Abstract
Autoimmune gastritis is the underlying pathological lesion of pernicious anemia in humans. The lesion is characterized by a chronic inflammatory infiltrate in the gastric mucosa with loss of parietal and zymogenic cells. It is associated with circulating autoantibodies to the gastric H/K-ATPase, the enzyme responsible for acidification of gastric juice. Experimental models of autoimmune gastritis have previously been produced in mice after a variety of manipulations, including thymectomy. Here we report for the first time a spontaneous mouse model of autoimmune gastritis in C3H/He mice. The spontaneous gastritis is also accompanied by circulating autoantibodies to the gastric H/K-ATPase. The spontaneous mouse model should be useful for studies directed toward the immunopathogenesis and treatment of autoimmune gastritis.
Autoimmune gastritis is the underlying cause of pernicious anemia in humans. 1,2 The pathological lesion is characterized by a chronic inflammatory infiltrate within the gastric mucosa and loss of parietal and zymogenic (chief) cells from the gastric glands. 3 The gastritis is associated with circulating autoantibodies specific for the α- and β-subunits of the gastric H/K-ATPase. 4,5
Experimental autoimmune gastritis can be induced in BALB/c mice by immunization with the gastric H/K-ATPase 6,7 or by a variety of manipulations that result in transient lymphopenia (reviewed in Ref. 8 ), including neonatal thymectomy, 9-11 adult thymectomy combined with cyclophosphamide treatment, 12 lymphoid irradiation, 13 and generation of single-chain TCR transgenic mice. 14 Murine autoimmune gastritis induced by neonatal thymectomy is CD4+ T cell mediated. 15,16 It is also characterized by mononuclear cell infiltrates within the gastric mucosa, loss of parietal and zymogenic cells from the gastric glands, 7,13,17,18 and parietal cell autoantibodies to the α- and β-subunits of the gastric H/K-ATPase, 19,20 which is predictive of gastritis. 6,11,21 Using transgenic mice, we have shown that a T-cell response to the β-subunit but not to the α-subunit of the gastric H/K-ATPase is required for the initiation of autoimmune gastritis. 6,21
Spontaneous animal models of a number of organ-specific autoimmune diseases in humans have previously been reported. For instance, the NOD (nonobese diabetic) mouse is a spontaneous model for autoimmune diabetes that shares features with human autoimmune diabetes. 22,23 Other models of organ-specific autoimmune diseases include autoimmune thyroiditis, 24 autoimmune hemolytic anemia, 25 and autoimmune thrombocytopenic purpura. 26 Indeed, spontaneous autoimmune gastritis has also previously been described in the BB rat, 27 although it does not appear to be a widely used model for autoimmune gastritis. Here we report for the first time the generation of spontaneous autoimmune gastritis in C3H/He mice. We show that C3H/He mice develop gastritis characterized by a mucosal infiltrate of mononuclear cells, loss of parietal and zymogenic cells, and circulating autoantibodies to the gastric H/K-ATPase, characteristics similar to that of human autoimmune gastritis and gastritis in BALB/c and BALB/cCrSlc mice induced by neonatal thymectomy. The spontaneous development of autoimmune gastritis in C3H/He mice more closely mimics the situation in humans and can be expected to be useful for studies of the immunopathogenesis and treatment of autoimmune gastritis.
Materials and Methods
Mice
C3H/He mice were obtained from the Walter and Elisa Hall Institute animal facilities (Melbourne, Australia), and BALB/cCrSlc mice were obtained from T. Masuda, Kyoto University, Japan. Mice were maintained under standard conditions in the Monash University Medical School animal facility. All procedures were performed in accordance with Monash University animal ethics guidelines for animal experimentation.
Neonatal Thymectomy
BALB/cCrSlc mice were subjected to neonatal thymectomy 3 days after birth as described previously. 11
Indirect Immunofluorescence
Sera from mice were assayed for circulating autoantibodies to gastric parietal cells by indirect immunofluorescence reactivity with paraffin-embedded mouse stomachs as described previously. 21 Autoantibodies reactive with stomach, thyroid, pancreas, ovary, or testis were assayed on 5-μm frozen sections.
Enzyme-Linked Immunosorbent Assay
The sera of mice were tested for immunoglobulin G (IgG) autoantibodies to the gastric H/K-ATPase by enzyme-linked immunosorbent assay (ELISA) as described previously. 21 Anti-H/K-ATPase titers were determined by serial twofold dilution of sera and taken as the highest dilution producing an optical density reading above baseline.
