Abstract
Previous studies have observed significant abnormalities in the fatty acid composition of peripheral tissues from drug-naïve first-episode schizophrenic (SZ) patients relative to normal controls, including deficits in omega-3 and omega-6 polyunsaturated fatty acids, which are partially normalized following chronic antipsychotic treatment. We hypothesized that postmortem cortical tissue from patients with SZ would also exhibit deficits in cortical docosahexaenoic acid (DHA, 22:6n-3) and arachidonic acid (AA; 20:4n-6) relative to normal controls, and that these deficits would be greater in drug-free SZ patients. We determined the total fatty acid composition of postmortem orbitofrontal cortex (OFC) (Brodmann area 10) from drug-free and antipsychotic-treated SZ patients (n=21) and age-matched normal controls (n=26) by gas chromatography. After correction for multiple comparisons, significantly lower DHA (−20%) concentrations, and significantly greater vaccenic acid (VA) (+12.5) concentrations, were found in the OFC of SZ patients relative to normal controls. Relative to age-matched same-gender controls, OFC DHA deficits, and elevated AA:DHA, oleic acid:DHA and docosapentaenoic acid (22:5n-6):DHA ratios, were found in male but not female SZ patients. SZ patients that died of cardiovascular-related disease exhibited lower DHA (−31%) and AA (−19%) concentrations, and greater OA (+20%) and VA (+17%) concentrations, relative to normal controls that also died of cardiovascular-related disease. OFC DHA and AA deficits, and elevations in oleic acid and vaccenic acid, were numerically greatest in drug-free SZ patients and were partially normalized in SZ patients treated with antipsychotic medications (atypical > typical). Fatty acid abnormalities could not be wholly attributed to lifestyle or postmortem tissue variables. These findings add to a growing body of evidence implicating omega-3 fatty acid deficiency as well as the OFC in the pathoaetiology of SZ, and suggest that abnormalities in OFC fatty acid composition may be gender-specific and partially normalized by antipsychotic medications.
Keywords: Schizophrenia, Postmortem brain, Docosahexaenoic acid (DHA), Arachidonic acid, Prefrontal cortex, Antipsychotic
1. Introduction
The principal omega-3 polyunsaturated fatty acid in the mammalian brain, docosahexaenoic acid (DHA, 22:6n-3) comprises ~15% of total fatty acid composition, and the principal omega-6 polyunsaturated fatty acid arachidonic acid (AA, 20:4n-6) comprises ~10% of total fatty acid composition. Both DHA and AA preferentially accumulate in synaptic membranes where they exert opposing effects on the phosphoinositide-protein kinase C signal transduction pathway and multiple clinically-relevant down-stream neurochemical processes (reviewed in McNamara et al., 2006b). Because mammals cannot synthesize omega-3 or omega-6 polyunsaturated fatty acids de novo, they are entirely dependent on dietary sources to procure and maintain adequate peripheral and central tissue concentrations. Cross-national and cross-sectional epidemiological surveys (Christensen & Christensen, 1988; Mellor et al., 1995; Peet et al., 2003) and intervention trials (Arvindakshan et al., 2003; Emsley et al., 2002; Mellor et al., 1995; Peet et al., 2001; Peet & Horrobin, 2002) suggest that dietary omega-3 fatty acid deficiency may increase symptom severity in SZ patients. Moreover, drug-naive first-episode psychotic patients exhibit significant deficits in omega-3 and omega-6 fatty acid concentrations in their red blood cell (RBC) membranes, suggesting that omega-3 and omega-6 fatty acid deficits precede illness onset, and both DHA and AA concentrations are partially normalized following chronic antipsychotic treatment leading to symptomatic improvement (Arvindakshan et al., 2003; Evans et al., 2003; Khan et al., 2002; Reddy et al., 2004).
Primate studies have demonstrated that RBC and cortical DHA concentrations both decrease in response to dietary deficits in omega-3 fatty acid intake, albeit at different rates (RBC≫>Cortex; Anderson et al., 2005; Connor et al., 1990). To date, three studies have investigated the fatty acid composition of postmortem brain tissue from SZ patients. Horrobin et al. (1991) found that DHA and AA concentrations were lower in the cholesterol ester fraction of the frontal cortex of antipsychotic-treated SZ patients. Yao et al. (2000) found that AA (−14%), but not DHA (−11%), concentrations were significantly lower in the postmortem caudate nucleus of antipsychotic-treated SZ patients. Landen et al. (2002) did not find significant alterations in individual fatty acid concentrations, including DHA or AA, in the postmortem cingulate cortex of antipsychotic-treated SZ patients. These postmortem studies have four notable limitations: (1) SZ patients were being treated with antipsychotic medications that have been found to partially normalize DHA and AA deficits in peripheral tissues of SZ patients (Arvindakshan et al., 2003; Evans et al., 2003; Khan et al., 2002), (2) normal controls were predominantly elderly subjects, and fatty acid concentrations have been found to vary as a function of age at death in human postmortem brain tissue (Carver et al., 2001; Gershbein et al., 1985; McNamara et al., 2006a), (3) these studies combined male and female SZ patients, and gender differences in brain fatty acid composition (Gershbein et al., 1985; McNamara et al., 2006a) and SZ epidemiology and psychopathology (Hafner, 2003) have been reported, and (4) these studies employed a relatively small number of SZ patients (n=7–11), and may have been underpowered to detect moderate changes in fatty acid concentrations.
In the present study, we determined the total fatty acid composition of postmortem orbitofrontal cortex (Brodmann area 10) from drug-free and antipsychotic-treated male and female SZ patients (n=21) and age-matched male and female healthy controls with no history of psychiatric illness (n=26). The OFC was selected as the region of interest because it has reciprocal connections with the amygdala, hippocampus, nucleus accumbens, and hypothalamus (Kringelbach & Rolls, 2004), and is thought to play an important role in cognitive and emotional processes relevant to SZ psychopathology (Bechara, 2004; Kringelbach, 2005; London et al., 2000). Previous neuroimaging studies have observed cortical thinning and/or reductions in OFC volume in patients with SZ relative to normal controls (Andreasen et al., 1997; Crespo-Facorro et al., 2000; Goldstein et al., 1999; Gur et al., 2000; Kuperberg et al., 2003; Pantelis et al., 2003), and postmortem studies have found alterations in the expression of multiple genes in the SZ OFC suggestive of synaptic pathology (Akil et al., 1999; Garey et al., 2006; Glantz & Lewis, 1997; Karson et al., 1999; Knable et al., 2004; Meador-Woodruff et al., 1997; Torrey et al., 2005). Our primary hypothesis was that DHA and AA concentrations would be significantly lower in the postmortem OFC of SZ patients, and that these deficits would be greater in drug-free SZ patients.
2. Methods
2.1 Postmortem brain tissues
Frozen, unfixed, postmortem OFC (Brodmann area 10) from normal (no psychiatric illness) male and female controls (n=26) and age-matched male and female patients with DSM-IV defined SZ (n=21) were used. Brain tissue was generously provided by the Stanley Research Foundation Neuropathology Consortium (Torrey et al., 2000) and the Harvard Brain Tissue Resource Center. Axis I DSM-IV diagnoses were made independently by senior psychiatrists based on medical records and interviews with family members. A comparison of subject and tissue variables is presented in Table 1. At time of death, n=3 SZ patients were drug-free, n=9 SZ patients were receiving typical antipsychotic medications (fluphenazine: n=1, thiothixene: n=1, chlorpromazine: n=2, thioridazine: n=2, haloperidol: n=3), and n=9 SZ patients were receiving atypical antipsychotic medications (olanzapine: n=1, risperidone: n=3, clozapine: n=5). There were n=5 SZ patients additionally receiving mood-stabilizers (valproic acid, carbamazepine, or lithium), and n=5 SZ patients additionally receiving antidepressant medications.
Table 1.
