Abstract
The LHX3 and LHX4 LIM-homeodomain transcription factors play essential roles in pituitary gland and nervous system development. Mutations in the genes encoding these regulatory proteins are associated with combined hormone deficiency diseases in humans and animal models. Patients with these diseases have complex syndromes involving short stature, and reproductive and metabolic disorders. Analyses of the features of these diseases and the biochemical properties of the LHX3 and LHX4 proteins will facilitate a better understanding of the molecular pathways that regulate the development of the specialized hormone-secreting cells of the mammalian anterior pituitary gland.
Keywords: transcription, growth, hormone, anterior pituitary
1. Introduction
Five differentiated cell types in the mammalian anterior pituitary gland secrete polypeptide hormones that regulate an array of developmental and physiological functions (Fig. 1). The cell types (and hormones) are corticotropes (producing adrenocorticotrophic hormone [ACTH] from the proopiomelanocortin gene); gonadotropes (follicle-stimulating hormone [FSH] and luteinizing hormone [LH]); thyrotropes (thyroid-stimulating hormone [TSH]); somatotropes (growth hormone [GH]); and lactotropes (prolactin [PRL]). Hormones produced by the anterior pituitary regulate growth and metabolism (GH and TSH), reproductive development and function (FSH, LH, PRL), thyroid physiology (TSH), lactation (PRL), and the stress response (ACTH). FSH, LH, and TSH are heterodimers of the alpha glycoprotein subunit (αGSU), and a distinct beta subunit (FSHβ, LHβ, TSHβ).
Figure 1.
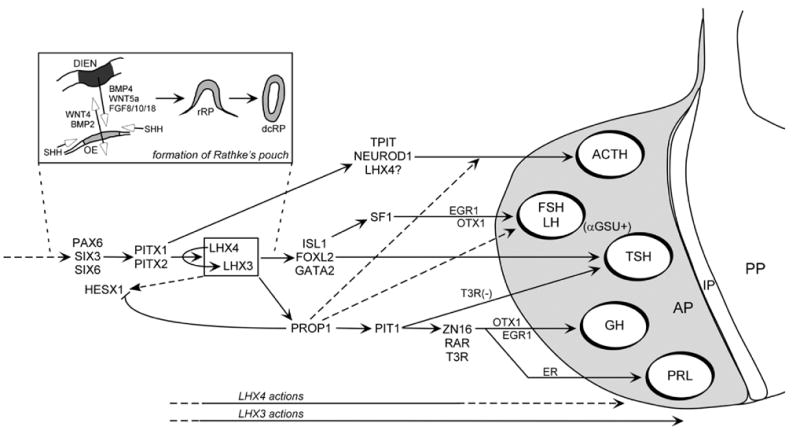
Regulation of anterior pituitary gland development by signaling proteins and transcription factors. Inductive signals between the ventral diencephalon (DIEN) and the oral ectoderm/anterior neural ridge (OE) precede formation of a rudimentary Rathke’s pouch (rRP, the precursor of the adenohypophysis from which the anterior pituitary develops). Subsequently, a definitive, closed Rathke’s pouch (dcRP) forms. The mature pituitary gland has three main components: the anterior pituitary lobe (AP), the intermediate pituitary (IP), and the posterior pituitary (PP).
During development, inductive events between Rathke’s pouch (the primordium of the anterior pituitary lobe) and the diencephalon prime the expression of transcription factors that orchestrate the establishment of the hormone-secreting cells of the anterior pituitary (Fig. 1) (Zhu et al., 2005). Analyses of rodent models with spontaneous and engineered mutations in pituitary transcription factor genes have delineated the roles of many of these genes in development of the gland. In addition, mutations in several of the transcription factor genes have been associated with pediatric hormone deficiency diseases (Dattani, 2005).
LHX3 and LHX4 are LIM-homeodomain (LIM-HD) transcription factors. The LIM domain, a multifunctional protein/protein interaction domain, was first recognized in several other members of this class of transcription factors: LIN11, ISL1, and MEC3 (Hunter and Rhodes, 2005). The large LIM protein superfamily also includes cytoskeletal proteins, signaling cascade transducers, and transcriptional coactivators. In mammals there are at least twelve LIM-HD genes encoding developmental regulatory proteins featuring two LIM domains and a DNA-binding HD (Fig. 2). The functions of LIM-HD proteins are impacted by interacting proteins, such as the NLI/LDB/CLIM, MRG1, SLB, and RLIM proteins (Bach, 2000). Of the mammalian LIM-HD proteins, ISL1, ISL2, LHX2, LHX3, and LHX4 have been implicated in pituitary development.
