Abstract
Using two models of acute lung inflammatory injury in rats (intrapulmonary deposition of immunoglobulin G immune complexes and systemic activation of complement after infusion of purified cobra venom factor), we have analyzed the requirements and patterns for upregulation of lung vascular P-selectin. In the immune complex model, upregulation of P-selectin was defined by Northern and Western blot analysis of lung homogenates, by immunostaining of lung tissue, and by vascular fixation of 125I-labeled anti-P-selectin. P-selectin protein was detected by 1 hour (long before detection of mRNA) and expression was sustained for the next 7 hours, in striking contrast to the pattern of P-selectin expression in the cobra venom factor model, in which upregulation was very transient (within the 1st hour). In the immune complex model, injury and neutrophil accumulation were P-selectin dependent. Upregulation of P-selectin was dependent on an intact complement system, and the presence of blood neutrophils was susceptible to the antioxidant dimethyl sulfoxide and required C5a but not tumor necrosis factor α. In contrast, in the cobra venom factor model, upregulation of P-selectin, which is C5a dependent, was also dimethyl sulfoxide sensitive but neutrophil independent. Different mechanisms that may explain why upregulation of lung vascular P-selectin is either transient or sustained are discussed.
In the acute inflammatory response in lung triggered by intra-alveolar deposition of immunoglobulin (Ig) G immune complexes, considerable gene activation occurs, resulting in engagement of a complicated array of mediator pathways. Activation of complement in the distal airway compartment results in generation of the anaphylatoxin C5a and is the prelude to expression of the early response cytokines (tumor necrosis factor (TNF)-α and interleukin-1). In turn, these cytokines are required for upregulation of lung vascular intercellular adhesion molecule (ICAM)-1 and E-selectin and for the subsequent recruitment of neutrophils. 1-3 The process of neutrophil recruitment also involves L-selectin, ICAM-1, and the β2 integrins CD11a/CD18 (leukotactic factor activity) and CD11b/CD18 (Mac-1). 2,4-7 Based on the use of selectin-IgG chimeric molecules, it has been suggested that P-selectin is not required for neutrophil recruitment in this inflammatory model. 8 This contrasts with lung injury induced by intravenous infusion of cobra venom factor (CVF) in which P-selectin plays an important and early role, based on findings with a cross-reactive anti-human P-selectin monoclonal antibody and the use of human P-selectin-IgG. 8,9 In the CVF inflammatory model, upregulation of lung vascular P-selectin is transient, dependent on availability of C5a, and platelet independent. 10 Mutant mice lacking the ability to express P-selectin display a series of defective inflammatory responses, including inflammatory peritoneal exudates and delayed-type hypersensitivity reactions, 11 although other studies suggest that the full development of peritoneal inflammatory exudates requires both L- and P-selectin. 12
In the following studies, we sought to explore the expression and role of P-selectin in inflammatory injury after intrapulmonary deposition of IgG immune complexes or CVF-induced systemic complement activation in rats using a monoclonal antibody to rat P-selectin. The data to be presented reveal that lung vascular upregulation of P-selectin in the immune complex model occurs early and is persistent over many hours. This upregulation is complement and neutrophil dependent and can be suppressed in the presence of the antioxidant dimethyl sulfoxide (DMSO). P-selectin blockade suppresses neutrophil recruitment and full development of injury in this model. The pattern of persistent expression of P-selectin upregulation is strikingly different from that found in the model of lung injury after systemic activation of complement. These data suggest that there may be different pathways that bring about in vivo upregulation of lung vascular P-selectin.
Materials and Methods
IgG Immune Complex-Induced Alveolitis
Male Long Evans (specific pathogen-free) rats (275 to 300 g, Charles River Breeding Laboratories, Inc., Portage, MI) were used in all studies. Intraperitoneal injections of ketamine (10 to 15 mg/100 g body weight) were given for sedation and anesthesia. Polyclonal rabbit IgG containing 2.5 mg of anti-bovine serum albumin purchased from Organon Teknika Corp. (West Chester, PA) was instilled (in 300 μl phosphate-buffered, pH 7.4, saline (PBS)) into the lungs via a tracheal cannula. Control animals received 2.5 mg of nonspecific rabbit IgG intratracheally. Ten mg of bovine serum albumin (together with trace amounts of 125I-labeled bovine serum albumin) was injected intravenously (in 0.5 ml of PBS) immediately after the intratracheal injections. The rats were sacrificed 4 hours later, unless otherwise indicated, and the permeability index was calculated as described elsewhere. 2
CVF Model of Acute Lung Injury
Animals were anesthetized as described above. CVF was isolated from lyophilized Naja kaouthia venom (Sigma Chemical Co., St. Louis, MO) by ion exchange and gel filtration chromatography. 13 CVF (3.5 units) diluted in a final volume of 0.3 ml of PBS was given in a bolus intravenous injection. Control animals received PBS only. Sacrifice of rats occurred at 30 minutes after the injections.
Measurement of Lung Vascular P-Selectin
As a marker for the expression of P-selectin, 5 μl (5 μg) of 125I-labeled mouse monoclonal antibody (IgG1) to human P-selectin (PB1.3) (Cytel Corp., San Diego, CA) or an irrelevant subclass-matched murine Ig, 125I-labeled MOPC-21, was injected intravenously 15 minutes before sacrifice. This antibody was used to make comparisons with previously published data dealing with upregulation of lung vascular P-selectin after systemic activation of complement. 10 At the time of sacrifice, 1.0 ml of blood was drawn from the posterior vena cava, and the lung vasculature was flushed through the pulmonary artery with 10 ml of PBS. The ratio of radioactivity remaining in the lung to that contained in the blood sample represented fixation of monoclonal antibody to rat lung (binding index). 125I-labeled MOPC-21 served as an indicator of nonspecific fixation (or leakage) of antibody to (from) the lung vasculature. Sacrifice of rats in the CVF model of lung injury occurred at 30 minutes after CVF injection (unless otherwise indicated), whereas in the IgG immune complex model, rats were killed at various time points (up to 8 hours) after immune complex deposition.
Immunostaining for Lung Vascular P-Selectin
Frozen sections of lung tissue were prepared as described previously. 1,2 Samples were fixed in methanol at −20°C for 10 minutes before immunostaining. Anti-P-selectin mouse monoclonal antibody (ARP2-4) (dilution 1/250 in PBS) was incubated at room temperature in a humidified chamber for 60 minutes. 14,15 Slides were washed in PBS and incubated for 60 minutes with peroxidase-conjugated goat anti-mouse IgG (Sigma) diluted 1/10,000 in PBS. P-selectin was visualized using diaminobenzidine substrate (Kirkegaard and Perry Inc., Gaithersburg, MD), and the tissues were counterstained with hematoxylin. Samples were examined by light microscopy.
Effects of DMSO or Neutrophil Depletion on Upregulation of Lung Vascular P-Selectin
To assess the extent to which upregulation of lung vascular P-selectin may be related to requirement for oxidants, 1.0 ml of DMSO (Burdick & Jackson Laboratories, Inc., Muskegon, MI) was injected intraperitoneally 10 minutes before initiation of lung inflammatory reactions. This protocol has been shown to be protective against oxidant-induced lung injury. 16,17 The protective effects of DMSO under such conditions appear to be related to its ability to function as a scavenger of the hydroxyl radical, 18-21 which has been incriminated as a toxic oxygen metabolite involved in neutrophil-mediated lung injury as well as in neutrophil-mediated injury in vitro of endothelial cells. 22-25 Neutrophil depletion was induced by the intraperitoneal injection of 0.5 ml of rabbit serum to rat neutrophils (Accurate Chemical & Scientific Corp., Westbury, NY) 12 hours before the onset of injury. Both models of lung injury are known to be neutrophil dependent. 16,17,26 The neutrophil counts in normal rat blood (2.21 × 103/mm3) dropped by 92% in animals treated with this antiserum, whereas there were no significant changes in counts of blood mononuclear cells and platelets (data not shown).
