Summary
Acetylation of histone H3 on lysine 56 occurs during mitotic and meiotic S phase in fungal species. This acetylation blocks a direct electrostatic interaction between histone H3 and nucleosomal DNA, and the absence of this modification is associated with extreme sensitivity to genotoxic agents. We show here that H3-K56 acetylation is catalyzed when Rtt109, a protein that lacks significant homology to known acetyltransferases, forms an active complex with either of two histone-binding proteins, Asf1 or Vps75. Rtt109 binds to both these cofactors but not to histones alone, forming enzyme complexes with kinetic parameters similar to those of known histone acetyltransferase (HAT) enzymes. Therefore, H3-K56 acetylation is catalyzed by a previously unknown mechanism that requires a complex of two proteins: Rtt109 and a histone chaperone. Additionally, these complexes are functionally distinct, with the Rtt109-Asf1 complex but not the Rtt109-Vps75 complex being critical for resistance to genotoxic agents.
Introduction
Eukaryotic genomes are assembled into a nucleoprotein complex called chromatin. The fundamental repeating unit of chromatin is the nucleosome. Nucleosomes are comprised of 146 bp of DNA wrapped around an octamer of core histone proteins, two H2A/H2B dimers flanking an (H3/H4)2 tetramer (Luger et al., 1997). In addition to their role in compacting the genome, core histones carry a wide variety of post-translational modifications. Many modifications occur on the N-terminal histone tails that protrude outside of DNA gyres of the nucleosome, and these often impact the biological activity of surrounding chromatin regions (Peterson and Laniel, 2004). In addition, mass spectrometric analyses have identified additional modifications within the globular domains of histones that are wrapped by DNA (Mersfelder and Parthun, 2006).
One such core domain modification is the acetylation of histone H3 lysine 56 (H3-K56) (Xu et al., 2005; Masumoto et al., 2005; Ozdemir et al., 2005; Hyland et al., 2005). This lysine interacts with the phospho-backbone of DNA at its entry and exit points of the nucleosome. Acetylation of H3-K56 neutralizes the positive charge on the lysine, disrupting this electrostatic interaction. Accordingly, an H3-K56Q alteration that mimics constitutive acetylation causes reduced superhelicity of plasmid chromatin and more rapid nuclease digestion, suggesting a more flexible wrapping of DNA at the nucleosome edge (Masumoto et al., 2005).
The synthesis and removal of H3-K56ac is regulated by cell cycle progression and the DNA damage checkpoint. H3-K56ac in yeasts is normally restricted to cells undergoing DNA replication, either during mitotic or meiotic cell cycles (Masumoto et al., 2005; Recht et al., 2006). However, the acetylation persists during DNA damage repair, because the Hst3 and Hst4 sirtuin family deacetylases that remove this acetyl group are tightly regulated at the transcriptional level, peaking after S phase but repressed upon checkpoint activation (Maas et al., 2006; Celic et al., 2006). These tight regulations reflect the fact that acetylation of H3-K56 is critical for genome stability. The inability to acetylate H3-K56, as in cells expressing only an H3-K56R mutant histone, causes substantial sensitivity to S phase-perturbing genotoxic agents such as hydroxyurea (HU), camptothecin (CPT) and methyl methane sulfonate (MMS) (Masumoto et al., 2005; Recht et al., 2006). However, constitutive H3-K56 acetylation, as observed in hst3 hst4 double mutants, also results in poor growth, spontaneous DNA damage, and chromosome loss, suggesting that too much of this modified histone negatively impacts chromosome structure (Celic et al., 2006; Maas et al., 2006). Therefore, the mechanisms of the synthesis and removal of H3-K56ac are of major impact to genome stability.
Unlike H3-K56ac deacetylation, the composition of HAT enzymes that synthesize H3-K56ac has not been defined. Cells lacking the histone chaperone Asf1 lack H3-K56 acetylation but display unchanged total H3 levels (Recht et al., 2006; Celic et al., 2006; Schneider et al., 2006; Adkins et al., 2007). Indeed, mutants that lack either Asf1 or H3-K56ac display similar DNA damage sensitivity phenotypes, suggesting that Asf1 and K56ac act in the same pathway. Consistent with this, an H3-K56Q mutation partially suppresses the DNA damage sensitivity and growth defects of asf1 cells (Recht et al., 2006). Notably, H3-K56ac depends on the Asf1-histone interaction, because residues that affect H3 K56ac cluster in a region overlapping the histone H3 binding site (Mousson et al., 2005; Recht et al., 2006; Antczak et al., 2006; English et al., 2006; Adkins et al., 2007). Therefore, loss of H3-K56ac is a primary cause of the DNA damage sensitivity phenotypes of asf1 cells, and the Asf1-H3 interaction is required for H3-K56 acetylation. Additionally, asf1 hst3 hst4 triple mutant cells also lack H3-K56ac, ruling out the possibility that Asf1’s role is to protect H3-K56ac groups from deacetylases (Celic et al., 2006).
However, although Asf1 is required for H3-K56ac, Asf1 is a small histone chaperone protein that lacks a candidate HAT catalytic domain (Daganzo et al., 2003). Moreover, the H3-K56 residue is far from the Asf1 binding site on histone H3 (Antczak et al., 2006; English et al., 2006). Therefore, other proteins are better candidates for being the H3-K56 histone acetyltransferase (HAT) enzyme. A biochemical survey of mutants lacking each known HAT enzyme showed that none display altered levels of H3-K56ac (Ozdemir et al., 2005). In contrast, a Global Proteomic Survey of yeast mutants detected a lack of H3-K56ac in rtt109 mutants, which also display sensitivity to genotoxic agents (Schneider et al., 2006). These data suggested that Rtt109 could be important for HAT activity, although it lacks a canonical enzymatic domain. Here, by analysis of recombinant Rtt109 protein, we confirm that this protein is essential for H3-K56 HAT activity. However, efficient catalytic activity also requires a histone chaperone protein, either Asf1 or Vps75. In contrast, Chromatin Assembly Factor-1 does not stimulate H3-K56 acetylation. Together, these data demonstrate that Rtt109 displays specificity regarding its histone-binding cofactors, and define a previously unknown two-subunit mechanism for histone acetylation.
Results
Asf1-dependent histone acetyltransferase activity
Detection of H3-K56ac groups in budding yeast cell lysates requires the Asf1 histone chaperone (Recht et al., 2006; Celic et al., 2006; Adkins et al., 2007), suggesting the presence of an H3-K56 acetyltransferase that requires Asf1 for activity. To test this idea, we made whole cell yeast extracts, incubated them with purified histones H3/H4 or Asf1/H3/H4 complexes, and analyzed the products on immunoblots. Using a commercially available antibody specific for the H3-K56ac modified residue, we indeed detected acetylation of H3-K56 that was largely Asf1-dependent (Figure 1A, lane 1 and 2). This observation did not result from degradation of the added H3 in the absence of Asf1 (Figure 1A, lower panels). Because the whole cell extracts contained a weak Asf1-independent activity, we fractionated these by ion exchange chromatography on Q sepharose, and found a fully Asf1-dependent activity was eluted at ~ 0.4 M NaCl (Figure 1A, lanes 3–6). This acetylation activity depended on addition of acetyl-Coenzyme A (AcCoA). Therefore, yeast extracts contain an Asf1-dependent H3-K56 acetyltransferase activity.
Figure 1. Detection and reconstitution of H3-K56 acetyltransferases.
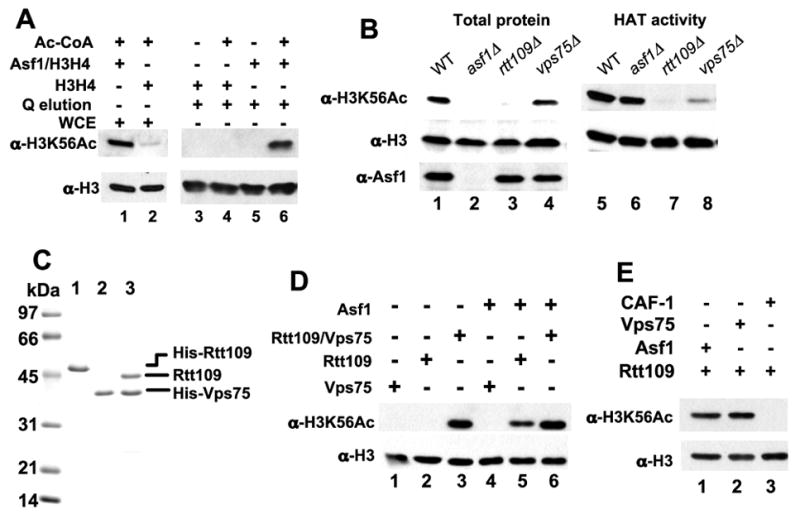
A. Asf1-dependent acetylation of histone H3-K56 in wild-type cell extracts. HAT assays were performed using 5 μg of wild-type yeast whole cell extract (WCE) proteins (lanes 1 and 2) or 2 μl of a Poros Q fraction from the extract (lanes 3–6). 6 pmol of chicken histones H3/H4 or Asf1/H3/H4 complexes were added, with or without 25μM AcCoA as indicated. The products were analyzed by immunoblotting with the indicated antibodies.
