Abstract
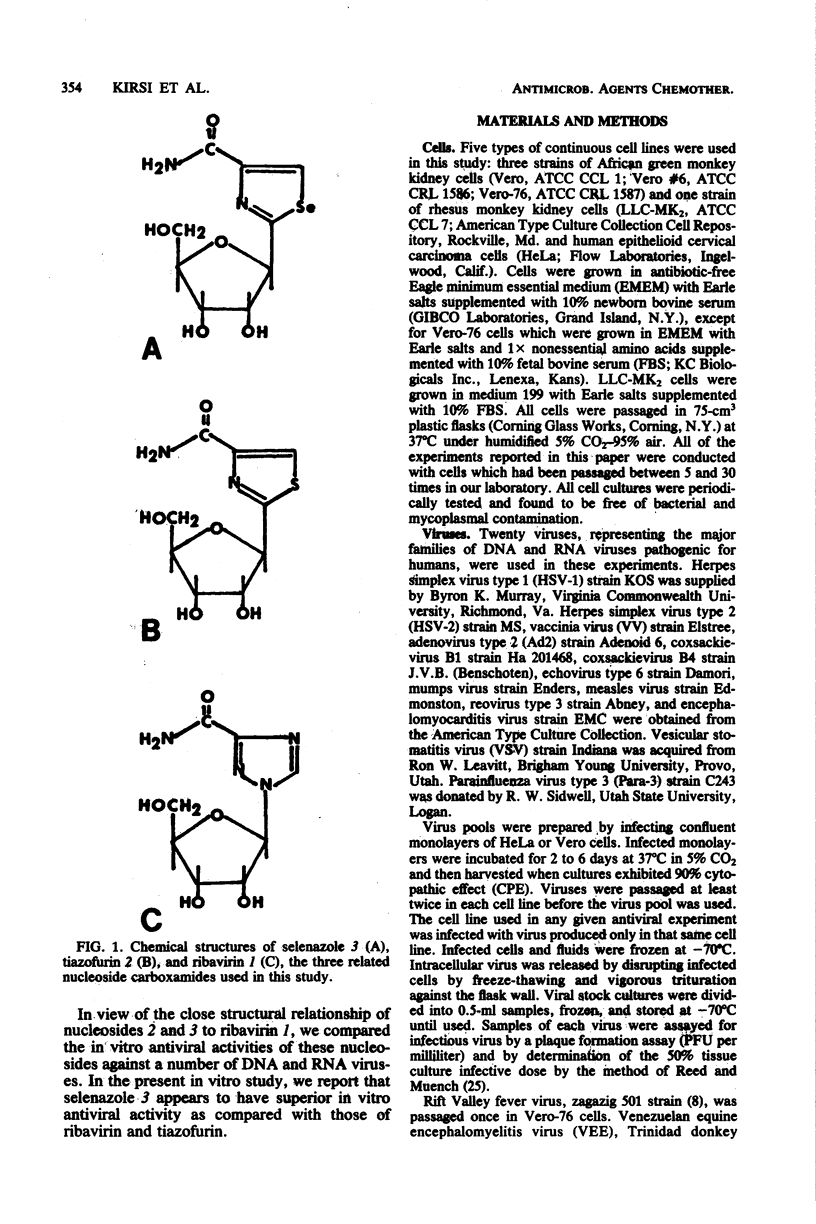
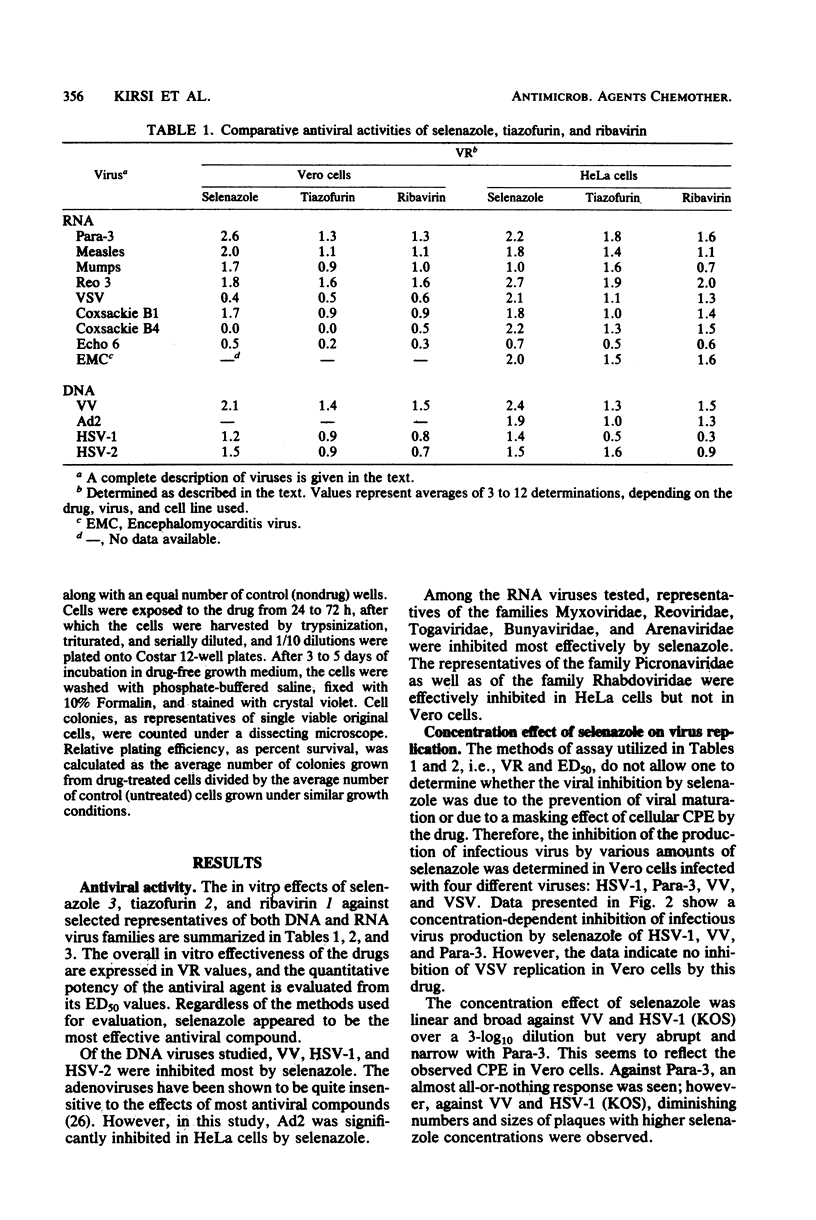
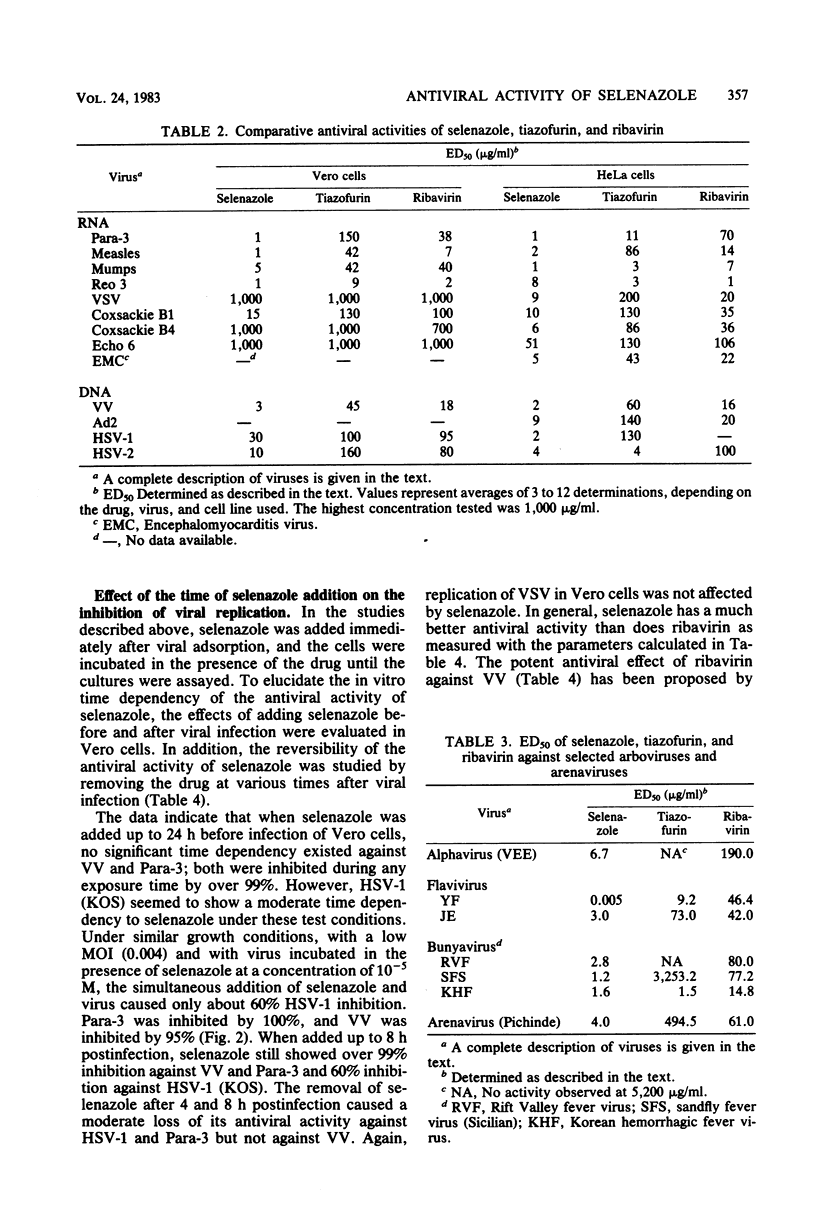
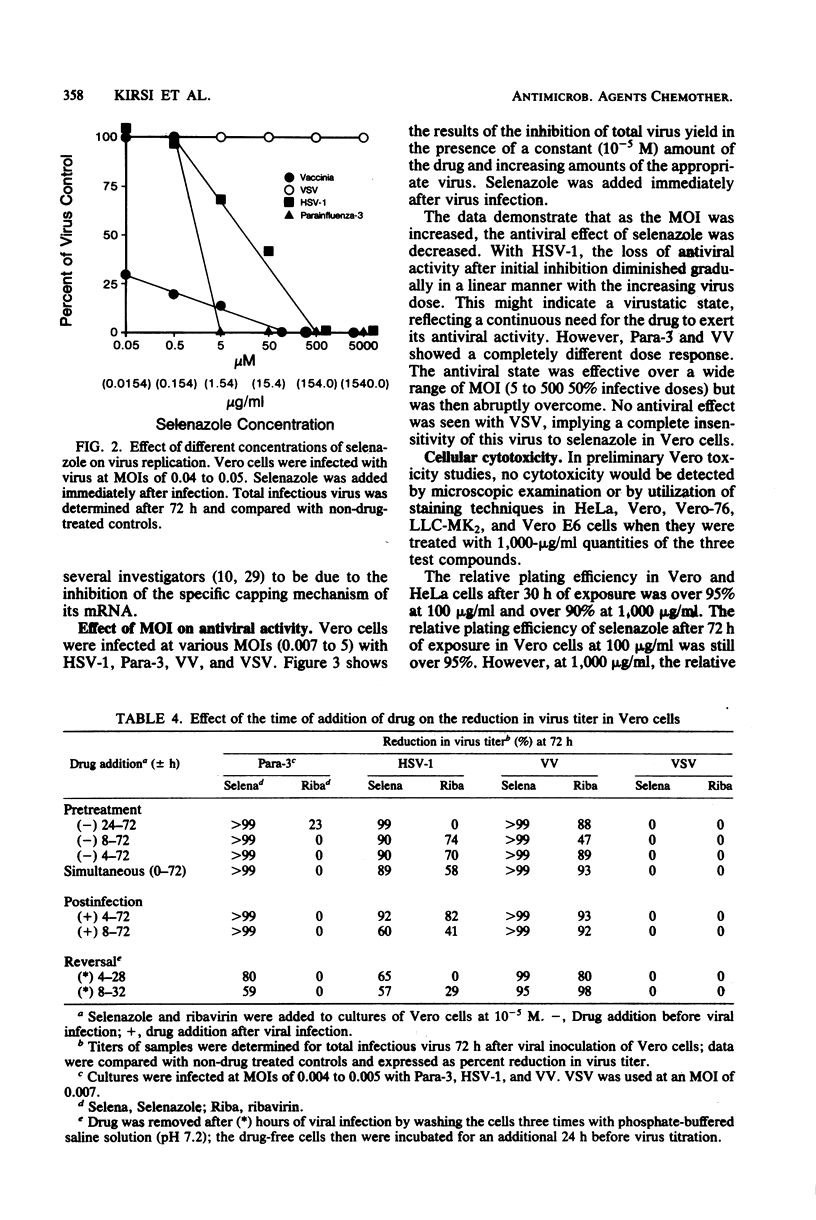
The relative in vitro antiviral activities of three related nucleoside carboxamides, ribavirin (1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide), tiazofurin (2-beta-D-ribofuranosylthiazole-4-carboxamide), and selenazole (2-beta-D-ribofuranosylselenazole-4-carboxamide), were studied against selected DNA and RNA viruses. Although the activity of selenazole against different viruses varied, it was significantly more potent than ribavirin and tiazofurin against all tested representatives of the families Paramyxoviridae (parainfluenza virus type 3, mumps virus, measles virus), Reoviridae (reovirus type 3), Poxviridae (vaccinia virus), Herpes-viridae (herpes simplex virus types 1 and 2), Togaviridae (Venezuelan equine encephalomyelitis virus, yellow fever virus, Japanese encephalitis virus), Bunyaviridae (Rift Valley fever virus, sandfly fever virus [strain Sicilian], Korean hemorrhagic fever virus), Arenaviridae (Pichinde virus), Picornaviridae (coxsackieviruses B1 and B4, echovirus type 6, encephalomyocarditis virus), Adenoviridae (adenovirus type 2), and Rhabdoviridae (vesicular stomatitis virus). The antiviral activity of selenazole was also cell line dependent, being greatest in HeLa, Vero-76, and Vero E6 cells. Selenazole was relatively nontoxic for Vero, Vero-76, Vero E6, and HeLa cells at concentrations of up to 1,000 micrograms/ml. The relative plating efficiency at that concentration was over 90%. The effects of selenazole on