Abstract
Alzheimer’s disease and frontotemporal dementia (FTD) can be difficult to differentiate clinically because of overlapping symptoms. Distinguishing the two dementias based on volumetric measurements of brain atrophy with MRI has been only partially successful. Whether MRI measurements of cortical thinning improve the differentiation between Alzheimer’s disease and FTD is unclear. In this study, we measured cortical thickness using a set of automated tools (Freesurfer) to reconstruct the brain’s cortical surface from T1-weighted structural MRI data in 22 patients with Alzheimer’s disease, 19 patients with FTD and 23 cognitively normal subjects. The goals were to detect the characteristic patterns of cortical thinning in these two types of dementia, to test the relationship between cortical thickness and cognitive impairment, to determine if measurement of cortical thickness is better than that of cortical volume for differentiating between these dementias and normal ageing and improving the classification of Alzheimer’s disease and FTD based on neuropsychological scores alone. Compared to cognitively normal subjects, Alzheimer’s disease patients had a thinner cortex primarily in bilateral, frontal, parietal, temporal and occipital lobes (P < 0.001), while FTD patients had a thinner cortex in bilateral, frontal and temporal regions and some thinning in inferior parietal regions and the posterior cingulate (P< 0.001). Compared to FTD patients, Alzheimer’s disease patients had a thinner cortex (P< 0.001) in parts of bilateral parietal and precuneus regions. Cognitive impairment was negatively correlated with cortical thickness of frontal, parietal and temporal lobes in Alzheimer’s disease, while similar correlations were not significant in FTD. Measurement of cortical thickness was similar to that of cortical volume in differentiating between normal ageing, Alzheimer’s disease and FTD. Furthermore, cortical thickness measurements significantly improved the classification between Alzheimer’s disease and FTD based on neuropsychological scores alone, including the Mini-Mental State Examination and a modified version of the Trail-Making Test. In conclusion, the characteristic patterns of cortical thinning in Alzheimer’s disease and FTD suggest that cortical thickness may be a useful surrogate marker for these types of dementia.
Keywords: Alzheimer’s disease, frontotemporal dementia, cortical thickness, cortical volume
Introduction
Alzheimer’s disease and frontotemporal dementia (FTD) are sometimes difficult to differentiate clinically because of overlapping symptoms (McKhann et al., 1984; Neary et al., 1998; Siri et al., 2001). Definite diagnosis requires histopathological examination of brain tissue. Although structural MRI data depict characteristic patterns of brain atrophy in Alzheimer’s disease and FTD, aiding a differential diagnosis between the dementias (Kitagaki et al., 1998; Frisoni et al., 1999; Laakso et al., 2000; Rosen et al., 2002; Gee et al., 2003; Grossman et al., 2004; Lipton et al., 2004; Whitwell et al., 2005), a complete division based on MRI has not been accomplished. Histopathological studies reported that Alzheimer’s disease and FTD pathologies are associated with damage to specific cortical layers, e.g. layer II of the entorhinal cortex and layer III of the neocortex in Alzheimer’s disease and layer III and V of frontal and temporal lobes in FTD (Pearson et al., 1985; Lewis et al., 1987; Gomez-Isla et al., 1996; Kersaitis et al., 2004). Although current MRI methods lack the power to resolve individual cortical layers, these histological observations raise the possibility that MRI-based examination of cortical thickness may be more specific than volumetric measurements for a differential diagnosis between Alzheimer’s disease and FTD. However, since the cortex is a highly folded structure and its surface is rarely positioned perpendicular to any of the cardinal axes, measurements of cortical thickness are difficult, especially in presence of pathological alterations. Techniques have been recently developed for measuring cortical thickness in MRI using automated surface reconstruction, transformation and high-resolution intersubject alignment procedures (Fischl et al., 1999; Dale et al., 1999; Fischl and Dale, 2000). In addition, a recent MRI study of cortical thickness showed thinning of the cortex in broad brain regions such as medial temporal lobe, frontal and parietal lobes in patients with Alzheimer’s disease when compared to cognitively normal (CN) subjects, consistent with the expected pathological pattern of Alzheimer’s disease (Lerch et al., 2005). However, to what extent these patterns in Alzheimer’s disease are dissociable from other dementias has not been established. Furthermore, MRI reports of cortical thinning in FTD are sparse. Therefore the main goal of this study was to determine the characteristic pattern of cortical thinning in FTD compared to CN and differences in cortical thickness between FTD and Alzheimer’s disease. The second goal was to explore the relationship between cortical thickness and severity of cognitive impairment in Alzheimer’s disease and FTD. Lastly, we compared the diagnostic value of assessing cortical thinning versus cortical volume loss for differentiating between normal ageing, Alzheimer’s disease and FTD and tested if measurement of cortical thickness improves the classification between Alzheimer’s disease and FTD based on neuropsychological scores alone.