B. Analysis of mutant cell lysates and extracts. (left) rtt109 and asf1 mutant cells lack H3-K56ac. Whole cell alkaline lysis extracts of the indicated mutant strains were analyzed by immunoblotting. (right) rtt109 mutant cell extracts lack HAT activity. HAT assays were performed using 6 pmol Asf1/H3/H4 and 5 μg of whole cell extracts of the indicated mutant strains.
C. Purified recombinant His6-Rtt109 (lane 1), His6-Vps75 (lane2), and Rtt109/His6-Vps75 proteins were analyzed on a 15% SDS-PAGE gel and stained with Coomassie Blue R250.
D. HAT activity of recombinant proteins. 0.3 pmol of recombinant Rtt109 and/or Vps75, 6 pmol of yeast H3/H4, and 3 pmol of Asf1 were assayed as indicated.
E. Asf1 and Vps75 but not CAF-1 stimulate Rtt109 activity. 0.9 pmol of Asf1, Vps75 or CAF-1 and the indicated amount of Rtt109 with 6 pmol of yeast H3/H4 were used in the HAT assay.
Recent data suggested that the Rtt109 protein is linked to H3-K56 HAT activity because rtt109 mutant cells lack this modification (Figure 1B) (Schneider et al., 2006). To test the roles of proteins implicated in H3-K56 acetylation, we made whole cell extracts from several mutant yeast strains. We observed that asf1 mutant cell extracts efficiently acetylated H3 in Asf1/H3/H4 complexes (Figure 1B) but not in free (H3/H4)2 tetramers (data not shown), consistent with Asf1 being a cofactor for the HAT enzyme. Whole cell lysates from cells lacking Vps75, a Rtt109-binding protein (Krogan et al., 2006), displayed wild-type levels of H3-K56ac, although extracts from vps75 mutant cells displayed reduced HAT activity. These data indicated that Vps75 is not essential for H3-K56 acetylation but may play an auxiliary role. Notably, extracts from rtt109 cells lacked activity regardless of the presence of Asf1 (Figure 1B and data not shown), suggesting that Rtt109 is required for Asf1-dependent H3-K56 HAT activity.
Activity of two different recombinant HAT complexes
To precisely determine the protein requirements for H3-K56 acetylation, we produced recombinant Rtt109 and Vps75 proteins separately in bacteria, and also coexpressed both subunits. We generated highly purified preparations of these proteins by metal affinity and ion exchange chromatography, and observed that the coexpressed Rtt109 and Vps75 subunits remained tightly associated during the purification (Figure 1C). Recombinant Rtt109 did not acetylate free (H3/H4)2 tetramers, nor did Vps75 (Figure 1D, lanes 1 and 2). In contrast, the Rtt109/Vps75 complexes displayed robust activity either in the presence or absence of Asf1 (Figure 1D, lanes 3 and 6). Because Vps75 has homology to the Nap1/Set family of histone binding proteins (Supplemental Figure 1), we hypothesized that multiple histone binding cofactors could stimulate activity. Indeed, Asf1 stimulated H3-K56 acetylation by Rtt109 in the absence of Vps75 (Figure 1D, lane 5). In contrast, yeast Chromatin Assembly Factor-1, a three-subunit complex important for DNA replication-linked histone deposition (Kaufman et al., 1997), did not support acetylation (Figure 1E). Together, these data indicate that Rtt109 is part of a histone chaperone-dependent HAT enzyme complex, and that only a subset of histone binding proteins can contribute to an active complex.
Protein complex formation by enzyme subunits
Because Vps75 stimulates histone acetylation by Rtt109, we analyzed the interaction of Vps75 and histones H3/H4 by velocity sedimentation on glycerol gradients (Figure 2A-C). Vps75 and histones H3/H4 together formed faster-sedimenting complexes, demonstrating interaction in solution. In contrast, Rtt109 did not appear to stably interact with histones H3/H4 (Figure 2D-E). Vps75 is clearly a high affinity Rtt109-binding protein (Krogan et al., 2006; Figure 1C), but an interaction between Rtt109 and its other cofactor, Asf1, had not been described. To examine this, we used a FLAG epitope-tagged version of Asf1 (Sharp et al., 2001) to test for interactions with Rtt109, Vps75 and Rtt109/Vps75 complexes by coimmunoprecipitation (Figure 2F). Notably, Rtt109 was efficiently coprecipitated with Asf1-FLAG. In contrast, Vps75 alone or the Rtt109/Vps75 complex were not efficiently retained. Thus, Asf1 and Vps75 are both histone binding proteins that form active complexes with Rtt109. However, Vps75 appears to block the Asf1-Rtt109 interaction.
Figure 2. Protein interactions that govern H3-K56 acetylation.
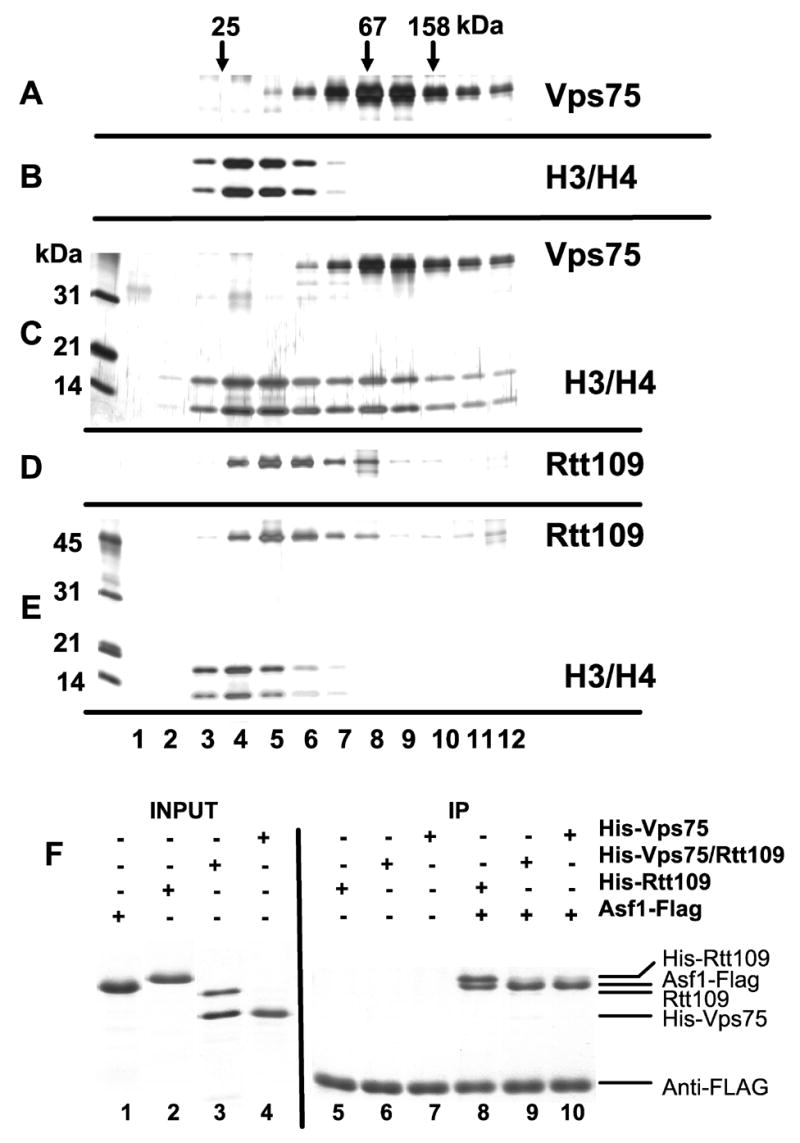
A–E. Vps75 is a histone binding protein. 1 μg of the following proteins were incubated on ice for 1hr and separated on a 5 ml 15–35 % glycerol gradient centrifuged at 49 Krpm (225 K x g) for 24 hrs at 4°C: A: Vps75, B: yeast histones H3/H4, C: Vps75 + H3/H4, D: Rtt109, E: Rtt109 + H3/H4. 350 μl of each fraction were TCA-precipitated and analyzed on silver-stained 15% SDS-PAGE gels.