Figure 2.
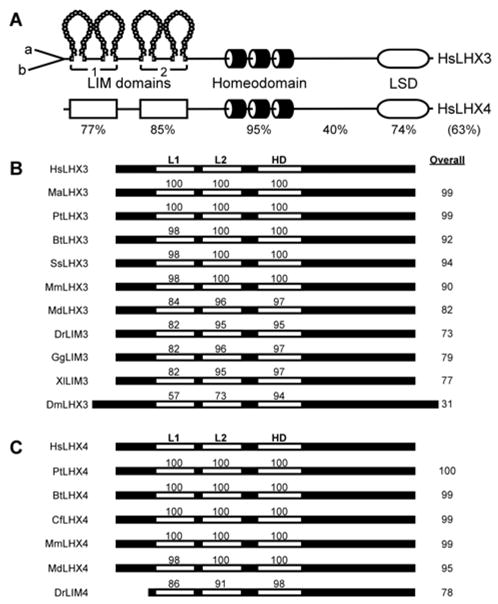
The LHX3/LHX4 LIM-homeodomain protein family. A. The domain structure of human (Hs) LHX3a/b proteins and comparison to human LHX4. Percent identity between key domains is shown and the overall identity is in parentheses. LSD = LIM3/4-specific domain. B. Conservation of LHX3/LIM3-class proteins. The overall structure and percent identity of key domains is shown for human, rhesus macaque (Ma), chimp (Pt), cow (Bt), pig (Ss), mouse (Mm), opossum (Md), zebrafish (Dr), chicken (Gg), African clawed frog (Xl), and fruit fly (Dm). L1, L2 = LIM domains; HD = homeodomain. Protein sequences were retrieved from the GenBank, Swiss-Prot, and TIGR databases. C. Conservation of LHX4 proteins. The overall structure and percent identity of key domains is shown for human, chimp, cow, dog (Cf), mouse, opossum, and zebrafish.
2. LHX3
The human LHX3 gene maps to chromosome 9 and is composed of seven coding exons and six introns that span ~8.5 kilobases (Netchine et al., 2000; Sloop et al., 2000a; Sloop et al., 2000b). Mammalian LHX3 genes produce two major mRNAs known as LHX3a and LHX3b (Zhadanov et al., 1995b; Sloop et al., 1999; Sloop et al., 2000a). Mouse Lhx3a and Lhx3b mRNAs have distinct temporal expression profiles during development and are differentially expressed in cell lines that model the specialized pituitary cell types (Zhadanov et al., 1995a; Sloop et al., 1999; Sloop et al., 2001a; West et al., 2004). During rodent embryogenesis, Lhx3 mRNAs can be detected in the ventral spinal cord, the pons, the medulla oblongata, the pineal gland, the lungs, and in the developing and established anterior/intermediate pituitary gland (Seidah et al., 1994; Bach et al., 1995; Zhadanov et al., 1995a; Meier et al., 1999; Weng et al., 2006). Similar expression patterns are seen during human development (Sloop et al., 1999; Schmitt et al., 2000; Sobrier et al., 2004).
Transcription of mammalian LHX3 genes is mediated by two TATA-less, GC-rich promoters upstream of exons Ia and Ib, and involves specificity protein-1 (Sp1) and nuclear factor I (NFI) actions (Yaden et al., 2006). Fibroblast growth factors, such as FGF8, in the diencephalon and Rathke’s pouch are involved in activation of Lhx3 (Fig. 1), and mice that fail to express FGF8 display a phenotype similar to Lhx3/Lhx4 double knockout mice (Takuma et al., 1998). Studies using cultured pituitary cells and analyses of mice with targeted gene mutations show that the Lhx4, Pitx1, and Pitx2 genes are also involved in activation of Lhx3 (Tremblay et al., 1998; Raetzman et al., 2002; Charles et al., 2005).