Histology
Mice were killed by cervical dislocation or C02 asphyxiation. Stomachs were removed and examined by histology by staining in hematoxylin and eosin or a modification of Maxwell’s staining method. 28,29 Gastritis was assessed by the presence of cellular infiltrate into the gastric glands and muscularis mucosa. 21 The modified Maxwell staining method allows identification of specialized cell types within the gastric mucosa. In normal mouse stomach sections, parietal cells stain blue-green, zymogenic (chief) cells stain red-purple, and areas of mucus secretion are stained yellow. 7,17
Results
Spontaneous Autoimmune Gastritis in C3H/He Mice
We have previously observed the presence of circulating antibodies to gastric parietal cells demonstrated by immunofluorescence in a small set of C3H/He mice. 30 To extend this observation, we examined 15 female and 20 male C3H/He mice, age 6 to 8 weeks, from the same animal facility as those reported in the earlier study. 30 Sera were collected from mice on the day of delivery to our animal facility and tested for autoantibodies reactive with the gastric H/K-ATPase by ELISA. One out of 15 female and 1 out of 20 male mice had autoantibodies to the gastric H/K-ATPase, giving an incidence of 2/35 (6%) (data not shown). These H/K-ATPase-reactive sera gave immunofluorescence staining of mouse gastric parietal cells (Figure 1A) ▶ , in a pattern identical to that seen with sera from BALB/cCrSlc mice with autoimmune gastritis induced by neonatal thymectomy 11 (Figure 1B) ▶ and from humans with pernicious anemia (Figure 1C) ▶ .
Figure 1.
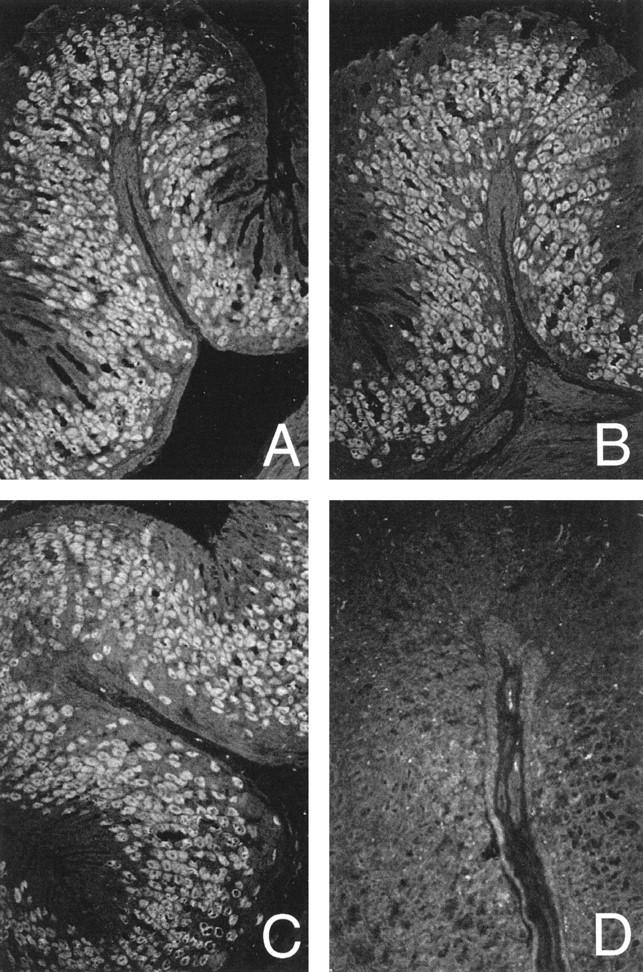
Indirect immunofluorescence staining on paraffin embedded-normal mouse stomach sections. Parietal cell reactivity in sera from a C3H/He mouse (A), a neonatally thymectomized BALB/cCrSlc mouse (B), a patient with pernicious anemia (C), and a C3H/He mouse without parietal cell reactivity (D). Magnification, objective ×20.
We have previously shown that the presence of circulating autoantibodies to parietal cells and the gastric H/K-ATPase correlates with the presence of gastritis in BALB/c and BALB/cCrSlc mice with autoimmune gastritis induced by neonatal thymectomy. 11,21 We wished to ascertain whether this is also the case for C3H/He mice with parietal cell autoantibodies. Indeed, we found that the two autoantibody-positive C3H/He mice (described above) had histological evidence of gastritis with mononuclear cell infiltrates in the submucosa and extending into the lamina propria of the gastric mucosa. (Figure 2A) ▶ . The pattern of cellular infiltration was similar to that seen in the gastritis induced by neonatal thymectomy in that pockets of mononuclear cell infiltrates were observed in the gastric mucosa of BALB/c mice (Figure 2B) ▶ . Gastritis was not observed in C3H/He mice without parietal cell autoantibodies (Figure 2C) ▶ .
Figure 2.
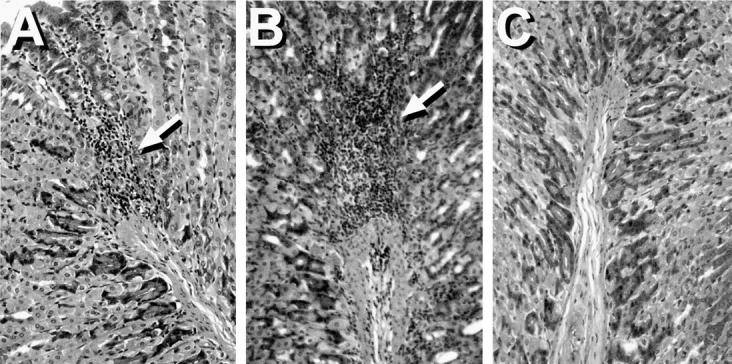
H&E-stained sections of paraffin-embedded mouse stomachs showing gastritis in C3H/He (A) and neonatally thymectomized BALB/c mice (B). Areas of mononuclear cell infiltration are indicated by arrows. (C) Stained section of a nongastritic C3H/He mouse stomach. Magnification, objective ×25.