Comparison of Subject and Brain Tissue Characteristics
| Normal (n=26) | Schizophrenia (n=21) | p-value1 | |
|---|---|---|---|
| Patient Characteristics: | |||
| Age at death, mean ± S.D. (range) | 46.8 ± 10.7 (29–65) | 44.2 ± 12.8 (25–65) | 0.450 |
| Male | 46.2 ± 9.21 (35–62) | 40.6 ± 12.4 (25–65) | 0.149 |
| Female | 48.1 ± 14.3 (29–65) | 51.4 ± 11.2 (30–62) | 0.630 |
| Gender | 18M, 8F | 14M, 7F | |
| Race2 | 19C,7UN | 17C,4A | |
| Cause of death | |||
| Suicide | 0 | 5 | |
| Cardiopulmonary | 19 | 10 | |
| Accident | 3 | 3 | |
| Other | 4 | 3 | |
| Age at disease onset (mean years ± S.D.) | - | 23.2 ± 7.9 | |
| Duration of disease (mean years ± S.D.) | - | 20.3 ± 11.8 | |
| Cigarette Smoker (yes/no)3 | 2/3 | 7/5 | |
| Alcohol abuse severity (low/high)3 | 12/1 | 4/9 | |
| Substance abuse severity (low/high)3 | 13/0 | 11/4 | |
| Tissue Characteristics: | |||
| Brain hemisphere | 10R/16L | 9R/12L | |
| Brain mass (mean grams ± S.D.) | 1459 ± 161 | 1443 ± 27 | 0.704 |
| Postmortem interval (mean hours ± S.D.) | 23.6 ± 7.7 | 29.9 ± 14.0 | 0.076 |
| Storage time (mean days ± S.D.) | 330 ± 228 | 621 ± 233 | 0.002 |
| Tissue pH (mean ± S.D.) | 6.3 ± 0.3 | 6.2 ± 0.3 | 0.312 |
Student’s t-test (2-tail)
C = Caucasian, A = Asian, UN = Unknown
Smoking status, alcohol abuse severity, and substance abuse severity could not be ascertained for the remaining subjects
2.2. Gas chromatography
The method for saponification and methylation of fatty acids for gas chromatographic (GC) analysis follows that originally reported by Mecalfe et al (1966). Frozen ~100 mg cortical samples (predominantly gray matter) were placed in a 20 ml glass vial into which 4 ml of 0.5N methanolic sodium hydroxide was added, and the sample heated at 80°C for 5 min. Following a 10 min cooling period, 3 ml of BF3 in methanol was added to methylate the sample. After an additional five minutes of heating in the water bath (80°C), the sample vial was allowed to cool, and 2 ml of a saturated solution (6.2 M) of sodium chloride and 10 ml of hexane was added. The samples were then mixed by vortex for one minute. The hexane fraction was then transferred into a 20 ml vial containing 10 mg of sodium sulfate to dry the sample. The hexane solution was then removed for GC analysis. An injection volume of 1 μL of the hexane solution was analyzed. Samples were analyzed with a Shimadzu GC-17A GC equipped with auto-injector (Shimadzu Scientific Instruments Inc., Columbia MD). Analysis of fatty acid methyl esters is based on areas calculated with Shimadzu Class VP 4.3 software. The column was a DB-23 (123–2332): 30m (length), I.D. (mm) 0.32 wide bore, film thickness of 0.25 μM (J&W Scientific, Folsom CA). Fatty acid identification was determined using retention times of authenticated fatty acid methyl ester standards (Matreya LLC Inc., Pleasant Gap PA). The GC conditions were: column temperature ramping by holding at 120ºC for one minute followed by an increase of 5ºC/min from 120–240ºC. The temperature of the injector and flame ionization detector was 250ºC. A split (8:1) injection mode was used. The carrier gas was helium with a column flow rate of 2.5 ml/min. All samples were processed by a technician blinded to treatment conditions.
In our GC analysis, we set the threshold at an area of 1000, which corresponds to 1 μg/mL of sample. The amount injected on the GC therefore allows us to detect 1 ng injected into the instrument, and we are able to detect 10 μg of an individual fatty acid in a 100 mg sample of tissue. At the outset of the experiment, analyses were conducted to determine between-sample reliability, and it was determined that our assays had an overall Cronbach’s reliability coefficient alpha of 0.935, and alphas ≥0.60 for individual fatty acids, indicating very good between-sample reliability. Accordingly, statistical (Student’s t-tests, 2-tail) comparison of duplicate values did not find significant differences for any fatty acid (ps>0.05). Based on this finding and limited tissue quantity, only a subset of brain samples (n=15 normals, n=15 SZ patients) were run in duplicate. Duplicate values were averaged for all subsequent analyses.
2.3. Statistical analysis
We restricted our primary analysis to the principal saturated fatty acids (palmitic, stearic, myristic), monounsaturated fatty acids (oleic acid, OA, vaccenic acid, VA), omega-6 polyunsaturated fatty acids (arachidonic acid, AA; docosatetraenoic acid, DTA; docosapentaenoic acid, DPA), and omega-3 polyunsaturated fatty acid (docosahexaenoic acid, DHA). Together these 9 fatty acids comprise ~90% of total fatty acids in postmortem brain tissue, and the remaining 10% is comprised of fatty acids that individually represent >2% of total fatty acids. Analyses of variance (ANOVA) and covariance (ANCOVA) were performed using SAS (Version 9.1, SAS Institute Cary, NC) PROC MIXED procedure. The null hypothesis that fatty acid concentrations do not differ by illness state (Normal, SZ) was tested as the interaction term Illness x Fatty acid in a two-factor ANOVA. Post-hoc tests of simple effects were performed using the Bonferroni correction with a group-wise error rate of α =0.05 to evaluate illness state effects for individual fatty acids (α =0.05/9 = 0.0056). An ANCOVA repeated the steps above, adding Age (continuous), Gender (Male, Female), Treatment (typical antipsychotic, atypical antipsychotic, drug-free) and second-order interaction terms of all variables, after which stepwise regression using p=0.10 as a cutoff point determined which terms remained in the final model. Post-hoc tests of simple effects using the Bonferroni correction were also performed on the final ANCOVA model, with a group-wise error rate of α =0.05 to evaluate illness state effects for individual fatty acids (α =0.05/9 = 0.0056). Pearson product moment correlation analyses were performed using GB-STAT (Dynamic Microsystems, Inc., Silver Springs MD) to determine the interrelationship between fatty acid concentrations and subject characteristics (age at onset of illness, duration of illness, age at time of death) and tissue variables (brain pH, brain weight, postmortem interval, and days in freezer storage). All tests were 2-tailed and performed at α =0.05. Exploratory analyses of continuous variables were conducted using Student’s t-tests (2-tailed, α =0.05). Effect size was calculated using Cohen’s d, with small, medium, and large effect sizes being equivalent to d-values of 0.30, 0.50, and 0.80, respectively.
3. Results
3.1. OFC fatty acid composition in normal controls
In the OFC of normal controls, concentrations of individual saturated fatty acids, monounsaturated fatty acids, and polyunsaturated fatty acids (Fig. 1) are consistent with those previously reported for postmortem frontal cortex from a larger cohort (N=38) of normal adult male and female subjects (Carver et al., 2001). Normal females exhibited higher OFC AA (+10%, p=0.04) and DTA (+4%, p=0.02) concentrations relative to normal males. In the OFC of normal controls, DHA (r = −0.43, p=0.02), AA (r = −0.44, p=0.02), DPA (r = −0.54, p=0.004), and stearic acid (r = −0.64, p=0.0004) concentrations were negatively correlated, and OA (r = +0.52, p=0.006) and VA (r = +0.40, p=0.04) concentrations were positively correlated, with age at death.
Figure 1.
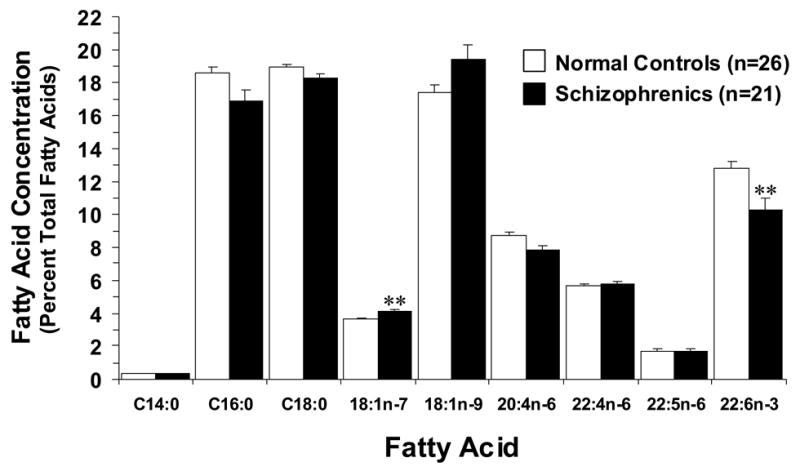
Comparison of principal fatty acid concentrations (mean ± S.E.M. percent total fatty acid composition) in the OFC of male and female normal controls (N)(n=26) and drug-free and antipsychotic-treated male and female SZ patients (n=21). After correction for multiple comparisons (α =0.05/9), DHA (22:6n-3) concentrations were significantly lower (p=0.0051), and vaccenic acid (18:1n-7) concentrations significantly higher (p=0.003), in the OFC of SZ patients relative to normal controls (**P ≤ 0.0056 vs. Normal controls). Saturated fatty acids (C14:0 - myristic acid; C16:0 - palmitic acid; C18:0 - stearic acid), monounsaturated fatty acids (18:1n-9 - oleic acid; 18:1n-7 - vaccenic acid), omega-6 polyunsaturated fatty acids arachidonic acid (20:4n-6), docosatetraenoic acid (22:4n-6), and docosapentaenoic acid (22:5n-6), and omega-3 fatty acid docosahexaenoic acid (22:6n-3 - DHA).
3.2. OFC fatty acid composition in normals vs. SZ patients
The overall ANOVA found a significant Illness x Fatty Acid Interaction, F(8,377)=3.87, p=0.0002, and a significant main effect of Fatty Acid, F(8,377)=255, p ≤ 0.0001, but not Illness F(1,377)=2.46, p=0.117. A significant Age x Fatty Acid Interaction, F(8,377)=6.35, p ≤ 0.0001, was found, and the main effect of Age, F(1,377)=1.14, p=0.286, and the Illness x Age Interaction, F(1,377)=0.20, p=0.659, were not significant. After correction for multiple comparisons, significantly lower DHA (−20%, p=0.0051) concentrations, and significantly greater vaccenic acid (VA) (+12.5, p=0.003) concentrations, were found in the OFC of SZ patients relative to normal controls (Fig. 1). Negative trends for AA (−10%, p=0.012), palmitic acid (−9%, p=0.01), and stearic acid (−4%, p=0.04), and a positive trend for oleic acid (OA)(+11%, p=0.03), were observed in the OFC of SZ patients relative to normal controls (Fig. 1). In the OFC of SZ patients, DHA concentrations were inversely correlated with OA concentrations (r = −0.93, p<0.0001), VA concentrations (r = −0.83, p<0.0001), and docosatetraenoic acid concentrations (r = −0.46, p=0.001), and were positively correlated with AA (r = +0.86, p<0.0001), palmitic acid (r = +0.81, p<0.0001), and stearic acid (r = +0.74, p<0.0001) concentrations. These findings suggest that DHA loss is predominantly compensated for by elevations in OA and VA. Elevations in the AA:DHA (+17%, p=0.01), OA:DHA (+41%, p=0.01), DPA:DHA (+28%, p=0.09), and palmitic acid:VA (−19%, p=0.003) ratios were observed in SZ patients relative to normal controls (Fig. 2). Total saturates (∑ MA+PA+SA) (−6%, p=0.01) and total polyunsaturates (∑AA+DPA+DT+DHA) (−11%, p=0.01) were lower, and total monounsaturates (∑OA+VA) were higher (+11%, p=0.02), in SZ patients relative to normal controls. The ∑saturate: ∑polyunsaturate (+7%, p=0.04) and ∑monunsaturate: ∑polyunsaturate (+24%, p=0.01) ratios were higher, and the ∑saturate: ∑monounsaturate (−15%, p=0.01) ratio lower, in SZ patients relative to normal controls.