LHX3/LIM3/P-Lim proteins are well conserved in evolution and homologs can be found in many species, including birds, fish, amphibians, and insects (Fig. 2B). At least three distinct proteins are produced from mammalian LHX3 mRNAs. The LHX3a and LHX3b isoforms, are identical for most of their sequences (LIMs, HD, and carboxyl) but differ in their distinct amino termini (Fig. 2A) (Zhadanov et al., 1995a; Sloop et al., 1999; Sloop et al., 2001a). A third protein isoform, M2-LHX3, is generated by translation of the second in-frame methionine codon of the LHX3a mRNA (Sloop et al., 2001a). M2-LHX3 lacks the amino terminus and LIM domains found in LHX3a and LHX3b. The three LHX3 isoforms display different biochemical and functional properties (Sloop et al., 1999; Parker et al., 2000; Bridwell et al., 2001; Sloop et al., 2001a; Parker et al., 2005; Yaden et al., 2005).
Molecular studies have demonstrated that LHX3 proteins can bind to regulatory elements in the promoters/enhancers of pituitary genes and cause increased transcription of the FSHβ, αGSU, PRL, TSHβ, gonadotropin releasing hormone receptor, and Pit1 promoters (e.g. Bach et al., 1995; Sloop et al., 1999; West et al., 2004; McGillivray et al., 2005; Granger et al., 2006). Recently, LHX3 has been found to be an upstream activator of FOXL2, a transcription factor with predicted roles in the differentiation of cells expressing αGSU (Ellsworth et al., 2006). For activation of some promoters in the pituitary and nervous system, LHX3 can participate in complex transcriptional interactions with other factors such as PIT1 and ISL1 (Bach et al., 1995; Thaler et al., 2002; Granger et al., 2006).
The phenotype of mice with a homozygous deletion of Lhx3 reflects the importance of the gene in pituitary and nervous system development (Sheng et al., 1996; Sheng et al., 1997; Sharma et al., 1998). In Lhx3 null mice, which die shortly after birth, a definitive Rathke’s pouch forms but fails to develop further and lacks four of the five hormone-secreting cell types, containing only a small population of corticotropes. LHX3, therefore, is critical for both early structural events and for the specification of the lactotrope, somatotrope, gonadotrope, and thyrotrope cell lineages. Rathke’s pouch appears normal in the Lhx3−/− mouse at embryonic day 11.5 (e11.5), but by e12.5, expansion of the pouch is arrested. The posterior lobe is present, however the anterior lobe is missing and the intermediate lobe shows a reduction in size. Lhx3+/− heterozygous mice have sufficient LHX3 for normal specification of the pituitary cell lineages and development. Lhx3Cre/Cre mice have reduced expression of LHX3 in the pituitary, but near normal expression in the developing nervous system (Zhao et al., 2006). In contrast to Lhx3+/− mice, Lhx3Cre/Cre mice display a pituitary phenotype similar to the null mouse. In these mice with reduced LHX3 action there is increased cell apoptosis in the ventral portion of Rathke’s pouch, but similar levels of cell proliferation to wild type animals. Increased apoptosis also is noted in Pitx1/Pitx2 null mice which lack detectable LHX3 expression (Charles et al., 2005).
To date, three types of autosomal recessive mutations of LHX3 have been documented in humans. All characterized patients have combined pituitary hormone deficiency (CPHD) lacking GH, PRL, FSH, LH, and TSH, with normal ACTH levels (Netchine et al., 2000; Bhangoo et al., 2006). This is a similar phenotype to the Lhx3 null mice that lack most hormone-secreting cell types but retain some ACTH-secreting corticotropes. In addition, LHX3 mutation patients have a rigid cervical spine and limited neck rotation presumably related to the role of LHX3 in motoneuron development. Further, as observed for the Lhx3+/− mouse, heterozygous family members are unaffected. The first classes of LHX3 mutations were described in two unrelated consanguineous families (Netchine et al., 2000). One mutation causes a substitution of a tyrosine residue in the highly conserved LIM2 domain with a cysteine; the other mutation involves a homozygous deletion that results in the loss of the HD. Pituitary morphology in these patients was variable with patients displaying hypoplastic or enlarged pituitaries. Molecular analyses of these mutations revealed that the LHX3 proteins encoded by these mutant genes have compromised abilities to trans-activate pituitary hormone gene promoters (Howard and Maurer, 2001; Sloop et al., 2001b). A third human LHX3 mutation involves a single-base pair deletion in exon II producing a truncated protein lacking all functional domains and having no predicted function (Bhangoo et al., 2006). This mutant protein likely is not produced because of nonsense mediated decay of the LHX3 message. In addition to the previously described phenotypes of LHX3 patients, this patient also shows a hypointense pituitary lesion consistent with a microadenoma, and neurological features of mental retardation, speech difficulties, and possible focal amyotrophy. These symptoms widen the phenotype of patients with LHX3 mutation and thus warrant further investigation of the mechanisms of these neurological findings.
