggest a unique role for β1C in controlling epithelial cell proliferation under normal and pathological conditions.
Figure 8.
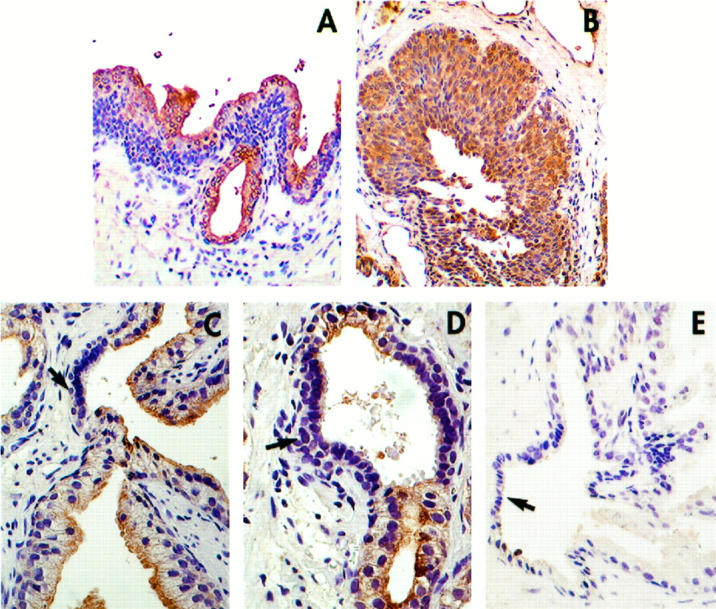
β1C is down regulated in regenerative areas of prostate glands. Prostate tissue sections from a hyperplastic specimen were analyzed by immunohistochemistry using 3.75 μg/ml affinity-purified antibody to β1C (A, C, and D); 2 μg/ml affinity-purified antibody to β1A (B); of 3.75 μg/ml nonimmune rabbit IgG (E). Magnification, ×200 (A and B) and ×400 (C through E) Regenerative areas displaying hyperchromatic nuclei and cellular crowding. (arrows, C and D), downregulate β1C, suggesting a selective loss of expression of β1C in proliferative areas. The results also show that β1C and β1A are differentially expressed in prostate epithelial cells; specifically, basal cells fail to express β1C (A) but strongly express β1A (B).
Discussion
The results described here show that the β1C integrin, an alternatively spliced variant of the β1 subunit, 25 is expressed in vivo in a cell type-specific manner, predominantly in nonproliferating differentiated epithelial cells, and that is an early marker of differentiation of prostate epithelium. We also show that β1C inhibits in vitro growth of malignant epithelial cells from the prostate.
Our in vivo study shows a selective expression of β1C in a subset of epithelial cells that does overlap with the expression of β1A. This analysis of β1C distribution was made possible by the availability of highly specific antibodies to the β1C cytoplasmic domain and emphasizes the importance of using antibodies specific to the different variants when analyzing β1 distribution. The β1C distribution is unique among the β1 variants. The β1B isoform has been found to be restricted to skin and liver tissues, 33 whereas the β1D subunit is expressed in striated muscles, where it replaces β1A 19,34 in a developmentally regulated manner. 35
Functional differences have been described for the β1 variants. β1D is functionally similar to β1A, in that both are localized at focal contacts and can stimulate focal adhesion kinase activation, 19,34 whereas β1B inhibits it. 15 This suggests that modulation of splicing patterns of β1 mRNA may provide an accessory mechanism to regulate signaling pathways initiated by integrins. A recent study shows that the ability of β1C to inhibit cell proliferation is shared by β1D36; however, the downstream signaling pathways by these variants are unknown and may be different.
The mechanisms that regulate epithelial cell proliferation and differentiation in developing embryonal and fetal tissue are largely unknown. In general terms during organogenesis, a proliferative phase is followed by one in which the cells progressively differentiate and acquire the phenotype required for their highly specialized functions. 37 The results of our study indicate that β1C is expressed at the transition between these phases and suggest that its expression might be an important mechanism by which cellular proliferation is inhibited in differentiating fetal epithelia in specific organs.
Altered expression of integrins in epithelial cells has been shown to generate pathological phenotypes. 32 Abnormal expression of β1 in the suprabasal epidermal layers of the skin causes epidermal hyperproliferation, as shown in studies performed using transgenic mice, thus confirming that incorrect integrin distribution can be a trigger for increased in vivo growth rate. 38,39 In normal skin, β1 integrins are found only in the basal layer of the epidermis, whereas during wound healing and psoriasis, which are associated with hyperproliferation, they also appear in the suprabasal layers of the skin. 40 Integrin expression affects the growth of tumor cells in vitro and tumor growth and metastasis in vivo. 41-50 On the basis of the different effects of β1C and β1A on cell proliferation, we would predict that replacement of the β1C with β1A could also lead to abnormal proliferation in epithelium.