Material and methods
Subjects
Twenty-three CN subjects, 22 patients diagnosed with Alzheimer’s disease and 19 patients diagnosed with FTD were included in the study (Table 1). The majority of subjects in this study are identical to those reported in our previous perfusion study (Du et al., 2006) except for two patients with Alzheimer’s disease, two patients with FTD and two CN subjects, who had MRI of inferior quality, not suitable for reliable tissue segmentation and spatial normalization processing with Freesurfer (https://surfer.nmr.mgh.harvard.edu). The patients with FTD and Alzheimer’s disease were recruited from the Memory and Aging Center of the University of California, San Francisco as described in detail in our previous paper (Du et al., 2006). All patients were diagnosed based upon information obtained from an extensive clinical history and physical examination. FTD was diagnosed according to the consensus criteria established by Neary et al. (1998). Patients with FTD who had motor neuron disease-related symptoms were excluded. Patients with Alzheimer’s disease were diagnosed according to the criteria of the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association (NINCDS/ADRDA) (McKhann et al., 1984). All subjects received a standard battery of neuropsychological tests, including assessment of global cognitive impairment using the Mini-Mental State Examination (MMSE) (Folstein et al., 1975) scores and global functional impairment using the Clinical Dementia Rating (CDR) scale (Morris, 1993). A modified version of the Trail-Making Test (TMT) was used to assess executive functions (Rosen et al., 2004). MRI data were visually inspected by a radiologist to rule out major neuropathologies other than neurodegeneration, such as tumour, stroke and severe white matter disease. All subjects or their guardians gave written informed consent before participating in the study, which was approved by the Committees of Human Research at the University of California and the VA Medical Center at San Francisco.
Table 1.
Demographics
| Controls | AD | FTD | |
|---|---|---|---|
| Number (F/M) | 23 (14/9) | 22(8/14) | 19 (3/16) |
| Age (years) | 61.9 ± 6.3 | 62.8 ± 7.0 | 61.7 ± 7.5 |
| MMSE | 29.9 ± 0.3 | 19.2 ± 5.5* | 25.1 ± 5.7*† |
| CDR box score | 0 ± 0 | 5.0 ± 2.8* | 6.3 ± 3.7* |
| Modified trail | 35.6 ± 6.2 | 6.0 ± 8.0* | 17.3 ± 11.7† |
P < 0.01 between AD vs CN or FTD vs CN;
P < 0.01 between Alzheimer’s disease and FTD. In units of number of corrected lines per minute.
Data acquisition and processing
MRI data were obtained on a 1.5 T Siemens Vision™ System (Siemens Inc., Iselin NJ), using a standard quadrature head coil. Structural MRI data were acquired using a double spin echo (DSE) sequence and a volumetric magnetization-prepared rapid gradient echo (MPRAGE) T1-weighted sequence. The parameters of DSE images were: TR/TE1/TE2 = 2500/20/80 ms timing, 1.00 × 1.25 mm2 in-plane resolution, and about 50 contiguous 3.00 mm thick axial slices oriented along the optic nerve as seen from a sagittal scout MR image. The parameters of MPRAGE T1-weighted images were: TR/TE/TI = 10/7/300 ms timing, 15° flip angle, 1.00 × 1.00 mm2 in-plane resolution, and 1.40 mm thick coronal partitions and oriented orthogonal to the image planes of DSE.
