Abstract
Desensitization of guanine nucleotide binding protein-coupled receptors is a ubiquitous phenomenon characterized by declining effector activity upon persistent agonist stimulation. The luteinizing hormone/choriogonadotropin receptor (LH/CGR) in ovarian follicles exhibits desensitization of effector adenylyl cyclase activity in response to the mid-cycle surge of LH. We have previously shown that uncoupling of the agonist-activated LH/CGR from the stimulatory G protein (Gs) is dependent on GTP and attributable to binding of β-arrestin present in adenylyl cyclase-rich follicular membrane fraction to the third intracellular (3i) loop of the receptor. Here, we report that LH/CGR-dependent desensitization is mimicked by ADP ribosylation factor nucleotide-binding site opener, a guanine nucleotide exchange factor of the small G proteins ADP ribosylation factors (Arfs) 1 and 6, and blocked by synthetic N-terminal Arf6 peptide, suggesting that the GTP-dependent step of LH/CGR desensitization is receptor-dependent Arf6 activation. Arf activation by GTP and ADP ribosylation factor nucelotide-binding site opener promotes the release of docked β-arrestin from the membrane, making β-arrestin available for LH/CGR; Arf6 but not Arf1 peptides block β-arrestin release from the membrane. Thus, LH/CGR appears to activate two membrane delimited signaling cascades via two types of G proteins: heterotrimeric Gs and small G protein Arf6. Arf6 activation releases docked β-arrestin necessary for receptor desensitization, providing a feedback mechanism for receptor self-regulation.
The luteinizing hormone/choriogonadotropin receptor (LH/CGR) belongs to the seven transmembrane family of receptors that signal by the activation of guanine nucleotide binding (G) proteins and downstream effectors including adenylyl cyclase (AC) (1–4). Characteristic of virtually all G protein-coupled receptors (GPCRs) (5), LH- and human (h) CG-stimulated AC activities wane in response to persistent stimulation of the LH/CGR by saturating agonist concentrations (6–8). We have previously shown that desensitization of the endogenous ovarian follicular LH/CGR in a cell-free membrane model is GTP dependent (Km ≈70 nM) (9–11). Based on the ability of anti-arrestin antibodies to abrogate LH/CGR desensitization, we concluded that endogenous plasma membrane-bound β-arrestin mediates desensitization of the follicular LH/CGR (12). Using synthetic peptides, we have also shown that on LH/CGR activation, β-arrestin specifically binds to the 3i loop of the activated LH/CGR, blocking its interaction with Gs (13). Here, we investigate the molecular basis of the GTP dependence of LH/CGR desensitization and the mechanism of the release of the membrane-bound β-arrestin required for LH/CGR desensitization.
Materials and Methods
Materials.
Recombinant β-arrestin (14), ADP ribosylation factor (Arf) nucleotide-binding site opener (ARNO) and ARNO mutant proteins (15), and Clostridium difficile and Clostridium sordelli toxins (16) were expressed and purified as described. Myristoylated (Myr)-Arf6(2–13), non-Myr-Arf6(2–13), and Myr-Arf1(2–17) N-terminal peptides were synthesized as described previously (17). Brefeldin A was purchased from Sigma; all other chemicals were from sources previously described (12, 13).
Desensitization, AC Assay, and Western Blotting.
A partially purified membrane fraction enriched in AC activity was isolated from preovulatory-sized porcine ovarian follicles and stored at −70°C (12). The two-stage desensitization reaction (13) is summarized in Fig. 1 legend. Western blotting with anti-β-arrestin (Transduction Laboratories, Lexington, KY) and mAb A7, made to a synthetic peptide conserved among glycoprotein hormone receptors (3, 18), was also as described previously (12). Results were analyzed using Student's t test (P < 0.05) (19).
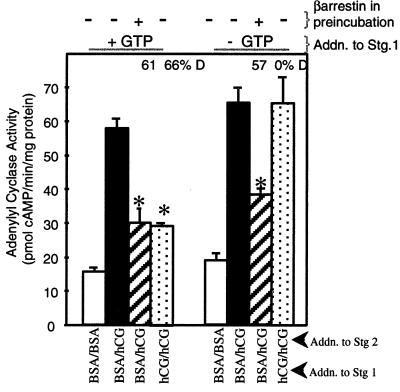
Figure 1.
LH/CGR desensitization promoted by exogenous β-arrestin is independent of GTP. Membranes were preincubated (≈30 μg protein in 20 μl) in the presence of 40 nM recombinant, purified β-arrestin or water for 30 min at 4° C. Following preincubation, the two-stage desensitization incubation was conducted. The presence of BSA in stages 1 and 2 measures basal AC activity; BSA in stage 1 and hCG in stage 2 measures full hCG-stimulated AC activity; hCG in stages 1 and 2 measures desensitization of hCG-stimulated AC activity. The percent reduction of full hCG-stimulated AC activity above basal AC activity is expressed as percent desensitization (D). Results are means ± SEM of quadruplicate determinations from a single experiment and are representative of two separate experiments. ∗, P < 0.05 between BSA and hCG and indicated additions.
Immunofluorescence and Cellular Fractionation.
