Abstract
Objective
Depression, apathy (amotivation), and cognitive impairment are common comorbidities in hip fracture patients, which may adversely affect functional outcome of rehabilitation. We examined whether post-fracture measures of mood, motivation, or cognition are associated with rehabilitation outcome (defined as functional improvement) in inpatient rehabilitation facilities (IRFs), as compared to skilled nursing facilities (SNFs).
Methods
This prospective study examined elderly patients who received surgical fixation for hip fracture and then received post-acute rehabilitation at an IRF or a SNF. Subjects were characterized at baseline for depression using the Hamilton Rating Scale for Depression, apathy/amotivation using the Apathy Evaluation Scale, and mild-moderate cognitive impairment using the Mini-Mental Status Examination. Functional recovery was measured over 12-week follow-up using the Functional Independence Measure.
Results
58 subjects were discharged from acute care to an IRF and 39 to a SNF. Patients with depression, apathy, or cognitive impairment who received rehabilitation at an IRF had significantly better functional outcomes than similarly-impaired patients at SNFs, and similar outcomes as nondepressed, motivated, and cognitively intact elderly at IRFs.
Conclusion
These findings suggest that depression, amotivation, or mild-moderate cognitive impairment after hip fracture do not reduce the benefit of post-acute rehabilitation in an IRF.
Keywords: geriatric depression, motivation, cognition, hip fracture, rehabilitation
INTRODUCTION
Hip fracture is a leading physical rehabilitation condition among older adults. As patients are often unable to return home after surgical fixation, [1–2] rehabilitation provides functional restoration through medical, exercise and activity-based therapies. Perhaps the critical rehabilitation interval occurs between acute hospital discharge and the patient’s return to home (or placement). Two major sites for rehabilitation exist for this interval: Inpatient rehabilitation facilities (IRFs), where patients receive comprehensive medical rehabilitative services, including at least three hours of combined PT and OT per day; and, skilled nursing facilities (SNFs), where patients typically receive no more than two sessions daily and less nursing physician contact than IRFs. [3–4]
IRF rehabilitation is more expensive, [3–5] despite decreasing length of stay, [6] raising the question of whether it provides advantages over SNF rehabilitation that would justify the cost. Two naturalistic studies, one by our group, found that hip fracture patients have better functional outcomes at IRFs than SNFs, [4,7] while two found no such benefit. [3,5] From these studies it is unclear which patients are more ideally treated in an IRF vs. a SNF after hip fracture, and decisions regarding appropriateness for IRF admission appear to be evolving in response to reimbursement guidelines designed for cost containment. [8] For example, stemming from the consensus that IRF patients should be able to tolerate three hours per day of therapy, [9] InterQual guidelines (proprietary rehabilitation guidelines for many insurance providers in the U.S.) include requirements such as “ability to follow commands” and “ability to fully participate and tolerate intensive rehabilitation”, [10]. These guidelines potentially exclude cognitively impaired or amotivated patients after hip fracture from an IRF. [3] Because depression is associated with apathy (or amotivation), [11] patients with depression after hip fracture may also be excluded from IRFs.
Yet it is unclear whether these commonly-observed impairments in cognition, mood, or motivation predict less benefit from IRFs. Although cognitive impairment predicts poor functional outcome after hip fracture, [12–13] mildly demented hip fracture patients [14] and those with lower Mini-Mental Status Examination (MMSE) scores [13,15] benefit from IRFs. Many patients with cognitive impairment after hip surgery have delirium, a reversible condition. [16] Similarly, some have argued that elderly persons with low motivation for therapy after hip fracture should be assigned to a less intensive setting, [17] and there is some evidence that depression after hip fracture is associated with poorer functional recovery, possibly due to lower motivation. [18–19] Yet, depression and amotivation may be temporary [20–21]; depression may have no negative effect on IRF outcome [13] and may improve with rehabilitation. [22–23]
Thus, studies have not clarified whether impairments in cognition, mood, or motivation after hip fracture define a group less likely to benefit from an IRF. We previously demonstrated in this sample an overall benefit of IRF (that is, IRF functional outcome was superior to SNF functional outcome for patients living in the community at the time of fracture). [7] In this analysis we examined whether this benefit of IRF rehabilitation (i.e., superior functional outcome compared to SNF) was seen in patients with depression, amotivation, or cognitive impairment. As an additional examination of this question, we examined whether IRF patients with these impairments had similar vs. poorer functional outcome as those without these impairments.
