Abstract
Inspired by the effect of norrisolide on the Golgi complex, we synthesized norrisolide probes that contain: the perhydroindane core of the parent natural product for Golgi localization, a crosslinking unit (aryl azide or epoxide) for covalent binding to the target and a tag (biotin or iodine) for subsequent target purification. We found that biotin-containing probes 14, 20 and 24 induced inefficient Golgi vesiculation. However, the iodinated probe 25 induced extensive and irreversible Golgi fragmentation. This probe can be used for the isolation of the cellular target of norrisolide.
The intracellular transport and sorting of biomolecules, a process referred to as the secretory pathway, is essential to cell function and survival.1 Central to this process is the Golgi complex, an assembly of membranes, whose function involves the post-translational modification of newly synthesized proteins and lipids, their sorting into secretory vesicles and their trafficking to the sites of function.2 It has also been shown that during mitosis the Golgi membranes undergo complete fragmentation to small vesicles.3 However, the mechanisms governing these vesiculation processes are currently poorly understood.4 Small molecules that affect Golgi vesiculation can be useful as probes for exploring this event and as leads for controlling disease pathogenesis.5 For instance, brefeldin A (1, Figure 1) was found to cause fusion of Golgi with endoplasmic reticulum (ER) and helped in unraveling the Golgi to ER retrograde pathway.6 Secramine (2) was shown to block protein transport from Golgi to the plasma membrane,7 while CCL-19 (4) blocks the exit of proteins from Golgi and induces Golgi fragmentation.8 The marine sesquiterpene ilimaquinone (3) was found to induce a reversible vesiculation of the Golgi and led to the identification of Protein Kinase D as a component of the secretion machinery.9 More recently, norrisolide (5)10 was shown to fragment the Golgi complex in an irreversible manner.11
Figure 1.
Structures of selected Golgi-disturbing agents.
Recent studies with norrisolide have suggested that the hydrocarbon-containing perhydroindane core of this natural product binds to a receptor at the Golgi membranes and induces reversible vesiculation.12 The vesiculation becomes irreversible when this core is decorated with electrophilic residues. Our efforts to explore this concept led to identification of compound 6 (Figure 1) that was shown to induce an identical phenotype with 5. We proposed that this effect is due to covalent reaction of the epoxide units of 6 with its biological receptor.12 Based on these findings we sought to synthesize and study trifunctional norrisolide probes that would contain the following functional groups: (a) the perhydroindane core for Golgi localization; (b) a crosslinking reagent for covalent binding to its putative target and (c) a tag for subsequent protein purification and isolation.
The first probe that we evaluated was compound 14 in which the perhydroindane motif is attached to an aryl azide unit and biotin. It was expected that light-activation of the aryl azide would produce a reactive nitrene intermediate that would react covalently with its receptor and thus mimic the irreversible Golgi vesiculation observed with the natural product.13 The biotin tag could then allow the isolation and purification of the target via affinity chromatography on resin-bound streptavidin. The synthesis of 14 is summarized in Scheme 1.14 Coupling of 715 with 816 in the presence of EDC and DMAP produced ester 9 in 77% yield. The phenol functionality of 9 was then alkylated with N-Boc iodopropane (10) in the presence of CsCO3 in DMF to form compound 11 (93% yield). TFA-induced Boc deprotection of 11, followed by coupling of the resulting amine with biotin (13), gave rise to trifunctional probe 14.17 The bifunctional probe 15,17 containing the aryl azide functionality and the biotin but not the perhydroindane motif, was prepared in a similar manner and was used as a control.
Scheme 1.
Reagents and conditions: (a) 7 (1.0 equiv), 8 (1.1 equiv), EDC (1.2 equiv), DMAP (1.2 equiv), CH2Cl2, 25 °C, 24 h, 77%; (b) 10 (1.3 equiv), CsCO3 (2.0 equiv), DMF, 25 °C, 4 h, 93%; (c) TFA (5.0 equiv), CH2Cl2, 25 °C, 0.5 h, then (d) 13 (1.3 equiv), DIEA (3.0 equiv), EDC (1.4 equiv), DMF/CH2Cl2: 1/1, 25 °C, 4 h, 89%.