The construction cortical surface was based on 3D MPRAGE images using Freesurfer (http://surfer.nmr.mgh.harvard.edu/) software. The detailed procedure for the surface construction with Freesurfer has been described and validated in previous papers (Fischl et al., 1999; Dale et al., 1999; Fischl and Dale, 2000). In brief, the procedure involves segmentation of white matter, tessellation of the grey/white matter junction, inflation of the folded surface tessellation patterns and automatic correction of topological defects in the resulting manifold. This surface is then used as the starting point for a deformable surface algorithm designed to find the grey/white and pial surfaces with submillimetre precision. This method uses both intensity and continuity information from the surfaces in the deformation procedure in order to interpolate surface locations for regions in which the MRI image is ambiguous. For each subject, thickness of the cortical ribbon was computed on a uniform grid with 1 mm spacing across both cortical hemispheres, with the thickness being defined by the shortest distance between the grey/white and pial surface models (Fischl and Dale, 2000), providing in essence estimates of submillimetre differences. Thickness measures were mapped to the inflated surface of each subject’s brain reconstruction, allowing visualization of data across the entire cortical surface. All images were aligned to a common surface template using a high-resolution surface-based averaging technique that aligned cortical folding patterns. Regions of interest (ROI) on a standard brain (Desikan et al., 2006) were mapped back to each participant’s native image space using a high-dimensional spherical morphing procedure to find the homologous regions across subjects. Then volume and mean thickness of cortical grey matter in each ROI were determined. Finally, cortical thickness was smoothed with a 10-mm full width at half height Gaussian kernel to reduce local variations in the measurements for further analysis.
Statistics
The comparison of regional cortical thickness variations between groups was tested regionally unbiased vertex-by-vertex using analysis of covariance (ANCOVA) with adjustments for age and sex effects. Significance was set to a P-value of < 0.001 without correction for family-wise errors. In addition to the regionally unbiased approach, ROIs measured cortical thickness in frontal, parietal and temporal lobes were also compared between the groups using ANCOVA with age and sex as covariates. Furthermore, the ROI measurements were used to test the relationship between cortical thickness in frontal, parietal and temporal lobe regions and cognitive function. Relationships between neuropsychological scores and MRI measures were tested using Pearson correlation statistics. The discriminatory powers of cortical thickness, cortical volume and neuropsychological tests were tested by leave-one-out cross-validations of the logistic regressions. In addition, the comparison of cortical thickness and volume for differentiating between CN, Alzheimer’s disease and FTD was tested by comparing the area under receiver operator characteristic analysis with Mann–Whitney tests (DeLong et al., 1988). The significance level of the tests was α < 0.05.
Results
The comparison of cortical thickness in Alzheimer’s disease and FTD versus CN
Figure 1 depicts the regional pattern of cortical thinning in patients with Alzheimer’s disease compared to CN subjects, showing for the Alzheimer’s disease significant cortical thinning in broad regions of bilateral frontal, parietal, temporal and occipital lobes (P < 0.001), while the sensor-imotor cortical regions are spared. The most significant thinning in Alzheimer’s disease involved bilateral medial temporal, temporoparietal regions, the posterior cingulate and the precuneus. There was no brain region in patients with Alzheimer’s disease where the cortex was significantly thicker than in CN subjects. Figure 2 depicts the regional pattern of cortical thinning in patients with FTD compared to CN subjects, showing for the FTD significant cortical thinning in bilateral frontal and temporal regions, and in some inferior parietal regions and the posterior cingulate (P < 0.001). The most significant thinning in FTD involved bilateral prefrontal regions, the anterior and posterior cingulate and right anterior temporal region. Similar to Alzheimer’s disease, there was no brain region in FTD patients where the cortex was significantly thicker than in CN subjects.
Fig. 1.
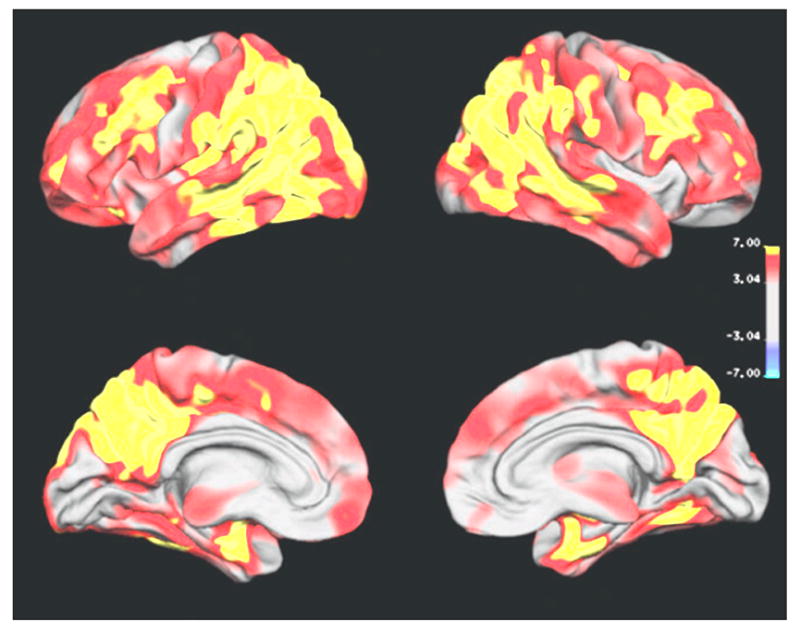
Regional variation of cortical thickness in Alzheimer’s disease compared to controls. The colour-code for P-values is on a logarithmic scale of 1–7. Warmer colours (positive values) represent cortical thinning; cooler colours (negative values) represent cortical thickening.
