Abstract
Gastric carcinomas with DNA replication errors (RER phenotype) display a particular clinicopathologic profile and carry a putative favorable prognosis. The RER phenotype has been identified as microsatellite instability in noncoding regions, as well as in repeat sequences within exons of several “target genes”: TGFβ RII, IGFII R, and BAX. In an attempt to find out whether the RER status is a significant prognostic factor in gastric carcinoma in a multivariate analysis and whether the clinicopathological features of the RER+ tumors are associated with mutations in the “target genes,” we evaluated a series of 152 cases of sporadic gastric carcinoma. Five or six microsatellite loci and/or BAT 26, a poly(A) tract, were analyzed in each case using polymerase chain reaction and electrophoresis. Thirty-five cases (23.0%) were RER+. The RER phenotype was closely associated with a low pTNM stage and carried a significantly better prognosis. The repeat sequences of the target genes were screened for mutations in 28 RER+ and 13 RER− tumors. Mutations in TGFβ RII occurred in 67.9% of the RER+ tumors and were significantly associated with the glandular histotype. IGFII R and BAX mutations occurred, respectively, in 25.0% and 32.1% of the cases; there was a trend toward an association between mutations in these genes and decreased nodal metastization and wall invasiveness, respectively. We conclude that the RER status is a significant prognostic indicator in gastric carcinoma and that such prognostic influence may be mediated by mutations in TGFβ RII, IGFII R, and BAX genes.
Microsatellite instability is a hallmark of the DNA replication error (RER) phenotype caused by the inactivation of mismatch repair genes. 1 The RER-positive (RER+) phenotype has been defined by frequent somatic variations in the size of microsatellites in tumor DNA. 2,3 Recently, Hoang et al 1 showed that using the poly(A) tract BAT 26, as a sole marker, it was possible to determine the RER status of 159 out of 160 colorectal tumors and cell lines (99.4% efficiency). In a previous study we observed, as did Thibodeau et al 2 and Risinger et al 4 in colon and endometrial cancer, respectively, a close relationship between RER+ phenotype and some clinicopathological features of sporadic gastric carcinomas. We observed also that RER+ carcinomas carried a significantly better prognosis in a univariate analysis. 5,6 It remains to be evaluated the importance of RER+ phenotype as a prognostic factor in a multivariate analysis and to find out whether some of the clinicopathological characteristics observed in RER+ tumors are mediated by mutations in the so-called “target genes” that are affected in repetitive coding regions, leading to inactivation of their function. This latter type of instability has been observed in transforming growth factor (TGF) receptor II (TGFβ RII), insulin-like growth factor II receptor (IGFII R), and BAX genes, in different types of RER+ tumors. 7-13
Most of the mutations within TGFβ RII occur in two microsatellites of the coding region. One of these microsatellites is a poly(A)10 tract, and mutations within this region consist of 1- or 2-base deletions or insertions; the other is a poly(GT)3 microsatellite that was found to have an insertion of an extra GT. 13-15 These mutations cause frameshifts of TGFβ RII and result in truncation or substitution of conserved residues of the predicted protein product. 16 The growth-inhibitory effect of TGF-β is primarily mediated by a heteromeric complex of two distantly related transmembrane serine/threonine kinases (receptors I and II); the inactivation of either receptor subtype can result in TGF-β resistance. 7,17 Loss of responsiveness to TGF-β is common in human cancers and is thought to be an important step in tumorigenesis. 18,19
The IGFII R gene contains several microsatellites within its coding region, one of which is an 8-deoxyguanosine repeat that is frequently mutated and comprises 1- or 2-bp deletions or insertions, causing frameshifts and premature stop codons. 9 IGFII R also inhibits cell proliferation mediated by the IGFII ligand, itself a potent growth stimulant, by internalizing and degrading this protein. 20 Thus, IGFII R, by antagonizing the growth-stimulatory effect of IGFII and activating the growth-inhibitory effect of TGF-β, serves as a growth suppressor gene. 9
The human BAX gene contains a tract of eight consecutive deoxyguanosines in the third coding exon; insertions or deletions of one nucleotide are the most frequent mutations in this microsatellite and lead to alterations in the function of the protein. 11 BCL2 and BAX proteins are encoded by a family of genes that take part in the maintenance of the balance between cell proliferation and programmed cell death, in multicellular organisms. The BAX gene acts as a promoter of cell death by opposing the death protector effect of the BCL2 gene. 21 BAX homodimerizes, as well as heterodimerizes with BCL2, and it was suggested that the ratio of BCL2 to BAX determines survival or death after an apoptotic stimulus. 22
We decided to search for microsatellite instability in a series of 152 thoroughly studied cases of sporadic gastric carcinoma and for the presence of mutations in TGFβ RII, IGFII R, and BAX genes in a subset of 28 RER+ and 13 RER− carcinomas, with the following aims: 1) to analyze the prognostic meaning of the RER status in a multivariate analysis along with the most important clinicopathological features and 2) to determine the frequency of mutations in each of the three target genes and to find out whether the occurrence of such mutations is related with any clinicopathological feature(s) of RER+ gastric carcinomas.
Materials and Methods
Subjects
Patients, Tissue Samples, and DNA Extraction
We analyzed the surgical specimens from 152 gastric carcinomas consecutively resected at Hospital of S. João (Porto, Portugal) from 1988 through 1995. Radical extended gastrectomy was performed in the 26 patients with carcinomas involving the cardia. Radical total gastrectomy was performed in the following 60 cases: 36 patients with carcinomas located in the proximal stomach regardless of the histological type, 22 patients with diffuse carcinomas located in the antrum, and 2 patients whose stomachs had been operated on for peptic ulcer. Curative subtotal distal gastrectomy was performed in 66 patients with intestinal or atypical carcinomas located in the antrum. 23 In all cases the surgical therapy was performed to achieve a resection without leaving behind macroscopic or microscopic disease. No patient had received preoperative chemo- or radiotherapy. A family history was obtained in every case; none of the patients included in the present study had a family history suggestive of hereditary nonpolyposis colorectal cancer. Follow-up information was obtained in all but five cases. Two cases were excluded from the survival analysis, because they had been in the study for less than 3 years. The range of follow-up was 3 to 9 years (median, 70 months). The six deaths occurring within the 1st month of surgery were considered postoperative deaths.