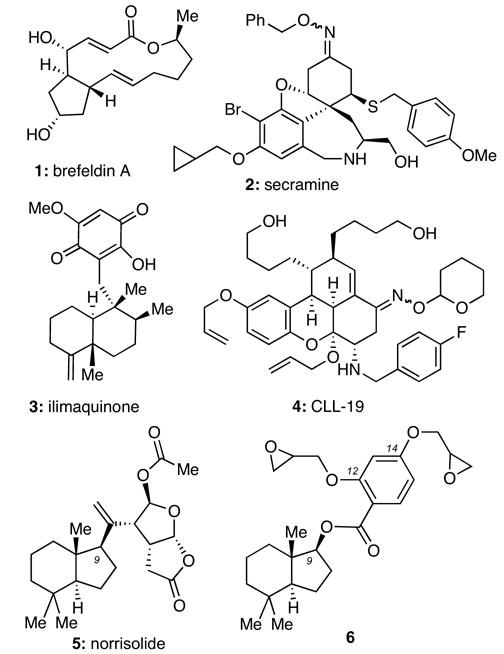
The effect of compounds 14 and 15 in Golgi vesiculation was evaluated using Normal Rat Kidney (NRK) cells. Cells grown on coverslips in complete growth medium were treated with 14 and 15 (30 μM) for 60 minutes at 37 °C.18 The cells were then fixed and processed for immunofluorescence microscopy.18 The Golgi complex is shown in red and the cell nucleus is shown in blue. Figure 2 highlights the results of this study. Comparison of Figures 2a and 2b indicate that compound 14 caused Golgi fragmentation in 50% of the cell population. In contrast, compound 15 did not lead to any fragmentation (data not shown), supporting the notion that the perhydroindane motif is essential for norrisolide activity. As expected, the fragmentation induced by 14 was completely reversible upon washing when the photoactivation of the aryl azide unit did not take place (Figure 2c). UV-irradiation for 15 min caused an irreversible Golgi fragmentation in a small percent of the cells (Figure 2d).18
Figure 2.
Effect of 14 on Golgi membranes. NRK cells were treated with 14 for 0 min (a) and 60 min (b). Cells were then exposed to photoactivation (d) and subsequently washed and incubated for 60 min. Control cells not subjected to UV irradiation are shown in (c). Golgi is shown in red; nuclei in blue.
Despite the low extent of irreversibility, we attempted isolation of the protein target of compound 14. After UV irradiation, the cells were lysed and analyzed by Western blot to identify proteins containing biotin.18 As a negative control, we used probe 15, containing biotin but not the norrisolide core. The results, shown in Figure 3, indicate that both compounds 14 and 15 led to isolation of the same protein bands. This suggests that these protein bands are due to non-specific interactions of biotin with cellular proteins.19
Figure 3.
Immunoblot of total protein lysate of cells treated with 14 (a) and 15 (b) and subjected to photo activation. Biotinylated proteins are visualized by Streptavidin-HRP.
In parallel with the above studies, we considered an alternative design of trifunctional probes in which the photoactivated aryl azide unit would be replaced by an epoxide functionality. This design was inspired by the observation of compound 6 induces an identical phenotype to that of norrisolide in Golgi membranes.12 We hypothesized that a probe that combines the perhydroindane motif with an epoxide unit and biotin would ensure the irreversible Golgi vesiculation and allow the isolation of its target protein. This hypothesis led us to synthesize and evaluate probes 20 and 24.
The synthesis of compound 20 is shown in Scheme 2. Alkylation of 16 with 1,3-dibromopropane (1.1 equiv) in refluxing acetone using K2CO3 as the base produced selectively the C14-monoalkylated adduct that, after treatment with NaN3, gave rise to azide 17 (83% yield over two steps). The C-12 phenolic oxygen was then benzylated and the adjacent methyl ester was saponified to afford carboxylic acid 18 (94% yield over two steps). Coupling of 18 with alcohol 7, followed by Staudinger reduction20 of the pendant azide group produced amine 19 (75% combined yield) that was coupled with biotin (13). Hydrogenolysis of the C12 benzyl ether of 19, followed by alkylation of the resulting alcohol with epibromohydrin using CsCO3 as the base, produced probe 20 (59% yield over three steps).21
Scheme 2.
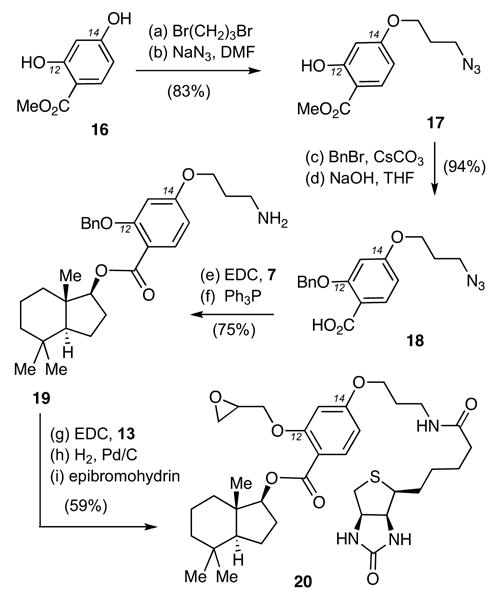
Reagents and conditions: (a) 1,3-dibromopropane (1.1 equiv), K2CO3 (2.0 equiv), acetone, 60 °C, 4 h, 87%; (b) NaN3 (1.5 equiv), DMF, 25 °C, 16 h, 95%; (c) BnBr (1.6 equiv), CsCO3 (2.0 equiv), DMF, 25 °C, 16 h, 95%; (d) NaOH (1 N) in THF (1:1) 50 °C, 4 h, 99%; (e) 7 (1.0 equiv), 18 (2.0 equiv), EDC (2.0 equiv), DMAP (2.0 equiv), CH2Cl2, 25 °C, 24 h, 83%; (f) PPh3 (1.3 equiv), H2O (1.3 equiv), THF, 25 °C, 24 h, 90%; (g) 13 (1.3 equiv), EDC (1.3 equiv), CH2Cl2/DMF: 1/1, 25 °C, 12 h, 92%; (h) H2 (1 atm), 10% Pd/C (0.2 equiv), AcOEt, 25 °C, 6 h, 79%; (i) epibromohydrin (1.3 equiv), CsCO3 (1.3 equiv), DMF, 25 °C, 12 h, 81%.