Figure 2.
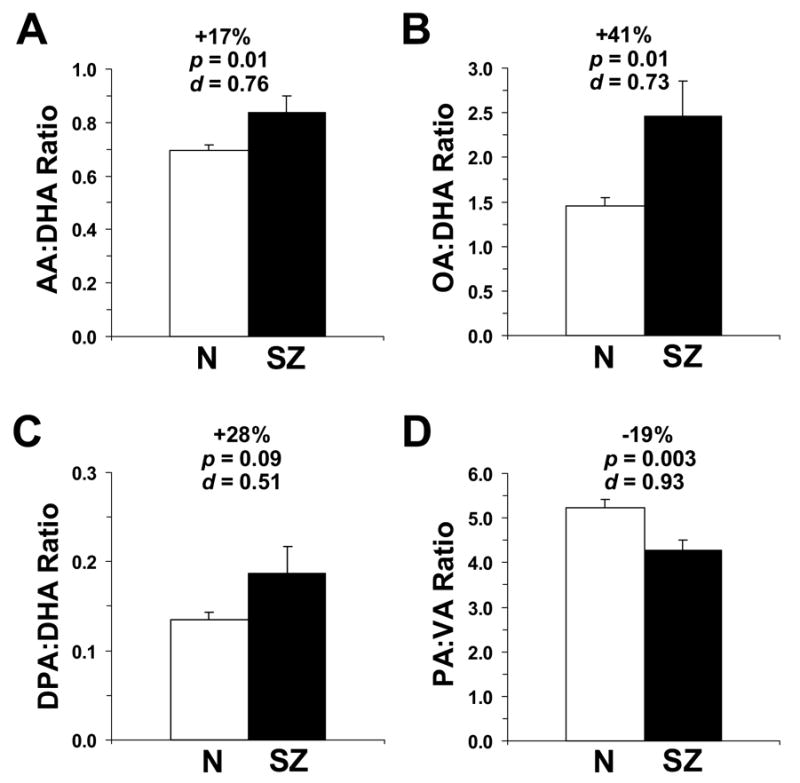
Comparison of the arachidonic acid (AA):DHA ratio (A), oleic acid (OA):DHA ratio (B), docosapentaenoic acid (DPA):DHA ratio (C), and palmitic acid (PA):vaccenic acid (VA) ratio (D) in the OFC of normal controls (N)(n=26) and SZ patients (n=21). Data are expressed as mean ± S.E.M. percent total fatty acids. Effect size is expressed as mean percent difference from normal controls, associated p-values (Student t-test, two-tail), and Cohen’s d-values.
SZ patients that died of cardiovascular-related disease (n=8) exhibited lower DHA (−31%, p=0.002) and AA (−19%, p=0.002) concentrations, and greater OA (+20%, p=0.006) and VA (+17%, p=0.003) concentrations, relative to normal controls that also died of cardiovascular-related disease (n=17). However, SZ patients that committed suicide (n=5) did not exhibit significantly lower DHA (−17%, p=0.07) or AA (−4%, p=0.57) concentrations, or greater OA (+6%, p=0.43) and VA (+7%, p=0.27) concentrations, relative to normal controls that died of cardiovascular-related disease (n=17).
We found a significant Illness x Fatty Acid x Gender Interaction, F(8,377)=5.37, p ≤ 0.0001, whereas the main effect of Gender, F(1,377)=0.07, p=0.793, and the Illness x Gender Interaction, F(1,377)=0.97, p=0.325, were not significant. OFC DHA concentrations did not differ significantly between normal male and female controls (p=0.158), or between SZ male and female patients (p=0.213). However, relative to age-matched same-gender controls, male SZ patients exhibited greater OFC DHA deficits (−27%, p=0.001) than did female SZ patients (−2%, p=0.91)(Fig. 3A). Similar OFC AA deficits were observed in male (−11%, p=0.03) and female (−7%, p=0.31) SZ patients, and the resulting AA:DHA ratio was greater in male SZ patients (+25%, p=0.003) than female SZ patients (−4%, p=0.57) relative to age-matched same-gender controls (Fig. 3B). Conversely, male SZ patients exhibited greater OFC OA concentrations (+15%, p=0.01) than female SZ patients (+0.5%, p=0.95), and the resulting OA:DHA ratio was greater in male SZ patients (+52%, p=0.01) than female SZ patients (+5%, p=0.83) (Fig. 3C). OFC DPA concentrations did not differ between male and female SZ patients (p=0.055), and the resulting DPA:DHA ratio was greater in male (+39%, p=0.04) than female (−15%, p=0.19) SZ patients relative to age-matched same-gender controls (Fig. 3D).
Figure 3.
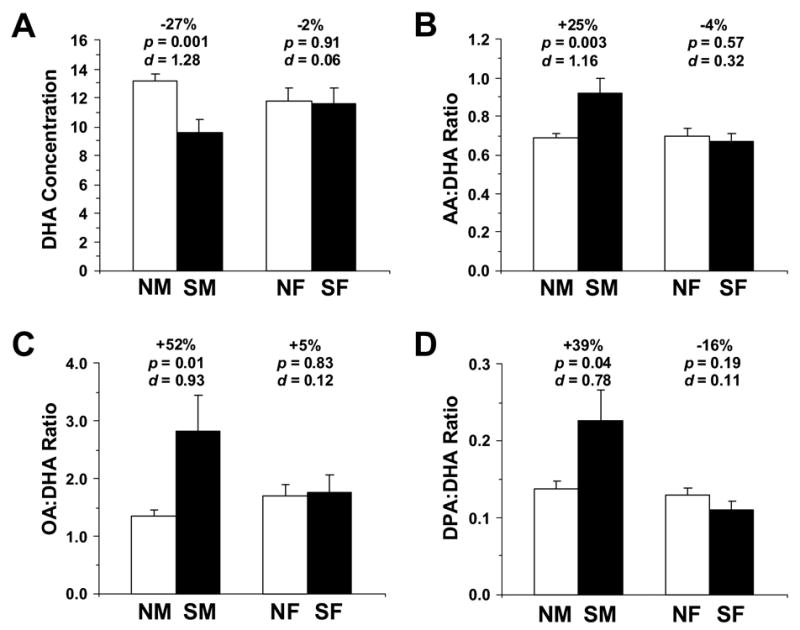
Comparison of DHA concentrations (A), AA:DHA ratio (B), OA:DHA ratio (C), and DPA:DHA ratio (D) in the OFC of normal males (NM, n=19), normal females (NF, n=8), male SZ patients (n=9), and female SZ patients (n=6). Note that DHA concentrations are lower, and AA:DHA, OA:DHA, and DPA:DHA ratios are higher, in male SZ patients relative to female SZ patients relative to same-gender controls. Effect size is expressed as mean percent difference from normal controls, associated p-values (Student t-test, two-tail), and Cohen’s d-values.
3.3. Effect of antipsychotic medications on OFC fatty acid composition in SZ patients
Analysis by Treatment (Normals, n=26; atypical antipsychotic, n=9; typical antipsychotic, n=9; drug-free, n=3) found a significant Treatment x Fatty Acid Interaction, F(24,315)=2.07, p=0.0028, and significant Age x Treatment x Fatty Acid, F(24,315)=2.67, p ≤ 0.0001, and Gender x Treatment x Fatty Acid, F(24,315)=1.72, p=0.02, Interactions. After adjustment for Age and Gender, the OFC DHA deficit observed in drug-free SZ patients (−33% vs. normal controls, p=0.008) was numerically greater than the DHA deficit observed in SZ patients treated with typical (−26% vs. normal controls, p=0.003) or atypical (−8% vs. normal controls, p=0.84) antipsychotic medications (Fig. 4A). OFC DHA concentrations in drug-free SZ patients did not differ significantly from OFC DHA concentrations in SZ patients treated with typical (p=0.73) or atypical (p=0.21) antipsychotic medications. The OFC AA deficit observed in drug-free SZ patients (−26% vs. normal controls, p=0.006) was numerically greater than the AA deficit observed in SZ patients treated with typical (−12% vs. normal controls, p=0.002) or atypical (−3% vs. normal controls, p=0.92) antipsychotic medications (Fig. 4B). OFC AA concentrations in drug-free SZ patients did not differ significantly from OFC AA concentrations in SZ patients treated with typical antipsychotic medications (p=0.21), but were significantly lower relative to SZ patients treated with atypical antipsychotic medications (p=0.01). The OFC AA:DHA ratio observed in drug-free SZ patients (+29% vs. normal controls, p=0.01) was greater than the ratio observed in SZ patients treated with typical (+21% vs. normal controls, p=0.003) or atypical (+7% vs. normal controls, p=0.14) antipsychotic medications (Fig. 4C). The OFC AA:DHA ratio in drug-free SZ patients did not differ significantly from the OFC AA:DHA ratio in SZ patients treated with typical (p=0.70) or atypical (p=0.25) antipsychotic medications. Finally, the OFC PA deficit observed in drug-free SZ patients (−11% vs. normal controls, p=0.003) was numerically greater than the PA deficits observed in SZ patients treated with atypical antipsychotic medications (−1% vs. normal controls, p=1.00), and were similar to SZ patients treated with typical antipsychotic medications (−17% vs. normal controls, p=0.0003).