viral replication were greatest when this agent was present at the time of viral infection. The removal of selenazole from the medium of infected cells did not reverse the antiviral effect against vaccinia virus, but there was a gradual resumption of viral replication in cells infected with parainfluenza type 3 or herpes simplex virus type 1 (strain KOS). However, the antiviral activity of ribavirin against the same viruses was reversible when the drug was removed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartelloni P. J., Tesh R. B. Clinical and serologic responses of volunteers infected with phlebotomus fever virus (Sicilian type). Am J Trop Med Hyg. 1976 May;25(3):456–462. doi: 10.4269/ajtmh.1976.25.456. [DOI] [PubMed] [Google Scholar]
- Browne M. J. Comparative inhibition of influenza and parainfluenza virus replication by ribavirin in MDCK cells. Antimicrob Agents Chemother. 1981 May;19(5):712–715. doi: 10.1128/aac.19.5.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E. Comparative efficacy of antiherpes drugs in different cell lines. Antimicrob Agents Chemother. 1982 Apr;21(4):661–663. doi: 10.1128/aac.21.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson B., Helgstrand E., Johansson N. G., Larsson A., Misiorny A., Norén J. O., Philipson L., Stenberg K., Stening G., Stridh S. Inhibition of influenza virus ribonucleic acid polymerase by ribavirin triphosphate. Antimicrob Agents Chemother. 1977 Jun;11(6):946–951. doi: 10.1128/aac.11.6.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedson D. S. Prospects for broad-spectrum chemotherapy of serious viral respiratory infections. JAMA. 1983 May 20;249(19):2689–2690. [PubMed] [Google Scholar]