A similar strategy was devised for the construction of probe 24 (Scheme 3).21 Compound 21 was selectively alkylated first at the C14 phenol (epibromohydrin, K2CO3) and then at the C12 center using EZ-link PEO-iodoacetylbiotin (23)22 and CsCO3, to form 24 in 55% yield (over two steps).
Scheme 3.
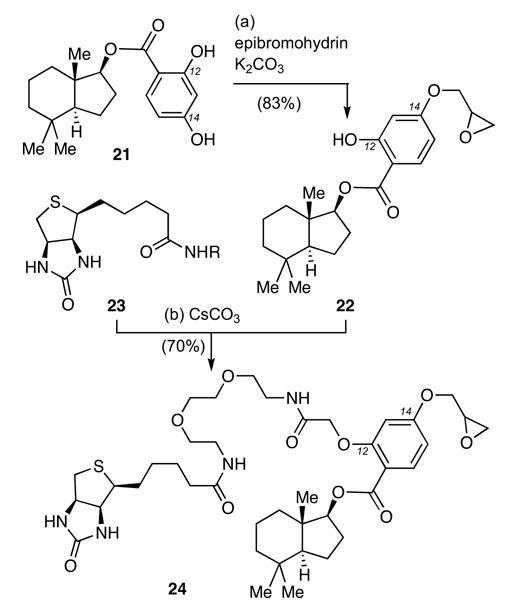
Reagents and conditions: (a) epibromohydrin (1.3 equiv), K2CO3 (1.3 equiv), acetone, 60 °C, 2 h, 83%; (b) 22 (1.0 equiv), 23 (R= (CH2CH2O)2CH2CH2NHCOCH2I) (1.3 equiv), CsCO3 (1.5 equiv), DMF, 25 °C, 16 h, 70%.
Evaluation of probes 20 and 24 in NRK cells was performed using the conditions discussed above. Incubation of NRK cells with 20 induced less than 10% of Golgi fragmentation in the entire cell population (Figure 4a). Moreover, this effect was fully reversible after cell washing (Figure 4b). Similar results were obtained with compound 24 (data not shown). The Golgi fragmentation induced by probes 20 and 24 was clearly less effective than that observed using norrisolide and compound 6 that, under identical conditions, led to complete and irreversible vesiculation in more than 90% of the cell population.12
Figure 4.
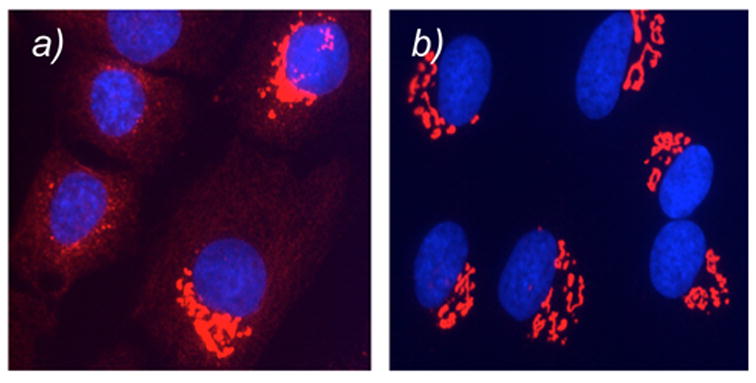
NRK cells treated with 20 for 60 min (a); then washed and incubated in fresh medium for 60 min (b). Golgi is shown in red; nuclei in blue. Similar results are obtained with probe 24.
We hypothesized that the low extent of Golgi vesiculation induced by probes 20 and 24 is due to the presence of biotin tag. It is possible that the steric hindrance caused by biotin in combination with its intrinsic affinity for biotin receptors leaves insufficient amounts of these probes on the Golgi membranes thus dramatically reducing their effect on the Golgi complex. In other words, the biotin tag could interfere with the ability of the perhydroindane motif to localize these probes on the Golgi. This hypothesis is also supported by the results obtained with probe 14 that again led to a decreased extent of Golgi vesiculation. This analysis led us to reconsider our overall strategy and design an alternative probe in which the biotin unit would be replaced by a radioisotope. Isolation of the norrisolide target(s) could then be pursued via radiolabeling.
