Abstract
Polyethyleneimine (PEI) has been used previously as a nonviral DNA transfer vector. In this article, we demonstrate its use as a vehicle for transmembrane delivery of proteins in cell culture conditions. Linking proteins to PEI required no other treatment beyond mixing them with PEI. The bond between PEI and protein combined at optimal ratios was maintained in electrophoresis, even in the presence of 2.5% sodium dodecyl sulfate (SDS). The optimal time for delivery of proteins was determined to be 24 h. We have successfully delivered an Alexa 488-labeled avidin protein into human glioblastoma cells. A functional antibody against the nuclear protein lamin was delivered into human fibroblasts and reacted with lamin inside live cells. PEI-based delivery of antibodies and fluorescently labeled proteins can be used for fluorescent detection, tracking, and evaluation of cellular protein function in vivo.
Keywords: Polyethyleneimine, PEI, Protein delivery, Avidin, Alexa–avidin conjugates, Lamin, Glial cells, Fibroblasts, Antibody delivery, Protein carrier, Fluorescent detection
Although many carriers have been developed for the transmembrane delivery of nucleic acids, there are very few protein delivery vectors. Currently available strategies are time-consuming, require crosslinking to the target peptide, or are expensive [1–3].
The ability to cross biological membranes independently of specific receptors was initially demonstrated for a number of small peptides called protein transduction domains (PTDs).1 Several peptides, such as the third alpha-helix of Antennapedia homeodomain [4], TAT protein from human immunodeficiency virus (HIV-1) [3], and VP22 protein from herpes simplex virus [5], have been shown to efficiently cross biological membranes and can serve as transporters of other peptides into cells. However, these PTD-based vectors deliver proteins that must be covalently linked to the carrier.
Pep-1, a new protein delivery vehicle based on a short amphipathic peptide carrier, was introduced recently [1]. It does not require covalent linkage of the vector to the delivered protein, but its commercially available version (Chariot transfection reagent) is expensive. More important, the described protein delivery vectors are themselves peptides and, therefore, can induce or increase antigen responses.
The advent of proteomics and protein therapy has created a need for efficient and inexpensive approaches for protein transportation into cells. In vivo delivery of antibodies and fluorescently labeled proteins, such as avidin, used in immunohistochemistry offers an additional advantage in that it permits fluorescent labeling of intracellular peptides and direct observation of their interactions. In this study, we report the successful application of a known non-peptide-based DNA transfer agent, polyethyleneimine (PEI), for transmembrane delivery of a functional antibody against the nuclear protein lamin and of a fluorescently labeled protein, avidin, into human cells. We expand the utility of PEI and report its successful use as a protein delivery vehicle in cell cultures of human fibroblasts and glioma cells. A previously unreported property of PEI, namely that unlabeled PEI can be observed and analyzed using agarose gels and UV illumination, is described as well. This permits rapid assessment of various PEI/protein preparations.
PEI is an inexpensive and efficient DNA transfection vehicle that, until now, has been used exclusively for the delivery of nucleic acids. It demonstrates very high transfection efficiencies in various cell cultures and in vivo gene transfer [6–8]. The PEI polymer comes in two forms: linear and branched. The branched form was used in these experiments because it is the standard form used for cell transfection [6].
The work presented here demonstrates that PEI is an efficient and cost-effective vehicle for transmembrane delivery of antibodies and fluorescently labeled proteins into human fibroblasts and glial cells.
Materials and methods
Creation complexes of avidin–Alexa 488 with PEI
PEI was diluted in water to make a stock solution of 10 mg/ml and then was mixed with avidin–Alexa 488 solution (Molecular Probes) at ratios ranging from 10,000:1 to 1:5 (PEI/avidin). No other treatment was necessary to link PEI to the protein.
Creation complexes of anti-lamin antibody with PEI
The monoclonal anti-lamin antibody was used in experiments (cat. no. sc-7292, Santa Cruz). The antibody reacts with lamin A and lamin C of human and porcine origin with signal localization in the nuclear envelope area. PEI was diluted in water to make a stock solution of 10 mg/ml and then was mixed with anti-lamin antibody solution (Santa Cruz) at ratios ranging from 1:3 to 3:1 (PEI/antibody). No other treatment was necessary to link PEI to the antibody.