Parietal, zymogenic, and mucus-secreting cells in normal stomachs can be readily distinguished from each other by a modified Maxwell’s stain 7,17 (Figure 3A) ▶ . In gastritic stomachs, parietal and zymogenic cells were reduced in number with an increase in the population of mucus-secreting cells (Figure 3B) ▶ . These observations are similar to those reported for autoimmune gastritis in BALB/cCrSlc mice induced by neonatal thymectomy 17 or by immunization with mouse H/K-ATPase. 7
Figure 3.
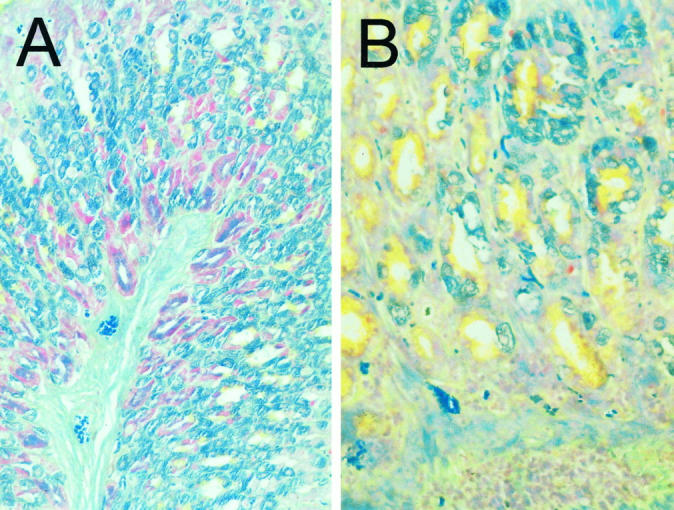
Modified Maxwell’s staining of paraffin-embedded mouse stomachs from nongastritic (A) and gastritic (B) C3H/He mice. Parietal cells stain blue-green, zymogenic (chief) cells stain red-purple, and mucus-secreting cells stain yellow. Note the change in cellular composition in C3H/He mice with gastritis. Magnification, objective ×25.
Establishing a C3H/He Mouse Line with a High Incidence of Spontaneous Autoimmune Gastritis
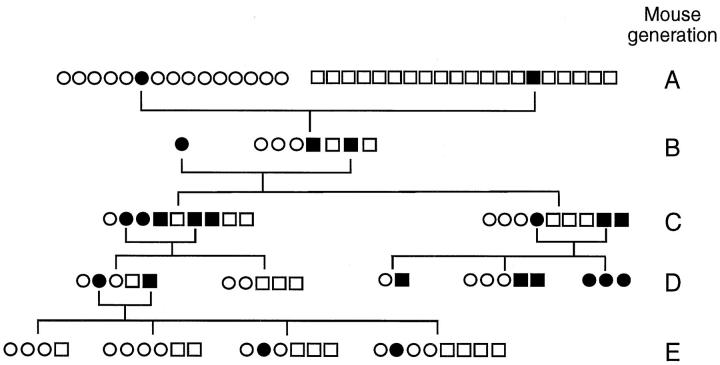
The incidence of autoimmune gastritis in the 35 mice that were originally introduced into our animal facility was 2/35 (6%). To establish a line of C3H/He mice that might display a higher incidence of spontaneous autoimmune gastritis, we began by mating mice that were parietal cell and H/K ATPase autoantibody positive. Part of the mating strategy is outlined in Figure 4 ▶ . C3H/He mice derived from these matings were screened for parietal cell autoantibodies by indirect immunofluorescence and H/K-ATPase, and IgG antibodies were screened by ELISA. As shown in Figure 4 ▶ , early generations (generations B and C) from this breeding strategy indicated that the incidence of autoimmune gastritis had increased. Compared with the initial group of 35 mice (Figure 4 ▶ , generation A) with an incidence of 2/35, subsequent litters (Figure 4 ▶ , generations B and C) had incidences of between 28.5% and 55%. However, in some litters (Figure 4 ▶ , generations D and E) there was no evidence of parietal cell antibodies, even though these mice had been derived from three generations of antibody-positive parents. However, overall the incidence of parietal cell antibodies in C3H/He mice bred in our facility (Figure 4 ▶ , generations B through E) was substantially increased compared with mice that were obtained from outside sources (Figure 4 ▶ , generation A) (2/35 versus 21/70; P = 0.0102). The absence of parietal cell autoantibodies in some litters suggests that a direct genetic factor is not involved in the increased incidence of autoimmune gastritis observed in our col- ony. This is not surprising given that these mice are an inbred strain and genetically identical. In addition, we have also screened litters that were generated by mating gastric H/K ATPase antibody-negative mice, and in one study, 6/13 (46%) mice had evidence of H/K ATPase antibodies by ELISA (data not shown). Because these mice were introduced to our conventional mouse facility from a specific pathogen-free facility, it may suggest that some environmental factor(s) may be associated with the increased incidence of autoimmune gastritis in C3H/He mice.