Figure 4.
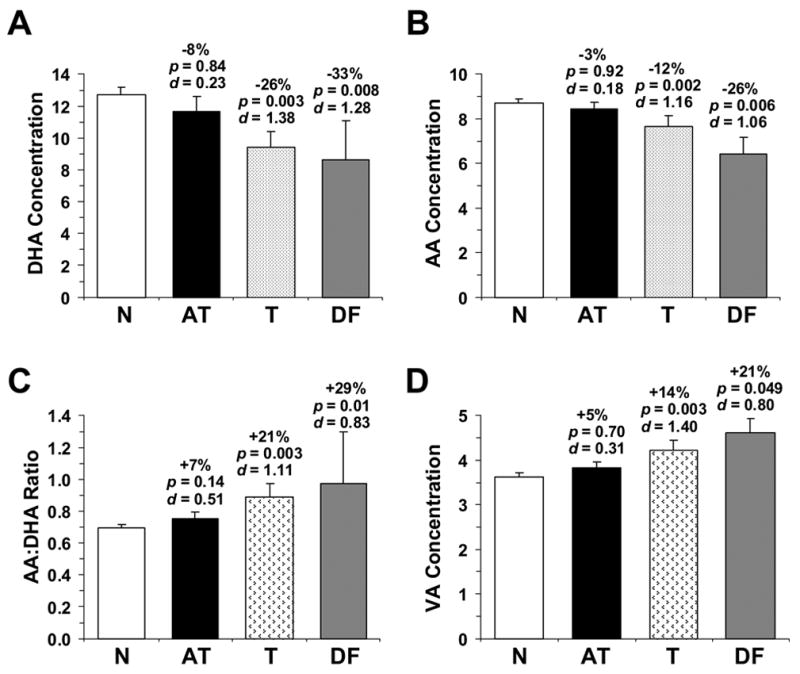
OFC DHA concentrations (A), AA concentrations (B), AA:DHA ratio (C), and VA concentrations (D) (% total fatty acid composition) in normal controls (n=26), drug-free SZ patients (DF, n=3), and SZ patients treated with typical antipsychotic medications (T) (n=9) or atypical antipsychotic medications (AT) (n=9). Note that AA and DHA deficits, and elevated AA:DHA ratio and VA concentrations, are greater in drug-free SZ patients, and partially normalized in SZ patients treated with atypical antipsychotic medications, and to a lesser extent typical antipsychotic medications. Data are expressed as mean ± S.E.M. percent total fatty acids. Effect size is expressed as mean percent difference from normal controls and associated age- and gender-adjusted p-values and Cohen’s d-values.
Additionally, elevated OFC VA concentrations observed in drug-free SZ patients (+21% vs. normal controls, p=0.049) were numerically greater than the elevation observed in SZ patients treated with typical (+14% vs. normal controls, p=0.003) or atypical (+5% vs. normal controls, p=0.70) antipsychotic medications (Fig. 4D). OFC VA concentrations in drug-free SZ patients did not differ significantly from OFC AA concentrations in SZ patients treated with typical antipsychotic medications (p=0.40), but were greater relative to SZ patients treated with atypical antipsychotic medications (p=0.01). Similarly, elevated OFC OA concentrations observed in drug-free SZ patients (+24% vs. normal controls, p=0.0003) were numerically greater than the elevation observed in SZ patients treated with typical (+13% vs. normal controls, p=0.003) or atypical (+3% vs. normal controls, p=0.99) antipsychotic medications.
The negative correlation between age at death and OFC DHA concentrations in normal controls (r = −0.43, p=0.02) was also observed in typical antipsychotic-treated SZ patients (r = −0.68, p=0.04) but not in atypical antipsychotic-treated SZ patients (r = +0.49, p=0.17)(Fig. 5). Similarly, the negative correlation between age at death and OFC AA concentrations in normal controls (r = −0.44, p=0.02) was also observed in typical antipsychotic-treated SZ patients (r = −0.79, p=0.01) but not in atypical antipsychotic-treated SZ patients (r = +0.23, p=0.55)(Fig. 5). Conversely, the positive correlation between age at death and OFC OA concentrations found in normal controls (r = +0.52, p=0.006) was also observed in typical antipsychotic-treated SZ patients (r = +0.72, p=0.02) but not in atypical antipsychotic-treated SZ patients (r = −0.37, p=0.32). Similarly, the positive correlation between age at death and OFC VA concentrations found in normal controls (r = +0.40, p=0.04) was also observed in typical antipsychotic-treated SZ patients (r = +0.77, p=0.01) but not in atypical antipsychotic-treated SZ patients (r = −0.32, p=0.40).
Figure 5.
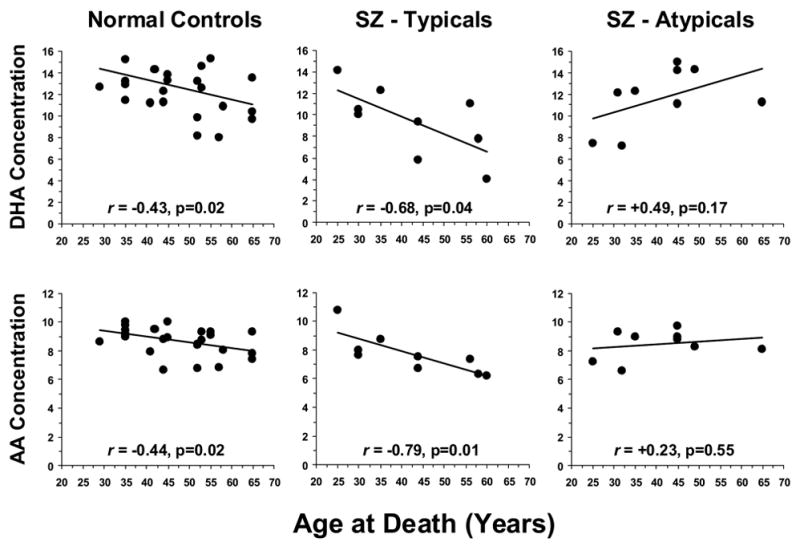
Correlations between age at death and DHA and AA concentrations in normal controls, and SZ patients treated with typical (SZ – Typicals) or atypical antipsychotic (SZ - Atypicals) medications at time of death. Note that the negative correlations between age at death and OFC DHA and AA concentrations in normal controls are observed in SZ patients treated with typical antipsychotic medications but not in SZ patients treated with atypical antipsychotic medications.
The duration of illness was negatively correlated with OFC DHA concentrations in typical antipsychotic-treated SZ patients (r = −0.67, p=0.04), but was positively correlated in atypical antipsychotic-treated SZ patients (r = +0.88, p=0.05). Similarly, the duration of illness was negatively correlated with OFC AA concentrations in typical antipsychotic-treated SZ patients (r = −0.71, p=0.03), but was positively correlated in atypical antipsychotic-treated SZ patients (r = +0.61, p=0.05). Conversely, the duration of illness was positively correlated with OFC OA concentrations in typical antipsychotic-treated SZ patients(r = +0.62, p=0.07), but was negatively correlated in atypical antipsychotic-treated SZ patients (r = −0.88, p=0.05). Similarly, the duration of illness was positively correlated with OFC VA concentrations in typical antipsychotic-treated SZ patients (r = +0.84, p=0.004), but was negatively correlated in atypical antipsychotic-treated SZ patients (r = −0.83, p=0.17).
3.4. Effects of lifestyle variables on OFC fatty acid concentrations
OFC DHA (p=0.264), AA (p=0.313), OA (p=0.347), and VA (p=0.121) concentrations did not differ between SZ patients positively identified as cigarette smokers (n=5) relative to SZ patients that did not smoke (n=7). OFC DHA (p=0.197), AA (p=0.241), and OA (p=0.239) concentrations did not differ between normals+SZ patients that smoked cigarettes (n=9) relative to normals+SZ patients that did not smoke (n=8), whereas OFC VA concentrations were higher in normals+SZ smokers (+11%, p=0.02). OFC DHA (p=0.676), AA (p=0.806), OA (p=0.908), and VA (p=0.916) concentrations did not differ between SZ patients with low alcohol abuse severity (n=9) relative to SZ patients with high alcohol abuse severity (n=4). OFC DHA (p=0.179), AA (p=0.445), OA (p=0.427), and VA (p=0.249) concentrations did not differ between normals+SZ patients with low alcohol abuse severity (n=21) relative to SZ patients with high alcohol abuse severity (n=5). OFC DHA (p=0.663), AA (p=0.368), OA (p=0.445), and VA (p=0.601) concentrations did not differ between SZ patients with low substance abuse severity (n=11) relative to SZ patients with high substance abuse severity (n=4). OFC DHA (p=0.869), AA (p=0.896), OA (p=0.773), and VA (p=0.923) concentrations did not differ between normals+SZ patients with low substance abuse severity (n=23) relative to normals+SZ patients with high substance abuse severity (n=4).