For intact cell studies, rats were injected with serum gonadotropin of pregnant mares to promote follicular maturation (20); 48 h later, granulosa cells were isolated and placed in culture (21). Cells were treated 40 min with vehicle or 1 IU human chorionic gonadtrophin (hCG) (Organon). For subcellular fractionation, cells were homogenized with a Dounce homogenizer in a magnesium-containing buffer (22) with protease inhibitors and homogenate was centrifuged at 200 × g to remove nuclei, then at 10,000 × g, generating a pellet and supernatant fraction. For immunofluorescence (IF), cells plated on coverslips were treated as above, then fixed with 3.7% formaldehyde and permeabilized with 1% Triton X-100, washed, and incubated for 1 h at 37°C with anti-Arf6 antibody (1:20 dilution) in PBS containing 5% normal goat serum. Coverslips were then washed and incubated for 1 h at 37°C with fluorescein isothiocynate-conjugated goat anti-rabbit secondary antibody (Jackson ImmunoResearch). Cells on coverslips were washed and mounted on slides in diazabicyclo[2.2.2]octane anti-fading medium (23). Slides were analyzed by a Zeiss Axiovert 100M confocal microscope.
Results
Effect of GTP on β-Arrestin-Dependent Desensitization of hCG-Stimulated AC Activity.
Porcine ovarian follicular membranes were preincubated with or without 40 nM β-arrestin and then subjected to a two-stage AC reaction (13), consisting of a stage 1 incubation (40 min, 30°C) under conditions that do (plus hCG) or do not (plus BSA) promote development of desensitization of hCG-stimulated AC activity, and a subsequent 5-min AC assay (with or without hCG). The stage 1 AC incubation was conducted with or without 10 μM GTP in the presence of 1 mM adenylyl 5′-imidodiphosphate, a nonhydrolyzable ATP analog that functions to inhibit both GTP degradation (10) and ATP hydrolysis (24). When membranes were incubated with GTP and hCG in stage 1, 66% desensitization of hCG-stimulated AC activity was achieved (Fig. 1, left panels, compare solid and dotted bars). Consistent with our previous findings (9, 10), no hCG-stimulated desensitization of LH/CGR activity was observed when GTP was omitted from stage 1 (Fig. 1, right panels). Addition of exogenous β-arrestin (hatched bars) lowered full hCG-stimulated AC activity regardless of the presence of GTP to levels of hCG-desensitized AC activity (dotted bar, left panels). Therefore, hCG-stimulated desensitization of the LH/CGR is GTP dependent, whereas exogenous β-arrestin-dependent desensitization is not. These results suggest that the GTP-dependent step of LH/CGR desensitization occurs upstream of β-arrestin binding to the receptor, possibly at the level of β-arrestin release from a membrane-docking site.
Effect of Inhibitors of Small G Protein Activation on Desensitization of the LH/CGR.
Although LH/CGR activates Gs, Gi, Gq/11, and G13 in follicular membranes (25, 26), LH/CGR desensitization is independent of the activation of these heterotrimeric G proteins (27). The GTP dependence might therefore reflect a role for small G proteins in regulating LH/CGR desensitization. We first evaluated the effect on LH/CGR desensitization of the active fragments of the large clostridial toxins that have been shown to possess full transferase activity for modification of the Rho and Ras families of GTPases in vitro (28, 29). Preincubation of follicular membranes with C. difficile toxin B, which blocks activity of the Rho-related GTPases, and C. sordelli lethal toxin, which inactivates Ras, Rap, and Rac GTPases (30), did not affect any parameter of AC activity (Table 1). Second, we evaluated the effect on LH/CGR desensitization of brefeldin A, a fungal metabolite that inhibits guanine nucleotide exchange on Arfs 1–5 (31) but not on Arf6 (32). Preincubation of follicular membranes with 50 or 200 μM brefeldin A did not affect AC activities (Table 1). These results suggest that the GTP-dependent step of LH/CGR desensitization is mediated neither by G proteins comprising the Ras, Rac, Rho, and Rap families nor by Arfs 1–5.
Table 1.
Effect of Clostridium toxins and Brefeldin A on LH/CGR desensitization
| Treatment* | % Desensitization |
|---|---|
| A | |
| Water | 57 |
| Toxin B, 10 μg/ml | 59 |
| Lethal toxin, 10 μg/ml | 61 |
| B | |
| Water | 62 |
| Brefeldin A, 50 μM | 61 |
| Brefeldin A, 200 μM | 63 |
Membranes were preincubated with toxins or brefeldin A, followed by the two-stage AC reaction (see Fig. 1), except that 1 mM ATP was substituted for adenylyl 5′-imidodiphosphate in stage 1. Results are means of quadruplicate determinations from a single experiment and are representative of two separate experiments.
ARNO Promotes LH/CGR Desensitization.