- Goswami B. B., Borek E., Sharma O. K., Fujitaki J., Smith R. A. The broad spectrum antiviral agent ribavirin inhibits capping of mRNA. Biochem Biophys Res Commun. 1979 Aug 13;89(3):830–836. doi: 10.1016/0006-291x(79)91853-9. [DOI] [PubMed] [Google Scholar]
- HARDY F. M. The growth of Venezuelan equine encephalomyelitis virus in various tissue cultures. Am J Hyg. 1959 Jul;70(1):21–27. doi: 10.1093/oxfordjournals.aje.a120061. [DOI] [PubMed] [Google Scholar]
- Hall C. B., Walsh E. E., Hruska J. F., Betts R. F., Hall W. J. Ribavirin treatment of experimental respiratory syncytial viral infection. A controlled double-blind study in young adults. JAMA. 1983 May 20;249(19):2666–2670. [PubMed] [Google Scholar]
- Hruska J. F., Morrow P. E., Suffin S. C., Douglas R. G., Jr In vivo inhibition of respiratory syncytial virus by ribavirin. Antimicrob Agents Chemother. 1982 Jan;21(1):125–130. doi: 10.1128/aac.21.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman J. H., Sidwell R. W., Khare G. P., Witkowski J. T., Allen L. B., Robins R. K. In vitro effect of 1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide (virazole, ICN 1229) on deoxyribonucleic acid and ribonucleic acid viruses. Antimicrob Agents Chemother. 1973 Feb;3(2):235–241. doi: 10.1128/aac.3.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaram H. N., Dion R. L., Glazer R. I., Johns D. G., Robins R. K., Srivastava P. C., Cooney D. A. Initial studies on the mechanism of action of a new oncolytic thiazole nucleoside, 2-beta-D-ribofuranosylthiazole-4-carboxamide (NSC 286193). Biochem Pharmacol. 1982 Jul 15;31(14):2371–2380. doi: 10.1016/0006-2952(82)90532-9. [DOI] [PubMed] [Google Scholar]