The structure of epoxide 22 offers the unique advantage of facile incorporation of radioactive iodine at the aromatic ring at the C15 center. The synthesis of the non-radioactive probe 2521 is highlighted in Scheme 4 and involves iodination of 22 with NaI and chloramine T,23 followed by alkylation of the C12 phenol with epibromohydrin (74% yield over two steps).
Scheme 4.
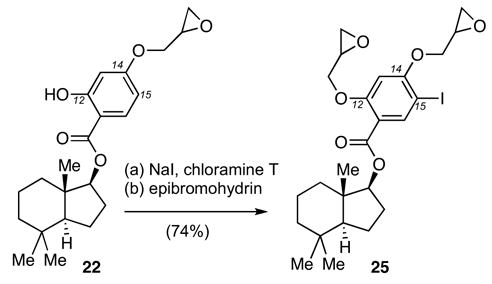
Reagents and conditions: (a) NaI (1.2 equiv), chloramine T (1.1 equiv), CH3CN/H2O: 10:1, 25 °C, 0.5 h, 89%; (b) epibromohydrin (2.0 equiv), CsCO3 (1.5 equiv), DMF, 25 °C, 12 h, 83%.
Incubation of NRK cells with compound 25 induced Golgi vesiculation at concentrations similar to those used for norrisolide and 6. Importantly, this fragmentation was found to occur in more than 80% of cells and was irreversible after standard washing (Figure 5). In fact, probe 25 induced comparable Golgi vesiculation to that obtained with analogue 6. This result suggests that a probe 25 armed with a radioactive isotope 125I could be used to tag the protein target and lead to its isolation radiochromatography.24
Figure 5.
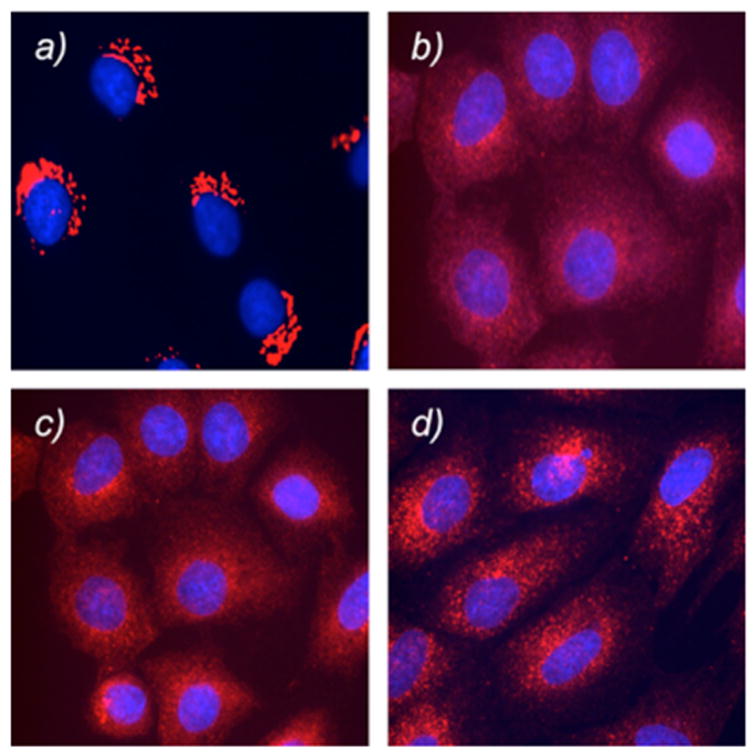
Effect of 6 and 25 on Golgi membranes. Untreated NRK cells (a) and NRK cells treated for 60 min with 6 (b) and 25 (c). Cells treated as in (c) are then washed and allowed to recover for 60 min in fresh medium (d).
In conclusion, we present herein the results of a study aiming to develop norrisolide-based trifunctional probes that could be used to identify the cellular target of this unusual natural product. Inspired by the finding that norrisolide and its analogue 6 induce an irreversible fragmentation of the Golgi membranes, we attempted to conjugate this structure with biotin and isolate its target by affinity chromatography. None of the biotin-based probes induced significant Golgi fragmentation to justify further studies. The only proteins isolated with these probes were non-specific biotin targets. However, iodinated probe 25 induces sufficient Golgi vesiculation at comparable concentrations to that of the natural product. Thus, this probe represents an appropriate reagent for further biological studies aiming to isolate the cellular target of norrisolide.