3.5. Effects of patient and postmortem tissue variables on OFC fatty acid composition
Among all SZ patients (n=21), there were no significant correlations between DHA concentrations and age at death (r = −0.15, p=0.51), age at onset of illness (r = 0.10, p=0.71), duration of illness (r = −0.29, p=0.26), fluphenazine mg equivalents (r = +0.42, p=0.12), brain pH (r = +0.22, p=0.34), brain weight (r = −0.38, p=0.08), postmortem interval (r = −0.27, p=0.25), or freezer storage duration (r = −0.39, p=0.15), nor were there significant correlations between other individual fatty acid concentrations and these variables. Separate analyses of fluphenazine mg equivalents in SZ patients treated with typical (r = +0.19, p=0.65) or atypical (r = +0.74, p=0.25) did not find significant correlations. Within normal controls, there were no significant correlations between OFC DHA concentrations and brain pH (r = +0.25, p=0.22), brain weight (r = −0.05, p=0.79), postmortem interval (r = −0.14, p=0.49), or freezer storage duration (r = −0.12, p=0.69), nor were there significant correlations between other fatty acid concentrations and these variables. The absence of correlations between freezer storage duration and DHA concentrations in notable in view of the greater freezer storage duration in SZ patients relative to normal controls (Table 1).
4. Discussion
Based on previous findings of DHA and AA deficits in the RBC of drug-naïve first-episode SZ patients, and primate studies suggesting a correlation between RBC and cortical DHA and AA concentrations, we hypothesized that the postmortem OFC from SZ patients would exhibit deficits in DHA and AA. In the present study, we found that OFC DHA concentrations (−20%), but not AA concentrations (−10%), were significantly lower in combined drug-free + antipsychotic-treated male and female SZ patients relative to age-matched male and female normal controls after correction for multiple comparisons. These fatty acid alterations were associated with elevations in AA:DHA (+17%) and OA:DHA (+41%) ratios, and a reduction in the PA:VA (−19%) ratio. Analysis by gender found that male SZ patients, but not female SZ patients, exhibited significantly lower OFC DHA concentrations, and elevated AA:DHA, OA:DHA, and DPA:DHA ratios, relative to age-matched same-gender controls. Elevated membrane AA:DHA and OA:DHA ratios are significant in view of previous in vitro studies finding that DHA inhibits, and AA and OA stimulate, protein kinase C (PKC), a major synaptic signal transduction molecule activated by phosphoinositide-coupled receptors (reviewed in McNamara et al., 2006b), and previous studies finding elevated indices of phosphoinositide metabolism in the postmortem prefrontal cortex of SZ patients (Jope et al., 1998; Lin et al., 1999). SZ patients that died of cardiovascular-related disease exhibited lower DHA (−31%) and AA (−19%) concentrations, and greater OA (+20%) and VA (+17%) concentrations, relative to normal controls that also died of cardiovascular-related disease. OFC DHA deficits were not significantly correlated with postmortem tissue variables, and could not be wholly attributed to alcohol abuse severity, substance abuse severity, or cigarette smoking.
The present postmortem study has four important limitations. First, there is no information available regarding the diets of SZ patients or normal controls in the months preceding their death in order to investigate its contribution to the present findings. Indeed, the precise cause of OFC DHA deficits in SZ patients cannot be determined from the present study, and may be attributable to a combination of state factors (diet, substance, alcohol and tobacco abuse) and/or trait factors, including deficits in brain DHA transport mechanisms and/or impaired DHA biosynthesis from dietary precursors. Second, positive and negative symptom severity at the time of death is not known, and may correlate with DHA concentrations (e.g., Mellor et al., 1995). Third, because SZ is a heterogeneous disorder, the small number of SZ patients, particularly in the drug-free group (n=3), may not be representative of all patients with SZ. Fourth, RBCs were not available to determine the interrelationship between peripheral and central tissue fatty acid composition (e.g., Carver et al., 2001).
In the present study, male SZ patients exhibited greater OFC DHA deficits (−27%) than did female SZ patients (−2%) relative to age-matched same-gender controls. This gender difference is consistent with epidemiological data indicating a gender difference in age at onset and illness course among SZ patients (reviewed in Hafner, 2003). Moreover, the conversion of α-linolenic acid to DHA is positively regulated by estrogen (Burdge & Wooten, 2002; Giltay et al., 2004), and a recent study found reduced levels of circulating estrogen levels in male first-episode psychotic patients (Huber et al., 2005). In contrast with the present findings, we previously found that OFC DHA deficits were greater in female (−32%) than male patients (−16%) with unipolar depression relative to age-matched same-gender controls (McNamara et al., 2006a), a pattern also consistent with epidemiological data indicating higher (2:1) prevalence rates of unipolar depression among females versus males (Kuehner et al., 2003). Elucidation of the interrelationship between circulating estrogen levels, tissue DHA concentrations, and symptom type and severity may provide important insight into the mechanisms conferring gender differences in psychopathology.
A previous study of adult monozygotic twin pairs discordant for SZ found that affected twins had significantly lower plasma DHA concentrations than unaffected twins (Bates et al., 1992), suggesting environmental rather than genetic determinants. For example, SZ patients smoke cigarettes at higher rates than the general population (McCloughen, 2003), and cigarette smoking is associated with elevated indices of lipid peroxidation and lower DHA and AA concentrations in peripheral tissues of female, but not male, SZ patients (Hibbeln et al., 2003) and in normal subjects (Leng et al., 1994). However, in the present study greater OFC DHA deficits were observed in male than female SZ patients, and we did not observe differences in OFC DHA concentrations between SZ patients that smoked cigarettes relative to those that did not smoke cigarettes, or between normal+SZ patients that smoked relative to normal+SZ patients that did not smoke. In a separate analysis, there were also no differences in OFC DHA concentrations in combined normals, SZ, bipolar, and depressed patients that smoked (n=22) relative to normals, SZ, bipolar, and depressed patients that did not smoke (n=17) (p=0.981)(McNamara, unpublished observation). Together these data suggest that cigarette smoking cannot wholly account for deficits in OFC DHA concentrations in SZ patients, but may nevertheless be a contributing factor in conjunction with other lifestyle factors. We also found that there were no differences between OFC DHA concentrations in SZ patients with low versus high alcohol abuse severity. However, in a separate analysis it was found that OFC DHA concentrations were significantly lower in combined normals, SZ, bipolar, and depressed patients with high alcohol abuse severity (n=17) relative to combined normals, SZ, bipolar, and depressed patients with low alcohol abuse severity (n=36) (−25%, p=0.006) (McNamara, unpublished observation). This finding is consistent with a previous primate study finding 20–30% reductions in cortical DHA concentrations following chronic alcohol intake (Pawlosky et al., 2001), and suggests that alcohol abuse may also contribute in part to DHA deficits in the human OFC.
In the present study, OFC DHA and AA deficits, and elevated OA and VA concentrations, were numerically greater in drug-free SZ patients relative to SZ patients treated with antipsychotic medications (atypical > typical). The latter finding is consistent with the previous finding that DHA deficits in RBC of drug-naive SZ patients are partially normalized following chronic antipsychotic treatment (Arvindakshan et al., 2003; Evans et al., 2003; Khan et al., 2002), as well as the more moderate DHA deficits (Yao et al., 2000), or no differences in DHA or AA concentrations (Landen et al., 2002), in postmortem brain tissues of antipsychotic-treated SZ patients relative to normal controls. Moreover, these findings suggest that the OFC DHA deficits observed in antipsychotic-treated SZ patients are not a consequence of chronic antipsychotic exposure which is consistent with preclinical findings (Levant et al., 2006; Parikh et al., 2003). Furthermore, the OFC AA deficit (−10%) observed in combined drug-free and antipsychotic-treated SZ patients (n=21) is similar to that found by Yao et al. (2000) in postmortem caudate nucleus of antipsychotic-treated SZ patient (−14%), and the 10% AA deficit reported by Horrobin et al. (1991). Together these findings suggest that antipsychotic treatments that are efficacious in reducing symptom severity in SZ patients partially normalize peripheral as well as central tissue fatty acid abnormalities in drug-free SZ patients. Future studies examining the fatty acid composition of central or peripheral tissues from SZ patients will therefore need to account for antipsychotic treatment as a confounding variable.
Antipsychotic medications have a high affinity for dopamine D2 and/or serotonin 5-HT2A/C receptors, and both serotonin 5-HT2A/C receptors (Basselin et al., 2005a; Garcia & Kim, 1997; Qu et al., 2003) and dopamine D2 receptors (Basselin et al., 2005b; Nilsson et al., 1998; Piomelli et al., 1991), are coupled to phospholipase A2 (PLA2), which mediates the cleavage of membrane-bound AA (calcium-dependent PLA2) and DHA (calcium-independent PLA2). It is off interest, therefore, that some studies (Gattaz et al., 1990, 1995; Lasch et al., 2003; Noponen et al., 1993; Ross et al., 1997; Smesny et al., 2005), but not all (Albers et al., 1993), have observed elevated PLA2 activity in the serum/platelets of drug-free SZ patients which are attenuated following chronic antipsychotic treatment. Moreover, preclinical studies have found that chronic blockade of D2 receptors with haloperidol significantly decreases PLA2 activity in rat brain (Myers et al., 2001; Ross et al., 1999; Trzeciak et al., 1995). Furthermore, a postmortem study observed normal calcium-independent PLA2 activity, and blunted calcium-stimulated PLA2 activity, in the postmortem OFC (Brodmann area 10) of antipsychotic-treated SZ patients (Ross et al., 1999). Collectively, these findings suggest that elevations in 5-HT2A/C and/or dopamine D2 receptor-generated PLA2 activity may contribute to the observed abnormalities in fatty acid composition. It is of interest, therefore, that dietary-induced DHA deficits in rat frontal cortex that are comparable to those observed in the OFC of drug-free SZ patients are associated with both an elevation in the 5-HT2A:D2 receptor binding density ratio (McNamara et al., 2006c) as well as elevations in calcium-dependent PLA2 expression and activity (Rao et al., 2006). However, elevated PLA2 activity and abnormalities in OFC fatty acid composition may also be a consequence of elevated lipid peroxidation, indices of which are increased in the plasma of drug-naïve first-episode SZ patients and reduced following chronic treatment with antipsychotic medications (Arvindakshan et al., 2003; Evans et al., 2003; Khan et al., 2002). However, a preclinical study found that elevations in lipid peroxidation following chronic treatment with haloperidol does not alter brain fatty acid composition, suggesting that these are dissociable events (Parikh et al., 2003).