- Knight V., McClung H. W., Wilson S. Z., Waters B. K., Quarles J. M., Cameron R. W., Greggs S. E., Zerwas J. M., Couch R. B. Ribavirin small-particle aerosol treatment of influenza. Lancet. 1981 Oct 31;2(8253):945–949. doi: 10.1016/s0140-6736(81)91152-1. [DOI] [PubMed] [Google Scholar]
- Knight V., Wilson S. Z., Alling D. W., Moore R. V., Longoria R. M. Lack of interference of guanosine with ribavirin aerosol treatment of influenza A infection in mice. Antimicrob Agents Chemother. 1981 Oct;20(4):477–480. doi: 10.1128/aac.20.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuttan R., Robins R. K., Saunders P. P. Inhibition of inosinate dehydrogenase by metabolites of 2-beta-D-ribofuranosyl thiazole-4-carboxamide. Biochem Biophys Res Commun. 1982 Aug;107(3):862–868. doi: 10.1016/0006-291x(82)90602-7. [DOI] [PubMed] [Google Scholar]
- Magnussen C. R., Douglas R. G., Jr, Betts R. F., Roth F. K., Meagher M. P. Double-blind evaluation of oral ribavirin (Virazole) in experimental influenza A virus infection in volunteers. Antimicrob Agents Chemother. 1977 Oct;12(4):498–502. doi: 10.1128/aac.12.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCammon J. R., Riesser V. W. Effects of ribavirin on BHK-21 cells acutely or persistently infected with mumps virus. Antimicrob Agents Chemother. 1979 Mar;15(3):356–360. doi: 10.1128/aac.15.3.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung H. W., Knight V., Gilbert B. E., Wilson S. Z., Quarles J. M., Divine G. W. Ribavirin aerosol treatment of influenza B virus infection. JAMA. 1983 May 20;249(19):2671–2674. [PubMed] [Google Scholar]
