Abstract
We recently reported that peritoneal fluid mainly contains two proteoglycans; one is the interstitial proteoglycan referred to as decorin, and the other an uncharacterized small chondroitin sulfate proteoglycan. In the present study, we have used a two-step process to isolate the small chondroitin sulfate proteoglycan free of decorin. The purified molecule ran as a single band on sodium dodecyl sulfate-polyacrylamide gel electrophoresis with apparent molecular mass 50 kd made up of a chondroitin-4-sulfate glycosaminoglycan chain and a 30-kd core protein. NH2-terminal analysis of the core protein showed significant sequence homology with bikunin, a component of the human inter-α-trypsin inhibitor (IαI) family. A Western blot analysis using anti-human inter-α-trypsin inhibitor confirmed the identity of the small chondroitin sulfate proteoglycan as bikunin, and a trypsin inhibitor counterstain assay confirmed its anti-trypsin activity. Examination of serum from patients receiving continuous peritoneal dialysis suggests that free bikunin in peritoneal fluid may be the result of leakage of serum proteins into the peritoneum. Our findings further show that the interaction of serum with peritoneal mesothelial cells offers a new and novel explanation for the presence of bikunin in peritoneal fluid.
The introduction of continuous ambulatory peritoneal dialysis (CAPD) has led to a heightened interest in the biological and physical properties of the peritoneum and, in particular, the mesothelial cell that covers the visceral and parietal peritoneum with a simple epithelial monolayer. The function of these cells has not been clearly defined, but evidence to date suggests that they act as a selective barrier regulating the transport of fluids and solutes between the circulation and the body cavity. 1 Mesothelial cells also produce macromolecules that serve as lubricants for the surface of the mesothelium and that serve to prevent adhesions. 2-4 In addition, they synthesize a number of other proteins and glycoproteins that also are released directly into the peritoneal fluid. 5,6 These molecules include a number of different proinflammatory cytokines; growth factors; and a variety of extracellular matrix components including fibronectin, laminin, and type I and type IV collagens. Our studies suggest that human peritoneal mesothelial cells also contribute several different proteoglycans (PGs) to the peritoneal fluid. 7,8
PGs are a heterogeneous group of glycoconjugates that are found on cell surfaces, in basement membranes and extracellular matrices, and in secretary granules and are widely distributed in tissue. 9,10 The common factor that distinguishes these molecules from other glycoconjugates is the substitution of the protein core with glycosaminoglycan (GAG) chains, either chondroitin sulfate (CS) or heparan sulfate chains. PGs have been implicated in a diverse range of biological functions such as cell adhesion and recruitment, cell differentiation and proliferation, and the flow of cells and proteins between bloodstream and tissues. They are also involved in the control of proteolytic events in tissues through their ability to enhance the inhibitory activity of serine proteinase inhibitors such as antithrombin and heparan cofactor II, which are involved in blood coagulation. 11 PGs, or more specifically their GAG side chains, also interact with other serine proteinase inhibitors such as human neutrophil elastase and protease nexin-1 and thus possibly modulate the extracellular proteolysis in the pericellular environment. 12,13 In other serine proteinase inhibitors GAG chains, although part of the subunit structure, are not responsible for the inhibitory activity. This is exemplified by inter-α-trypsin inhibitor (IαI), which consists of an inhibitory unit, referred to as bikunin, and two heavy chains (H chains) covalently joined by a CS chain. 14 It has also been shown that the ectodomain of syndecan-1 and syndecan-4, cell surface heparan sulfate PGs, reduces the proteolytic activities of elastase and cathepsin G in wound fluid. 15 The detection of PGs in peritoneal fluid may indicate that these molecules play a role in the control of proteinases within the peritoneum.
Analysis of the PGs in peritoneal fluid obtained from patients receiving CAPD suggests that CS was the predominant GAG in this fluid, some of which was associated with a protein core immunologically related to decorin. In addition, however, a high proportion of CS eluted with a molecular size suggesting the presence of free GAG chains or their degradation products. The objective of this investigation was to identify this CSPG. We present biochemical and immunological evidence that the CS GAGs are not in fact free chains but are covalently linked to a small peptide of molecular mass 30 kd that is immunologically related to bikunin. We also present evidence that the presence of free bikunin in the peritoneal fluid is due in part to an interaction between peritoneal mesothelial cells and IαI in serum.
Materials and Methods
Materials
Uninfected heparin-free peritoneal fluid was obtained from patients receiving continuous peritoneal ambulatory dialysis therapy for end-stage renal failure. The underlying renal diseases included cystinosis, acute and chronic renal failure, polycystic kidney, immunoglobulin A nephropathy, anti-glomerular basement membrane glomerulonephritis, hypertension, vasculitis, and type I diabetes mellitus. Human omentum was obtained with consent from nonuremic patients undergoing abdominal surgery; normal human serum was obtained with consent from healthy laboratory staff with no clinical signs of renal impairment. Medium 199, fetal calf serum, penicillin, streptomycin, glutamine, insulin, transferrin and hydrocortisone were purchased from Life Technologies, Inc. (Paisley, UK); cetyltrimethylammonium bromide (CTAB), guanidine HCl, Triton X-100 was from Aldrich (Gillingham, Dorset, UK); diethylaminoethyl-Sephacel was from Pharmacia Ltd. (Uppsala, Sweden); bovine serum albumin, Alcian Blue 8GX, N-acetyl-dl-phenylalanine β-napthyl ester, N,N-dimethylformamide, and tetrazolatized o-dianisidine were from Sigma Chemical Co. (Poole, Dorset, UK); proteinase-free chondroitin ABC lyase and CS disaccharide standards were from ICN (Theme, UK); and EconoPac Q columns, chemicals, and apparatus for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were from Bio-Rad (Hemel Hemstead, UK). The following antibodies were used: rabbit anti-human decorin (LF-30) and anti-human biglycan (LF-15) (the kind gift of Dr. L. W. Fisher, National Institute of Dental Health, Bethesda, MD); rabbit anti-bovine versican (the kind gift of Prof. D. Heinegard, Lund, Sweden); 16 and mouse monoclonal antibodies (mAbs) 2B6 and 3B3 (the kind gift of Prof. B. Caterson, University of Wales College of Cardiff, Cardiff, UK). mAbs 2B6 and 3B3, respectively, recognize the unsaturated C4- and C6-sulfate disaccharides remaining on the core protein of CSPGs after depolymerization of the GAG chains with chondroitin ABC lyase. 17 Additionally, rabbit anti-human IαI was obtained from DAKO Ltd. (High Wycombe, Bucks, UK). This antiserum recognizes both the heavy (H1, H2, and H3) and light (bikunin) chains of IαI. Purified samples of tissue inhibitor of metalloproteinase TIMP-1 and TIMP-2 were kindly donated by Prof. T. E. Cawston (Newcastle, UK).
Isolation of PGs from Peritoneal Dialysate Fluid
Peritoneal fluid was centrifuged at 5000 × g for 15 minutes at 4°C to remove cells and insoluble debris, and the PGs in the supernatant were concentrated either by passage over diethylaminoethyl-Sephacel anion-exchange chromatography as previously reported by us 8 or by precipitation with CTAB according to the method of Meyer et al, 18 as described by Dietrich et al, 19 for the isolation of GAGs from human urine. Briefly, for the latter method, the peritoneal fluid (∼2 L) was adjusted to pH 6.0 by the addition of 6 mol/L HCl, CTAB (34 ml of 5% w/v solution in water) was then added, and the mixture was incubated overnight at 4°C. The precipitate formed was collected by centrifugation at 5000 × g for 15 minutes, washed with 3 volumes of 95% v/v ethanol, dried under N2, and then extracted with 4 mol/L guanidine-HCl containing 0.5% v/v Triton X-100 and 50 mmol/L sodium acetate, pH 6.0. This extract was subjected to three further precipitation cycles with 1.3% w/v potassium acetate in 95% w/v ethanol. The final pellet was incubated with 20 ml of 2 mol/L NaCl overnight at 4°C, insoluble material was removed by centrifugation at 15,000 × g for 30 minutes at 4°C, and then the PGs in the supernatant were precipitated with 3 volumes of potassium acetate ethanol and dried under N2. Finally, the pellet was dissolved in 20 ml of 50 mmol/L Tris-HCl, pH 7.4 (Tris buffer), filtered through a 0.2-μm Millipore (Bedford, MA) membrane. The PGs prepared by either diethylaminoethyl-Sephacel or CTAB precipitation were further purified using an EconoPac Q column, pre-equilibrated with Tris buffer, and interfaced with a fast protein liquid chromatography system (Pharmacia). Nonbound material was removed by washing the column with three volumes of Tris buffer, and the bound PGs eluted stepwise with NaCl in the same buffer.