3. LHX4
The human LHX4 gene on chromosome 1 has a similar exon/intron organization to LHX3 but longer introns result in a gene length of >45 kilobases (Machinis et al., 2001; Sloop et al., 2001c). In mice, the Lhx4 (or Gsh4) gene is expressed in the developing hindbrain, cerebral cortex, pituitary gland, and spinal cord (Li et al., 1994; Liu et al., 2002). The human LHX4 protein shares >95% identity with orthologs in other mammals demonstrating sequence conservation throughout the protein (Fig. 2C). LHX4 also shows significant similarity to LHX3 (Fig. 2A), consistent with this pair of LIM-HD genes having diverged relatively recently during the evolution of this subclass of HD transcription factors (Hunter and Rhodes, 2005). Like LHX3a, the LHX4 mRNA has an internal methionine codon located at an equivalent position in the LIM2-coding region and has the capacity to produce a short (M2-LHX4) isoform lacking the LIM domains (Sloop et al., 2001a). In gene regulation experiments, the LHX4 protein exhibits similar activities to LHX3a in assays using pituitary hormone promoter reporter genes, consistent with the similar biochemical properties of the proteins (e.g. Sloop et al., 2001a; Kawamata et al., 2002; West et al., 2004).
Although LHX3 and LHX4 share marked similarities in protein structure, the genes have different expression patterns, and their overlapping but distinct roles in development have been revealed by single and combined gene targeting in mice. Mice with a homozygous Lhx4 gene disruption die shortly after birth from lung defects; heterozygous animals are apparently unaffected (Li et al., 1994). In both Lhx3 and Lhx4 single gene knockout animals, a definitive Rathke’s pouch develops, but development of the pituitary arrests in this pouch stage (Sheng et al., 1996; Sheng et al., 1997). Proliferation appears to be impaired and the anterior pituitary is severely hypoplastic (Sheng et al., 1997). However, unlike the Lhx3−/− mice, Rathke’s pouch of Lhx4−/− mice contains all of the differentiated cell types. Further analyses revealed that the hypocellularity of the anterior pituitary in Lhx4 mutants is primarily due to apoptosis of pituitary precursor cells (Raetzman et al., 2002). Further, LHX3 expression is impaired in the Lhx4 mutants and absent in Lhx4/Prop1 double knockouts, indicating that Lhx4 is required for proper expression of LHX3 which is aided by Prop1 (Raetzman et al., 2002). The observation that Lhx4 is required for cell survival and LHX3 expression in Rathke’s pouch indicates that Lhx4 is required for proper expansion of the pouch during development. The Lhx3 and Lhx4 genes have some overlapping roles. For example, Lhx3 and Lhx4 are both required during early stages of pituitary development: in mice lacking both genes Rathke’s pouch does not progress beyond an early rudimentary stage. However, in mice lacking either Lhx3 or Lhx4, Rathke’s pouch is able to progress from its early rudimentary structure to the more definitive pouch, indicating expression of either Lhx3 or Lhx4 is required for the development of a definitive pouch (Fig. 1) (Sheng et al., 1997). Like Lhx3, the Lhx4 gene also is important in ventral motor neuron differentiation (Sharma et al., 1998).
Recent studies of a mouse pituitary cell side population containing pituitary stem/progenitor cells have revealed that these cells express Lhx4. By contrast, Lhx3, which unlike Lhx4 is not down-regulated after anterior pituitary development, is restricted to the main population of pituitary cells, suggesting that the two genes play discrete roles in pituitary stem cell function and gland maintenance (Chen et al., 2005).
