The adverse consequences of comorbidity pose a major clinical challenge in the care of older cancer patients. While the burden of comorbidity is clearly a major prognostic factor for long-term survival, the underlying mechanisms are not well understood. Health services research that focuses on specific comorbidities and their effects in a cancer patient’s clinical trajectory can produce new insights into the optimal diagnosis, treatment, and long-term surveillance of cancer patients with comorbid disease. This information can then be used to design interventions that improve prognosis. We propose a conceptual model of comorbidity and cancer that can guide this research. The model illustrates the potential impact of a specific comorbidity at multiple points of a patient’s clinical trajectory, from cancer detection through diagnosis and treatment.
Comorbid illness is a significant concern in patients with cancer.1,2 For example, patients with severe underlying chronic obstructive pulmonary disease are not good candidates for resection of a lung malignancy, and therefore their chance of cure is decreased.3,4 Similarly, a diagnosis of congestive heart failure precludes some cancer treatments.5,6 Comorbid disease is also a competing cause of death. This is particularly true for older patients with cancer, who comprise the majority of new cancers diagnosed.
It has been customary in population-based studies to use a summary measure of a patient’s comorbid conditions, include this number in a multivariate analysis along with other patient characteristics, and calculate a patient’s risk of death according to the multivariate model (Fig 1).7–9 This model reflects the common observation that the overall survival of cancer populations decreases as the burden of comorbid disease increases.10 Information provided by this model is useful in estimating the prognosis of individual patients and also in risk stratification for comparison of outcomes across providers or institutions.
Fig 1.
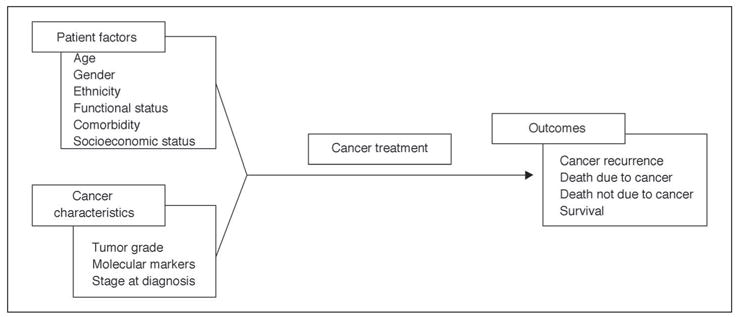
Prevailing model of comorbidity and cancer.
However, this model does not provide information on mechanisms. How does a given comorbidity lead to decreased survival? Does it affect stage at diagnosis, choice of treatment, compliance with the therapeutic regimen, treatment response, or perhaps all of these points in the patient’s care? Does the cancer or cancer treatment affect the comorbidity? Such mechanistic questions are important. Some of these potential effects might be mitigated by changes in systems of cancer care. Investigation of individual comorbid conditions and their effects on a cancer patient’s clinical trajectory could produce new insights into the optimal strategies for diagnosis, treatment and long-term surveillance of cancer patients with comorbid disease. In this article we discuss types of comorbidity measures, sources of data on comorbidity, and the complexities involved in studying comorbidity in cancer patients. We conclude with the presentation of a conceptual model and suggest types of studies and data sets that would be suitable to investigate individual comorbidities and at specific points in the cancer clinical trajectory.