Isolation and Culture of Human Peritoneal Mesothelial Cells
Human peritoneal mesothelial cells were isolated by enzymatic disaggregation of human omentum and cultured as reported by us previously. 7,20 The cells were maintained and propagated in Medium 199 supplemented with 10% fetal calf serum and containing 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mmol/L glutamine, 5 μg/ml insulin, 5 μg/ml transferrin, and 0.4 μg/ml hydrocortisone (Medium 199) and incubated at 37°C with 5% CO2 in humidified air. For the tissue culture experiments, cells (2 × 105/ml) were seeded onto 35-mm plastic petri dishes and grown in the above medium for 20 hours. The medium was then removed, the cells thoroughly washed with phosphate-buffered saline and then grown in the same medium, but with the fetal calf serum replaced by 10% heat-inactivated human serum, at 37°C for 24 hours. The medium was collected and the cell layers extracted with 1% SDS at room temperature for 2 hours. In control experiments medium 199 containing 10% heat-treated human serum was incubated in the absence of cells. To prepare conditioned medium, cells (1 × 106/ml) were cultured in Medium 199 without serum for 24 hours, after which the medium was removed under aseptic conditions and centrifuged at 1000 × g for 10 minutes and the supernatant was frozen and stored at −20°C until subsequent use.
SDS-PAGE
SDS-PAGE was carried out under reducing conditions according to the procedure of Laemmli 21 on either 3 to 12% or 5 to 15% gradient gels using a Bio-Rad minigel system. Aliquots for analysis were diluted 1:1 with a solution of 2% SDS, 20% glycerol, 0.005% (w/v) bromphenol blue, and 0.125 mmol/L Tris, pH 6.0 with 10% (v/v) 2-mercaptoethanol and then heated at 100°C for 5 minutes. After electrophoresis gels were stained with either Coomassie brilliant blue (BDH, Poole, UK) to visualize the protein bands or Alcian blue 8GX (Sigma) to visualize GAGs. 22 For Western blot analysis, the separated proteins from a second gel run under identical conditions were transferred to nitrocellulose membrane (Schleicher & Schuell, Keene, NH). The membrane was blocked with Tris-buffered saline containing 5% nonfat powdered milk for 1 hour and then incubated with the primary antibody in Tris-buffered saline containing 1% bovine serum albumin and 0.05% Tween 80 (Tris-buffered saline-Tween) for 1 hour at room temperature. The blots were subsequently washed in Tris-buffered saline-Tween and then incubated with the appropriate secondary antibody. Proteins were visualized either using streptavidin/biotinylated alkaline phosphatase complex (Boehringer Mannheim, Mannheim, Germany) or enhanced chemiluminescence (Amersham, Little Chalfont, UK) according to the manufacturer’s instructions.
Trypsin Inhibitor Counterstain (TIC) Assay
To visualize trypsin proteinase inhibitory activity, the method described by Enghild et al 23 was used. Briefly, nonreduced samples were electrophoresed on 3 to 12% gradient SDS-PAGE, and the gels were equilibrated in 0.1 mol/L sodium phosphate buffer, pH 7.8, at 37°C for 15 minutes followed by the same buffer containing 40 μg/ml trypsin for a further 15 minutes. The buffer was decanted, and the gels were rinsed with water (50 ml, two times) and then incubated with solution prepared by adding 10 ml of N-acetyl-dl-phenylalanine β-napthyl ester (2.5 mg/ml in N,N-dimethylformamide) and 50 ml of tetrazolatized o-dianisidine (1 mg/ml in 50 mmol/L sodium phosphate buffer, pH 7.0) for 30 minutes at 25°C. Active proteins are defined as the clear area on the TIC-stained gels.
Other Analytical Methods
GAGs were assayed by the dimethylmethylene blue binding method 24 using shark CS as the standard. Depolymerization of CS and dermatan sulfate chains was carried out by incubating samples with 100 mU of proteinase-free chondroitin ABC lyase (ICN) at 37°C for 20 hours as described previously by us. 25
Analysis of CS GAG by Capillary Electrophoresis
The analysis of CS GAG chains depolymerized by incubation with chondroitin ABC lyase was carried out on an Applied Biosystems 270A HT CE apparatus following the methods of Carney and Osborne. 26 Electrophoresis was carried out on a 72-cm, 50 μm-diameter capillary column using either 15 kV at 40°C for 30 minutes in 40 mmol/L sodium phosphate-10 mmol/L sodium borate buffer, pH 9.0, containing 40 mmol/L SDS, or reverse polarity at −15 kV in 200 mmol/L orthophosphoric acid, pH 3.0, at 40°C for 30 minutes. The material eluted was monitored at 232 nm and compared with standard disaccharides (ICN).
Results
Isolation of a CSPG of Molecular Mass 50 kd
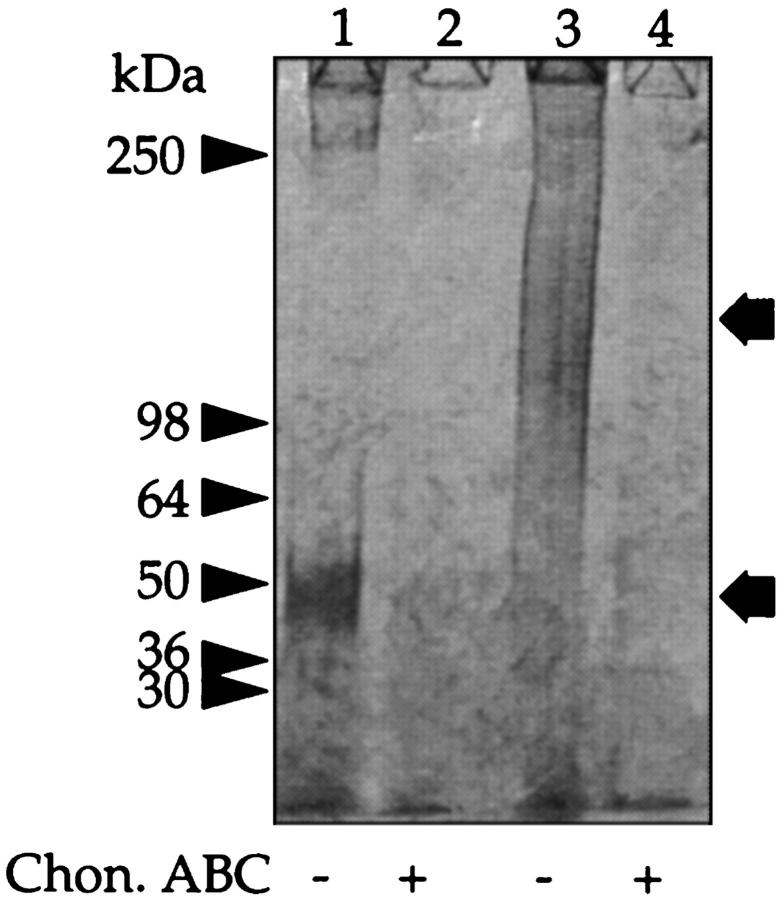
In initial experiments the PGs in overnight dwell fluid were concentrated as described previously 8 and then analyzed by ion exchange chromatography on a EconoPac Q column. Assay of the individual fractions for PGs using the Farndale et al 24 assay revealed two populations: a low-charge pool (designated F1) and a more highly charged pool (F2). Analysis by SDS-PAGE of these pools showed that F1 contained a PG with an apparent molecular mass of 50 kd (Figure 1 ▶ lane 1), whereas the PG in F2 ran as a diffuse band of molecular mass ∼150 kd (lane 3). Analysis of the material in F2 by Western blot using three different polyclonal antisera that recognize the core proteins of decorin (LF-30), biglycan (LF-15), and versican indicated that these CSPGs were only present in F2 (Table 1) ▶ .
Figure 1.
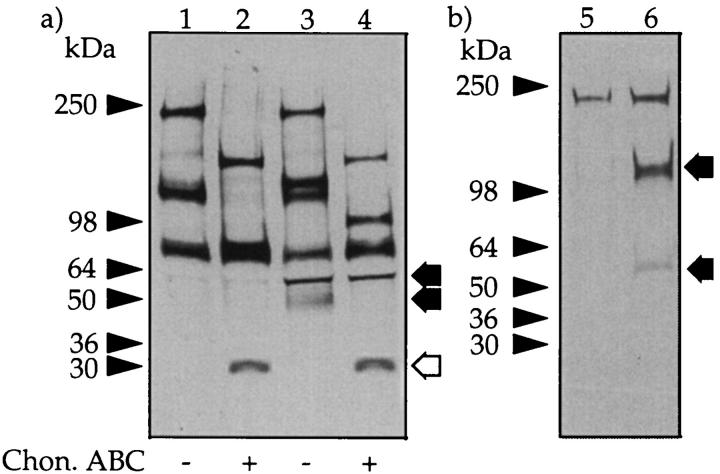
SDS-PAGE of peritoneal fluid PGs. Peritoneal fluid was passed over an EconoPac Q column, and the nonbound material and GAGs were eluted with increasing concentrations of NaCl (see Materials and Methods). The materials that eluted with 0.4 mol/L NaCl (F1) and 0.6 mol/L NaCl (F2) were separately pooled. Aliquots of F1 (lanes 1 and 2) and F2 (lanes 3 and 4) were incubated with buffer alone (lanes 1 and 3) or chondroitin ABC lyase (lanes 2 and 4) and subjected to SDS-PAGE under reducing conditions on a 3 to 12% gradient gel and stained for GAG with Alcian blue. The prestained molecular mass markers are indicated with arrowheads, and the resolved PGs are indicated with arrows.
Table 1.