Hematoxylin and eosin-stained sections were used to classify the tumors according to the classifications of Laurén, 23 Ming, 24 and Carneiro. 25 The pathological staging was achieved using the unified 1987 TNM system for gastric carcinoma. Orcein-stained sections were used for the detection of vascular invasion. Lymphoid infiltration was subjectively scored into absent/minimal and moderate/abundant. Immunohistochemistry was used to classify the lymphocytic infiltration of the tumors, according to the predominance of T or B lymphocytes, using UCHL1 and L26 antibodies (Dako, Glostrup, Denmark), respectively. From each case, tissue fragments from primary tumors and nonneoplastic mucosa were immediately frozen in liquid nitrogen and stored at −70°C until use. High molecular weight DNA was isolated using standard methods 26 in a total section of the tumors wherever tumor cells occupied more than 50% of tumor tissue or in microdissected areas where at least 50% of tumor cells were present as evaluated by concurrent cryostat sections.
RER Assays
The 152 gastric carcinomas were studied for microsatellite instability using a panel of five or six dinucleotide repeat sequences, as described by Santos et al 5 (n = 104), and/or using a primer set localized on intron 5 of the hMSH2 gene, that amplifies an adenine monomorphic mononucleotide repeat, BAT 26, as described by Hoang et al 1 (n = 152). Fifty of the 152 tumors were previously reported by Santos et al. 5 The polymerase chain reaction (PCR) products were labeled by [α-32P]dCTP during amplification reaction, separated by electrophoresis in 6% denaturing polyacrylamide gels, and visualized through autoradiography.
Cases were considered as having an RER-positive phenotype whenever they expressed high frequency of microsatellite instability (≥40%) using the dinucleotide repeat markers and/or BAT 26 positivity. In the 104 cases in which we have used both methods, the consistency of the results was total (100%).
All cases were screened, at least twice, by independent PCRs and independent electrophoretic run. All the scorings were done independently by two observers.
Amplification of the Target Genes (TGFβ RII,IGFII R, and BAX) and the “Control Gene” (HPRT)
Detection of mutations in target genes was restricted, in the present study, to the 28 RER+ cases in which constitutional and tumoral DNA was available. We analyzed, furthermore, 13 tumors with an RER− phenotype that were randomly selected from the series of 117 RER− carcinomas.
TGFβ RII
Poly(A)10 microsatellite sequence in nucleotides 709 to 718 of the TGFβ RII gene was amplified by radioactive PCR using the following set of primers: (RIIU1) 5′-AGA TGC TGC TTC TCC AAA GTG C-3′ and (RIID1) 5′-TTG CAC TCA TCA GAG CTA CAG G-3′. These primers amplify a 90-bp target sequence from nucleotides 677 to 766. The primer sequences for the (GT)3 microsatellite sequence in nucleotides 1931 to 1936 were (RIIU2) 5′-ACT GAG TGC TGG GAC CAC G-3′ and (RIID2) 5′-AGG AAT CTT CTC CTC CGA GC-3′, which amplify the 123-bp target sequence from nucleotides 1887 to 2009.
IGFII R
The 110-bp sequence within IGFII R that contains an 8-deoxyguanosine repeat from nucleotides 4030 to 4140 was amplified by PCR using primer set R4, consisting of (IGFII R1) 5′-GCA GGT CTC CTG ACT CAG AA-3′ and (IGFII R2) 5′-GAA GAA GAT GGC TGT GGA GC-3′.
BAX
The 94-bp region encompassing the (G)8 tract of the BAX gene was amplified by PCR with the following primers: (BAX1) 5′-ATC CAG GAT CGA GCA GGG CG-3′ and (BAX2) 5′-ACT CGC TCA GCT TCT TGG TG-3′, from nucleotides 90 to 184.
HPRT
The 160-bp region within HPRT, which contains a 6-deoxyguanosine repeat in exon 3, was amplified by PCR with the following primers: (HPRT1) 5′-GAC TGA ACG TCT TGC TCG AGA TG-3′ and (HPRT2) 5′-AAT CTA CAG TCA TAG GAA TGG A-3′.
The PCR products of the different primer sets, described above, were denaturated in a solution containing 95% formamide for 5 minutes at 94°C and then run in denaturing 6% polyacrylamide sequencing gels. TGFβ RII and BAX genes were run in polyacrylamide gels with 32.5% formamide and 6.88 mol/L urea, and IGFII R was run in a gel without formamide and with a lower concentration of urea (6 mol/L). After electrophoresis, the gels were exposed to X-ray film for 4 to 12 hours.
The tumors were considered as having a mutation whenever they showed abnormal bands or shifts in bands in comparison with the respective normal tissue.
Statistical Analysis
The statistical analysis of the results was performed using the χ 2 test with Yates correction, Fisher’s exact test, or Student’s t-test with Statview 4.01 software. Of the 152 patients, 139 were considered for survival analysis; five patients were lost to follow-up, 2 patients were excluded because they had been on the study for less than 3 years, and 6 patients died in the postoperative period. The relationship between RER status and survival rate of patients was assessed by univariate and multivariate analysis (BMDP statistical software, Cork, Ireland). The following parameters were taken into consideration in the survival analysis: age, sex, tumor site, gross appearance, histological classification, depth of wall penetration, venous and lymphatic invasion, pathological staging (pTNM), and RER status. Survival curves were calculated according to Berkson’s actuarial method and compared using the Generalized Savage (Mantel-Cox) test. The evaluation of the prognostic significance of the clinicopathological factors was performed by multivariate regression techniques (Cox’s proportional hazards model) in 136 of the 139 patients; three cases were excluded from this analysis because of missing values on size (n = 2) and gross appearance (n = 1). A P value of <0.05 was considered statistically significant, and a P value of <0.1 was considered suggestively significant.
Results
The mean age (± standard deviation (SD)) of the 152 patients was 60.5 ± 12.5 years. Male/female ratio was 1.6:1. Data on location, size, gross appearance, Laurén’s 23 classification, Carneiro’s 25 classification, Ming’s 24 classification, lymphoid infiltration, wall invasion, lymph node metastases, vascular invasion, and pTNM stage are summarized in Table 1 ▶ .