Gel electrophoresis
PEI/avidin–Alexa 488 complexes (10 μl) prepared at ratios ranging from 10,000:1 to 1:5 (PEI/avidin) were loaded on 1% agarose gels and run for 1 h at 72 V. PEI was observed using a transilluminator with 312 nm UV light illumination. Gel images were taken using a digital camera and were processed in MetaMorph 6.0 (Princeton Scientific). In some of the series, sodium dodecyl sulfate (SDS) was added to the particle preparations at a 2.5% final concentration prior to loading onto the gel.
Anti-lamin antibody detection in agarose gels
The monoclonal anti-lamin antibody (5 μl, cat. no. sc-7292, Santa Cruz) and its complexes with PEI were loaded onto 1% agarose gels. The gels were run for 20 min at 100 V, rinsed with phosphate-buffered saline (PBS), and then soaked in 1:200 solution of secondary biotinylated goat anti-mouse antibody (cat. no. 553999, BD PharMingen) in PBS for 30 min, rinsed, and incubated in 1:500 solution of streptavidin–fluorescein isothiocyanate (FITC) in PBS for 20 min.
Avidin–Alexa 488 delivery into human U87 cells
U87 human glioblastoma cells were obtained from American Type Culture Collection (ATCC) and were cultured according to ATCC recommendations. The line was derived from tissue removed from a patient undergoing operation for glioblastoma. As recommended by ATCC, the cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and pH adjusted to 7.35 prior to filtration. For our experiments, all cells were subcultured in 24-well cell culture plates (Fisher). PEI/avidin complexes were added directly into the wells of the 24-well cell culture plates. Cells were incubated with the complexes for 0 h (washed within 5 min after the addition of PEI particles), 2, 4, and 24h and then were washed 2 × 5 min in PBS and taken for microscopy or processed for fluorescence measurements.
Microscopy of U87 cell cultures and image acquisition
Microscopy of U87 cells was performed directly in cell culture plates using an Olympus IX-70 fluorescent microscope. Consecutive nonfluorescent and fluorescent images of live cell cultures were captured by a MicroMax digital video camera (Princeton Instruments), attached to the microscope, using both visible light illumination and fluorescence. For Alexa 488 fluorescence detection, a Chroma Technology bandpass filter set was used (excitation D490/40, emission 520/10). Images were superimposed in MetaMorph 6.1 (Advanced Scientific), and composite images were created in Adobe Photoshop 6.0.
Fluorescence measurements in U87 cells using microplate reader
Fluorescence of Alexa 488 delivered by PEI particles was measured using a GENios Plus Multi-Detection Microplate Reader with Enhanced Fluorescence (Tecan). Cells were fixed in 100% methanol, and Alexa fluorescence was measured directly in 24-well plates using a Chroma Technology bandpass filter set (excitation D490/40, emission 520/10). The reactions were repeated three times. The results were analyzed using Microsoft Excel and were represented as graphs of average values and standard errors. In additional controls, avidin–Alexa 488 was added to cell cultures without PEI. Fluorescent signal intensity in controls did not differ from the signal in untreated cell cultures where no avidin–Alexa 488 was used.
Anti-lamin antibody detection in cell cultures of human fibroblasts
Normal neonatal human foreskin HCA 2 fibroblasts (PD32) were obtained from the laboratory of Olivia Smith-Pereira. The cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum medium and pH adjusted to 7.4 prior to filtration. For the experiments, all cells were subcultured in 24-well cell culture plates.
After incubation with PEI/anti-lamin antibody complexes for 2, 4, and 24 h, the cells were fixed in methanol and anti-lamin antibody detection was performed using a secondary biotinylated goat anti-mouse antibody (1:25) for 30 min, with subsequent incubation with streptavidin–FITC conjugates (1:500, Molecular Probes) for 45 min. Then the cells were rinsed with sodium bicarbonate and covered with Vectashield with DAPI (Vector Laboratories). Fluorescent images were captured by a MicroMax digital video camera, attached to the microscope, using both visible light illumination and fluorescence. For fluorescence detection, a Chroma Technology bandpass filter set was used (FITC excitation D490/40, emission 520/10; DAPI excitation D360/ 40, emission 460/20). Images were superimposed in MetaMorph 6.1, and composite images were created in Adobe Photoshop 6.0.