Inventory of Peritoneal Fluid PGs
| Fraction from ion exchange chromatography | ||
|---|---|---|
| F1 | F2 | |
| PG size | 50 kd | 100 to 200 kd |
| Total GAG | 240 mg | 180 mg |
| CS GAG | 100% | 81% |
| Decorin | ND | +++ |
| Biglycan | ND | + |
| Versican | ND | + |
GAGs were assayed before and after incubation with chondroitin ABC lyase using the Farndale assay 24 with shark CS GAGs as standards. The PG size was determined by SDS-PAGE (see Figure 1 ▶ ). The presence of decorin, biglycan, and versican was determined using Western blot analysis. ND, not detected.
Characterization of the 50-kd CSPG
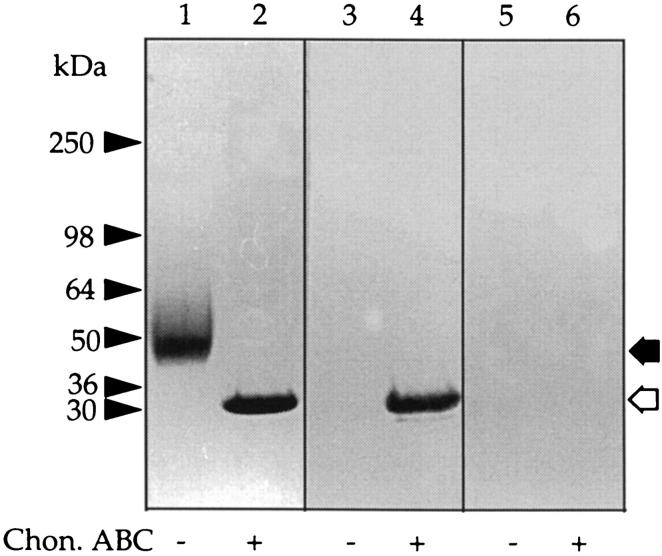
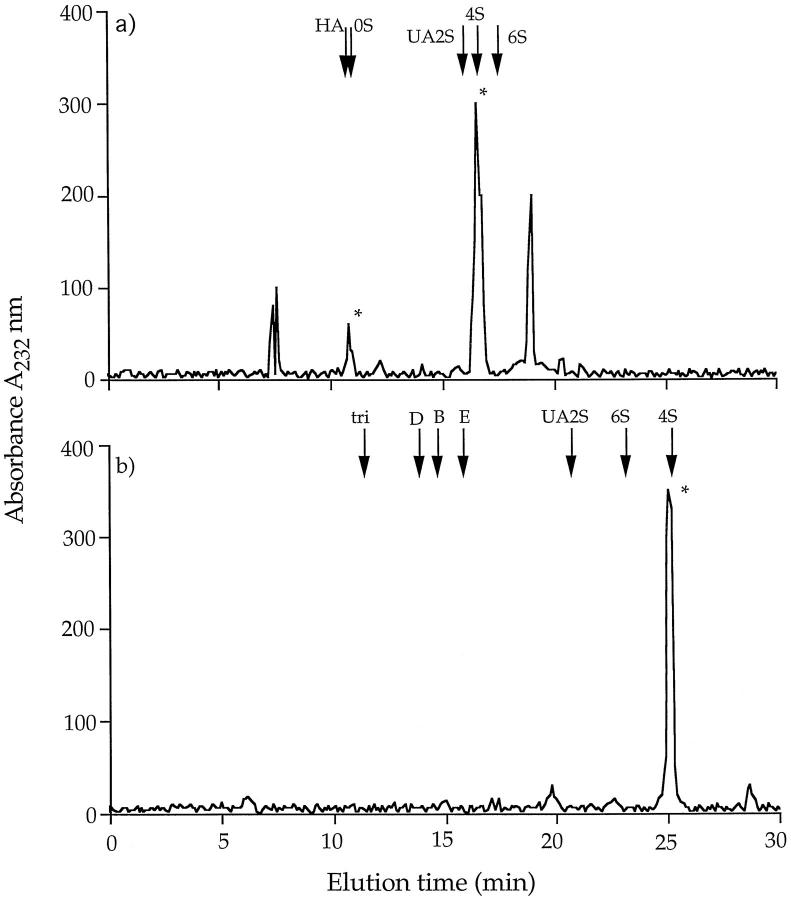
To investigate the 50-kd CSPG in F1 in more detail, the PGs in the overnight dwell fluid from 10 different patients were precipitated with CTAB, and the precipitates were bulked and fractionated into low- and high-charge species on an EconoPac Q column as above. The amount of GAG present in F1 from overnight dwell was 302 ± 146 μg per 2 L (n = 10). Aliquots of F1 were separated on gradient gels before and after digestion with chondroitin ABC lyase, and the gels were either stained directly with Coomassie blue or transferred to nitrocellulose membranes and Western blotted using two mAbs (2B6 and 3B3) that recognize the unsaturated disaccharides in the CS “stubs” that remain associated with the core protein after digestion with chondroitin ABC lyase. 17 Staining with Coomassie blue revealed a single band at molecular mass 50 kd, which after chondroitin ABC lyase-treatment was reduced to a single new protein band with an apparent molecular mass of ∼30 kd (Figure 2 ▶ , compare lanes 1 and 2). In a Western blot, the 30-kd band was also identified with mAb 2B6, which recognizes chondroitin-4-sulfate stubs (Figure 2 ▶ , lanes 3 and 4), but not mAb 3B3 (which recognizes chondroitin-6-sulfates) (Figure 2 ▶ , lanes 5 and 6). Capillary electrophoresis analysis of the disaccharides derived from the digestion of F1 with the same lyase confirmed that this CSPG contained mainly chondroitin-4-sulfated disaccharide together with a small amount of nonsulfated disaccharide but no chondroitin-6-sulfate (Figure 3) ▶ .
Figure 2.
Western blot analysis of the 50-kd CSPG. Peritoneal fluid was concentrated by precipitation with CTAB and F1 obtained as outlined in Figure 1 ▶ . Aliquots were either incubated with buffer alone (lanes 1, 3, and 5) or chondroitin ABC lyase (lanes 2, 4, and 6) and run on a 3 to 12% gradient gel. The gel was cut as indicated and stained for either protein with Coomassie brilliant blue (lanes 1 and 2) or blotted and probed with mAb 2B6 (lanes 3 and 4) or mAb 3B3 (lanes 5 and 6). The prestained molecular mass markers are indicated with arrowheads and the resolved PGs with arrows. The open arrows indicate the core protein released after incubation with chondroitin ABC lyase.
Figure 3.
Capillary electrophoresis. An aliquot (1 mg/ml) of the 50-kd CSPG was incubated with chondroitin ABC lyase, and the released disaccharides were electrophoresed under normal polarity (15 kV) (a) or reverse polarity (−15 kV) (b) at 40°C for 30 minutes. The peaks were monitored at 232 nm and compared with standard disaccharides: HA, hyalurono-ΔDi-0S; 0S, chondro-ΔDi-0S; 4S, chondro-ΔDi-4S; 6S, chondro-ΔDi-6S; 2S, chondro-ΔDi-UA2S; B, dermato-ΔDi-di-4S, UA2S; D, chondro-ΔDi-di6S, UA2S; E, chondro-ΔDi-di-4,6S; and tri, chondro-ΔDi-tri-4,6S, UA2S.
To identify the 30-kd PG, samples of the 50-kd CSPG were treated with chondroitin ABC lyase and after SDS-PAGE the products transferred to a polyvinylidene difluoride membrane for NH2-terminal amino acid sequence analysis. This indicated a 13-amino acid sequence, A V L P Q E E E G (G) G G G (Table 2) ▶ , which is 92% in agreement with the published amino acid sequence of the Kunitz-type serine proteinase inhibitor bikunin, the inhibitory component of several members of the IαI family. 23,27 Thus, to confirm the identity of the 50-kd PG as bikunin, samples either before or after digestion with chondroitin ABC lyase were subjected to SDS-PAGE followed by Western blot analysis using rabbit anti-human IαI immunoglobulin G or to inhibition studies using the TIC assay. 23 The antiserum identified both the intact 50-kd PG (Figure 4 ▶ , lane 1) and its 30-kd core protein (Figure 4 ▶ , lane 2), whereas the results from the TIC assay (Figure 5) ▶ showed that both intact PG (Figure 5 ▶ , lane 1) and its core protein (Figure 5 ▶ , lane 2) inhibited trypsin activity. No other new peptide or inhibitory bands were observed even on overloaded gels (data not shown). From the above studies, we conclude that the 50-kd PG is immunologically related to bikunin.
Table 2.
Amino Acid Sequence of the 50-kd proteoglycan
| Amino acid sequence | |
|---|---|
| 50 kd PG | AVLPQEEEG[G]GGG |
| Bikunin* | AVLPQEEEG[ ]GGG |
| Bikunin† | AVLPQEEEG[S]GGG |
The 50-kd CSPG isolated as described in Figure 2 ▶ was incubated with chondroitin ABC lyase and the protein core electrophoresed on a 3 to 12% gradient gel and electroblotted onto a polyvinylidene difluoride membrane for amino acid sequencing using the Edmund N-terminal procedure. Amino acid NH2-terminal sequences are consistent with the cDNA analysis of bikunin. 57 The failure to recognize serine 10 is due to its glycosylation as the GAG attachment site for this proteoglycan.