Types of Comorbidity Measures
A comorbidity is an illness other than the principal diagnosis that influences the outcome of treatment.11 Much prior work on comorbidity employs summary comorbidity measures that attempt to assess the combined impact of different diseases. These summary measures can be divided into two groups: general measures intended for use in multiple disease populations, and disease-specific measures.12 The most commonly used general comorbidity measure is the Charlson Index.13 It was developed to predict 1-year mortality in medical inpatients, and was subsequently validated in a population of breast cancer patients.13 Nineteen comorbid conditions are assigned weights of 1, 2, 3, or 6, based on the ratio of the mortality risk for patients with the comorbidity of interest versus the mortality risk for those without. The sum of the weights for all of the conditions is calculated to create a comorbidity index for each patient. Klabunde refined the Charlson Index by incorporating diagnostic and procedure data in physician Medicare claims, in a study predicting 2-year noncancer mortality in cancer patients.14 Another example of a general comorbidity measure is the Adult Comorbidity Evaluation 27 (ACE-27), which requires the review of medical records to collect data on the presence and severity of 27 comorbid conditions in cancer patients.10
Disease-specific comorbidity measures are developed and tested in a single disease population, and intended for use only in that disease. Several disease-specific comorbidity measures have been created for breast cancer, for example.15–17
One weakness of general measures is in the assumption that each constituent comorbidity in the measure has the same impact in different diseases and populations. One should expect that a general comorbidity measure will not explain as much variance in the outcome of interest as a disease-specific comorbidity measure could.12 In addition, general comorbidity measures may include conditions that actually arise as manifestations of the disease under study, rather than as independent comorbidities. For example, anemia, weight loss, pneumonia, and electrolyte disorders may all occur in the months before a diagnosis of certain cancers. This issue would normally be addressed in the design of disease-specific comorbidity measures.
Disease-specific comorbidity measures have a conceptual advantage in that specific treatment and outcome issues unique to the population of interest are considered in their development. Conversely, disease-specific comorbidity measures may not always perform better than general measures in the prediction of outcomes. For example, Piccirillo found that the Charlson and Klabunde Indices performed as well as two disease-specific comorbidity measures in predicting overall survival in patients with head and neck cancer.18
Both general and disease-specific measures are limited by the sources of data used in their generation. For example, most of these measures do not account for the severity of any particular comorbid illness, because such information was not available in the data sets used to develop and validate the measures. Sources of data are considered in the following section.
Sources of Comorbidity Data
Data sources for assessing the impact of comorbidity on cancer care include clinical trial data sets, cohort studies that prospectively interview patients or retrospectively review medical records, and administrative data that contain bills from health care providers, which may be linked to tumor registry data. There are strengths and limitations to each of the above data sources, as summarized in Table 1.
Table 1.
Relative Strengths of Comorbidity Data Sources
| Data Feature | Existing Clinical Trial Data | Prospective Cohorts of Cancer Patients | Retrospective Chart Review | Administrative Data | Linked Tumor Registry Administrative Data |
|---|---|---|---|---|---|
| Representative data set (reference No.) | Meyerhardt et al (20) | Prostate Cancer Outcomes Study (25) | Adult Comorbidity Evaluation-27 (10) | State and other hospital discharge data sets (31,32) | SEER-Medicare (33) |
| Captures severity of illness | ++ | +++ | +++ | + | ++ |
| Information on comorbidity | ++ | +++ | +++ | + | + |
| Complete cancer information | ++++ | +++ | +++ | - | +++ |
| Captures other patient information (functional status, social support) | ++ | +++ | + | +/− | +/− |
| Generalizability | + | ++ | ++ | ++++ | ++++ |
| Cost | + | ++++ | ++ | + | + |
NOTE. + indicates “least”; ++++ indicates “greatest.”
Abbreviation: SEER, Surveillance, Epidemiology, and End Results.
Existing clinical trial data sets that contain detailed information about their participants and their cancer treatments are theoretically the best data to evaluate comorbid conditions as possible risk factors for adverse outcomes, including cancer-specific outcomes such as relapse or disease progression.19,20 This is because their design precludes selection biases that may confound an association between comorbidity and outcome. A secondary analysis of such data would be less expensive than most primary data collection efforts. However, only 3% to 5% of all adult cancer patients are enrolled in clinical trials,21 and their results are often not broadly generalizable to the general population of cancer patients.22 This is particularly true for patients with serious comorbidity, and elderly patients in general, who tend to be excluded from clinical trials.23,24
Cancer-specific prospective cohorts provide the opportunity to collect comorbidity and self-rated health information directly from cancer patients.25 Patient ratings of their own health predict survival and outcomes for a variety of diseases, including cancer,26–28 even after adjustment for extensive physiologic, health, and performance measures.29 These data are relatively costly to collect but may be necessary to explore differences in treatment provision that persist despite adjustment for patient characteristics such as age, ethnicity, comorbidity, and cancer characteristics. Retrospective chart review may also be used to collect detailed data on comorbidity and its severity.10 Prospective cohorts and studies utilizing retrospective chart review will offer greater generalizability than those using clinical trial data.
