Background
The first observations of programmed cell death were made more than a century ago, and the term “apoptosis” was coined for this widely occurring phenomenon as early as 2 decades ago 1,2. However, it is only during the past few years that serious mechanistic studies on it have been launched, leading to a revelation of its basic molecular intricacies. Along with this, investigators also working with clinical tumor material have obtained new analytical tools to study the role of apoptosis in cancer. Indeed, during the past years, there have been numerous papers in which the occurrence and extent of apoptosis and its association with the growth and progression of cancer have been studied in various types of neoplasms. This is not surprising, because, in essence, tumor growth is the net result of cell proliferation and cell loss. Rather, one may wonder why it is that, after so many years of meticulous use of not only mitotic count but also of more sophisticated proliferation markers as pointers of cell growth, it is only now that “apoptotic index” is becoming to be included among the parameters used to measure tumor growth.
In the following, we first give a brief overview of the molecular mechanisms of apoptosis. We then look at the role of apoptosis in cancer. Finally, we review studies done on the occurrence and extent of apoptosis in various types of tumors, and on the apoptotic index as a prognostic marker. We also reflect on, as extracted from the literature and based on our own experience, the currently used methods to determine the number of apoptotic cells in tumor samples and their limitations.
Introduction
Apoptosis is a complex, tightly regulated, and active cellular process whereby individual cells are triggered to undergo self-destruction in a manner that will neither injure neighboring cells nor elicit any inflammatory reaction. 3-6 Three phases can be discerned in apoptosis: initiation phase, effector phase, and degradation phase. 7 In the initiation phase, the cells receive a stimulus triggering the apoptotic process. In the effector phase, apoptotic machinery is activated, but the process is still reversible. 7 In the degradation phase, a point of no return is reached, beyond which the cell disintegrates. 7
The duration of the process of apoptotic cell death depends on the stimulus and the cell type and is usually estimated to take from 12 to 24 hours. 8 Visible changes in cell morphology last for 2 to 3 hours and are associated with the degradation phase. 7,8 The characteristic oligonucleosomal DNA fragmentation, manifesting itself as a ladder pattern in gel electrophoresis, is also a late event. 7,8
Molecular Mechanisms of Apoptosis
Caenorhabditis elegans and the CED Genes
Much of our knowledge about the molecular mechanisms and regulation of apoptosis comes from the studies on Caenorhabditis elegans. 9,10 During development, 131 of its 1090 cells are lost through apoptosis. 9,10 This process is mainly regulated by three genes, CED3, CED4, and CED9, of which CED3 and CED4 are positive regulators of apoptosis, whereas CED9 is antiapoptotic. 9,10 Homologous genes are found in higher organisms. The apoptosis-inducing caspases and apaf-1 correspond to CED3 and CED4, respectively, whereas the well-known antiapoptotic bcl-2 corresponds to CED9. 11-13
Induction of Apoptosis
A large number of stimuli can induce apoptosis in a cell type-dependent manner. 6,14 General inducers that act on most types of cells include various chemotherapeutic agents, ultraviolet and γ-irradiation, heat, osmotic imbalance, high calcium, and nitrogen oxide. 6,14 A selective induction, on the other hand, is seen, eg, in thymocytes that undergo massive apoptosis when exposed to glucocorticoids. 15 Also, ablation of a supply of a trophic hormone or a growth factor leads to apoptosis of only those cells that harbor the corresponding receptor. 16,17
Depending on the triggering factor and the cell type, there are multiple signaling pathways that lead to activation of the apoptotic machinery. A few of them are briefly mentioned here. Apoptosis induced by cytotoxic lymphocytes is mediated either by a nonsecretory, ligand-induced, and receptor-mediated mechanism or by a secretory perforin and granzyme-mediated mechanism. 18 In cases of DNA damage, apoptosis is initiated via p53-dependent pathway leading to activation of mediators such as bax and KILLER/DR5. 19,20 Withdrawal of a trophic factor, such as nerve growth factor in the case of neural cells or serum in the case of fibroblasts, triggers an apoptotic pathway mediated by p38 mitogen-activated protein kinase. 21 Changes in the composition of membrane phospholipids may also initiate apoptosis. 22-25 Radiation, for instance, leads to an activation of sphingomyelinase resulting in the degradation of sphingomyelin to ceramide. 22,25 Ceramide-mediated apoptosis is launched also by several other factors, such as serum deprivation, interleukin 1, tumor necrosis factor α, 1,25-dihydroxyvitamin D3, and nerve growth factor withdrawal. 23
One of the best-known apoptotic pathways is the one emanating from APO-1/FAS/CD95 receptor. 7,18,26-28 It belongs to a family of tumor necrosis factor-related receptor proteins and serves as a receptor for APO-L, a ligand present on cytotoxic T cells. 18,28 Its engagement leads to an ushering of cells toward apoptotic death. 18,28 Downstream of APO-1 is FADD/MORT1, which binds to the cytoplasmic end of APO-1. 28 Together, these two proteins form a death-inducing signaling complex with FLICE, a member of a large family of caspase proteins. 28,29 It is caspases that during the past few years have been extensively studied for their role in apoptosis. Indeed, they have been identified as a common final pathway of the execution of apoptosis in highly divergent systems. 28
Caspases
Caspases are cysteine proteases that cleave their target proteins at aspartic acid residues in a defined consensus sequence context. 30,31 Presently, at least 12 caspases are known. 30 They are expressed as precursors and are activated in a cascade-like cleavage parade. It involves cleaving the molecule to 10- and 20-kd subunits, which then heterodimerize and associate into tetramers that constitute the active enzyme. 31,32
The noncaspase target proteins include, eg, proteins of the DNA repair system, cytoskeletal or structural proteins, and oncoproteins. 33 Among the DNA repair enzymes is poly(ADP-ribose)-polymerase, which, after DNA damage, catalyzes attachment of ADP-ribose to nuclear proteins, such as histones. 33,34 Cytoskeletal or structural substrates include nuclear lamins, fodrin, cytokeratin 18, actin, and catenin β. 33,35-37 Best-known oncoproteins degraded by caspases are the retinoblastoma protein (Rb) and mdm2. 38,39 Recently, caspases have also been shown to activate DNase leading to chromosomal breakage of DNA during apoptosis. 40
The bcl-2 Family
The bcl-2 family is another group of closely related proteins that plays a major role in apoptosis. It includes death-promoting and death-inhibiting members. 5,6,11,41-43 In a sense, they can be considered to operate at checkpoints in which it is determined whether a cell is ushered toward survival or death. They act upstream of caspases. 44
Apoptosis-inhibiting members of the bcl-2 family include bcl-2, bcl-xL, bcl-w, bfl-1, brag-1, mcl-1, and A1. 5,6,41-43 Apoptosis-promoting members are bax, bak, bcl-xS, bad, bid, bik, and Hrk. 5,6,41-43 Instrumental for their action is homo- and heterodimerization, which occurs through their conserved domains. 5,6,41-43,45-47 They regulate apoptosis in a rheostatic manner; in an excess of bax, for instance, bax homodimers predominate, which favors apoptosis. 5,6 Conversely, in an excess of bcl-2, bcl-2/bax heterodimers are formed, which leads to inhibition of apoptosis. 5,6 Competition between family members also has an effect. bcl-xL, for example, inhibits apoptosis by binding and sequestering bax. 6 By binding bcl-2 and bcl-xL, bad, on the other hand, releases bax, which leads to bax homodimerization and promotion of apoptosis. 6
bcl-2 is the epitome of an antiapoptotic or survival gene. Attesting to its role in an apoptosis checkpoint, it counteracts apoptosis initiated by quite disparate signals, such as chemotherapeutic drugs, oxidative stress, viral infections, and p53. 6 In lymphoid cells, for instance, bcl-2 inhibits apoptosis induced by glucocorticoids and growth factor withdrawal. 6 Indeed, in many cases, actions of bcl-2 underlie the well-known survival functions of hormones and growth factors. Thus, for example, in breast epithelial cells, estrogen stimulation leads to upregulation of bcl-2 and resistance to apoptosis. 48 Upregulation of bcl-2 and bcl-xL is also effected by interleukins. 49,50
Many members of the bcl-2 family, such as bcl-2, bcl-xL, and bax, are resident proteins of the mitochondrial membranes, endoplasmic reticulum, and nuclear envelope in which they are inserted via their carboxy-terminal ends. 11,43,51 In mitochondria, they form pores and act as ion channels. 43,52-54 This is probably the key to their function in apoptosis. Namely, induction of apoptosis is almost invariably accompanied by disruption of the mitochondrial transmembrane potential and release of caspase-activating substances, such as cytochrome c and apoptosis-inducing factor, from the mitochondria. 43,52-54 Consistent with their role as negative regulators of apoptosis, induction of expression of bcl-2 and bcl-xL effectively counteracts the flow of these molecules to cytosol, whereas bax promotes it. 55,56 bcl-2 and bcl-xL counteract induction of apoptosis also by binding to apaf-1, which prevents it from activating pro-caspase-3. 12,13 A very recent study has demonstrated that caspase-3 is able to cleave the loop domain of bcl-2 at Asp, 34 and that the carboxyl-terminal cleavage product triggers and accelerates apoptosis. 57 It remains to be seen whether in tumors showing a positive association between bcl-2 expression and the extent of apoptosis, bcl-2 is present in a cleaved, and thus, in fact, apoptosis-promoting form.
Ever since its discovery as an upregulated gene in t(14;18) translocation in follicular lymphoma, bcl-2 has been considered as an “oncogene.” 58 Now it has been revealed that its apoptosis-promoting countervail bax is a bona fide tumor suppressor gene. This is implied by a recent study of Rampino et al, 59 who have shown that more than 50% of colon cancers exhibiting the microsatellite mutator phenotype contain disabling somatic mutations in the Bax gene. 59 None of the microsatellite mutator phenotype-negative tumors showed any mutations. Given the role of bax in apoptosis, this strongly supports the notion that paralysis of the death machinery is, by way of leaving genetically injured cells uneliminated, an important step in the progression of cancer.