*Amino acid NH2-terminal sequence determined by Enghild et al. 23
†Amino acid NH2-terminal sequence determined by a combination of NH2-terminal sequencing and amino acid analysis. 27
Figure 4.
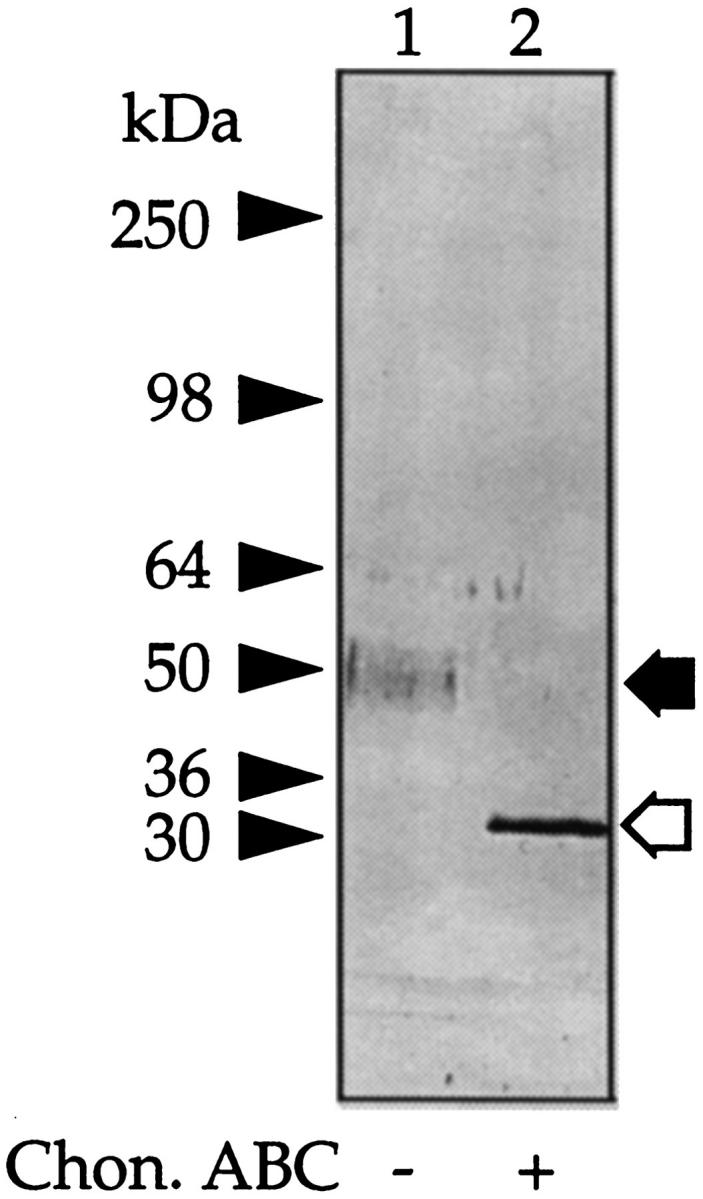
Identification of the 50-kd CSPG as bikunin. Aliquots of the 50-kd CSPG were incubated with buffer alone (lane 1) or chondroitin ABC lyase (lane 2), electrophoresed on 3 to 12% gels, blotted onto nitrocellulose, and probed with an antiserum raised to human serum IαI. The prestained molecular mass markers are indicated with arrowheads and the resolved PGs with arrows. The open arrows indicate the core protein released after incubation with chondroitin ABC lyase.
Figure 5.
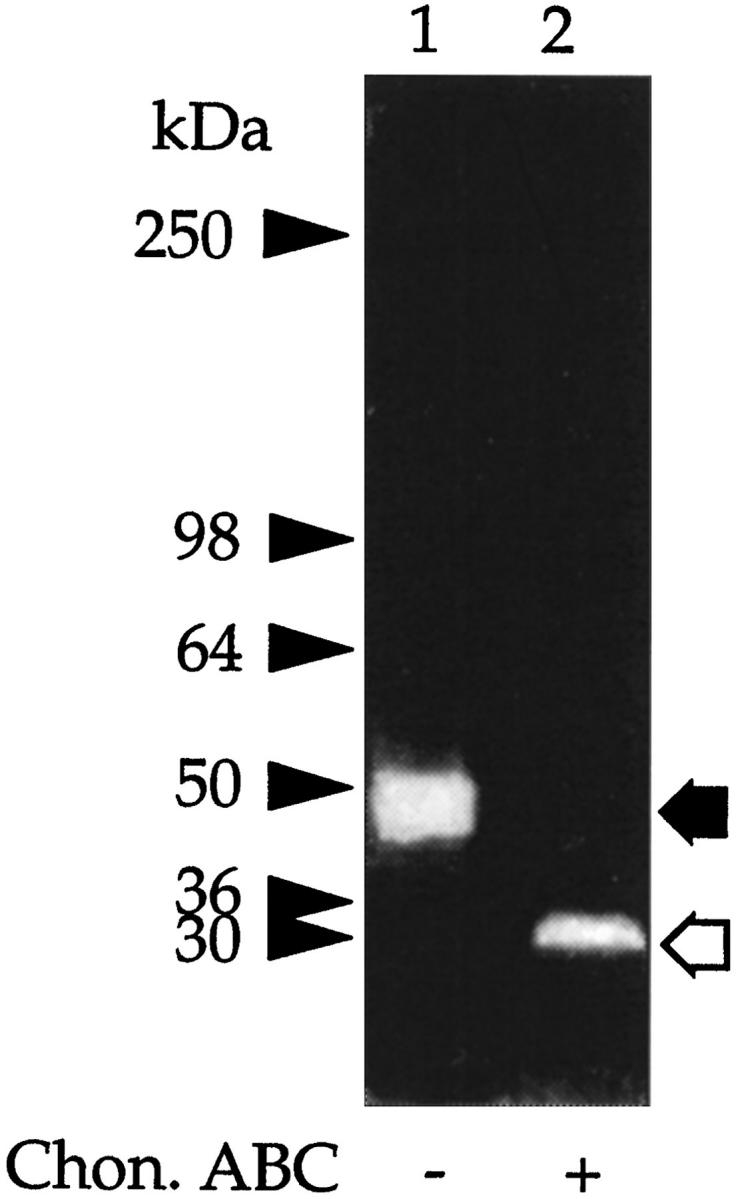
Trypsin inhibitory activity of the 50-kd CSPG. Aliquots of the CSPG were incubated with buffer alone (lane 1) or chondroitin ABC lyase (lane 2) and electrophoresed under nonreducing conditions on 3 to 12% gels, and the trypsin inhibitory activity was determined using the TIC assay. The clear areas on the gel represent trypsin inhibitory activity. The prestained molecular mass markers are indicated with arrowheads and the resolved PGs with arrows. The open arrows indicate the core protein released after incubation with chondroitin ABC lyase.
Detection of IαI in Peritoneal Fluid
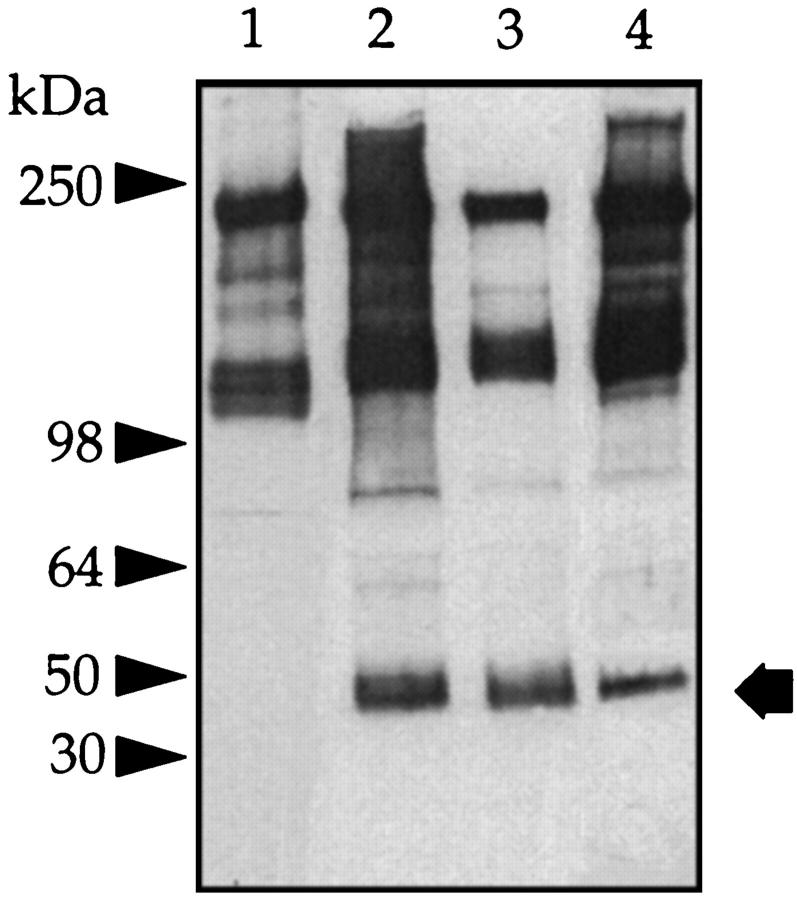
In serum, the majority of bikunin is covalently linked to at least one of four peptides referred to as H chains. Therefore, we next investigated whether in peritoneal fluid bikunin is also present complexed to H chains. Peritoneal fluid was subjected to SDS-PAGE, and the gels were analyzed as above. Western blot with anti-IαI revealed, in addition to the expected band at 50 kd, major protein bands of molecular masses 300 kd, 220 kd, and 125 kd, as well as two minor bands of ∼70 kd each (Figure 6 ▶ , lane 1). Incubation with chondroitin ABC lyase resulted in the loss of the 300-kd, 220-kd, 125-kd, and 50-kd bands and the appearance of new peptide bands with apparent molecular masses of 150 kd and 30 kd (Figure 6 ▶ , lane 2) and an enhancement of the 70-kd bands. TIC gel analysis of fresh peritoneal fluid also showed intense inhibitory bands at 50 kd and, in addition, bands at molecular masses 220 kd and 125 kd (Figure 7 ▶ lane 1). After digestion with chondroitin ABC lyase, only a single inhibitory band was shown at molecular mass 30 kd (Figure 7 ▶ , lane 2).