F. Rtt109 binds Asf1. 40 pmol (1.2 μg) Asf1-FLAG protein was incubated with 20 pmol of the indicated His6-recombinant proteins and then precipitated with anti-FLAG antibody beads. Washed beads were analyzed on a 15% SDS-PAGE gel stained with Coomassie Blue G.
Vps75 shares significant similarity with the NAP/SET of histone chaperones (Supplemental Figure 1). Because Nap1 binds to both H2A/H2B dimers and H3/H4 complexes (McBryant et al., 2003), we determined whether Vps75 shares this property. Indeed, we observed that Vps75 does form complexes with H2A/H2B during velocity sedimentation (Supplemental Figure 4). However, histones are poorly recovered during affinity purification of epitope-tagged Vps75-containing complexes from yeast (data not shown), suggesting that Vps75-histone interactions are very dynamic in vivo.
Inhibition of HAT activity by DNA
The previous experiments were performed using histone substrates in the absence of DNA. H3-K56ac modifications are observed on nascent protein complexes (Masumoto et al., 2005; Zhou et al., 2006) but it was not known whether acetylation occurred on histones already deposited onto DNA. We therefore examined histone octamer and (H3/H4)2 tetramer complexes on DNA as substrates, testing for H3-K56 acetylation by immunoblotting of SDS-PAGE gels to detect proteins (Figure 3A) or native gels to separate histone-DNA complexes (Supplemental Figure 2). Neither Asf1 nor Vps75 in combination with Rtt109 could modify H3 bound to DNA, either in tetramers or octamers. Similar results were observed whether the DNA template was a multimer of Xenopus 5S DNA or a synthetic sequence selected for high nucleosome affinity (Lowary and Widom, 1998; data not shown). These data suggest that the preferred substrates of both Rtt109-containing enzyme complexes are likely to be nascent histones.
Figure 3. Biochemcial analyses of substrate specificity.
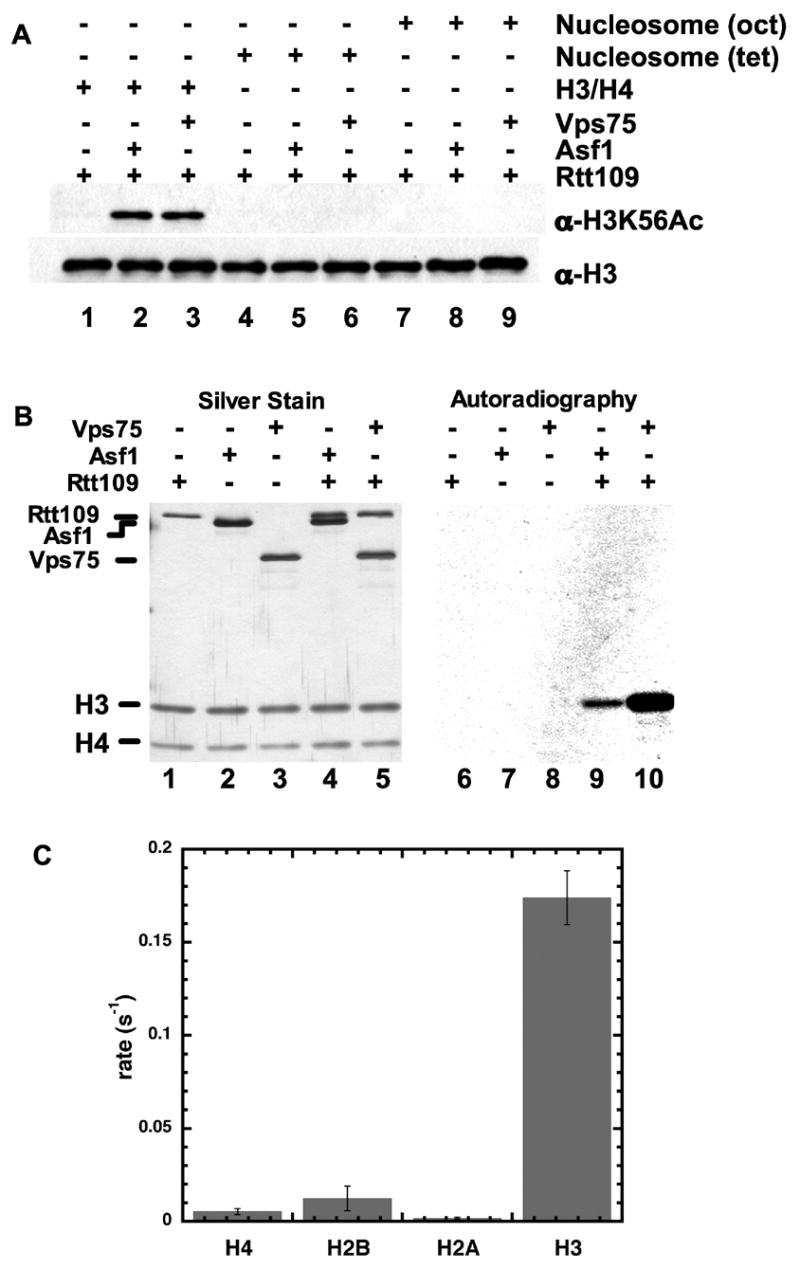
A. HAT assays were performed with 0.3 pmol of Rtt109 and 3 pmol of Asf1 or Vps75 where indicated. Substrates were 2 pmol of either chicken (H3/H4)2 tetramers in solution (lanes 1–3), tetramers deposited onto arrays of 5S DNA (lanes 4–6), or complete nucleosomes on 5S DNA arrays (lanes 7–9). Products were analyzed by SDS-PAGE and immunoblotting.
B. SDS-PAGE analysis of HAT reactions performed using 2.0 μM [3H]-AcCoA, 3 pmol of Asf1 or Vps75, 1.0 pmol of Rtt109, and 6 pmol of yeast histones H3/H4. Products were analyzed by silver staining (left) or autoradiography for 6 days (right).
C. Rate of Rtt109/Vps75 labeling of individual core histones. Filter binding assays were performed with 75 μM Acetyl-CoA, 20 μM histone, 0.4–0.8 μM Rtt109/Vps75 complex at 25 ºC. Rates are averages from triplicate experiments with standard deviation shown.
Site specificity of recombinant HAT complexes
To more thoroughly analyze the substrate specificity of the different enzyme complexes, we performed reactions using [3H]-AcCoA and histone (H3/H4)2 tetramer substrates (Figure 3B). This experiment demonstrated that both the Asf1- and Vps75-stimulated reactions resulted in preferential labeling of histone H3. Additionally, the Rtt109-Vps75 complex was incubated with AcCoA and each core histone (H3, H4, H2A and H2B) individually, and H3 was greatly preferred by 15 to 100-fold over the other core histones (Figure 3C). We also subjected the reaction products to mass spectrometric analyses. Upon incubation of Rtt109 and Asf1 with recombinant yeast histone (H3/H4)2 tetramers, we observed a single new species dependent on the addition of AcCoA (Figure 4A). This material had the mass of 15265 Da, indicative of a single acetylation on the recombinant yeast H3 used here, which has an expected mass of 15,225 Da. No alteration of the mass of H4 was observed. Tandem mass spectrometry (MS/MS) analysis of these reaction products detected acetylation on H3 lysine 56 (Figure 4B). We also analyzed products formed by the other complex, Rtt109/Vps75, and detected one acetylated residue, H3 lysine 56 (Figure 4C). We conclude that Rtt109 can form two different H3-K56 acetyltransferase complexes with different histone chaperone cofactors.
Figure 4. Mass spectrometric analyses of substrate specificity.