Cohorts that are assembled retrospectively using data such as those in the SEER (Surveillance, Epidemiology, and End Results) tumor registries, and administrative data such as state discharge data sets, are often large enough to evaluate even uncommon cancers, treatments, and comorbidities.30–32 These data sets will cover the largest populations of cancer patients and thus their study results will be the most generalizable. The SEER-Medicare data are the combination of detailed data on cancer diagnosis and initial treatment from tumor registries, with administrative data that include Medicare bills for procedures and treatments and International Classification of Diseases, Clinical Modification (ICD-9-CM) codes for cancer and noncancer diagnoses.33 As such, they reflect community-based, usual care for the elderly and include ethnic minorities in sufficient numbers for evaluation of variations in care across these groups. However, under-recording of diagnoses, including comorbidities, is a well-recognized limitation of administrative data.11,34 Retrospective cohorts based on claims (with diagnoses recorded in the form of ICD-9-CM codes) also do not contain sufficient information for accurate measurement of the severity of individual comorbid conditions. Thus, administrative data generally are the weakest source of comorbidity information, and this is reflected in their ratings in Table 1.
In summary, there is no one perfect data set for evaluating the range of comorbid conditions and cancers. Investigators must weigh the strengths and weaknesses of the comorbidity data available to them and also consider further complexities in measuring comorbidity, as noted in the next section.
Complexities in Assessing the Role of Comorbidity in Cancer Patients
We see five levels of complexity in evaluating comorbidity in cancer patients. First, for any given cancer, different comorbid conditions will have unique effects. Second, the severity of the comorbidity will influence how that comorbidity affects the cancer patient. Third, the effect of a comorbidity will vary across cancers and specific treatments. Fourth, a given comorbidity can affect cancer care at multiple points in the cancer care trajectory. Fifth, the effect of a comorbidity on cancer care is modified by other patient characteristics such as age, sex, ethnicity, socioeconomic status, and other comorbidities. These complexities are briefly discussed in the following paragraphs.
First, a given cancer will be affected differently by different comorbidities. For example, in patients with early-stage lung cancer, the presence of chronic obstructive pulmonary disease will have a major impact on receipt of potentially curative therapy (surgical removal), while renal disease would have a larger effect in those for whom chemotherapy would be appropriate. Many studies of the impact of comorbidity on treatment selection in cancer patients use scores that assign a unit weight to each of many comorbid conditions, as is done with the Charlson Index.13 This assumes that similarly weighted comorbidities have the same impact on treatment choice, which appears not to be the case when a given cancer and individual comorbid conditions are studied.6,17,35
At a second level, comorbid conditions vary in their severity. Mild chronic obstructive pulmonary disease is common among patients with lung cancer but does not preclude definitive treatment, whereas more severe chronic obstructive pulmonary disease will make a patient inoperable. Most comorbidity studies do not contain information on the severity of individual comorbid conditions, and clinical trials usually exclude patients with severe comorbid disease, so we are mostly lacking information on the impact of severe comorbidities on cancer diagnosis, treatment, and outcomes.
Third, a given comorbidity might have a major impact on the trajectory of a patient’s care with some cancers but not others. For example, some chronic illnesses appear to increase a patient’s likelihood of early cancer diagnosis, perhaps due to more regular contact with the health care system.16,35,36 This effect should be most apparent for those cancers identifiable through screening, such as breast and colon cancer, but not for cancers for which screening is not commonly performed, such as lung, ovarian, or brain malignancies.
Fourth, the influence of a given comorbid condition will vary depending on where a patient is located within the cancer care trajectory. For example, diabetes mellitus appears to increase the risk of developing breast cancer, but does not appear to worsen breast cancer-specific outcomes.37
The fifth level of complexity is introduced by the heterogeneity among patients in all of their other characteristics. Age, sex, ethnicity, and socioeconomic status influence cancer patient preferences for treatment and treatment outcomes. A comorbid condition can interact with these characteristics. An important example is the cancer patient’s access to health care. A comorbidity such as mild dementia might be expected to have a greater impact on the cancer care trajectory of a socially isolated patient than one with a support structure of family or friends that can assist the patient in seeking care.38–40 Also, many patients have multiple comorbid conditions, and the occurrence and impact of specific combinations of comorbidities has not been studied extensively.