Figure 4.
Breeding program used to generate C3H/He mouse line with higher incidence of autoimmune gastritis. Mice with parietal cell autoantibodies from each generation were selected for breeding. Sequential generations of mice are labeled A through E. ○, females without parietal cell reactivity; •, females with parietal cell reactivity; □, males without parietal cell reactivity; ▪, males with parietal cell reactivity.
It has recently been suggested that many of the existing models of experimental organ-specific autoimmunity, including autoimmune gastritis, are associated with a state of T-cell lymphopenia. 8 We have addressed this by numerating the lymphocyte populations in C3H/He mice and comparing these with BALB/c mice, which do not develop experimental autoimmune gastritis unless thymectomized. The total number of thymocytes and splenocytes in C3H/He mice were 1.6 × 10 8 ± 2.5 × 10 7 and 8.1 × 10 7 ± 2.3 × 107, respectively. This was similar to BALB/c total thymocyte and splenocyte counts of 1.5 × 10 8 ± 7.0 × 10 7 and 4.5 × 10 7 ± 1.5 × 107, respectively. In addition, the CD4+/CD8+ T-cell ratio of thymocytes and splenocytes from C3H/He mice was within the expected ratio of 2:1 to 3:1 (data not shown). This indicates that an obvious lymphopenia is not associated with autoimmune gastritis in C3H/He mice, although it does not discount the potential for functional lymphocytic abnormalities in these mice, such as those recently described by Hammond et al 31 and Baxter et al 32 in the NOD mouse model for insulin-dependent diabetes mellitus.
Autoantibodies to Gastric Parietal Cells in C3H/He Mice Are Reactive with the Gastric H/K ATPase and Associated with Histological Evidence of Gastritis
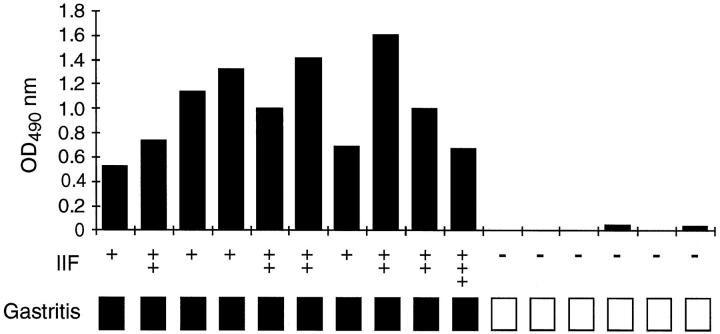
Autoimmune gastritis in the neonatal thymectomy model is associated with the presence of parietal cell autoantibodies and gastritis. In addition, anti-parietal cell reactivity is associated with autoantibodies to the gastric H/K ATPase and indicative of gastritis. 21 We have confirmed that parietal cell reactivity observed in C3H/He mice was associated with autoantibodies to the gastric H/K ATPase and gastritis. Sixteen mice were selected from our colony based on gastric H/K ATPase reactivity and screened for parietal cell reactivity and histological evidence of gastritis. As shown in Figure 5 ▶ , all 10 sera reactive with gastric H/K-ATPase by ELISA were also positive for parietal cell reactivity and had histological evidence of gastritis (Figure 5) ▶ . However, the level or degree of infiltration was often not as pronounced in gastritic C3H/He mice compared with thymectomized mice and may reflect a difference in the pathogenesis of these two models. Mice that were not reactive by ELISA or immunofluorescence did not have gastritis (Figure 5) ▶ . This confirms that the presence of parietal cell reactivity in C3H/He mice is associated with gastric H/K ATPase IgG autoantibodies, which are predictive of gastritis. This association has also been described in the neonatal thymectomy model of experimental autoimmune gastritis. 21 This suggests that the mechanisms associated with the autoimmune response in C3H/He mice and the neonatal thymectomy model are similar.
Figure 5.
Incidence of gastritis in C3H/He mice with parietal cell- and H/K-ATPase-reactive autoantibodies. Anti-H/K-ATPase reactivity was detected by ELISA (filled bars). Parietal cell autoantibodies were detected by indirect immunofluorescence (IIF) on paraffin-embedded stomach sections. Anti-parietal cell reactivity was scored as follows: −, negative; +, weakly positive; ++, positive; and +++, strongly positive. Gastritis was assessed by histology and indicated by a filled square. Mice without gastritis are indicated by an open square.
Autoimmune gastritis and pernicious anemia is one of a group of autoimmune diseases that target the stomach and other endocrine organs. As such, autoimmune diseases may often be associated with subclinical autoimmune responses to other organs. This is also true in animal models for autoimmune diseases. For example, in neonatally thymectomized BALB/c mice, apart from autoimmune gastritis, mice may also develop other autoimmune diseases such as oophoritis (17%) in females and orchitis (4%) in males. 9 Different autoimmune diseases may or may not be overlapping in individual mice. 21 To assay for the presence of autoimmune responses in C3H/He mice other than autoimmune gastritis, 28 sera (from 15 females and 13 males; of the 28 sera, 14 were positive for gastric H/K ATPase reactivity by ELISA) were screened by indirect immunofluorescence on frozen sections of stomach, ovary, testis, thyroid, and pancreas. Parietal cell staining in stomach sections correlated with H/K ATPase reactivity, but none of the sera demonstrated specific staining of ovary, testis, thyroid, or pancreas (data not shown). These data suggest that the incidence of autoimmune diseases other than autoimmune gastritis is not a major association with C3H/He mice. If such autoimmune diseases are present in C3H/He mice, then the frequency would appear to be quite low.