Previous neuroimaging studies have observed cortical thinning and/or reductions in OFC volume in patients with SZ relative to normal controls (Andreasen et al., 1997; Crespo-Facorro et al., 2000; Goldstein et al., 1999; Gur et al., 2000; Kuperberg et al., 2003; Pantelis et al., 2003), and postmortem studies have found alterations in the expression of multiple genes in the SZ OFC suggestive of synaptic pathology or agenesis (Akil et al., 1999; Garey et al., 2006; Glantz & Lewis, 1997; Karson et al., 1999; Knable et al., 2004; Meador-Woodruff et al., 1997; Torrey et al., 2005). It is not known whether the observed OFC DHA deficits contribute to, or are a consequence of, OFC neuropathology. In support of DHA contributing to OFC cortical shrinkage or agenesis, preclinical studies have found that perinatal deficits in cortical DHA accrual are associated with reductions in neuronal arborization (Calderon & Kim, 2004) and neuronal size (Ahmad et al., 2002a,b) in rat brain. Moreover, OFC DHA concentrations (present results; McNamara et al., 2006a), OFC neuronal size (Cotter et al., 2005), and OFC volume (Tisserand et al., 2002) are all inversely correlated with age in normal subjects. Finally, preterm delivery, a risk factor for SZ (Ichiki et al., 2000), is associated with deficits in cortical DHA accrual (Clandinin et al., 1980), and children born preterm exhibit cortical volume reductions relative to term-born children (Peterson et al., 2000). However, a recent study found that the postmortem OFC of SZ patients does not exhibit reductions in neuronal size (Cotter et al., 2005), and because DHA preferentially accumulates in synaptic membranes (Suzuki et al., 1997), DHA deficits may simply reflect reductions in the number of synapses in the OFC of SZ patients. It will therefore be of interest to determine whether chronic omega-3 fatty acid treatment can normalize OFC volume deficits in SZ patients.
Preclinical studies have found that brain DHA concentrations are relatively resistance to loss following long-term dietary omega-3 fatty acid deficiency in the adult rat brain (Bourre et al., 1992; DeMar et al., 2004), suggesting that OFC DHA deficits may originally stem from perinatal deficits in DHA accrual that were not subsequently corrected by diet (reviewed in McNamara & Carlson, 2006). Because primate PFC DHA deficits can be normalized with long-term dietary omega-3 fatty acid treatment (Anderson et al., 2005), normalization of cortical DHA concentrations may contribute to reductions in symptom severity in SZ patients following chronic treatment with either omega-3 fatty acids (Arvindakshan et al., 2003; Emsley et al., 2002; Mellor et al., 1995; Peet et al., 2001; Peet & Horrobin, 2002) or antipsychotic medications (Arvindakshan et al., 2003; Evans et al., 2003; Khan et al., 2002). The present data therefore have implications for future clinical trials because they would suggest, for example, that drug-free male SZ patients would exhibit the greatest benefit from omega-3 fatty acid treatment whereas atypical antipsychotic-treated female SZ patients would exhibit the least benefit.
Acknowledgments
This work was supported in part by National Institute of Mental Health grants MH073704 and MH074858 to R.K.M., and by the Department of Veterans Affairs Medical Research Service (NMR). Postmortem brain tissue was donated by The Stanley Medical Research Institute's Brain Collection courtesy of Drs. Michael B. Knable, E. Fuller Torrey, Maree J. Webster, Serge Weis, and Robert H. Yolken, and the Harvard Brain Tissue Resource Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad A, Moriguchi T, Salem N. Decrease in neuron size in docosahexaenoic acid-deficient brain. Pediatr Neurol. 2002a;26:210–218. doi: 10.1016/s0887-8994(01)00383-6. [DOI] [PubMed] [Google Scholar]
- Ahmad A, Murthy M, Greiner RS, Moriguchi T, Salem N. A decrease in cell size accompanies a loss of docosahexaenoate in the rat hippocampus. Nutr Neurosci. 2002b;5:103–113. doi: 10.1080/10284150290018973. [DOI] [PubMed] [Google Scholar]
- Akil M, Pierri JN, Whitehead RE, Edgar CL, Mohila C, Sampson AR, Lewis DA. Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psychiatry. 1999;156:1580–1589. doi: 10.1176/ajp.156.10.1580. [DOI] [PubMed] [Google Scholar]
- Albers M, Meurer H, Marki F, Klotz J. Phospholipase A2 activity in serum of neuroleptic-naive psychiatric inpatients. Pharmacopsychiatry. 1993;26(3):94–98. doi: 10.1055/s-2007-1014349. [DOI] [PubMed] [Google Scholar]
- Anderson GJ, Neuringer M, Lin DS, Connor WE. Can prenatal N-3 fatty acid deficiency be completely reversed after birth? Effects on retinal and brain biochemistry and visual function in rhesus monkeys. Pediatr Res. 2005;58(5):865–872. doi: 10.1203/01.pdr.0000182188.31596.5a. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O'Leary DS, Flaum M, Nopoulos P, Watkins GL, Boles Ponto LL, Hichwa RD. Hypofrontality in schizophrenia: distributed dysfunctional circuits in neuroleptic-naive patients. Lancet. 1997;349(9067):1730–1734. doi: 10.1016/s0140-6736(96)08258-x. [DOI] [PubMed] [Google Scholar]
- Arvindakshan M, Ghate M, Ranjekar PK, Evans DR, Mahadik SP. Supplementation with a combination of omega-3 fatty acids and antioxidants (vitamins E and C) improves the outcome of schizophrenia. Schizophr Res. 2003;62(3):195–204. doi: 10.1016/s0920-9964(02)00284-0. [DOI] [PubMed] [Google Scholar]
- Arvindakshan M, Sitasawad S, Debsikdar V, Ghate M, Evans D, Horrobin DF, Bennett C, Ranjekar PK, Mahadik SP. Essential polyunsaturated fatty acid and lipid peroxide levels in never-medicated and medicated schizophrenia patients. Biol Psychiatry. 2003;53(1):56–64. doi: 10.1016/s0006-3223(02)01443-9. [DOI] [PubMed] [Google Scholar]
- Basselin M, Chang L, Seemann R, Bell JM, Rapoport SI. Chronic lithium administration to rats selectively modifies 5-HT2A/2C receptor-mediated brain signaling via arachidonic acid. Neuropsychopharmacology. 2005;30:461–472. doi: 10.1038/sj.npp.1300611. [DOI] [PubMed] [Google Scholar]
- Basselin M, Chang L, Bell JM, Rapoport SI. Chronic lithium chloride administration to unanesthetized rats attenuates brain dopamine D2-like receptor-initiated signaling via arachidonic acid. Neuropsychopharmacology. 2005;30(6):1064–1075. doi: 10.1038/sj.npp.1300671. [DOI] [PubMed] [Google Scholar]
- Bates C, Horrobin DF, Ells K. Fatty acids in plasma phospholipids and cholesterol esters from identical twins concordant and discordant for schizophrenia. Schizophr Res. 1991;6(1):1–7. doi: 10.1016/0920-9964(91)90014-i. [DOI] [PubMed] [Google Scholar]
- Bechara A. The role of emotion in decision-making: evidence from neurological patients with orbitofrontal damage. Brain Cogn. 2004;55(1):30–40. doi: 10.1016/j.bandc.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Bourre JM, Dumont OS, Piciotti MJ, Pascal GA, Durand GA. Dietary alpha-linolenic acid deficiency in adult rats for 7 months does not alter brain docosahexaenoic acid content, in contrast to liver, heart and testes. Biochim Biophys Acta. 1992;1124:119–122. doi: 10.1016/0005-2760(92)90087-c. [DOI] [PubMed] [Google Scholar]
- Burdge GC, Wootton SA. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br J Nutr. 2002;88:411–420. doi: 10.1079/BJN2002689. [DOI] [PubMed] [Google Scholar]
- Calderon F, Kim HY. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J Neurochem. 2004;90:979–988. doi: 10.1111/j.1471-4159.2004.02520.x. [DOI] [PubMed] [Google Scholar]
- Carver JD, Benford VJ, Han B, Cantor AB. The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects. Brain Res Bull. 2001;56(2):79–85. doi: 10.1016/s0361-9230(01)00551-2. [DOI] [PubMed] [Google Scholar]
- Christensen O, Christensen E. Fat consumption and schizophrenia. Acta Psychiatr Scand. 1988;78(5):587–591. doi: 10.1111/j.1600-0447.1988.tb06388.x. [DOI] [PubMed] [Google Scholar]
- Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW. Intrauterine fatty acid accretion rates in human brain: implications for fatty acid requirements. Early Hum Dev. 1980;4:121–129. doi: 10.1016/0378-3782(80)90015-8. [DOI] [PubMed] [Google Scholar]
- Connor WE, Neuringer M, Lin DS. Dietary effects on brain fatty acid composition: the reversibility of n-3 fatty acid deficiency and turnover of docosahexaenoic acid in the brain, erythrocytes, and plasma of rhesus monkeys. J Lipid Res. 1990;31(2):237–247. [PubMed] [Google Scholar]