Cancer Genes and Apoptosis
One facet of the intertwinement of apoptosis and cancer is the involvement of many oncogene and tumor suppressor gene products in the regulation and execution of apoptosis. Among them are p53, Rb, ras, raf, and myc. p53, because of its role in apoptosis, has earned the name “guardian of the genome.” 60 It monitors the state of DNA, and, in case of DNA damage, stalls the cell cycle. 60-62 This takes place through the induction of CIP/WAF1/p21, a protein that prevents phosphorylation of cyclin-dependent kinases, the well-known positive regulators of the cell cycle. 63,64 In the absence of phosphorylated, active cyclin-dependent kinases, also another regulator of the cell cycle, Rb, remains inactive (unphoshorylated), and, hence, the cell cycle halts. 65 This then leads to activation of DNA repair machinery. If the DNA repair fails, p53 takes over again and triggers apoptosis in a process that involves upregulation of the apoptosis-inducing bax and down-regulation of the anti-apoptotic bcl-2. 19,60,66,67 p53 also upregulates KILLER/DR5, a novel 45-kd apoptosis-inducing member of the tumor necrosis factor receptor family. 20,68 Analogous to the APO-1/FAS/CD95 receptor system, its activation also leads to a FLICE-mediated caspase activation. 20,68
Proto-oncogenes myc and ras are also part of the apoptotic machinery. The role of myc is capricious, because it depends critically on how the cell is “conditioned” by other factors. Thus, in the presence of growth factors, it induces proliferation, whereas in their absence, it acts apoptotic. 69
Overexpression of ras may lead to increased or decreased apoptosis. 70-72 It is negatively regulated by bcl-2. 73 Phosphorylation of bcl-2, however, invalidates its capacity to protect cells from ras-induced apoptosis. 73
Morphology of Apoptosis
Several light and electron microscopically detectable changes characterize apoptosis. 1,3,4 They include, most conspicuously, condensation of the chromatin to sharply delineated granular masses along the nuclear envelope, shrinking of the cells, convolution of the cellular and nuclear outlines, and fragmentation of the nucleus. 1,3,4 Finally, the cell disintegrates into membrane-bound apoptotic bodies that contain, eg, nuclear remains, and that are quickly removed by neighboring macrophages. 1,4 Throughout this process, the cell membrane and the membrane encasing the apoptotic fragments retain their integrity. 1,4 Also, the lysosomes remain intact and, hence, lysosomal enzymes are not released to the surrounding tissues. 1,4 Consequently, there is no associated inflammation in apoptosis. 1,4
Apoptosis and Necrosis
Necrosis, in contrast to apoptosis, is considered to be a passive and a much more vaguely regulated event, the nature of which is dictated more by the type of the external injurious agent than by the internal workings of the cell. 4 The most obvious difference is that, whereas necrosis leads to a destruction of a large group of cells in the same area, in apoptosis, only scattered cells are involved. 4 The basic mechanistic difference is that in necrosis, because of membrane damage, there is swelling of the cytoplasm and bursting of the cell, which leads to a release of lysosomal enzymes and to inflammation. 4 In apoptosis, the outer cell membrane remains intact and the entire process is “contained” without any harm done to the neighboring cells. 4
There are also differences in cellular morphology. Unlike in apoptosis, in necrosis, the chromatin is never marginated. Rather, it is unevenly distributed as clumps that are irregular and poorly defined. 4 Moreover, there is no nuclear fragmentation, cellular shrinking, or “body” formation in necrosis. 4
Although quite distinct by appearance and considered antithetical, necrosis and apoptosis have been recently shown to be mechanistically related, eg, in the following ways. 8,52 First, in vitro, certain stimuli are apoptotic at low doses but bring about necrosis when present in high doses. 8 Second, many stimuli, such as heat shock, hypoxia, viruses, radiation, nitric oxide etc, can induce both apoptosis and necrosis. 8 Third, at a tissue level, areas of necrosis are surrounded by a zone of apoptotic cells, suggesting that they are associated phenomena. 74 Relatedness between apoptosis and necrosis is also seen at a biochemical level. Depletion of intracellular ATP in human T cells shifts cell death from apoptosis to necrosis. 75 Furthermore, caspases 8 and 10, which are located upstream in the signaling pathway, can also provoke necrosis. 8 This, on the other hand, can be inhibited by the anti-apoptotic bcl-2 protein, suggesting that at least part of the signaling machinery is shared. 8
The dual nature of the death process has remained enigmatic. One way to settle this “apoptotic paradox” is to view the death process as a dichotomous event, the direction of which is highly context dependent. 52 Experimental evidence for this includes observations indicating that stimuli that under normal conditions lead to apoptosis may initiate necrosis under conditions of low intracel- lular ATP. 8,75 Also, the availability of apoptotic proteases, eg, activated caspases, direct the cell death pathway such that sudden and extensive damage may exhaust the apoptotic machinery, causing it to yield to necrosis. 52
Morphological Detection of Apoptosis
Detection of apoptotic cells in tissue sections in, eg, tumors is possible because of characteristic morphological features that are manifest even in routinely stained sections. 1,3,4 Recently, more refined techniques have also been developed for tissue studies, which are based on the detection of apoptosis-specific biochemical changes or expression of apoptosis-associated proteins directly on tissue sections. 76-78 Thus, for instance, the fragmentation of DNA into 180 to 200-bp fragments, the biochemical hallmark of apoptosis, is being used in morphological analysis of apoptosis. 4 Such techniques make use of radioactive or nonradioactive labeling of the free ends of the DNA, allowing accurate identification of single apoptotic cells. 76,78 This technique, called in situ 3′-end labeling method, can be divided in two variants. In the first, DNA polymerase or its Klenow fragment is used to incorporate labeled nucleotides into fragmented DNA by in situ nick translation. 79,80 In terminal deoxytransferase-mediated dUTP nick-end labeling (TUNEL), on the other hand, terminal transferase is used to add labeled nucleotides into the 3′-end of the DNA. 80 For the detection of the radioactive label, autoradiography is used, whereas with nonradioactive labeling, usually an appropriate chromogen reaction is used.
Apoptotic Index
In histological tumor material, apoptotic index is used as a measure of the extent of apoptosis. Most often it is defined as a percentage of apoptotic cells and bodies per all tumor cells. Some authors, however, use it to denote the number of apoptotic cells per 1000 tumor cells. 81 Furthermore, in some investigations, apoptosis is measured as number of apoptotic cells per 10 high-power fields. 82
Table 1 ▶ shows a comprehensive list of studies with apoptotic indexes reported for different types of tumors by using either DNA end-labeling techniques (TUNEL) or plain morphology. In all listed cases, the apoptotic index is given as a percentage of apoptotic cells in tumor cell population.
Table 1.
The Extent of Apoptosis in Various Types of Tumors
| Tumor type | Apoptotic index % | Method | References |
|---|---|---|---|
| Non-Hodgkin’s lymphoma | |||
| High grade | 8.80, 1.44, 3.23 | T | 96,97,98 |
| Low grade | 2.40, 0.38, 0.71 | T | 96,97,98 |
| Lung carcinoma | |||
| NSCLC | 0.37 | T | 137 |
| 0.95, 1.00 | M | 135,162 | |
| SQCLC | 2.30, 2.10 | M | 136,141 |
| 1.20, 2.30 | T | 134,163 | |
| AC | 1.10, 1.50 | M | 135,141 |
| 1.20, 1.30 | T | 134,163 | |
| SCLC | 0.10, 10.9, 2.65 | T | 140,163,164 |
| 10.6 | M | 141 | |
| Colon carcinoma | 3.50, 1.90, 5.10 | T | 127,165,166 |
| 3.60, 4.70 | M | 141, 167 | |
| Endometrial carcinoma | 0.89–1.29, 1.18–5.15 | M | 111,112 |
| Prostate carcinoma | 5.40 | T | 119 |
| 0.87, 0.41, 0.80 | M | 121,141,168 | |
| Gastric carcinoma | 1.12–1.26, 1.48–4.83 | M | 81,126 |
| 2.80–5.10, 3.69–4.10 | T | 81,150 | |
| Breast carcinoma | 1.20 | M | 141 |
| 0.76 | T | 106 | |
| Thyroid carcinoma | 0.20–1.40 | T | 122 |
| Liver carcinoma | 0.73 | T | 129 |
| Pancreatic carcinoma | 0.59 | T | 130 |
| Bladder carcinoma | 0.54–1.24 | T | 142 |
| Salivary gland tumors | |||
| Benign | 0.01 | T | 94 |
| Malignant | 0.42 | T | 94 |
NSCLC, non-small-cell lung carcinoma; SQCLC, squamous cell lung carcinoma; AC, adenocarcinoma; SCLC, small cell lung carcinoma. T, TUNEL assessment; M, light microscopic assessment.
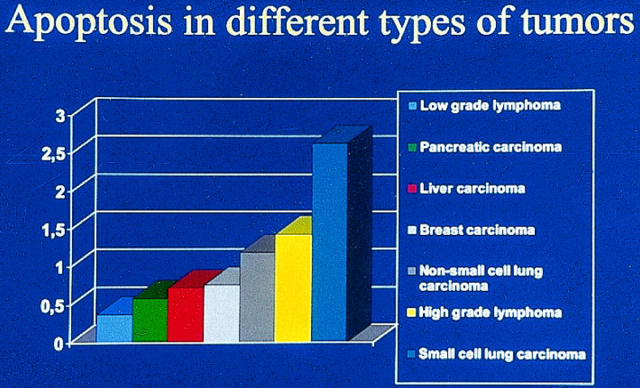
It is readily apparent that there is a wide variation in the extent of apoptosis not only between different tumors but also within a tumor type (Table 1 ▶ and Figure 1 ▶ ). In high-grade non-Hodgkin’s lymphomas, for instance, the average apoptotic index varies between 1.4 and 8.8% and in small cell lung carcinoma between 0.1 and 10.9%. Even though there may be biological variations within the tumor groups (in carcinomas the relative number of grade I, II, and III lesions may vary, and in lymphomas different types of histological lesions may be variably represented), it is quite unlikely that it could solely account for the observed variability. Indeed, there are a number of methodological and technical factors that may influence the determination of the apoptotic index.
Figure 1.
Variation in apoptotic index in different types of tumors in materials processed and analyzed in a similar manner (data are from Refs. 98, 106, 129, 130, 134, and 140 ). Of the tumors shown, low-grade malignant lymphomas have the lowest and small cell lung carcinomas the highest apoptotic index. The values presented represent averages, and wide variations between studies (as noted in Table 1 ▶ ) are common.
Detection of Apoptotic Cells
The extent of apoptosis may vary in different areas of the tumor and, frequently, apoptotic cells appear in clusters. 83 Consequently, to avoid erroneous results, care should be taken to include enough fields in the analysis. It is estimated that at least 20 microscopic fields of 1000× magnification (containing an average of 1500 cells each) should be examined to guarantee representativeness. 83
The identification of apoptotic cells also depends on the magnification used. With a lower magnification, fewer apoptotic cells are detected, and there is an increase in interobserver variability 83 Therefore, a high-power lens should be used.
One obvious cause for interobserver variation is error in counting the number of tumor cells in a given field. An erroneous estimate of the total number of tumor cells in the field easily changes the apoptotic index severalfold.
The duration of the morphologically detectable phase of apoptosis may vary and influence the apoptotic index. 84 In tumors in which detectable apoptotic changes take a longer time, the apoptotic index is higher, even though the actual number of cells undergoing apoptosis would be the same. 84 Because of this, the apoptotic index cannot be correlated with an actual “death index,” even though technical problems with measuring the apoptotic index could be solved.