Table 1.
Summary of the Clinicopathological Features of 152 Sporadic Gastric Carcinomas According to RER Status
| Clinicopathological features | No. of cases | RER+ (n = 35) | RER− (n = 117) | Total (n = 152) | P value |
|---|---|---|---|---|---|
| Age (mean± SD) | 152 | 64.5 ± 12.8 | 59.3± 12.1 | 60.5± 12.5 | 0.03 |
| Male/female | 152 | 18/17 | 75/42 | 93/59 | 0.18 |
| Location | 150* | ||||
| Antrum | 30 | 58 | 88 | ||
| Body | 3 | 33 | 36 | 0.0003 | |
| Cardia | 1 | 25 | 26 | ||
| Size (mean ± SD) (cm) | 149† | 8.3 ± 3.6 | 6.7± 3.7 | 9.2 ± 3.4 | 0.02 |
| Gross appearance | 151† | ||||
| Fungating/ulcerofungating | 18 | 52 | 70 | ||
| Ulcerating/ulceroinfiltrative | 17 | 55 | 72 | 0.23 | |
| Infiltrative | 0 | 9 | 9 | ||
| Laurén 23 classification | 152 | ||||
| Intestinal | 18 | 65 | 83 | ||
| Diffuse | 1 | 32 | 33 | 0.0002 | |
| Atypical | 16 | 20 | 36 | ||
| Carneiro 25 classification | 151† | ||||
| Glandular | 19 | 52 | 71 | ||
| Isolated cells | 0 | 9 | 9 | 0.05 | |
| Solid | 7 | 11 | 18 | ||
| Mixed | 8 | 45 | 53 | ||
| Ming 24 classification | 150† | ||||
| Expanding | 28 | 44 | 72 | 0.0001 | |
| Infiltrative | 6 | 72 | 78 | ||
| Lymphoid infiltrate | 149† | ||||
| Absent/minimal | 20 | 75 | 95 | 0.50‡ | |
| Moderate/abundant | 14 | 40 | 54 | ||
| Wall invasion | 152 | ||||
| Mucosa+ submucosa | 3 | 13 | 16 | 0.67 | |
| Muscular+ serosa | 32 | 104 | 136 | ||
| Lymph node metastases | 151† | ||||
| Absent | 16 | 32 | 48 | 0.03 | |
| Present | 18 | 85 | 103 | ||
| Vascular invasion | 149† | ||||
| Absent | 14 | 51 | 65 | 0.74 | |
| Present | 20 | 64 | 84 | ||
| pTNM stage | 152 | ||||
| IA | 2 | 10 | 12 | ||
| IB | 14 | 19 | 33 | ||
| II | 4 | 27 | 31 | 0.05 | |
| IIIA | 10 | 41 | 51 | ||
| IIIB | 5 | 15 | 20 | ||
| IV | 0 | 5 | 5 |
*Two operated stomachs.
†The missing cases (1 to 3) were not classifiable for technical reasons.
‡No significant differences were observed either when lymphoid cells were separated into B and T lineage (data not shown).
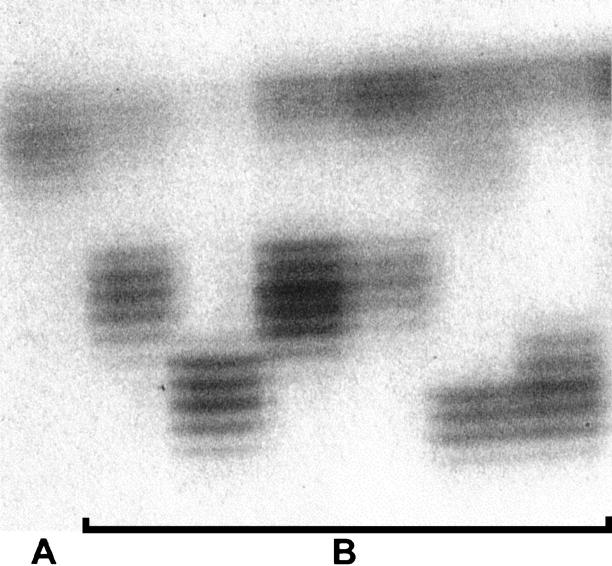
Thirty-five of the 152 tumors (23.0%) were classified as RER+ and 117 as RER− (77.0%). Figure 1 ▶ shows some examples of BAT 26 analysis with unstable shortened alleles. The comparison of the clinicopathological features of the cases with RER+ and RER− carcinomas is summarized in Table 1 ▶ . Patients with RER+ tumors were significantly (P = 0.03) older (64.5 ± 12.8 years) than patients with RER− tumors (59.3 ± 12.1 years). Significant associations were also found between RER+ phenotype and distal location of the tumors (P = 0.0003), Laurén’s 23 intestinal and atypical histotypes (P = 0.0002), Carneiro’s 25 glandular type (P = 0.05), and Ming’s 24 expansive pattern of growth (P = 0.0001). The percentage of cases with lymph node metastases was significantly lower (P = 0.03) in the group of RER+ carcinomas (17.5%) than in the group of RER− carcinomas (82.5%) (Table 1) ▶ .
Figure 1.
A: Example of a case with stable alleles with BAT 26 analysis. B: Examples of cases with unstable shortened alleles in RER+ gastric carcinomas.
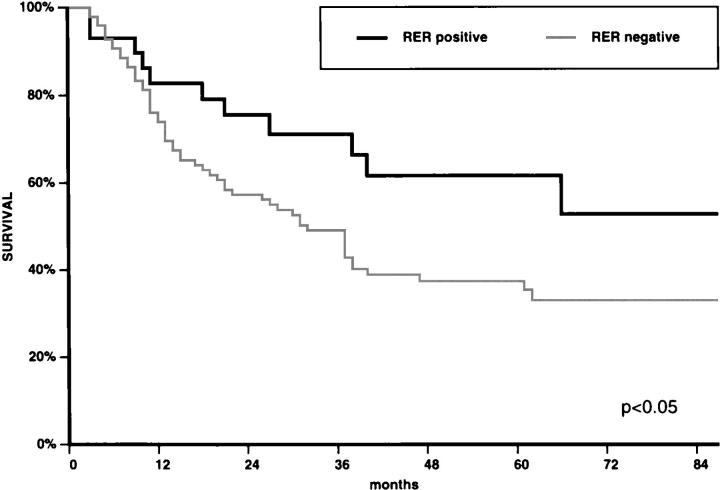
In univariate analysis, the survival curve of patients with RER+ carcinomas was significantly better (P = 0.046) than that of patients with RER− carcinomas (Figure 2) ▶ . The other clinicopathological features significantly associated with the survival of the patients, in univariate analysis, were: Carneiro’s 25 histological classification (P = 0.008), pTNM stage (P = 0.012), and vascular invasion (P = 0.032). The multivariate analysis showed that pTNM stage was the strongest prognostic factor, followed by wall invasion, vascular invasion, Carneiro’s histological classification, and RER status (P = 0.048) (Table 2) ▶ .