The Conceptual Model of the Cancer Patient Clinical Trajectory
Figure 2 outlines a model of the cancer patient’s clinical trajectory that is proposed to facilitate consideration of the many ways comorbidity can impact the cancer patient. The five columns reflect primary care and screening for cancer, evaluation to establish the cancer diagnosis and stage, treatment selection and adherence, surveillance for recurrence or progression, and end of life. As one focuses in on each discrete step in Figure 2, the use of a summary comorbidity measure that represents the total burden of comorbid diseases becomes less attractive. Instead, it would seem more appropriate to consider specific comorbidities individually to assess how they might impact the cancer care trajectory at specific steps. For example, some comorbid illnesses such as rheumatoid arthritis are associated with increased use of cancer screening41 while other comorbidities, such as dementia, are associated with decreased screening.42 A summary measure including both of these conditions would not provide complete information on the impact of comorbidities on stage at diagnosis. Use of the model also allows not only for the assessment of the overall impact of a given comorbidity on an outcome, such as survival, but also the piecing together of the specific mechanisms underlying how the comorbidity exerts its impact. For example, preexisting depression is associated with decreased survival after a cancer diagnosis.43 Figure 2 helps separate out the various components in the trajectory where depression may be influential (eg, increased stage at diagnosis and decreased likelihood of receipt of definitive treatment).41 The model also can be used in comparing strengths and limitations of various data sets, depending on the components of the cancer trajectory that are targeted for examination.
Fig 2.
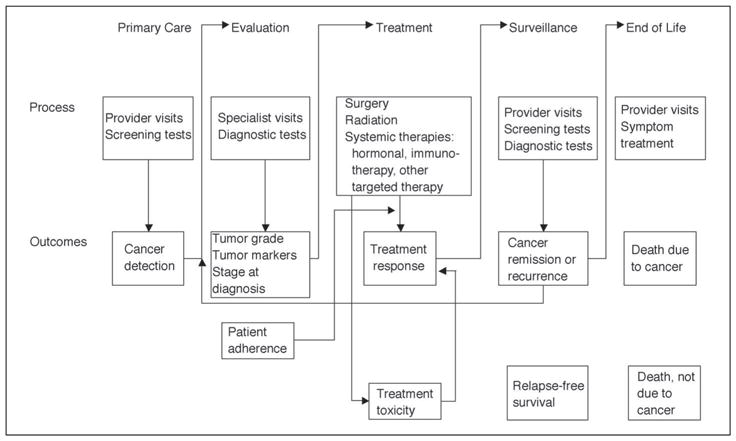
The cancer patient clinical trajectory.
There are few studies of variations in surveillance patterns after cancer treatment, but it is reasonable to suppose that patient characteristics and specific comorbidities could also impact this.44,45 Finally, as the proportion of cancer patients with advanced cancer survive longer due to the increasing use of palliative treatments, understanding how to manage comorbid illnesses in these patients will become increasingly important.46
Recommendations for Future Studies
Investigators examining a question about the cancer patient’s clinical trajectory should consider the complexities of comorbidity measurement and how these may interact with the process of care and outcomes of interest. This will then guide the selection of the data needed for study. Consideration of this trajectory will likely lead to greater attention to the impact of individual comorbidities at different stages in the cancer care trajectory. An important component of these studies will be measures of severity of the comorbidity under study.
Important questions remain about how comorbidity and other patient characteristics influence cancer patient diagnosis, treatment, and outcome. Are patients with substantial comorbid disease refusing or are they not being offered certain cancer treatments? Collection of data directly from patients will improve our ability to interpret treatment and outcome patterns.47 Such information should include comorbid conditions that may be underrepresented in existing data sets because they are under-diagnosed by physicians in everyday practice, as is the case with dementia,48,49 or because they are not recorded in claims data, as with impaired functional status and self-reported health. This effort will be particularly fruitful for older cancer patients, who often simultaneously have comorbid conditions, impaired functional status, and decreased social support as potential causes for poor outcomes.40,50 Finally, qualitative studies should explore the decision-making process as it unfolds for patients, their families, and their physicians, potentially providing new information that sheds light on the variations in treatment patterns seen in many cancer populations.