Results and discussion
In the first set of experiments, we tested PEI-based intracellular delivery of avidin conjugated to Alexa 488 fluorophore (green fluorescence). Avidin is widely used in a variety of labeling schemes to detect biotin-labeled biomolecules. It is a highly cationic, 66,000-Da glycoprotein with an isoelectric point of approximately 10.5 [9]. We were interested in seeing whether a cationic protein could be transported by a cationic carrier, namely PEI. However, we found that although avidin per se was cationic, its conjugate with Alexa 488 had anionic properties, likely due to the charge of the fluorophore. Avidin was selected because if the experiments demonstrated a successful transfer of fluorescent conjugates of avidin into live cells, the approach could be used later for visualizing biotin-labeled targets such as DNA and RNA probes inside cells. In addition, various biotin-labeled proteins, such as antibodies, could also be visualized. The choice of the fluorophore was dictated by the fact that Alexa 488 has a superior fluorescence output per protein conjugate, surpassing that of all spectrally similar fluorophores [9]. Its fluorescence spectrum is nearly identical to that of fluorescein, with excitation and emission maxima of 491 and 515 nm, respectively, but Alexa is much more photostable [9].
In the second set of experiments, we used an antibody against lamin to be delivered by PEI. We chose this antibody so as to deliver a protein different from avidin in charge, size, and properties. An important factor in using PEI-based delivery is the preservation of protein function. Maintenance of the correct protein conformation is critical for antibody reaction with its target protein. This ability is easily lost if an antibody is denatured, is damaged by proteases, or loses its active conformation. To test the ability of a protein to maintain its proper conformation and activity, after delivery using PEI, we chose an antibody with characteristic target localization on the nuclear envelope. In addition to the fact that nuclear lamins are critical in maintaining the integrity and function of the nuclear envelope and cellular morphology in general, we could easily observe the morphological consequences of their reaction with antibody. Nuclear localization of antibody signal, as opposed to generalized cytoplasmic labeling, would indicate an active antibody that reacted with its target protein on delivery.
Electrophoresis-based testing of PEI and its complexes with fluorescent avidin and anti-lamin antibody
We found that unlabeled PEI can be observed in agarose gels under UV illumination as a weakly fluorescent, light blue smear. Being strongly cationic, PEI moves to the negative electrode (Fig. 1A). No additional staining was necessary to visualize PEI. When illuminated by a 312-nm UV light, 50 μg of PEI could be observed on a regular 1% agarose gel. Because this technique permits reliable and rapid separation of avidin–Alexa conjugates linked with PEI from the free conjugates, which are anionic, we used agarose electrophoresis as a new approach for the evaluation of PEI/protein preparations.
Fig. 1.
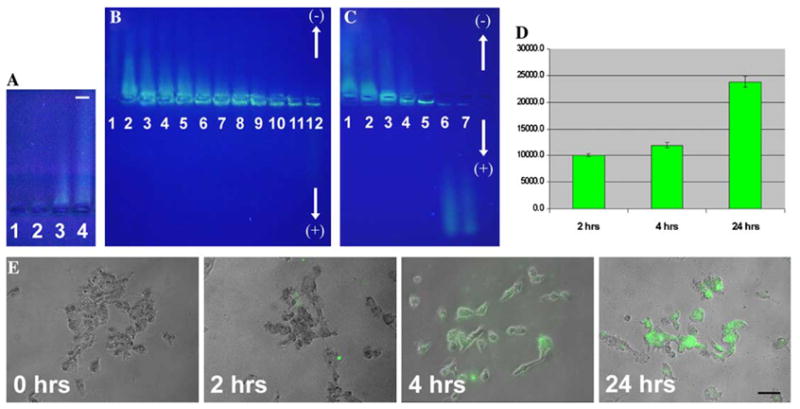
Testing PEI complexes with avidin–Alexa 488 in agarose gels and in live cells. (A) Visualization of unlabeled PEI in agarose gels by UV illumination. Unlabeled PEI can be observed in agarose gels under UV illumination (UV wavelength = 312 nm). It moves to the negative electrode (50 μg PEI can be easily detected). Lane 1, 1 μg PEI; lane 2, 10 μg PEI; lane 3, 50 μg PEI; lane 4, 100 μg PEI. (B) Agarose gel electrophoresis of PEI complexes with avidin–Alexa 488: Optimization of PEI/avidin–Alexa ratios. Ratios (w/w) of PEI/protein tested were as follows: 10,000:1 (lane 2), 8000:1 (lane 3), 6000:1 (lane 4), 4000:1 (lane 5), 2000:1 (lane 6), 1000:1 (lane 7), 500:1 (lane 8), 100:1 (lane 9), 20:1 (lane 10), 1:1 (lane 11), and 1:5 (lane 12). Lane 1 shows unlabeled PEI. Increasing the PEI in the ratio results in stronger compaction of complexed protein and its better penetration into the gel. The optimal ratio