Nonneoplastic apoptotic cells may also pose a problem for the estimation of the apoptotic index. This is the case especially in lymphomas, in which apoptotic macrophages and lymphocytes may resemble apoptotic neoplastic cells. Moreover, neoplastic cells may even stimulate apoptosis in reactive cells. This can take place if tumor cells express FAS-L on their surface. 85,86 The ligand may associate with the APO-1/FAS/CD95 receptor of the invading normal T lymphocytes and trigger apoptosis in them. 85,86 Apoptotic T lymphocytes mistakenly counted as apoptotic tumor cells would give a distorted view of the relationship between apoptosis and tumor growth.
False Positives and Negatives
Other sources of misinterpretation lie in the differences in the sensitivities and specificities of the end-labeling techniques and in such technicalities as, for instance, the type of pretreatment, the type of labeling enzyme, the method of tissue processing, and incubation times. 79,87,88
It seems to be a common experience that performance of TUNEL labeling depends greatly on the tissue pretreatment, concentration of the terminal transferase enzyme, and the type and concentration of the fixative (Table 2) ▶ . 80,87,89,90 Ethanol fixation, for example, is associated with diminished staining intensity and increased background staining compared with 10% buffered formalin. 88 Of the tissue pretreatments, microwave treatment of the formalin-fixed tissue raises the number of labeled cells twofold compared with proteinase K pretreatment. 80
Table 2.
Factors Reported to Influence DNA End Labeling
| Fixative used |
|---|
| Concentration of the fixative |
| Pretreatment used affects the results |
| Tissue drying |
| DNA strand breaks are not solely associated with apoptosis (fragmentation may be present in necrosis or may be caused by DNA-damaging agents) |
| Long fixation times (more than 3 weeks) may influence apoptosis |
| Delay in fixation |
| Duration and concentration of the polymerase or transferase enzyme treatment influence the number of nuclei stained |
In the in situ 3′-end labeling method by in situ nick translation, a high concentration of or a prolonged incubation with polymerase leads to a gradual increase of the labeling of the morphologically normal nuclei. 79 The number and intensity of the labeled nuclei also depends on the duration of the tissue pretreatment with pepsin or proteinase K; a brief treatment leaves many apoptotic cells unlabeled, whereas too long a pretreatment results in labeling of morphologically nonapoptotic nuclei. 79,87 In the studies referred to in Table 1 ▶ , proteinase K at a concentration of 20 to 40 μg/ml for 15 minutes was usually used. In many studies, however, there is no mention of the proteinase K concentration.
A further caveat of the end labeling methods is that cells other than apoptotic cells can also become labeled. Such false positives are seen especially in cases with DNA damage, autolysis, tissue drying, and necrosis. 87,89,91 Conversely, formalin fixation of a long duration can give rise to “false negatives” because of a decreased labeling of apoptotic cells. 92
The lowest numbers of apoptotic cells are usually scored in light microscopy based solely on morphology (Table 1) ▶ . This may be due to the fact that morphological manifestations of apoptosis, such as shrinking of the nucleus, are of such a short duration or inconspicuous that they could go partially undetected. Results obtained by “plain” morphology show, however, a good correlation with DNA end labeling methods. 93,94 Thus, morphology alone, although less sensitive, is a fairly reliable and inexpensive method for the detection of apoptosis.
Comparison of in situ nick translation and TUNEL shows that TUNEL is more sensitive. 87,88 This could be at least partially due to the ability of terminal transferase (used in TUNEL) to label both double- and single-stranded DNA breaks, whereas polymerase I (of in situ nick translation) only labels single-stranded breaks. 80,88 Also, the kinetics of the enzymes are different; DNA polymerase I is slower than terminal transferase in incorporating nucleotides. 87 In investigations on clinical tumor material, the TUNEL technique has almost exclusively been used.
Occurrence of Apoptosis in Human Tumors
Lymphomas
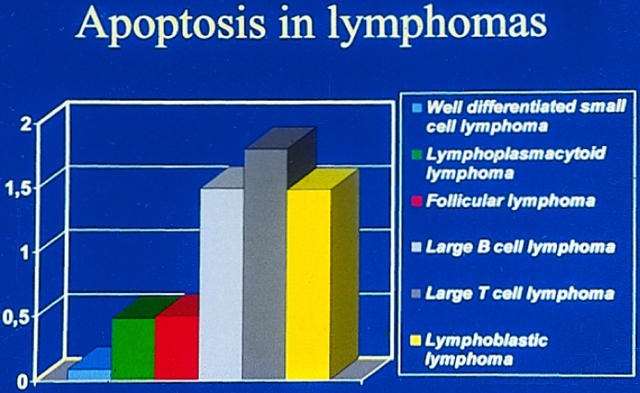
High-grade malignant non-Hodgkin’s lymphomas show a significantly higher apoptotic index than low-grade lymphomas (Figure 2) ▶ . 95-98 This correlates nicely with the occurrence of antiapoptotic bcl-2, which is overexpressed in low-grade follicular lymphomas due to a translocation that takes the bcl-2 locus to a highly active chromosomal environment. 58 In fact, there is an inverse association between the immunohistochemical expression of bcl-2 and the apoptotic index. 95,98 Also, other members of the bcl-2 group, such as the apoptosis-promoting bax and apoptosis-inhibiting mcl-1, are expressed in lymphomas. 98,99 They are also seen in Reed-Sternberg cells of Hodgkin’s disease. 100,101 A potentially important factor is Epstein-Barr virus, which is found in Hodgkin’s disease, in some other lymphomas, and also in some nonlymphoid neoplasms such as gastric and nasopharyngeal carcinomas. 102-104 Its latent membrane protein 1 upregulates bcl-2 and can, in this way, influence the extent of apoptosis. 105
Figure 2.
Difference in the extent of apoptosis between high-grade and low-grade lymphomas (data from Ref. 98 ). High-grade lymphomas display a significantly higher extent of apoptosis than do low-grade lymphomas.
Breast Carcinomas
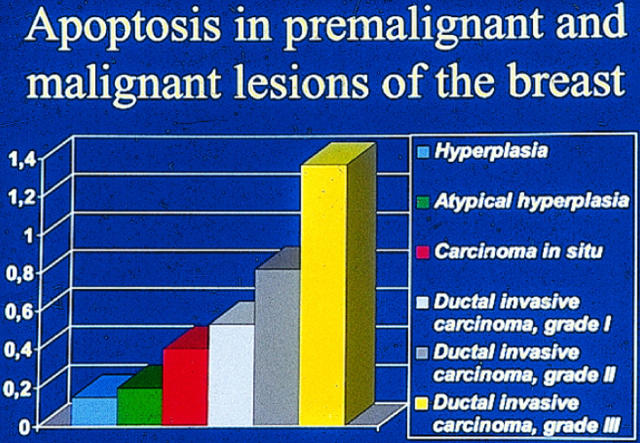
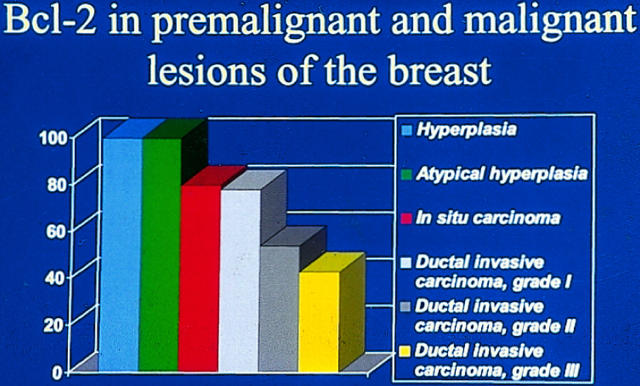
In breast carcinomas, a high extent of apoptosis is associated with a poor prognosis, and more apoptosis is seen in tumors of high grade (Figures 3 and 4) ▶ ▶ . 82,106 This is probably due to a loss of receptors for hormones that act as survival factors. Interestingly, in breast carcinomas, concurrent expression of progesterone or estrogen receptors and of antiapoptotic bcl-2 can be seen. 82,107 This correlates with the cell culture studies that show that stimulation of the estrogen receptors leads to upregulation of bcl-2. 108 bcl-2 expression is seen in 70% of breast carcinomas, and its expression is inversely associated with the apoptotic index and with a better prognosis. 82,106,109,110
Figure 3.
Relation of the extent of apoptosis with different types of breast lesions (data from Ref. 106 ). The extent of apoptosis increases parallel with the neoplastic potential of the breast lesion.
Figure 4.
Relation of bcl-2 expression with different types of breast lesions (data from Ref. 106 ). bcl-2 is inversely related to the neoplastic potential of the breast lesion.
Endometrial Carcinomas
Apoptosis is increased in high-grade endometrial adenocarcinomas and is more pronounced in tumor areas with a solid growth pattern. 111,112 To some extent, this correlates inversely with the expression of bcl-2, which is strongly expressed in epithelial cells of the normal endometrium but reduced in atypical hyperplasias and carcinomas. 113,114 The expression of bcl-2 is associated with a lower extent of apoptosis. 112
Prostate Carcinoma
In prostate cancer as well, hormones acting as survival factors and their receptors play a role. It is well known that androgen deprivation leads to apoptosis of normal prostate epithelial cells and tumor cells. 115,116 Characteristic of prostatic carcinoma cells is that further down in the course of progression, they tend to gain resistance to the apoptosis-inducing hormone withdrawal. 117 High bcl-2 expression is found in androgen-independent prostate tumors, suggesting that bcl-2 upregulation contributes to the survival of neoplastic cells in a hormonally deprived environment. 118 In line with this, the extent of apoptosis was found to be lower in recurrent than primary tumors. 119 bcl-2 positivity is found in only 25% of prostate carcinomas and is reported to be higher in high-grade tumors (Table 3 ▶ and Figure 5 ▶ ). 120 Another antiapoptotic factor, mcl-1, is expressed in 81% of prostate carcinomas and is also more frequently seen in high-grade tumors. 120 Even though there is a decrease in apoptosis in recurring tumors, and the expression of antiapoptotic bcl-2 and mcl-1 is higher in high-grade tumors, increased apoptosis has been associated with a poor prognosis. 121
Table 3.