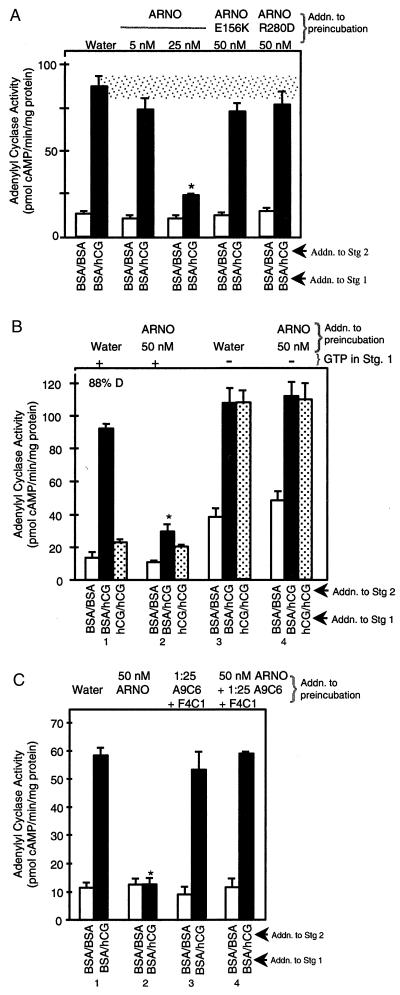
Because Arf6 is highly expressed in ovaries and uniquely localized to plasma membranes of an ovarian-derived cell line (32), we tested whether ARNO promotes LH/CGR desensitization. ARNO catalyzes the exchange of GTP for GDP on Arfs 1 and 6 (15, 33). Preincubation of follicular membranes with ARNO yielded a dose-dependent reduction in hCG-stimulated AC activity (Fig. 2A, solid bars) without affecting forskolin-stimulated AC activity (data not shown). To ascertain whether the effects of ARNO on LH/CGR desensitization require its activity, we tested two ARNO mutants: a catalytically inactive mutant E156K which does not promote GTP exchange at Arf because of a point mutation in the Sec7 domain but which retains its ability to bind membrane phosphoinositides via its PH domain (34), and catalytically active R280D mutant which does not bind phosphoinositides (35) because of a point mutation in its PH domain. Neither E156K nor R280D ARNO mutant affects hCG-stimulated AC activity (P > 0.05) (Fig. 2A, solid bars). Thus, Arf activation by ARNO mimics hCG-induced desensitization of the LH/CGR. To determine whether this activation requires GTP, membranes were preincubated with ARNO and then subjected to stage 1 desensitization reaction in the absence or presence of 10 μM GTP; saturating concentrations of GTP were then added to the 5-min AC assay. When GTP was present in stage 1, ARNO significantly lowered full hCG-stimulated AC activity (P < 0.05) (Fig. 2B, solid bars in groups 1 and 2). However, when GTP was omitted from the stage 1 incubation, ARNO no longer attenuated hCG-stimulated AC activity (solid bars in groups 3 and 4). Consistent with results of Fig. 1, hCG-dependent desensitization of the LH/CGR failed to occur when GTP was omitted from stage 1 (Fig. 2B, dotted bars in groups 3 and 4 versus 1). Thus, the ability of exogenous ARNO to promote LH/CGR desensitization requires GTP. Our results are therefore consistent with the hypothesis that the GTP-dependent step of LH/CGR desensitization is the activation of Arf by activated LH/CGR.
Figure 2.
ARNO mimics LH/CGR agonist and promotes LH/CGR desensitization; catalytically or PH domain-inactive ARNO mutants do not promote LH/CGR desensitization. Membranes were preincubated with indicated concentrations of ARNO or ARNO mutant proteins with or without anti-arrestin antibodies followed by the two-stage AC reaction (see Fig. 1). When indicated, GTP (at 10 μM) was omitted from stage 1. For B, 1 mM adenylyl 5′-imidodiphosphate was substituted for 1 mM ATP. Stage 2 AC assay was always conducted in the presence of 100 μM GTP. Results are means ± SEM of quadruplicate determinations from a single experiment and are representative of three separate experiments. Results equivalent to those seen with 25 nM ARNO were seen with 50 nM ARNO in separate experiments. *, P < 0.05 for BSA/hCG compared with water control.
Activation of Arf by ARNO Causes β-Arrestin Release.
Since LH/CGR desensitization requires endogenous β-arrestin (12) and since desensitization induced by exogenous β-arrestin does not require GTP (Fig. 1), GTP-dependent activation of Arf by ARNO may bring about the release of β-arrestin from follicular membranes. To test this hypothesis, follicular membranes were preincubated with ARNO and the neutralizing arrestin antibodies A9C6 and F4C1, which bind to the C- and N-terminal domains of arrestins, respectively (36), and block arrestin binding to the LH/CGR (12), followed by a two-stage AC reaction. Preincubation of membranes with ARNO significantly (P < 0.05) lowered hCG-stimulated AC activity (Fig. 2C, solid bars in groups 1 and 2). The ability of ARNO to promote LH/CGR desensitization was abrogated when membranes were preincubated with ARNO along with neutralizing anti-arrestin antibodies (Fig. 2C, solid bars in group 4 versus 2). The ability of the neutralizing arrestin antibodies to reverse ARNO-stimulated desensitization suggests that ARNO is promoting the release of β-arrestin from a membrane docking site.