Requirements for Complement, C5a, and TNF-α in Upregulation of Lung Vascular P-Selectin
The requirements for complement, C5a, and TNF-α in upregulation of lung vascular P-selectin were determined. Complement depletion was induced by repeated intraperitoneal injections of CVF. Twenty units of CVF were given 36, 24, and 12 hours before the onset of injury. 9,10 The requirements for TNF-α and C5a were determined by intratracheal instillation (at the onset of injury) of 400 μg of rabbit IgG reactive with TNF-α or C5a. 27
Western Blot Analysis of Homogenates from Whole Lung
The IgG immune complex model was used as described above. At the times indicated, the lungs were flushed with 10 ml of PBS through the pulmonary artery, surgically dissected, and immediately frozen in liquid nitrogen. Mechanical homogenization at 5°C was performed using a homogenization buffer containing 0.6% Nonidet P-40, 10 mmol/L HEPES, pH 7.9, 150 mmol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid, and 0.5 mmol/L phenylmethylsulfonylfluoride (Sigma). The tissue homogenates were sonicated for 30 seconds and centrifuged at 2500 rpm for 30 minutes at 4°C. The resulting supernatant fluids were used for analysis. To load equal amounts of protein onto the gel, a bicinchoninic protein assay (Pierce Co., Rockford, IL) was performed. The samples were analyzed on a 7.5% sodium dodecyl sulfate polyacrylamide gel electrophoresis (National Diagnostics, Inc., Atlanta, GA) together with a high molecular weight standard (Life Technologies, Inc., Gaithersburg, MD). The gel was transferred on Westran polyvinylidene difluoride membrane (Schleicher & Schuell Co., Keene, NH) using protocols described elsewhere (R & D Systems, Inc., Minneapolis, MN). The blot was blocked overnight in 5% bovine powdered milk at 4°C. The primary reagent, monoclonal antibody to rat P-selectin (described below), was added at a 1:1000 dilution in 1% milk and incubated for 1 hour at room temperature. The blot was washed three times for 5 minutes each in Tris-buffered saline containing 0.5% polyoxyethylene sorbitan monolaurate (Tween 20) (Sigma). Immunostaining was performed using rabbit anti-mouse IgG horseradish peroxidase conjugate (Amersham, Inc., Arlington Heights, IL) at a 1:20,000 dilution in 1% milk. After a 1-hour incubation, the blot was washed three times for 5 minutes each in Tris-buffered saline containing 0.5% Tween 20. The blot was put on plastic foil and covered with enhanced chemiluminescence (Amersham). Western blotting detection reagents were applied for 1 minute. The fluids were poured off the membrane, and the blot was wrapped in plastic foil and placed into a film cassette. Hyperfilm (Amersham) was exposed overnight.
Northern Blot Analysis of mRNA for P-Selectin and TNF-α
Acute lung injury was induced in rats by IgG immune complex deposition as described above. Rats were sacrificed at the times indicated after induction of injury. Total RNA was isolated from lungs using acid-guanidium-phenol-chloroform. Fifteen μg of RNA were loaded per lane and subjected to electrophoretic separation on a 1% agarose/formaldehyde gel. The RNA was then transferred onto a GeneScreen nylon membrane (NEN, Inc., Wilmington, DE) by the capillary method. Before hybridization, the transferred RNA was cross-linked to the membrane by ultraviolet-light irradiation. Hybridization was performed overnight at 42°C in a buffer containing 50% formamide, 5× standard saline sulfate (750 nmol/L NaCl and 75 mmol/L sodium citrate), 1% sodium dodecyl sulfate, 2× Denhardt’s solution, and 0.1 mg/ml denatured salmon sperm DNA. 32P-labeled cDNA probes for rat P-selectin and mouse TNF-α as well as human β-actin were then applied. The cDNA probe for rat P-selectin is described in recent reports. 14,15 After the hybridization, the filter was washed twice in 2× standard saline sulfate at room temperature for 10 minutes followed by addition of 2× standard saline sulfate and 0.1% sodium dodecyl sulfate at 65°C for 30 minutes. Then the filter was exposed to X-ray film.
Lung Neutrophil Content
The neutrophil content of lung was measured as neutrophil counts in bronchoalveolar lavage (BAL) fluids. After sacrifice, 5 ml of sterile PBS was instilled into the lungs via an intratracheal cannula. The liquid was aspirated, and the procedure was repeated twice. BAL fluids were centrifuged for 15 minutes at 1500 rpm. NaCl (0.9%) was added to the samples to reproduce the initial volumes of the BAL fluids. Ten μl of each sample were analyzed using conventional microcytometry.
Effects on IgG Immune Complex-Induced Lung Injury by Anti-P-Selectin
Together with the bovine serum albumin, 400 μg of mouse monoclonal antibody against rat-P-selectin (ARP2-4) (Sumitomo Pharmaceuticals, Inc., Osaka, Japan) 14,15 were injected intravenously at the indicated time points. Companion positive control animals received 400 μg of MOPC-21 instead of ARP2-4.
Statistical analysis
All values were expressed as mean ± standard error of the mean, unless otherwise indicated. Data sets were examined with one- or two-way analysis of variance, and individual group means were then compared by Student’s t-test.