Development of Gastric H/K ATPase Autoantibodies in C3H/He Mice
We have previously observed in day 3 thymectomy-induced autoimmune gastritis that gastric H/K ATPase antibodies may be detected as early as 4 weeks of age and peaks at 8 to 10 weeks 17 (F. Alderuccio et al, unpublished data). To assess the development of H/K ATPase antibodies in C3H/He mice, sera from mice 4 to 10 weeks of age were assayed by ELISA for gastric H/K ATPase IgG antibody reactivity. From a group of 15 mice examined, 4 of 15 (26%) had gastric H/K ATPase antibodies by 10 weeks of age. Within the group of 4 mice, 3 had evidence of H/K ATPase reactivity as early as 4 weeks of age (Figure 6A ▶ , optical density reading 0.5 or greater). At 6 weeks of age, sera from all 4 mice were reactive with gastric H/K ATPase by ELISA (Figure 6A) ▶ . Reactivity with the H/K ATPase was confirmed by indirect immunofluorescence reactivity with parietal cells in mouse stomach sections (data not shown).
Figure 6.
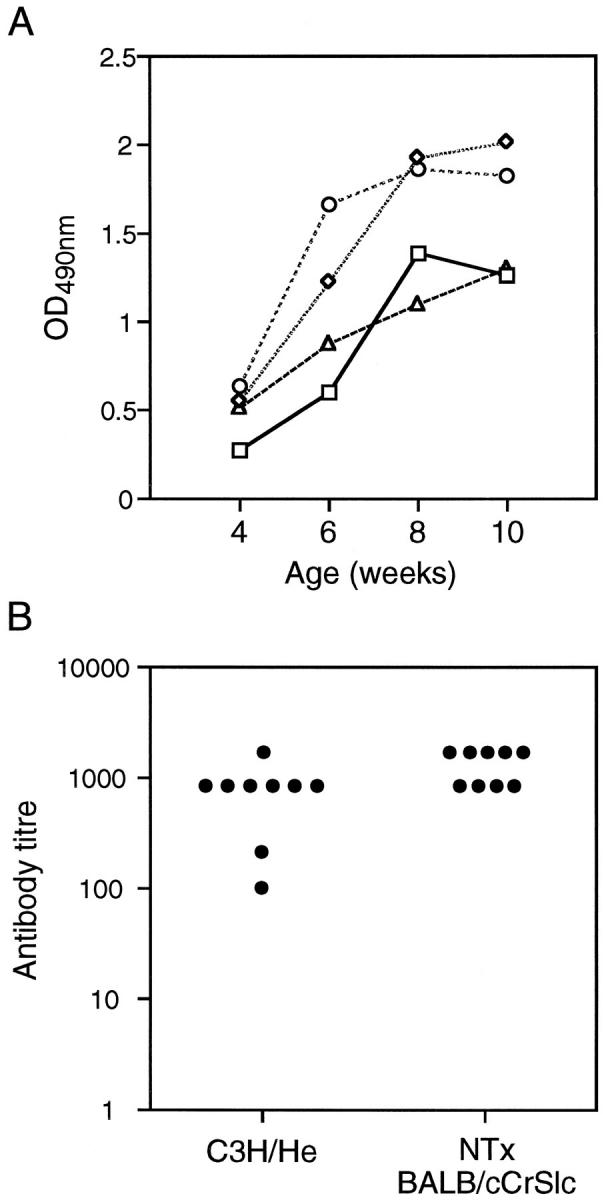
A: Development of anti-H/K-ATPase antibodies in C3H/He mice. Sera were collected from four C3H/He mice at 4, 6, 8, and 10 weeks of age and assayed for gastric H/K ATPase reactivity by ELISA. Each symbol represents the antibody reactivity with gastric H/K ATPase for an individual mouse over the course of the study. B: Serum titer of antibodies to the gastric H/K-ATPase in C3H/He mice and neonatally thymectomized (NTx) BALB/cCrSlc mice. Serum antibody titers were determined by ELISA on gastric H/K-ATPase-coated plates and taken as the highest dilution that produced an optical density reading above baseline.
Although an autoantibody response to the gastric H/K ATPase is clearly present in C3H/He mice, we were interested to compare this response with the more established neonatal thymectomy model. IgG antibody titers to the gastric H/K-ATPase in nine C3H/He mice (4 to 6 months of age) with spontaneous autoimmune gastritis and nine BALB/cCrSlc mice (2 to 3 months of age) with autoimmune gastritis induced by neonatal thymectomy were determined by ELISA (Figure 6B) ▶ . In general, the antibody titers from both sets of mice were similar, with titers of 800 or more, with the exception of two C3H/He sera that had lower titers of H/K-ATPase antibodies (Figure 6B) ▶ . Lower titers in these mice were not due to the age of the C3H/He mice and may indicate that the severity of disease or kinetics of disease induction and progression may be different in the two mouse models.