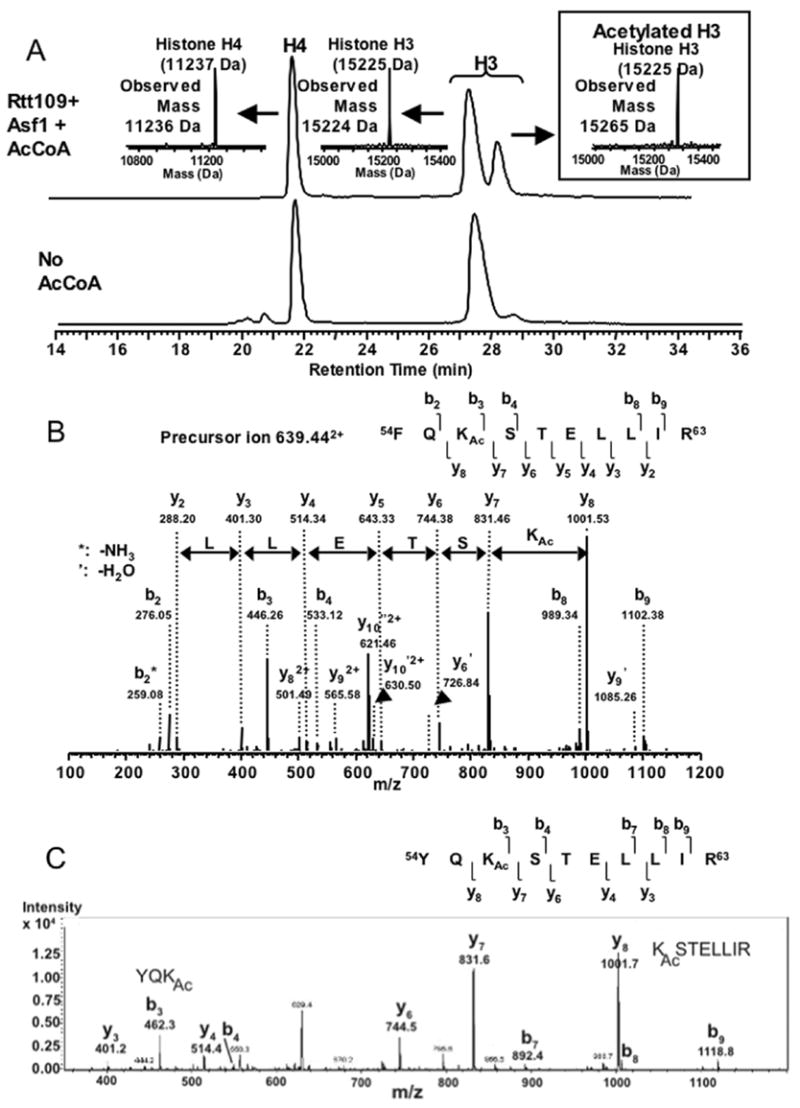
A. Single acetylation of H3 by the Rtt109/Asf1 complex. Upper panel: Rtt109/Asf1 was incubated with recombinant yeast histones H3/H4 in the presence of AcCoA. Reaction products were analyzed by LCMS and a portion of the chromatograph is shown. Peaks were analyzed by MS/MS, resulting in the indicated mass observations. The expected mass of recombinant yeast H3 is 15,225 Da and H4 is 11,237 Da. Lower panel: As above, except that AcCoA was omitted.
B. H3-K56 is the preferred substrate for the Rtt109/Asf1 complex. MS/MS Fragmentation ions from the selected acetylated peptide shown above.
C. H3-K56 is the preferred substrate for the Rtt109/Vps75 complex. MS/MS Fragmentation ions from the selected acetylated peptide. No additional acetylated peptides were identified.
Kinetic analysis and identification of essential Rtt109 residues
To provide detailed biochemical evidence that the Rtt109-Vps75 and Rtt109-Asf1 complexes are bona fide HATs, we determined the steady-state kinetics of AcCoA-dependent histone acetylation. The initial rates of acetylation were determined at varied levels of AcCoA and histones (Figure 5), and the rate data were fitted to the Michaelis-Menten equation to determine kcat, Km, and kcat/Km kinetic constants. Both AcCoA and histone substrates displayed saturation kinetics, a diagnostic behavior of an enzyme-catalyzed reaction. In the AcCoA titration, the turnover number (kcat) determined for Rtt109-Vps75 was 0.19 ± 0.01 s−1, with a Km value for AcCoA of 1.0 ± 0.2 μM, and a kcat/Km of 1.9 ± 0.4 x 105 M−1 s−1 (Figure 5A). An AcCoA Km value of 1 μM is similar to those determined for the well-characterized HATs Gcn5, p/CAF, Esa1/piccolo NuA4, and p300/CBP, suggesting that, despite the absence of a typical AcCoA binding motif (R/QXXGXG/A), Rtt109-Vps75 binds AcCoA in a manner similar to other known HATs (Tanner et al., 1999; Tanner et al., 2000a; Tanner et al., 2000b; Lau et al., 2000; Thompson et al., 2001; Berndsen et al., 2007). Moreover, these data demonstrate that Rtt109-Vps75 can bind acetyl-CoA with high efficiency, something that has not been shown for Spt10, a putative acetyltransferase also implicated to acetylate H3-K56 (Neuwald and Landsman, 1997; Shen et al., 2002; Hess et al., 2004; Xu et al., 2005). The Km of the enzyme for AcCoA was in the micromolar range in the presence of either the Asf1 or Vps75 cofactor (Supplemental Figure 3). In contrast to the efficient enzyme complexes, time course analysis of H3 acetylation revealed that Rtt109 alone displays almost negligible acetyltransferase activity (Figure 5B).
Figure 5. Enzymatic characterization of Rtt109.
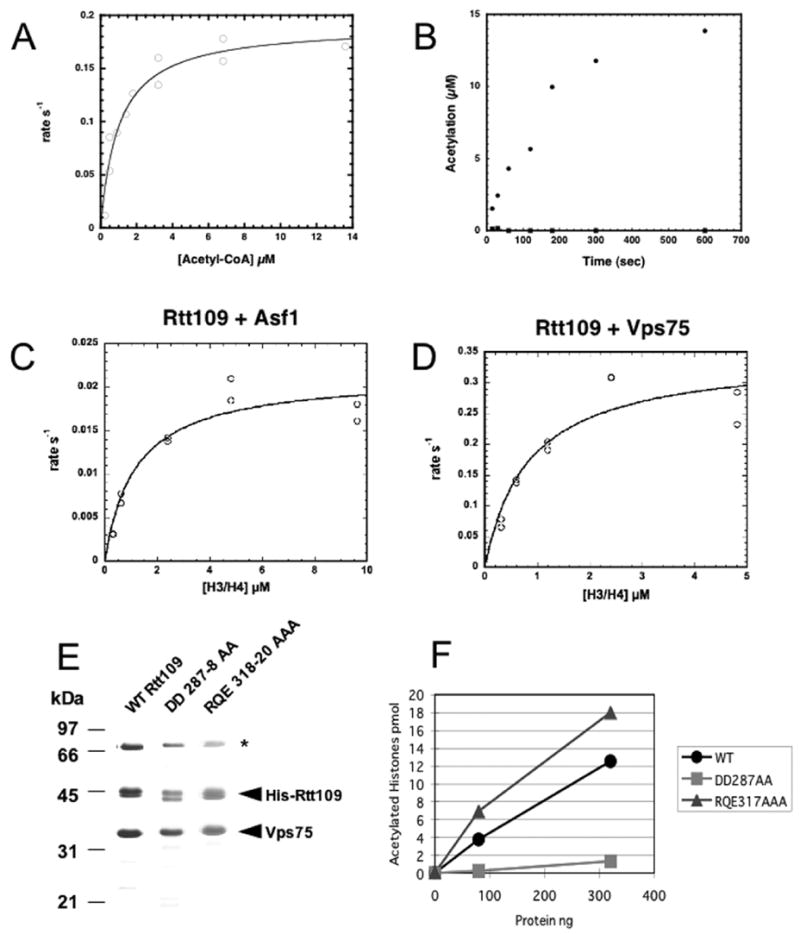
A. Acetyl-CoA saturation curve using the preformed Rtt109-Vps75 complex. Acetyl-CoA was varied from 0.25 to 13.5 μM at 30 μM histone H3 in filter binding assays containing 0.1 μM Rtt109-Vps75 complex. The kcat was 0.19 ± 0.01 s−1, with a Km for acetyl-CoA of 1 ± 0.2 μM and a kcat/Km =1.9 ± 0.4 x 105 M−1 s−1. Data from duplicate samples are shown.
B. Time course of activity of Rtt109 on H3. Filter binding assay was performed with 75 μM Acetyl-CoA, 20 μM histone H3 at 25 ºC. Either 1 μM Rtt109 (squares) or 0.46 μM Rtt109-Vps75 complex (circles) was used in assays. Initial velocity for the Rtt109-Vps75 complex was 0.13 s−1 with background levels of activity for Rtt109 alone. Experiments with a higher concentration of H3 showed similar results (data not shown).
C. H3/H4 saturation curve using Rtt109 with Asf1. The rate of acetylation was determined at H3/H4 concentrations (as dimer) from 0.3 to 9.6 or 4.8 μM at 4.8 μM acetyl-CoA. Filter binding assays contained 0.06 μM Rtt109 and 0.3 μM of Asf1. kcat was 0.021 ± 0.002 s−1, with a Km for histones H3/H4 of 1.19 ± 0.34 μM, and a kcat/Km value of 2.0 ± 0.5 x 104 M−1 s−1. Data from duplicate samples are shown.