Acknowledgments
Financial support by the NIH (CA 086079 to EAT and GM 46224 and GM 53747 to VM) is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Glick BS. In: Protein Targeting, Transport & Translocation. RE Dalbey, Von Heijne G., editors. New York: Academic Press; 2002. pp. 358–376. For selected reviews on the secretory pathway see: [Google Scholar]; Ostermann J, Stauber T, Nilsson T. In: Protein Targeting, Transport & Translocation. Dalbey RE, Von Heijne G, editors. New York: Academic Press; 2002. pp. 377–401. [Google Scholar]; Trombetta ES, Parodi A. J Ann Rev Cell & Develop Biol. 2003;19:649–676. doi: 10.1146/annurev.cellbio.19.110701.153949. [DOI] [PubMed] [Google Scholar]; Harter C, Reinhard C. Subcell Biochem. 2000;34:1–38. doi: 10.1007/0-306-46824-7_1. [DOI] [PubMed] [Google Scholar]; Haigh NG, Johnson AE. In: Protein Targeting, Transport & Translocation. Dalbey RE, Von Heijne G, editors. New York: Academic Press; 2002. pp. 74–106. [Google Scholar]
- 2.Roth J. Chem Rev. 2002;102:285–303. doi: 10.1021/cr000423j. For selected reports on this topic see: [DOI] [PubMed] [Google Scholar]; Arnold SM, Kaufman RJ. New Compreh Biochem. 2003;38:411–432. [Google Scholar]; van Vliet C, Thomas EC, Merino-Trigo A, Teasdale RD, Gleeson PA. Progr Biophys & Mol Biol. 2003;83:1–45. doi: 10.1016/s0079-6107(03)00019-1. [DOI] [PubMed] [Google Scholar]; Schulein R. Rev Physiol Biochem & Pharmacol. 2004;151:45–91. doi: 10.1007/s10254-004-0022-8. [DOI] [PubMed] [Google Scholar]; Sifers RN. Science. 2003;299:1330–1331. doi: 10.1126/science.1082718. [DOI] [PubMed] [Google Scholar]
- 3.Maag RS, Hicks SW, Machamer CE. Curr Opin Cell Biol. 2003;15:456–461. doi: 10.1016/s0955-0674(03)00075-9. For selected reports on the effect of Golgi during cell cycle see: [DOI] [PubMed] [Google Scholar]; Lippincott-Schwartz J, Zaal KJM. Histochem Cell Biol. 2000;114:93–103. doi: 10.1007/s004180000176. [DOI] [PubMed] [Google Scholar]; Colanzi A, Deerinck TJ, Ellisman MH, Malhotra V. J Cell Biol. 2000;149:331–339. doi: 10.1083/jcb.149.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nelson WJ. J Cell Biol. 2000;149:243–248. doi: 10.1083/jcb.149.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sutterlin C, Lin CY, Feng Y, Ferris DK, Erikson RL, Malhotra V. Proc Natl Acad Sci USA. 2001;98:9128–9132. doi: 10.1073/pnas.161283998. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lin CY, Madsen ML, Yarm FR, Jang YJ, Liu X, Erikson RL. Proc Natl Acad Sci USA. 2000;97:12589–12594. doi: 10.1073/pnas.220423497. [DOI] [PMC free article] [PubMed] [Google Scholar]; Rios RM, Bornens M. Curr Opin Cell Biol. 2003;15:60–66. doi: 10.1016/s0955-0674(02)00013-3. [DOI] [PubMed] [Google Scholar]
- 4.Colanzi A, Suetterlin C, Malhotra V. Curr Opin Cell Biol. 2003;15:462–467. doi: 10.1016/s0955-0674(03)00067-x. [DOI] [PubMed] [Google Scholar]; Warren G, Malhotra V. Curr Opin Cell Biol. 1998;10:493–498. doi: 10.1016/s0955-0674(98)80064-1. [DOI] [PubMed] [Google Scholar]; Glick BS. Curr Opin Cell Biol. 2000;12:450–456. doi: 10.1016/s0955-0674(00)00116-2. [DOI] [PubMed] [Google Scholar]; Shorter J, Warren G. Rev Cell & Develop Biol. 2002;18:379–420. doi: 10.1146/annurev.cellbio.18.030602.133733. [DOI] [PubMed] [Google Scholar]
- 5.Dinter A, Berger EG. Histochem Cell Biol. 1998;109:371–390. doi: 10.1007/s004180050256. [DOI] [PubMed] [Google Scholar]; Ludwig JW, Richards ML. Curr Topics Med Chem. 2006;6:165–178. doi: 10.2174/156802606775270314. [DOI] [PubMed] [Google Scholar]
