Abstract
Background
The publication of results from randomized clinical trials can have a dramatic effect on treatment patterns, but the impact of oral presentations at national scientific meetings is unknown. We investigated the temporal association between the oral presentation of the results from the Cancer and Leukemia Group B (CALGB) Study 9344 at the May 1998 meeting of the American Society of Clinical Oncology, which showed that paclitaxel improves survival of women with lymph node – positive breast cancer, and use of taxane chemotherapy for breast cancer.
Methods
We studied chemotherapy use in 3341 women identified through the Surveillance, Epidemiology, and End Results–Medicare database who were diagnosed with stage I–III breast cancer in 1994–1999 at age 65 years or older and received adjuvant chemotherapy, as identified through claims data, within 1 year of diagnosis. We assessed the temporal association between the CALBG presentation and taxane use with piecewise regression analysis. Multivariable logistic regression analysis was used to determine which patient characteristics were associated with taxane use.
Results
The use of taxanes increased substantially after the CALGB presentation, with absolute rates of taxane use of 5.2% before May 1998 and 23.6% in May 1998 and later. Initially, this increase was confined to patients with lymph node–positive disease (40% of whom were receiving taxanes by the end of 1999), but over time it extended to patients with lymph node–negative disease (15% of whom were receiving taxanes by the end of 1999). In multivariable analysis, patients who were treated in May 1998 or later were statistically significantly more likely to have received a taxane than patients treated before this date (estimated relative risk = 6.84, 95% confidence interval = 5.71 to 8.07). Younger patient age, larger number of lymph nodes involved, higher tumor grade, and larger tumor size were also independently associated with adjuvant taxane use.
Conclusions
The oral presentation of a single study at a national conference was temporally associated with an increase in the use of taxanes for primary breast cancer, even before study publication or Food and Drug Administration approval.
The publication of results from clinical trials can have a rapid and dramatic effect on treatment patterns (1–3). For example, the publication of the results from the Women’s Health Initiative, which showed that substantial harms are associated with hormone replacement therapy use in postmenopausal women, was followed by a rapid decline in the use of hormone replacement therapy (3). Although the publication of study results in peer-reviewed journals remains the standard method for disseminating medical information, many studies are presented at national or international meetings in advance of study publication. Studies presented at medical meetings, whether as posters or oral presentations, are rarely subject to the same rigor of peer review as published manuscripts, but the results may still be widely disseminated.
In this study, we examined the impact of a single oral presentation at a large medical conference on national practice patterns. We chose the Cancer and Leukemia Group B (CALGB) Study 9344, which was the first randomized trial to evaluate the treatment effect of adjuvant paclitaxel in women with lymph node–positive breast cancer (4,5). Paclitaxel, a chemotherapeutic drug in the taxane family, was approved by the U.S. Food and Drug Administration (FDA) in April 1994 for the treatment of women with metastatic breast cancer. The CALGB study was designed to determine whether adjuvant paclitaxel is of benefit in women with primary breast cancer that has spread to axillary lymph nodes. The initial results of this study, which were presented in an oral presentation at the American Society of Clinical Oncology (ASCO) meeting in May 1998, showed that treatment with paclitaxel statistically significantly improved survival of women with lymph node–positive breast cancer (overall survival at 18 months was 97% in the paclitaxel arm versus 95% in the placebo arm; P = .039). The data from this study were submitted to the FDA in April 1999, and approval for the use of paclitaxel for the treatment of lymph node–positive primary breast cancer was granted in October 1999. The updated results of the study, with longer follow-up, were published in a peer-reviewed journal in 2003 (5) (Fig. 1).
Fig. 1.
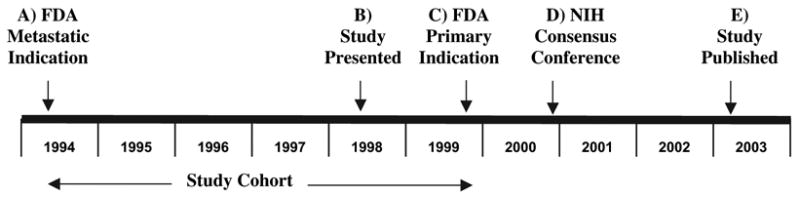
Timeline of major events around the approval of paclitaxel for primary breast cancer. The U.S. Food and Drug Administration (FDA) approved the use of paclitaxel for metastatic breast cancer in April 1994 (A). Results from Cancer and Leukemia Group B (CALGB) Study 9344 were presented at the American Society of Clinical Oncology Annual Meeting in May 1998 (B). FDA approved paclitaxel for lymph node–positive primary breast cancer I October 1999 (C). In November 2000, a National Institutes of Health Consensus Conference stated that “Currently available data regarding the use of taxanes in the adjuvant treatment of women with node-positive breast cancer are inconclusive, ” (21) (D). The CALGB study was published in March 2003 (E). The position of the study cohort relative to these events is shown below the timeline.