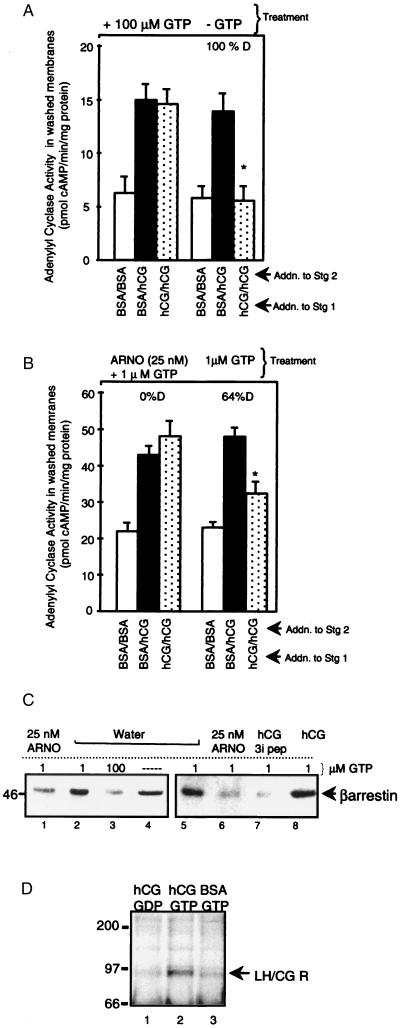
Therefore, next we tested whether the addition of ARNO in the presence of GTP causes β-arrestin release from follicular membranes. Membranes were treated with or without 100 μM GTP to bypass receptor and activate G proteins, washed to remove any released proteins, and a two-stage AC reaction was then performed on washed membranes. When membranes were treated in the absence of GTP, normal hCG-induced desensitization of hCG-stimulated AC activity was observed (Fig. 3A, compare solid and dotted bars, right panels). When membranes were first treated with 100 μM GTP, washed, and then subjected to a two-stage reaction, hCG no longer induced desensitization (Fig. 3A, left panels). We next determined whether ARNO plus 1 μM GTP mimicked the effect of 100 μM GTP. When membranes were treated with ARNO and 1 μM GTP, washed, and subjected to a two-stage desensitization reaction, hCG did not promote LH/CGR desensitization (Fig. 3B, left panels). This result suggests that the pool of β-arrestin, which can be released by the activated LH/CGR, is depleted by ARNO. Incubation of membranes with 1 μM GTP alone followed by washing of membranes and two-stage desensitization reaction preserved the ability of hCG to promote desensitization (Fig. 3B, right panels). These results are consistent with the hypothesis that β-arrestin is released from a membrane-docking site on G protein activation by 100 μM GTP and upon ARNO-stimulated Arf activation. Released β-arrestin presumably does not bind to inactive LH/CGR and can therefore be washed away. As a result, hCG can no longer promote LH/CGR desensitization in membranes treated with ARNO or 100 μM GTP. To test this hypothesis, we compared the amount of β-arrestin in untreated membranes versus those treated with 100 μM GTP or ARNO plus 1 μM GTP, all of which were then washed. The results of this experiment (Fig. 3C) confirm our hypothesis. Both 100 μM GTP (lane 3) and ARNO plus 1 μM GTP (lane 1) reduced β-arrestin content in the membranes. Notably, the β-arrestin retained in the membranes after incubation with 100 μM GTP or 25 nM ARNO plus 1 μM GTP and washing is not accessible to the activated LH/CGR, since in these membranes hCG no longer promotes LH/CGR desensitization (Fig. 3 A and B, left panels). Based on our evidence that β-arrestin binds to the 3i loop of the LH/CGR to cause desensitization (13), with LH/CGR activation by hCG β-arrestin should now bind to the 3i loop of the receptor and not be released by membrane washing. Results (Fig. 3C, lane 8) show that activation of the LH/CGR exposes a binding site for β-arrestin, resulting in retention of β-arrestin in washed membranes. Consistent with this conclusion, competing LH/CGR 3i peptide releases β-arrestin from the receptor and β-arrestin is washed away (lane 7). Our hypothesis presumes that β-arrestin does not bind to the inactive LH/CGR. To test this assumption, membranes were subjected to the stage 1 desensitization reaction in the presence of hCG to activate the LH/CGR and either 100 μM guanosine 5′-O-(2-thiodiphosphate) to maintain G proteins in an inactive conformation or 100 μM GTP to activate G proteins. Membranes were pelleted, proteins were solubilized, β-arrestin was immunoprecipitated, and blots were probed with a glycoprotein hormone receptor-specific antibody (18). Results (Fig. 3D) show GTP-released β-arrestin preferentially associates with activated LH/CGR.
Figure 3.
Arf activation by 100 μM GTP or ARNO plus 1 μM GTP promotes β-arrestin release from the AC-rich membrane and, when membranes are subsequently washed, a loss of the ability of LH/CGR agonist to promote LH/CGR desensitization. (A) Membranes were treated (30°C, 30 min in the presence of buffer, MgCl2, EGTA/EDTA with/without 100 μM GTP), diluted ≈25-fold with 10 mM Tris·HCl, and pelleted. Washed membranes were then subjected to two-stage AC desensitization reaction. Results are means ± SEM of quadruplicate determinations from a single experiment and are representative of two separate experiments. *, P < 0.05 compared with BSA/hCG. (B) Membranes were treated as indicated, diluted, pelleted, and subjected to two-stage AC reaction as in A. Results are means ± SEM of quadruplicate determinations from a single experiment and are representative of three separate experiments. (C) Membranes were treated as indicated, with 10 μg/ml hCG (to activate LH/CGR) and 15 μM 3i peptide (13), diluted, and pelleted, pellets were mixed with SDS/PAGE stop and subjected to SDS/PAGE, proteins were transferred to Immobilon P and subjected to Western blot analysis using anti-β-arrestin antibody (Transduction Laboratories). (D) Membranes (100 μg) were subjected to a stage 1 AC desensitization reaction in the presence of hCG or BSA and 100 μM 5′-O-(2-thiodiphosphate) (GDP) or GTP, as indicated. Membranes were diluted and pelleted as in A, and membrane proteins in pellets were solubilized in 1% Triton X-100 (25), β-arrestin and associated proteins were immunoprecipitated with anti-β-arrestin antibody, proteins were separated by SDS/PAGE and transferred to Immobilon, and blots probed with mAb A7 made to a synthetic peptide were conserved among glycoprotein hormone receptors. Results are representative of two separate experiments.