Figure 6.
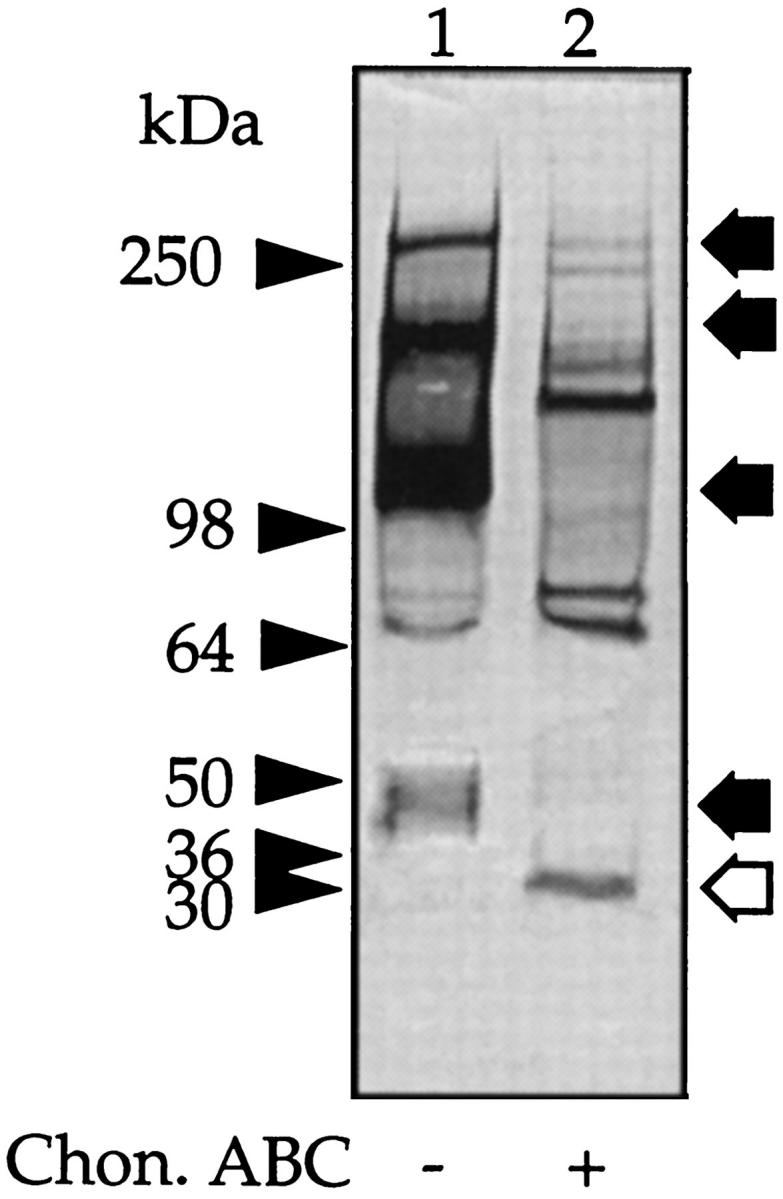
Western blot of peritoneal fluid. Peritoneal fluid (5 μl) was incubated with buffer alone (lane 1) or with chondroitin ABC lyase (lane 2), and a Western blot was generated with anti-human IαI antibody. The prestained molecular mass markers are indicated with arrowheads and the resolved PGs with arrows. The open arrows indicate the core protein released after incubation with chondroitin ABC lyase.
Figure 7.
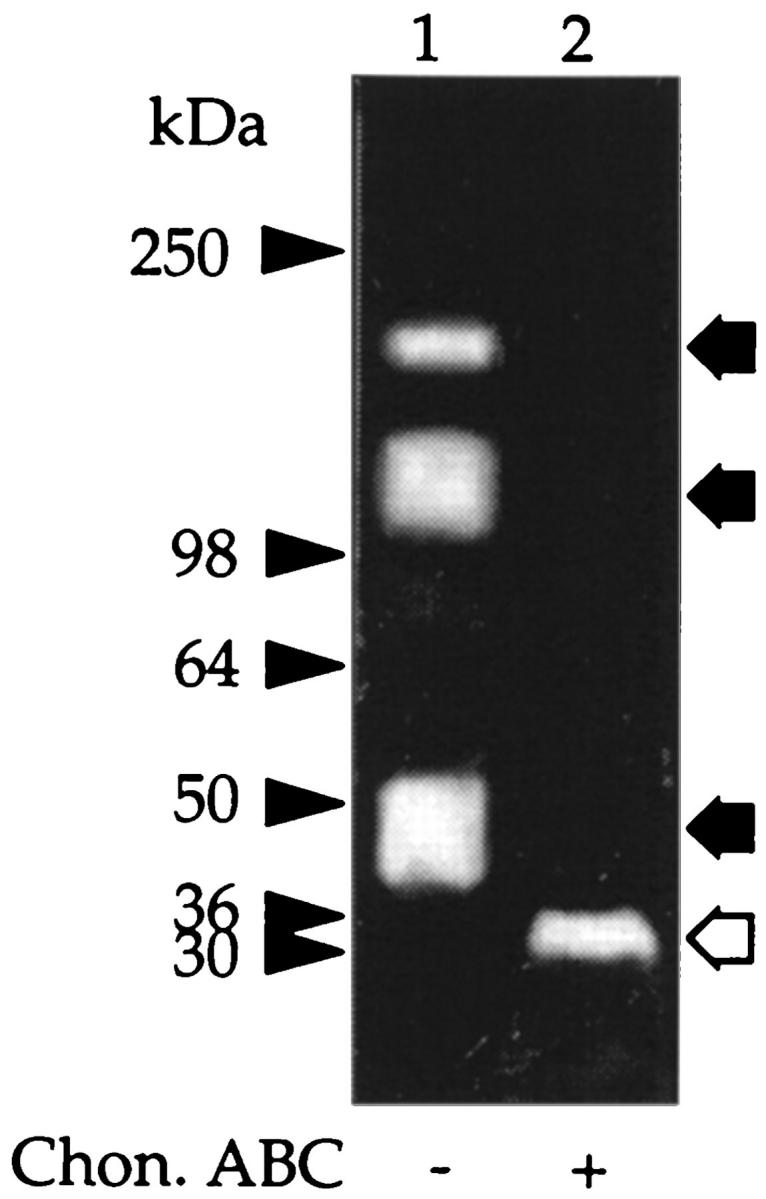
Trypsin inhibitory activity in peritoneal fluid. Fresh peritoneal fluid was concentrated as outlined in Figure 1 ▶ . Aliquots were incubated with buffer alone (lane 1) or with chondroitin ABC lyase (lane 2), electrophoresed under nonreducing conditions on 3 to 12% gels, and the trypsin inhibitory activity was determined using the TIC assay. The prestained molecular mass markers are indicated with arrowheads and the resolved PGs with arrows. The open arrows indicate the core protein released after incubation with chondroitin ABC lyase.
Source of the Bikunin in the Peritoneum
The peritoneal fluid contains proteins because of the leakage of serum into the peritoneal cavity. In our experiments, the peritoneal fluids contained serum proteins (1.53 ± 0.61 mg/ml; n = 20), which therefore represent a source of bikunin. To investigate this possibility we compared serum from healthy donors (n = 10) and serum and fluid from patients receiving CAPD (n = 6). Figure 8 ▶ shows that normal serum contains little or no free bikunin (Figure 8 ▶ , lane 1), whereas it is clearly present in the serum from patients receiving CAPD (Figure 8 ▶ , lane 2). Bikunin was also detected in serum of patients with impaired renal function (creatinine clearance >630 μmol/L) but not receiving replacement therapy (Figure 8 ▶ , lane 4).
Figure 8.
IαI of normal and uremic serum. Aliquots of normal serum (0.5 μl) (lane 1), serum from a patient receiving peritoneal dialysis (0.5 μl) (lane 2), peritoneal fluid from the same patient (5 μl) (lane 3), and serum from a patient with advanced renal failure not receiving CAPD (0.5 μl) (lane 4) were run on 3 to 12% gels, and a Western blot was generated with anti-human IαI antibody. Arrow: Free bikunin; arrowheads: prestained molecular mass markers.