METHODS
The study recruited consecutive hip fracture admissions to an acute care hospital from March 2002–October 2004. Subjects received surgical repair and were aged 60 or older, able to provide informed consent, and free of metastatic cancer. This study was approved by the university’s Institutional Review Board.
Subjects were assessed at the end of their acute hospital stay (i.e., at baseline) with the 17-item Hamilton Depression Rating Scale (Ham-D) [24] to measure depressive symptoms. A Ham-D score of 10 or greater indicates clinically significant depressive symptoms. [25] Interrater reliability was 0.86–0.96 (Intraclass Correlation Coefficient). The Apathy Evaluation Scale (AES) [26] was used to measure signs and symptoms of apathy, or amotivation. An AES score of 38 or greater indicates clinically significant apathy. Cronbach’s alpha for the measure in this study was 0.93. Cognitive impairment was measured with the MMSE [27]; scores of 24 or less indicate cognitive impairment. [28] Interrater reliability was 0.92–0.93. Because of the requirement that subjects provide consent for the study, those with severe cognitive impairment were excluded.
Functional status was measured at the end of the surgical stay and two and 12 weeks later, using the 13 motor items of the Functional Independence Measure (FIM™-motor) [29], a measure of dependence on assistive devices or other persons for motor and ADL tasks. At the end of the surgical stay and 2 weeks later, FIM-motor data were collected from occupational and physical therapy practitioners (or from patients at week 12) by telephone by a Uniform Data System certified occupational therapist with over 10 years experience using a standardized interview[s1]. At 12 weeks, data were collected from patients and caregivers by telephone by the same rater using the same standardized interview.
We examined the decision-making process by which patients were referred to IRFs or SNFs. Disposition was based in part on orthopedic surgeon preference, which appeared to be idiosyncratic, and patient and family preference, which appeared to be based on geographic proximity to the patient’s or family’s home. Additionally, we examined whether patients received mental health services at IRFs or SNFs; no patient received either a psychiatric consultation or a psychological evaluation. This analysis included only subjects who received rehabilitation at an IRF or SNF and with at least a week 2 FIM-motor score and comprised patients discharged to eight different IRFs and 12 different SNFs.
To characterize subjects for baseline variables that may influence functional recovery, demographic variables were gathered as well as characteristics of the fracture, surgery, and length of surgical stay. Additionally, number and frequency of social supports was measured using the Social Network Index, [30] and medical comorbidity was measured using the Cumulative Illness Rating Scale for Geriatrics. [31]
The first goal was to examine whether in patients with impairments in mood, motivation, and cognition there was a superior outcome in IRFs vs. SNFs, and the second goal was to examine whether patients with these impairments had poorer IRF outcomes than those without such impairments. Thus, we carried out a mixed model repeated measures analysis of FIM-motor scores, comparing IRF to SNF patients in three ways: dichotomizing by baseline (1)Ham-D scores ≥10 vs. <10, (2)AES scores ≥38 vs. <38, and (3)MMSE scores ≥25 vs. <25. As an added test of these goals, we compared week 12 estimated (least square mean) FIM-Motor scores in IRF and SNF patients, divided into group (e.g., depressed vs. nondepressed). To examine the potential confound of baseline covariates, those that varied significantly (at p<.10) between IRF and SNF were added to the mixed models to determine whether they were predictors of FIM-motor outcome. Only age and social support were predictors; the mixed models were rerun including these two covariates.
RESULTS
Study sample
The study approached 139 subjects; 12 refused the study, 14 dropped out before week 2, and 16 did not receive rehabilitation at an IRF or SNF. Thus, the sample includes 97 subjects: 58 at IRFs and 39 at SNFs.
Baseline characteristics of the subjects are: age mean 81.7 (SD 8.8); 81.4% female; 93.8% White; (post-fracture) FIM-motor mean 46.5 (SD 8.2); Ham-D mean 9.4 (SD 4.8); MMSE mean 25.2 (SD 3.5); AES mean 33.5 (SD 9.4, n=95), 39.2% (n=38) subcapital fracture (remainder inter- or sub-trochanteric); surgical length of stay mean 5.7 (SD 2.7) days.