Immunohistochemical bcl-2 Expression in Carcinomas
| Tumor type | bcl-2 | Reference |
|---|---|---|
| Lung carcinoma | ||
| SQCLC | 35% | 136 |
| 28% | 134 | |
| 36% | 169 | |
| 25% | 170 | |
| AC | 0% | 136 |
| 8% | 134 | |
| 12% | 170 | |
| SCLC | 93.70% | 169 |
| Colon carcinoma | 67% | 127 |
| 5.3% | 167 | |
| Endometrial carcinoma | 50–78% | 112 |
| 47% | 113 | |
| Prostate carcinoma | 25% | 120 |
| Gastric carcinoma | 43% | 126 |
| Breast carcinoma | 62% | 106 |
| Thyroid carcinoma | 74% | 122 |
| Liver carcinoma | ||
| Hepatocellular | 0% | 133 |
| 15% | 132 | |
| Cholangiocarcinoma | 60% | 133 |
| Pancreatic carcinoma | 55% | 171 |
| Bladder carcinoma | 24.7% | 143 |
SQCLC, squamous cell lung carcinoma; AC, adenocarcinoma; SCLC, small cell lung carcinoma.
Figure 5.
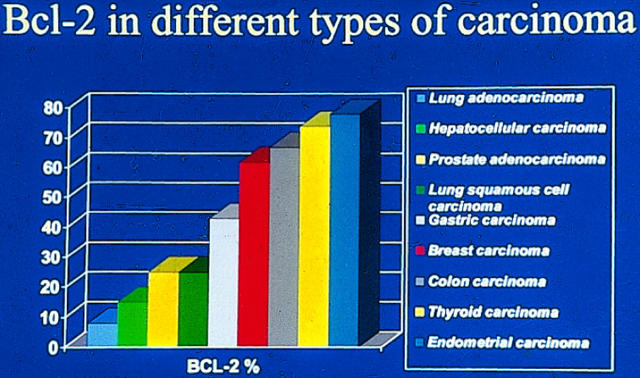
Variation in bcl-2 expression in different types of carcinomas (data are from references shown in Table 3 ▶ ). Highest bcl-2 expression is seen in thyroid and endometrial carcinomas, whereas lowest expression is seen in lung adenocarcinoma and hepatocellular carcinoma.
Thyroid and Adrenal Tumors
More apoptosis is found in thyroid carcinomas with a low degree of differentiation. 122 There is an inverse association between the bcl-2 expression and apoptosis in papillary thyroid carcinomas but not thyroid cancers of other histological types. 122
In adrenal cortical tumors, apoptosis is reported to be lower or at the same level as in nonneoplastic tissues. 123,124 In cell culture studies, bovine adrenocortical cells undergo apoptosis in adrenocorticotropic hormone-free culture conditions. 125
Gastrointestinal Carcinomas
In gastric and colon carcinomas, lower apoptotic indexes have been reported in early than in advanced-stage lesions, but there are also opposing results. 81,126 Positive bcl-2 immunoreactivity is found in 43 and 67% of the gastric and colon carcinomas, respectively, and its occurrence seems to be inversely associated with apoptosis (Table 3 ▶ and Figure 5 ▶ ). 126,127 Stronger bcl-2 expression was associated with gastric carcinomas of the diffuse type. 128 On the other hand, expression of bax, mcl-1, and bcl-X was more frequent in the intestinal type. 128
In pancreatic and hepatocellular carcinomas, no association is found between apoptosis and the grade of tumor or survival of the patients. 129,130 Among liver carcinomas, hepatocellular carcinomas show a much lower bcl-2 expression than cholangiocarcinomas (Table 2) ▶ . 131-133 This is analogous to bcl-2 expression in the nonneoplastic liver; normal hepatocytes do not express bcl-2, whereas a high degree of expression is seen in small bile ducts. 131,133
Lung Carcinomas
In non-small cell lung carcinomas (NSCLCs), no association between apoptosis and survival or advanced stage of the tumor is usually found, but again, there are also conflicting reports. 134-137 In NSCLCs, bcl-2 expression can be seen in only 8 to 30% of the cases, whereas in small cell lung carcinomas, it is present in 90%. 138,139 Curiously enough, small cell lung carcinomas have a higher apoptotic index than NSCLCs, even though their bcl-2 expression is high. 140,141
Urogenital Carcinomas
In transitional cell carcinomas of the bladder, high-grade tumors display a higher apoptotic index. 142 bcl-2 expression is low, ranging from 5 to 25% in different reports. 142,143 A similar low frequency of bcl-2 expression is also found in transitional cell carcinomas of the renal pelvis and in renal cell adenocarcinoma. 144,145
Other Tumors
Other tumor groups have been less extensively studied, and in many cases only single reports are available. In brain tumors, more apoptosis is seen in grade II gliomas compared with grade III lesions, suggesting that apoptosis contributes to a better prognosis. 146 Glioblastomas, however, have a higher apoptotic index than better-differentiated tumors. 146
Also in germ cell tumors, apoptosis varies in histologically different types of tumors, being highest in the more aggressive and less-differentiated ones, such as embryonal carcinomas. 147 Interestingly, bcl-2 expression was only found in teratocarcinomas but not in other germ cell tumors. 147
Apoptosis in sarcomas and mesenchymal tumors has not been studied extensively. The apoptotic index in most sarcomas is generally between 0 and 6%. 141
Premalignant Lesions
In atypical hyperplasias and in in situ carcinomas of the breast, a rise in the apoptotic index and a decrease in the bcl-2 immunoreactivity is associated with the grade of the lesion. 106 In endometrial atypical hyperplasias and in esophageal Barrett’s metaplasia or dysplasia, a decrease in bcl-2 immunoreactivity was also seen. 148 Thus, in these cases, the idea of less apoptosis promoting the early steps of carcinogenesis is not sustained, at least in its simplistic form. Rather, in light of these examples, a high degree of apoptosis in premalignant lesions can be considered to be a reflection of an ardent effort to eliminate genetically damaged cells. This, in fact, has been suggested in studies on apoptosis in gastric premalignant lesions and in dysplasias of the oral cavity, which show that the apoptotic index may be even higher in dysplastic lesions than in the corresponding invasive carcinomas. 149,150
On the other hand, in dysplastic lesions of the colon and stomach, there is an increase of bcl-2 immunoreactivity compared with invasive tumors. 127,151,152 This finding has been appropriately interpreted to mean that dysplastic, most likely genetically compromised, cells thus become saved from apoptosis, which contributes to the neoplastic progression. Clearly, further studies are needed to get a comprehensive view on apoptosis and its regulating proteins in preneoplastic lesions.
Why Is There Increased Apoptosis in Cancer?
It is obvious from the considerations above that apoptosis is generally increased in cancer. In fact, there are only a few tumors, such as follicular B-cell lymphomas, in which inhibition of apoptosis has been convincingly shown to play a decisive role in the development of neoplasia. 58 On the other hand, occurrence of apoptosis does not show any single rule in its relation to tumor stage, grade, or progression. Rather, a high degree of tumor-dependent variability is seen.
Why then is it that apoptosis is so often increased in tumors? Part of the explanation probably lies in the activation of cancer genes in the process of neoplastic development, some of which also influence apoptosis. In such cases, the degree of apoptosis is a reflection of the internal functioning of the death machinery. In other cases, apoptosis is due to extrinsic factors such as activated T cells that launch a FAS-mediated apoptosis in tumor cells.
Loss of Cell Adhesion and Hypoxia Enhance Apoptosis
One cell biological explanation for apoptosis in tumor cells is the increased sensitivity to apoptosis of cells that have lost their matrix attachment or cell-cell contacts. 153 This could be due, for instance, to a loss of an expression of cell adhesion molecules from the surface of the neoplastic cells. Especially important in this sense are integrin and cadherin molecules. 154 Still another factor that is conducive to apoptosis in tumors are the hypoxic conditions that prevail in tumors. For instance, in experimental brain necrosis of rats, apoptosis is seen primarily in the area bordering the ischemic zone. 74 Also in tumors, an increased number of apoptotic cells are seen adjacent to necrotic areas. 155
Apoptosis and Proliferation Are Mechanistically Linked
A consistent feature in many studies is the positive correlation or association between apoptosis and proliferation, suggesting that they are mechanistically linked (see Table 4 ▶ ). One link relates to the fact that although apoptosis may be initiated in any phase of the cell cycle, the majority of cells undergo apoptosis primarily in the G1 phase of cycling cells. 156 In fact, many proteins that operate in the cell cycle checkpoints are also regulators and inducers of apoptosis. Examples of such are p53 and Rb proteins, which act on the G1/S checkpoint. Also, overexpression of cyclins, such as cyclins D1, A, and B, can induce apoptosis. 157-161
Table 4.
Apoptotic Index and Proliferation
| Tumor type | Method | Reference |
|---|---|---|
| Apoptotic index associated with proliferation | ||
| Non-Hodgkin’s lymphoma | Histone mRNA in situ labeling | 96 |
| MIB1 | 97 | |
| NSCLC | Ki-67 | 136 |
| Mitotic count | 135 | |
| Colon carcinoma | Ki-67 | 165 |
| Mitotic count | 167 | |
| Gastric carcinoma | Mitotic count | 126 |
| Endometrial carcinoma | Mitotic count | 112 |
| Breast carcinoma | Mitotic count | 82 |
| Bladder carcinoma | Ki-67 | 142 |
| Apoptotic index not associated with proliferation | ||
| Gastric carcinoma | MIB1 | 81 |
| Small cell carcinoma | PCNA | 140 |
| Pancreatic carcinoma | PCNA | 130 |
| NSCLC | PCNA | 134 |
PCNA, proliferating cell nuclear antigen.
Concluding Remarks
Even though there is a high degree of variability in the apoptotic index reported by different authors for the same types of tumors, some generalizations on apoptosis and its associations with some clinical and biological parameters can be made. In lymphomas and hormone-dependent epithelial tumors, such as breast, endometrial, or thyroid carcinomas, a higher extent of apoptosis is associated with tumors of a higher grade. This is in contrast to other epithelial tumors, in which association with tumor grade is variable and less evident. Another feature is that the apoptotic index in most tumors is associated with cell proliferation, suggesting a mechanistic link between these two mechanisms.
Because proliferation and apoptosis contribute to tumor growth, some authors have created compound indexes taking into consideration the influence of both proliferation and apoptosis and even necrosis. 129,143,150 In these studies, the ratio between apoptotic and mitotic index was higher in dysplasias than in invasive carcinomas, suggesting that apoptosis is overwhelmed by cell proliferation in invasive lesions. 145,149 In hepatocellular carcinomas, tumors showing a high extent of proliferation and a low extent of apoptosis and necrosis had a significantly worse prognosis than other tumors. 129 Thus, it seems to be more reasonable to combine apoptosis and proliferation, and perhaps also include necrosis, to a common index when evaluating their impact on tumor growth and prognosis in various neoplasias. Indeed, when evaluated alone, the extent of apoptosis does not generally associate with survival (see Table 5 ▶ ).
Table 5.