The Mesothelium as a Source of Free Bikunin
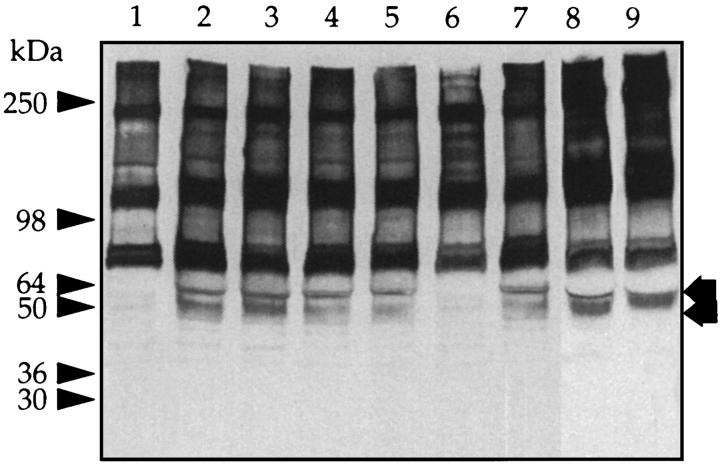
Recently it has been reported that murine granulosa cells release a factor that catalyzes the interaction of the H chains of IαI and hyaluronan with the release of free bikunin. 28 This report raises the possibility that the presence of bikunin in peritoneal fluid could also be explained by the cleavage of serum-derived IαI by the mesothelial cells that line the peritoneum. A second possibility is that the mesothelial cells in vitro themselves synthesize and release bikunin. To investigate these possibilities, human peritoneal mesothelial cells were cultured in Medium 199 alone or in the presence of heat-treated normal human serum. As a further control, a sample of the serum was incubated with culture medium alone. After incubation, aliquots of the SDS-extract of the cell layer and culture medium were electrophoresed, blotted, and probed with the IαI antiserum. The medium derived from cells incubated without serum contained no detectable IαI or bikunin when analyzed on Western blot (data not shown). Furthermore, serum incubated in culture medium alone showed no detectable cleavage of IαI or release of free bikunin (Figure 9 ▶ , lane 1). In contrast, analysis of the culture medium of serum incubated with mesothelial cells revealed a slight reduction of the 220-kd band and new bands at 130 kd and 50 kd (Figure 9 ▶ , lane 3). The failure of chondroitin ABC lyase to alter the molecular mass of the 60-kd band (Figure 9 ▶ , lane 4) suggests that it was a modified free H chain. In contrast, the band at 50 kd was sensitive to chondroitin ABC lyase, indicating that it was free bikunin (Figure 9 ▶ , compare lanes 3 and 4). These experiments indicated that peritoneal mesothelial cells have the potential to cleave IαI with the subsequent release of free bikunin and H chains into the culture medium. Furthermore, the examination of the Western blot from the cell layer from this experiment (Figure 9 ▶ , lanes 5 and 6) suggests that the 120-kd protein, together with a small amount of the modified free H chain, possibly equivalent to the 60-kd band present in the medium, binds to the cell membrane of mesothelial cells.
Figure 9.
Mesothelial cells generate bikunin from serum IαI. Heat-treated human serum (10%) was incubated in Medium 199 in the presence and absence of confluent human peritoneal mesothelial cells at 37°C for 24 hours. The supernatants were collected, and the cell layer was extracted with 1% SDS. Samples of the supernatant before and after digestion with chondroitin ABC lyase were electrophoresed on a 5 to 15% gel, and a Western blot was generated with anti-human IαI antibody. a: The supernatants obtained in the absence (lanes 1 and 2) or presence (lanes 3 and 4) of cells. Prestained molecular mass markers are indicated with arrowheads and released H chains (60 kd) and free bikunin (50 kd) with arrows. The open arrows indicate the core protein released after incubation with chondroitin ABC lyase. b: Control incubation (lane 5) and cell extract of mesothelial cells incubated with serum (lane 6). Prestained molecular mass markers are indicated with arrowheads and bound proteins with arrows.
To determine the nature of the causal agent, mesothelial cells were incubated in serum-free medium for 24 hours; the conditioned medium was removed, and then the cells were incubated with serum as above. Again, the IαI remained intact when incubated with Medium 199 alone over 24 hours (Figure 10 ▶ , lane 1). In contrast, after incubation of serum with medium conditioned by mesothelial cells, an immunoreactive band corresponding to bikunin was detected by Western blot (Figure 10 ▶ , lane 2). In addition, the IαI antiserum detected a protein of 60 kd, which corresponds to the modified H chain described in Figure 9 ▶ (lane 3). The formation of both of these products was prevented by the addition of ethylenediaminetetraacetic acid (Figure 10 ▶ , lane 6) but not by group-specific inhibitors. Heat treatment of the conditioned medium resulted in complete loss of activity.
Figure 10.
Mesothelial cell-conditioned medium releases bikunin from serum IαI. Conditioned medium was obtained from mesothelial cells as described in Materials and Methods and incubated in Medium 199 containing 10% heat-inactivated normal human serum in the absence (lane 2) or presence (lanes 3 to 7 of the following proteinase inhibitors at 37°C for 20 hours: 1 mmol/L phenylmethylsulfonyl fluoride (lane 3), 1 mmol/L benzamidine (lane 4), 0.01 mmol/L leupeptin (lane 5), 10 mmol/L ethylenediaminetetraacetic acid (lane 6), 0.36 mmol/L pepstatin (lane 7), 250 ng/ml TIMP-1 (lane 8), and 250 ng/ml TIMP-2 (lane 9). Lane 1: Serum incubated with Medium 199 alone. Aliquots were electrophoresed on 5 to 15% gels, and a Western blot was generated with anti-human IαI antibody. Prestained molecular mass markers are indicated with arrowheads and released H chains (60 kd) and free bikunin (50 kd) with arrows.
Discussion
Bikunin proteins are members of the pancreatic trypsin inhibitory family. This family, in addition to bikunin, is made up of at least four other different gene products that are referred to as H chains (see Ref. 29 for review and suggested nomenclature). Three of these chains (H1, H2, and H3), in various combinations, are covalently linked to bikunin via a chondroitin-4-sulfate glycan bond. 27 At present, five such combinations of mature proteins have been identified in the serum, of which IαI (H1 plus H2 plus bikunin) and pre-α-trypsin inhibitor (PαI) (H3 plus bikunin) are the best characterized.
In the present study, we have isolated and characterized from peritoneal fluid a small CSPG of molecular mass ∼50 kd. Several biochemical and immunological observations clearly indicate that this molecule represents free bikunin. This result therefore extends the inventory of PGs identified in peritoneal dialysis fluid and means CS accounts for more than 90% of the GAGs in this fluid. Of this figure, we estimate that bikunin accounts for ∼65%, of which 43% is free and the remainder covalently linked to H chains in the form of IαI or PαI.
The liver is the principal site of synthesis of the IαI and bikunin, and, apart from the brain and placenta, 30 no other tissue to date has been reported to express mRNA for bikunin or any of the different H chains. The different proteins of the IαI family are the products of separate genes, and the current view is that once transcribed, their association into a mature protein complex takes place in the liver and to a lesser extent in the brain. It is uncertain to what extent free H peptide chains or bikunin are released from the liver directly into the circulation. 29,31 The presence of free bikunin in normal human serum is controversial. Huang et al, 32 on the basis of SDS-PAGE analysis of serum published by several different authors, 33-35 argue that serum contains free bikunin. Examination of the published gels, however, fails to reveal a band at ∼50 kd that was sensitive to chondroitin ABC lyase. This interpretation is in agreement with a number of other reports that free bikunin is not a component of normal serum. 23,31,36,37 The presence of bikunin in normal serum is also likely to be very low, given that, despite its similarity in charge and hydrodynamic size to serum albumin, it has a relatively high glomerular clearance. 38-40 This would explain the presence of free bikunin (urinary trypsin inhibitor), but not free H peptides in normal urine. 41,42 We were unable to detect in normal human serum using SDS-PAGE a ∼50-kd band that resembled bikunin. We can, however, report that this proteinase inhibitor is present in the serum of patients receiving CAPD as well as individuals with raised serum creatinine levels, ie, advanced renal failure, but not receiving replacement treatment. Thus, the appearance of free bikunin in serum is probably related to loss of renal function, and its relatively high levels in peritoneal fluid are the result of its leakage together with other serum proteins into the peritoneum. The same explanation would also account for the presence in peritoneal fluid of IαI and PαI, a finding that is in keeping with the general understanding that the peritoneal membrane constitutes a size-selective, but probably not a charge-selective, barrier for the transport of serum proteins between blood and dialysate during stable CAPD. 43 It remains to be determined whether there is a correlation of serum bikunin levels with the degree of renal failure or peritoneal levels with the efficiency of peritoneal dialysis.