Defining depression as Ham-D≥10, 22/58 (38%) IRF and 17/39 (44%) SNF patients were depressed at baseline (exact p=0.67). Defining amotivation as AES≥38, 14/57 (25%) IRF patients and 21/38 (55%) SNF patients had clinically significant levels of amotivation (exact p=0.004), and 18/58 (31%) IRF and 20/39 (51%) SNF patients had MMSE≤25, showing cognitive impairment (exact p=0.06). Figure 1 shows that subjects with a given impairment often had one or both other impairments. For example, of the 39 depressed patients, 24 (62%) also had either amotivation, cognitive impairment, or both.
Figure 1.

Impairment in depression, apathy and cognition scores in 95 hip fracture patients
Functional improvements over 12 weeks
The models displayed in Figure 2a–c display results from the mixed effect models. As each model shows, there is a main effect of rehabilitation site, indicating a better functional (FIM) outcome in IRFs compared to SNFs, a main effect of time, indicating that patients improved over time in functional outcome, and a rehabilitation site by time interaction, indicating that patients in IRFs improve at a differential rate (more early recovery, less late recovery) than patients in SNFs. These results have been reported previously. [7]
Figure 2.
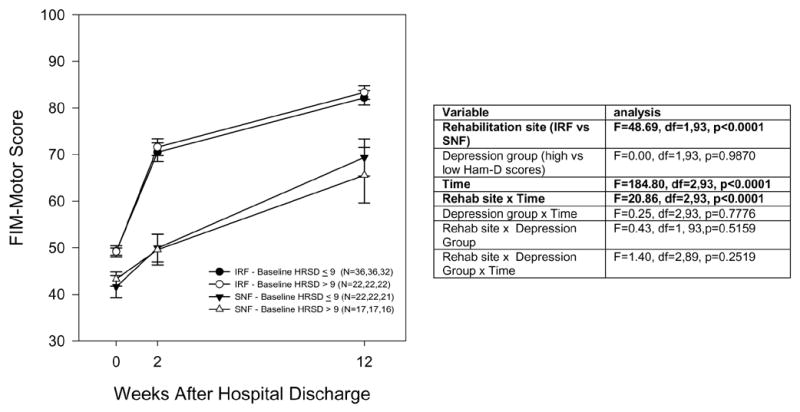
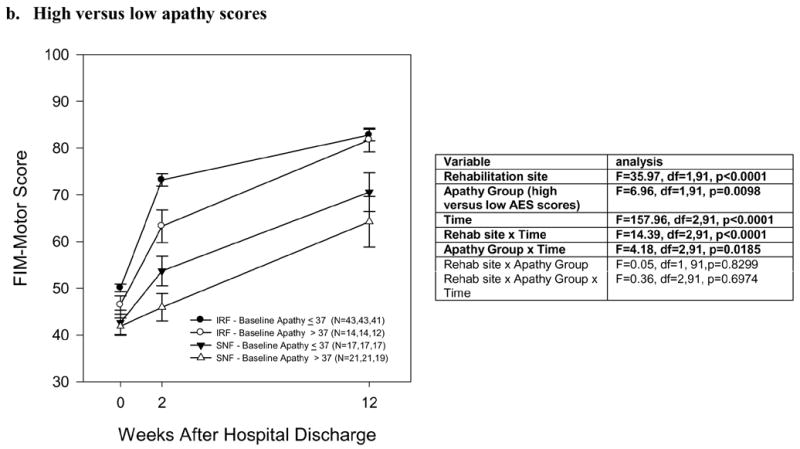

Functional recovery over 12 weeks after hip fracture, in an inpatient rehabilitation facility (IRF) or a skilled nursing facility (SNF), among patients divided by (a) Depressed versus nondepressed (Ham-D ≥10 versus ≤9), (b) High versus low apathy scores (AES ≥38 versus ≤37), and (c) Impaired versus unimpaired cognition (MMSE ≤24 versus ≥25). a. Depressed versus nondepressed b. High versus low apathy scores c. Cognitively impaired versus unimpaired
a. Associations with depression
Figure 2a shows a comparison of FIM-motor changes for IRF versus SNF patients, subdivided into depressed and nondepressed groups. There was no overall association of depression status with functional outcome (nonsignificant depression group effect and depression group x time interaction) and no evidence that the superior functional outcome in IRFs was altered by depression status (nonsignificant three-way interaction of rehabilitation site x depression group x time). In other words, the data with Figure 2a suggest that depressed elderly were as likely to show benefit from IRF (compared to SNF rehabilitation) as nondepressed elderly. These results did not change when controlling for age and social support (data not shown).