Results
Upregulation of Lung Vascular P-Selectin in IgG Immune Complex-Induced Lung Injury
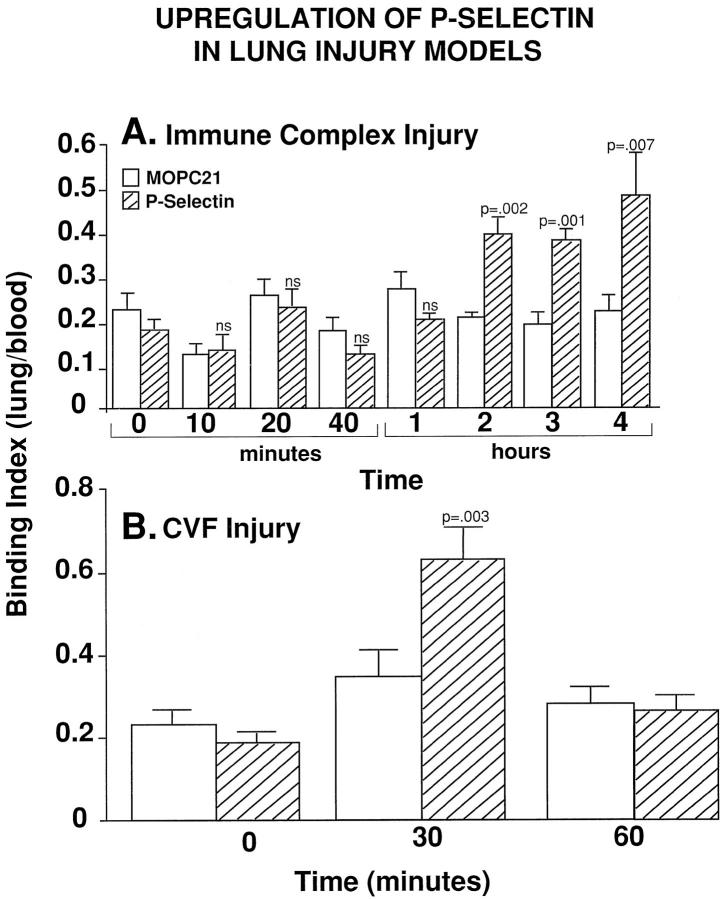
Using fixation (expressed as the binding index) to lung of 125I-labeled monoclonal antibody to P-selectin, significant upregulation of P-selectin was found at 2 hours (P = 0.002), persisting at 3 hours (P = 0.001) and 4 hours (P = 0.007) after initiation of lung inflammatory reactions (Figure 1A) ▶ . By 4 hours, the binding index had risen by 2.6-fold when compared with the value obtained at time 0. No significant increase in binding of 125I-labeled MOPC was observed at any of the time points studied (Figure 1A) ▶ . For the sake of comparison, upregulation of P-selectin was also evaluated in the model of acute lung injury occurring after systemic activation of complement. As expected, significant upregulation of P-selectin was found 30 minutes after infusion of CVF, as shown in Figure 1B ▶ . The index for 125I-labeled anti-P-selectin was 0.63 ± 0.08, approximately 3.5-fold above the level found at time 0 (P = 0.003). No significant increase in bound 125I-labeled MOPC was observed at the two intervals of time. At 60 minutes after infusion of CVF, there was no difference between the lung binding of MOPC-21 and anti-P-selectin (Figure 1B) ▶ . It has been recently shown that upregulation of P-selectin in the CVF model is C5a dependent and essential for neutrophil accumulation in lung and attendant full expression of lung injury, even though upregulation of P-selectin under these circumstances is very transient, disappearing within 30 minutes. 9,10 Thus, in the two models of injury, expression of P-selectin is transient in one and prolonged in the other.
Figure 1.
Time-dependent upregulation of P-selectin, as determined by the binding index using 125I-labeled anti-P-selectin in the IgG immune complex-induced model of lung injury (A) and in the CVF model of lung injury (B). For each column, n ≥ 4.
Western Blot Analysis of Lung P-Selectin during IgG Immune Complex Deposition
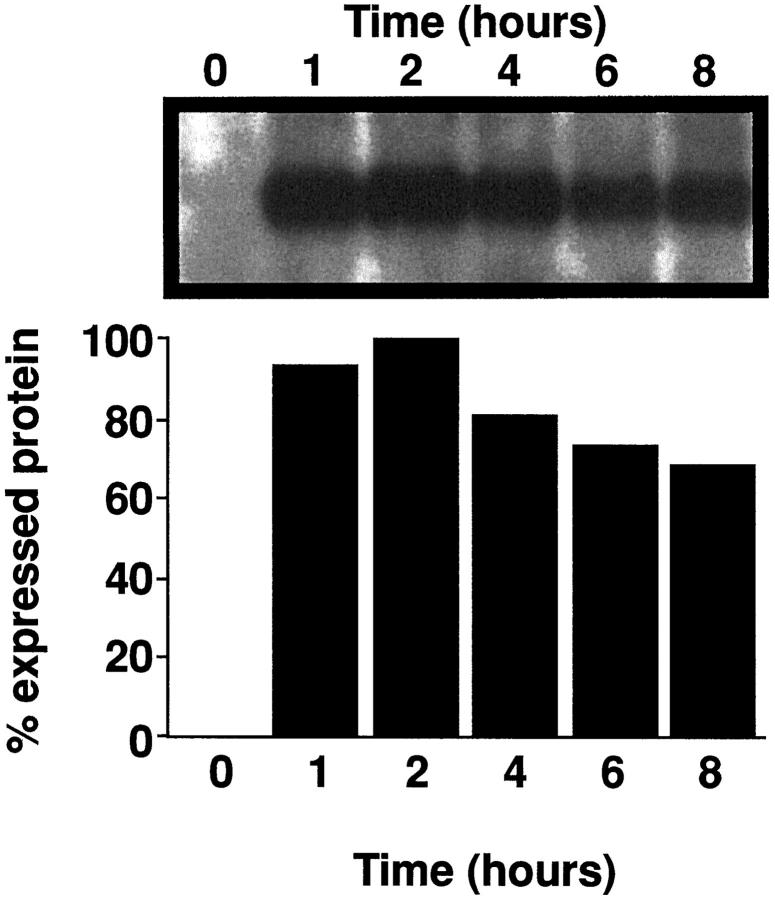
The presence of P-selectin in homogenates of whole lung during intrapulmonary deposition of IgG immune complexes was determined using a Western blot technique. There was little, if any, detectable P-selectin in lung homogenates at time 0 (Figure 2) ▶ . However, P-selectin was readily detectable in homogenates of lung by 1 hour after initiation of the lung inflammatory reactions, persisting at 2, 4, 6, and 8 hours (Figure 2) ▶ .
Figure 2.
Western blot analysis for P-selectin in whole lung homogenates from IgG immune complex-injured rat lungs. Indicated are times (hours) at which lung homogenates were obtained relative to the time of initiation of lung inflammatory reactions (time 0). The lower part of the frame represents densitometric analysis of the blot.
Immunostaining of Lung for P-Selectin
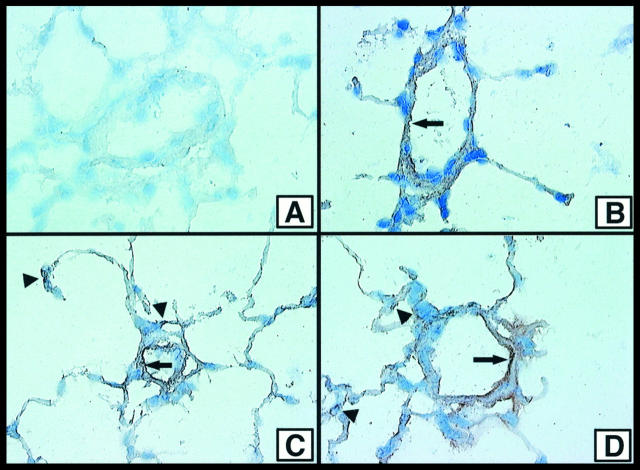
Lungs of rats undergoing IgG immune complex deposition were evaluated by immunostaining techniques at 0, 1, 2, and 4 hours after initiation of the reaction. At time 0, no staining for P-selectin was detectable in the lung vasculature (Figure 3A) ▶ . However, by 1 hour (Figure 3B) ▶ , products of immunostaining were visible along venular surfaces (Figure 3B ▶ , arrow) and in the interstitium in what appeared to be interstitial capillaries. At the 2- and 4-hour intervals (Figure 3, C and D) ▶ , there was evidence of immunostaining for P-selectin in a similar distribution.
Figure 3.
Immunostaining of frozen sections of lung for P-selectin after intrapulmonary deposition of IgG immune complexes. Tissues were obtained 0 (A), 1 (B), 2 (C), and 4 (D) hours after initiation of reactions. Arrows indicate venular staining, and arrowheads indicate what appears to be interstitial capillary staining. Magnification, A to D: ×200; counterstaining with hematoxylin.