- 6.Pelham HR. Cell. 1991;67:449–451. doi: 10.1016/0092-8674(91)90517-3. [DOI] [PubMed] [Google Scholar]; Pelham HR. Trends Biochem Sci. 1990;15:483–486. doi: 10.1016/0968-0004(90)90303-s. [DOI] [PubMed] [Google Scholar]; Klausner RD, Donaldson JG, Lippincott-Schwartz J. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]; Reaves B, Banting G. J Cell Biol. 1992;116:85–94. doi: 10.1083/jcb.116.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]; Schiaky N, Presley J, Smith C, Zaal KJM, Cole N, Moreira JE, Terasaki M, Siggia E, Lippincott-Schwartz J. JCell Biol. 1997;139:1137–1155. doi: 10.1083/jcb.139.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pelish HE, Ciesla W, Tanaka N, Reddy K, Shair MD, Kirchhausen T, Lencer WI. Biochem Pharmacol. 2006;71:1720–1726. doi: 10.1016/j.bcp.2006.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pelish HE, Peterson JR, Salvarezza SB, Rodriguez-Boulan E, Chen JL, Stammes M, Macia E, Feng Y, Shair MD, Kirchhausen T. Nature Chem Biol. 2006;2:1, 39–46. doi: 10.1038/nchembio751. [DOI] [PubMed] [Google Scholar]; Pelish HE, Westwood NJ, Feng Y, Kirchhausen T, Shair MD. J Am Chem Soc. 2001;123:6740–6741. doi: 10.1021/ja016093h. [DOI] [PubMed] [Google Scholar]
- 8.Goess BC, Hannoush RN, Chan LK, Kirchhausen T, Shair MD. J Am Chem Soc. 2006;126:5391–5403. doi: 10.1021/ja056338g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takizawa PA, Yucel JK, Veit B, Faulkner JD, Deernick T, Soto G, Ellisman M, Malhotra V. Cell. 1993;73:1079–1090. doi: 10.1016/0092-8674(93)90638-7. [DOI] [PubMed] [Google Scholar]; Veit B, Yucel JK, Malhotra V. J Cell Biol. 1993;122:1197–1206. doi: 10.1083/jcb.122.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]; Radeke HS, Digits CA, Casaubon RL, Snapper ML. Chem & Biol. 1999;6:639–647. doi: 10.1016/s1074-5521(99)80115-x. [DOI] [PubMed] [Google Scholar]; Radeke HS, Snapper ML. Bioorg & Med Chem. 1998;6:1227–1232. doi: 10.1016/s0968-0896(98)00100-x. [DOI] [PubMed] [Google Scholar]; Casaubon R, Snapper ML. Bioorg & Med Chem Lett. 2001;11:133–136. doi: 10.1016/s0960-894x(00)00617-x. [DOI] [PubMed] [Google Scholar]; Jamora C, Takizawa PA, Zaarour RF, Denesvre C, Faulkner DJ, Malhotra V. Cell. 1999;91:617–626. doi: 10.1016/s0092-8674(00)80449-3. [DOI] [PubMed] [Google Scholar]
- 10.Hochlowski JE, Faulkner DJ, Matsumoto J, Clardy J. J Org Chem. 1983;48:1141–1142. [Google Scholar]
- 11.Brady TP, Wallace EK, Kim SH, Guizunnti G, Malhotra V, Theodorakis EA. Bioorg Med Chem Lett. 2004;14:5035–5039. doi: 10.1016/j.bmcl.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Guizzunti G, Brady TP, Malhotra V, Theodorakis EA. J Am Chem Soc. 2006;128:4190–4191. doi: 10.1021/ja058259a. [DOI] [PubMed] [Google Scholar]
- 13.Schnapp KA, Poe R, Leyva E, Soundararajan N, Platz MS. Bioconj Chem. 1993;4:172–177. doi: 10.1021/bc00020a010. [DOI] [PubMed] [Google Scholar]; Chen Y, Ebright RH. J Mol Biol. 1993;230:453–460. doi: 10.1006/jmbi.1993.1162. [DOI] [PubMed] [Google Scholar]; Chavan AJ, Rychlik W, Blaas D, Luechler E, Watt DS, Rhoads RE. Biochem. 1990;29:5521–5529. doi: 10.1021/bi00475a016. [DOI] [PubMed] [Google Scholar]
- 14.All new compounds exhibited satisfactory spectroscopic and analytical data. Yields refer to spectroscopically and chromatographically homogeneous materials.