An alternative explanation to account for bikunin in dialysate arises from the observation that the addition of hyaluronic acid (HA) to human or bovine serum under physiological conditions results in the formation of a HA-H chain complex, devoid of bikunin or CS. 32,44,45 The same complex is found in human synovial fluid 45,46 and mouse follicular fluid, 47 both which are rich in HA. Peritoneal fluid contains significant amounts of HA, which is considerably enhanced in peritonitis. 7 Thus, conditions exist within the peritoneum for the formation of the HA-H chain complex and the concomitant release of bikunin. The precise manner in which this complex is formed is not known, but it is Ca2+ dependent and clearly involves the formation of a covalent bond between HA and one H chain of either IαI or PαI. Energy considerations for such a reaction suggest that a catalyst is required to bring about the exchange between these two molecules. Indeed, Huang et al 32 suggest that such a catalyst is present in serum. More recently, work by Chen et al 28 have shown that a factor(s) is released by granulosa cells, which catalyzes a transesterification between HA and the CS chain at the C-terminal asparagine of an H chain. Our results also show that when human peritoneal mesothelial cells or mesothelial cell-conditioned medium are cultured in the presence of fresh human serum as a source of IαI/PαI, free bikunin was generated. In our system the release of free bikunin was achieved in the absence of added HA. We have, however, previously shown that under such culture conditions human peritoneal mesothelial cells are stimulated to secrete significant amounts of HA, the majority of which is located in the culture medium. The factor produced by the peritoneal mesothelial cells was sensitive to ethylenediaminetetraacetic acid but not several other proteinase inhibitors, including TIMP-1 and TIMP-2, thus suggesting a Ca2+-dependent mechanism. The identification of this factor is a major aim of our current studies.
IαI is one of several plasma proteins that could play an important role in the control of proteolytic activity within the peritoneum, in particular the inhibition of elastase and cathepsin G released from activated neutrophils. 48,49 The principal inhibitors of these serine proteinases in serum and dialysate are probably α-1-proteinase and α-1 antichymotrypsin, which belong to the serpin supergene family and which account for more than 90% of the total serum proteinase inhibitory activity. In contrast to these two serpins, IαI and bikunin, although present in human serum in relatively high amounts (0.45 mg/ml) account for less than 5% of the serine inhibitory activity. 41 In addition, IαI and bikunin bind weakly with serine proteinases and consequently even at high enzyme/inhibitor ratios they have little effect on elastase and cathepsin G activity. 50 This has prompted speculation that the function of IαI may not be related directly to its inhibitory properties. 50 Indeed, current opinion supports the idea that IαI is involved in the stabilization of the extracellular matrix of cells, thus playing a role in controlling cell growth, migration, and differentiation.
In summary, the present study provides evidence that free bikunin is the major CSPG of dialysate from patients receiving CAPD. How this fact relates to the function of the peritoneum is not known, but it could well be related to the presence of HA within this cavity. HA is an important component of the pericellular matrix that surrounds many different cell types, including normal pleural mesothelial cells. 51 The formation of the HA-containing coat requires the presence of serum IαI and involves a reaction in which HA is covalently linked to H1 or H2. 32,45,52 A similar interaction is also required to stabilize the cumulus-oocyte extracellular matrix in the process of oocyte maturation after ovulatory stimulus. 47,53,54 Preliminary data from our laboratories indicate that human peritoneal mesothelial cells in vitro also contain a HA-rich glycocalyx (S. Yung, unpublished data). Because the dialysate from patients receiving CAPD contains significant levels of HA that is synthesized by the resident mesothelial cells, 7 it is an attractive proposition that the IαI in the peritoneal fluid in concert with HA is involved in the maintenance and organization of the peritoneal serous membrane. The possible clinical relevance of such an interaction is highlighted by recent studies with an experimental system of peritoneal dialysis, which suggest a role for hyaluronan in the control of fluid balance within the peritoneum. 55,56
Acknowledgments
We thank Professor G. A. Coles for helpful discussion.
Footnotes
Address reprint requests to Dr. Malcolm Davies, Institute of Nephrology, Royal Infirmary, Cardiff, CF2 1SZ, Wales, UK. E-mail: daviesm6@cf.ac.uk.
Supported by a grant from the Wellcome Foundation and the Kidney Research Foundation for Wales.
References
- 1.Dobbie JW: New concepts in molecular biology and ultrastructural pathology of the peritoneum: their significance for peritoneal dialysis. Am J Kidney Dis 1990, 15:97-109 [DOI] [PubMed] [Google Scholar]
- 2.Dobbie JW, Pavlina T, Lloyd J, Johnson RC: Phosphatidylcholine synthesis by peritoneal mesothelium: its implications for peritoneal dialysis. Am J Kidney Dis 1988, 12:31-36 [DOI] [PubMed] [Google Scholar]
- 3.Beavis J, Harwood J, Coles G, Williams J: Synthesis of phospholipids by human peritoneal mesothelial cells. Perit Dial Int 1994, 14:348-355 [PubMed] [Google Scholar]
- 4.Mizusawa Y, Thomas K, Hills B, Burke J, Mizushima W, Rigby R, Crawford C, Freeman J: Proteolipid in peritoneal effluent of CAPD patients. Perit Dial Int 1998, 18:225-228 [PubMed] [Google Scholar]
- 5.Douvdevani A, Rapoport J, Konforty A, Argov S, Ovnat A, Chaimovitz C: Human peritoneal mesothelial cells synthesize IL-1α and IL-1β. Kidney Int 1994, 46:993-1001 [DOI] [PubMed] [Google Scholar]
- 6.Perfumo F, Altieri P, Degglinnocenti ML, Ghiggeri GM, Caridi G, Trivelli A, Gusmano R: Effects of peritoneal effluents on mesothelial cells in culture-cell proliferation and extracellular-matrix regulation. Nephrol Dial Transplant 1996, 11:1803-1809 [PubMed] [Google Scholar]
- 7.Yung S, Coles GA, Williams JD, Davies M: The source and possible significance of hyaluronan in the peritoneal cavity. Kidney Int 1994, 46:527-533 [DOI] [PubMed] [Google Scholar]
- 8.Yung S, Thomas GJ, Stylianou E, Coles GA, Williams JD, Davies M: The source of peritoneal proteoglycans: human peritoneal mesothelial cells synthesize and secrete mainly small dermatan sulfate proteoglycans. Am J Pathol 1995, 146:520-529 [PMC free article] [PubMed] [Google Scholar]
- 9.Ruoslahti E: Proteoglycans in cell regulation. J Biol Chem 1989, 264:13369-13372 [PubMed] [Google Scholar]
- 10.Iozzo RV, Murdoch AD: Proteoglycans of the extracellular environment: clues from the gene and protein side offer novel perspectives in molecular diversity and function. FASEB 1996, 10:598-614 [PubMed] [Google Scholar]
- 11.Bourin M-C, Lindahl U: Glycosaminoglycans and the regulation of blood coagulation. Biochem J 1993, 289:313-330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Twining SS: Regulation of proteolytic activity in tissues. Crit Rev Biochem Mol Biol 1994, 29:315-383 [DOI] [PubMed] [Google Scholar]
- 13.Hornebeck W, Lafuma C, Robert L, Moczar M, Moczar E: Heparin and its derivatives modulate serine proteinases (SERPS) serine proteinase inhibitors (SERPINS) balance: physiopathological relevance. Pathol Res Pract 1994, 190:895-902 [DOI] [PubMed] [Google Scholar]
- 14.Bost F, Diarra-Mehrpour M, Martin J-P: Inter-α-trypsin inhibitor proteoglycan family: a group of proteins binding and stabilizing the extracellular matrix. Eur J Biochem 1998, 252:339-346 [DOI] [PubMed] [Google Scholar]
- 15.Kainulainen V, Wang H, Schick C, Bernfield M: Syndecans, heparan sulfate proteoglycans, maintain the proteolytic balance of acute wound fluids. J Biol Chem 1998, 273:11563-11569 [DOI] [PubMed] [Google Scholar]
- 16.Heinegard D, Bjorne-Persson A, Coster L, Franzen A, Gardell S, Malmstrom A, Paullson M, Sandfalk R, Vogel K: The core proteins of a large and small interstitial proteoglycan from various connective tissues form distinct subgroups. Biochem J 1985, 230:181-194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Couchman JR, Caterson B, Christner JE, Baker JR: Mapping by monoclonal antibody detection of glycosaminoglycans in connective tissue. Nature 1984, 307:650-652 [DOI] [PubMed] [Google Scholar]