The expression of β1C completely inhibited thymidine incorporation of prostate cancer epithelial cells in response to serum. Consistent with these in vitro results, β1C appeared to be downregulated in prostate glands that exhibit regenerative features in benign hyperplastic epithelium. These observations, and previous findings showing that β1C is downregulated in prostate cancer, 26 suggest that the loss of β1C expression may contribute malignant progression in prostate cells.
Acknowledgments
We thank Drs. Simon W. Hayward, Matvey E. Lukashev, Robert M. Pytela, and Kenneth M. Yamada for reagents; Rocco Carbone for support in performing flow cytometric analysis; Mary Helie and Linda Gutierrez de Castejon in the Department of Pathology for technical advice; and Nancy Bennett for assisting with the preparation of the manuscript.
Footnotes
Address reprint requests to Dr. Lucia R. Languino, Department of Pathology, Yale University School of Medicine, P.O. Box 208023, 310 Cedar Street, New Haven, CT 06520. E-mail: lucia.languino@yale.edu.
Supported in part by National Institutes of Health Grants CA 71870 and DK 52670, Donaghue Medical Research Foundation grant 95-006, a Milheim Foundation Award (to LRL), a Donaghue Medical Research Foundation Fellowship Award (to MF), and a fellowship from the American-Italian Cancer Foundation (to MM).
MF and MM contributed equally to this report.
MM’s present address: Department of Pathology, European Institute of Oncology, Milan, Italy.
References
- 1.Brakebusch C, Hirsch E, Potocnik A, Fassler R: Genetic analysis of β1 integrin function: confirmed, new and revised role for a crucial family of cell adhesion molecules. J Cell Sci 1997, 110:2895-2904 [DOI] [PubMed] [Google Scholar]
- 2.Damsky CH, Werb Z: Signal transduction by integrin receptors for extracellular matrix: cooperative processing of extracellular information. Curr Opin Cell Biol 1992, 4:772-781 [DOI] [PubMed] [Google Scholar]
- 3.Ruoslahti E, Reed JC: Anchorage dependence, integrins and apoptosis. Cell 1994, 77:477-478 [DOI] [PubMed] [Google Scholar]
- 4.Varner JA, Cheresh DA: Integrins and cancer. Curr Opin Cell Biol 1996, 8:724-730 [DOI] [PubMed] [Google Scholar]
- 5.Haas TA, Plow EF: Integrin-ligand interactions: a year in review. Curr Opin Cell Biol 1994, 6:656-662 [DOI] [PubMed] [Google Scholar]
- 6.Hynes RO: Integrins: versatility, modulation and signaling in cell adhesion. Cell 1992, 69:11-25 [DOI] [PubMed] [Google Scholar]
- 7.Schwartz MA, Schaller MD, Ginsberg MH: Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol 1995, 11:549-599 [DOI] [PubMed] [Google Scholar]
- 8.Fornaro M, Languino LR: Alternatively spliced variants: a new view of the integrin cytoplasmic domain. Matrix Biol 1997, 16:185-193 [DOI] [PubMed] [Google Scholar]
- 9.Williams MJ, Hughes PE, O’Toole TE, Ginsberg MH: The inner world of cell adhesion: integrin cytoplasmic domains. Trends Cell Biol 1994, 4:109-112 [DOI] [PubMed] [Google Scholar]
- 10.Hemler ME, Weitzman JB, Pasqualini R, Kawaguchi S, Kassner PD, Berdichevsky FB: Structure, biochemical properties, and biological functions of integrin cytoplasmic domains. Takada Y eds. Integrins: The Biological Problems. 1995, :pp 1-35 CRC Press, Ann Arbor [Google Scholar]
- 11.Marcantonio EE, Hynes RO: Antibodies to the conserved cytoplasmic domain of the integrin β1 subunit react with proteins in vertebrates, invertebrates and fungi. J Cell Biol 1988, 106:1765-1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fornaro M, Zheng DQ, Languino LR: The novel structural motif Gln795-Gln802 in the integrin β1C cytoplasmic domain regulates cell proliferation. J Biol Chem 1995, 270:24666-24669 [DOI] [PubMed] [Google Scholar]
- 13.Meredith J, Jr, Takada Y, Fornaro M, Languino LR, Schwartz MA: Inhibition of cell cycle progression by the alternatively spliced integrin β1C. Science 1995, 269:1570-1572 [DOI] [PubMed] [Google Scholar]