D. As in C, with 0.3 μM Vps75 as the cofactor. kcat was 0.34± 0.04 s−1 with Km for histones H3/H4 of 0.84 ± 0.28 μM, and a kcat/Km value of 4.4 ± 0.9 x 105 M−1 s−1. Data from duplicate samples are shown.
E. Mutant Rtt109 proteins bind Vps75. Wild-type or mutant His6-Rtt109 proteins containing residues 287–288 or 318–320 changed to alanines were coexpressed in bacteria with untagged Vps75. Purified complexes were analyzed on a 15% SDS-PAGE gel and stained with Coomassie Blue. These preparations contain a greater amount of a bacterial heat shock protein (*) than do the Rtt109/Vps75-His6 preparations shown in Figure 1C.
F. HAT activity of mutant enzyme complexes. Filter binding assays were performed with 0, 80 or 320 ng of recombinant WT or mutant His6-Rtt109/Vps75 complexes, 24 pmol of yeast histones H3/H4 and 3.2 μM [3H]-AcCoA.
In experiments where the concentrations of histone H3/H4 substrates were varied, we observed that both complexes displayed a Km value for histones of ~1.0 μM (Figures 5C–D). For the Rtt109-Asf1 complex, kcat was 0.021 ± 0.002 s−1, with a kcat/Km value of 2.0 ± 0.5 x 104 M−1 s−1 (Figure 5C). For the Rtt109-Vps75 complex, kcat was 0.34± 0.04 s−1, with a kcat/Km value of 4.4 ± 0.9 x 105 M−1 s−1 (Figure 5D). The Km and kcat/Km values for histones were also in line with those reported for authenticated HATs (Tanner et al., 1999; Tanner et al., 2000a; Tanner et al., 2000b; Lau et al., 2000; Thompson et al., 2001; Berndsen et al., 2007), providing strong evidence that both the Rtt109-Vps75 and Rtt109-Asf1 complexes are bona fide histone acetyltransferases.
Examination of protein databases reveals homologs of S. cerevisiae Rtt109 only among other fungal species (Supplemental Figure 5). Alignment of Rtt109 homologs from distantly related fungi shows only a small number of invariant residues. We hypothesized that highly conserved charged or polar residues were likely candidates for important catalytic roles, and we mutated two clusters of these to alanines. We found that both mutant Rtt109 proteins bound to and copurified with Vps75 coexpressed in bacteria, demonstrating that they were not grossly misfolded (Figure 5E). However, the Rtt109(DD287–288AA) double mutant protein formed complexes that lacked HAT activity, whereas the Rtt109(318–320AAA) mutant protein formed functional HAT complexes (Figure 5F). These data indicate that some conserved residues are essential for catalytic activity, and emphasize the primary importance of the Rtt109 subunit for HAT activity.
Histone substrate requirements
To provide insight into the requirement for the Vps75- and Asf1-dependence of Rtt109 HAT activity, we also assessed the acetylation efficiency on free H3 in the absence of H4 under a variety of conditions. Figure 6A shows a representative saturation plot of free histone H3 with Rtt109-Vps75. The kcat value was 0.21 ± 0.04 s−1 with a Km of 5.9 ± 0.8 μM and a kcat/Km of 3.5 ± 0.9 x 104 M−1 s−1. The moderately increased Km value suggests that H4 makes a small contribution to histone H3 modification by Rtt109-Vps75. In contrast, addition of Asf1 to Rtt109 yielded a complex incapable of acetylation on free H3 (Figure 6B). However, when H4 was added to the reaction of H3 and Rtt109-Asf1 (Figure 6C), efficient acetylation was observed. Interestingly, Asf1 does not significantly increase the rate of H3 acetylation when added to a pre-formed complex of Rtt109-Vps75 (Figure 6B), consistent with its poor binding to the Rtt109-Vps75 complex (Figure 2). Collectively, these data suggest that Rtt109 is essential for HAT activity and that binding of either Vps75 or Asf1 creates a highly efficient and specific HAT that acetylates lysine 56 of H3. In the case of Asf1, inclusion of histone H4 is required for this robust acetylation.
Figure 6. H4 is required for Rtt109/Asf1 activity.
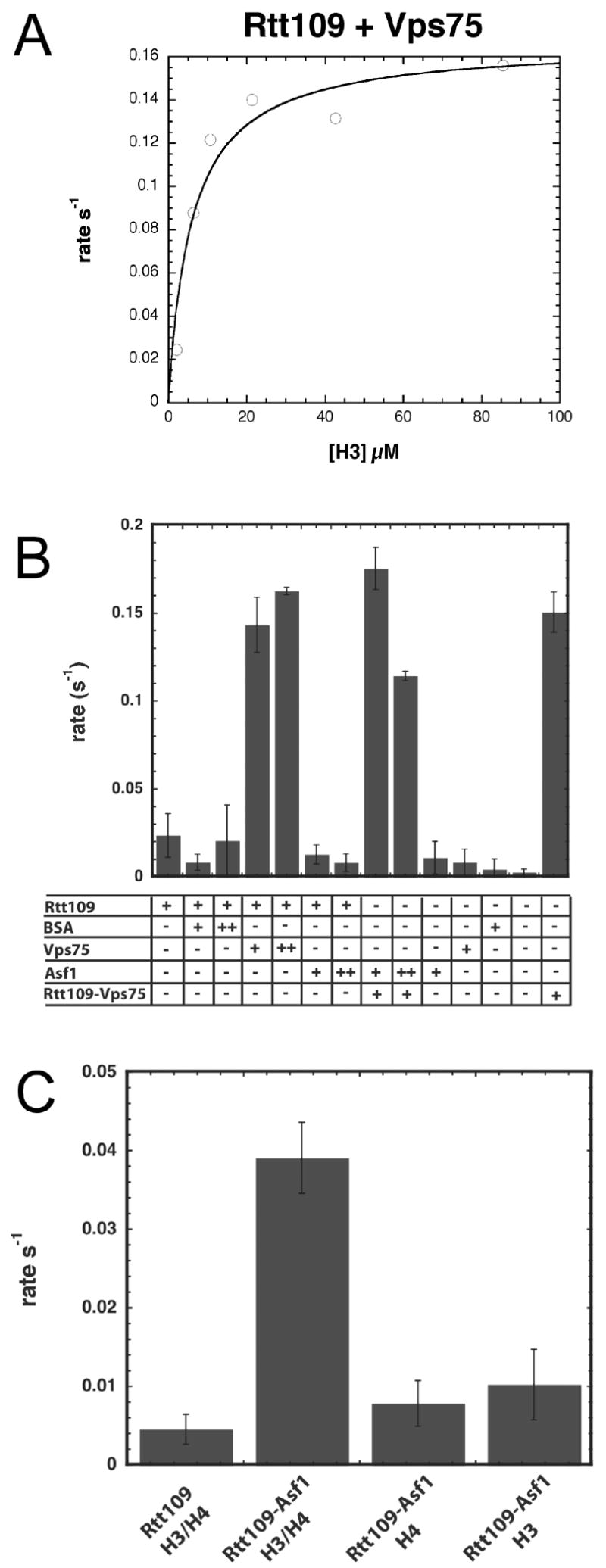
A. Free H3 saturation curve with the Rtt109-Vps75 complex. The rate of acetylation was determined at H3 concentrations from 0.8 to 86 μM at 75 μM acetyl-CoA. Filter binding assays contained 0.1 to 0.3 μM Rtt109-Vps75 complex. kcat was determined to be 0.21± 0.04 s−1, with a Km for histone H3 of 5.9 ± 0.8 μM, and a kcat/Km value of 3.5 ± 0.9 x 104 M−1 s−1. Experiments were done in triplicate with representative data shown.
B. Vps75 but not Asf1 can stimulate acetylation of free H3. Filter binding assays contained 75 μM acetyl-CoA, 20 μM H3. The concentration of Rtt109 (alone or in the coexpressed Rtt109-Vps75 complex) was 0.4 μM, Asf1 was 0.5 μM (+) or 1 μM (++), and Vps75 was 0.7 (+) or1.4 μM (++). 5 μg (+) or 10 μg (++)BSA was used as a control for interaction specificity. Data are averages from two experiments with standard deviation shown.
C. Asf1 stimulates H3 acetylation only in the presence of H4. Acetylation was monitored in filter binding assays with 75 μM acetyl-CoA, 20 μM each of H3 and/or H4, and 0.1 μM Rtt109. 0.7 μM Asf1 was added where noted. Data are averages from two experiments with standard deviation shown.