Determinants of Upregulation of P-Selectin in Lung Inflammatory Models
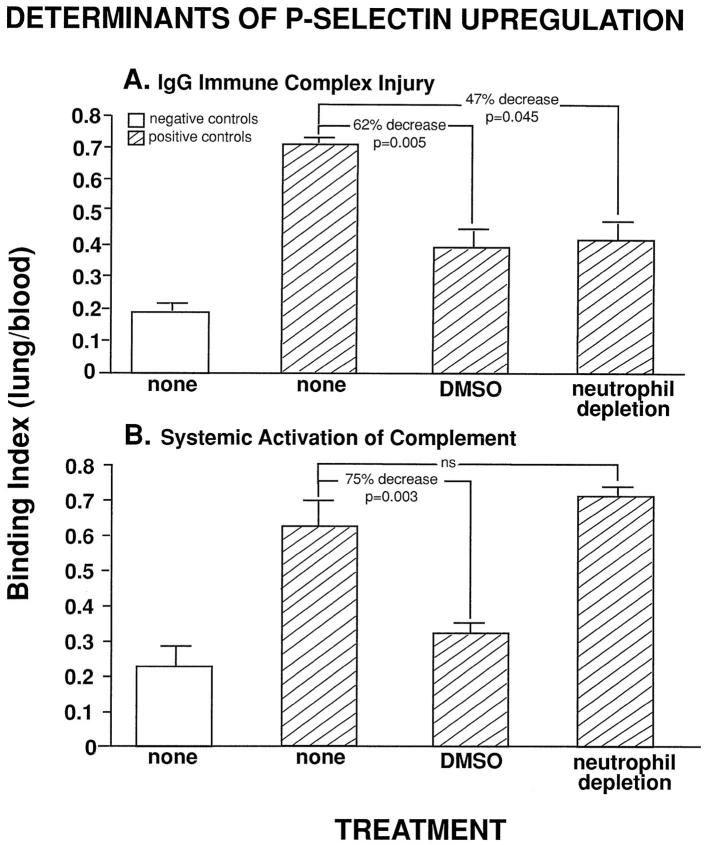
In this series of studies employing the IgG immune complex-induced injury of lung, we sought to determine whether neutrophils, oxidants, complement C5a, and TNF-α were required for the upregulation of lung vascular P-selectin, using the binding index for P-selectin as the endpoint. In turn, these findings were compared with those in the lung model of injury after systemic activation of complement. Upregulation of lung vascular P-selectin was measured by binding of 125I-labeled ARP2-4. Intraperitoneal injection of the antioxidant DMSO is known to be effective in reducing neutrophil- and oxidant-mediated injury in both models of lung injury. 16,17,26 In the IgG immune complex model of lung injury, treatment of rats with DMSO reduced P-selectin upregulation by 62% (P = 0.005), whereas neutrophil depletion reduced this upregulation by 47% (P = 0.045) (Figure 4A) ▶ . Companion experiments were done using the model of systemic activation of complement after intravenous infusion of CVF. In this model, it has recently been shown that upregulation of lung vascular P-selectin can be greatly attenuated in rats that are complement depleted or treated with anti-C5a. 10,27 In the current studies and similar to findings in the immune complex model, treatment with DMSO reduced lung vascular upregulation of P-selectin by 75% (P = 0.003) (Figure 4B) ▶ , whereas neutrophil depletion failed to affect upregulation of P-selectin after intravenous infusion of CVF (Figure 4C) ▶ . Thus, in these two models of lung injury, upregulation of P-selectin shows a strikingly different time course and appears to be oxidant mediated, but the source of the oxidants may be different, based on the differing effects of neutrophil depletion.
Figure 4.
Effects of the antioxidant DMSO and neutrophil depletion on upregulation of P-selectin in IgG immune complex-induced lung injury (A) and in CVF-induced lung injury (B). For each column, n ≥ 4.
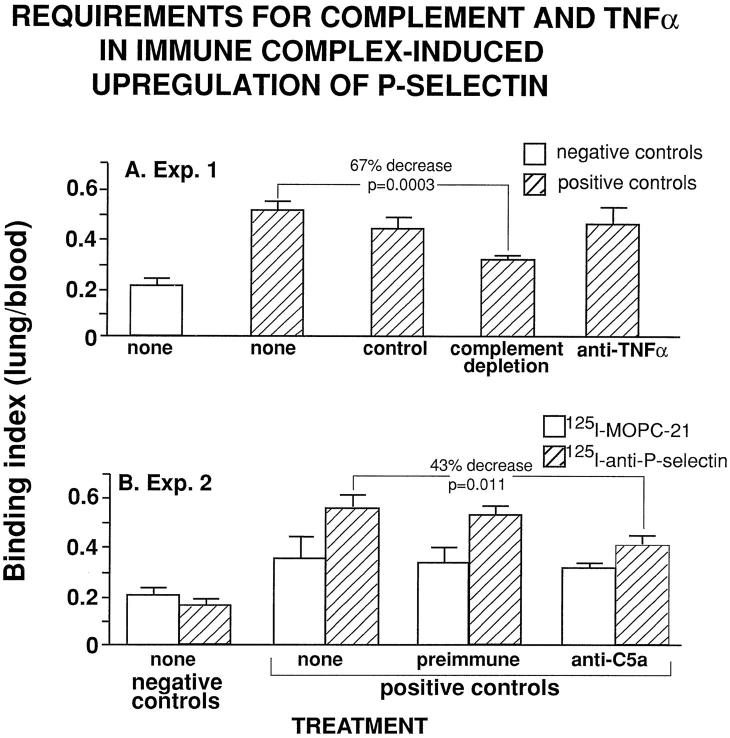
As pointed out above, both C5a and TNF-α have been shown to play key roles in neutrophil recruitment and full development of lung injury in the IgG immune complex-induced model of lung injury, being ultimately linked to upregulation of lung vascular ICAM-1 and E-selectin. 1-3,7 We assessed to what extent upregulation of lung vascular P-selectin, 4 hours after intrapulmonary deposition of IgG immune complexes, would be affected by complement depletion or by treatment with anti-C5a or anti-TNF-α. The results of these studies are shown in Figure 5 ▶ . In rats depleted of complement, upregulation of lung vascular P-selectin was decreased by 67% (P = 0.0003). Intratracheal instillation of 400 μg of anti-rat C5a IgG reduced upregulation of P-selectin by 43% (P = 0.001) when compared with the effects of similar treatment with 400 μg of preimmune IgG (Figure 5B) ▶ . In contrast, treatment of rats with anti-TNF-α failed to affect upregulation of P-selectin (Figure 5A) ▶ , although the same treatment caused a pronounced reduction in upregulation of lung vascular ICAM-1, in neutrophil recruitment, and in the degree of lung injury. 2,3 Accordingly, it is clear that upregulation of P-selectin and ICAM-1 in the immune complex model of lung injury occurs via different mechanisms.
Figure 5.
Effects of complement depletion or treatment with anti-C5a or anti-TNF-α on upregulation of lung vascular P-selectin after intrapulmonary deposition of IgG immune complexes in experiment 1 (A) and experiment 2 (B). For each column, n ≥ 4.