Discussion
To our knowledge, spontaneous autoimmune gastritis has not previously been reported in any strain of mice. Kojima and Prehn 9 examined the incidence of gastritis in C3H mice after neonatal thymectomy. Gastritis was observed in 10% of C3HeB/FeJ and 2% of C3H/HeJ mice after neonatal thymectomy; however, the incidence of gastritis in the nonthymectomized C3H substrains was not reported.
Experimental autoimmune gastritis can be induced in BALB/c and BALB/cCrSlc by neonatal thymectomy at age 2 to 4 days. 11,33 Autoimmune gastritis in thymectomized mice is characterized by circulating autoantibodies to the α- and β-subunits of the gastric H/K-ATPase. 6,19,20 Histological examination of stomachs from mice with H/K-ATPase antibodies reveals a mononuclear cell infiltrate within the gastric submucosa that extends into the lamina propria of the mucosa. 10,17,33 The extension of the mononuclear infiltrate into the lamina propria is accompanied by the loss of parietal and zymogenic cells from the gastric glands and an expansion of an immature cell population. 7,17,18
In this study, we have described features of autoimmune gastritis that occur spontaneously in C3H/He mice. In several instances, we have directly compared the autoimmune response in C3H/He mice with that seen in the well characterized neonatal thymectomy model. As we have shown, the major parameters that define autoimmune gastritis in the thymectomy model are also present in the C3H/He model. First, C3H/He mice spontaneously develop circulating autoantibodies to the gastric H/K-ATPase. This is the major autoantigen associated with induction of experimental autoimmune gastritis in thymectomized BALB/c mice. However, it still remains to be shown in C3H/He mice that an immune response to the β-subunit of the gastric H/K ATPase is required for disease induction, which has been shown in the neonatal thymectomy model. 21 Autoantibodies in C3H/He mice could be detected in mice as early as 4 weeks of age, and ultimately, the antibody titers to the H/K-ATPase in C3H/He mice are similar to those observed in thymectomized BALB/cCrSlc mice. Secondly, histological examination of the stomachs confirmed the presence of gastritis in C3H/He mice that had circulating autoantibodies to the gastric H/K-ATPase. The gastritis was similar to that seen in BALB/cCrSlc mice after neonatal thymectomy in which there is also a tight correlation between the presence of circulating autoantibodies and underlying gastritis. 11 The mucosal infiltration of mononuclear cells is also accompanied by the loss of parietal and chief cells and an increase in the population of mucous cells.
Initially, our strategy was to develop a substrain of C3H/He with a high incidence of autoimmune gastritis by sequential mating of H/K ATPase antibody-positive mice. Although the incidence of autoimmune gastritis has significantly increased in our colony, it does not appear to be due solely to a genetic effect, which may be a limitation to this model. This was highlighted by examples of litters from H/K ATPase antibody-positive parents in which none of the offspring developed H/K ATPase antibodies. In addition, we have also observed the development of H/K ATPase antibodies in litters derived from mating mice without H/K ATPase antibodies. The mechanism(s) associated with the development of autoimmune gastritis in C3H/He mice is unknown. It is possible that environmental factors may be involved in the increased incidence of gastritis. This is fueled by the observation that the increase in gastritis occurred in mice that were transported from a specific pathogen-free environment to our conventional animal facility. We are exploring this possibility further. It has recently been described that a feature common to many models of organ-specific autoimmunity is a state of lymphopenia. 8 From our studies, C3H/He mice were not lymphopenic when compared with BALB/c mice or abnormal in the CD4+/CD8+ T-cell ratios. However, it is possible that more subtle or functional abnormalities, which are yet to be defined, may exist in the lymphocyte populations.
In summary, we have described a spontaneous model of autoimmune gastritis in C3H/He mice. Spontaneous gastritis with loss of parietal and zymogenic cells and autoantibodies to the gastric H/K-ATPase are hallmarks of human autoimmune gastritis and pernicious anemia. 3,34 In humans, a tight correlation between the presence of circulating parietal cell autoantibodies and the presence of underlying gastritis has also been observed. 35,36 We believe that autoimmune gastritis in C3H/He mice is an excellent model for human autoimmune gastritis. It represents one of very few animal models in which the target autoantigens have been defined and shown to be identical to that in the corresponding human disease. The existence of a spontaneous model for autoimmune gastritis that occurs without immunization or the need for manipulating the immune system will be a significant contribution to the field of autoimmunity research.
Acknowledgments
We thank Ms. Louise Judd for performing the modified Maxwell staining procedure.
Footnotes
Address reprint requests to Dr. Frank Alderuccio, Department of Pathology and Immunology, Monash University Medical School, Commercial Road, Prahran, Melbourne, Victoria, 3181, Australia. E-mail: frank.alderuccio@med.monash.edu.au.