Roles of the chaperone cofactors in vivo
To examine how Rtt109 and Asf1 are related to the genome stability function of H3-K56 acetylation, we performed genetic epistasis tests. Because asf1 and rtt109 cells are both very sensitive to high concentrations of genotoxic agents (Schneider et al., 2006), we tested growth in the presence of a low concentration of CPT to facilitate detection of synthetic phenotypes. Deletion of the ASF1 gene or a histone H3-K56R mutation cause similar sensitivity to low concentrations of CPT (Figure 7A). Additionally, rtt109 asf1 and rtt109 H3-K56R double mutants displayed the same sensitivity as the single mutants, suggesting all these factors function in the same process. In contrast, vps75 mutant cells did not display the CPT-sensitive phenotype observed in rtt109 cells (Figure 7B).
Figure 7. Asf1 and Vps75 have functionally different roles in vivo.
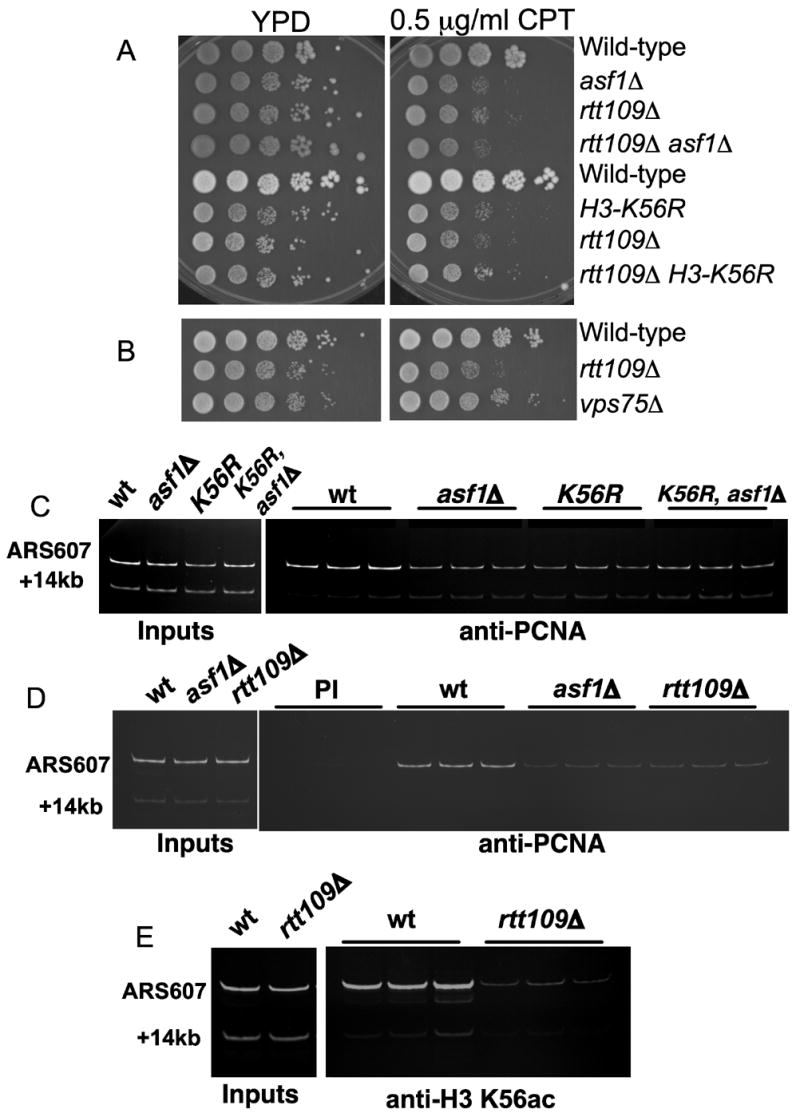
A. Similar sensitivity to CPT caused by asf1Δ, rtt109Δ and H3-K56R mutations. Equal numbers of log-phase cells of the indicated genotypes were plated on rich, non-selective media (YPD) or on the same media containing 0.5 μg/ml CPT. Plates were grown at 30°C for 3 days prior to photography.
B. As in A, comparing rtt109Δ and vps75Δ mutants.
C. Chromatin immunoprecipitation analysis of PCNA enrichment at an early replication origin (ARS607). α factor-arrested cells were released into 0.2 M HU for 30 minutes and crosslinked with formaldehyde. Triplicate IP reactions with an anti-PCNA antibody were carried out using extracts from the indicated strains. PCR was used to detect the amount of ARS607 DNA immunoprecipitated relative to the +14kb band, which represents a DNA probe distal from the origin.
D. As in C, comparing rtt109Δ and asf1Δ mutants. PI indicates immunoprecipitation with pre-immune control sera.
E. As above, except that IPs were performed with anti-H3-K56ac antibodies.
Previous data showed that asf1 mutant cells display defects in the stability of DNA replication proteins at HU-stalled forks (Franco et al., 2005). To determine whether other mutations that affect H3-K56 metabolism share this phenotype, we performed chromatin immunoprecipitation (ChIP) experiments to examine retention of PCNA, the DNA polymerase processivity clamp, at an early replication origin termed ARS607. As expected, PCNA levels in wild-type cells were enriched at ARS607 compared to a distal region 14kb away. However, PCNA levels were reduced at the origin DNA in asf1 cells, and similar reductions were observed in H3-K56R or rtt109 mutant cells (Figure 7C–D). Together, these data indicate that the Rtt109/Asf1 complex but not the Rtt109/Vps75 complex is important for genome stability.
Additionally, by ChIP we compared levels of H3-K56ac at stalled forks in wild-type and rtt109 cells (Figure 7E). We observed that wild-type cells display significant enrichment of this modification at stalled forks, and that this is lost in rtt109 mutant cells. Therefore, rtt109 cells not only display global deficits in H3-K56 acetylation in cell extracts (Figure 1; (Schneider et al., 2006)) and at specific gene promoters (Schneider et al., 2006), but also lack this modification at replication forks. These data are consistent with the role of Rtt109 and H3-K56ac in genome stability.
Discussion
We report here the identification and characterization of a new class of histone acetyltransferase enzymes, formed by complexes of budding yeast Rtt109 with two different histone chaperones. There are three prominent characteristics of these complexes. First, they both have a strong preference for histone H3 lysine 56 as their substrate. Second, Rtt109 bears no obvious homology to the many well-characterized HAT enzymes previously described. Third, enzymatic activity of these complexes requires a histone binding cofactor, and only a subset of histone binding proteins suffice for this role.
We show that both Vps75 and Asf1 can function as cofactors, but generate functionally distinct complexes with Rtt109. Most notably, the Rtt109/Vps75 complex efficiently acetylates free histone H3, and is not dramatically stimulated by H4. In contrast, the Rtt109/Asf1 complex does not efficiently acetylate free H3, and only becomes a robust enzyme in the presence of H4. These data suggest that the direct Asf1-H4 contacts (English et al., 2006) are important for the proper binding and/or positioning of H3 in the enzyme complex. In contrast, the interaction between the Rtt109/Vps75 complex and H3 is sufficient for efficient enzyme activation.
We demonstrate that Vps75, a Rtt109 binding partner and a member of the NAP/SET family is indeed a histone H3/H4 and H2A/H2B binding protein (Figure 2 and Supplemental Figure 4). The crystal structure of the related yeast Nap1 protein demonstrates that this protein has a subdomain containing a beta-stranded region reminiscent of the immunoglobulin fold of the Asf1 globular domain (Park and Luger, 2006; Daganzo et al., 2003; English et al., 2006). This region is conserved among the Nap1 and Vps75 family members (Supplemental Figure 1). Notably, Asf1 binds to a histone H3/H4 dimer in a manner that leaves the H3-K56 residue accessible to solution (Antczak et al., 2006; English et al., 2006), and this geometry is likely important for substrate recognition by Rtt109.
We demonstrate that Asf1 and Vps75 both bind Rtt109 and form active enzyme complexes. We propose that these histone chaperone proteins act as a scaffold to bring together the histones and Rtt109, which do not interact tightly on their own (Figure 2). The chaperones may also present the histones in a favorable orientation for acetylation. We note that Vps75 appears to inhibit the interaction between Asf1 and Rtt109. Future studies will be required to determine how the interaction of Rtt109 with multiple cofactors may be regulated in vivo. Such regulation is likely critical because the two Rtt109 cofactors are functionally distinct. Specifically, Asf1 is the functionally relevant cofactor for providing the H3-K56 acetylated molecules that confer genome stability (Figure 7). Therefore, we hypothesize that Asf1 directs formation of an active complex with Rtt109 for the purpose of modifying newly synthesized histone H3 molecules during S phase, and that Rtt109-Vps75 complexes have other functions yet to be determined.