Northern Blot Analysis of mRNA for P-Selectin and TNF-α
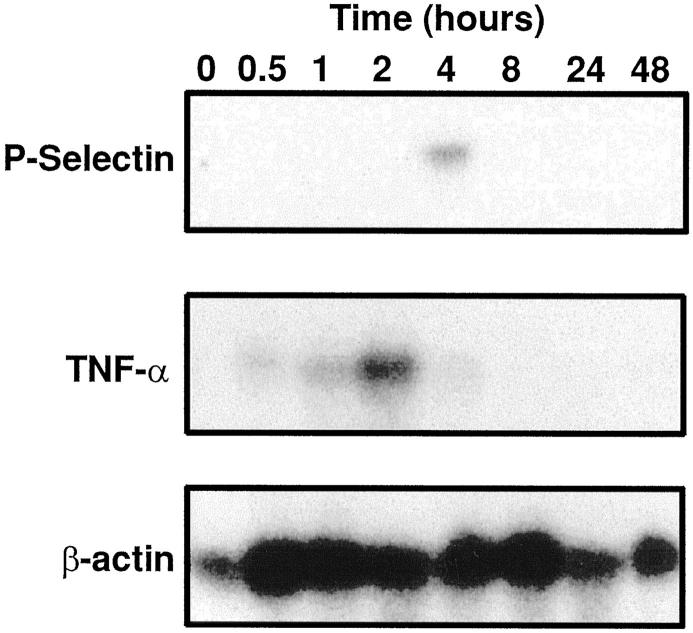
Expression of mRNA for TNF-α and P-selectin in lungs was investigated by Northern blot analysis of lung homogenates obtained at various time points in animals undergoing IgG immune complex-induced lung injury. The expression of mRNA for P-selectin was preceded by expression of mRNA for TNF-α. As shown in Figure 6 ▶ (middle frame), mRNA for TNF-α was first detected as early as 0.5 hours after the induction of injury, with a peak at 2 hours, followed by a decline thereafter. In contrast, mRNA for P-selectin could only be found at 4 hours (considerably after detectable P-selectin protein in lung homogenates), with disappearance thereafter (Figure 6 ▶ , top frame). Rehybridization of the membrane with a β-actin mRNA probe as an internal control confirmed that approximately equal quantities of mRNA samples had been loaded into each lane (Figure 6 ▶ , bottom frame).
Figure 6.
Northern blot analysis of mRNA for P-selectin and TNF-α in lung homogenates after IgG immune complex-induced injury. Shown are times (hours) when RNA extracts were obtained after initiation of the inflammatory reactions. Loading was defined by β actin measurements of extracts, as shown in the bottom frame.
Attenuation of IgG Immune Complex-Induced Injury by anti-P-Selectin
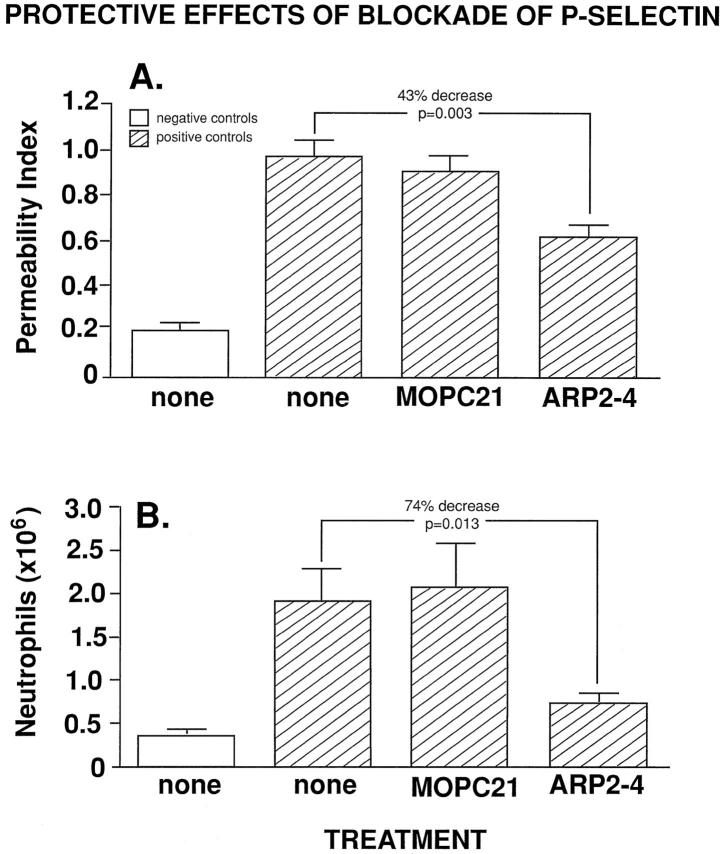
The following experiments indicate that the appearance of P-selectin in lung after deposition of IgG immune complexes is important for neutrophil recruitment and full development of lung injury (Figure 7) ▶ . The intravenous infusion of 400 μg of mouse monoclonal anti-rat P-selectin (ARP2-4) at the onset of the injury (time 0) was associated with significantly protective effects after IgG immune complex deposition. The vascular permeability index, which rose nearly fivefold in the positive control group when compared with the negative control group, decreased by 43% (falling to a value of 0.63 ± 0.05, P = 0.003) in animals treated with anti-P-selectin when compared with positive control animals without further treatment or positive control animals treated intravenously with 400 μg of MOPC-21 (Figure 7A) ▶ . BAL fluids were evaluated for neutrophil content in the negative control group, in the positive control groups otherwise untreated (Figure 7A ▶ , “none” columns), and in the positive control group infused intravenously at time 0 with 400 μg of MOPC-21 or ARP2-4. The results are shown in Figure 7B ▶ . Neutrophil content (× 106)in BAL fluids rose from 0.37 ± 0.06 in the negative control group to 1.94 ± 0.39 in the positive control groups not otherwise treated (P < 0.001) and to 2.14 ± 0.50 in the positive control group treated with 400 μg of MOPC-21 (P < 0.001). In positive control animals treated intravenously with 400 μg of ARP2-4, there was a 74% decrease (P = 0.013) in numbers of BAL neutrophils falling to 0.77 ± 0.12 neutrophils (× 106)/ml (Figure 7B) ▶ . Thus, blockade of P-selectin dramatically reduces recruitment of neutrophils into the lung and protects against the full development of lung injury.
Figure 7.
Protective effects of 400 μg of monoclonal anti-P-selectin (ARP2-4) or MOPC-21 mouse IgG, intravenously administered, in IgG immune complex-induced lung injury, as measured by vascular permeability (A) and neutrophils in BAL fluids (B). For each column, n ≥ 5.