The ASCO meeting draws a large national and international audience; more than 18 000 participants attended in 1998 (6). The results of the CALGB 9344 trial were widely reported in the media in the days and weeks following the presentation, with articles appearing in high-circulation newspapers and magazines, including the New York Times (7), the Wall Street Journal (8), Business Week (9), Maclean’s (10), and U.S. News and World Report (11). Because treatment patterns have been shown to be influenced by other highly publicized events, such as Nancy Reagan’s mastectomy (12), we hypothesized that the results of CALGB 9344 would have had an impact on national practice patterns before FDA approval of paclitaxel for early breast cancer and publication of the peer-reviewed manuscript. To investigate this question, we studied chemotherapy use in a population-based sample of women who were diagnosed in the period spanning the May 1998 report of the trial.
Methods
Data Source
We used the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) – Medicare linked database for this study. The SEER program is a national population-based tumor registry that collects information on incident cancer cases. Medicare is the primary health insurer for 97% of the United States population aged 65 years and older. Under an agreement between the National Cancer Institute and the Center for Medic-aid and Medicare Services, SEER data are linked with data from Medicare records for those SEER subjects who are eligible for Medicare. Of persons who are reported by SEER as having been diagnosed with cancer at age 65 years or older, 93% can be matched with their Medicare enrollment records (13). As of 2005, Medicare record linkages have been created for SEER subjects who were diagnosed with cancer from 1973 to 1999, and complete Medicare claims are available from 1991 to 2002 for such subject. Data on patient demographics, dates of diagnosis, extent of disease, and surgical treatment are available through the SEER registry data and are found in the SEER–Medicare Patient Entitlement and Diagnosis Summary File. Chemotherapy use can be ascertained through the presence of Common Procedural Terminology (CPT) J codes in the SEER–Medicare Outpatient, Physician/Supplier, and Durable Medical Equipment files.
Study Population
The study population consisted of women aged 65 years or older who were diagnosed with stage I – III breast cancer (14) from 1994 through 1999, treated with definitive surgery, and had a claim for chemotherapy between April 1994 (date of FDA approval of paclitaxel for metastatic breast cancer) and December 1999. Patients were excluded if the breast cancer diagnosis was not the patient’s first cancer or if she was diagnosed with a second cancer within 1 year after her breast cancer diagnosis. Patients who did not have full coverage under both Medicare Part A and Part B or who were members of health maintenance organizations were excluded because their claims data may be incomplete. A total of 27 408 women met these criteria. Adjuvant chemotherapy use within 1 year of diagnosis was identified through the CPT J codes in SEER–Medicare files. The following J codes indicated claims for chemotherapy: J8510, J8520, J8521, J8530 – J8999, and J9000–J9999. We excluded codes J9202 (goserelin), J9209 (mesna), J9212–J9214 (interferon), and J9217–J9218 (leuprolide acetate) because these drugs are not cytotoxic chemotherapeutic agents. A total of 3341 patients were identified as having received chemotherapy between April 1994 and December 1999. A patient was defined as having received a taxane if there was a claim for paclitaxel (J9265) or docetaxel (J9170).
Statistical Analysis
The proportion of patients who received a taxane was computed for each month or quarter of the study period based on the date of the first claim of chemotherapy use. A piecewise regression analysis with one knot point was used to fit the curve of taxane use over the period, and the location of the knot point was estimated with nonlinear least-squares regression (15–17). Multivariable logistic regression analyses were performed to determine which patient characteristics were associated with taxane use. The variables evaluated in the regression models were age (categorical 65–69 years, 70–74 years, 75–79 years, ≥80 years) race (white, black, other), census tract education level, census tract poverty level, tumor size in the largest dimension (0–2.0 cm, 2.1–5.0 cm, >5.0 cm), tumor grade (low, intermediate, high, unknown), number of positive lymph nodes (0, 1 – 3, 4 – 9, ≥10, unknown), estrogen receptor status (negative, positive, unknown), and date of treatment (April 1994 through April 1998 or May 1998 through December 1999, i.e., before or after presentation of the data at the ASCO meeting). A backward logistic regression approach was used to identify cofactors that showed a statistically significant (P<.05) association with taxane use (18). Race, poverty, and education level were excluded from the final models because they were not statistically significant cofactors in this analysis. Logistic regression models stratified by time were also performed to determine factors associated with taxane use before and after May 1998. Estimated relative risks were calculated using the method of Zhang & Yu (19). SAS software (SAS Institute, Inc., Cary, NC) was used for all statistical analyses. All statistical tests were two-sided. This study was reviewed by the institutional review board of the University of Texas M. D. Anderson Cancer Center and was deemed to be exempt from approval.