of 2000:1 permits retention of all protein using minimal amounts of PEI. (C) Agarose gel electrophoresis of PEI and its complexes with fluorescent avidin using 2.5% SDS pretreatment. Ratios (w/w) of PEI/protein tested were as follows: 4000:1 (lane 1), 2000:1 (lane 2), 1000:1 (lane 3), 500:1 (lane 4), 100:1 (lane 5), 20:1 (lane 6), and 1:1 (lane 7). The complexes at the low PEI/protein ratios were disrupted by SDS and released the captured protein, which moved in the direction opposite to that of the PEI (lanes 6 and 7). (D) Detection of intracellular avidin–Alexa 488 delivered into glioma U87 cells with the help of PEI. A 2000:1 ratio of PEI/avidin–Alexa 488 was used. The most significant increase of fluorescence was observed after 24 h of incubation with PEI. A Chroma Technology bandpass filter set was used (excitation D490/40, emission 520/10). The reactions were repeated three times, and averages and standard errors are shown. The y axis shows arbitrary GENios Plus readings of fluorescence. (E) Delivery of fluorescent avidin into glioma U-87 cells. The earliest fluorescent signal was seen 2 h after the addition of PEI particles carrying Alexa 488-labeled avidin (green fluorescence). At 4 and 24 h, all cells in the field of view were labeled, demonstrating an efficient transmembrane delivery of the fluorescently labeled protein. Bar = 50 μm.
We combined avidin–Alexa 488 conjugates with PEI at different ratios and subsequently evaluated them using agarose gels. In these experiments, PEI/avidin–Alexa ratios ranged from 10,000:1 to 1:5. In preliminary studies, we found that avidin–Alexa 488 conjugates without PEI could be easily observed in agarose gels and moved toward the positive electrode (data not shown). The final charge of the complexes of avidin–Alexa with PEI depended on the PEI/avidin ratio, so that at the 20:1, 1:1, and 1:5 ratios the complexes stayed in the wells and did not have sufficient charge to enter the gel (Fig. 1B, lanes 10–12). Increasing the ratio from 100:1 to 10,000:1 resulted in an increase of the positive charge of the complexes and their subsequent movement to the cathode (Fig. 1B, lanes 2–9).
To establish the most stable preparation, we increased the stringency of electrophoretic testing by adding 2.5% SDS to the PEI/avidin complexes prior to loading them onto gels. SDS is a strong chaotropic agent with the capability to completely eliminate many types of weak bonds. The addition of SDS destabilized the complexes, so that at the lower ratios of PEI/avidin, such as 1:5 and 1:1, avidin–Alexa conjugates were able to escape and move to the anode (Fig. 1C, lanes 6 and 7). The PEI/avidin–Alexa ratios of 4000:1 and 2000:1 demonstrated sufficient stability, maintaining a strong positive charge necessary for transporting maximal amounts of avidin–Alexa into the gel (Fig. 1C, lanes 1 and 2). Theoretically, these stable complexes with a strong positive charge should have high efficiency of transfection. We chose the smallest of these ratios (2000:1) for experiments with live cells to reduce the possible toxic effects of PEI.
Anti-lamin antibody was tested in agarose gels before and after mixing with PEI. The antibody was detected by gel incubation with a secondary biotinylated antibody and subsequent signal visualization by streptavidin–FITC. The antibody, per se showed anionic properties by moving to the anode in the direction opposite that of PEI. When PEI was added, the antibody/PEI complex moved in the same direction as PEI due to formation of noncovalent complexes with PEI (data not shown). PEI linked much more efficiently with the antibody, as compared with avidin, likely due to the opposing charges. Bonds between a protein and PEI are noncovalent and are maintained due to charge interactions; thus, anionic proteins carrying multiple negative charges are generally expected to have higher protein/PEI ratios in the complexes. Because of this, they are likely to be transferred through cellular membranes more efficiently and to have stronger bonds with PEI inside cells as compared with the proteins carrying less negative charges.