The easily detected homologs of budding yeast Rtt109 are only found in fungal species (Supplemental Figure 5). However, H3-K56 acetylation has been detected as an abundant modification in other eukaryotic organisms, including Drosophila (Xu et al., 2005; Schneider et al., 2006) and Tetrahymena (Garcia et al., 2006). Additionally, it appears at low levels in cultured human cells (Garcia et al., 2006). Therefore, non-fungal organisms that display H3-K56 acetylation may harbor highly diverged Rtt109 family members, or possess uncharacterized enzymes that perform this reaction. It remains to be determined whether this modification is formed in mammalian cells at limited times during the cell cycle or in a tissue specific manner.
While the revised version of this paper was in review, two reports were published describing links between Rtt109, H3-K56 acetylation and genome stability (Driscoll et al., 2007; Han et al., 2007).
Experimental Procedures
Proteins
Recombinant Rtt109 and Vps75 were generated by cloning the open reading frames into pET28a+ (kanamycin selection, Novagen) or pET3a-Tr (Tan, 2001) (ampicillin selection), respectively. E. coli Rosetta (Novagen) cells were transformed with either or both of these plasmids and selected with the appropriate drugs. Cultures were grown in LB media at 27°C and protein expression was induced by addition of 0.5 mM IPTG at a density of A600 ~0.6. Cells were harvested after 4 hours of induction, washed with 1 x phosphate-buffered saline + 0.1 mM PMSF, and frozen in liquid nitrogen and stored at -80°C. Cells were resuspended in 1/100th culture volume 1x Ni-NTA Buffer (50 mM Na phosphate, pH 8.0, 300 mM NaCl, 5% glycerol, 0.01 % NP40, 10 mM imidazole and 10 mM beta-mercaptoethanol) and lysed by sonication. Extracts were clarified by centrifugation at 100,000 x g for 30 minutes and incubated with Ni-NTA metal affinity resin (Qiagen). The resin was washed extensively with 1x Ni-NTA Buffer, and recombinant proteins were eluted with the same buffer containing 200 mM imidazole. Eluted proteins were dialyzed into Buffer A (25 mM Tris-Cl, pH 7.5, 1 mM EDTA, 10% glycerol, 0.01% NP40) + 50 mM NaCl and further purified by chromatography on Poros HS cation exchange resin by salt gradient elution from 50 mM to 1.0 M NaCl.
Histones were purified from either chicken erythrocyte nuclei (von Holt et al., 1989), or recombinant yeast or Xenopus histones were generated in bacteria as described (Luger et al., 1999; Gelbart et al., 2001). Yeast Asf1 and Asf1/H3/H4 complexes were generated as described (Sharp et al., 2001). Recombinant yeast CAF-1 was overproduced in insect cells as described (Krawitz et al., 2002).
Wild-type and mutant yeast extracts for enzyme assays were prepared by grinding log phase cells in a porcelain mortar under liquid nitrogen for 20–30 minutes until a fine powder was generated. The powder was collected and resuspended in an equal volume of 2x Buffer A. Whole cell lysates for immunoblotting were generated by alkali lysis as described (Kushnirov, 2000).
Antibodies
Anti-H3K56ac (Upstate) and anti-H3 (Abcam) were from commercial suppliers. Anti-Asf1 polyclonal antibodies have been described (Daganzo et al., 2003).
Histone acetyltransferase assays
Reactions analyzed by immunoblotting were performed in 10 μl in HAT assay buffer (50 mM Tris-acetate, 50 mM Bis-Tris-acetate, 100 mM Na acetate, pH 7.0, 1 mM DTT) with 25 μM AcCoA (Sigma) except as indicated, 3 pmol of Asf1/H3/H4 complex, and a final adjusted NaCl concentration of 20–30 mM. Reactions were incubated at 30°C for 30 min and stopped by addition of protein gel sample buffer. Reactions analyzed by filter binding and scintillation counting of products (Berndsen and Denu, 2005) were performed in 50 mM Tris-Cl, pH 7.5, 1 mM DTT, using 2 μM [3H]-acetyl Coenzyme A (2000 cpm/pmol, Sigma or Pharmacia) except where noted. Data were fit to the Michaelis-Menten equation in Kaleidagraph to determine kinetic constants, which are reported as average values +/− standard deviations.
Protein interaction and other assays
20 pmol of His-Rtt109, His-Vps75/Rtt109 or His-Vps75 were incubated with or without 40 pmol of Asf1-FLAG in the presence of anti-FLAG M2 agarose affinity resin (Sigma) in Buffer A + 150 mM NaCl + 1 mM DTT for 12 hrs at 4°C with gentle rotation. The resin was collected by centrifugation at 5 Krpm and washed twice with 500 μl Buffer A + 150 mM NaCl + 1 mM DTT. Washed beads were analyzed on a 15% SDS-PAGE gel stained with Coomassie Blue G.
Chromatin immunoprecipitation was performed as described (Franco et al., 2005). Additional methods for mass spectrometry and assembly of histone-DNA complexes are found in the Supplementary Material.
Supplementary Material
Acknowledgments
The authors state that they have no competing financial interests. The authors would like to thank Judith Recht, Nevan Krogan, and David Allis for communication of data prior to publication. This work was supported by the following grants: NIH GM55712, NSF MCB-0549131 (PDK), NIH GM059785 (JMD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adkins MW, Carson JJ, English CM, Ramey CJ, Tyler JK. The histone chaperone anti-silencing function 1 stimulates the acetylation of newly synthesized histone H3 in S-phase. J Biol Chem. 2007;282:1334–1340. doi: 10.1074/jbc.M608025200. [DOI] [PubMed] [Google Scholar]
- Antczak AJ, Tsubota T, Kaufman PD, Berger JM. Structure of the yeast histone H3-ASF1 interaction: Implications for chaperone mechanism, species-specific interactions, and epigenetics. BMC Struct Biol. 2006;6:26. doi: 10.1186/1472-6807-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndsen CE, Albaugh BN, Tan S, Denu JM. Catalytic Mechanism of a MYST family Histone Acetyltransferase. Biochemistry. 2007;46:623–629. doi: 10.1021/bi602513x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndsen CE, Denu JM. Assays for mechanistic investigations of protein/histone acetyltransferases. Methods. 2005;36:321–331. doi: 10.1016/j.ymeth.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Celic I, Masumoto H, Griffith WP, Meluh P, Cotter RJ, Boeke JD, Verreault A. The sirtuins hst3 and hst4p preserve genome integrity by controlling histone h3 lysine 56 deacetylation. Curr Biol. 2006;16:1280–1289. doi: 10.1016/j.cub.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Daganzo SM, Erzberger JP, Lam WM, Skordalakes E, Zhang R, Franco AA, Brill SJ, Adams PD, Berger JM, Kaufman PD. Structure and function of the conserved core of histone deposition protein Asf1. Curr Biol. 2003;13:2148–2158. doi: 10.1016/j.cub.2003.11.027. [DOI] [PubMed] [Google Scholar]
- Driscoll R, Hudson A, Jackson SP. Yeast Rtt109 promotes genome stability by acetylating histone H3 on lysine 56. Science. 2007;315:649–652. doi: 10.1126/science.1135862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English CM, Adkins MW, Carson JJ, Churchill ME, Tyler JK. Structural basis for the histone chaperone activity of asf1. Cell. 2006;127:495–508. doi: 10.1016/j.cell.2006.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco AA, Lam WM, Burgers PM, Kaufman PD. Histone deposition protein Asf1 maintains DNA replisome integrity and interacts with replication factor C. Genes Dev. 2005;19:1365–1375. doi: 10.1101/gad.1305005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia BA, Hake SB, Diaz RL, Kauer M, Morris SA, Recht J, Shabanowitz J, Mishra N, Strahl BD, Allis CD, Hunt DF. Organismal differences in post-translational modifications in histones H3 and H4. J Biol Chem. 2006 doi: 10.1074/jbc.M607900200. epub Dec. 28. [DOI] [PubMed] [Google Scholar]
- Gelbart ME, Rechsteiner T, Richmond TJ, Tsukiyama T. Interactions of Isw2 chromatin remodeling complex with nucleosomal arrays: analyses using recombinant yeast histones and immobilized templates. Mol Cell Biol. 2001;21:2098–2106. doi: 10.1128/MCB.21.6.2098-2106.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Zhou H, Horazdovsky B, Zhang K, Xu RM, Zhang Z. Rtt109 acetylates histone H3 lysine 56 and functions in DNA replication. Science. 2007;315:653–655. doi: 10.1126/science.1133234. [DOI] [PubMed] [Google Scholar]