Acknowledgments
We thank two anonymous reviewers whose comments led to significant improvements in the manuscript.
Footnotes
Authors’ Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
J.S.G. and J.L.F. are supported by grants P-50-CA CA105631, RO-1-CA104949, and RO1-CA72076 from the US Public Health Service.
References
- 1.Ogle KS, Swanson GM, Woods N, et al. Cancer and comorbidity. Cancer. 2000;88:653–663. doi: 10.1002/(sici)1097-0142(20000201)88:3<653::aid-cncr24>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 2.Yancik R, Ganz PA, Varricchio CG, et al. Perspectives on comorbidity and cancer in older patients: Approaches to expand the knowledge base. J Clin Oncol. 2001;19:1147–1151. doi: 10.1200/JCO.2001.19.4.1147. [DOI] [PubMed] [Google Scholar]
- 3.Beckles MA, Spiro SG, Colice GL, et al. The physiologic evaluation of patients with lung cancer being considered for resectional surgery. Chest. 2003;123:105S–114S. doi: 10.1378/chest.123.1_suppl.105s. [DOI] [PubMed] [Google Scholar]
- 4.Bogart JA, Scalzetti E, Dexter E. Early stage medically inoperable non-small cell lung cancer. Curr Treat Options Oncol. 2003;4:81–88. doi: 10.1007/s11864-003-0034-7. [DOI] [PubMed] [Google Scholar]
- 5.Holmes CE, Muss HB. Diagnosis and treatment of breast cancer in the elderly. CA Cancer J Clin. 2003;53:227–244. doi: 10.3322/canjclin.53.4.227. [DOI] [PubMed] [Google Scholar]
- 6.Shavers VL, Brown ML, Potosky AL, et al. Race/ethnicity and the receipt of watchful waiting for the initial management of prostate cancer. J Gen Intern Med. 2004;19:146–155. doi: 10.1111/j.1525-1497.2004.30209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du X, Freeman JL, Nattinger AB, et al. Survival of women after breast conserving surgery for early stage breast cancer. Breast Cancer Res Treat. 2002;72:23–31. doi: 10.1023/a:1014908802632. [DOI] [PubMed] [Google Scholar]
- 8.Kryzanowska MK, Weeks JC, Earle CC. Treatment of locally advanced pancreatic cancer in the real world: Population-based practices and effectiveness. J Clin Oncol. 2003;21:3409–3414. doi: 10.1200/JCO.2003.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Hershman D, Fleischauer AT, Jacobson JS, et al. Patterns and outcomes of chemotherapy for elderly patients with stage II ovarian cancer: A population-based study. Gynecol Oncol. 2004;92:293–299. doi: 10.1016/j.ygyno.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Piccirillo JF, Tierhey RM, Costas I, et al. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 11.Klabunde CN, Warren JL, Legler JM. Assessing comorbidity using claims data: An overview. Med Care. 402002:IV-26–IV-35. doi: 10.1097/00005650-200208001-00004. [DOI] [PubMed] [Google Scholar]
- 12.Nitz NM. Comorbidity. In: Kane RL, editor. Understanding health care outcomes research. Gaithersburg, MD: Aspen Publishers Inc; 1997. pp. 153–174. [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 14.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 15.Satariano WA, Raglund DR. The effect of comorbidity on 3-year survival of women with primary breast cancer. Ann Intern Med. 1994;120:104–110. doi: 10.7326/0003-4819-120-2-199401150-00002. [DOI] [PubMed] [Google Scholar]
- 16.Vaeth PAC, Satariano WA, Raglund DR. Limiting comorbid conditions and breast cancer stage at diagnosis. J Gerontol. 2000;55A:M593–M600. doi: 10.1093/gerona/55.10.m593. [DOI] [PubMed] [Google Scholar]