Fig. 2.
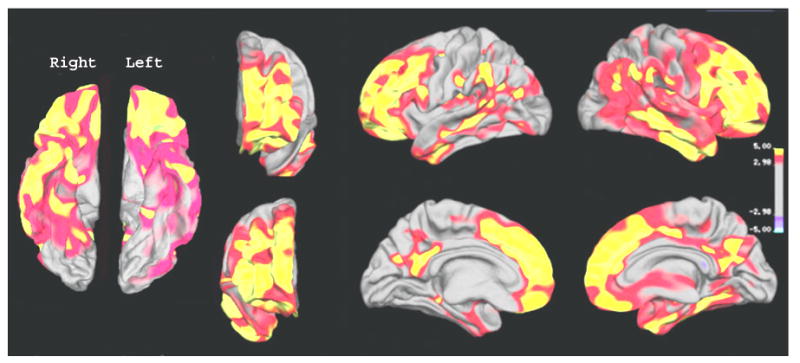
Regional variation of cortical thickness in FTD compared to controls. The colour-coding for P-values is on a logarithmic scale of 1–5. Warmer colours (positive values) represent cortical thinning; cooler colours (negative values) represent cortical thickening.
Comparison of cortical thickness between Alzheimer’s disease and FTD
Figure 3 depicts regional differences of cortical thickness between patients with Alzheimer’s disease and FTD. Compared to patients with FTD, patients with Alzheimer’s disease had a thinner cortex (P < 0.001) in parts of bilateral parietal and precuneus regions and in left temporal and occipital regions. In contrast, patients with FTD exhibited no significant regions of cortical thinning when compared to patients with Alzheimer’s disease.
Fig. 3.
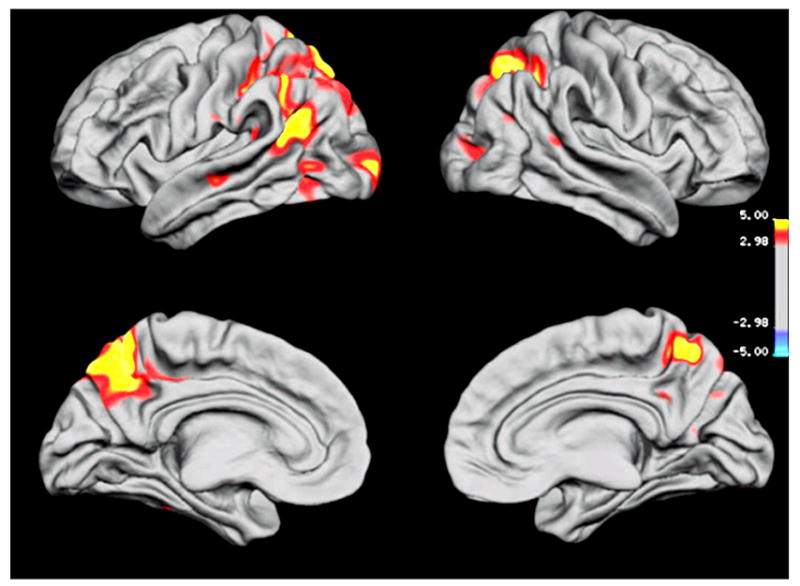
Regional variations of cortical thickness between Alzheimer’s disease and FTD. The colour-coding is identical to that shown in Fig. 2.