hCG-Dependent β-Arrestin Release Is Inhibited by Arf6 Peptide.
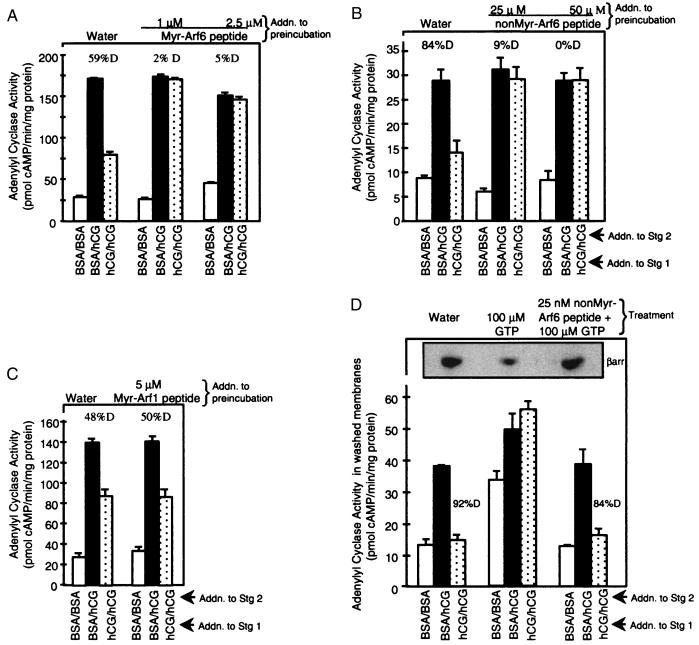
Our results point to Arf6 as the small G protein involved in LH/CGR desensitization based on the insensitivity of LH/CGR desensitization to brefeldin A (32) and activation by ARNO (15, 33). To confirm its identification as Arf6 (and not Arf1), membranes were preincubated with synthetic N-terminal Arf peptides reported to block Arf activity (17, 37). Both Myr- and non-Myr-Arf6 peptides but not Myr-Arf1 peptide blocked desensitization of hCG-stimulated AC activity (Fig. 4 A–C). Desensitization of LH/CGR activity was more sensitive to inhibition by the Myr-Arf6 peptide compared with non-Myr-Arf6, consistent with their relative activity in intact cells (38). Higher concentrations of both Myr-Arf6 and -Arf1 peptides, but not the non-Myr-Arf6 peptide, reduced hCG- and forskolin-stimulated AC activities in a 5-min AC assay, suggesting that Myr-Arf peptides interfered directly with AC activation (data not shown). We hypothesized that both Arf6 peptides blocked desensitization by inhibiting β-arrestin release from its membrane-docking site. To test this hypothesis, membranes were treated in the presence of 100 μM GTP (to activate G proteins and promote β-arrestin release from its docking site) with Arf6 peptides, washed to remove released proteins and peptides, and a two-stage AC reaction was performed. Both non-Myr-Arf6 (Fig. 4D) and Myr-Arf6 (data not shown) peptides blocked β-arrestin release stimulated by 100 μM GTP, thereby rescuing the desensitization response to hCG (compare dotted bars). These results provide direct evidence that the G protein that binds β-arrestin (directly or indirectly) is Arf6.
Figure 4.
Arf6 peptides inhibit LH/CGR desensitization by reducing β-arrestin release stimulated by 100 μM GTP. Membranes were preincubated with indicated concentrations of Myr-Arf6(2–13) peptide (A), non-Myr-Arf6(2–13) peptide (B), or Myr-Arf1(2–17) peptide (C) followed by the two-stage AC reaction. Results are means ± SEM of quadruplicate determinations from a single experiment and are representative of two or three separate experiments. For D, membranes were incubated with indicated treatments, diluted, and pelleted (see Fig. 3A legend); washed membranes were subjected to a two-stage AC reaction and to SDS/PAGE and Western blot analyses (see Fig. 3C legend). Results are means ± SEM of quadruplicate determinations from a single experiment and are representative of three separate experiments. Different AC activities reflect, in part, different membrane preparations.
Arf6 Is Localized to the Plasma Membrane.