- 17.Fleming ST, Rastogi A, Dmitrienko A, et al. A comprehensive prognostic index to predict survival based on multiple comorbidities: A focus on breast cancer. Med Care. 1999;37:601–614. doi: 10.1097/00005650-199906000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Piccirillo JF, Spitznagel EL, Vermani N, et al. Comparison of comorbidity indices for patients with head and neck cancer. Med Care. 2004;42:482–486. doi: 10.1097/01.mlr.0000124254.88292.a1. [DOI] [PubMed] [Google Scholar]
- 19.Weiser MA, Cabanillas ME, Konopleva M, et al. Relation between the duration of remission and hyperglycemia during induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone/methotrexate-cytarabine regimen. Cancer. 2004;100:1179–1185. doi: 10.1002/cncr.20071. [DOI] [PubMed] [Google Scholar]
- 20.Meyerhardt JA, Catalano PJ, Haller DG, et al. Impact of diabetes mellitus on outcomes in patients with colon cancer. J Clin Oncol. 2003;21:433–440. doi: 10.1200/JCO.2003.07.125. [DOI] [PubMed] [Google Scholar]
- 21.Cassileth BR. Clinical trials: Time for action [editorial] J Clin Oncol. 2003;21:765–766. doi: 10.1200/JCO.2003.12.070. [DOI] [PubMed] [Google Scholar]
- 22.Satariano WA, Silliman RA. Comorbidity: Implications for research and practice in geriatric oncology. Crit Rev Oncol Hematol. 2003;48:239–248. doi: 10.1016/j.critrevonc.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Goodwin JS, Hunt WC, Key CR, et al. Cancer treatment protocols: Who gets chosen? Arch Intern Med. 1988;148:2258–2260. [PubMed] [Google Scholar]
- 24.Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21:1383–1389. doi: 10.1200/JCO.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Hoffman RM, Harlan LC, Klabunde CN, et al. Racial differences in initial treatment for clinically localized prostate cancer. Results from the Prostate Cancer Outcomes study. J Gen Intern Med. 2003;18:845–853. doi: 10.1046/j.1525-1497.2003.21105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mandleblatt JS, Bierman AS, Gold K, et al. Constructs of burden of illness in older patients with breast cancer: A comparison of measurement methods. Health Serv Res. 2001;36:1085–1107. [PMC free article] [PubMed] [Google Scholar]
- 27.Shadbolt B, Barresi J, Craft P. Self-rated health as a predictor of survival among patients with advanced cancer. J Clin Oncol. 2002;20:2514–2519. doi: 10.1200/JCO.2002.08.060. [DOI] [PubMed] [Google Scholar]
- 28.Benjamins MR, Hummer RA, Eberstein IW, et al. Self-reported health and adult mortality risk: An analysis of cause-specific mortality. Soc Sci Med. 2004;59:1297–1306. doi: 10.1016/j.socscimed.2003.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Fried LP, Kronmal RA, Newman AB, et al. Risk factors for 5-year mortality in older adults: The Cardiovascular Health Study. JAMA. 1998;279:585–592. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- 30.Menzin J, Lang K, Earle CC, et al. The outcomes and costs of acute myeloid leukemia among the elderly. Arch Intern Med. 2002;162:1597–1603. doi: 10.1001/archinte.162.14.1597. [DOI] [PubMed] [Google Scholar]
- 31.Hwang JP, Lam TP, Cohen DS, et al. Hematopoietic stem cell transplantation among patients with leukemia of all ages in Texas. Cancer. 2004;101:2230–2238. doi: 10.1002/cncr.20628. [DOI] [PubMed] [Google Scholar]
- 32.Virnig BA, McBean M. Administrative data for public health surveillance and planning. Annu Rev Public Health. 2001;22:213–230. doi: 10.1146/annurev.publhealth.22.1.213. [DOI] [PubMed] [Google Scholar]