ROI analysis of cortical thickness in frontal, parietal and temporal lobes
Average cortical thickness in the frontal, temporal and parietal lobes is listed by group in Table 2. Consistent with the vertex-by-vertex analysis, patients with Alzheimer’s disease and FTD had thinner cortices in the frontal, temporal and parietal lobes than CN subjects (all P < 0.001), independent of age and gender. Furthermore, patients with Alzheimer’s disease had a thinner parietal cortex than patients with FTD (P < 0.001), while differences between Alzheimer’s disease and FTD in the frontal and temporal cortices were not significant. Similar to the vertex-by-vertex analysis, FTD patients exhibited no significant regions of cortical thinning when compared to patients with Alzheimer’s disease.
Table 2.
Average cortical thickness (in mm) and standard deviation of frontal, parietal and temporal lobes in CN, Alzheimer’s disease and FTD
| Frontal | Temporal | Parietal | |
|---|---|---|---|
| CN | 2.4 ± 0.2 | 2.8 ± 0.2 | 2.1 ± 0.2 |
| Alzheimer’s disease | 2.0 ± 0.2* | 2.2 ± 0.3* | 1.5 ± 0.2* |
| FTD | 1.9 ± 0.4* | 2.3 ± 0.4* | 1.9 ± 0.2*† |
P < 0.001 between Alzheimer’s disease vs CN or between FTD vs CN;
P < 0.001 between Alzheimer’s disease and FTD.
Since in the clinical FTD group, there is a greater risk that older patients could be false positive where the true pathological diagnosis is actually Alzheimer’s disease and parietal cortical thinning is considered a feature of Alzheimer’s disease, we tested in FTD separately the extent to which age explains parietal cortical thinning, but found no significant age effect (P = 0.17). Furthermore, we compared the neuropsychological profile of FTD patients having a cortical thickness thicker than the median parietal cortical thickness (1.83 mm) with those having a cortical thickness thinner than the median parietal cortical thickness, but found no significant difference in MMSE, CDR or TMT scores (all P > 0.8) between the two groups.
Relationship between cortical thickness and cognitive function
The relationship between cortical thickness and cognitive functions were tested including only Alzheimer’s disease and FTD patients. MMSE scores were positively correlated with cortical thickness of the frontal (r = 0.50, P < 0.05), temporal (r = 0.48, P < 0.05) and parietal lobes (r = 0.46, P < 0.05) in Alzheimer’s disease, but not in FTD (all P > 0.5). In Alzheimer’s disease, CDR box scores were negatively correlated with cortical thickness of parietal lobe (r = −0.45, P < 0.05), but not that of frontal and temporal lobes, in contrast to MMSE scores. In FTD, no significant correlations were found between CDR box scores and cortical thickness, similar to the results for MMSE. Since the distributions of both MMSE and CDR box scores were skewed towards higher dementia severity, we repeated the analyses of correlations for log-transformed MMSE and CDR data but obtained similar results. Furthermore, executive function, as measured with TMT, was not significantly correlated with cortical thickness of frontal, parietal or temporal lobe in patients with FTD (all regions P > 0.16).
Classification between groups
Results of group classifications using either volume or thickness of frontal, temporal or parietal cortex are summarized in Table 3. This shows that Alzheimer’s disease patients could be distinguished from controls with 87 to 99% accuracy, based on either volume or thickness of frontal, parietal and temporal cortex. In comparison, FTD patients could be distinguished from controls with 74 to 94% accuracy based on either volume or thickness of frontal, parietal and temporal cortex. Moreover, Alzheimer’s disease and FTD patients could be distinguished from each other with 76 to 83% accuracy, based on either volume or thickness of parietal cortex. However, whether volume or thickness was used for classification made no significant difference (all P > 0.3). Note, volume and thickness of frontal and temporal cortex were not used for classification, because differences in these regions between Alzheimer’s disease and FTD were not significant. Using MMSE scores alone correctly (P < 0.01) separated FTD and Alzheimer’s disease patients with an overall classification of 72 ± 2%. Using TMT scores alone correctly (P < 0.01) separated FTD and Alzheimer’s disease patients with an overall classification of 76 ± 2%. Adding parietal cortical thickness to the MMSE scores significantly improved (P < 0.01) overall classification between Alzheimer’s disease and FTD to 78 ± 2%. Similarly, adding parietal cortical thickness to TMT scores significantly improved (P = 0.02) overall classification between Alzheimer’s disease and FTD to 84 ± 2%.
Table 3.