Apoptotic Index and Survival
| Tumor type | References |
|---|---|
| Prognosis not associated with a high apoptotic index | |
| NSCLC | 135, 137 |
| Small cell carcinoma | 140 |
| Gastric carcinoma | 126 |
| Thyroid carcinoma | 122 |
| Hepatocellular carcinoma | 129 |
| Poor prognosis associated with a high apoptotic index | |
| NSCLC | 134 |
| Breast carcinoma | 82 |
| Poor prognosis associated with a low apoptotic index | |
| Colon carcinoma | 167 |
Finally, the studies show that in the evaluation of the apoptotic index there are technical and methodological problems. To reduce interobserver variations, a consent on the criteria of how to define and calculate the “apoptotic index” is needed. As far as the methodological factors are concerned, at least some standard protocols on tissue processing, protease pretreatments, incubations, etc., should be pursued. Also, applying more than one method to determine apoptosis would improve the accuracy and reliability. For instance, inclusion of a plain morphological evaluation of apoptosis with the 3′-end labeling method would be an inexpensive and straightforward complement to the investigation.
Footnotes
Address reprint requests to Dr. Ylermi Soini, Department of Pathology, University of Oulu, Kajaanintie 52 D, FIN-90220 Oulu, Finland. E-mail: msoini@cc.oulu.fi.
References
- 1.Kerr JFR, Wyllie AH, Currie AR: Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 1972, 26:239-257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Majno G, Joris I: Apoptosis, oncosis, and necrosis: an overview of cell death. Am J Pathol 1995, 146:3-15 [PMC free article] [PubMed] [Google Scholar]
- 3.Cummings MC, Winterford CM, Walker NI: Apoptosis. Am J Surg Pathol 1997, 21:88-101 [DOI] [PubMed] [Google Scholar]
- 4.Kerr JFR, Winterford CM, Harmon BV: Apoptosis: its significance in cancer and cancer therapy. Cancer 1994, 73:2013-2026 [DOI] [PubMed] [Google Scholar]
- 5.White E: Life, death, and the pursuit of apoptosis. Gene Dev 1996, 10:1-15 [DOI] [PubMed] [Google Scholar]
- 6.Yang E, Korsmeyer SJ: Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood 1996, 88:386-401 [PubMed] [Google Scholar]
- 7.Susin SA, Zamzami N, Castedo M, Daugas E, Wang H-G, Geley S, Fassy F, Reed JC, Kroemer G: The central executioner of apoptosis: multiple connections between protease activation and mitochondria in Fas/APO-1/CD95- and ceramide-induced apoptosis. J Exp Med 1997, 186:25-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leist M, Nicotera P: The shape of cell death (breakthroughs and views). Biochem Biophys Res Commun 1997, 236:1-9 [DOI] [PubMed] [Google Scholar]
- 9.Hengartner MO, Horvitz HR: Programmed cell death in Caenorhabditis elegans. Curr Opin Genet Dev 1994, 4:581-586 [DOI] [PubMed] [Google Scholar]
- 10.Horvitz HR, Shaham S, Hengartner MO: The genetics of programmed cell death in the nematode Caenorhabditis elegans. Cold Spring Harbor Symp Quant Biol 1994, 59:377-385 [DOI] [PubMed] [Google Scholar]
- 11.Jacobson MD: Apoptosis: Bcl-2 related proteins get connected. Curr Biol 1997, 7:R277-R281 [DOI] [PubMed] [Google Scholar]
- 12.Vaux DL: CED-4: the third horseman of apoptosis. Cell 1997, 90:389-390 [DOI] [PubMed] [Google Scholar]
- 13.Zou H, Henzel WJ, Liu X, Lutschg A, Wang X: Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell 1997, 90:405-413 [DOI] [PubMed] [Google Scholar]
- 14.Thompson CB: Apoptosis in the pathogenesis and treatment of disease. Science 1995, 267:1456-1462 [DOI] [PubMed] [Google Scholar]
- 15.Cory S: Regulation of lymphocyte survival by the bcl-2 gene family. Annu Rev Immunol 1995, 13:513-543 [DOI] [PubMed] [Google Scholar]
- 16.Colombel M, Olsson CA, Ng P-Y, Buttyan R: Hormone-regulated apoptosis results from reentry of differentiated prostate cells onto a defective cell cycle. Cancer Res 1992, 52:4313-4319 [PubMed] [Google Scholar]
- 17.Wilson JW, Wakeling AE, Morris ID, Hickman JA, Dive C: MCF-7 human mammary adenocarcinoma cell death in vitro in response to hormone-withdrawal and DNA damage. Int J Cancer 1995, 61:502-508 [DOI] [PubMed] [Google Scholar]
- 18.Berke G: The CTL’s kiss of death. Cell 1995, 81:9-12 [DOI] [PubMed] [Google Scholar]
- 19.Miyashita T, Reed JC: Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 1995, 80:293-299 [DOI] [PubMed] [Google Scholar]
- 20.Wu GS, Burns TF, McDonald ER, III, Jiang W, Meng R, Krantz ID, Kao G, Gan D-D, Zhou J-Y, Muschel R, Hamilton SR, Spinner NB, Markowitz S, Wu G, El-Deiry WS: KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nat Genet 1997, 17:141-143 [DOI] [PubMed] [Google Scholar]
- 21.Kummer JL, Rao PK, Heidenreich KA: Apoptosis induced by withdrawal of trophic factors is mediated by p38 mitogen-activated protein kinase. J Biol Chem 1997, 272:20490-20494 [DOI] [PubMed] [Google Scholar]
- 22.Haimovitz-Friedman A, Kan C-C, Ehleiter D, Persaud RS, McLoughlin M, Fuks Z, Kolesnick RN: Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J Exp Med 1994, 180:525-535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hannun YA: The sphingomyelin cycle and the second messenger function of ceramide. J Biol Chem 1994, 269:3125-3128 [PubMed] [Google Scholar]
- 24.Hannun YA: Functions of ceramide in coordinating cellular responses to stress. Science 1996, 274:1855-1859 [DOI] [PubMed] [Google Scholar]
- 25.Santana P, Peña LA, Haimovitz-Friedman A, Martin S, Green D, McLoughlin M, Cordon-Cardo C, Schuchman EH, Fuks Z, Kolesnick R: Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell 1996, 86:189-199 [DOI] [PubMed] [Google Scholar]
- 26.Fraser A, Evan G: A license to kill. Cell 1996, 85:781-784 [DOI] [PubMed] [Google Scholar]
- 27.Henkart PA: ICE family proteases: mediators of all apoptotic cell death? Immunity 1996, 4:195-201 [DOI] [PubMed] [Google Scholar]
- 28.Nagata S: Apoptosis by death factor. Cell 1997, 88:355-365 [DOI] [PubMed] [Google Scholar]
- 29.Muzio M, Chinnaiyan AM, Kischkel FC, O’Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, Mann M, Krammer PH, Peter ME, Dixit VM: FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell 1996, 85:817-827 [DOI] [PubMed] [Google Scholar]
- 30.Barge RMY, Willemze R, Vandenabeele P, Fiers W, Beyaert R: Differential involvement of caspases in apoptosis of myeloid leukemic cells induced by chemotherapy versus growth factor withdrawal. FEBS Lett 1997, 409:207-210 [DOI] [PubMed] [Google Scholar]
- 31.Harvey NL, Butt AJ, Kumar S: Functional activation of Nedd2/ICH-1 (caspase-2) is an early process in apoptosis. J Biol Chem 1997, 272:13134-13139 [DOI] [PubMed] [Google Scholar]
- 32.Thornberry NA, Peterson EP, Zhao JJ, Howard AD, Griffin PR, Chapman KT: Inactivation of interleukin-1β converting enzyme by peptide (acyloxy)methyl ketones. Biochemistry 1994, 33:3934-3940 [DOI] [PubMed] [Google Scholar]
- 33.Patel T, Gores GJ, Kaufmann SH: The role of proteases during apoptosis. FASEB J 1996, 10:587-597 [DOI] [PubMed] [Google Scholar]
- 34.Eliasson MJL, Sampei K, Mandir AS, Hurn PD, Traystman RJ, Bao J, Pieper A, Wang Z-Q, Dawson TM, Snyder SH, Dawson VL: Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med 1997, 3:1089-1095 [DOI] [PubMed] [Google Scholar]
- 35.Rao L, Perez D, White E: Lamin proteolysis facilitates nuclear events during apoptosis. J Cell Biol 1996, 135:1441-1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brancolini C, Lazarevic D, Rodriguez J, Schneider C: Dismantling cell-cell contacts during apoptosis is coupled to a caspase-dependent proteolytic cleavage of β-catenin. J Cell Biol 1997, 139:759-771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caulín C, Salvesen GS, Oshima RG: Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J Cell Biol 1997, 138:1379-1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen L, Marechal V, Moreau J, Levine AJ, Chen J: Proteolytic cleavage of the mdm2 oncoprotein during apoptosis. J Biol Chem 1997, 272:22966-22973 [DOI] [PubMed] [Google Scholar]
- 39.Tan X, Martin SJ, Green DR, Wang JYJ: Degradation of retinoblastoma protein in tumor necrosis factor- and CD95-induced cell death. J Biol Chem 1997, 272:9613-9616 [DOI] [PubMed] [Google Scholar]
- 40.Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S: A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 1998, 391:43-50 [DOI] [PubMed] [Google Scholar]
- 41.Hockenbery DM: Bcl-2 in cancer, development and apoptosis. J Cell Sci 1994, 18(Suppl):51-55 [DOI] [PubMed] [Google Scholar]
- 42.Reed JC: Bcl-2 and the regulation of programmed cell death. J Cell Biol 1994, 124:1-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kroemer G: The proto-oncogene bcl-2 and its role in regulating apoptosis. Nat Med 1997, 3:614-620 [DOI] [PubMed] [Google Scholar]
- 44.Chinnaiyan AM, Orth K, O’Rourke K, Duan H, Poirier GG, Dixit VM: Molecular ordering of the cell death pathway: Bcl-2 and bcl-xL function upstream of the ced-3-like apoptotic proteases. J Biol Chem 1996, 271:4573-4576 [DOI] [PubMed] [Google Scholar]
- 45.Oltvai ZN, Milliman CL, Korsmeyer SJ: Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 1993, 74:609-619 [DOI] [PubMed] [Google Scholar]