- Cotter D, Hudson L, Landau S. Evidence for orbitofrontal pathology in bipolar disorder and major depression, but not in schizophrenia. Bipolar Disord. 2005;7:358–369. doi: 10.1111/j.1399-5618.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro B, Kim J, Andreasen NC, O'Leary DS, Magnotta V. Regional frontal abnormalities in schizophrenia: a quantitative gray matter volume and cortical surface size study. Biol Psychiatry. 2000;48(2):110–119. doi: 10.1016/s0006-2332(00)00238-9. [DOI] [PubMed] [Google Scholar]
- DeMar JC, Ma K, Bell JM, Rapopo SI. Half-lives of docosahexaenoic acid in rat brain phospholipids are prolonged by 15 weeks of nutritional deprivation of n-3 polyunsaturated fatty acids. J Neurochem. 2004;91:1125–1137. doi: 10.1111/j.1471-4159.2004.02789.x. [DOI] [PubMed] [Google Scholar]
- Emsley R, Myburgh C, Oosthuizen P, van Rensburg SJ. Randomized, placebo-controlled study of ethyl-eicosapentaenoic acid as supplemental treatment in schizophrenia. Am J Psychiatry. 2002;159(9):1596–1598. doi: 10.1176/appi.ajp.159.9.1596. [DOI] [PubMed] [Google Scholar]
- Evans DR, Parikh VV, Khan MM, Coussons C, Buckley PF, Mahadik SP. Red blood cell membrane essential fatty acid metabolism in early psychotic patients following antipsychotic drug treatment. Prostaglandins Leukot Essent Fatty Acids. 2003;69(6):393–399. doi: 10.1016/j.plefa.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Garcia MC, Kim HY. Mobilization of arachidonate and docosahexaenoate by stimulation of the 5-HT2A receptor in rat C6 glioma cells. Brain Res. 1997;768(1–2):43–48. doi: 10.1016/s0006-8993(97)00583-0. [DOI] [PubMed] [Google Scholar]
- Garey LJ, Von Bussmann KA, Hirsch SR. Decreased numerical density of kainate receptor-positive neurons in the orbitofrontal cortex of chronic schizophrenics. Exp Brain Res. 2006 doi: 10.1007/s00221-006-0396-8. [DOI] [PubMed] [Google Scholar]
- Gattaz WF, Hubner CV, Nevalainen TJ, Thuren T, Kinnunen PK. Increased serum phospholipase A2 activity in schizophrenia: a replication study. Biol Psychiatry. 1990;28(6):495–501. [PubMed] [Google Scholar]
- Gattaz WF, Schmitt A, Maras A. Increased platelet phospholipase A2 activity in schizophrenia. Schizophr Res. 1995;16(1):1–6. doi: 10.1016/0920-9964(94)00060-l. [DOI] [PubMed] [Google Scholar]
- Gershbein LL, Baburao K, Pedroso AF, Rao KC, Arellano G, Sumikoshi K. Regression analysis of total fatty acids from human whole brain according to age and sex. J Appl Biochem. 1985;7(1):55–63. [PubMed] [Google Scholar]
- Giltay EJ, Gooren LJ, Toorians AW, Katan MB, Zock PL. Docosahexaenoic acid concentrations are higher in women than in men because of estrogenic effects. Am J Clin Nutr. 2004;80:1167–1174. doi: 10.1093/ajcn/80.5.1167. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Reduction of synaptophysin immunoreactivity in the prefrontal cortex of subjects with schizophrenia. Regional and diagnostic specificity. Arch Gen Psychiatry. 1997;54(7):660–669. doi: 10.1001/archpsyc.1997.01830190088009. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Goodman JM, Seidman LJ, Kennedy DN, Makris N, Lee H, Tourville J, Caviness VS, Faraone SV, Tsuang MT. Cortical abnormalities in schizophrenia identified by structural magnetic resonance imaging. Arch Gen Psychiatry. 1999;56(6):537–547. doi: 10.1001/archpsyc.56.6.537. [DOI] [PubMed] [Google Scholar]
- Gur RE, Cowell PE, Latshaw A, Turetsky BI, Grossman RI, Arnold SE, Bilker WB, Gur RC. Reduced dorsal and orbital prefrontal gray matter volumes in schizophrenia. Arch Gen Psychiatry. 2000;57(8):761–768. doi: 10.1001/archpsyc.57.8.761. [DOI] [PubMed] [Google Scholar]
- Hafner H. Gender differences in schizophrenia. Psychoneuroendocrinology Suppl. 2003;2:17–54. doi: 10.1016/s0306-4530(02)00125-7. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR, Makino KK, Martin CE, Dickerson F, Boronow J, Fenton WS. Smoking, gender, and dietary influences on erythrocyte essential fatty acid composition among patients with schizophrenia or schizoaffective disorder. Biol Psychiatry. 2003;53(5):431–441. doi: 10.1016/s0006-3223(02)01549-4. [DOI] [PubMed] [Google Scholar]
- Horrobin DF, Manku MS, Hillman H, Iain A, Glen M. Fatty acid levels in the brains of schizophrenics and normal controls. Biol Psychiatry. 1991;30(8):795–805. doi: 10.1016/0006-3223(91)90235-e. [DOI] [PubMed] [Google Scholar]
- Huber TJ, Tettenborn C, Leifke E, Emrich HM. Sex hormones in psychotic men. Psychoneuroendocrinology. 2005;30(1):111–114. doi: 10.1016/j.psyneuen.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Ichiki M, Kunugi H, Takei N, Murray RM, Baba H, Arai H, Oshima I, Okagami K, Sato T, Hirose T, Nanko S. Intra-uterine physical growth in schizophrenia: evidence confirming excess of premature birth. Psychol Med. 2000;30:597–604. doi: 10.1017/s003329179900210x. [DOI] [PubMed] [Google Scholar]
- Jope RS, Song L, Grimes CA, Pacheco MA, Dilley GE, Li X, Meltzer HY, Overholser JC, Stockmeier CA. Selective increases in phosphoinositide signaling activity and G protein levels in postmortem brain from subjects with schizophrenia or alcohol dependence. J Neurochem. 1998;70(2):763–771. doi: 10.1046/j.1471-4159.1998.70020763.x. [DOI] [PubMed] [Google Scholar]
- Karson CN, Mrak RE, Schluterman KO, Sturner WQ, Sheng JG, Griffin WS. Alterations in synaptic proteins and their encoding mRNAs in prefrontal cortex in schizophrenia: a possible neurochemical basis for 'hypofrontality'. Mol Psychiatry. 1999;4(1):39–45. doi: 10.1038/sj.mp.4000459. [DOI] [PubMed] [Google Scholar]
- Khan MM, Evans DR, Gunna V, Scheffer RE, Parikh VV, Mahadik SP. Reduced erythrocyte membrane essential fatty acids and increased lipid peroxides in schizophrenia at the never-medicated first-episode of psychosis and after years of treatment with antipsychotics. Schizophr Res. 2002;58(1):1–10. doi: 10.1016/s0920-9964(01)00334-6. [DOI] [PubMed] [Google Scholar]
- Knable MB, Barci BM, Webster MJ, Meador-Woodruff J, Torrey EF Stanley Neuropathology Consortium. Molecular abnormalities of the hippocampus in severe psychiatric illness: postmortem findings from the Stanley Neuropathology Consortium. Mol Psychiatry. 2004;9(6):609–620. doi: 10.1038/sj.mp.4001471. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72(5):341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6(9):691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kuehner C. Gender differences in unipolar depression: an update of epidemiological findings and possible explanations. Acta Psychiatr Scand. 2003;108:163–74. doi: 10.1034/j.1600-0447.2003.00204.x. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams SC, van der Kouwe AJ, Salat DH, Dale AM, Fischl B. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60(9):878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Landen M, Davidsson P, Gottfries CG, Mansson JE, Blennow K. Reduction of the synaptophysin level but normal levels of glycerophospholipids in the gyrus cinguli in schizophrenia. Schizophr Res. 2002;55(1–2):83–88. doi: 10.1016/s0920-9964(01)00197-9. [DOI] [PubMed] [Google Scholar]
- Lasch J, Willhardt I, Kinder D, Sauer H, Smesny S. Fluorometric assays of phospholipase A2 activity with three different substrates in biological samples of patients with schizophrenia. Clin Chem Lab Med. 2003;41(7):908–914. doi: 10.1515/CCLM.2003.138. [DOI] [PubMed] [Google Scholar]
- Leng GC, Horrobin DF, Fowkes FG, Smith FB, Lowe GD, Donnan PT, Ells K. Plasma essential fatty acids, cigarette smoking, and dietary antioxidants in peripheral arterial disease. A population-based case-control study. Arterioscler Thromb. 1994;14(3):471–478. doi: 10.1161/01.atv.14.3.471. [DOI] [PubMed] [Google Scholar]
- Levant B, Crane JF, Carlson SE. Sub-chronic antipsychotic drug treatment does not alter brain phospholipid fatty acid composition in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(4):728–732. doi: 10.1016/j.pnpbp.2005.11.034. [DOI] [PubMed] [Google Scholar]
- London ED, Ernst M, Grant S, Bonson K, Weinstein A. Orbitofrontal cortex and human drug abuse: functional imaging. Cereb Cortex. 2000;10(3):334–342. doi: 10.1093/cercor/10.3.334. [DOI] [PubMed] [Google Scholar]