Results
A total of 3341 women with stage I–III breast cancer who received adjuvant chemotherapy were included in this study (Table 1). The median age was 71.5 years. Approximately 66% of the patients were treated with mastectomy, and approximately 70% of patients had lymph node involvement. The percentage of women who received adjuvant chemotherapy increased over this period, from 9.5% (441 of 4660) in 1994 to 16.3% (744 of 4562) in 1999 (P<.001).
Table 1.
Characteristics of patients in the cohort*
| Characteristic | No. of patients | % |
|---|---|---|
| Total | 3341 | 100% |
| Age, y | ||
| 65–69 | 1293 | 38.7 |
| 70–74 | 1167 | 34.9 |
| 75–79 | 663 | 19.8 |
| ≥80 | 218 | 6.5 |
| Race | ||
| White | 2849 | 85.3 |
| Black | 216 | 6.5 |
| Other | 276 | 8.3 |
| Census tract education, %† | ||
| <10 | 800 | 23.9 |
| 10–15.9 | 735 | 22.0 |
| 16–23.9 | 840 | 25.1 |
| ≥24 | 966 | 28.9 |
| Census tract poverty level, %‡ | ||
| <3 | 736 | 22.0 |
| 3–5.9 | 868 | 26.0 |
| 6–10.9 | 824 | 24.7 |
| ≥11 | 913 | 27.3 |
| SEER region | ||
| San Francisco | 165 | 4.9 |
| Connecticut | 468 | 14.0 |
| Detroit | 638 | 19.1 |
| Hawaii | 101 | 3.0 |
| Iowa | 441 | 13.2 |
| New Mexico | 113 | 3.4 |
| Seattle | 359 | 10.8 |
| Utah | 194 | 5.8 |
| Atlanta | 218 | 6.5 |
| San Jose | 144 | 4.3 |
| Los Angeles | 500 | 15.0 |
| AJCC* stage | ||
| I | 549 | 16.4 |
| II | 2183 | 65.3 |
| III | 609 | 18.2 |
| Positive lymph nodes, No. | ||
| 0 | 914 | 27.4 |
| 1–3 | 1088 | 32.6 |
| 4–9 | 660 | 19.8 |
| ≥10 | 379 | 11.3 |
| Unknown | 300 | 9.0 |
| Tumor size, cm | ||
| 0–2.0 | 1399 | 41.9 |
| 2.1–5.0 | 1327 | 39.7 |
| >5 | 505 | 15.1 |
| Unknown | 110 | 3.3 |
| Estrogen receptor status | ||
| Negative | 1185 | 35.5 |
| Positive | 1665 | 49.8 |
| Unknown | 491 | 14.7 |
| Tumor grade | ||
| Low | 234 | 7.0 |
| Intermediate | 1029 | 30.8 |
| High | 1716 | 51.4 |
| Unknown | 362 | 10.8 |
| Surgery | ||
| Breast conserving | 1143 | 34.2 |
| Mastectomy | 2198 | 65.8 |
| Received taxane | 390 | 11.7 |
Patients were identified through the Surveillance, Epidemiology and End Results (SEER)–Medicare linked database. All patients were diagnosed with Stage I–III Breast Cancer in April 1994 through December 1999 and received definitive surgery and chemotherapy within 1 year of diagnosis. AJCC = American Joint Committee on Cancer (14).
Percentage of adults in the census tract with less than 12 years of education.
Percentage of census tracts residents living below poverty.
Analysis of the percentage of patients who were treated with a taxane as part of their chemotherapy regimen by date of first chemotherapy claim (Fig. 2) showed that the percentage of such patients remained relatively low and steady, at less than 10% from 1994 through early 1998. Starting in early 1998, the percentage of patients who received a taxane increased. Piecewise regression analysis showed that the knot point—i.e., the point at which the use of taxanes began to rise—was in February 1998 (95% confidence interval [CI] = October 1997 to June 1998). Before the knot point, the percentage of patients who received a taxane increased by 0.15% (95% CI = 0.08% to 0.21%) per month. After February 1998, this percentage increased by 1.07% (95% CI = 0.80% to 1.35%) per month. Thus, the rate of increase of taxane use over time increased by more than sevenfold after the February 1998 knot (P<.001). Absolute rates of taxane use increased from 5.2% before May 1998 to 23.6% in May 1998 and later.
Fig. 2.