- 15.Brady TP, Kim SH, Wen K, Theodorakis EA. Angew Chem Int Ed. 2004;43:739–742. doi: 10.1002/anie.200352868. [DOI] [PubMed] [Google Scholar]; Brady TP, Kim SH, Wen K, Kim C, Theodorakis EA. Chemistry Eur J. 2005;11:7175–7190. doi: 10.1002/chem.200500513. [DOI] [PubMed] [Google Scholar]
- 16.Hasinoff BB, Chee GL, Day BW, Avor KS, Barnabe N, Thampatty P, Yalowich JC. Bioorg Med Chem. 2001;9:1765–1771. doi: 10.1016/s0968-0896(01)00102-x. [DOI] [PubMed] [Google Scholar]; Gano KW, Monbouquette HG, Myles DC. Tetrahedron Lett. 2001;42:2249–2251. [Google Scholar]
- 17.Spectroscopic and analytical data for compounds 14–15. Compound 14: [α]25D= +48.7 (c= 0.45, CH2Cl2); Rf= 0.2 (3% MeOH in CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.99 (m, 1H), 7.95 (d, J= 8.4 Hz, 1H), 6.67 (d, J= 8.4 Hz, 1H), 6.54 (s, 1H), 5.92 (bs, 1H), 4.68 (t, J= 8 Hz, 1H), 4.84 (m, 1H), 4.29 (m, 1H), 4.12 (m, 2H), 3.51 (m, 2H), 3.12 (m, 1H), 2.89 (m,1H), 2.86 (m, 1H), 2.72 (d, J= 12.4 Hz, 1H), 2.38 (t, J= 7.2 Hz, 2H), 2.25– 2.20 (m, 2H), 2.05 (t, J= 4.8 Hz, 2H), 1.78–1.42 (m, 11H), 1.25–1.03 (m, 4H) 0.99 (s, 3H), 0.90 (s, 3H), 0.87 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 173.9, 164.4, 160.5, 146.0, 133.7, 115.2, 110.4, 103.2, 83.7, 69.4, 61.7, 55.4, 52.8, 42.9, 41.4, 40.5, 38.4, 37.9, 35.3, 33.1, 32.9, 28.5, 28.2, 28.1, 27.0, 26.7, 25.9, 20.8, 20.4, 19.4, 13.1; HRMS, calcd for C32H46N6O5S [M+Na+]: 649.31478, found 649.31469. Compound 15: Rf= 0.2 (3% MeOH in CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.92 (d, J= 8.4 Hz, 1H), 7.82 (t, J= 4.8 Hz, 1H), 6.65 (dd, J= 8.4, 2 Hz, 1H), 6.53 (d, J= 1.6 Hz, 1H), 6.10 (bs, 1H), 5.57 (bs, 1H), 4.56 (m, 1H), 4.27 (m, 1H), 4.11 (t, J= 5.2 Hz, 2H), 3.85 (s, 3H), 3.51 (q, J= 5.2 Hz, 2H), 3.12 (m, 1H), 2.86 (dd, J= 12.8, 5.2 Hz, 1H), 2.72 (d, J= 12.8 Hz, 1H), 2.35 (t, J= 7.6 Hz, 2H), 2.04 (m, 2H), 1.77–1.60 (m, 4H), 1.44 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 173.8, 165.1, 163.7, 160.4, 146.1, 133.7, 114.7, 110.4, 103.2, 69.2, 61.7, 60.1, 55.4, 51.9, 40.5, 38.2, 35.4, 28.5, 28.2, 28.1, 25.9; HRMS, calcd for C21H28N6O5S [M+Na+]: 499.17396, found 499.1742.
- 18.Reagents and cells: NRK cells were grown on coverslips as described in reference 11. Stock solution (5 mg/ml) of norrisolide analogues were made in DMSO and stored at –20 °C. The working concentration of the compounds was 30 μM for each coverslip and cells were incubated at 37 °C for 60 min. An equal volume of DMSO was used as a negative control for each compound. For the crosslinking reaction, cells were irradiated for 15 min with UV light using a UVL-21 hand lamp (1mW/cm2 at 366 nm) placed at 5 cm distance from the cells. To test the irreversibility of Golgi fragmentation, cells were washed 4 times with phosphate-buffered saline (PBS) (150 mM NaCl, 1.8 mM NaH2PO4, 8.4 mM Na2HPO4) and then incubated in fresh complete medium at 37°C for 90 minutes. Immunofluorescence microscopy was performed as described in reference 11. Immunoblot: Cells treated with 14 and 15 for 60 min and irradiated with UV light were collected and lysated with Loading Buffer (60 mM Tris pH 6.8, 5% 2-Mercaptoethanol, 2% SDS, 0.01% Bromophenol Blue, 10% glycerol). Proteins in the lysate were separated by SDS-PAGE electrophoresis using a 10% running gel. Proteins were transferred on a nitrocellulose membrane (Western Blot) (60 min, 350 mA) that was then kept in blocking buffer (50 mN Tris pH 7.5, 150 mM NaCl, 0.05% Tween20, 1% BSA) for 30 min. The membrane was incubated for 1 h at room temperature with Streptavidin-HRP (BD Pharmingen) diluted in blocking buffer. After washing 3 times with PBS, it was added the reagent for ECL (Perkin Elmer). Kodak Biomax films were used for exposure.
- 19.Chapman-Smith A, Cronan JE., Jr Biomol Eng. 1999;16:119–125. doi: 10.1016/s1050-3862(99)00046-7. [DOI] [PubMed] [Google Scholar]
- 20.Liu L, Hong ZY, Wong CH. ChemBioChem. 2006;7:429–432. doi: 10.1002/cbic.200500437. [DOI] [PubMed] [Google Scholar]; Soellner MB, Nillson BL, Raines RT. J Am Chem Soc. 2006;128:8820–8828. doi: 10.1021/ja060484k. [DOI] [PubMed] [Google Scholar]
- 21.Pierce Biotechnology, Inc. Rockford, IL: Compound 23 is commercially available.