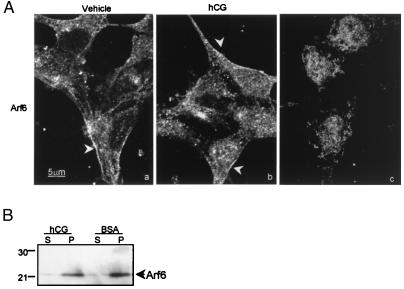
Arf6 is described to relocate to the plasma membrane on activation (39, 40). Yet, LH/CGR desensitization occurs in our membrane delimited model, suggesting that participating Arf6 is already membrane associated and does not need to translocate to the membrane to mediate LH/CGR desensitization. We tested this hypothesis by determining the cellular distribution of Arf6 by both IF and subcellular fractionation. Arf6 in vehicle and hCG-treated granulosa cells exhibited a predominant association with the cell periphery (arrowheads) (Fig. 5A), consistent with previous reports (15, 32, 41), which was blocked with competing peptide (c), and was associated with the membrane pellet fraction (Fig. 5B).
Figure 5.
Effect of hCG on the localization of Arf6 in granulosa cells. Preovulatory rat granulosa cells were treated with vehicle or 1 IU hCG for 40 min and then subjected to IF using anti-Arf6 antibody or to subcellular fractionation. (A) Distribution of Arf6 in vehicle and hCG-treated cells (a and b) was evaluated by IF using confocal microscopy. Results are representative of five separate experiments for each treatment. Edge staining was greatly reduced by incubating antibody with 500 μg of synthetic peptide overnight at 4°C before addition to fixed cells (c). (B) Western blots were performed on supernatant (S) and pellet (P) fractions using anti-Arf6 antibody. Protein load of supernatant fraction was ≈90 μg; pellets were resuspended in the same volume as supernatants and load volume was that of the corresponding supernatant. Results are representative of two separate experiments. Rat follicular membranes, like porcine follicular membranes, exhibit GTP- and β-arrestin-dependent LH/CGR desensitization (data not shown).
Discussion
Taken together these results suggest that agonist-induced desensitization of the LH/CGR depends upon activation of the membrane-localized small G protein Arf6 in response to LH/CGR activation, release of β-arrestin from a membrane-docking site, and its subsequent binding to the agonist-activated LH/CGR. Arf6 is a ubiquitously expressed protein (32). It has been shown to mediate insulin-stimulated glucose transport and GLUT4 translocation (41), to participate in regulated exocytosis by activating phospholipase D (17, 38), to play a regulatory role in receptor-mediated endocytosis (39), to remodel the actin cytoskeleton (34, 42), and to be required for phagocytosis in macrophage (43). Our results show that Arf6 also participates in GPCR desensitization. Indeed, the GTP-dependent step in LH/CGR desensitization, originally identified by Salomon and coworker (44, 45), appears to be the LH/CGR-dependent activation of Arf6, triggering β-arrestin release from a membrane-docking site.
Purified nonvisual arrestins also bind with high affinity to clathrin, AP-2 adaptor, and other proteins (46–48). Yet, it appears that in the cell, these interactions are not constitutive but occur in a strictly timed and regulated fashion. Our finding that β-arrestin is docked to the plasma membrane and released as the result of LH/CGR-dependent activation of Arf6 suggests one of the mechanisms regulating the availability of β-arrestin to its interaction partners, including the receptor itself. LH/CGR apparently initiates at least two distinct signaling cascades: one via a heterotrimeric G protein and another via the small G protein Arf6. Interestingly, this latter cascade ultimately leads to the release of β-arrestin necessary for receptor desensitization, providing a negative feedback loop whereby activated LH/CGR regulates its own shut-off. The GTP-dependent release of β-arrestin from a membrane-docking site upon LH/CGR activation may be unique to the LH/CGR. It is possible that activation of other GPCRs may also activate GTP-dependent mechanisms that regulate the release of arrestin proteins (which may be docked at the plasma membrane or elsewhere) necessary for their desensitization. Since desensitization of most GPCRs has been evaluated in intact cells, the GTP-dependent protein interactions we have observed in our isolated membrane model have not been possible to observe in this more common setting. Thus, there may be a more universal role for small G proteins like Arf6 in triggering the release of docked β-arrestin to mediate GPCR desensitization.
Acknowledgments
We thank Joshua E. Cottom for his expert technical assistance, Dr. Paul A. Randazzo for his helpful discussions, and members of the Hunzicker-Dunn laboratory for their advice throughout the progress of these studies. We gratefully acknowledge the gifts of mAb A7 from Dr. J. Paul Banga (King's College, School of Medicine and Dentistry, London, England), and arrestin antibodies from Dr. Larry Donoso (Wills Eye Hospital Research Division, Philadelphia, PA). This work was supported by National Institutes of Health Grants P01 HD 21921 and R01 HD 38060 (to M.H.D.), R01 EY 09339 (to K.P.), R01 EY 11500 (to V.V.G.), R01 AI 32991 (to J.E.C.) and by a Lalor Foundation Fellowship (to S.M.).