Figure 2.
Survival curves of patients with RER+ (n = 31) and RER− (n = 108) gastric carcinomas. For details see text.
Table 2.
Summary of the Results Obtained with the Multivariate Analysis of Prognostic Factors with Cox’s Model in 136 Cases*
| Factors | P value |
|---|---|
| Age | 0.200 |
| Sex | 0.200 |
| Site | 0.169 |
| Size | 0.287 |
| Gross appearance | 0.688 |
| Laurén 23 classification | 0.844 |
| Carneiro 25 classification | 0.023 |
| Ming 24 classification | 0.558 |
| Lymphoid infiltrate | 0.306 |
| Wall invasion | 0.008 |
| Lymph node metastases | 0.124 |
| Vascular invasion | 0.017 |
| pTNM stage | 0.0007 |
| RER status | 0.0481 |
*The following 16 cases were excluded from this analysis: Lost to follow-up, 5 cases; too short follow-up period, 2 cases; postoperative death, 6 cases; and excluded because of missing values, 3 cases. For details see Materials and Methods.
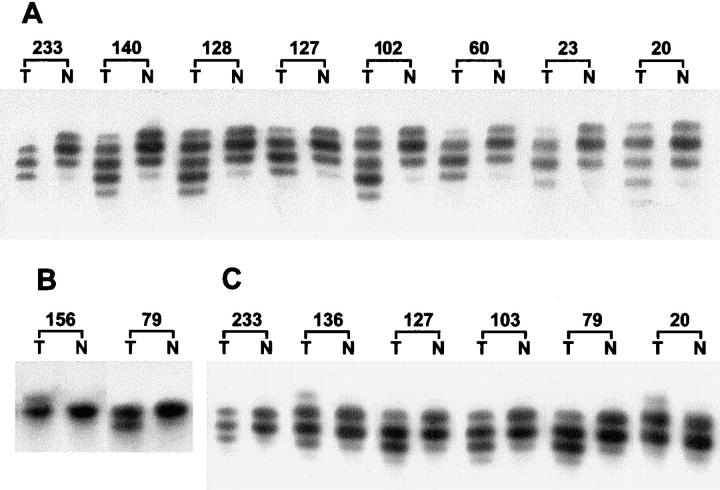
There was a significant association (P = 0.0001) between the RER+ phenotype and mutations in the “target genes.” Twenty-two of the 28 RER+ carcinomas had mutations in one or more of the “target genes” (Figure 3) ▶ , whereas 1 of the 13 RER− carcinomas had mutations in IGFII R and BAX genes (Table 3) ▶ . In the repeat sequence of HPRT gene, no mutations were found in any of the cases. From the 23 tumors that presented mutations in the target genes, 11 tumors (47.8%) displayed mutations in only one gene, and 12 (52.2%) in more than one gene (Table 3) ▶ . TGFβ RII was the most frequently affected gene. Nineteen of the 28 RER+ tumors (67.9%) presented alterations within this gene. Eight of the 19 tumors displayed mutations only in TGFβ RII, whereas in the remaining 11 tumors the mutations in TGFβ RII were associated with mutations in other gene(s) (Table 3) ▶ . In the series of the RER+ tumors, we found that mutations in the TGFβ RII gene were significantly associated (P = 0.02) with the glandular histotype (Table 4) ▶ .
Figure 3.
Representative examples of frameshift mutations detected in mononucleotide tracts of the various coding regions. T, tumor; N, normal. A: Mutations within the poly(A)10 tract of TGFβ RII. B: Mutations within the poly(G)8 tract of IGFII R. C: Mutations within the poly(G)8 tract of BAX.
Table 3.
Summary of the Clinicopathological Features of the 23 Gastric Carcinomas with Mutations in the Microsatellite Sequences of the Coding Regions of TGFβ RII, IGFII R, and BAX genes (All But One Case, No. 79, Displayed the RER+ Phenotype)
| Case no. (n = 23) | Histological type | Lymph node metastasis | Depth of invasion | RER status* | TGFβ RII† poly(A)10 | IGFII R† poly(G)8 | BAX† poly(G)8 |
|---|---|---|---|---|---|---|---|
| Cases with one mutation | |||||||
| 16 | Intestinal | Present | Muscular + serosa | + | −1/W | W | W |
| 23 | Intestinal | Absent | Muscular+ serosa | + | −1/W | W | W |
| 31 | Atypical | Absent | Muscular+ serosa | + | W | −1/W | W |
| 36 | Atypical | Absent | Muscular+ serosa | + | −1/W | W | W |
| 65 | Intestinal | Present | Muscular+ serosa | + | −1/W | W | W |
| 136 | Intestinal | Absent | Mucosa+ submucosa | + | W | W | +1/W |
| 140 | Atypical | Present | Muscular+ serosa | + | −1/W | W | W |
| 183 | Atypical | Present | Muscular+ serosa | + | W | W | +1/W |
| 191 | Intestinal | Present | Muscular+ serosa | + | −1/W | W | W |
| 192 | Atypical | Present | Muscular+ serosa | + | −1/W | W | W |
| 199 | Atypical | Absent | Muscular+ serosa | + | −1/W/+1 | W | W |
| Cases with more than one mutation | |||||||
| 20 | Intestinal | Present | Muscular+ serosa | + | −1/W | W | +1/W |
| 37 | Intestinal | Present | Muscular+ serosa | + | −1/W | −1/W | W |
| 46 | Intestinal | Absent | Muscular+ serosa | + | +1/W | −1/W | W |
| 54 | Intestinal | Absent | Muscular+ serosa | + | −1/W | −1/W | W |
| 60 | Atypical | Absent | Muscular+ serosa | + | −1/W | +1/W | −1/W |
| 79 | Intestinal | Absent | Muscular+ serosa | − | W | −1/W | −1/W |
| 102 | Intestinal | Present | Muscular+ serosa | + | −2/W | −1/W | +1/W |
| 103 | Atypical | Present | Muscular+ serosa | + | −1/W | W | −1/W |
| 127 | Atypical | Present | Muscular+ serosa | + | −1/W | W | −1/W |
| 128 | Intestinal | Present | Muscular+ serosa | + | −2/W | W | −1/W |
| 156 | Intestinal | Absent | Muscular+ serosa | + | −1/W/+1 | +1/W | W |
| 233 | Atypical | Mucosa+ submucosa | + | −1/W | W | −1/W |
*+, microsatellite instability; −, no instability.