This work was supported by grants from the National Health and Research Council and the Alfred Healthcare Group.
References
- 1.Gleeson PA, Toh BH: Molecular targets in pernicious anaemia. Immunol Today 1991, 12:233-238 [DOI] [PubMed] [Google Scholar]
- 2.Toh BH, van Driel IR, Gleeson PA: Pernicious anemia. N Engl J Med 1997, 337:1441-1448 [DOI] [PubMed] [Google Scholar]
- 3.Strickland R, Mackay IR: A reappraisal of the nature and significance of chronic atrophic gastritis. Am J Digest Dis 1973, 18:426-440 [DOI] [PubMed] [Google Scholar]
- 4.Toh BH, Gleeson PA, Simpson RJ, Moritz RL, Callaghan JM, Goldkorn I, Jones CM, Martinelli TM, Mu F-T, Humphris DC, Pettitt JM, Mori Y, Masuda T, Sobieszczuk P, Weinstock J, Mantamadiotis T, Baldwin GS: The 60- to 90-kd parietal cell autoantigen associated with autoimmune gastritis is a β subunit of the gastric H+/K+-ATPase (proton pump). Proc Natl Acad Sci USA 1990, 87:6418-6422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callaghan JM, Khan MA, Alderuccio F, van Driel IR, Gleeson PA, Toh BH: α and β subunits of the gastric H+/K+-ATPase are concordantly targeted by parietal cell autoantibodies associated with autoimmune gastritis. Autoimmunity 1993, 16:289-295 [DOI] [PubMed] [Google Scholar]
- 6.Alderuccio F, Gleeson PA, Berzins SP, Martin M, van Driel IR, Toh BH: Expression of the gastric H/K ATPase α-subunit in the thymus may explain the dominant role of the β-subunit in the pathogenesis of autoimmune gastritis. Autoimmunity 1997, 25:167-175 [DOI] [PubMed] [Google Scholar]
- 7.Scarff KJ, Pettitt JM, van Driel IR, Gleeson PA, Toh BH: Immunization with gastric H+/K+-ATPase induces a reversible autoimmune gastritis. Immunology 1997, 92:91-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gleeson PA, Toh BH, van Driel IR: Organ-specific autoimmunity induced by lymphopenia. Immunol Rev 1996, 149:97-125 [DOI] [PubMed] [Google Scholar]
- 9.Kojima A, Prehn RT: Genetic susceptibility to post-thymectomy autoimmune diseases in mice. Immunogenetics 1981, 14:15-27 [DOI] [PubMed] [Google Scholar]
- 10.Tung KSK, Smith S, Teuscher C, Cook C, Anderson RE: Murine autoimmune oophoritis, epididymoorchitis, and gastritis induced by day 3 thymectomy: immunopathology. Am J Pathol 1987, 126:293-302 [PMC free article] [PubMed] [Google Scholar]
- 11.Alderuccio F, Toh BH, Gleeson PA, van Driel IR: A novel method for isolating mononuclear cell from the stomachs of mice with experimental autoimmune gastritis. Autoimmunity 1995, 21:215-221 [DOI] [PubMed] [Google Scholar]
- 12.Barrett SP, Toh BH, Alderuccio F, van Driel IR, Gleeson PA: Organ-specific autoimmunity induced by adult thymectomy and cyclosphamide-induced lymphopenia. Eur J Immunol 1995, 25:238-244 [DOI] [PubMed] [Google Scholar]
- 13.Sakaguchi N, Miyai K, Sakaguchi S: Ionizing radiation and autoimmunity: induction of autoimmune disease in mice by high dose fractionated total lymphoid irradiation and its prevention by inoculating normal T cells. J Immunol 1994, 152:2586-2595 [PubMed] [Google Scholar]
- 14.Sakaguchi S, Ermak TH, Toda M, Berg LJ, Ho W, Fazekas de St. Groth B, Peterson PA, Sakaguchi N, Davis MM: Induction of autoimmune disease in mice by germline alteration of the T cell receptor gene expression. J Immunol 1994, 152:1471-1484 [PubMed] [Google Scholar]
- 15.Smith H, Lou YH, Lacy P, Tung KSK: Tolerance mechanisms in experimental ovarian and gastric autoimmune diseases. J Immunol 1992, 149:2212-2218 [PubMed] [Google Scholar]
- 16.De Silva HD, van Driel IR, La Gruta N, Toh BH, Gleeson PA: CD4+ T cells, but not CD8+ T cells, are required for the development of experimental autoimmune gastritis. Immunology 1998, 93:405-408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinelli TM, van Driel IR, Alderuccio F, Gleeson PA, Toh BH: Analysis of mononuclear cell infiltrate and cytokine production in murine autoimmune gastritis. Gastroenterology 1996, 110:1791-1802 [DOI] [PubMed] [Google Scholar]
- 18.Kojima A, Taguchi O, Nishizuka Y: Experimental production of possible autoimmune gastritis followed by macrocytic anemia in athymic nude mice. Lab Invest 1980, 42:387-395 [PubMed] [Google Scholar]