- Hess D, Liu B, Roan NR, Sternglanz R, Winston F. Spt10-dependent transcriptional activation in Saccharomyces cerevisiae requires both the Spt10 acetyltransferase domain and Spt21. Mol Cell Biol. 2004;24:135–143. doi: 10.1128/MCB.24.1.135-143.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland EM, Cosgrove MS, Molina H, Wang D, Pandey A, Cottee RJ, Boeke JD. Insights into the role of histone H3 and histone H4 core modifiable residues in Saccharomyces cerevisiae. Mol Cell Biol. 2005;25:10060–10070. doi: 10.1128/MCB.25.22.10060-10070.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman PD, Kobayashi R, Stillman B. Ultraviolet radiation sensitivity and reduction of telomeric silencing in Saccharomyces cerevisiae cells lacking chromatin assembly factor-I. Genes Dev. 1997;11:345–357. doi: 10.1101/gad.11.3.345. [DOI] [PubMed] [Google Scholar]
- Krawitz DC, Kama T, Kaufman PD. Chromatin assembly factor I mutants defective for PCNA binding require Asf1/Hir proteins for silencing. Mol Cell Biol. 2002;22:614–625. doi: 10.1128/MCB.22.2.614-625.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogan NJ, Cagney G, Yu H, Zhong G, Guo X, Ignatchenko A, Li J, Pu S, Datta N, Tikuisis AP, et al. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature. 2006;440:637–643. doi: 10.1038/nature04670. [DOI] [PubMed] [Google Scholar]
- Kushnirov VV. Rapid and reliable protein extraction from yeast. Yeast. 2000;16:857–860. doi: 10.1002/1097-0061(20000630)16:9<857::AID-YEA561>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Lau OD, Courtney AD, Vassilev A, Marzilli LA, Cotter RJ, Nakatani Y, Cole PA. p300/CBP-associated factor histone acetyltransferase processing of a peptide substrate. Kinetic analysis of the catalytic mechanism. J Biol Chem. 2000;275:21953–21959. doi: 10.1074/jbc.M003219200. [DOI] [PubMed] [Google Scholar]
- Lowary PT, Widom J. New DNA sequence rules for high affinity binding to histone octamer and sequence-directed nucleosome positioning. J Mol Biol. 1998;276:19–42. doi: 10.1006/jmbi.1997.1494. [DOI] [PubMed] [Google Scholar]
- Luger K, Mäder AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8Å resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- Luger K, Rechsteiner TJ, Richmond TJ. Expression and purification of recombinant histones and nucleosome reconstitution. Methods Mol Biol. 1999;119:1–16. doi: 10.1385/1-59259-681-9:1. [DOI] [PubMed] [Google Scholar]
- Maas NL, Miller KM, Defazio LG, Toczyski DP. Cell cycle and checkpoint regulation of histone h3 k56 acetylation by hst3 and hst4. Mol Cell. 2006;23:109–119. doi: 10.1016/j.molcel.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Masumoto H, Hawke D, Kobayashi R, Verreault A. A role for cell-cycle-regulated histone H3 lysine 56 acetylation in the DNA damage response. Nature. 2005;436:294–298. doi: 10.1038/nature03714. [DOI] [PubMed] [Google Scholar]
- McBryant SJ, Abernathy SM, Laybourn PJ, Nyborg JK, Luger K. Preferential binding of the histone (H3–H4)2 tetramer by NAP1 is mediated by the amino terminal histone tails. J Biol Chem. 2003;278:44574–44583. doi: 10.1074/jbc.M305636200. [DOI] [PubMed] [Google Scholar]
- Mersfelder EL, Parthun MR. The tale beyond the tail: histone core domain modifications and the regulation of chromatin structure. Nucleic Acids Res. 2006;34:2653–2662. doi: 10.1093/nar/gkl338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousson F, Lautrette A, Thuret JY, Agez M, Courbeyrette R, Amigues B, Becker E, Neumann JM, Guerois R, Mann C, Ochsenbein F. Structural basis for the interaction of Asf1 with histone H3 and its functional implications. Proc Natl Acad Sci U S A. 2005;102:5975–5980. doi: 10.1073/pnas.0500149102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwald AF, Landsman D. GCN5-related histone N-acetyltransferases belong to a diverse superfamily that includes the yeast SPT10 protein. Trends Biochem Sci. 1997;22:154–155. doi: 10.1016/s0968-0004(97)01034-7. [DOI] [PubMed] [Google Scholar]
- Ozdemir A, Spicuglia S, Lasonder E, Vermeulen M, Campsteijn C, Stunnenberg HG, Logie C. Characterization of lysine 56 of histone H3 as an acetylation site in Saccharomyces cerevisiae. J Biol Chem. 2005;280:25949–25952. doi: 10.1074/jbc.C500181200. [DOI] [PubMed] [Google Scholar]
- Park YJ, Luger K. The structure of nucleosome assembly protein 1. Proc Natl Acad Sci U S A. 2006;103:1248–1253. doi: 10.1073/pnas.0508002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson CL, Laniel MA. Histones and histone modifications. Curr Biol. 2004;14:R546–551. doi: 10.1016/j.cub.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Recht J, Tsubota T, Tanny JC, Diaz RL, Berger JM, Zhang X, Garcia BA, Shabanowitz J, Burlingame AL, Hunt DF, et al. Histone chaperone Asf1 is required for histone H3 lysine 56 acetylation, a modification associated with S phase in mitosis and meiosis. Proc Natl Acad Sci U S A. 2006;103:6988–6993. doi: 10.1073/pnas.0601676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J, Bajwa P, Johnson FC, Bhaumik SR, Shilatifard A. Rtt109 is required for proper H3K56 acetylation: A chromatin mark associated with the elongating RNA polymerase II. J Biol Chem. 2006;281:37270–37274. doi: 10.1074/jbc.C600265200. [DOI] [PubMed] [Google Scholar]
- Sharp JA, Fouts ET, Krawitz DC, Kaufman PD. Yeast Histone Deposition Protein Asf1p Requires Hir Proteins and PCNA for Heterochromatic Silencing. Current Biology. 2001;11:463–473. doi: 10.1016/s0960-9822(01)00140-3. [DOI] [PubMed] [Google Scholar]
- Shen CH, Leblanc BP, Neal C, Akhavan R, Clark DJ. Targeted histone acetylation at the yeast CUP1 promoter requires the transcriptional activator, the TATA boxes, and the putative histone acetylase encoded by SPT10. Mol Cell Biol. 2002;22:6406–6416. doi: 10.1128/MCB.22.18.6406-6416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S. A modular polycistronic expression system for overexpressing protein complexes in Escherichia coli. Protein Expr Purif. 2001;21:224–234. doi: 10.1006/prep.2000.1363. [DOI] [PubMed] [Google Scholar]
- Tanner KG, Langer MR, Denu JM. Kinetic mechanism of human histone acetyltransferase P/CAF. Biochemistry. 2000a;39:11961–11969. doi: 10.1021/bi001272h. [DOI] [PubMed] [Google Scholar]
- Tanner KG, Langer MR, Kim Y, Denu JM. Kinetic mechanism of the histone acetyltransferase GCN5 from yeast. J Biol Chem. 2000b;275:22048–22055. doi: 10.1074/jbc.M002893200. [DOI] [PubMed] [Google Scholar]
- Tanner KG, Trievel RC, Kuo MH, Howard RM, Berger SL, Allis CD, Marmorstein R, Denu JM. Catalytic mechanism and function of invariant glutamic acid 173 from the histone acetyltransferase GCN5 transcriptional coactivator. J Biol Chem. 1999;274:18157–18160. doi: 10.1074/jbc.274.26.18157. [DOI] [PubMed] [Google Scholar]
- Thompson PR, Kurooka H, Nakatani Y, Cole PA. Transcriptional coactivator protein p300. Kinetic characterization of its histone acetyltransferase activity. J Biol Chem. 2001;276:33721–33729. doi: 10.1074/jbc.M104736200. [DOI] [PubMed] [Google Scholar]
- von Holt C, Brandt WF, Greyling HJ, Lindsey GG, Retief JD, Rodrigues JD, Schwager S, Sewell BT. Isolation and characterization of histones. Methods Enzymol. 1989;170:431–523. doi: 10.1016/0076-6879(89)70061-6. [DOI] [PubMed] [Google Scholar]
- Xu F, Zhang K, Grunstein M. Acetylation in histone H3 globular domain regulates gene expression in yeast. Cell. 2005;121:375–385. doi: 10.1016/j.cell.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Zhou H, Madden BJ, Muddiman DC, Zhang Z. Chromatin assembly factor 1 interacts with histone H3 methylated at lysine 79 in the processes of epigenetic silencing and DNA repair. Biochemistry. 2006;45:2852–2861. doi: 10.1021/bi0521083. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