†Number of inserted (+) or deleted (−) base pairs on each allele; W, wild type.
Table 4.
Summary of the Clinicopathological Features of 28 RER+ Carcinomas According to the Presence of Mutations in TGFβ RII Gene
| Clinicopathological features | No. of cases | TGFβ RII+ (n = 19) | TGFβ RII− (n = 9) | Total (n = 28) | P value |
|---|---|---|---|---|---|
| Age (mean± SD) | 28 | 63.2± 14.6 | 66.1± 10.8 | 64.5 ± 12.8 | 0.59 |
| Male/female | 28 | 8/11 | 6/3 | 1/1 | 0.23 |
| Location | 27* | ||||
| Antrum | 16 | 8 | 24 | 0.69 | |
| Body | 1 | 1 | 2 | ||
| Cardia | 1 | 0 | 1 | ||
| Size (mean± SD) (cm) | 28 | 9.7 ± 3.6 | 8.2± 3.0 | 9.2 ± 3.4 | 0.31 |
| Gross appearance | 28 | ||||
| Fungating/ulcerofungating | 10 | 5 | 15 | 0.89 | |
| Ulcerating/ulceroinfiltrative | 9 | 4 | 13 | ||
| Infiltrative | 0 | 0 | 0 | ||
| Laurén 23 classification | 28 | ||||
| Intestinal | 11 | 2 | 13 | 0.10 | |
| Diffuse | 0 | 1 | 1 | ||
| Atypical | 8 | 6 | 14 | ||
| Carneiro 25 classification | 27* | ||||
| Glandular | 12 | 2 | 14 | ||
| Isolated cells | 0 | 0 | 0 | 0.02 | |
| Solid | 1 | 4 | 5 | ||
| Mixed | 6 | 2 | 8 | ||
| Ming 23 classification | 27* | ||||
| Expanding | 16 | 5 | 21 | 0.32 | |
| Infiltrative | 3 | 3 | 6 | ||
| Lymphoid infiltrate | 27* | ||||
| Absent/minimal | 11 | 5 | 16 | >0.99† | |
| Moderate/abundant | 8 | 3 | 11 | ||
| Wall invasion | 28 | ||||
| Mucosa+ submucosa | 1 | 1 | 2 | 0.55 | |
| Muscular+ serosa | 18 | 8 | 26 | ||
| Lymph node metastases | 28 | ||||
| Absent | 7 | 3 | 10 | >0.99 | |
| Present | 12 | 6 | 18 | ||
| Vascular invasion | 27* | ||||
| Absent | 7 | 3 | 10 | >0.99 | |
| Present | 12 | 5 | 17 | ||
| pTNM stage | 28 | ||||
| IA | 0 | 1 | 1 | ||
| IB | 7 | 2 | 9 | ||
| II | 4 | 0 | 4 | 0.27 | |
| IIIA | 6 | 3 | 9 | ||
| IIIB | 2 | 3 | 5 | ||
| IV | 0 | 0 | 0 |
*One operated stomach.
†No significant differences were observed either when lymphoid cells were separated into B and T lineage (data not shown).
IGFII R was mutated in 7 of the 28 RER+ tumors (25.0%). Just 1 tumor showed mutation in IGFII R as a sole alteration; the remaining 6 cases presented mutations in IGFII R in association with any of the other two target genes (Table 3) ▶ . A suggestive association (P = 0.06) was found in the setting of RER+ cases between IGFII R mutations and lower prevalence of lymph node metastases: RER+ carcinomas with IGFII R mutations had lower prevalence of lymph node metastases (11.8%) than RER+ carcinomas without mutations in this gene (88.2%).
BAX mutations were present in 9 of the 28 RER+ tumors (32.1%). Two of the 9 tumors had BAX mutations as a sole alteration; the remaining 7 tumors presented mutations in BAX gene in association with mutations in TGFβ RII and/or IGFII R genes. We observed a significantly higher (P = 0.005) percentage of females in the group of cases with BAX mutations (77.8%) than in the group of cases without BAX mutations (31.6%). A suggestive association (P = 0.09) was found between BAX mutations and the degree of wall invasion: 2 of the 9 cases with mutations in BAX were limited to the superficial layers (T1 or T2) of the gastric wall, whereas all 19 carcinomas without BAX mutations invaded the deep layers (T3 or T4) of the stomach.
No other associations were found between the clinicopathological features of the tumors and IGFII R or BAX mutations (data not shown).