- 46.Yin X-M, Oltvai ZN, Korsmeyer SJ: BH1, and BH2 domains of bcl-2, are required for inhibition of apoptosis, and heterodimerization with bax. Nature 1994, 369:321-323 [DOI] [PubMed] [Google Scholar]
- 47.Ottilie S, Diaz J-L, Chang J, Wilson G, Tuffo KM, Weeks S, McConnell M, Wang Y, Oltersdorf T, Fritz LC: Structural and functional complementation of an inactive bcl-2 mutant by bax truncation. J Biol Chem 1997, 272:16955-16961 [DOI] [PubMed] [Google Scholar]
- 48.Lu Q-L, Abel P, Foster CS, Lalani E-N: Bcl-2: role in epithelial differentiation and oncogenesis. Hum Pathol 1996, 27:102-110 [DOI] [PubMed] [Google Scholar]
- 49.Kinoshita T, Yokota T, Arai K-i, Miyajima A: Regulation of bcl-2 expression by oncogenic ras protein in hematopoietic cells. Oncogene 1995, 10:2207-2212 [PubMed] [Google Scholar]
- 50.Leverrier Y, Thomas J, Perkins GR, Mangeney M, Collins MKL, Marvel J: In bone marrow derived baf-3 cells, inhibition of apoptosis by IL-3 is mediated by two independent pathways. Oncogene 1997, 14:425-430 [DOI] [PubMed] [Google Scholar]
- 51.Hockenbery D, Nuñez G, Milliman C, Schreiber RD, Korsmeyer SJ: Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 1990, 348:334-336 [DOI] [PubMed] [Google Scholar]
- 52.Hirsch T, Marchetti P, Susin SA, Dallaporta B, Zamzami N, Marzo I, Geuskens M, Kroemer G: The apoptosis-necrosis paradox: apoptogenic proteases activated after mitochondrial permeability transition determine the mode of cell death. Oncogene 1997, 15:1573-1581 [DOI] [PubMed] [Google Scholar]
- 53.Minn AJ, Vélez P, Schendel SL, Liang H, Muchmore SW, Fesik SW, Fill M, Thompson CB: Bcl-xL forms an ion channel in synthetic lipid membranes. Nature 1997, 385:353-357 [DOI] [PubMed] [Google Scholar]
- 54.Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng T-I, Jones DP, Wang X: Prevention of apoptosis by bcl-2: release of cytochrome c from mitochondria blocked. Science 1997, 275:1129-1132 [DOI] [PubMed] [Google Scholar]
- 55.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD: The release of cytochrome c from mitochondria: a primary site for bcl-2 regulation of apoptosis. Science 1997, 275:1132-1136 [DOI] [PubMed] [Google Scholar]
- 56.Manon S, Chaudhuri B, Guérin M: Release of cytochrome c and decrease of cytochrome c oxidase in bax-expressing yeast cells, and prevention of these effects by coexpression of bcl-xL. FEBS Lett 1997, 415:29-32 [DOI] [PubMed] [Google Scholar]
- 57.Cheng EH-Y, Kirsch DG, Clem RJ, Ravi R, Kastan MB, Bedi A, Ueno K, Hardwick JM: Conversion of bcl-2 to a bax-like death effector by caspases. Science 1997, 278:1966-1968 [DOI] [PubMed] [Google Scholar]
- 58.Tsujimoto Y, Cossman J, Jaffe E, Croce CM: Involvement of the bcl-2 gene in human follicular lymphoma. Science 1985, 228:1440-1443 [DOI] [PubMed] [Google Scholar]
- 59.Rampino N, Yamamoto H, Ionov Y, Li Y, Sawai H, Reed JC, Perucho M: Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science 1997, 275:967-969 [DOI] [PubMed] [Google Scholar]
- 60.Lane DP: p53, guardian of the genome. Nature 1992, 358:15-16 [DOI] [PubMed] [Google Scholar]
- 61.Michalovitz D, Halevy O, Oren M: Conditional inhibition of transformation and of cell proliferation by a temperature-sensitive mutant of p53. Cell 1990, 62:671-680 [DOI] [PubMed] [Google Scholar]
- 62.Martinez J, Georgoff I, Martinez J, Levine AJ: Cellular localization and cell cycle regulation by a temperature-sensitive p53 protein. Genes Dev 1991, 5:151-159 [DOI] [PubMed] [Google Scholar]
- 63.El-Deiry WS, Tokino T, Velculescu VE, Levy BD, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B: WAF1, a potential mediator of p53 tumor suppression. Cell 1993, 75:817-825 [DOI] [PubMed] [Google Scholar]
- 64.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ: The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 1993, 75:805-816 [DOI] [PubMed] [Google Scholar]
- 65.Reed SI, Bailly E, Dulic V, Hengst L, Resnitzky D, Slingerland J: G1 control in mammalian cells. J Cell Sci 1994, 18(Suppl):69-73 [DOI] [PubMed] [Google Scholar]
- 66.Miyashita T, Krajewski S, Krajewska M, Wang HG, Lin HK, Liebermann DA, Hoffman B, Reed JC: Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene 1994, 9:1799-1805 [PubMed] [Google Scholar]
- 67.Yonish-Rouach E, Resnitzky D, Lotem J, Sachs L, Kimchi A, Oren M: Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature 1991, 352:345-347 [DOI] [PubMed] [Google Scholar]
- 68.Kastan M: On the TRAIL from p53 to apoptosis? Nat Genet 1997, 17:130-131 [DOI] [PubMed] [Google Scholar]
- 69.Evan GI, Wyllie AH, Gilbert CS, Littlewood TD, Land H, Brooks M, Waters CM, Penn LZ, Hancock DC: Induction of apoptosis in fibroblasts by c-myc protein. Cell 1992, 69:119-128 [DOI] [PubMed] [Google Scholar]
- 70.Trent JC, II, McConkey DJ, Loughlin SM, Harbison MT, Fernandez A, Ananthaswamy HN: Ras signaling in tumor necrosis factor-induced apoptosis. EMBO J 1996, 15:4497-4505 [PMC free article] [PubMed] [Google Scholar]
- 71.Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G: Suppression of c-myc-induced apoptosis by ras signalling through PI(3)K and PKB. Nature 1997, 385:544-548 [DOI] [PubMed] [Google Scholar]
- 72.Ward RL, Todd AV, Santiago F, O’Connor T, Hawkins NJ: Activation of the K-ras oncogene in colorectal neoplasms is associated with decreased apoptosis. Cancer 1997, 79:1106-1113 [PubMed] [Google Scholar]
- 73.Chen C-Y, Faller DV: Phosphorylation of bcl-2 protein and association with p21Ras in Ras-induced apoptosis. J Biol Chem 1996, 271:2376-2379 [DOI] [PubMed] [Google Scholar]
- 74.Li Y, Chopp M, Jiang N, Zhang ZG, Zaloga C: Induction of DNA fragmentation after 10 to 120 minutes of focal cerebral ischemia in rats. Stroke 1995, 26:1252-1257 [DOI] [PubMed] [Google Scholar]
- 75.Leist M, Single B, Castoldi AF, Kühnle S, Nicotera P: Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med 1997, 185:1481-1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gavrieli Y, Sherman Y, Ben-Sasson SA: Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 1992, 119:493-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gold R, Schmied M, Rothe G, Zischler H, Breitschopf H, Wekerle H, Lassmann H: Detection of DNA fragmentation in apoptosis: application of in situ nick translation to cell culture systems and tissue sections. J Histochem Cytochem 1993, 41:1023-1030 [DOI] [PubMed] [Google Scholar]
- 78.Wijsman JH, Jonker RR, Keijzer R, van de Velde CJH, Cornelisse CJ, van Dierendonck JH: A new method to detect apoptosis in paraffin sections: in situ end-labeling of fragmented DNA. J Histochem Cytochem 1993, 41:7-12 [DOI] [PubMed] [Google Scholar]
- 79.Ansari B, Coates PJ, Greenstein BD, Hall PA: In situ end-labelling detects DNA strand breaks in apoptosis, and other physiological, and pathological states. J Pathol 1993, 170:1-8 [DOI] [PubMed] [Google Scholar]
- 80.Negoescu A, Lorimier P, Labat-Moleur F, Drouet C, Robert C, Guillermet C, Brambilla C, Brambilla E: In situ apoptotic cell labeling by the TUNEL method: improvement and evaluation on cell preparations. J Histochem Cytochem 1996, 44:959-968 [DOI] [PubMed] [Google Scholar]
- 81.Shinohara T, Ohshima K, Murayama H, Kikuchi M, Yamashita Y, Shirakusa T: Apoptosis and proliferation in gastric carcinoma: the association with histological type. Histopathology 1996, 29:123-129 [DOI] [PubMed] [Google Scholar]
- 82.Lipponen P, Aaltomaa S, Kosma V-M, Syrjänen K: Apoptosis in breast cancer as related to histopathological characteristics and prognosis. Eur J Cancer 1994, 30A:2068-2073 [DOI] [PubMed] [Google Scholar]
- 83.van de Schepop HAM, de Jong JS, van Diest PJ, Baak JPA: Counting of apoptotic cells: a methodological study in invasive breast cancer. J Clin Pathol Mol Pathol 1996, 49:M214-M217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Potten CS: What is an apoptotic index measuring? (a commentary). Br J Cancer 1996, 74:1743-1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hahne M, Rimoldi D, Schröter M, Romero P, Schreier M, French LE, Schneider P, Bornand T, Fontana A, Lienard D, Cerottini J-C, Tschopp J: Melanoma cell expression of Fas(APO-1/CD95) ligand: implications for tumor immune escape. Science 1996, 274:1363-1366 [DOI] [PubMed] [Google Scholar]
- 86.O’Connell J, O’Sullivan GC, Collins JK, Shanahan F: The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med 1996, 184:1075-1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Migheli A, Cavalla P, Marino S, Schiffer D: A study of apoptosis in normal and pathologic nervous tissue after in situ end-labeling of DNA strand breaks. J Neuropathol Exp Neurol 1994, 53:606-616 [DOI] [PubMed] [Google Scholar]
- 88.Mundle SD, Gao XZ, Khan S, Gregory SA, Preisler HD, Raza A: Two in situ labeling techniques reveal different patterns of DNA fragmentation during spontaneous apoptosis in vivo, and induced apoptosis in vitro. Anticancer Res 1995, 15:1895-1904 [PubMed] [Google Scholar]
- 89.Petito CK, Roberts B: Effect of postmortem interval on in situ end-labeling of DNA oligonucleosomes. J Neuropathol Exp Neurol 1995, 54:761-765 [DOI] [PubMed] [Google Scholar]
- 90.Sasano H: In situ end labeling, and its applications to the study of endocrine disease: How can we study programmed cell death in surgical pathology materials? Endocrinol Pathol 1995, 6:87-89 [Google Scholar]