Discussion
The selectins (P-, E-, and L-selectin) have a similar primary structure consisting of an N-terminal lectin-like domain, an epidermal growth factor-like domain, a variable number of units homologous to the repeat structures of complement regulatory proteins, a transmembrane spanning region, and a short cytoplasmic COOH-terminal domain. 28-34 L-selectin is constitutively expressed on leukocytes. 29-31 E-selectin is transcriptionally induced (within 1 to 3 hours) by endothelial cell contact with TNF-α or interleukin-1, with protein expression peaking at 4 hours and returning to background levels by 16 to 24 hours. Constitutive P-selectin is stored in Weibel-Palade bodies of endothelial cells and is transported to the cell surface within minutes on stimulation of endothelial cells. 34,35 Histamine-induced upregulation of P-selectin in endothelial cells in vitro is transient, peaking at 10 to 15 minutes and then rapidly disappearing, presumably because of endocytic removal of P-selectin. Upregulation of P-selectin on endothelial cells also occurs after contact with H202, but in this case P-selectin upregulation is stably expressed, lasting several hours, 36 perhaps because H202 has damaged the endocytic apparatus, resulting in prolonged surface expression of P-selectin. It is also known that, under certain conditions, transcriptional upregulation of P-selectin can occur in vivo. 37-39
Both P-selectin and L-selectin seem to play a role in lipopolysaccharide-induced leukocyte rolling in the rat mesentery, but only P-selectin seems to be required for lipopolysaccharide-induced firm leukocyte adhesion to the endothelium. 39 In the lung injury model after systemic activation of complement by CVF, very early and transient (15 to 20 minutes) upregulation of P-selectin in the lung vasculature occurs, followed by rapid down-regulation. 9 This pattern mimics the well known transient pattern of in vitro expression (10 to 15 minutes) of P-selectin in human umbilical vein endothelial cells stimulated by histamine, thrombin, H202, or C5a. 35,36,40 In the CVF model of lung injury, in vivo upregulation of P-selectin is blocked in the presence of anti-C5a. In the CVF model of lung injury, the requirement for P-selectin for full development of lung injury has been documented by the protective effects of anti-P-selectin or human P-selectin-Ig. 8,9 In earlier studies involving the IgG immune complex model of lung injury, it was concluded that P-selectin was not involved because of the failure of P-selectin-Ig to protect against neutrophil accumulation and full development of lung injury. 8 However, the current studies clearly show upregulation of P-selectin (by Western blot analysis, by in vivo fixation of 125I-labeled antibody, and by immunostaining). The use of monoclonal antibody to rat P-selectin also revealed protective effects in vivo. In this model, the very early but persistent appearance of P-selectin (as determined by immunostaining, tissue fixation of 125I-labeled anti-P-selectin, and Western blot analysis) long before the appearance of P-selectin mRNA suggests that the source of P-selectin may be constitutive.
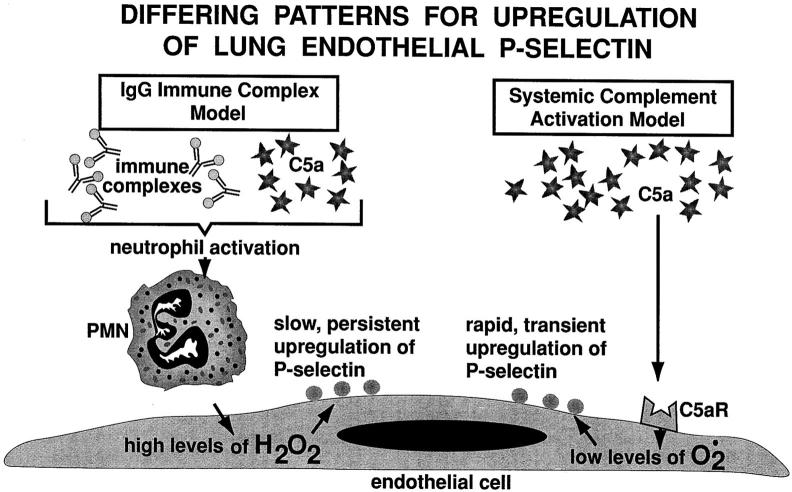
As emphasized above, the onset of upregulation of P-selectin and its transient or persistent nature in rat lung are shown in striking contrast in the two lung models used (Figure 8) ▶ . In the case of intrapulmonary deposition of IgG immune complexes, C5a, which is generated in the vicinity of the immune complex deposits, 27 may lead to activation of intravascular neutrophils, which then become more rigid (less deformable) and entrapped within the pulmonary microvasculature. Under these conditions, activated neutrophils could deliver significant amounts of H202 to adjacent endothelial cells, causing persistent upregulation of P-selectin on endothelial cell surfaces. This might be analogous to the well described in vitro exposure of human umbilical vein endothelial cells to H202, which results in sustained surface expression of P-selectin. 36 The appearance of P-selectin, together with TNF-α-induced upregulation of ICAM-1 and E-selectin, 41 may set the stage for neutrophil adhesion to lung vascular endothelial cells and subsequent migration into the interstitial and alveolar compartments. It should be pointed out that, although neutrophils activated by intravascular infusion of formyl met-leu-phe or zymosan-activated plasma accumulate within the lung vasculature, this does not lead to injury nor does it lead to transmigration of neutrophils. 42,43 Clearly, activation of neutrophils is not sufficient to injure the lung vasculature. Endothelial adhesion molecules are also required to facilitate events that lead to injury of lung endothelial and alveolar epithelial cells.
Figure 8.
Proposed pathways showing different patterns for upregulation of lung vascular P-selectin.
In the case of CVF-induced systemic activation of complement, which leads to lung injury with endothelial accumulation of neutrophils in the absence of transmigration, 26 it seems likely that extensive generation of C5a within the vascular compartment sets the stage for direct activation by C5a of lung vascular endothelial cells, resulting in transient expression of P-selectin (Figure 8) ▶ . This event is crucial in promoting intimate adhesive contacts between C5a-activated blood neutrophils and the activated endothelium. Blockade of P-selectin or C5a in this model by the use of antibody interferes with adhesive interactions between neutrophils and endothelial cells, resulting in diminished accumulation of neutrophils and reduced intensity of lung damage. 10 This transient expression of endothelial P-selectin would be similar to what has been found in vitro with C5a-stimulated endothelial cells, as described above. These events are in sharp contrast to the pattern of upregulation of lung vascular P-selectin after intrapulmonary deposition of IgG immune complexes. In this case, the appearance of P-selectin is delayed, only being measurable after the first 60 minutes. However, P-selectin expression is persistent for up to the 8-hour interval.
The two different pathways of P-selectin expression may be related to why the first is neutrophil dependent whereas the second is neutrophil independent. In both cases the presence of DMSO suggests that oxidants delivered to or generated within endothelial cells are required for P-selectin expression. DMSO is known to be a scavenger for the hydroxyl radical, 18-21 which could suggest that neutrophil-derived H202 and/or endothelial cell-derived superoxide are ultimately converted to the hydroxyl radical (HO·), which causes upregulation of P-selectin, although there is no direct proof for such a conclusion. There is evidence that in vitro expression of P-selectin by endothelial cells is dependent on nuclear factor κB activation and requires participation of intracellular oxidants, as assessed by the inhibitory effects of N-acetylcysteine or pyrrolidine dithiocarbamate. 44 However, the precise source and the role of endothelial intracellular oxidants await definition. In the immune complex model of lung injury, the requirement of neutrophils for upregulation of P-selectin may be due to the delivery to endothelial cells of H202 generated by the large number of neutrophils accumulating in the interstitial and alveolar compartments. The combination of C5a together with immune complexes would represent a powerful stimulus for neutrophil activation in the lung. The diffusion of H202 into nearby endothelial cells may, as in the in vitro situation in which addition of H202 leads to sustained expression of endothelial P-selectin, lead to long-lasting expression of P-selectin on vascular endothelial surfaces of lung.
The data in this report suggest that in the IgG immune complex model of lung injury, P-selectin plays an important role in events that contribute to the recruitment of neutrophils into the lung. The prior attempts to define the role of P-selectin in this model of injury, using P-selectin-Ig (which would compete with endogenous P-selectin), failed to suggest a role for P-selectin. 8 However, a single infusion of P-selectin-Ig at time 0 may have been insufficient to saturate binding sites on neutrophils, namely, P-selectin glycoprotein ligand-1. The ability of antibody to rat P-selectin to reduce injury in the IgG immune complex model of lung injury, together with the clear evidence for the sustained appearance of P-selectin in lung during development of the inflammatory response, documents a role for P-selectin in this model of lung injury. It is also clear that the appearance of P-selectin protein occurs hours before mRNA expression can be detected in lung. It seems likely that endogenous endothelial P-selectin is the source for P-selectin in this model of lung injury, the source(s) of constitutive P-selectin being described above.