For that reason, in the case of anti-lamin antibody and unlike the case of avidin, gel electrophoresis testing of different ratios was not useful because the much stronger PEI/antibody link could not be disrupted by our electrophoretic interventions. Instead, we determined the optimal PEI/antibody ratio by using cell cultures.
Cell culture testing of fluorescently labeled avidin delivery
We used a 2000:1 ratio of PEI/avidin–Alexa when preparing complexes for delivery into human glioma cells in culture. The complex was added to glioma cells grown in microplates. It was determined elsewhere that PEI attaches to the plasma membrane within 30 min of administration and enters the cell by endocytosis within 2–3 h [7,10]. We evaluated the results of protein delivery at 2, 4, and 24 h after the addition of the PEI particles (Figs. 1D and E). The fluorescence intensity of intracellular Alexa 488 was measured in three independent experiments by a microplate reader after the cell cultures were washed to eliminate all extracellular avidin–Alexa PEI particles. The fluorescence intensities, measured in relative fluorescence units (RFU), were as follows: 0 h, 6464 ± 346; 2 h, 10,039 ± 235; 4 h, 11,939 ± 585; 24 h, 23,822 ± 1377. Therefore, successful transmembrane delivery of the protein was observed at the earliest time tested, namely 2 h. By 4 h, the intracellular fluorescence of Alexa increased 1.8 times compared with 0 h, and after 24 h it increased 3.7 times compared with 0 h (Fig. 1D). Fluorescent conjugates of avidin–Alexa 488 were localized uniformly inside every cell in the field of view (Fig. 1E). No signs of toxicity were observed at the concentrations of PEI used.
These experiments demonstrated that PEI particles carrying fluorescently labeled protein are transported efficiently through cellular membranes.
Cell culture testing of anti-lamin antibody delivery
We tested PEI-based intracellular delivery of anti-lamin antibody into human fibroblasts using various antibody/PEI ratios ranging from 1:15 to 2:1. In our initial work, we found that PEI concentrations 0.5–5 μg/ml did not produce any toxic effects in cultured human fibroblasts (data not shown). Therefore, we used a concentration of PEI in the middle of this range, 3 μg/ml, for the antibody delivery. Increasing amounts of antibody corresponding to the ratios of 1:15, 1:3, 2:3, and 4:3 were added to cells combined with the same amount of PEI to achieve a final PEI concentration of 3 μg/ml in cell culture media. Cells were fixed after 2, 4, and 24 h, and antibody detection was performed using a secondary biotinylated antibody and streptavidin–FITC conjugates.
We found that a 2:3 antibody/PEI ratio produced the best results. In this case, the first weak signal was detected after 4 h of incubation (data not shown) and a much stronger signal was observed after 24 h (Figs. 2C and D). The antibody was detected in its characteristic location inside the nuclear envelope (Figs. 2C and D). Staining patterns were the same as those achieved by using conventional labeling approaches [11]. Interestingly, the cells with strong antibody labeling showed changes in their morphology such as formation of micronuclei (Fig. 2C). Similar changes were described in cells with antisense-suppressed synthesis of lamins [12]. These changes were not observed in the avidin–Alexa protein delivery (Fig. 1E) or when either PEI or anti-lamin antibody alone was added into cell cultures (Figs. 2A and B). Therefore, we concluded that the morphological changes were the result of the direct antibody labeling of lamins on the nuclear membrane by PEI-based transmembrane delivery. We believe that the micronucleation resulted from a partial blockage of the lamin function due to the reaction with the antibody. This property of the antibody has not been reported previously and is potentially useful for its application in blocking lamin function.
Fig. 2.
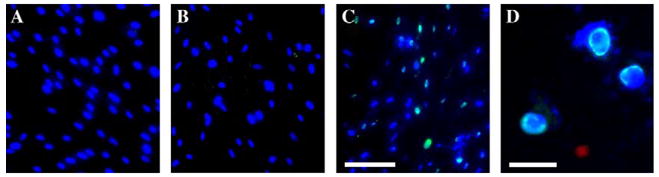
PEI-based delivery of anti-lamin antibody into cultured human fibroblasts. Fibroblast nuclei in cell culture were visualized using DAPI (blue fluorescence). Antibody to lamin was detected using secondary biotinylated antibody and streptavidin–FITC (green fluorescence). (A) Incubation (24 h) with antibody to lamin without PEI. (B) Incubation (24 h) with PEI (3 μg/ml) without antibody. (C) Incubation (24 h) with PEI–antibody complexes. Images show delivery of anti-lamin antibody by PEI into human fibroblasts. Note antibody signal detected exclusively in the area of lamin localization in cell nuclei. Only part of cells (~one-fourth of all cell nuclei) are stained, and the signal is of uneven intensity, indicating different numbers of antibody molecules per cell. Bar = 50 μm. (D) High-magnification image of cells after 24 h incubation with PEI–antibody complexes. Note precise antibody staining of the nuclear envelope. Bar = 20 μm.