In depressed patients in IRFs, week 12 FIM-motor was 80.1 (least square mean, controlling for baseline FIM-motor, age and social support; standard error 3.0), which was not significantly different than non-depressed patients in IRFs (mean 81.1 [standard error 2.6], t=0.24, df=84, p=0.81) and was greater than depressed patients in SNFs (67.9 [standard error 3.5], t=2.65, df=84, p=0.01).
b. Associations with apathy/amotivation
Figure 2b shows the same analysis for patients divided into high apathy scores (≥38) versus low (≤37). There is a significant main effect of apathy as well as an apathy group x time interaction, as the data with Figure 2b show: higher apathy scores are associated with lower week 2 functional outcome in the IRF group, and lower week 2 and week 12 functional outcome in the SNF group. There was no evidence, though, that apathy alters the greater functional outcome in IRF vs. SNF patients (non-significant three-way interaction). These results did not change when controlling for age and social support.
In high-apathy patients in IRFs, the week 12 FIM-motor was 77.9 (least square mean, controlling for baseline FIM-motor, age and social support; standard error 4.0), which was not significantly different than low-apathy patients in IRFs (mean 81.6 [standard error 2.3], t=0.81, df=82, p=0.42) and was greater than high-apathy patients in SNFs (67.3 [standard error 3.4], t=1.99, df=82, p=0.05).
c. Associations with cognitive impairment
Figure 2c shows patients divided into cognitively impaired versus intact elderly. There was no overall effect of cognitive impairment on functional outcomes (nonsignificant cognitive group effect and cognitive group x time interaction), nor an effect of cognition on superior functional outcome in IRFs compared with SNFs (non-significant three-way interaction). These results did not change when controlling for age and social support.
In cognitively impaired patients in IRFs, the week 12 FIM-motor was 81.2 (least square mean, controlling for baseline FIM-motor, age and social support; standard error 3.3), which was not significantly different than cognitively intact patients in IRFs (mean 80.3 [standard error 2.4], t=− 0.22, df=84, p=0.83) but was also not significantly different than cognitively impaired patients in SNFs (73.1 [standard error 3.5], t=1.69, df=84, p =0.10).
DISCUSSION
The consensus in geriatric rehabilitation is that patients admitted to IRFs should be able to tolerate three hours daily of physical and occupational therapy, [8] raising the question of whether post-hip fracture evidence of depression, apathy, or mild-moderate cognitive impairment should be used to determine appropriateness for an IRF. We found that these baseline measures had little or no bearing on IRF outcomes. We found that functional outcomes at 12 weeks were similar in IRF patients with these impairments, to those without such impairments. Further, we found significantly better week 12 functional outcomes after IRF rehabilitation (compared to SNF) for the depressed and apathetic subgroups (the respective analysis of the cognitively impaired group was non-significant likely due to insufficient power).
One caveat is that we did find that apathy was associated with poorer functional outcome in the overall sample, although IRF outcomes in apathetic patients were superior to SNF outcomes. In these apathetic patients in IRFs, functional gains were poorer at week 2 (compared to non-apathetic) but not at week 12, suggesting that functional benefit is delayed but not diminished. This possibility is consistent with a previous finding by our group that rehabilitation participation improves as patients spend more time in IRFs, [32] suggesting that a longer rehabilitation process may be necessary. Given the small sample size of this subgroup, further research would be needed to examine this hypothesis.
Some limitations should be noted. First, this was in one geographic region. Second, we excluded those with severe cognitive impairment (i.e., could not reliably follow commands and understand information); thus, our findings can’t address whether those with severe cognitive impairment should go to an IRF. Third, the higher proportion of SNF patients with apathy suggest that apathetic patients were selected for SNFs; randomization to IRF vs. SNF would be necessary to make a clearer inference that superior outcomes in IRFs are not due to selection of less ideal patients for SNFs.