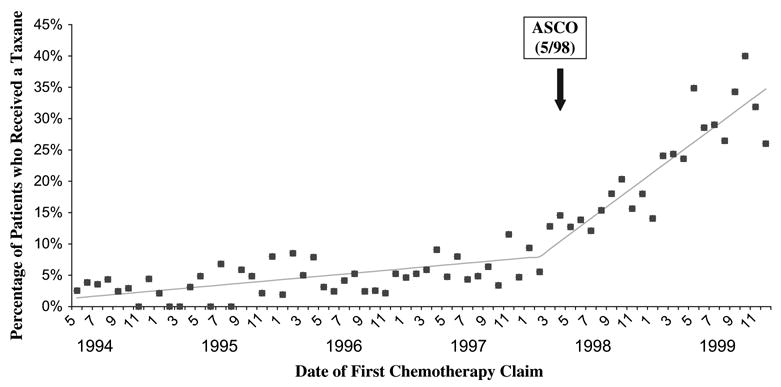
Percentage of patients on chemotherapy who received a taxane as a part of their regimen, by calendar month of first chemotherapy claim. The lines show the estimated slope of taxane use, with the knot point at February 1998. The date of the ASCO meeting at which CALGB 9344 results were presented is indicated.
Taxanes are typically administered after approximately 3 months of anthracycline-based chemotherapy. Therefore, we calculated the time between date of first chemotherapy claim and date of first taxane claim for our patient population. The median time from date of first claim for chemotherapy to date of first claim for taxane was 87 days (interquartile range = 64.5 to 129 days). Therefore, patients who received a taxane in May 1998 would have started chemotherapy in February 1998—the time of the knot point. Indeed, when we plotted the number of taxane claims by date of taxane administration (data not shown), the knot point was calculated as April 1998 (95% CI = February 1998 to June 1998). Thus, both of these analyses demonstrated a close temporal association between the presentation at the meeting and the dramatic increase in taxane use.
Because the CALBG 9344 study results applied specifically to women with lymph node–positive breast cancer, we examined the use of taxanes according to lymph node status (Fig. 3). The increase in taxane use starting in early 1998 was confined almost entirely to women with lymph node–positive breast cancer. By the fourth quarter of 1999, 40% of women with lymph node–positive disease received a taxane as a component of their chemotherapy regimen. Taxanes were used in a smaller percentage of lymph node–negative women throughout the study period; however, by the fourth quarter of 1999, 15% of women with lymph node–negative breast cancer were given a taxane as part of their adjuvant chemotherapy.
Fig. 3.
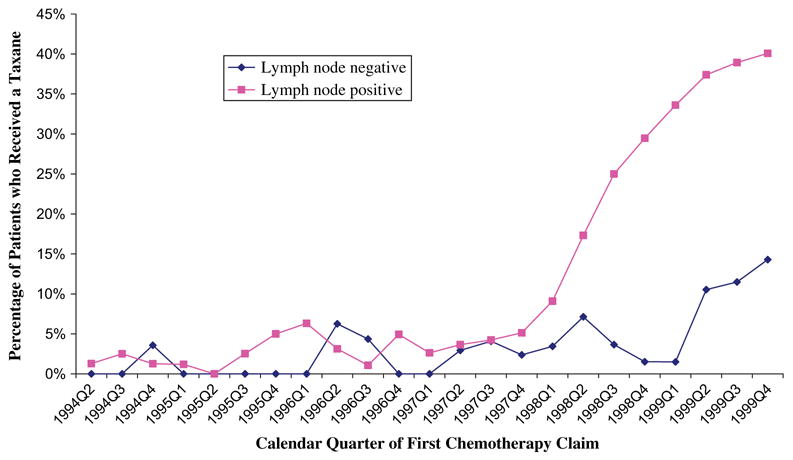
Percentage of patients on chemotherapy who received a taxane as part of their regimen by calendar quarter of first chemotherapy claim, according to axillary lymph node status.
We then used multivariable regression models to examine the effect of period of treatment on taxane use, adjusting for potential confounders. In a model that included period of chemotherapy administration (i.e., April 1994 through April 1998 and May 1998 through December 1999), age, number of positive lymph-nodes, tumor size, tumor grade, and estrogen receptor status, patients who started chemotherapy in or after May 1998 were more likely to have received a taxane (estimated relative risk = 6.84, 95% CI = 5.71 to 8.07) than patients who started chemotherapy before May 1998. We next performed a multivariable analysis stratified by treatment period (Table 2). Among patients treated in April 1994 through April 1998, number of positive lymph nodes showed a weak association with taxane use. However, among patients treated in May 1998 through December 1999, number of positive lymph nodes was strongly associated with taxane use. Younger patient age, larger tumor size, and higher grade of disease were also statistically significantly associated with taxane use in May 1998 or after (Table 2).
Table 2.