In conclusion, PEI represents an efficient, inexpensive, and cost-effective system for transportation of fluorescently labeled protein molecules through cellular membranes in cell culture conditions and can be a valuable tool for in vivo visualization of protein interactions.
Acknowledgments
We thank Benxiao Zhang for valuable technical assistance with these experiments. This research was supported by grants from the DeBakey Medical Foundation (V.V. Didenko), the Baylor College of Medicine (V.V. Didenko), the Texas Higher Education Coordinating Board (D.S. Baskin and V.V. Didenko), the Taub Foundation (D.S. Baskin), and the Henry J.N. Taub Fund for Neurosurgical Research (D.S . Baskin).
Abbreviations used
- PTD
protein transduction domain
- HIV
human immunodeficiency virus
- PEI
polyethyleneimine
- SDS
sodium dodecyl sulfate
- PBS
phosphate-buffered saline
- FITC
fluorescein isothiocyanate
- ATCC
American Type Culture Collection
- RFU
relative fluorescence units
References
- 1.Morris MC, Depollier J, Mery J, Heitz F, Divita G. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat Biotechnol. 2001;19:1173–1176. doi: 10.1038/nbt1201-1173. [DOI] [PubMed] [Google Scholar]
- 2.Schwarze SR, Dowdy SF. In vivo protein transduction: intracellular delivery of biologically active proteins, compounds, and DNA. Trends Pharmacol Sci. 2000;21:45–48. doi: 10.1016/s0165-6147(99)01429-7. [DOI] [PubMed] [Google Scholar]
- 3.Fawell S, Seery J, Daikh Y, Moore C, Chen LL, Pepinsky B, Barsoum J. Tat-mediated delivery of heterologous proteins into cells. Proc Natl Acad Sci USA. 1994;91:664–668. doi: 10.1073/pnas.91.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Derossi D, Joliot AH, Chassaings G, Prochiantz A. The third helix of the antennapedia homeodomain translocates through biological membranes. J Biol Chem. 1994;269:10444–10450. [PubMed] [Google Scholar]
- 5.Elliott G, O’Hare P. Intercellular trafficking and protein delivery by a Herpes virus structural protein. Cell. 1997;88:223–233. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- 6.Lemkine GF, Demeneix BA. Polyethylenimines for in vivo gene delivery. Curr Opin Mol Ther. 2001;3:178–182. [PubMed] [Google Scholar]
- 7.Godbey WT, Wu KK, Mikos AG. Poly(ethylenimine) and its role in gene delivery. J Control Release. 1999;60:149–160. doi: 10.1016/s0168-3659(99)00090-5. [DOI] [PubMed] [Google Scholar]
- 8.Schatzlein AG. Non-viral vectors in cancer gene therapy principles and progress. Anticancer Drugs. 2001;12:275–304. doi: 10.1097/00001813-200104000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Haugland RP. Handbook of Fluorescent Probes and Research Products. Molecular Probes; Eugene, OR: 2002. [Google Scholar]
- 10.Godbey WT, Wu KK, Mikos AG. Tracking the intracellular path of poly(ethylenimine)/DNA complexes for gene delivery. Proc Natl Acad Sci USA. 1999;96:5177–5181. doi: 10.1073/pnas.96.9.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerace L, Blum A, Blobel G. Immunocytochemical localization of the major polypeptides of the nuclear pore complex–lamina fraction: interphase and mitotic distribution. J Cell Biol. 1978;79:546–566. doi: 10.1083/jcb.79.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harborth J, Elbashir SM, Bechert K, Tuschl T, Weber K. Identification of essential genes in cultured mammalian cells using small interfering RNAs. J Cell Sci. 2001;114:4557–4565. doi: 10.1242/jcs.114.24.4557. [DOI] [PubMed] [Google Scholar]