- 18.Meyer K, Grumbach MM, Linker A, Hoffman P: Excretion of sulfated mucopolysaccharides in gargoylism. Proc Soc Exp Biol Med 1958, 97:275-281 [DOI] [PubMed] [Google Scholar]
- 19.Dietrich CP, Martins JRM, Sampaio LO, Nader HB: Anomalous structure of urinary chondroitin sulfate from cancer patients. Lab Invest 1993, 68:439-445 [PubMed] [Google Scholar]
- 20.Stylianou E, Jenner LA, Davies M, Coles GA, Williams JD: Isolation, culture, and characterization of human peritoneal mesothelial cells. Kidney Int 1990, 37:1563-1570 [DOI] [PubMed] [Google Scholar]
- 21.Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227:680-685 [DOI] [PubMed] [Google Scholar]
- 22.Kruger RC, Schwartz NB: An improved method of sequential Alcian blue and ammonical silver staining of chondroitin sulfate proteoglycans in polyacrylamide gels. Anal Biochem 1987, 167:295-300 [DOI] [PubMed] [Google Scholar]
- 23.Enghild JJ, Thorgersen IB, Pizzo SV, Salvesen G: Analysis of inter-α-trypsin inhibitor and a novel trypsin inhibitor, pre-α-trypsin inhibitor, from human plasma. J Biol Chem 1989, 264:15975-15981 [PubMed] [Google Scholar]
- 24.Farndale RW, Sayers CA, Barrett AJ: A direct spectrophotometric microassay for sulphated glycosaminoglycans in cartilage cultures. Connect Tissue Res 1982, 9:247-248 [DOI] [PubMed] [Google Scholar]
- 25.Thomas GJ, Jenner L, Mason RM, Davies M: Human glomerular epithelial cell proteoglycans. Arch Biochem Biophys 1990, 278:11-20 [DOI] [PubMed] [Google Scholar]
- 26.Carney SL, Osborne DJ: The separation of chondroitin sulphate disaccharides and hyaluronan oligosaccharides by capillary zone electrophoresis. Anal Biochem 1991, 195:132-140 [DOI] [PubMed] [Google Scholar]
- 27.Enghild JJ, Salvesen G, Hefta SA, Thorgersen IB, Rutherford S, Pizzo SV: Chondroitin-4-sulfate covalently cross-links the chains of the human blood protein pre-α-inhibitor. J Biol Chem 1991, 266:747-751 [PubMed] [Google Scholar]
- 28.Chen L, Zhang H, Powers RW, Russell PT, Larsen WJ: Covalent linkage between proteins of the inter-α-inhibitor family and hyaluronic acid is mediated by a factor produced by granulosa cells. J Biol Chem 1996, 271:19409-19414 [DOI] [PubMed] [Google Scholar]
- 29.Salier J-P, Rouet P, Raguenez G, Daveau M: The inter-α-inhibitor family: from structure to regulation. Biochem J 1996, 315:1-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delaria KA, Muller DK, Marlor CW, Brown JE, Das RC, Roczniak SO, Tamburini PP: Characterization of placental bikunin, a novel human serine protease inhibitor. J Biol Chem 1997, 272:12209-12214 [DOI] [PubMed] [Google Scholar]
- 31.Thorgersen IB, Enghild JJ: Biosynthesis of bikunin proteins in the human carcinoma cell line HepG2 and in primary human hepatocytes: polypeptide assembly by glycosaminoglycans. J Biol Chem 1995, 270:18700-18709 [DOI] [PubMed] [Google Scholar]
- 32.Huang L, Yoneda M, Kimata K: A serum derived hyaluronan associated protein (SHAP) is the heavy chain of the inter-α-trypsin inhibitor. J Biol Chem 1993, 268:26723-26730 [PubMed] [Google Scholar]
- 33.Odum L: Inter-α-trypsin inhibitor: a plasma proteinase inhibitor with a unique chemical structure. Int J Biochem 1990, 22:925-930 [DOI] [PubMed] [Google Scholar]
- 34.Rouet P, Daveau M, Salier JP: Electrophoretic pattern of the inter-α-inhibitor family of proteins in human serum, characterized by chain specific antibodies. Biol Chem Hoppe-Seyler 1992, 373:1019-1024 [DOI] [PubMed] [Google Scholar]
- 35.Castillo GM, Templeton DM: Subunit structure of bovine ESF (extracellular-matrix stabilizing factor(s)): a chondroitin sulfate proteoglycan with homology to human α-1 trypsin inhibitors. FEBS Lett 1993, 318:292-296 [DOI] [PubMed] [Google Scholar]
- 36.Daveau M, Rouet P, Scotte M, Faye L, Hiron M, Lebreton J-P, Salier J-P: Human inter-α-inhibitor family in inflammation: simultaneous synthesis of positive and negative acute-phase proteins. Biochem J 1993, 292:485-492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blom A, Thuveson M, Fries E: Intracellular coupling of bikunin and the heavy chain of rat pre-α inhibitor in cos-1 cells. Biochem J 1997, 328:185-189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugiki H, Sumi H, Maruyama M, Yosihda E, Mihara H: Clearance and distribution of acid-stable trypsin inhibitor (ASTI). Enzyme 1989, 42:31-38 [DOI] [PubMed] [Google Scholar]
- 39.Sjoberg EM, Blom A, Larsson BS, Alston-Smith J, Sjoquist W, Fries E: Plasma clearance of rat bikunin: evidence for receptor-mediated uptake. Biochem J 1995, 308:881-887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindstrom KE, Blom A, Johnsson E, Haraldsson B, Fries E: High glomerular permeability of bikunin despite similarity in charge to albumin and hydrodynamic size to serum albumin. Kidney Int 1997, 51:1053-1058 [DOI] [PubMed] [Google Scholar]
- 41.Gebhard W, Hochstrasser K: Inter-α-trypsin inhibitor, and its close relatives. Barrett AJ Salvesen G eds. Proteinase Inhibitors. 1986, :pp 375-388 Elsevier, Amsterdam [Google Scholar]
- 42.Wachter E, Hochstrasser K, Bretzel G, Heindl S: Kunitz type proteinase inhibitors derived from limited proteolysis of the α trypsin inhibitor. II. Characterization of a second inhibitory inactive domain by amino acid sequence determination. Biol Chem Hoppe-Seyler 1979, 360:1297-1303 [DOI] [PubMed] [Google Scholar]
- 43.Buis B, Koomen GCM, Imholz ALT, Struijk DG, Reddingius RE, Arisz L, Krediet RT: Of electric charge on the transperitoneal transport of plasma-proteins during CAPD. Nephrol Dial Transplant 1996, 11:1113-1120 [PubMed] [Google Scholar]
- 44.Yoneda M, Susuki S, Kimata K: Hyaluronic acid associated with the surfaces of cultured fibroblasts is linked to a serum-derived 85-kd protein. J Biol Chem 1990, 265:5247-5257 [PubMed] [Google Scholar]
- 45.Zhao M, Yoneda M, Ohashi Y, Curono S, Iwata H, Ohnuki Y, Kimata K: Evidence for the covalent binding of SHAP, heavy chains of inter-α-trypsin inhibitor, to hyaluronan. J Biol Chem 1995, 270:26657-26663 [DOI] [PubMed] [Google Scholar]
- 46.Jessen TE, Odum L, Johnson AH: In vivo binding of human inter α trypsin inhibitor free heavy chains to hyaluronic acid. Biol Chem Hoppe-Seyler 1994, 375:521–526 [DOI] [PubMed]
- 47.Chen L, Moa JT, Larsen WJ: Identification of a factor in fetal bovine serum that stabilizes the cumulus extracellular matrix. J Biol Chem 1992, 267:12380-12386 [PubMed] [Google Scholar]
- 48.Carrell R, Travis J: α-1 anti-trypsin, and the serpins: variations and counter variations. Trends Biochem Sci 1985, 10:20-24 [Google Scholar]
- 49.Potempa J, Korzus E, Travis J: The serpin superfamily of proteinase inhibitors: structure, function, and regulation. J Biol Chem 1994, 269:15957-15960 [PubMed] [Google Scholar]
- 50.Potempa J, Kwon K, Chawla R, Travis J: Inter-α-trypsin inhibitor: inhibition spectrum of native and derived forms. J Biol Chem 1989, 264:15109-15114 [PubMed] [Google Scholar]
- 51.Heldin P, Pertoft H: Synthesis and assembly of the hyaluronan-coats around normal human mesothelial cells. Exp Cell Res 1993, 208:422-429 [DOI] [PubMed] [Google Scholar]
- 52.Blom A, Pertoft H, Fries E: Inter-α-inhibitor is required for the formation of the hyaluronan-containing coat on fibroblasts, and mesothelial cells. J Biol Chem 1995, 270:9698-9701 [DOI] [PubMed] [Google Scholar]
- 53.Chen L, Moa SJT, McLean LR, Powers RW, Larsen WJ: Proteins of the inter-α-trypsin inhibitor family stabilize the cumulus extracellular matrix through their direct binding with hyaluronic acid. J Biol Chem 1994, 269:28282-28287 [PubMed] [Google Scholar]
- 54.Camaioni A, Hascall VC, Yanagishita M, Salustri A: Effects of exogenous hyaluronic acid and serum on matrix organization and stability in the mouse cumulus cell-oocyte complex. J Biol Chem 1993, 268:20473-20481 [PubMed] [Google Scholar]
- 55.Wang T, Chen C, Heimburger O, Waniewski J, Bergstrom J, Lindholm B: Hyaluronan decreases peritoneal fluid absorption in peritoneal dialysis. J Am Soc Nephrol 1997, 8:1915-1920 [DOI] [PubMed] [Google Scholar]
- 56.Wang T, Cheng H-H, Heimburger O, Waniewski J, Bergstrom J, Lindholm B: Hyaluronan prevents the decreased net ultrafiltration caused by increased peritoneal dialysate fill volume. Kidney Int 1998, 53:496-502 [DOI] [PubMed] [Google Scholar]
- 57.Kaumeyer JF, Polazzi JO, Kotick MP: The messenger RNA for a proteinase inhibitor related to the HI-30 domain of inter-α-trypsin inhibitor also encodes α-1-microglobulin. Nucleic Acids Res 1986, 14:7839-7850 [DOI] [PMC free article] [PubMed] [Google Scholar]