- 91.Grasl-Kraupp B, Ruttkay-Nedecky B, Koudelka H, Bukowska K, Bursch W, Schulte-Hermann R: In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: a cautionary note. Hepatology 1995, 21:1465-1468 [DOI] [PubMed] [Google Scholar]
- 92.Davison FD, Groves M, Scaravilli F: The effects of formalin fixation on the detection of apoptosis in human brain by in situ end-labelling of DNA. Histochem J 1995, 27:983-988 [PubMed] [Google Scholar]
- 93.Hawkins NJ, Lees J, Ward RL: Detection of apoptosis in colorectal carcinoma by light microscopy and in situ end labelling. Anal Quant Cytol Histol 1997, 19:227-232 [PubMed] [Google Scholar]
- 94.Soini Y, Törmänen U, Pääkkö P: Apoptosis is inversely related to bcl-2 but not to bax expression in salivary gland tumours. Histopathology 1998, 32:28-34 [DOI] [PubMed] [Google Scholar]
- 95.Leoncidi L, Del Vecchio MT, Spina D, Megha T, Barbini P, Sabattini E, Pileri S, Tosi P, Kraft R, Laissue JA, Cottier H: Presence of the bcl-2 protein and apoptosis in non-Hodgkin lymphomas with diffuse growth pattern. Int J Cancer 1995, 61:826-831 [DOI] [PubMed] [Google Scholar]
- 96.Kiberu SW, Pringle JH, Sobolewski S, Murphy P, Lauder I: Correlation between apoptosis, proliferation and bcl-2 expression in malignant non-Hodgkin’s lymphoma. J Clin Pathol Mol Pathol 1996, 49:M268-M272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gisbertz IAM, Schouten HC, Bot FJ, Arends J-W: Proliferation and apoptosis in primary gastric B-cell non-Hodgkin’s lymphoma. Histopathology 1997, 30:152-159 [DOI] [PubMed] [Google Scholar]
- 98.Soini Y, Raunio H, Pääkkö P: High grade malignant non-Hodgkin’s lymphomas differ from low grade lymphomas in the extent of apoptosis and their expression of bcl-2, mcl-1, bax, and p53. Tumor Biol 1998, 19:176-185 [DOI] [PubMed] [Google Scholar]
- 99.Schlaifer D, Krajewski S, Galoin S, Rigal-Huguet F, Laurent G, Massip P, Pris J, Delsol G, Reed JC, Brousset P: Immunodetection of apoptosis-regulating proteins in lymphomas from patients with and without human immunodeficiency virus infection. Am J Pathol 1996, 149:177-185 [PMC free article] [PubMed] [Google Scholar]
- 100.Hell K, Lorenzen J, Fischer R, Hansmann M-L: Hodgkin cells accumulate mRNA for bcl-2. Lab Invest 1995, 73:492-496 [PubMed] [Google Scholar]
- 101.Rigal-Huguet F, Gopas J, Prinsloo I, Pris J, Delsol G, Reed JC, Schlaifer D, Brousset P, Benharroch D, Krajewski S, Laurent G, Meggetto F: Frequent expression of the cell death-inducing gene bax in Reed-Sternberg cells of Hodgkin’s disease. Blood 1996, 87:2470-2475 [PubMed] [Google Scholar]
- 102.zur Hausen H, Schulte-Holthausen HS, Klein G, Henle W, Henle G, Clifford P, Santesson L: EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature 1970, 228:1056-1058 [DOI] [PubMed] [Google Scholar]
- 103.Weiss LM, Movahed LA, Warnke RA, Sklar J: Detection of Epstein-Barr viral genomes in Reed-Sternberg cells of Hodgkin’s disease. N Engl J Med 1989, 320:502-506 [DOI] [PubMed] [Google Scholar]
- 104.Burke AP, Yen TS, Shekitka KM, Sobin LH: Lymphoepithelial carcinoma of the stomach with Epstein-Barr virus demonstrated by polymerase chain reaction. Mod Pathol 1990, 3:377-380 [PubMed] [Google Scholar]
- 105.Henderson S, Rowe M, Gregory C, Croom-Carter D, Wang F, Longnecker R, Kieff E, Rickinson A: Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell 1991, 65:1107-1115 [DOI] [PubMed] [Google Scholar]
- 106.Mustonen M, Raunio H, Pääkkö P, Soini Y: The extent of apoptosis is inversely associated with bcl-2 expression in premalignant and malignant breast lesions. Histopathology 1997, 31:347-354 [DOI] [PubMed] [Google Scholar]
- 107.Bhargava V, Kell DL, van de Rijn M, Warnke RA: Bcl-2 immunoreactivity in breast carcinoma correlates with hormone receptor positivity. Am J Pathol 1994, 145:535-540 [PMC free article] [PubMed] [Google Scholar]
- 108.Huang Y, Ray S, Reed JC, Ibrado AM, Tang C, Nawabi A, Bhalla K: Estrogen increases intracellular p26Bcl-2 to p21Bax ratios and inhibits taxol-induced apoptosis of human breast cancer MCF-7 cells. Breast Cancer Res Treat 1997, 42:73-81 [DOI] [PubMed] [Google Scholar]
- 109.Joensuu H, Pylkkänen L, Toikkanen S: Bcl-2 protein expression and long-term survival in breast cancer. Am J Pathol 1994, 145:1191-1198 [PMC free article] [PubMed] [Google Scholar]
- 110.Hellemans P, van Dam PA, Weyler J, van Oosterom AT, Buytaert P, Van Marck E: Prognostic value of bcl-2 expression in invasive breast cancer. Br J Cancer 1995, 72:354-360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Heatley MK: Association between the apoptotic index and established prognostic parameters in endometrial adenocarcinoma. Histopathology 1995, 27:469-472 [DOI] [PubMed] [Google Scholar]
- 112.Saegusa M, Kamata Y, Isono M, Okayasu I: Bcl-2 expression is correlated with a low apoptotic index and associated with progesterone receptor immunoreactivity in endometrial carcinomas. J Pathol 1996, 180:275-282 [DOI] [PubMed] [Google Scholar]
- 113.Chhieng DC, Ross JS, Ambros RA: Bcl-2 expression and the development of endometrial carcinoma. Mod Pathol 1996, 9:402-406 [PubMed] [Google Scholar]
- 114.Henderson GS, Brown KA, Perkins SL, Abbott TM, Clayton F: Bcl-2 is down-regulated in atypical endometrial hyperplasia and adenocarcinoma. Mod Pathol 1996, 9:430-438 [PubMed] [Google Scholar]
- 115.Sandford NL, Searle JW, Kerr JF: Successive waves of apoptosis in the rat prostate after repeated withdrawal of testosterone stimulation. Pathology 1984, 16:406-410 [DOI] [PubMed] [Google Scholar]
- 116.Kyprianou N, English HF, Isaacs JT: Programmed cell death during regression of PC-82 human prostate cancer following androgen ablation. Cancer Res 1990, 50:3748-3753 [PubMed] [Google Scholar]
- 117.McDonnell TJ, Troncoso P, Brisbay SM, Logothetis C, Chung LWK, Hsieh J-T, Tu S-M, Campbell ML: Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res 1992, 52:6940-6944 [PubMed] [Google Scholar]
- 118.Colombel M, Symmans F, Gil S, O’Toole KM, Chopin D, Benson M, Olsson CA, Korsmeyer S, Buttyan R: Detection of the apoptosis-suppressing oncoprotein bcl-2 in hormone-refractory human prostate cancers. Am J Pathol 1993, 143:390-400 [PMC free article] [PubMed] [Google Scholar]
- 119.Koivisto P, Visakorpi T, Rantala I, Isola J: Increased cell proliferation activity and decreased cell death are associated with the emergence of hormone-refractory recurrent prostate cancer. J Pathol 1997, 183:51-56 [DOI] [PubMed] [Google Scholar]
- 120.Krajewska M, Krajewski S, Epstein JI, Shabaik A, Sauvageot J, Song K, Kitada S, Reed JC: Immunohistochemical analysis of bcl-2, bax, bcl-X and mcl-1 expression in prostate cancers. Am J Pathol 1996, 148:1567-1576 [PMC free article] [PubMed] [Google Scholar]
- 121.Aihara M, Scardino PT, Truong LD, Wheeler TM, Goad JR, Yang G, Thompson TC: The frequency of apoptosis correlates with the prognosis of Gleason grade 3 adenocarcinoma of the prostate. Cancer 1995, 75:522-529 [DOI] [PubMed] [Google Scholar]
- 122.Basolo F, Pollina L, Fontanini G, Fiore L, Pacini F, Baldanzi A: Apoptosis and proliferation in thyroid carcinoma: correlation with bcl-2 and p53 protein expression. Br J Cancer 1997, 75:537-541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sasano H, Imatani A, Shizawa S, Suzuki T, Nagura H: Cell proliferation and apoptosis in normal and pathologic human adrenal. Mod Pathol 1995, 8:11-17 [PubMed] [Google Scholar]
- 124.Wolkersdörfer GW, Marx C, Brown JW, Scherbaum WA, Bornstein SR: Evaluation of apoptotic parameters in normal and neoplastic human adrenal. Endocr Res 1996, 22:411-419 [DOI] [PubMed] [Google Scholar]
- 125.Negoescu A, Labat-Moleur F, Defaye G, Mezin P, Drouet C, Brambilla E, Chambaz EM, Feige JJ: Contribution of apoptosis to the phenotypic changes of adrenocortical cells in primary culture. Mol Cell Endocrinol 1995, 110:175-184 [DOI] [PubMed] [Google Scholar]
- 126.Koshida Y, Saegusa M, Okayasu I: Apoptosis, cell proliferation, and expression of Bcl-2, and Bax in gastric carcinomas: immunohistochemical and clinicopathological study. Br J Cancer 1997, 75:367-373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Baretton GB, Diebold J, Christoforis G, Vogt M, Müller C, Dopfer K, Schneiderbanger K, Schmidt M, Löhrs U: Apoptosis and immunohistochemical bcl-2 expression in colorectal adenomas and carcinomas: aspects of carcinogenesis and prognostic significance. Cancer 1996, 77:255-264 [DOI] [PubMed] [Google Scholar]
- 128.Krajewska M, Fenoglio-Preiser CM, Krajewski S, Song K, Macdonald JS, Stemmerman JS, Reed JC: Immunohistochemical analysis of bcl-2 family proteins in adenocarcinomas of the stomach. Am J Pathol 1996, 149:1449-1457 [PMC free article] [PubMed] [Google Scholar]
- 129.Soini Y, Virkajärvi N, Lehto V-P, Pääkkö P: Hepatocellular carcinomas with a high proliferation index and a low degree of apoptosis and necrosis are associated with a shortened survival. Br J Cancer 1996, 73:1025-1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Virkajärvi N, Pääkkö P, Soini Y: Association between p53 overexpression, cell proliferation, tumor necrosis and extent of apoptosis in operated pancreatic adenocarcinoma. APMIS 1997, 105:765-772 [DOI] [PubMed] [Google Scholar]