- 14.Baudoin C, Goumans M-J, Mummery C, Sonnenberg A: Knockout and knockin of the β1 exon D define distinct roles for integrin splice variants in heart function and embryonic development. Genes Dev 1998, 12:1202-1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balzac F, Retta SF, Albini A, Melchiorri A, Koteliansky VE, Geuna M, Silengo L, Tarone G: Expression of β1B integrin isoform in CHO cells results in a dominant negative effect on cell adhesion and motility. J Cell Biol 1994, 127:557-565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LaFlamme SE, Thomas LA, Yamada SS, Yamada KM: Single subunit chimeric integrins as mimics and inhibitors of endogenous integrin functions in receptor localization, cell spreading and migration, and matrix assembly. J Cell Biol 1994, 126:1287-1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcantonio EE, Guan J-L, Trevithick JE, Hynes RO: Mapping of the functional determinants of the integrin β1 cytoplasmic domain by site-directed mutagenesis. Cell Regul 1990, 1:597-604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reszka AA, Hayashi Y, Horwitz AF: Identification of amino acid sequences in the integrin β1 cytoplasmic domain implicated in cytoskeleton association. J Cell Biol 1992, 117:1321-1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belkin AM, Zhidkova NI, Balzac F, Altruda F, Tomatis D, Maier A, Tarone G, Koteliansky VE, Burridge K: β1D integrin displaces β1A isoform in striated muscles: localization at junctional structures and signaling potential in non-muscle cells. J Cell Biol 1996, 132:211-226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei J, Shaw LM, Mercurio AM: Regulation of mitogen-activated protein kinase activation by the cytoplasmic domain of the α6 integrin subunit. J Biol Chem 1998, 273:5903-5907 [DOI] [PubMed] [Google Scholar]
- 21.Akiyama SK, Yamada SS, Yamada KM, LaFlamme SE: Transmembrane signal transduction by integrin cytoplasmic domains expressed in single-subunit chimeras. J Biol Chem 1994, 269:15961-15964 [PubMed] [Google Scholar]
- 22.Lukashev ME, Sheppard D, Pytela R: Disruption of integrin function, and induction of tyrosine phosphorylation, by the autonomously expressed β1 integrin cytoplasmic domain. J Biol Chem 1994, 269:18311-18314 [PubMed] [Google Scholar]
- 23.Schaller MD, Parsons JT: Focal adhesion kinase and associated proteins. Curr Opin Cell Biol 1994, 6:705-710 [DOI] [PubMed] [Google Scholar]
- 24.Zhang H, Tan SM, Lu J: cDNA cloning reveals two mouse β5 integrin transcripts distinct in cytoplasmic domains as a result of alternative splicing. Biochem J 1998, 331:631-637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Languino LR, Ruoslahti E: An alternative form of the integrin β1 subunit with a variant cytoplasmic domain. J Biol Chem 1992, 267:7116-7120 [PubMed] [Google Scholar]
- 26.Fornaro M, Tallini G, Bofetiado CJM, Bosari S, Languino LR: Down-regulation of β1C integrin, an inhibitor of cell proliferation, in prostate carcinoma. Am J Pathol 1996, 149:765-773 [PMC free article] [PubMed] [Google Scholar]
- 27.Hayward SW, Cunha GR, Bartek J, Deshpande N, Narayan P: Establishment and characterization of an immortalized but not transformed human prostate epithelial cell line: BPH-1. In Vitro Cell Dev Biol Anim 1995, 31:14-24 [DOI] [PubMed] [Google Scholar]
- 28.Languino LR, Duperray A, Joganic KJ, Fornaro M, Thornton GB, Altieri DC: Regulation of leukocyte-endothelium interaction and leukocyte transendothelial migration by ICAM1-fibrinogen recognition. Proc Natl Acad Sci USA 1995, 92:1505-1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Languino LR, Gehlsen KR, Wayner E, Carter WG, Engvall E, Ruoslahti E: Endothelial cells use α2β1 integrin as a laminin receptor. J Cell Biol 1989, 109:2455-2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mao P, Angrist A: The fine structure of the basal cells of human prostate. Lab Invest 1966, 15:1768-1782 [PubMed] [Google Scholar]
- 31.Xue Y, Smedts F, Debruyne FM, de la Rosette JJ, Schalken JA: Identification of intermediate cell types by keratin expression in the developing human prostate. Prostate 1998, 34:292-301 [DOI] [PubMed] [Google Scholar]