Multivariable analysis of characteristics associated with taxane use before and after the presentation of results from the Cancer and Leukemia Group B Study 9344 in May 1998, among women with stage I–III breast cancer who were treated with adjuvant chemotherapy*
| Before May 1998 (N = 1177)
|
After May 1998 (N = 2164)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Patients who received taxane (%) | OR (95% CI) | P† | Estimated RR‡ (95% CI) | Patients who received taxane (%) | OR (95% CI) | P† | Estimated RR‡ (95% CI) |
| Age, y | ||||||||
| 65–69 | 5.9 | 1.00 (referent) | 1.00 (referent) | 27.5 | 1.00 (referent) | 1.00 (referent) | ||
| 70–74 | 3.9 | 0.62 (0.39 to 1.00) | .050 | 0.63 (0.40 to 1.00) | 23.1 | 0.66 (0.46 to 0.95) | .027 | 0.73 (0.54 to 0.96) |
| 75–79 | 5.5 | 0.83 (0.49 to 1.42) | .493 | 0.84 (0.51 to 1.39) | 17.7 | 0.32 (0.20 to 0.50) | <.001 | 0.39 (0.26 to 0.58) |
| ≥80 | 6.9 | 0.80 (0.37 to 1.71) | .564 | 0.81 (0.38 to 1.64) | 25.0 | 0.41 (0.22 to 0.74) | .003 | 0.49 (0.28 to 0.80) |
| Positive lymph nodes, No. | ||||||||
| None | 2.7 | 1.00 (referent) | 1.00 (referent) | 6.3 | 1.00 (referent) | 1.00 (referent) | ||
| 1–3 | 2.1 | 0.77 (0.36 to 1.65) | .501 | 0.77 (0.37 to 1.62) | 20.8 | 4.31 (2.65 to 7.01) | <.001 | 3.57 (2.40 to 5.08) |
| 4–9 | 5.7 | 2.16 (1.09 to 4.27) | .027 | 2.09 (1.09 to 3.92) | 52.7 | 18.19 (10.78 to 30.71) | <.001 | 8.73 (6.67 to 10.69) |
| ≥10 | 6.7 | 2.31 (1.10 to 4.84) | .027 | 2.23 (1.10 to 4.39) | 49.5 | 16.63 (9.03 to 30.63) | <.001 | 8.38 (6.00 to 10.68) |
| Tumor size, cm | ||||||||
| 0–2.0 | 2.6 | 1.00 (referent) | 1.00 (referent) | 16.8 | 1.00 (referent) | 1.00 (referent) | ||
| 2.1–5.0 | 4.8 | 1.88 (1.10 to 3.21) | .021 | 1.84 (1.10 to 3.04) | 22.2 | 1.19 (0.84 to 1.68) | .341 | 1.15 (0.86 to 1.51) |
| ≥5.0 | 11.5 | 3.18 (1.80 to 5.60) | <.001 | 3.01 (1.76 to 5.00) | 46.7 | 2.91 (1.87 to 4.53) | <.001 | 2.20 (1.63 to 2.84) |
| Tumor grade | ||||||||
| Low | 4.7 | 1.00 (referent) | 1.00 (referent) | 11.3 | 1.00 (referent) | 1.00 (referent) | ||
| Intermediate | 3.8 | 0.71 (0.28 to 1.82) | .474 | 0.72 (0.29 to 1.75) | 24.3 | 2.09 (1.04 to 4.20) | .039 | 1.86 (1.04 to 3.08) |
| High | 4.0 | 0.84 (0.34 to 2.08) | .712 | 0.85 (0.35 to 1.98) | 25.9 | 2.39 (1.20 to 4.77) | .013 | 2.07 (1.17 to 3.34) |
| Estrogen receptor status | ||||||||
| Negative | 6.6 | 1.00 (referent) | 1.00 (referent) | 21.3 | 1.00 (referent) | 1.00 (referent) | ||
| Positive | 4.1 | 0.56 (0.35 to 0.88) | .012 | 0.58 (0.37 to 0.89) | 24.9 | 0.82 (0.56 to 1.19) | .288 | 0.85 (0.62 to 1.14) |
OR = Odds ratio; CI = confidence interval; RR = relative risk.
P values (two-sided) were calculated by Wald chi-square test.
Estimated relative risks were calculated using the method of Zhang and Yu (19).
Discussion
We have shown that, coincident with the presentation of the results of CALGB 9344 at the ASCO annual meeting in May 1998, the use of taxanes as part of chemotherapy for primary breast cancer in the United States rose dramatically. This increase was seen well before the data were published in a peer-reviewed journal and even before FDA granted approval for the use of paclitaxel for early-stage breast cancer.