Discussion
We detected an RER+ phenotype in 23.0% of the 152 unselected sporadic cases of gastric carcinoma. This percentage fits with those previously reported in the literature, which vary from 9 to 25%. 5,6,8,12,13,27-29
The present study of 35 RER+ sporadic gastric carcinomas confirms most of the clinicopathological data previously obtained in a series of 12 RER+ carcinomas. 5 RER+ gastric carcinomas tend to occur as large and expanding tumors of the distal stomach in relatively old patients; they display usually an intestinal (glandular) or atypical (solid) histotype and often do not give rise to lymph node metastases, regardless of the degree of wall invasion (they usually occur with low pTNM stages). Similar clinicopathological features had been previously pointed out in RER+ carcinomas of other organs. 2 The striking differences between RER+ and RER− carcinomas with regard to site and histotype of the tumors (RER+ carcinomas are extremely rare in the cardia and almost never display a pure diffuse (isolated cell) pattern) support the assumption that the etiopathogenesis of cardiac and diffuse carcinomas of the stomach differ from those of antral and intestinal types. 29-32
At variance with our previous results, 5 we did not find, in the present study, a significantly higher lymphoid infiltration in RER+ carcinomas, despite a trend to more abundant lymphoid cells in this group of tumors. We also did not find any significant difference between the two groups of carcinomas regarding the abundance and relative distribution of B and T lymphocytes (data not shown). It remains therefore questionable whether unrepaired errors of certain genes in the setting of RER phenotype may lead to the appearance of new surface molecules and, in this way, trigger an immune response. 5,33
In keeping with most of the on-record studies in gastric and colorectal carcinomas, 5,26,34 the RER status was found to be significantly related to survival in univariate analysis. We have shown, moreover, for the first time, to the best of our knowledge, that the same holds true regarding multivariate analysis. The significant association between RER+ phenotype and low prevalence of lymph node metastasization and low pTNM stages indicates that the good outcome of patients with RER+ carcinomas may be ascribed, partly at least, to the close relationship between RER status and staging.
The search for mutations in the three target genes, TGFβ RII, IGFII R, and BAX, yielded positive results, in contrast to the absence of mutations in HPRT, a constitutional gene present in all types of cells. This finding supports the assumption that only mutations in target genes that have a direct role in carcinogenesis confer a clonal advantage to the neoplastic cells. 16 Mutations in the target genes were almost exclusively detected in RER+ tumors, thus confirming that the involvement of such genes is most likely due to a mismatch repair deficiency.
The higher incidence of mutations in TGFβ RII gene suggests that the alterations of the TGFβ RII gene occur as an earlier event than those of IGFII R or BAX gene in gastric carcinogenesis. We found just one case with mutations in IGFII R and BAX in the control series composed of RER− tumors. Similar findings were reported by Renault et al, 29 Myeroff et al, 28 and Akiyama et al. 10 As in the series of Ouyang et al, 8 the RER− tumor of our series with mutations of IGFII R and BAX genes is an advanced tumor (T3); this finding points to the possibility that such mutations may represent a genetic change occurring during progression, rather than a crucial event in the early steps of tumor development.
In all cases with mutations, we found bands corresponding to the wild-type sequences of the target genes; however, in most of the primary tumors, the band representing the wild-type allele was decreased in intensity, thus suggesting that both alleles were mutant (the residual wild-type signal probably arose from contaminating nonneoplastic cells within the tumor specimens). This assumption was confirmed in xenografts derived from cases 199 and 233 of our series (data not shown), as it had been previously shown in colon cancer. 16,28 Alternatively, heterozygous mutations may contribute to tumor progression, as it was described for TGFβ RII. 35 Rampino et al 11 also suggested that reduction of wild-type BAX, because of the inactivation of one allele, facilitates escape from apoptosis by diminishing the BAX-BCL2 ratio.
In the present study, TGFβ RII was affected by mutations in 67.9% of the 28 RER+ carcinomas, a percentage that fits within the range of those on record (50 to 92.4%). 8,12,13,28,29,35,36 Because the TGFβ RII poly(A)10 tract is a mutation target in cells with genetic instability, it is tempting to advance the existence of a carcinogenic pathway in which TGFβ RII mutations would confer growth advantage, and would be selected for, in RER+ gastric carcinomas. 12,13,37-39 The significant association between TGFβ RII mutations and the RER+ phenotype fits with this possibility. Our finding of a close relationship between TGFβ RII mutations and the glandular histotype supports, moreover, the role played by TGFβ/TGFβ RII in the development of glandular-type gastric carcinoma, in a similar way as in colon cancer. 12,15,28,40
We found IGFII R mutations in 25% of the RER+ sporadic gastric carcinomas. This percentage is similar to those reported by other groups (range, 23 to 33%). 8,9,12,13 We observed, furthermore, a trend toward an association between mutations in IGFII R and low prevalence of lymph node metastases. We still do not know the meaning of this finding, although it fits with the association between RER phenotype and less clinically aggressive tumors. Curiously, the only IGFII R mutated tumor with an RER− phenotype in our series did not display lymph node metastases despite invading the wall of the stomach widely (data not shown). Our results are in accordance with the role on cell motility and metastasization advanced by Minniti et al 41 for IGFII R in rhabdomyosarcoma cells. A larger series is necessary to confirm whether or not there is a relationship between IGFII R mutations and decreased nodal metastatic ability of gastric carcinoma.
BAX mutations were detected in 32.1% of the RER+ sporadic gastric carcinomas. This percentage is lower than that observed previously in colon cancer 11 and lies in between those reported by Chung et al 12 in a series of 6 gastric carcinomas (66%) and by Wu et al 13 in a series of 13 gastric carcinomas (15.4%). We found a significantly larger percentage of cases with BAX mutations in women than in men. We do not know the reason behind this finding, which cannot be linked to a particular cancer histotype, because it is known that women tend to have diffuse (isolated cell) carcinoma, 25 and this type of gastric carcinoma is extremely rare in the setting of RER+ phenotype in most of the series on record. 5,6,13,29,36,42-44 We also found evidence suggesting a putative association (P = 0.09) between the presence of BAX mutations and diminished penetration of the gastric wall by the neoplastic cells; this finding fits with the overall impression on the low clinical aggressiveness of many RER+ carcinomas, but the association is too weak and the series is too small to allow a definitive conclusion on this issue. In conclusion, this retrospective study demonstrates that RER status is a significant prognostic indicator in gastric carcinoma. It shows, moreover, that such prognostic meaning may be mediated by mutations in several “target genes” exhibiting microsatellite instability.
Footnotes
Address reprint requests to Dr. Manuel Sobrinho-Simões, Institute of Molecular Pathology and Immunology of the University of Porto, Rua Dr. Roberto Frias s/n, 4200 Porto, Portugal.
Supported by PRAXIS XXI (project 2/2.1/BIA/250/94).