Overall classification between CN, FTD and AD based on logistic regressions and leave-one-out cross validations using either cortical thickness or volumes of frontal, parietal and temporal lobes
| Cortical measure | Alzheimer’s disease vs CN (%) | FTD vs CN (%) | Alzheimer’s disease vs FTD (%) | |
|---|---|---|---|---|
| Volume | Frontal | 93 ± 3 | 89 ± 5 | –a |
| Parietal | 95 ± 4 | 81 ± 7 | 79 ± 3 | |
| Temporal | 95 ± 3 | 85 ± 7 | –a | |
| Frontal | 91 ± 4 | 88 ± 6 | –a | |
| Thickness | Parietal | 96 ± 3 | 82 ± 7 | 82 ± 1 |
| Temporal | 93 ± 3 | 85 ± 6 | –a |
No difference between AD vs FTD.
Discussion
The major findings of this study are: (i) Alzheimer’s disease is associated with cortical thinning primarily in the frontal, parietal, temporal and occipital lobes, while a different regional pattern of cortical thinning is found in FTD, involving primarily the frontal and temporal lobes. The pattern of cortical thinning in each disease is consistent with previous MRI studies using volumetric measurements of brain atrophy and also with histopathological findings of the brain’s selective vulnerability to Alzheimer’s disease and FTD; (ii) dementia severity is negatively correlated with cortical thickness in Alzheimer’s disease, while comparable correlations in FTD were not significant; (iii) measurement of cortical thickness provided similar accuracy as that of cortical volume for differentiating between CN, Alzheimer’s disease and FTD and significantly improved the classification between Alzheimer’s disease and FTD based on neuropsychological scores alone.
The finding of characteristic patterns of cortical thinning in Alzheimer’s disease replicates the previous study (Lerch et al., 2005) and is consistent with the pattern of tissue loss reported by histopathological and volumetric MRI studies (Braak and Braak, 1995, 1998; Baron et al., 2001). Furthermore, we found that FTD is associated with a characteristic regional pattern of cortical thinning in frontal, temporal regions and parietal lobe, and also with greatest cortical atrophy in prefrontal regions, and less cortical atrophy in parietal regions. The findings in FTD are consistent with previous pathological reports and volumetric MRI studies (Rosen et al., 2002; Broe et al., 2003; Grossman et al., 2004; Whitwell et al., 2005). In particular, prominent thinning of frontal and temporal cortex in FTD is in agreement with autopsy findings from macroscopic examinations of FTD brains showing consistently frontal and temporal and, less commonly, parietal atrophy (Dickson, 2001; Kersaitis et al., 2004). Furthermore, this study showed that FTD was associated with the cortical thinning in both orbital and medial frontal cortices with a similar severity, which is also consistent with the previous pathological study that FTD is associated with the orbital and medial frontal cortices in the early stage (Broe et al., 2003). However, the previous VBM studies (Rosen et al., 2005; Williams et al., 2005) have shown that in FTD, the behaviour change is related to grey matter loss in the medial frontal cortex other than the orbital frontal cortex. More studies may be needed to elucidate the relationship between the behaviour change and cortical atrophy with cortical thickness measurement in FTD. While patients with Alzheimer’s disease had thinner cortices on MRI than FTD patients, specifically in the precuneus and the parietal lobe regions, no cortical region in FTD was significantly thinner than in Alzheimer’s disease. The finding that Alzheimer’s disease and FTD patients showed similar levels of cortical thinning in the frontal lobe is surprising, given that greater brain atrophy in frontal regions in FTD than Alzheimer’s disease has previously been reported (Grossman et al., 2004). Different demographics of patients including stage and severity of cognitive impairment may explain the different findings. For example, in the previous MRI study (Grossman et al., 2004), patients with Alzheimer’s disease or FTD were matched for dementia severity based solely on MMSE scores, which assesses predominantly memory impairments but less executive functions. In fact, a previous neuropsychological study demonstrated that FTD patients could be more impaired in judgement and problem solving than Alzheimer’s disease patients when MMSE scores of the patients are matched (Rosen et al., 2004). It is therefore conceivable that previous findings of more frontal lobe atrophy in FTD than in Alzheimer’s disease can be explained at least in part by greater executive dysfunction in some of FTD patients than in Alzheimer’s disease patients. It is also possible that Alzheimer’s disease heterogeneity may lead to different results, depending whether the frontal cortex is involved in the disease process or not. Although there was no difference in cortical thinning in the frontal regions between Alzheimer’s disease and FTD in this study, we found that Alzheimer’s disease was associated with a thinner cortex in the dorsolateral frontal cortex than the orbital and medial frontal cortices (Fig. 1), while FTD was associated with a similar cortical thinning in the dorsolateral, orbital and medial frontal cortices (Fig. 2). Taken together, these results suggest that while there are areas of overlap between the atrophic patterns of the two dementias, their patterns are dissociable and measurements of cortical thickness may be a useful surrogate marker for Alzheimer’s disease and FTD.