Our results raise several questions. First, why did practice patterns change so rapidly? Breast cancer is a high-visibility disease and the most common malignancy among women in the United States (20). This study therefore received intensive media coverage, and the findings would have reached both patients and health care providers (7–11). Consistent with a general tendency of the popular press to exaggerate both positive and negative medical reports, the tone of the media coverage was highly optimistic. For example, the following excerpt from an article in the Wall Street Journal illustrates the enthusiasm with which this study was received: “ ‘This is going to change the standard of care for people with node-positive breast cancer,’ says Larry Norton, director of the breast-cancer service at Memorial Sloan-Kettering Cancer Center. ‘God’s smiling on us this year.’ He says the drug also holds promise for women whose cancer hasn’t advanced into lymph nodes” (8). In addition to the media influence, it is likely the findings of this study were disseminated to community oncologists by pharmaceutical representatives. Finally, the credibility of these data—that is, the fact that they came from a multicenter randomized trial conducted by a respected and established research group—would also have encouraged practice change.
A second question raised by our findings is whether rapid change in practice patterns following dissemination of data presented at a medical meeting is of concern. Rapid dissemination of new data is important to optimize patient care and outcomes. In the case of breast cancer, women are facing a potentially fatal disease, and rapid dissemination of scientific advances such as the findings of the CALGB trial can save lives. Certainly, for oncologists to have failed to add taxanes to standard therapy for breast cancer for 5 years until the published article became available does not seem reasonable.
However, there is the inherent danger that rapid changes in practice patterns may, at times, be premature. In this example of taxane use for primary breast cancer, median follow-up at time of presentation of the initial results was only 18 months, and considerable controversy still surrounded the use of adjuvant taxanes. Indeed, results of the National Surgical Adjuvant Project for Breast and Bowel Cancers B-28 trial, which had a design similar to that of the CALGB trial, showed no overall survival benefit of adjuvant paclitaxel (23). In fact, when these data were presented to the 2000 NIH Consensus Conference on adjuvant therapy of breast cancer, the conference concluded that there were still insufficient data to recommend taxanes for the adjuvant treatment of breast cancer (21). Since then, and with the benefit of more studies (22–24), the oncology community has reached a consensus that taxanes do improve survival among women with lymph node–positive breast cancer (25).
However, these uncertainties highlight the potential risk of exposing women to toxic agents before a benefit has been clearly established. That is, although it took years to definitively establish the benefit of taxanes for adjuvant treatment of breast cancer, paclitaxel produced clear toxic effects (grade 3 or higher myelo-suppression in 21% of patients, neuropathy in 5% of patients, and pain in 5% of patients). Thus, paclitaxel’s toxic effects were evident earlier than its benefits.
Also, an abstract or a short oral presentation may be inadequate to fully communicate efficacy, toxic effects, subgroup differences, and limitations of a particular study. For example, the abstract of the CALGB study did not include information on the age of patients or on potential interactions between taxane efficacy and age, number of lymph nodes, or tumor size. Moreover, we found that the predictors of taxane use were not completely consistent with the CALGB study findings. That is, although most patients in our study received taxanes for lymph node–positive breast cancer, taxanes were used in some patients with lymph node–negative breast cancer, despite a lack of data in this patient population. Also, the patients in our study population were substantially older than those in the CALGB study. The application of the CALGB data to this population of older women is of more than theoretical concern because the efficacy of chemotherapy for breast cancer declines with age, and the benefit of chemotherapy with taxanes in this population of older women was not established (26). However, we were reassured to find that the data from the CALGB study were applied appropriately in most patients, in that the presence of lymph node metastases was the strongest predictor of use of taxanes.
The findings of our study therefore suggest that a process for the rapid dissemination of detailed data is essential, particularly for practice-changing trials. Over the past decade, this process has improved. For example, many major medical journals offer rapid review and publication of clinical trials that are likely to affect practice, which can greatly reduce the potential interval from submission to publication. Early online publication also reduces publication delays. However, although we are not in a position to know the reasons for the long delay before publication of the CALGB paper, we speculate that the delay was probably due mainly to factors other than the speed of the review and publication process. Several recent practice-changing trials in breast cancer (27,28) have been initially presented as publications rather than as oral presentations at meetings. ASCO has also provided improved access to data from studies presented at its annual meeting. For example, since 1999 a Virtual Meeting has been available online that provides audio or video recordings and slide sets from oral presentations, as well as access to posters and abstracts. Therefore, health care providers have available to them more information than is provided by the media or pharmaceutical representatives.
Our study has several limitations. One is that the generaliz-ability of our findings is unknown. We took advantage of a unique situation that permitted a rigorous study of practice change: Presentation of data at the meeting was separated in time from publication of the study, the results were from a single study that prompted a new FDA indication for an established drug, and the use of the treatment could be tracked in an administrative database. To address whether other oral presentations could have a rapid impact on treatment patterns, we analyzed changes in the use of gonadotropin-releasing hormone analogs after an oral presentation of a randomized phase III trial at the 1995 ASCO meeting (29) that reported improved progression-free survival in men with locally advanced prostate cancer who were treated with goserelin. We found a rapid increase in use of gonadotropin-releasing hormone analogs after the ASCO presentation (data not shown), suggesting that other ASCO presentations can influence treatment patterns. However, although we have found that meeting presentations can be associated with dramatic changes in clinical practice, we were not able to address the characteristics of the trial or meeting that determine the degree of influence on treatment patterns. Other studies will be needed to specifically address these issues.