- 19.Kontani K, Taguchi O, Takahashi T: Involvement of the H+/K+-ATPase α subunit as a major antigenic protein in autoimmune gastritis induced by neonatal thymectomy in mice. Clin Exp Immunol 1992, 89:63-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones CM, Callaghan Gleeson PA, Mori Y, Masuda T, Toh BH: The parietal cell autoantibodies recognised in neonatal thymectomy-induced murine gastritis are the α and β subunits of the gastric proton pump. Gastroenterology 1991, 101:287-294 [DOI] [PubMed] [Google Scholar]
- 21.Alderuccio F, Toh BH, Tan SS, Gleeson PA, van Driel IR: An autoimmune disease with multiple molecular targets abrogated by the transgenic expression of a single autoantigen in the thymus. J Exp Med 1993, 178:419-426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castano L, Eisenbarth GS: Type-1 diabetes: a chronic autoimmune disease of human, mouse and rat. Annu Rev Immunol 1990, 8:647-679 [DOI] [PubMed] [Google Scholar]
- 23.O’Reilly LA, Cooke A: Animal models of insulin dependent diabetes mellitus. Bona CA Siminovitch KA Zanetti M Theofilopoulos AN eds. The Molecular Pathology of Autoimmune Diseases. 1993, :pp 619-625 Harwood Academic Publishers Camberwell, Victoria, Australia [Google Scholar]
- 24.Bigazzi PE: Autoimmunity in Hashimoto’s disease. Bona CA Siminovitch KA Zanetti M Theofilopoulos AN eds. The Molecular Pathology of Autoimmune Diseases. 1993, :pp 493-510 Harwood Academic Publishers Camberwell, Victoria, Australia [Google Scholar]
- 25.Calkins CE, Caulfield MJ: Experimental models of autoimmune hemolytic anaemia. Bona CA Siminovitch KA Zanetti M Theofilopoulos AN eds. The Molecular Pathology of Autoimmune Diseases. 1993, :pp 451-470 Harwood Academic Publishers Camberwell, Victoria, Australia [Google Scholar]
- 26.Ikehara S, Mizutani H, Kawamura M, Kurata Y: Experimental autoimmune thrombocytopenia purpura. Bona CA Siminovitch KA Zanetti M Theofilopoulos AN eds. The Molecular Pathology of Autoimmune Diseases. 1993, :pp 487-492 Harwood Academic Publishers Camberwell, Victoria, Australia [Google Scholar]
- 27.Jaworski MA, Honore L, Jewell LD, Mehta JG, McGuire-Clark P, Schoul JJ, Yap WY: Cyclosporin prophylaxis induces long-term prevention of diabetes, and inhibits lyphocytic infiltration in multiple target tissues in the high-risk BB rat. Diabetes Res 1986, 3:1-6 [PubMed] [Google Scholar]
- 28.Maxwell A: The alcain dyes applied to gastric mucosa. Stain Technol 1963, 38:286-287 [PubMed] [Google Scholar]
- 29.Beinborn M, Giebel J, Linck M, Cetin Y, Schwenk M, Sewing KF: Isolation, identification, and quantitative evaluation of specific cell types from the mammalian gastric mucosa. Cell Tissue Res 1993, 274:229-240 [DOI] [PubMed] [Google Scholar]
- 30.Sakagami T, Dixon M, O’Rourke J, Howlett R, Alderuccio F, Vella J, Shimoyama T, Lee A: Atrophic gastric changes in both H. felis and H. pylori infected mice are host dependent and separate from antral gastritis. Gut 1996, 39:639-648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammond KJL, Poulton LD, Palmisano LJ, Silveira PA, Godfrey DI, Baxter AG: α/β-T cell receptor (TCR)+CD4−CD8− (NKT) thymocytes prevent insulin-dependent diabetes mellitus in nonobese diabetic (NOD)/Lt mice by the influence of interleukin (IL)-4 and/or IL-10. J Exp Med 1998, 187:1047-1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baxter AG, Kinder SJ, Hammond KJL, Scollay R, Godfrey DI: Association between αβTCR+CD4-CD8- T-cell deficiency and IDDM in NOD/Lt mice. Diabetes 1997, 46:572-582 [DOI] [PubMed] [Google Scholar]
- 33.Fukuma K, Sakaguchi S, Kuribayashi K, Chen W-L, Morishita R, Sekita K, Uchino H, Masuda T: Immunologic and clinical studies in murine experimental autoimmune gastritis induced by neonatal thymectomy. Gastroenterology 1988, 94:274-283 [DOI] [PubMed] [Google Scholar]
- 34.Toh BH, van Driel IR, Gleeson PA: Autoimmune gastritis: tolerance and autoimmunity to the gastric H+/K+ ATPase (proton pump). Autoimmunity 1992, 13:165-172 [DOI] [PubMed] [Google Scholar]
- 35.Taylor KB, Roitt IM, Doniach D, Couchman KG, Shapland C: Autoimmune phenomena in pernicious anaemia: gastric antibodies. Br Med J 1962, 24:1347-1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strickland R: Gastritis. Springer Semin Immunopathol 1990, 12:203-217 [DOI] [PubMed] [Google Scholar]