- Lin XH, Kitamura N, Hashimoto T, Shirakawa O, Maeda K. Opposite changes in phosphoinositide-specific phospholipase C immunoreactivity in the left prefrontal and superior temporal cortex of patients with chronic schizophrenia. Biol Psychiatry. 1999;46(12):1665–1671. doi: 10.1016/s0006-3223(99)00036-0. [DOI] [PubMed] [Google Scholar]
- McCloughen A. The association between schizophrenia and cigarette smoking: a review of the literature and implications for mental health nursing practice. Int J Ment Health Nurs. 2003;12(2):119–129. doi: 10.1046/j.1440-0979.2003.00278.x. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Carlson SE. Role of omega-3 fatty acids in brain development and function: Potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot Essent Fatty Acids. 2006;74(4–5):329–349. doi: 10.1016/j.plefa.2006.07.010. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Hahn C-G, Jandacek R, Rider T, Tso P, Stanford K, Richtand NM. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol Psychiatry. 2006a doi: 10.1016/j.biopsych.2006.08.026. (in press) [DOI] [PubMed] [Google Scholar]
- McNamara RK, Ostrander M, Abplanalp W, Richtand NM, Benoit S, Clegg D. Modulation of phosphoinositide-protein kinase C signal transduction by omega-3 fatty acids: Implications for the pathophysiology and treatment of recurrent neuropsychiatric illness. Prostaglandins Leukot Essent Fatty Acids. 2006b;74(4–5):237–257. doi: 10.1016/j.plefa.2006.07.009. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Richtand NM, Levant B. Omega-3 fatty acid deficiency decreases dopamine D2 receptor binding and increases serotonin 5-HT2A receptor binding in the adult rat prefrontal cortex. Biol Psychiatry. 2006c;59:S146. [Google Scholar]
- Meador-Woodruff JH, Haroutunian V, Powchik P, Davidson M, Davis KL, Watson SJ. Dopamine receptor transcript expression in striatum and prefrontal and occipital cortex. Focal abnormalities in orbitofrontal cortex in schizophrenia. Arch Gen Psychiatry. 54(12):1089–1095. doi: 10.1001/archpsyc.1997.01830240045007. [DOI] [PubMed] [Google Scholar]
- Mellor JE, Laugharne JD, Peet M. Schizophrenic symptoms and dietary intake of n-3 fatty acids. Schizophr Res. 1995;18(1):85–86. doi: 10.1016/0920-9964(95)00084-4. [DOI] [PubMed] [Google Scholar]
- Metcalfe LD, Schmitz AA, Pelka JR. Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Anal Chem. 1966;38:514–515. [Google Scholar]
- Myers CS, Contreras MA, Chang MC, Rapoport SI, Appel NM. Haloperidol downregulates phospholipase A(2) signaling in rat basal ganglia circuits. Brain Res. 2001;896(1–2):96–101. doi: 10.1016/s0006-8993(01)02014-5. [DOI] [PubMed] [Google Scholar]
- Nilsson CL, Hellstrand M, Ekman A, Eriksson E. Direct dopamine D2-receptor-mediated modulation of arachidonic acid release in transfected CHO cells without the concomitant administration of a Ca2+-mobilizing agent. Br J Pharmacol. 1998;124(8):1651–1658. doi: 10.1038/sj.bjp.0702025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noponen M, Sanfilipo M, Samanich K, Ryer H, Ko G, Angrist B, Wolkin A, Duncan E, Rotrosen J. Elevated PLA2 activity in schizophrenics and other psychiatric patients. Biol Psychiatry. 1993;34(9):641–649. doi: 10.1016/0006-3223(93)90157-9. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, Yung AR, Bullmore ET, Brewer W, Soulsby B, Desmond P, McGuire PK. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361(9354):281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Parikh V, Khan MM, Mahadik SP. Differential effects of antipsychotics on expression of antioxidant enzymes and membrane lipid peroxidation in rat brain. J Psychiatr Res. 2003;37(1):43–51. doi: 10.1016/s0022-3956(02)00048-1. [DOI] [PubMed] [Google Scholar]
- Pawlosky RJ, Bacher J, Salem N. Ethanol consumption alters electroretinograms and depletes neural tissues of docosahexaenoic acid in rhesus monkeys: nutritional consequences of a low n-3 fatty acid diet. Alcohol Clin Exp Res. 2001;25:1758–1765. [PubMed] [Google Scholar]
- Peet M, Brind J, Ramchand CN, Shah S, Vankar GK. Two double-blind placebo-controlled pilot studies of eicosapentaenoic acid in the treatment of schizophrenia. Schizophr Res. 2001;49(3):243–251. doi: 10.1016/s0920-9964(00)00083-9. [DOI] [PubMed] [Google Scholar]
- Peet M, Horrobin DF E-E Multicentre Study Group. A dose-ranging exploratory study of the effects of ethyl-eicosapentaenoate in patients with persistent schizophrenic symptoms. J Psychiatr Res. 2002;36(1):7–18. doi: 10.1016/s0022-3956(01)00048-6. [DOI] [PubMed] [Google Scholar]
- Peet M. Nutrition and schizophrenia: an epidemiological and clinical perspective. Nutr Health. 2003;17(3):211–219. doi: 10.1177/026010600301700304. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC, Katz KH, Westerveld M, Sparrow S, Anderson AW, Duncan CC, Makuch RW, Gore JC, Ment LR. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284:1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- Piomelli D, Pilon C, Giros B, Sokoloff P, Martres MP, Schwartz JC. Dopamine activation of the arachidonic acid cascade as a basis for D1/D2 receptor synergism. Nature. 1991;353(6340):164–167. doi: 10.1038/353164a0. [DOI] [PubMed] [Google Scholar]
- Qu Y, Chang L, Klaff J, Balbo A, Rapoport SI. Imaging brain phospholipase A2 activation in awake rats in response to the 5-HT2A/2C agonist (+/−)2,5-dimethoxy-4-iodophenyl-2-aminopropane (DOI) Neuropsychopharmacology. 2003;28(2):244–252. doi: 10.1038/sj.npp.1300022. [DOI] [PubMed] [Google Scholar]
- Rao JS, Ertley RN, Demar JC, Rapoport SI, Bazinet RP, Lee HJ. Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Mol Psychiatry. 2006 doi: 10.1038/sj.mp.4001887. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Reddy RD, Keshavan MS, Yao JK. Reduced red blood cell membrane essential polyunsaturated fatty acids in first episode schizophrenia at neuroleptic-naive baseline. Schizophr Bull. 2004;30(4):901–911. doi: 10.1093/oxfordjournals.schbul.a007140. [DOI] [PubMed] [Google Scholar]
- Remmers AE, Nordby GL, Medzihradsky F. Modulation of opioid receptor binding by cis and trans fatty acids. J Neurochem. 1990;55(6):1993–2000. doi: 10.1111/j.1471-4159.1990.tb05787.x. [DOI] [PubMed] [Google Scholar]
- Ross BM, Hudson C, Erlich J, Warsh JJ, Kish SJ. Increased phospholipid breakdown in schizophrenia. Evidence for the involvement of a calcium-independent phospholipase A2. Arch Gen Psychiatry. 1997;54(5):487–494. doi: 10.1001/archpsyc.1997.01830170113015. [DOI] [PubMed] [Google Scholar]
- Ross BM, Turenne S, Moszczynska A, Warsh JJ, Kish SJ. Differential alteration of phospholipase A2 activities in brain of patients with schizophrenia. Brain Res. 1999;821(2):407–413. doi: 10.1016/s0006-8993(99)01123-3. [DOI] [PubMed] [Google Scholar]
- Smesny S, Kinder D, Willhardt I, Rosburg T, Lasch J, Berger G, Sauer H. Increased calcium-independent phospholipase A2 activity in first but not in multiepisode chronic schizophrenia. Biol Psychiatry. 2005;57(4):399–405. doi: 10.1016/j.biopsych.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Manabe S, Wada O, Crawford MA. Rapid incorporation of docosahexaenoic acid from dietary sources into brain microsomal, synaptosomal and mitochondrial membranes in adult mice. Int J Vitam Nutr Res. 1997;67(4):272–278. [PubMed] [Google Scholar]
- Tisserand DJ, Pruessner JC, Sanz Arigita EJ, van Boxtel MP, Evans AC, Jolles J, Uylings HB. Regional frontal cortical volumes decrease differentially in aging: an MRI study to compare volumetric approaches and voxel-based morphometry. Neuroimage. 2002;17:657–669. [PubMed] [Google Scholar]
- Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knab MB. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry. 2005;57(3):252–260. doi: 10.1016/j.biopsych.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Webster M, Knable M, Johnston N, Yolken RH. The Stanley Foundation Brain collection and Neuropathology Consortium. Schizophr Res. 2000;44(2):151–155. doi: 10.1016/S0920-9964(99)00192-9. [DOI] [PubMed] [Google Scholar]
- Trzeciak HI, Kalacinski W, Malecki A, Kokot D. Effect of neuroleptics on phospholipase A2 activity in the brain of rats. Eur Arch Psychiatry Clin Neurosci. 1995;245(3):179–182. doi: 10.1007/BF02193092. [DOI] [PubMed] [Google Scholar]
- Yao JK, Leonard S, Reddy RD. Membrane phospholipid abnormalities in postmortem brains from schizophrenic patients. Schizophr Res. 2000;42(1):7–17. doi: 10.1016/s0920-9964(99)00095-x. [DOI] [PubMed] [Google Scholar]