Our study has several further limitations. First, although we have shown the association between the presentation of the CALGB findings and a rise in use of taxanes as adjuvant breast cancer therapy, we cannot prove causality. However, given that this study was the only one with survival data on women receiving adjuvant taxane therapy, a causal association seems likely. Also, the temporal connection suggests causality. We were not able to explore the impact of subsequent events, such as the 2000 NIH Consensus Conference or publication of the trial, on taxane use because the dataset we used is limited to patients diagnosed through 1999. Another limitation is that we evaluated the use of taxanes in women aged 65 years and older. The use of taxanes among women across all ages is likely to be higher than depicted in our findings because a substantial body of evidence shows that older breast cancer patients are treated less aggressively than younger patients (30–34). Thus, this study probably underestimates the increase in use of taxanes after the meeting presentation. In addition, the generalizability of our data to women of all ages is uncertain. Still another possible limitation is that we ascertained chemotherapy use from CPT J codes, which are subject to coding errors or underreporting and which are not used when chemotherapy is administered in an inpatient setting. However, we suspect that coding problems were not substantial given that clinics have an incentive to correctly submit claims because the codes are tied to reimbursement.
In conclusion, we found that a single oral presentation at a large medical conference was associated with a dramatic change in national practice patterns. Although in many ways this example represents a best-case scenario, in which the meeting report of a multicenter randomized trial turns out to have stimulated the adoption of a treatment that has eventually become part of evidence-based practice, it also illustrates the enormous power of highly publicized meeting presentations. Investigators should be aware of the potential impact of their presentations and exercise appropriate caution and judgment in their interpretation of research findings. We encourage rapid processes for study peer review and publication, especially for practice-changing trials. Otherwise, the danger of premature application or misapplication of trial data may be substantial.
Footnotes
Supported by NCI 1K07CA109064-01 (SHG). Also supported, in part, by the University of Texas–Medical Branch Center on Population Health and Health Disparities, grant NIH P50CA105631. The funding sources had no role in the study design, conduct, data analysis, or manuscript preparation. Dr. Giordano had full access to the data and takes responsibility for the integrity of the data, the accuracy of the data analysis, and the decision to submit for publication.
References
- 1.Jackevicius CA, Anderson GM, Leiter L, Tu JV. Use of the statins in patients after acute myocardial infarction: does evidence change practice? Arch Intern Med. 2001;161:183–8. doi: 10.1001/archinte.161.2.183. [DOI] [PubMed] [Google Scholar]
- 2.Stafford RS, Furberg CD, Finkelstein SN, Cockburn IM, Alehegn T, Ma J. Impact of clinical trial results on national trends in alpha-blocker prescribing, 1996–2002. JAMA. 2004;291:54–62. doi: 10.1001/jama.291.1.54. [DOI] [PubMed] [Google Scholar]
- 3.Haas JS, Kaplan CP, Gerstenberger EP, Kerlikowske K. Changes in the use of postmenopausal hormone therapy after the publication of clinical trial results. Ann Intern Med. 2004;140:184–8. doi: 10.7326/0003-4819-140-3-200402030-00009. [DOI] [PubMed] [Google Scholar]
- 4.Henderson IC, Berry D, Demetri GD, Cirrincione C, Goldstein LJ, Martino S, et al. Improved disease-free and overall survival from the addition of sequential paclitaxel but not from the escalation of doxorubicin dose in the adjuvant chemotherapy of patients with node-positive primary breast cancer. Proc ASCO. 1998;17:101a. [Google Scholar]
- 5.Henderson IC, Berry DA, Demetri GD, Cirrincione CT, Goldstein LJ, Martino S, et al. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–83. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 6.American Society of Clinical Oncology. [[Last accessed: January 4, 2005.]];ASCO Profile. Available at: http://www.asco.org.