The sources of P-selectin in the IgG immune complex model of injury would most likely be endothelial cells (Weibel-Palade granules) or platelets. The late appearance of mRNA for P-selectin (at 4 hours) suggests that expression of P-selectin in lung as early as 1 hour is likely to involve constitutive rather than inducible P-selectin. The immunostaining pattern suggests that endothelial cells may be the source, although we cannot rule out that platelets adherent to the endothelium could account for this finding. In this model, we have found no evidence of platelet adhesion to the endothelium on the basis of transmission electron microscopy (P. A. Ward, unpublished observations). Platelet depletion experiments have been used to address the issue of the source of P-selectin. However, because injury induced by intrapulmonary deposition of IgG immune complexes is inherently hemorrhagic, under conditions of platelet depletion the outcome in the lung is extensive intrapulmonary hemorrhage, which makes platelet depletion procedures unusable (N. M. Bless and P.A. Ward, unpublished observations).
In the immune complex model of lung injury, the ultimate pathway leading to endothelial injury appears to involve the conversion of neutrophil-derived H202 within endothelial cells to HO· in the presence of Fe2+. 45 In the CVF model in which large amounts of C5a are generated, 46 there may be early and intense but transient expression of P-selectin on endothelial surfaces, sufficient to facilitate selectin-dependent adhesive interactions, followed by β2 integrin-dependent and ICAM-1-dependent adhesive interactions. Details regarding the different patterns of lung vascular expression of P-selectin remain to be defined.
Acknowledgments
The authors thank Shigeaki Morooka, Masaji Hayashi and Masako Ohnishi for helpful discussions and Hironobu Tan for TNF-α DNA probe. The authors also thank Ms. Beverly Schumann and Ms. Peggy Otto for their assistance in the preparation of the manuscript.
Footnotes
Address reprint requests to Dr. Peter A. Ward, Department of Pathology, The University of Michigan Medical School, M5240 Medical Science I, Box 0602, 1301 Catherine Road, Ann Arbor, MI 48109-0602. E-mail: pward@umich.edu.
Supported by National Institutes of Health grants HL-31963 and AI-33189.
References
- 1.Mulligan MS, Varani J, Dame MK, Lane CL, Smith CW, Anderson DC, Ward PA: Role of endothelial-leukocyte adhesion molecule 1 (ELAM-1) in neutrophil-mediated lung injury in rats. J Clin Invest 1991, 88:1396-1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mulligan MS, Vaporciyan AA, Miyasaka M, Tamatani T, Ward PA: Tumor necrosis factor α regulates in vivo intrapulmonary expression of ICAM-1. Am J Pathol 1995, 142:1739-1749 [PMC free article] [PubMed] [Google Scholar]
- 3.Warren JS, Yabroff KR, Remick DG, Kunkel SL, Chensue SW, Kunkel RG, Johnson KJ, Ward PA: Tumor necrosis factor participates in the pathogenesis of acute immune complex alveolitis in the rat. J Clin Invest 1989, 84:1873-1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mulligan MS, Varani J, Warren JS, Till GO, Smith CW, Anderson DC, Todd RF, III, Ward PA: Roles of β2 integrins of rat neutrophils in complement- and oxygen radical-mediated acute inflammatory injury. J Immunol 1992, 148:1847-1857 [PubMed] [Google Scholar]
- 5.Mulligan MS, Vaporciyan AA, Warner RL, Jones ML, Foreman KE, Miyasaka M, Todd RF, III, Ward PA: Compartmentalized roles for leukocyte adhesion molecules in lung inflammatory injury. J Immunol 1995, 154:1350-1363 [PubMed] [Google Scholar]
- 6.Mulligan MS, Miyasaka M, Tamatani T, Jones ML, Ward PA: Requirements for L-selectin in neutrophil-mediated lung injury in rats. J Immunol 1994, 152:832-840 [PubMed] [Google Scholar]
- 7.Mulligan MS, Wilson GP, Todd RF, Smith CW, Anderson DC, Varani J, Issekutz T, Miyasaka M, Tamatani T, Rusche JR, Vaporciyan AA, Ward PA: Role of β1, β2 integrins and ICAM-1 in lung injury following deposition of IgG and IgA immune complexes. J Immunol 1993, 150:2407-2417 [PubMed] [Google Scholar]
- 8.Mulligan MS, Watson SR, Fennie C, Ward PA: Protective effects of selectin chimeras in neutrophil-mediated lung injury. J Immunol 1993, 151:6410-6417 [PubMed] [Google Scholar]
- 9.Mulligan MS, Polley MJ, Bayer RJ, Nunn MF, Paulson JC, Ward PA: Neutrophil-dependent acute lung injury: requirement for P-selectin (GMP-140). J Clin Invest 1992, 90:1600-1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mulligan MS, Schmid E, Till GO, Hugli TE, Friedl HP, Roth RA, Ward PA: C5a-dependent up-regulation in vivo of lung vascular P-selectin. J Immunol 1997, 158:1857-1861 [PubMed] [Google Scholar]
- 11.Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD: Leukocyte rolling and extravasation are severely compromised in P-selectin-deficient mice. Cell 1993, 74:541-554 [DOI] [PubMed] [Google Scholar]
- 12.Bosse R, Vestweber D: Only simultaneous blocking of the L- and P-selectin completely inhibits neutrophil migration into mouse peritoneum. Eur J Immunol 1994, 24:3019-3024 [DOI] [PubMed] [Google Scholar]
- 13.Ballow M, Cochrane CG: Two anti-complementary factors in cobra venom: hemolysis of guinea pig erythrocytes by one of them. J Immunol 1969, 103:944-952 [PubMed] [Google Scholar]
- 14.Tojo SJ, Yokota S, Koike H, Schultz J, Hamazume Y, Misugi E, Yamada K, Hayashi M, Paulson JC, Morooka S: Reduction of rat myocardial ischemia and reperfusion injury by sialyl-Lewis X oligosaccharide and anti-rat P-selectin antibodies. Glycobiology 1996, 6:463-469 [DOI] [PubMed] [Google Scholar]
- 15.Ohnishi M, Koike H, Kawamura N, Tojo SJ, Hayashi M, Morooka S: Role of P-selectin in the early stage of the Arthus reaction. Immunopharmacology 1996, 34:161-170 [DOI] [PubMed] [Google Scholar]
- 16.Till GO, Ward PA: Oxygen radicals in complement and neutrophil-mediated acute lung injury. J Free Radical Biol Med 1985, 1:163-168 [DOI] [PubMed] [Google Scholar]
- 17.Ward PA, Till GO, Kunkel R, Beauchamp C: Evidence for role of hydroxyl radical in complement and neutrophil-dependent tissue injury. J Clin Invest 1983, 72:789-801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castro GD, Lopez A, Castro JA: Evidence for hydroxyl free radical formation during paraquat but not for nifurtimox liver microsomal biotransformation: a dimethyl-sulfoxide scavenging study. Arch Toxicol 1988, 62:355-358 [DOI] [PubMed] [Google Scholar]