- 131.Charlotte F, L’Herminé A, Martin N, Geleyn Y, Nollet M, Gaulard P, Zafrani ES: Immunohistochemical detection of bcl-2 protein in normal and pathological human liver. Am J Pathol 1994, 144:460-465 [PMC free article] [PubMed] [Google Scholar]
- 132.Zhao M, Zhang N-X, Economou M, Blaha I, Laissue JA, Zimmermann A: Immunohistochemical detection of bcl-2 protein in liver lesions: Bcl-2 protein is expressed in hepatocellular carcinomas but not in liver cell dysplasia. Histopathology 1994, 25:237-245 [DOI] [PubMed] [Google Scholar]
- 133.Skopelitou A, Hadjiyannakis M, Alexpoulou V, Krikoni O, Kamina S, Agnantis N: Topographical immunohistochemical expression of bcl-2 protein in human liver lesions. Anticancer Res 1996, 16:975-978 [PubMed] [Google Scholar]
- 134.Törmänen U, Eerola A-K, Rainio P, Vähäkangas K, Soini Y, Sormunen R, Bloigu R, Lehto V-P, Pääkkö P: Enhanced apoptosis predicts shortened survival in non-small cell lung carcinoma. Cancer Res 1995, 55:5595-5602 [PubMed] [Google Scholar]
- 135.Komaki R, Fujii T, Perkins P, Ro JY, Allen PK, Mason KA, Mountain CF, Milas L: Apoptosis and mitosis as prognostic factors in pathologically staged N1 nonsmall cell lung cancer. Int J Radiat Oncol Biol Phys 1996, 36:601-605 [DOI] [PubMed] [Google Scholar]
- 136.O’Neill AJ, Staunton MJ, Gaffney EF: Apoptosis occurs independently of bcl-2 and p53 over-expression in non-small cell lung carcinoma. Histopathology 1996, 29:45-50 [DOI] [PubMed] [Google Scholar]
- 137.Stammler G, Volm M: Apoptosis in non-small cell lung cancer as related to drug resistance and prognosis. Apoptosis 1996, 1:95-99 [Google Scholar]
- 138.Ikegaki N, Katsumata M, Minna J, Tsujimoto Y: Expression of bcl-2 in small cell lung carcinoma cells. Cancer Res 1994, 54:6-8 [PubMed] [Google Scholar]
- 139.Jiang S-X, Kameya T, Sato Y, Yanase N, Yoshimura H, Kodama T: Bcl-2 protein expression in lung cancer and close correlation with neuroendocrine differentiation. Am J Pathol 1996, 148:837-846 [PMC free article] [PubMed] [Google Scholar]
- 140.Eerola A-K, Törmänen U, Rainio P, Sormunen R, Bloigu R, Vähäkangas K, Lehto V-P, Soini Y, Pääkkö P: Apoptosis in operated small cell lung carcinoma is inversely related to tumour necrosis and p53 immunoreactivity. J Pathol 1997, 181:172-177 [DOI] [PubMed] [Google Scholar]
- 141.Staunton MJ, Gaffney EF: Tumor type is a determinant of susceptibility to apoptosis. Am J Clin Pathol 1995, 103:300-307 [DOI] [PubMed] [Google Scholar]
- 142.King ED, Matteson J, Jacobs SC, Kyprianou N: Incidence of apoptosis, cell proliferation and bcl-2 expression in transitional cell carcinoma of the bladder: association with tumor progression. J Urol 1996, 155:316-320 [PubMed] [Google Scholar]
- 143.Shiina H, Igawa M, Urakami S, Honda S, Shirakawa H, Ishibe T: Immunohistochemical analysis of bcl-2 expression in transitional cell carcinoma of the bladder. J Clin Pathol 1996, 49:395-399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lipponen P, Eskelinen M, Syrjänen K: Expression of tumour-suppressor gene Rb, apoptosis-suppressing protein bcl-2 and c-myc have no independent prognostic value in renal adenocarcinoma. Br J Cancer 1995, 71:863-867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Furihata M, Sonobe H, Ohtsuki Y, Yamashita M, Morioka M, Yamamoto A, Terao N, Kuwahara M, Fujisaki N: Detection of p53 and bcl-2 protein in carcinoma of the renal pelvis and ureter including dysplasia. J Pathol 1996, 178:133-139 [DOI] [PubMed] [Google Scholar]
- 146.Patsouris E, Davaki P, Kapranos N, Davaris P, Papageorgiou K: A study of apoptosis in brain tumors by in situ end-labeling method. Clin Neuropathol 1996, 15:337-341 [PubMed] [Google Scholar]
- 147.Soini Y, Pääkkö P: Extent of apoptosis in relation to p53 and bcl-2 expression in germ cell tumors. Hum Pathol 1996, 27:1221-1226 [DOI] [PubMed] [Google Scholar]
- 148.Goldblum JR, Rice TW: Bcl-2 protein expression in the Barrett’s metaplasia-dysplasia-carcinoma sequence. Mod Pathol 1995, 8:866-869 [PubMed] [Google Scholar]
- 149.Birchall MA, Winterford CM, Allan DJ, Harmon BV: Apoptosis in normal epithelium, premalignant and malignant lesions of the oropharynx and oral cavity: a preliminary study. Oral Oncol Eur J Cancer 1995, 31B:380-383 [DOI] [PubMed] [Google Scholar]
- 150.Ishida M, Gomyo Y, Tatebe S, Ohfuji S, Ito H: Apoptosis in human gastric mucosa, chronic gastritis, dysplasia and carcinoma: analysis by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labelling. Virchows Arch 1996, 428:229-235 [DOI] [PubMed] [Google Scholar]
- 151.Flohil CC, Janssen PA, Bosman FT: Expression of bcl-2 protein in hyperplastic polyps, adenomas, and carcinomas of the colon. J Pathol 1996, 178:393-397 [DOI] [PubMed] [Google Scholar]
- 152.Lauwers GY, Scott GV, Hendricks J: Immunohistochemical evidence of aberrant bcl-2 protein expression in gastric epithelial dysplasia. Cancer 1994, 73:2900-2904 [DOI] [PubMed] [Google Scholar]
- 153.McGill G, Shimamura A, Bates RC, Savage RE, Fisher DE: Loss of matrix adhesion triggers rapid transformation-selective apoptosis in fibroblasts. J Cell Biol 1997, 138:901-911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bates RC, Lincz LF, Burns GF: Involvement of integrins in cell survival. Cancer Metastasis Rev 1995, 14:191-203 [DOI] [PubMed] [Google Scholar]
- 155.Arai E, Katayama I: Role of apoptosis in spontaneous regression of peripheral T-cell lymphoma arising in skin or subcutis. Hum Pathol 1997, 28:472-477 [DOI] [PubMed] [Google Scholar]
- 156.Thomaidou D, Mione MC, Cavanagh JFR, Parnavelas JG: Apoptosis and its relation to the cell cycle in the developing cerebral cortex. J Neurosci 1997, 17:1075-1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bortner DM, Rosenberg MP: Overexpression of cyclin A in the mammary glands of transgenic mice results in the induction of nuclear abnormalities and increased apoptosis. Cell Growth Differ 1995, 6:1579-1589 [PubMed] [Google Scholar]
- 158.Gao CY, Zelenka PS: Induction of cyclin B and H1 kinase activity in apoptotic PC12 cells. Exp Cell Res 1995, 219:612-618 [DOI] [PubMed] [Google Scholar]
- 159.Meikrantz W, Schlegel R: Apoptosis and the cell cycle. J Cell Biochem 1995, 58:160-174 [DOI] [PubMed] [Google Scholar]
- 160.Kranenburg O, van der Eb AJ, Zantema A: Cyclin D1 is an essential mediator of apoptotic neuronal cell death. EMBO J 1996, 15:46-54 [PMC free article] [PubMed] [Google Scholar]
- 161.Sofer-Levi Y, Resnitzky D: Apoptosis induced by ectopic expression of cyclin D1 but not cyclin E. Oncogene 1996, 13:2431-2437 [PubMed] [Google Scholar]
- 162.Kargi HA, Aktas S, Sağol Ö, Ermete S, Akpinar O, Akkoclu A: Apoptosis bcl-2 and p53 expression and their relation to tumour stage in non-small cell lung carcinomas (NSCLC). Cancer Lett 1997, 116:185-189 [DOI] [PubMed] [Google Scholar]
- 163.Gaffney EF, O’Neill AJ, Staunton MJ: In situ end-labelling, light microscopic assessment, and ultrastructure of apoptosis in lung carcinoma. J Clin Pathol 1995, 48:1017-1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Brambilla E, Negoescu A, Gazzeri S, Lantuejoul S, Moro D, Brambilla C, Coll J-L: Apoptosis-related factors p53, bcl2, and bax in neuroendocrine lung tumors. Am J Pathol 1996, 149:1941-1952 [PMC free article] [PubMed] [Google Scholar]
- 165.Tatebe S, Ishida M, Kasagi N, Tsujitani S, Kaibara N, Ito H: Apoptosis occurs more frequently in metastatic foci than in primary lesions of human colorectal carcinomas: analysis by terminal-deoxynucleotidyl-transferase mediated dUTP-biotin nick end labeling. Int J Cancer 1996, 65:173-177 [DOI] [PubMed] [Google Scholar]
- 166.Tsujitani S, Shirai H, Tatebe S, Sugamura K, Ohfuji S, Gomyo Y, Maeta M, Ito H, Kaibara N: Apoptotic cell death and its relationship to carcinogenesis in colorectal carcinoma. Cancer 1996, 77:1711-1716 [DOI] [PubMed] [Google Scholar]
- 167.Langlois NEI, Lamb J, Eremin O, Heys SD: Apoptosis in colorectal carcinoma occurring in patients aged 45 years and under: relationship to prognosis, mitosis, and immunohistochemical demonstration of p53, c-myc and bcl-2 protein products. J Pathol 1997, 182:392-397 [DOI] [PubMed] [Google Scholar]
- 168.Yang G, Wheeler TM, Kattan MW, Scardino PT, Thompson TC: Perineural invasion of prostate carcinoma cells is associated with reduced apoptotic index. Cancer 1996, 78:1267-1271 [DOI] [PubMed] [Google Scholar]
- 169.Jiang S-X, Sato Y, Kuwao S, Kameya T: Expression of bcl-2 oncogene protein is prevalent in small cell lung carcinomas. J Pathol 1995, 177:135-138 [DOI] [PubMed] [Google Scholar]
- 170.Pezzella F, Turley H, Kuzu I, Tungekar MF, Dunnill MS, Pierce CB, Harris A, Gatter KC, Mason DY: Bcl-2 protein in non-small-cell lung carcinoma. N Engl J Med 1993, 329:690-694 [DOI] [PubMed] [Google Scholar]
- 171.Sinicrope FA, Evans DB, Leach SD, Cleary KR, Fenoglio CJ, Lee JJ, Abbruzzese JL: bcl-2 and p53 expression in resectable pancreatic adenocarcinomas: association with clinical outcome. Clin Cancer Res 1996, 2:2015-2022 [PubMed] [Google Scholar]