Consistent with the importance of Lhx4 for pituitary development in the mouse, a heterozygous mutation in LHX4 has been identified in a family in which affected members presented with CPHD, including deficiencies of GH, TSH, and ACTH (LH and FSH not tested). Magnetic resonance imaging analyses of affected family members revealed a hypoplastic pituitary, small sella turcica, chiari malformation, and an ectopic posterior pituitary (Machinis et al., 2001). The observed LHX4 mutation is a G to C transversion at a splice acceptor site predicted to result in the production of either a protein with deletion of four conserved amino acids within the HD or a truncated protein. The aberrant proteins produced from this mutation in LHX4 are unable to bind to a putative binding site within the PIT1 promoter due to a decrease in DNA binding ability (Machinis and Amselem, 2005). The heterozygous nature of this mutation suggested a possible dominant negative action of the mutant allele, although haploinsufficiency or other mechanisms may be more likely alternate explanations. The deregulation of LHX4 has also been associated with chromosomal translocations involving several types of leukemia (Kawamata et al., 2002; Yamaguchi et al., 2003).
4. Conclusions
Analyses of LHX3 and LHX4 gene mutations in animal models and patients with hormone deficiency diseases have revealed that the genes play critical roles in pituitary development. These investigations have allowed the molecular diagnosis of new forms of pituitary hormone deficiency diseases and will facilitate more accurate diagnoses and treatment of patients with similar diseases and the genetic counseling of affected families. The early detection of mutations will lead to improvement in the developmental outcome of these children and prevention of complications. As the direct target genes of LHX3 and LHX4 during each stage of pituitary development are identified, and the mechanism of gene activation by these proteins is better characterized, novel targets for investigation will be revealed and a better comprehension of the functional genetics of complex human mutations (such as those found in the heterozygous state) involving these genes will be achieved.
Acknowledgments
The authors apologize to any colleagues whose work was not mentioned due to space constraints. SJR is supported by grants from the National Institutes of Health (HD42024) and the National Science Foundation (IBN 0131702).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bach I. The LIM domain: regulation by association. Mech Dev. 2000;91:5–17. doi: 10.1016/s0925-4773(99)00314-7. [DOI] [PubMed] [Google Scholar]
- Bach I, Rhodes SJ, Pearse RV, 2nd, Heinzel T, Gloss B, Scully KM, Sawchenko PE, Rosenfeld MG. P-Lim, a LIM homeodomain factor, is expressed during pituitary organ and cell commitment and synergizes with Pit-1. Proc Natl Acad Sci U S A. 1995;92:2720–2724. doi: 10.1073/pnas.92.7.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhangoo AP, Hunter CS, Savage JJ, Anhalt H, Pavlakis S, Walvoord EC, Ten S, Rhodes SJ. Clinical case seminar: a novel LHX3 mutation presenting as combined pituitary hormonal deficiency. J Clin Endocrinol Metab. 2006;91:747–753. doi: 10.1210/jc.2005-2360. [DOI] [PubMed] [Google Scholar]
- Bridwell JA, Price JR, Parker GE, McCutchan Schiller A, Sloop KW, Rhodes SJ. Role of the LIM domains in DNA recognition by the Lhx3 neuroendocrine transcription factor. Gene. 2001;277:239–250. doi: 10.1016/s0378-1119(01)00704-1. [DOI] [PubMed] [Google Scholar]