- 19.Rosenblum WI, El-Sabban F: Dimethyl sulfoxide (DMSO) and glycerol, hydroxyl radical scavengers, impair platelet aggregation within, and eliminate the accompanying vasodilation of, injured mouse pial arterioles. Stroke 1982, 13:35-39 [DOI] [PubMed] [Google Scholar]
- 20.Repine JE, Pfenninger OW, Talmage DW, Berger EM, Pettijohn DE: Dimethyl sulfoxide prevents DNA nicking mediated by ionizing radiation or iron/hydrogen peroxide-generated hydroxyl radical. Proc Natl Acad Sci USA 1981, 78:1001-1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wood DC, Wood J: Pharmacologic and biochemical considerations of dimethyl sulfoxide. Ann NY Acad Sci 1975, 243:7-19 [DOI] [PubMed] [Google Scholar]
- 22.Varani J, Fligeil SEG, Till GO, Kunkel RG, Ryan US, Ward PA: Pulmonary endothelial cell killing by human neutrophils: possible involvement of hydroxyl radical. Lab Invest 1985, 53:656-663 [PubMed] [Google Scholar]
- 23.Weiss SJ: Tissue destruction by neutrophils. N Engl J Med 1989, 320:365-376 [DOI] [PubMed] [Google Scholar]
- 24.Martin WJ: Neutrophils kill pulmonary endothelial cells by a hydrogen-peroxide dependent pathway: an in vitro model of neutrophil mediated lung injury. Am Rev Respir Dis 1984, 130:209-213 [DOI] [PubMed] [Google Scholar]
- 25.Sacks T, Moldow CF, Craddock PR, Bowers TK, Jacob HS: Oxygen radicals mediate endothelial cell damage by complement stimulated granulocytes on in vitro model of immune vascular damage. J Clin Invest 1978, 61:1161-1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Till GO, Johnson KJ, Kunkel R, Ward PA: Intravascular activation of complement and acute lung injury: dependency on neutrophils and toxic oxygen metabolites. J Clin Invest 1982, 69:1126-1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulligan MS, Schmid E, Beck-Schimmer B, Till GO, Friedl HP, Brauer RB, Hugli TE, Miyasaka M, Warner RL, Johnson KJ, Ward PA: Requirement and role of C5a in acute lung inflammatory injury in rats. J Clin Invest 1996, 98:503-512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foxall C, Watson SR, Dowbenko D, Fennie C, Lasky LA, Kiso M, Hasegawa A, Asa D, Brandley BK: The three members of the selectin receptor family recognize a common carbohydrate epitope, the sialyl Lewis(X)oligosaccharide. J Cell Biol 1992, 117:895-902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tedder TF, Isaacs CM, Ernst TJ, Demetri GD, Adler DA, Disteche CM: Isolation and chromosomal localization of cDNAs encoding a novel human lymphocyte cell surface molecule, LAM-1: homology with the mouse lymphocyte homing receptor and other human adhesion proteins. J Exp Med 1989, 170:123-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Camerini D, James SP, Stamenkovic I, Seed B: Leu-8/TQ1 is the human equivalent of the Mel-14 lymph node homing receptor. Nature 1989, 342:78-82 [DOI] [PubMed] [Google Scholar]
- 31.Weller A, Isenmann S, Vestweber D: Cloning of the mouse endothelial selectins. J Biol Chem 1992, 267:15176-15183 [PubMed] [Google Scholar]
- 32.Gallatin WM, Weissmann IL, Butcher EC: A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature 1983, 304:30-34 [DOI] [PubMed] [Google Scholar]
- 33.Bevilacqua MP, Butcher E, Furie B, Gallatin M, Gimbrone M, Harlan J, Kishimoto K, Lasky L, McEver R, Paulson J, Rosen S, Seed B, Siegelmann M, Springer T, Stoolman L, Tedder T, Varki A, Wagner D, Weissmann I, Zimmermann G: Selectins: a family of adhesion receptors. Cell 1991, 67:233. [DOI] [PubMed] [Google Scholar]
- 34.McEver RP, Beckstead JH, Moore KL, Marshall-Carlson L, Bainton DF: GMP-140, a platelet α-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Weibel-Palade bodies. J Clin Invest 1989, 84:92-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geng JG, Bevilacqua MP, Moore KL, McIntyre T, Newman B, Chi-Rosso G, Hamilton KH, Fugate RD, McEver RP: Rapid neutrophil adhesion to activated endothelium mediated by GMP-140. Nature 1990, 343:757-760 [DOI] [PubMed] [Google Scholar]
- 36.Patel KD, Zimmermann GA, Prescott SM, McEver RP, McIntyre TM: Oxygen radicals induce human endothelial cells to express GMP-140 and bind neutrophils. J Cell Biol 1991, 112:749-759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eppihimer MJ, Wolitzky B, Anderson DC, Labow MA, Granger DN: Heterogenity of expression of E- and P-selectins in vivo. Circ Res 1996, 79:560-569 [DOI] [PubMed] [Google Scholar]
- 38.Auchampach JA, Oliver MG, Anderson DC, Manning AM: Cloning, sequence comparison, and in vivo expression of the gene encoding rat P-selectin. Gene 1994, 145:251-255 [DOI] [PubMed] [Google Scholar]
- 39.Davenpeck KL, Bochner BS: Distinct but overlapping roles of P- and L-selectin in lipopolysaccharide (LPS)-induced leukocyte-endothelial interactions in the rat mesenteric microcirculation. FASEB J 1997, A594 [PubMed]
- 40.Foreman KE, Vaporciyan AA, Bonish BK, Jones MJ, Johnson KJ, Glovsky MM, Eddy SM, Ward PA: C5a-induced expression of P-selectin in endothelial cells. J Clin Invest 1994, 94:1147-1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mulligan MS, Vaporciyan AA, Miyasaka M, Tamatani T, Ward PA: Tumor necrosis factor α regulates in vivo intrapulmonary expression of ICAM-1. Am J Pathol 1993, 142:1739-1749 [PMC free article] [PubMed] [Google Scholar]
- 42.Doerschuk CM, Winn RK, Coxson HO, Harlan JM: CD18-dependent and -independent mechanisms of neutrophil emigration in the pulmonary and systemic microcirculation of rabbits. J Immunol 1990, 144:2320-2327 [PubMed] [Google Scholar]
- 43.O’Flaherty JT, Showell HJ, Ward PA: Neutropenia induced by systemic infusion of chemotactic factors. J Immunol 1977, 118:1586-1589 [PubMed] [Google Scholar]
- 44.Kilgore KS, Schmid E, Shanley TP, Flory CM, Maheswari V, Tramontini NL, Cohen H, Ward PA, Friedl HP, Warren JS: Sublytic concentrations of the membrane attack complex (MAC) of complement induce endothelial interleukin 8 (IL-8) and monocyte protein 1 (MCP-1) through nuclear factor kappa-B (NF-κB) activation. Am J Pathol 1997, 150:2019-2031 [PMC free article] [PubMed] [Google Scholar]
- 45.Varani J, Ward PA: Mechanisms of neutrophil-dependent and neutrophil-independent endothelial cell injury. Biol Signals 1994, 3:1-14 [DOI] [PubMed] [Google Scholar]
- 46.Schmid E, Warner RL, Crouch LD, Friedl HP, Till GO, Hugli TE, Ward PA: Neutrophil chemotactic activity and C5a following systemic activation of complement in rats. Inflammation 1997, 21:325-333 [DOI] [PubMed] [Google Scholar]