- 7.Pollack A. Drug for advanced breast cancer is also found effective in early treatment. New York Times. 1998 52298:Sect A14. [Google Scholar]
- 8.Burton T. Drug research alters standards for cancer care. Wall Street Journal. 1998 51998:Sect B1. [Google Scholar]
- 9.Arnst C. Dispatches from the cancer front. Business Week. 1998 6198:146. [Google Scholar]
- 10.Inroads in battle against cancer. Maclean’s. 1998 6198:52. [Google Scholar]
- 11.Marcus MB. Tracking a cancer cure. U.S. News and World Report. 1998 6198:52. [Google Scholar]
- 12.Nattinger AB, Hoffmann RG, Howell-Pelz A, Goodwin JS. Effect of Nancy Reagan’s mastectomy on choice of surgery for breast cancer by US women. JAMA. 1998;279:762–6. doi: 10.1001/jama.279.10.762. [DOI] [PubMed] [Google Scholar]
- 13.Potosky AL, Riley GF, Lubitz JD, Mentnech RM, Kessler LG. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31:732–48. [PubMed] [Google Scholar]
- 14.Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI, et al. Revision of the American Joint Committee on Cancer staging system for breast cancer. J Clin Oncol. 2002;20:3628–36. doi: 10.1200/JCO.2002.02.026. [DOI] [PubMed] [Google Scholar]
- 15.Marsh L, Cormier D. Spline regression models. Thousand Oaks (CA); Sage: 2001. [Google Scholar]
- 16.Marsh L, Mcglynn M, Chakraborty D. Interpreting complex nonlinear models. Proceedings of SAS User’s Group International. 1994;19:1185–9. [Google Scholar]
- 17.Marsh L. Estimating the number and location of knots in spline regression. J Appl Bus Res. 1986;3:60–70. [Google Scholar]
- 18.Hosmer D, Lemeshow S. Applied logistic regression. New York (NY): John Wiley & Sons; 2000. [Google Scholar]
- 19.Zhang J, Yu K. What’s the relative risk: a method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 20.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 21.J Natl Cancer Inst Monogr; National Institutes of Health Consensus Development Conference statement: adjuvant therapy for breast cancer; November 1–3, 2000; 2001. pp. 5–15. [PubMed] [Google Scholar]
- 22.Martin M, Pienkowski T, Mackey J, Pawlicki M, Guastalla JP, Weaver C, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352:2302–13. doi: 10.1056/NEJMoa043681. [DOI] [PubMed] [Google Scholar]
- 23.Mamounas EP, Bryant J, Lembersky B, Fehrenbacher L, Sedlacek SM, Fisher B, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol. 2005;23:3686–96. doi: 10.1200/JCO.2005.10.517. [DOI] [PubMed] [Google Scholar]
- 24.Heys SD, Hutcheon AW, Sarkar TK, Ogston KN, Miller ID, Payne S, et al. Neoadjuvant docetaxel in breast cancer: 3-year survival results from the Aberdeen trial. Clin Breast Cancer. 2002;3 (Suppl 2):S69–74. doi: 10.3816/cbc.2002.s.015. [DOI] [PubMed] [Google Scholar]
- 25.Clinical Practice Guidelines in Oncology. [[Last accessed: February 9, 2006.]];Breast cancer. Available at: http://www.nccn.org/professionals/physician_gls/default.asp.
- 26.Polychemotherapy for early breast cancer: an overview of the ran-domised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;352:930–42. [PubMed] [Google Scholar]
- 27.Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. [see comment] N Engl J Med. 2003;349:1793–802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 28.Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. [see comment] N Engl J Med. 2004;350:1081–92. doi: 10.1056/NEJMoa040331. [DOI] [PubMed] [Google Scholar]
- 29.Pilepich M, Caplan R, Byhardt R, Lawton C, Gallagher M, Mesic J, et al. Phase III trial of androgen suppression using goserelin in unfavorable prognosis carcinoma of the prostate treated with definitive radiotherapy (RTOG 85–31) Proc ASCO. 1995;14:239. doi: 10.1200/JCO.1997.15.3.1013. [DOI] [PubMed] [Google Scholar]
- 30.Greenfield S, Blanco DM, Elashoff RM, Ganz PA. Patterns of care related to age of breast cancer patients. JAMA. 1987;257:2766–70. [PubMed] [Google Scholar]
- 31.Goodwin JS, Hunt WC, Samet JM. Determinants of cancer therapy in elderly patients. Cancer. 1993;72:594–601. doi: 10.1002/1097-0142(19930715)72:2<594::aid-cncr2820720243>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 32.Silliman RA, Guadagnoli E, Weitberg AB, Mor V. Age as a predictor of diagnostic and initial treatment intensity in newly diagnosed breast cancer patients. J Gerontol. 1989;44:M46–50. doi: 10.1093/geronj/44.2.m46. [DOI] [PubMed] [Google Scholar]
- 33.Samet J, Hunt WC, Key C, Humble CG, Goodwin JS. Choice of cancer therapy varies with age of patient. JAMA. 1986;255:3385–90. [PubMed] [Google Scholar]
- 34.Mandelblatt JS, Hadley J, Kerner JF, Schulman KA, Gold K, Dunmore-Griffith J, et al. Patterns of breast carcinoma treatment in older women: patient preference and clinical and physical influences. Cancer. 2000;89:561–73. [PubMed] [Google Scholar]