Notwithstanding these limitations, this study supports the assertion that functional benefit of rehabilitation at an IRF after hip fracture is not diminished by impairments in mood, motivation, or cognition. Research examining factors differing between IRFs and SNFs, and their relationship with functional outcomes, could improve rehabilitation outcomes for more vulnerable elderly populations.
Acknowledgments
The investigators would like to acknowledge the staff of the UPMC Shadyside Hospital and UPMC Presbyterian Hospital for their efforts with this study. This research was supported by National Institute of Mental Health grants K23 MH64196, P30 MH52247, P30 MH71944, and the UPMC Endowment in Geriatric Psychiatry.
Footnotes
Sponsor's Role: The sponsor, NIMH, had no role in the design, methods, subject recruitment, data collection, analysis, or preparation of the paper.
Financial Disclosure(s): Dr. Lenze indicates he receives, or has received grant/research support from Pfizer, Johnson & Johnson, and Forest. Dr. Munin indicates he receives grant/research support from Allergan, Inc. Dr. Reynolds indicates he receives, or has received grant/research support from Glaxo Smith Kline, Forest, Pfizer, and Lilly. Drs. Skidmore, Dew, Butters, Rogers, and Ms Begley have no potential conflicts to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zuckerman JD. Current concepts: hip fracture. New Engl J Med. 1996;334:1519–1525. doi: 10.1056/NEJM199606063342307. [DOI] [PubMed] [Google Scholar]
- 2.Magaziner J, Simonsick EM, Kashner M, et al. Predictors of functional recovery on year following hospital discharge for hip fracture: a prospective study. J Geront A Biol Sci Med Sci. 1990;45:M101–M107. doi: 10.1093/geronj/45.3.m101. [DOI] [PubMed] [Google Scholar]
- 3.Kramer AM, Steiner JF, Schlenker RE, et al. Outcomes and costs after hip fracture and stroke. A comparison of rehabilitation settings. JAMA. 1997;277:396–404. [PubMed] [Google Scholar]
- 4.Kane RL, Chen Q, Finch M, et al. The optimal outcomes of post-hospital care under medicare. Health Services Research. 2000;35:615–661. [PMC free article] [PubMed] [Google Scholar]
- 5.Deutsch A, Granger CV, Fiedler RC, et al. Outcomes and reimbursement of inpatient rehabilitation facilities and subacute rehabilitation programs for Medicare beneficiaries with hip fracture. Med Care. 2005;43:892–901. doi: 10.1097/01.mlr.0000173591.23310.d5. [DOI] [PubMed] [Google Scholar]
- 6.Ottenbacher KJ, Smith PM, Ilig SB, et al. Trends in length of stay, living setting, functional outcome, and mortality following medical rehabilitation. JAMA. 2004;292:1687–95. doi: 10.1001/jama.292.14.1687. [DOI] [PubMed] [Google Scholar]
- 7.Munin MC, Seligman K, Dew MA, et al. Effect of rehabilitation site on functional recovery after hip fracture. Arch Phys Med Rehabil. 2005;86:367–372. doi: 10.1016/j.apmr.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Braddom RL. Medicare Funding for Inpatient Rehabilitation: How Did We Get to This Point and What Do We Do Now? Arch Phys Med Rehabil. 2005;86:1287–1292. doi: 10.1016/j.apmr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Medicare and Medicaid Services, Medicare Program; prospective payments for Medicare inpatient hospital services (1983) 48 Federal Register 39752.