- McCormick J. B., Sasso D. R., Palmer E. L., Kiley M. P. Morphological identification of the agent of Korean haemorrhagic fever (Hantaan virus)as a member of the Bunyaviridae. Lancet. 1982 Apr 3;1(8275):765–768. doi: 10.1016/s0140-6736(82)91812-8. [DOI] [PubMed] [Google Scholar]
- Patki S. A., Gupta P. Evaluation of ribavirin in the treatment of acute hepatitis. Chemotherapy. 1982;28(4):298–303. doi: 10.1159/000238094. [DOI] [PubMed] [Google Scholar]
- Povey R. C. In vitro antiviral efficacy of ribavirin against feline calicivirus, feline viral rhinotracheitis virus, and canine parainfluenza virus. Am J Vet Res. 1978 Jan;39(1):175–178. [PubMed] [Google Scholar]
- Robins R. K., Srivastava P. C., Narayanan V. L., Plowman J., Paull K. D. 2-beta-D-Ribofuranosylthiazole-4-carboxamide, a novel potential antitumor agent for lung tumors and metastases. J Med Chem. 1982 Feb;25(2):107–108. doi: 10.1021/jm00344a002. [DOI] [PubMed] [Google Scholar]
- Shuman S., Hurwitz J. Mechanism of mRNA capping by vaccinia virus guanylyltransferase: characterization of an enzyme--guanylate intermediate. Proc Natl Acad Sci U S A. 1981 Jan;78(1):187–191. doi: 10.1073/pnas.78.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidwell R. W., Huffman J. H., Khare G. P., Allen L. B., Witkowski J. T., Robins R. K. Broad-spectrum antiviral activity of Virazole: 1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide. Science. 1972 Aug 25;177(4050):705–706. doi: 10.1126/science.177.4050.705. [DOI] [PubMed] [Google Scholar]