Abbreviations
- LH/CGR
luteinizing hormone/choriogonadotropin receptor
- GPCR
guanine nucleotide-binding protein-coupled receptor
- AC
adenylyl cyclase
- 3i
third intracellular
- IF
immunofluorescence
- Arf
ADP ribosylation factor
- ARNO
Arf nucleotide-binding site opener
- Myr
myristoylated
- hCG
human chorionic gondotrophin
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.100127097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.100127097
References
- 1.Dohlman H G, Thorner J, Caron M G, Lefkowitz R J. Annu Rev Biochem. 1991;60:653–688. doi: 10.1146/annurev.bi.60.070191.003253. [DOI] [PubMed] [Google Scholar]
- 2.Dufau M L. Annu Rev Physiol. 1998;60:461–496. doi: 10.1146/annurev.physiol.60.1.461. [DOI] [PubMed] [Google Scholar]
- 3.Loosfelt H, Misrahi M, Atger M, Salesse R, Vuhai-Luuthi M T, Jolivet A, Guiochon-Mantel A, Sar S, Jallal B, Garnier J, Milgrom E. Science. 1989;245:525–528. doi: 10.1126/science.2502844. [DOI] [PubMed] [Google Scholar]
- 4.McFarland K C, Sprengel R, Phillips H S, Kohler M, Rosembilt N, Nikolics K, Segaloff D L, Seeburg P H. Science. 1989;245:494–499. doi: 10.1126/science.2502842. [DOI] [PubMed] [Google Scholar]
- 5.Hausdorff W P, Caron M G, Lefkowitz R J. FASEB J. 1990;4:2881–2889. [PubMed] [Google Scholar]
- 6.Marsh J M, Mills T M, Lemaire W J. Biochim Biophys Acta. 1973;304:197–202. doi: 10.1016/0304-4165(73)90128-1. [DOI] [PubMed] [Google Scholar]
- 7.Conti M, Harwood J P, Hsueh A J W, Dufau M L, Catt K J. J Biol Chem. 1976;251:7729–7731. [PubMed] [Google Scholar]
- 8.Hunzicker-Dunn M, Birnbaumer L. Endocrinology. 1976;99:185–197. doi: 10.1210/endo-99-1-185. [DOI] [PubMed] [Google Scholar]
- 9.Ekstrom R C, Hunzicker-Dunn M. Endocrinology. 1989;124:956–963. doi: 10.1210/endo-124-2-956. [DOI] [PubMed] [Google Scholar]
- 10.Ekstrom R C, Hunzicker-Dunn M. Endocrinology. 1989;125:2470–2474. doi: 10.1210/endo-125-5-2470. [DOI] [PubMed] [Google Scholar]
- 11.Ekstrom R C, Carney E M, Lamm M L G, Hunzicker-Dunn M. J Biol Chem. 1992;267:22183–22189. [PubMed] [Google Scholar]
- 12.Mukherjee S, Palczewski K, Benovic J L, Gurevich V V, Hunzicker-Dunn M. Proc Natl Acad Sci USA. 1999;96:493–498. doi: 10.1073/pnas.96.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukherjee S, Palczewski K, Gurevich V V, Hunzicker-Dunn M. J Biol Chem. 1999;274:12984–12989. doi: 10.1074/jbc.274.19.12984. [DOI] [PubMed] [Google Scholar]
- 14.Gurevich V V, Pals-Rylaarsdam R, Benovic J L, Hosey M M, Onorato J J. J Biol Chem. 1997;272:28849–289852. doi: 10.1074/jbc.272.46.28849. [DOI] [PubMed] [Google Scholar]
- 15.Frank S, Upender S, Hansen S H, Cassanova J E. J Biol Chem. 1998;273:23–27. doi: 10.1074/jbc.273.1.23. [DOI] [PubMed] [Google Scholar]
- 16.Kowluru A, Li G, Rabaglia M E, Seug V B, Hofmann F, Aktories K, Metz S A. Biochem Pharmacol. 1997;54:1097–1108. doi: 10.1016/s0006-2952(97)00314-6. [DOI] [PubMed] [Google Scholar]
- 17.Caumont A-S, Galas M-C, Vitale N, Aunis D, Bader M-F. J Biol Chem. 1998;273:1373–1379. doi: 10.1074/jbc.273.3.1373. [DOI] [PubMed] [Google Scholar]
- 18.Nicholson L B, Vlase H, Graves P, Nilsson M, Hyang G C, Morgenthaler N G, Davies T F, MacGregor A M, Banga J P. J Mol Endocrinol. 1996;16:159–170. doi: 10.1677/jme.0.0160159. [DOI] [PubMed] [Google Scholar]
- 19.Bender F E, Douglass L W, Kramer A. Statistical Methods for Food and Agriculture. Inc., Westport, CT: AVI Publishing Co.; 1982. pp. 87–107. [Google Scholar]
- 20.Hunzicker-Dunn M, Maizels E T, Kern L C, Ekstrom R C, Constantinou A I. Mol Endocrinol. 1989;3:780–789. doi: 10.1210/mend-3-5-780. [DOI] [PubMed] [Google Scholar]
- 21.Carr D W, DeManno D A, Atwood A, Hunzicker-Dunn M, Scott J D. J Biol Chem. 1993;268:20729–20732. [PubMed] [Google Scholar]
- 22.Gaschet J, Hsu V W. J Biol Chem. 1999;274:20040–20045. doi: 10.1074/jbc.274.28.20040. [DOI] [PubMed] [Google Scholar]