- 32.Scoazec JV: Expression of cell-matrix adhesion molecules in the liver and their modulation during fibrosis. J Hepatol 1995, 22:20-27 [PubMed] [Google Scholar]
- 33.Balzac F, Belkin AM, Koteliansky VE, Balabanov YV, Altruda F, Silengo L, Tarone G: Expression and functional analysis of a cytoplasmic domain variant of the β1 integrin subunit. J Cell Biol 1993, 121:171-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belkin AM, Retta SF, Pletjushkina OY, Balzac F, Silengo L, Fassler R, Koteliansky VE, Burridge K, Tarone G: Muscle β1D integrin reinforces the cytoskeleton-matrix link: modulation of integrin adhesive function by alternative splicing. J Cell Biol 1997, 139:1583-1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brancaccio M, Cabodi S, Belkin AM, Collo G, Koteliansky VE, Tomatis D, Altruda F, Silengo L, Tarone G: Differential onset of expression of α7 and β1D integrin during mouse heart and skeletal muscle development. Cell Adhes Commun 1998, 5:193-205 [DOI] [PubMed] [Google Scholar]
- 36.Belkin AM, Retta SF: β1D integrin inhibits cell cycle progression in normal myoblasts and fibroblasts. J Biol Chem 1998, 273:15234-15240 [DOI] [PubMed] [Google Scholar]
- 37.Moore KL, Persud TVN: The Developing Human: Clinically Oriented Embryology. Edited by KL Moore, TVN Persud. Philadelphia, WB Saunders Co, 1998
- 38.Carroll JM, Romero RM, Watt FM: Suprabasal integrin expression in epidermis of transgenic mice results in developmental defects and a phenotype resembling psoriasis. Cell 1995, 83:957-968 [DOI] [PubMed] [Google Scholar]
- 39.Frenette PS, Wagner DD: Molecular medicine: adhesion molecules, part I. N Engl J Med 1996, 334:1526-1529 [DOI] [PubMed] [Google Scholar]
- 40.Hertle MD, Kubler M-D, Leigh IM, Watt FM: Aberrant integrin expression during epidermal wound healing and in psoriatic epidermis. J Clin Invest 1992, 89:1892-1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Albelda SM: Biology of disease: role of integrins and other cell adhesion molecules in tumor progression and metastasis. Lab Invest 1993, 68:4-17 [PubMed] [Google Scholar]
- 42.Ruoslahti E: Integrins as signaling molecules and targets for tumor therapy. Kidney Int 1997, 51:1413-1417 [DOI] [PubMed] [Google Scholar]
- 43.Bonkhoff H, Stein U, Remberger K: Differential expression of α6 and α2 very late antigen integrins in the normal, hyperplastic, and neoplastic prostate: simultaneous demonstration of cell surface receptors and their extracellular ligands. Hum Pathol 1993, 24:243-248 [DOI] [PubMed] [Google Scholar]
- 44.Knox JD, Cress AE, Clark V, Manriquez L, Affinito KS, Dalkin BL, Nagle RB: Differential expression of extracellular matrix molecules and the α6-integrins in the normal and neoplastic prostate. Am J Pathol 1994, 145:167-174 [PMC free article] [PubMed] [Google Scholar]
- 45.Nagle RB, Hao J, Knox JD, Dalkin BL, Clark V, Cress AE: Expression of hemidesomosomal and extracellular matrix proteins by normal and malignant human prostate tissue. Am J Pathol 1995, 146:1498-1507 [PMC free article] [PubMed] [Google Scholar]
- 46.Stroeken PJ, van Rijthoven E, van der Valk MA, Roos E: Targeted disruption of the β1 integrin gene in a lymphoma cell line greatly reduces metastatic capacity. Cancer Res 1998, 58:1569-1577 [PubMed] [Google Scholar]
- 47.Felding-Habermann B, Cheresh DA: Vitronectin and its receptors. Curr Opin Cell Biol 1993, 5:864-868 [DOI] [PubMed] [Google Scholar]
- 48.Witkowski M, Rabinovitz I, Nagle RB, Affinito KD, Cress AE: Characterization of integrin subunits, cellular adhesion and tumorigenicity of four human prostate cell lines. Cancer Res Clin Oncol 1993, 119:637-644 [DOI] [PubMed] [Google Scholar]
- 49.Wewer UM, Shaw LM, Albrechtsen R, Mercurio AM: The integrin α6β1 promotes the survival of metastatic human breast carcinoma cells in mice. Am J Pathol 1997, 151:1191-1197 [PMC free article] [PubMed] [Google Scholar]
- 50.Chao C, Lotz MM, Clarke AC, Mercurio AM: A function for the integrin α6β4 in the invasive properties of colorectal carcinoma cells. Cancer Res 1996, 56:4811-4819 [PubMed] [Google Scholar]