- Charles MA, Suh H, Hjalt TA, Drouin J, Camper SA, Gage PJ. PITX genes are required for cell survival and Lhx3 activation. Mol Endocrinol. 2005;19:1893–1903. doi: 10.1210/me.2005-0052. [DOI] [PubMed] [Google Scholar]
- Chen J, Hersmus N, Van Duppen V, Caesens P, Denef C, Vankelecom H. The adult pituitary contains a cell population displaying stem/progenitor cell and early embryonic characteristics. Endocrinology. 2005;146:3985–3998. doi: 10.1210/en.2005-0185. [DOI] [PubMed] [Google Scholar]
- Dattani MT. Growth hormone deficiency and combined pituitary hormone deficiency: does the genotype matter? Clin Endocrinol (Oxf) 2005;63:121–130. doi: 10.1111/j.1365-2265.2005.02289.x. [DOI] [PubMed] [Google Scholar]
- Ellsworth BS, Egashira N, Haller JL, Butts DL, Cocquet J, Clay CM, Osamura RY, Camper SA. FOXL2 in the Pituitary: Molecular, Genetic and Developmental Analysis. Mol Endocrinol. 2006 2006 Jul 13; doi: 10.1210/me.2005-0303. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Granger A, Bleux C, Kottler ML, Rhodes SJ, Counis R, Laverriere JN. The LIM-homeodomain proteins Isl-1 and Lhx3 act with steroidogenic factor 1 to enhance gonadotrope-specific activity of the gonadotropin-releasing hormone receptor gene promoter. Mol Endocrinol. 2006;20:2093–2108. doi: 10.1210/me.2005-0184. [DOI] [PubMed] [Google Scholar]
- Howard PW, Maurer RA. A point mutation in the LIM domain of Lhx3 reduces activation of the glycoprotein hormone alpha-subunit promoter. J Biol Chem. 2001;276:19020–19026. doi: 10.1074/jbc.M101782200. [DOI] [PubMed] [Google Scholar]
- Hunter CS, Rhodes SJ. LIM-homeodomain genes in mammalian development and human disease. Mol Biol Rep. 2005;32:67–77. doi: 10.1007/s11033-004-7657-z. [DOI] [PubMed] [Google Scholar]
- Kawamata N, Sakajiri S, Sugimoto KJ, Isobe Y, Kobayashi H, Oshimi K. A novel chromosomal translocation t(1;14)(q25;q32) in pre-B acute lymphoblastic leukemia involves the LIM homeodomain protein gene, Lhx4. Oncogene. 2002;21:4983–4991. doi: 10.1038/sj.onc.1205628. [DOI] [PubMed] [Google Scholar]
- Li H, Witte DP, Branford WW, Aronow BJ, Weinstein M, Kaur S, Wert S, Singh G, Schreiner CM, Whitsett JA, et al. Gsh-4 encodes a LIM-type homeodomain, is expressed in the developing central nervous system and is required for early postnatal survival. Embo J. 1994;13:2876–2885. doi: 10.1002/j.1460-2075.1994.tb06582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machinis K, Amselem S. Functional relationship between LHX4 and POU1F1 in light of the LHX4 mutation identified in patients with pituitary defects. J Clin Endocrinol Metab. 2005;90:5456–5462. doi: 10.1210/jc.2004-2332. [DOI] [PubMed] [Google Scholar]
- Machinis K, Pantel J, Netchine I, Leger J, Camand OJ, Sobrier ML, Dastot-Le Moal F, Duquesnoy P, Abitbol M, Czernichow P, Amselem S. Syndromic short stature in patients with a germline mutation in the LIM homeobox LHX4. Am J Hum Genet. 2001;69:961–968. doi: 10.1086/323764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGillivray SM, Bailey JS, Ramezani R, Kirkwood BJ, Mellon PL. Mouse GnRH receptor gene expression is mediated by the LHX3 homeodomain protein. Endocrinology. 2005;146:2180–2185. doi: 10.1210/en.2004-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier BC, Price JR, Parker GE, Bridwell JL, Rhodes SJ. Characterization of the porcine Lhx3/LIM-3/P-Lim LIM homeodomain transcription factor. Mol Cell Endocrinol. 1999;147:65–74. doi: 10.1016/s0303-7207(98)00213-5. [DOI] [PubMed] [Google Scholar]
- Netchine I, Sobrier ML, Krude H, Schnabel D, Maghnie M, Marcos E, Duriez B, Cacheux V, Moers A, Goossens M, Gruters A, Amselem S. Mutations in LHX3 result in a new syndrome revealed by combined pituitary hormone deficiency. Nat Genet. 2000;25:182–186. doi: 10.1038/76041. [DOI] [PubMed] [Google Scholar]