In this study, we found that FTD was associated with cortical thinning in inferior parietal lobes and the posterior cingulate cortex, which are prominent regions affected by Alzheimer’s disease pathology. Other MRI studies using voxel-based morphometry found no significant structural abnormalities in these regions in FTD (Rosen et al., 2002; Gee et al., 2003; Whitwell et al., 2005). There are several possible explanations for the discrepancy. First, cortical thickness measurements may be more sensitive than voxel-based morphometry in detecting cortical alterations. Second, older patients with FTD may have concomitant Alzheimer’s disease pathology or could be false negatively classified as Alzheimer’s disease patients. However, we did not find systematic differences in brain atrophy between younger and older FTD patients; nor did we discover differences in the neurocognitive characteristics between FTD patients with more parietal atrophy and less parietal atrophy. Nonetheless, only autopsy can provide conclusive results for comorbidity of Alzheimer’s disease pathology in some patients with FTD symptoms.
Another finding is that regional cortical thinning correlated with dementia severity in Alzheimer’s disease but no significant correlations were seen in FTD. It is unclear why correlations show a disease selective effect. One reason could be that severity of FTD, which presents with predominantly behavioural problems, is not accurately reflected in MMSE and CDR tests, which assess primarily cognitive functions, though other studies have used MMSE and CDR together to compare dementia severity between Alzheimer’s disease and FTD (Likeman et al., 2005). Previous pathological studies showed that FTD impacts also white matter and several subcortical nuclei such as the thalamus in addition to cortical involvement (Mann and South, 1993; Broe et al., 2003; Schofield et al., 2003). Thus, damage of white matter and subcortical nuclei may play a role in cognitive impairment in FTD. However, both dementias are neuropathologically defined by cortical neuronal changes, thus involvement of white matter is not surprising, because neuronal damage implies damage to the white matter as the white matter reflects the neuronal axons and dendrites that are affected in Alzheimer’s disease as well as in FTD. More studies involving novel MRI techniques, such as diffusion imaging that is more sensitive to white matter changes than volumetric MRI are needed to evaluate the difference of white matter and subcortical nuclei between Alzheimer’s disease and FTD. In addition, the negative finding between cortical thickness and cognitive function may be due to the small range of MMSE and CDR scores in FTD patients, which makes detecting a relationship between cortical thickness and cognitive functions unreliable. More studies of FTD patients with a broader spread of dementia severity are needed to evaluate a potential relationship between cortical thickness and cognitive functions.
The best discriminator between Alzheimer’s disease and FTD was parietal lobe atrophy in Alzheimer’s disease. This is in agreement with a recent diagnostic MRI study, which reported for a range of pathology confirmed dementia cases that only posterior greater than anterior gradient of atrophy was highly specific for Alzheimer’s disease when compared to other dementias, including FTD (Likeman et al., 2005). Overall, however, we found no significant improvement in correctly classifying CN, Alzheimer’s disease and FTD from cortical thickness measurement as compared to volumetric measurements of the cortex. We expected that measurements of cortical thinning would be less confounded by underlying white matter atrophy than measurements of cortical volume. Our argument was based on the assumption that the inner surface area of grey matter, which is interfaced with the surface area of white matter, might shrink as a consequence of white matter atrophy or white matter lesions, hence impacting computations of volume measurements more than computations of cortical thickness. The negative outcome could be due to the fact that thickness measurements are intrinsically limited by the finite resolution of MRI, diminishing the advantage of assessing cortical thickness versus cortical volume. Studies at higher magnetic fields that can afford higher image resolution may overcome this limitation. Although cortical thickness and volume measurements provided similar divisions between Alzheimer’s disease and FTD, cortical thickness measurement significantly improved the classification between the two diseases based on neuropsychological scores alone. In addition, measurement of cortical thickness provided a similar distinction between Alzheimer’s disease and FTD patients than biomarkers such as CSF based tau and isoprostane and better classifications than CSF amyloid beta (1–42), as reported in the previous study (Grossman et al., 2005). Taken together, this suggests that cortical thickness is a useful marker for differentiating the illnesses.