- 22.Spectroscopic and analytical data for compounds 20, 24 and 25. Compound 20: Rf= 0.4 (5% MeOH in CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.84 (d, J= 8.8 Hz, 1H), 6.50 (m, 2H), 6.11 (bs, 1H), 5.78 (s, 1H), 5.10 (s, 2H), 4.86 (d, J= 8.8 Hz, 2H), 4.75 (t, J= 6.8 Hz, 1H), 4.50 (m, 2H), 4.31 (m, 2H), 4.29 (m, 2H), 4.04 (m, 2H), 3.42 (m, 2H), 3.14 (m, 1H), 2.91 (m, 3H), 2.71 (m, 1H), 2.34 (t, J= 6.8 Hz, 2H), 2.20 (m, 2H), 2.01 (m, 2H), 1.70–1.01 (m, 11H), 0.99 (s, 3H), 0.89 (s, 3H), 0.86 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 173.1, 165.4, 163.2, 162.9, 160.1, 133.7, 107.5, 106.0, 100.7, 83.4, 69.6, 61.8, 61.7, 60.0, 55.3, 55.2, 52.7, 50.2, 41.4, 40.5, 37.7, 36.8, 35.9, 33.6, 31.4, 29.7, 28.9, 28.3, 27.9, 26.5, 25.5, 20.8, 20.3, 19.4, 13.1; HRMS, calcd for C35H51N3O7S [M+Na+]: 680.33454, found 680.2249. Compound 24: [α]25D= +17.9 (c= 0.3, CH2Cl2); Rf= 0.3 (70% ether in hexanes); 1H NMR (400 MHz, CDCl3) δ 8.54 (bs, 1H), 7.88 (d, J= 8.8 Hz, 1H), 6.82 (m, 1H), 6.56 (dd, J= 8.8, 2.4 Hz, 1H), 6.48 (d, J= 2.4 Hz, 1H), 6.36 (s, 1H), 5.37 (s, 1H), 4.69 (t, J= 8 Hz, 1H), 4.57 (s, 2H), 4.48 (m, 1H), 4.32 (dd, J= 11.2, 2.8 Hz, 1H), 4.29 (m, 1H), 3.95 (dd, J= 10.8, 5.6 Hz, 1H), 3.67–3.53 (m, 12H), 3.43–3.34 (m, 2H), 3.11 (m, 1H), 2.95–2.86 (m, 3H), 2.78–2.68 (m, 2H) 2.21 (m, 2H), 2.01 (bs, 2H) 1.75–1.01(m, 13H), 0.99 (s, 3H), 0.90 (s, 3H), 0.87 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 173.3, 168.5, 164.6, 163.2, 159.6, 133.6, 112.5, 106.9, 100.8, 83.2, 70.2, 70.1, 69.9, 69.4, 69.1, 67.9, 61.7, 60.1, 55.2, 52.8, 49.9, 44.5, 43.8, 41.4, 40.5, 39.1, 38.9, 37.8, 35.9, 33.1, 32.9, 28.2, 28.0, 26.6, 25.6, 20.7, 20.3, 20.2, 19.4, 13.1; HRMS, calcd for C40H60N4O10S [M+H+]: 789.41084, found 789.4107. Compound 25: [α]25D= +22.2 (c= 0.1, CH2Cl2); Rf= 0.3 (70% ether in hexanes); 1H NMR (400 MHz, CDCl3) δ 8.24 (s, 1H), 6.60 (d, J= 6.4 Hz, 1H), 4.73 (t, J= 7.6 Hz, 1H), 4.44–4.33 (m, 2H), 4.09–3.99 (m, 2H), 3.38 (m, 2H), 2.95– 2.87 (m, 4H), 2.83 (m, 1H), 2.22 (m, 1H), 1.75–1.01 (m, 11H), 0.99 (s, 3H), 0.90 (s, 3H), 0.86 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 164.2, 160.8, 160.5, 142.3, 115.7, 99.3, 83.7, 70.3, 69.8, 52.7, 50.1, 50.0, 49.9, 44.7, 44.5, 41.4, 37.7, 33.0, 32.8, 29.6, 26.5, 20.7, 20.3, 19.4, 13.1; HRMS, calcd for C25H33IO6 [M+H+]: 557.1400, found 557.1412.
- 23.Kometani T, Watt DS, Ji T. Tetrahedron Lett. 1985;26:2043–2046. [Google Scholar]
- 24.Sun P, Wang GX, Furuta K, Suzuki M. Bioorg Med Chem Lett. 2006;16:2433–2436. doi: 10.1016/j.bmcl.2006.01.083. [DOI] [PubMed] [Google Scholar]; Hashimoto M, Kato Y, Hatanaka Y. Tetrahedron Lett. 2006;47:3391–3394. [Google Scholar]