- 23.Goldfinger L E, Stack M S, Jones J C R. J Cell Biol. 1998;141:255–265. doi: 10.1083/jcb.141.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benovic J L, Kuhn H, Weyand I, Codina J, Caron M G, Lefkowitz R J. Proc Natl Acad Sci USA. 1987;84:8879–8882. doi: 10.1073/pnas.84.24.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajagopalan-Gupta R M, Lamm M L, Mukherjee S, Rasenick M M, Hunzicker-Dunn M. Endocrinology. 1998;139:4547–4555. doi: 10.1210/endo.139.11.6302. [DOI] [PubMed] [Google Scholar]
- 26.Rajagopalan-Gupta R M, Rasenick M M, Hunzicker-Dunn M. Mol Endocrinol. 1997;11:538–549. doi: 10.1210/mend.11.5.9929. [DOI] [PubMed] [Google Scholar]
- 27.Rajagopalan-Gupta R M, Mukherjee S, Zhu X, Ho Y-K, Hamm H, Birnbaumer M, Birnbaumer L, Hunzicker-Dunn M. Endocrinology. 1999;140:1612–1621. doi: 10.1210/endo.140.4.6657. [DOI] [PubMed] [Google Scholar]
- 28.Hofmann F, Busch C, Prepens U, Just I, Aktories K. J Biol Chem. 1997;272:11074–11078. doi: 10.1074/jbc.272.17.11074. [DOI] [PubMed] [Google Scholar]
- 29.Busch C, Hofmann F, Selzer J, Munro S, Jeckel D, Aktories K. J Biol Chem. 1998;273:19566–10572. doi: 10.1074/jbc.273.31.19566. [DOI] [PubMed] [Google Scholar]
- 30.Aktories K. Trends Microbiol. 1997;5:282–288. doi: 10.1016/S0966-842X(97)01067-6. [DOI] [PubMed] [Google Scholar]
- 31.Donaldson J G, Finazzi D, Klausner R D. Nature (London) 1992;360:350–354. doi: 10.1038/360350a0. [DOI] [PubMed] [Google Scholar]
- 32.Cavenagh M M, Whitney J A, Carroll K, Zhang C-j, Boman A L, Rosenwald A G, Mellman I, Kahn R A. J Biol Chem. 1996;271:21767–21774. doi: 10.1074/jbc.271.36.21767. [DOI] [PubMed] [Google Scholar]
- 33.Chardin P, Paris S, Antonny B, Robineau S, Beraud-Dufour S, Jackson C L, Chabre M. Nature (London) 1996;384:481–484. doi: 10.1038/384481a0. [DOI] [PubMed] [Google Scholar]
- 34.Frank S R, Hatfield J C, Casanova J E. Mol Biol Cell. 1998;9:3133–3146. doi: 10.1091/mbc.9.11.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagel W, Schilcher P, Zeitlmann L, Kolanus W. Mol Biol Cell. 1999;9:1981–1994. doi: 10.1091/mbc.9.8.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knospe V, Donoso L A, Banga J P, Yue S, Kasp E, Gregerson D S. Eye Res. 1988;7:1137–1147. doi: 10.3109/02713688809001885. [DOI] [PubMed] [Google Scholar]
- 37.Kahn R A, Randazzo P, Serafini T, Weiss O, Rulka C, Clark J, Amherdt M, Roller P, Orci L, Rothman J E. J Biol Chem. 1992;267:13039–13046. [PubMed] [Google Scholar]
- 38.Galas M-C, Helms J B, Vitale N, Thierse D, Aunis D, Bader M-F. J Biol Chem. 1997;272:2788–2793. doi: 10.1074/jbc.272.5.2788. [DOI] [PubMed] [Google Scholar]
- 39.D'Souza-Schorey C, Li G, Colombo M I, Stahl P D. Science. 1995;267:1175–1178. doi: 10.1126/science.7855600. [DOI] [PubMed] [Google Scholar]
- 40.Peters P J, Hsu V W, Ooi C E, Finazzi D, Teal S B, Oorschot V, Donaldson J G, Klausner R D. J Cell Biol. 1995;128:1003–1017. doi: 10.1083/jcb.128.6.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Millar C A, Powell K A, Hickson G R X, Bader M-F, Gould G W. J Biol Chem. 1999;274:17619–17625. doi: 10.1074/jbc.274.25.17619. [DOI] [PubMed] [Google Scholar]
- 42.D'Souza-Schorey C, Boshans R L, McDonough M, Stahl P D, Vam Aelst L. EMBO J. 1997;16:5445–5454. doi: 10.1093/emboj/16.17.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Q, Cox D, Tseng C-C, Donaldson J G, Greenberg S. J Biol Chem. 1998;273:19977–19981. doi: 10.1074/jbc.273.32.19977. [DOI] [PubMed] [Google Scholar]
- 44.Ezra E, Salomon Y. J Biol Chem. 1980;255:653–658. [PubMed] [Google Scholar]
- 45.Ezra E, Salomon Y. J Biol Chem. 1981;256:5377–5382. [PubMed] [Google Scholar]
- 46.Goodman O B, Krupnick J G, Santini F, Gurevich V V, Penn R B, Gagnon A W, Keen J H, Benovic J L. Nature (London) 1997;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 47.Laporte S A, Oakley RlH, Zhang J, Holt J A, Ferguson S S G, Caron M G, Barak L S. Proc Natl Acad Sci USA. 1999;96:3712–3717. doi: 10.1073/pnas.96.7.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McDonald P H, Cote N L, In F-T, Premont R T, Pitcher J A, Lefkowitz R J. J Biol Chem. 1999;274:10677–10680. doi: 10.1074/jbc.274.16.10677. [DOI] [PubMed] [Google Scholar]