- 10.McKesson Health Solutions LLC; 2002. InterQual Level of care criteria rehabilitation adult. [Google Scholar]
- 11.Marin RS, Butters MA, Mulsant BH, et al. Apathy and executive function in depressed elderly. J Geriatr Psychiatry Neurol. 2003;16:112–6. doi: 10.1177/0891988703016002009. [DOI] [PubMed] [Google Scholar]
- 12.Gruber-Baldini AL, Zimmerman S, Morrison RS, et al. Cognitive impairment in hip fracture patients: timing of detection and longitudinal follow-up. J Am Geriatr Soc. 2003;51:1227–36. doi: 10.1046/j.1532-5415.2003.51406.x. [DOI] [PubMed] [Google Scholar]
- 13.Lenze EJ, Munin MC, Dew MA, et al. Adverse effects of depression and cognitive impairment on rehabilitation participation and recovery from hip fracture. Int J Geriatr Psychiatry. 2004;19:472–478. doi: 10.1002/gps.1116. [DOI] [PubMed] [Google Scholar]
- 14.Huusko TM, Karppi P, Avikainen V, et al. Randomised, clinically controlled trial of intensive geriatric rehabilitation in patients with hip fracture: subgroup analysis of patients with dementia. BMJ. 2000;321:1107–1111. doi: 10.1136/bmj.321.7269.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heruti RJ, Lusky A, Barell V, et al. Cognitive status at admission: does it affect the rehabilitation outcome of elderly patients with hip fracture? Arch Phys Med Rehabil. 1999;80:432–436. doi: 10.1016/s0003-9993(99)90281-2. [DOI] [PubMed] [Google Scholar]
- 16.Marcantonio ER, Flacker JM, Michaels M, et al. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48:618–624. doi: 10.1111/j.1532-5415.2000.tb04718.x. [DOI] [PubMed] [Google Scholar]
- 17.Folman Y, Gepstein R, Assaraf A, et al. Functional recovery after operative treatment of femoral neck fractures in an institutionalized elderly population. Arch Phys Med Rehabil. 1994;75:454–456. doi: 10.1016/0003-9993(94)90171-6. [DOI] [PubMed] [Google Scholar]
- 18.Mossey JM, Knott K, Craik R. The effects of persistent depressive symptoms on hip fracture recovery. J Geront A Biol Sci Med Sci. 1990;45:M163–M168. doi: 10.1093/geronj/45.5.m163. [DOI] [PubMed] [Google Scholar]
- 19.Holmes J, House A. Psychiatric illness predicts poor outcome after surgery for hip fracture: a prospective cohort study. Psychol Med. 2000;30:921–929. doi: 10.1017/s0033291799002548. [DOI] [PubMed] [Google Scholar]
- 20.Kamel HK, Mohammad AI, Mogallapu R, et al. Time to ambulation after hip fracture surgery: relation to hospitalization outcomes. J Gerontol A Biol Sci Med Sci. 2003;58:1042–1045. doi: 10.1093/gerona/58.11.m1042. [DOI] [PubMed] [Google Scholar]
- 21.Zimmerman SI, Smith HD. Short-term persistent depression following hip fracture: a risk factor and target to increase resilience in elderly people. Soc Work Res. 1999;23:187–196. [Google Scholar]
- 22.Barbisoni P, Bertozzi B, Franzoni S, et al. Mood improvement in elderly women after in-hospital physical rehabilitation. Arch Phys Med Rehabil. 1996;77:346–9. doi: 10.1016/s0003-9993(96)90082-9. [DOI] [PubMed] [Google Scholar]
- 23.Schubert DSP, Burns R, Paras W, Sioson E. Decrease of depression during stroke and amputation rehabilitation. Gen Hosp Psychiatry. 1992;14:135–141. doi: 10.1016/0163-8343(92)90039-d. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psych. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hedlund JL, Vieweg BW. The Hamilton Rating Scale for Depression: a comprehensive review. J Oper Psychiatr. 1979;10:149–165. [Google Scholar]
- 26.Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res. 1991;38:143–162. doi: 10.1016/0165-1781(91)90040-v. [DOI] [PubMed] [Google Scholar]
- 27.Folstein MF, Folstein SW, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 28.Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40:922–35. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 29.Linacre JM, Heinemann AW, Wright BD, et al. The structure and stability of the Functional Independence Measure. Arch Phys Med Rehabil. 1994;75:127–132. [PubMed] [Google Scholar]
- 30.Cohen S, Doyle WJ, Skoner DP, et al. Social ties and susceptibility to the common cold. JAMA. 1997;277:1940–1944. [PubMed] [Google Scholar]
- 31.Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41:237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 32.Lenze EJ, Munin MC, Quear T, et al. The Pittsburgh Rehabilitation Participation Scale: Reliability and Validity of a Clinician-Rated Measure of Participation in Acute Rehabilitation. Arch Phys Med Rehabil. 2004;85:380–384. doi: 10.1016/j.apmr.2003.06.001. [DOI] [PubMed] [Google Scholar]