- 33.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Medical Care. 2002;40:IV-3–IV-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 34.Quan H, Parsons GA, Ghali WA. Validity of information on comorbidity derived from ICD-9-CM administrative data. Med Care. 2002;40:675–695. doi: 10.1097/00005650-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Fleming ST, Pursley HG, Newman B, et al. Comorbidity as a predictor of stage of illness for patients with breast cancer. Med Care. 2005;43:132–140. doi: 10.1097/00005650-200502000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Reid BC, Warren JL, Rozier G. Comorbidity and early diagnosis of head and neck cancer in a Medicare population. Am J Prev Med. 2004;27:373–378. doi: 10.1016/j.amepre.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 37.Wolf I, Sadetzki S, Catane R, et al. Diabetes mellitus and breast cancer. Lancet Oncol. 2005;6:103–111. doi: 10.1016/S1470-2045(05)01736-5. [DOI] [PubMed] [Google Scholar]
- 38.Goodwin JS, Hunt WC, Samet JM. Determinants of cancer therapy in elderly patients. Cancer. 1993;72:594–601. doi: 10.1002/1097-0142(19930715)72:2<594::aid-cncr2820720243>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 39.Goodwin JS, Satish S, Anderson ET, et al. Effect of nurse case management on the treatment of older women with breast cancer. J Am Geriatr Soc. 2003;51:1252–1259. doi: 10.1046/j.1532-5415.2003.51409.x. [DOI] [PubMed] [Google Scholar]
- 40.Goodwin JS, Hunt WC, Samet JM. A population-based study of functional status and social support networks of elderly patients newly diagnosed with cancer. Arch Intern Med. 1991;151:366–370. [PubMed] [Google Scholar]
- 41.Solomon DH, Karlson FW, Curham GC. Cardiovascular care and cancer screening in women with and without arthritis. Arthritis Rheum. 2004;51:429–432. doi: 10.1002/art.20418. [DOI] [PubMed] [Google Scholar]
- 42.Marwill SL, Freund KM, Barry PP. Patient factors associated with breast cancer screening among older women. J Am Geriatr Soc. 1996;44:1210–1214. doi: 10.1111/j.1532-5415.1996.tb01371.x. [DOI] [PubMed] [Google Scholar]
- 43.Goodwin JS, Zhang D, Ostir GV. Effect of depression on diagnosis, treatment, and survival of older women with breast cancer. J Am Geriatr Soc. 2004;52:106–111. doi: 10.1111/j.1532-5415.2004.52018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schrag D, Hsieh LJ, Rabbani F, et al. Adherence to surveillance among patients with superficial bladder cancer. J Natl Cancer Inst. 2003;95:588–597. doi: 10.1093/jnci/95.8.588. [DOI] [PubMed] [Google Scholar]
- 45.Shavers VL, Brown M, Klabunde CN, et al. Race/ethnicity and the intensity of medical monitoring under “watchful waiting” for prostate cancer. Med Care. 2004;42:239–250. doi: 10.1097/01.mlr.0000117361.61444.71. [DOI] [PubMed] [Google Scholar]
- 46.Stevenson J, Abernethy AP, Miller C, et al. Managing comorbidities in patients at the end of life. BMJ. 2004;329:909–912. doi: 10.1136/bmj.329.7471.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silliman RA, Lash TL. Comparison of interview-based and medical-record based indices of comorbidity among breast cancer patients. Med Care. 1999;37:339–349. doi: 10.1097/00005650-199904000-00004. [DOI] [PubMed] [Google Scholar]
- 48.Valcour VG, Masaki KH, Curb JD, et al. The detection of dementia in the primary care setting. Arch Intern Med. 2000;160:2964–2968. doi: 10.1001/archinte.160.19.2964. [DOI] [PubMed] [Google Scholar]
- 49.Boise L, Neal MB, Kaye J. Dementia assessment in primary care: Results from a study in three managed care systems. J Gerontol A Biol Sci Med Sci. 2004;59:M621–M626. doi: 10.1093/gerona/59.6.m621. [DOI] [PubMed] [Google Scholar]
- 50.Balducci L, Beghe C. The application of the principles of geriatrics to the management of the older person with cancer. Crit Rev Oncol Hematol. 2000;35:147–154. doi: 10.1016/s1040-8428(00)00089-5. [DOI] [PubMed] [Google Scholar]