- Sidwell R. W., Huffman J. H. Use of disposable micro tissue culture plates for antiviral and interferon induction studies. Appl Microbiol. 1971 Nov;22(5):797–801. doi: 10.1128/am.22.5.797-801.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava P. C., Pickering M. V., Allen L. B., Streeter D. G., Campbell M. T., Witkowski J. T., Sidwell R. W., Robins R. K. Synthesis and antiviral activity of certain thiazole C-nucleosides. J Med Chem. 1977 Feb;20(2):256–262. doi: 10.1021/jm00212a014. [DOI] [PubMed] [Google Scholar]
- Srivastava P. C., Robins R. K. Synthesis and antitumor activity of 2-beta-D-ribofuranosylselenazole-4- carboxamide and related derivatives. J Med Chem. 1983 Mar;26(3):445–448. doi: 10.1021/jm00357a024. [DOI] [PubMed] [Google Scholar]
- Stephen E. L., Sammons M. L., Pannier W. L., Baron S., Spertzel R. O., Levy H. B. Effect of a nuclease-resistant derivative of polyriboinosinic-polyribocytidylic acid complex on yellow fever in rhesus monkeys (Macaca mulatta). J Infect Dis. 1977 Jul;136(1):122–126. doi: 10.1093/infdis/136.1.122. [DOI] [PubMed] [Google Scholar]
- Witkowski J. T., Robins R. K., Sidwell R. W., Simon L. N. Design, synthesis, and broad spectrum antiviral activity of 1- -D-ribofuranosyl-1,2,4-triazole-3-carboxamide and related nucleosides. J Med Chem. 1972 Nov;15(11):1150–1154. doi: 10.1021/jm00281a014. [DOI] [PubMed] [Google Scholar]
- Zimmerman T. P., Deeprose R. D. Metabolism of 5-amino-1-beta-D-ribofuranosylimidazole-4-carboxamide and related five-membered heterocycles to 5'-triphosphates in human blood and L5178Y cells. Biochem Pharmacol. 1978 Mar 1;27(5):709–716. doi: 10.1016/0006-2952(78)90508-7. [DOI] [PubMed] [Google Scholar]