References
- 1.Hoang JM, Cottu PH, Thuille B, Salmon RJ, Thomas G, Hamelin R: BAT-26, an indicator of the replication error phenotype in colorectal cancers cell lines. Cancer Res 1997, 57:300-303 [PubMed] [Google Scholar]
- 2.Thibodeau SN, Bren G, Schaid D: Microsatellite instability in cancer of the proximal colon. Science 1993, 260:816-819 [DOI] [PubMed] [Google Scholar]
- 3.Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M: Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 1993, 363:558-561 [DOI] [PubMed] [Google Scholar]
- 4.Risinger JI, Umar A, Barrett JC, Kunkel TA: A hPMS2 mutant cell line in defective in strand-specific mismatch repair. J Biol Chem 1995, 270:18183-18186 [DOI] [PubMed] [Google Scholar]
- 5.Santos NR, Seruca R, Constância M, Seixas M, Sobrinho-Simões M: Microsatellite instability at multiple loci in gastric carcinoma: clinicopathologic implications and prognosis. Gastroenterology 1996, 110:38-44 [DOI] [PubMed] [Google Scholar]
- 6.Seruca R, Santos NR, David L, Constância M, Barroca H, Carneiro F, Seixas M, Peltomäki P, Lothe R, Sobrinho-Simões M: Sporadic gastric carcinomas with microsatellite instability display a particular clinicopathologic profile. Int J Cancer 1995, 64:32-36 [DOI] [PubMed] [Google Scholar]
- 7.Lu S-L, Zhang W-C, Akiyama Y, Nomizu T, Yuasa Y: Genomic structure of the transforming growth factor β type II receptor gene and its mutations in hereditary nonpolyposis colorectal cancers. Cancer Res 1996, 56:4595-4598 [PubMed] [Google Scholar]
- 8.Ouyang H, Shiwaku HO, Hagiwara H, Miura K, Abe T, Kato Y, Ohtani H, Shiiba K, Souza RF, Meltzer SJ, Horii A: The insulin-like growth factor II receptor gene is mutated in genetically unstable cancers of the endometrium, stomach, and colorectum. Cancer Res 1997, 57:1851-1854 [PubMed] [Google Scholar]
- 9.Souza RF, Appel R, Yin J, Wang S, Smolinski KN, Abraham JM, Zou T-T, Shi Y-Q, Lei J, Cottrell J, Cymes K, Biden K, Simms L, Leggett B, Lynch PM, Frazier M, Powell SM, Harpaz N, Sugimura H, Young J, Meltzer SJ: Microsatellite instability in the insulin-like growth factor II receptor gene in gastrointestinal tumours. Nat Genet 1996, 14:255-257 [DOI] [PubMed] [Google Scholar]
- 10.Akiyama Y, Iwanaga R, Saitoh K, Shiba K, Ushio K, Ikeda E, Iwama T, Nomizu T, Yuasa Y: Transforming growth factor β type II receptor gene mutations in adenomas from hereditary nonpolyposis colorectal cancer. Gastroenterology 1997, 112:33-39 [DOI] [PubMed] [Google Scholar]
- 11.Rampino N, Yamamoto H, Ionov Y, Li Y, Sawai H, Reed JC, Perucho M: Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science 1997, 275:967-969 [DOI] [PubMed] [Google Scholar]
- 12.Chung Y-J, Park S-W, Song J-M, Lee K-Y, Seo E-J, Choi S-W, Rhyu M-G: Evidence of genetic progression in human gastric carcinomas with microsatellite instability. Oncogene 1997, 15:1719-1726 [DOI] [PubMed] [Google Scholar]
- 13.Wu M-S, Lee C-W, Shun C-T, Wang H-P, Lee W-J, Sheu J-C, Lin J-T: Clinicopathological significance of altered loci of replication error and microsatellite instability-associated mutations in gastric cancer. Cancer Res 1998, 58:1494-1497 [PubMed] [Google Scholar]
- 14.Souza RF, Lei J, Yin J, Appel R, Zou T-T, Zhou X, Wang S, Rhyu M-G, Cymes K, Chan O, Park W-S, Krasna MJ, Greenwald BD, Cottrell J, Abraham JM, Simms L, Leggett B, Young J, Harpaz N, Meltzer SJ: A transforming growth factor β1 receptor type II mutation in ulcerative colitis-associated neoplasms. Gastroenterology 1997, 112:40-45 [DOI] [PubMed] [Google Scholar]
- 15.Markowitz S, Wang J, Myeroff L, Parsons R, Sun L-Z, Lutterbaugh J, Fan RS, Zborowska E, Kinzler KW, Vogelstein B, Brattain M, Willson JKV: Inactivation of the type II TGFβ receptor in colon cancer cells with microsatellite instability. Science 1995, 268:1336-1338 [DOI] [PubMed] [Google Scholar]
- 16.Parsons R, Myeroff LL, Liu B, Willson JKV, Markowitz SD, Kinzler KW, Vogelstein B: Microsatellite instability and mutations of the transforming growth factor β type II receptor gene in colorectal cancer. Cancer Res 1995, 55:5548-5550 [PubMed] [Google Scholar]
- 17.Polyak K: Negative regulation of the cell growth by TGFβ. Biochim Biophys Acta 1996, 1242:185-199 [DOI] [PubMed] [Google Scholar]
- 18.Taetle R, Payne C, Santos BD, Russell M, Segarini P: Effects of transforming growth factor β1 on growth and apoptosis of human acute myelogenous leukemia cells. Cancer Res 1993, 53:3386-3393 [PubMed] [Google Scholar]
- 19.Jürgensmeier JM, Schmitt CP, Viesel E, Höfler P, Bauer G: Transforming growth factor-β-treated normal fibroblasts eliminate transformed fibroblasts by induction of apoptosis. Cancer Res 1994, 54:393-398 [PubMed] [Google Scholar]
- 20.Kornfeld S: Structure and function of the mannose 6-phosphate/insulin-like growth factor type II receptors. Annu Rev Biochem 1992, 61:307-330 [DOI] [PubMed] [Google Scholar]
- 21.Kapucuoglu N, Losi L, Eusebi V: Immunohistochemical localization of Bcl2 and Bax proteins in in situ and invasive duct breast carcinomas. Virchows Arch 1997, 430:17-22 [DOI] [PubMed] [Google Scholar]