- Parker GE, Sandoval RM, Feister HA, Bidwell JP, Rhodes SJ. The homeodomain coordinates nuclear entry of the Lhx3 neuroendocrine transcription factor and association with the nuclear matrix. J Biol Chem. 2000;275:23891–23898. doi: 10.1074/jbc.M000377200. [DOI] [PubMed] [Google Scholar]
- Parker GE, West BE, Witzmann FA, Rhodes SJ. Serine/threonine/tyrosine phosphorylation of the LHX3 LIM-homeodomain transcription factor. J Cell Biochem. 2005;94:67–80. doi: 10.1002/jcb.20287. [DOI] [PubMed] [Google Scholar]
- Raetzman LT, Ward R, Camper SA. Lhx4 and Prop1 are required for cell survival and expansion of the pituitary primordia. Development. 2002;129:4229–4239. doi: 10.1242/dev.129.18.4229. [DOI] [PubMed] [Google Scholar]
- Schmitt S, Biason-Lauber A, Betts D, Schoenle EJ. Genomic structure, chromosomal localization, and expression pattern of the human LIM-homeobox3 (LHX 3) gene. Biochem Biophys Res Commun. 2000;274:49–56. doi: 10.1006/bbrc.2000.3038. [DOI] [PubMed] [Google Scholar]
- Seidah NG, Barale JC, Marcinkiewicz M, Mattei MG, Day R, Chretien M. The mouse homeoprotein mLIM-3 is expressed early in cells derived from the neuroepithelium and persists in adult pituitary. DNA Cell Biol. 1994;13:1163–1180. doi: 10.1089/dna.1994.13.1163. [DOI] [PubMed] [Google Scholar]
- Sharma K, Sheng HZ, Lettieri K, Li H, Karavanov A, Potter S, Westphal H, Pfaff SL. LIM homeodomain factors Lhx3 and Lhx4 assign subtype identities for motor neurons. Cell. 1998;95:817–828. doi: 10.1016/s0092-8674(00)81704-3. [DOI] [PubMed] [Google Scholar]
- Sheng HZ, Moriyama K, Yamashita T, Li H, Potter SS, Mahon KA, Westphal H. Multistep control of pituitary organogenesis. Science. 1997;278:1809–1812. doi: 10.1126/science.278.5344.1809. [DOI] [PubMed] [Google Scholar]
- Sheng HZ, Zhadanov AB, Mosinger B, Jr, Fujii T, Bertuzzi S, Grinberg A, Lee EJ, Huang SP, Mahon KA, Westphal H. Specification of pituitary cell lineages by the LIM homeobox gene Lhx3. Science. 1996;272:1004–1007. doi: 10.1126/science.272.5264.1004. [DOI] [PubMed] [Google Scholar]
- Sloop KW, Dwyer CJ, Rhodes SJ. An isoform-specific inhibitory domain regulates the LHX3 LIM homeodomain factor holoprotein and the production of a functional alternate translation form. J Biol Chem. 2001a;276:36311–36319. doi: 10.1074/jbc.M103888200. [DOI] [PubMed] [Google Scholar]
- Sloop KW, Meier BC, Bridwell JL, Parker GE, Schiller AM, Rhodes SJ. Differential activation of pituitary hormone genes by human Lhx3 isoforms with distinct DNA binding properties. Mol Endocrinol. 1999;13:2212–2225. doi: 10.1210/mend.13.12.0395. [DOI] [PubMed] [Google Scholar]
- Sloop KW, Parker GE, Hanna KR, Wright HA, Rhodes SJ. LHX3 transcription factor mutations associated with combined pituitary hormone deficiency impair the activation of pituitary target genes. Gene. 2001b;265:61–69. doi: 10.1016/s0378-1119(01)00369-9. [DOI] [PubMed] [Google Scholar]
- Sloop KW, Parker GE, Rhodes SJ. Transcriptional regulation in mammalian pituitary development and disease. Current Genomics. 2001c;2:379–398. [Google Scholar]
- Sloop KW, Showalter AD, Von Kap-Herr C, Pettenati MJ, Rhodes SJ. Analysis of the human LHX3 neuroendocrine transcription factor gene and mapping to the subtelomeric region of chromosome 9. Gene. 2000a;245:237–243. doi: 10.1016/s0378-1119(00)00025-1. [DOI] [PubMed] [Google Scholar]
- Sloop KW, Walvoord EC, Showalter AD, Pescovitz OH, Rhodes SJ. Molecular analysis of LHX3 and PROP-1 in pituitary hormone deficiency patients with posterior pituitary ectopia. J Clin Endocrinol Metab. 2000b;85:2701–2708. doi: 10.1210/jcem.85.8.6706. [DOI] [PubMed] [Google Scholar]