- 22.Selvakumaran M, Lin H-K, Miyashita T, Wang HG, Krajewski S, Reed JC, Hoffman B, Leibermann D: Immediate early up-regulation of bax expression by p53 but not TGFβ1: a paradigm for distinct apoptosis pathways. Oncogene 1994, 9:1791-1798 [PubMed] [Google Scholar]
- 23.Laurén P: The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma: an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 1965, 64:31-49 [DOI] [PubMed] [Google Scholar]
- 24.Ming S-C: Gastric carcinoma: a pathobiological classification. Cancer 1977, 39:2475-2485 [DOI] [PubMed] [Google Scholar]
- 25.Carneiro F: Classification of gastric carcinomas. Curr Diagn Pathol 1997, 4:51-59 [Google Scholar]
- 26.Mullënbach R, Lagoda PJL, Welter C: An efficient salt-chloroform extraction of DNA from blood and tissues. Trends Genet 1989, 5:391. [PubMed] [Google Scholar]
- 27.Hayden JD, Cawkwell L, Quirke P, Dixon MF, Goldstone AR, Sue-Ling H, Johnston D, Martin IG: Prognostic significance of microsatellite instability in patients with gastric carcinoma. Eur J Cancer 1997, 33:2342-2346 [DOI] [PubMed] [Google Scholar]
- 28.Myeroff LL, Parsons R, Kim S-J, Hedrick L, Cho KR, Orth K, Mathis M, Kinzler KW, Lutterbaugh J, Park K, Bang Y-J, Lee HY, Park J-G, Lynch HT, Roberts AB, Vogelstein B, Markowitz SD: A transforming growth factor β receptor type II gene mutation common in colon and gastric but rare in endometrial cancers with microsatellite instability. Cancer Res 1995, 55:5545-5547 [PubMed] [Google Scholar]
- 29.Renault B, Calistri D, Buonsanti G, Nanni O, Amadori D, Ranzani GN: Microsatellite instability and mutations of p53 and TGF-β RII genes in gastric cancer. Hum Genet 1996, 98:601-607 [DOI] [PubMed] [Google Scholar]
- 30.Powell J, McConkey CC: Increasing incidence of adenocarcinoma of the gastric cardia and adjacent sites. Br J Cancer 1990, 62:440-443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tahara E: Molecular mechanism of stomach carcinogenesis. J Cancer Res Clin Oncol 1993, 119:265-272 [DOI] [PubMed] [Google Scholar]
- 32.Correa P, Shiao Y: Phenotypic and genotypic events in gastric carcinogenesis. Cancer Res 1994, 54(Suppl):1941-1943 [PubMed] [Google Scholar]
- 33.Bodmer W, Bishop T, Karran P: Genetic steps in colorectal cancer. Nat Genet 1994, 6:217-219 [DOI] [PubMed] [Google Scholar]
- 34.Kim HG, Jen J, Vogelstein B, Hamilton SR: Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol 1994, 145:148-156 [PMC free article] [PubMed] [Google Scholar]
- 35.Chang J, Park K, Bang Y-J, Kim WS, Kim D, Kim S-J: Expression of transforming growth factor β type II receptor reduces tumorigenicity in human gastric cancer cells. Cancer Res 1997, 57:2856-2859 [PubMed] [Google Scholar]
- 36.Chung Y-J, Song J-M, Lee J-Y, Jung Y-T, Seo E-J, Choi S-W, Rhyu M-G: Microsatellite instability-associated mutations associate preferentially with the intestinal type of primary gastric carcinomas in a high-risk population. Cancer Res 1996, 56:4662-4665 [PubMed] [Google Scholar]
- 37.Shibata D, Peinado MA, Ionov Y, Malkhosyan S, Perucho M: Genomic instability in repeated sequences is an early somatic event in colorectal tumorigenesis that persists after transformation. Nat Genet 1994, 6:273-281 [DOI] [PubMed] [Google Scholar]
- 38.Aaltonen LA, Peltomäki P, Leach FS, Sistonen P, Pylkkänen L, Mecklin J-P, Järvinen H, Powell SM, Jen J, Hamilton RS, Petersen GM, Kinzler KW, Vogelstein B, de la Chapelle A: Clues to the pathogenesis of familial colorectal cancer. Science 1993, 260:812-816 [DOI] [PubMed] [Google Scholar]
- 39.Young J, Leggett B, Gustafson C, Ward M, Searle J, Thomas L, Buttenshaw R, Chenevix-Trench G: Genomic instability occurs in colorectal carcinomas but not in adenocarcinomas. Hum Mutat 1993, 2:351-354 [DOI] [PubMed] [Google Scholar]
- 40.Boivin GP, Molina JR, Ormsby I, Stemmermann G, Doetschman T: Gastric lesions in transforming growth factor β-1 heterozygous mice. Lab Invest 1996, 74:513-518 [PubMed] [Google Scholar]
- 41.Minniti CP, Kohn EC, Grubb JH, Sly WS, Oh Y, Müller HL, Rosenfeld RG, Helman LJ: The insulin-like growth factor II (IGF-II)/mannose 6-phosphate receptor mediates IGF-II-induced motility in human rhabdomyosarcoma cells. J Biol Chem 1992, 267:9000-9004 [PubMed] [Google Scholar]
- 42.Keller G, Rotter M, Vogelsang H, Bischoff P, Becker K-F, Mueller J, Brauch H, Siewert J-R, Hofler H: Microsatellite instability in adenocarcinomas of the upper gastrointestinal tract: relation to clinicopathological data and family history. Am J Pathol 1995, 147:593-600 [PMC free article] [PubMed] [Google Scholar]
- 43.Ottini L, Palli D, Falchetti M, D’Amico C, Amorosi A, Saieva C, Calzolari A, Cimoli F, Tatarelli C, Marchis L, Masala G, Mariani-Constantini R, Cama A: Microsatellite instability in gastric cancer is associated with tumor location and family history in a high-risk population from Tuscany. Cancer Res 1997, 57:4523-4529 [PubMed] [Google Scholar]
- 44.Lin J-T, Wu M-S, Shun C-T, Lee W-J, Sheu J-C, Wang T-H: Occurrence of microsatellite instability in gastric carcinoma is associated with enhanced expression of erbB-2 oncoprotein. Cancer Res 1995, 55:1428-1430 [PubMed] [Google Scholar]