- Sobrier ML, Attie-Bitach T, Netchine I, Encha-Razavi F, Vekemans M, Amselem S. Pathophysiology of syndromic combined pituitary hormone deficiency due to a LHX3 defect in light of LHX3 and LHX4 expression during early human development. Gene Expr Patterns. 2004;5:279–284. doi: 10.1016/j.modgep.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Takuma N, Sheng HZ, Furuta Y, Ward JM, Sharma K, Hogan BL, Pfaff SL, Westphal H, Kimura S, Mahon KA. Formation of Rathke's pouch requires dual induction from the diencephalon. Development. 1998;125:4835–4840. doi: 10.1242/dev.125.23.4835. [DOI] [PubMed] [Google Scholar]
- Thaler JP, Lee SK, Jurata LW, Gill GN, Pfaff SL. LIM factor Lhx3 contributes to the specification of motor neuron and interneuron identity through cell-type-specific protein-protein interactions. Cell. 2002;110:237–249. doi: 10.1016/s0092-8674(02)00823-1. [DOI] [PubMed] [Google Scholar]
- Tremblay JJ, Lanctot C, Drouin J. The pan-pituitary activator of transcription, Ptx1 (pituitary homeobox 1), acts in synergy with SF-1 and Pit1 and is an upstream regulator of the Lim-homeodomain gene Lim3/Lhx3. Mol Endocrinol. 1998;12:428–441. doi: 10.1210/mend.12.3.0073. [DOI] [PubMed] [Google Scholar]
- Weng T, Chen Z, Jin N, Gao L, Liu L. Gene Expression Profiling Identifies Regulatory Pathways Involved in the Late Stage of Rat Fetal Lung Development. Am J Physiol Lung Cell Mol Physiol. 2006 doi: 10.1152/ajplung.00435.2005. [DOI] [PubMed] [Google Scholar]
- West BE, Parker GE, Savage JJ, Kiratipranon P, Toomey KS, Beach LR, Colvin SC, Sloop KW, Rhodes SJ. Regulation of the follicle-stimulating hormone beta gene by the LHX3 LIM-homeodomain transcription factor. Endocrinology. 2004;145:4866–4879. doi: 10.1210/en.2004-0598. [DOI] [PubMed] [Google Scholar]
- Yaden BC, Garcia M, 3rd, Smith TP, Rhodes SJ. Two promoters mediate transcription from the human LHX3 gene: involvement of nuclear factor I and specificity protein 1. Endocrinology. 2006;147:324–337. doi: 10.1210/en.2005-0970. [DOI] [PubMed] [Google Scholar]
- Yaden BC, Savage JJ, Hunter CS, Rhodes SJ. DNA recognition properties of the LHX3b LIM homeodomain transcription factor. Mol Biol Rep. 2005;32:1–6. doi: 10.1007/s11033-004-4069-z. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Yamamoto K, Miura O. Aberrant expression of the LHX4 LIM-homeobox gene caused by t(1;14)(q25;q32) in chronic myelogenous leukemia in biphenotypic blast crisis. Genes Chromosomes Cancer. 2003;38:269–273. doi: 10.1002/gcc.10283. [DOI] [PubMed] [Google Scholar]
- Zhadanov AB, Bertuzzi S, Taira M, Dawid IB, Westphal H. Expression pattern of the murine LIM class homeobox gene Lhx3 in subsets of neural and neuroendocrine tissues. Dev Dyn. 1995a;202:354–364. doi: 10.1002/aja.1002020405. [DOI] [PubMed] [Google Scholar]
- Zhadanov AB, Copeland NG, Gilbert DJ, Jenkins NA, Westphal H. Genomic structure and chromosomal localization of the mouse LIM/homeobox gene Lhx3. Genomics. 1995b;27:27–32. doi: 10.1006/geno.1995.1004. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Morales DC, Hermesz E, Lee WK, Pfaff SL, Westphal H. Reduced expression of the LIM-homeobox gene Lhx3 impairs growth and differentiation of Rathke's pouch and increases cell apoptosis during mouse pituitary development. Mech Dev. 2006;123:605–613. doi: 10.1016/j.mod.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Zhu X, Lin CR, Prefontaine GG, Tollkuhn J, Rosenfeld MG. Genetic control of pituitary development and hypopituitarism. Curr Opin Genet Dev. 2005;15:332–340. doi: 10.1016/j.gde.2005.04.011. [DOI] [PubMed] [Google Scholar]