A major limitation of this study is that the diagnosis of dementia and its type was made clinically without autopsy confirmation. Therefore, it is possible that some of the patients with FTD had also Alzheimer’s disease, vice versa or had other causes of dementia. The inclusion of patients at a more advanced stage of disease may reduce the relevance of our findings to the earliest detection of dementia and differentiation between Alzheimer’s disease and FTD. Studies including early stage patients may be more useful in evaluating the clinical diagnostic value of cortical thickness. The relatively young age of patients with Alzheimer’s disease, used to match the average age of the patients with FTD, may limit the projection of the findings to an older Alzheimer’s disease population.
Acknowledgments
This work was supported in part by a research applications grant of the Department of Veterans Affairs REAP, NIH grants AG10897, a Program Project Grant from the National Institute of Aging (PO1AG19724), ADRC (P50-AG023501) and a Network grant from the Hillblom Foundation. We also thank Dr Fischl et al. in Massachusetts General Hospital for assistance with use of Freesurfer.
Abbreviations
- FTD
frontotemporal dementia
- CN
cognitively normal
- MMSE
Mini-Mental State Examination
- CDR
Clinical Dementia Rating scale
- TMT
Trail-Making Test
References
- Baron JC, Chetelat G, Desgranges B, et al. In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer’s disease. Neuroimage. 2001;14:298–309. doi: 10.1006/nimg.2001.0848. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–8. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Evolution of neuronal changes in the course of Alzheimer’s disease. J Neural Transm Suppl. 1998;53:127–40. doi: 10.1007/978-3-7091-6467-9_11. [DOI] [PubMed] [Google Scholar]
- Broe M, Hodges JR, Schofield E, Shepherd CE, Kril JJ, Halliday GM. Staging disease severity in pathologically confirmed cases of frontotemporal dementia. Neurology. 2003;60:1005–11. doi: 10.1212/01.wnl.0000052685.09194.39. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dickson DW. Neuropathology of Pick’s disease. Neurology. 2001;56:S16–S20. doi: 10.1212/wnl.56.suppl_4.s16. [DOI] [PubMed] [Google Scholar]
- Du AT, Jahng GH, Hayasaka S, et al. Hypoperfusion in frontotemporal dementia and Alzheimer’s disease by arterial spin labeling MRI. Neurology. 2006;67:1215–20. doi: 10.1212/01.wnl.0000238163.71349.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Laakso MP, Beltramello A, et al. Hippocampal and entorhinal cortex atrophy in frontotemporal dementia and Alzheimer’s disease. Neurology. 1999;52:91–100. doi: 10.1212/wnl.52.1.91. [DOI] [PubMed] [Google Scholar]
- Gee J, Ding L, Xie Z, Lin M, DeVita C, Grossman M. Alzheimer’s disease and frontotemporal dementia exhibit distinct atrophy-behavior correlates: a computer-assisted imaging study. Acad Radiol. 2003;10:1392–401. doi: 10.1016/s1076-6332(03)00543-9. [DOI] [PubMed] [Google Scholar]
- Gomez-Isla T, Price JL, McKeel DW, Jr, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci. 1996;16:4491–500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, McMillan C, Moore P, et al. What’s in a name: voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer’s disease, frontotemporal dementia and corticobasal degeneration. Brain. 2004;127:628–49. doi: 10.1093/brain/awh075. [DOI] [PubMed] [Google Scholar]
- Grossman M, Farmer J, Leight S, et al. Cerebrospinal fluid profile in frontotemporal dementia and Alzheimer’s disease. Ann Neurol. 2005;57:721–9. doi: 10.1002/ana.20477. [DOI] [PubMed] [Google Scholar]
- Kersaitis C, Halliday GM, Kril JJ. Regional and cellular pathology in frontotemporal dementia: relationship to stage of disease in cases with and without Pick bodies. Acta Neuropathol (Berl) 2004;108:515–23. doi: 10.1007/s00401-004-0917-0. [DOI] [PubMed] [Google Scholar]
- Kitagaki H, Mori E, Yamaji S, et al. Frontotemporal dementia and Alzheimer disease: evaluation of cortical atrophy with automated hemispheric surface display generated with MR images. Radiology. 1998;208:431–9. doi: 10.1148/radiology.208.2.9680572. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Frisoni GB, Kononen M, et al. Hippocampus and entorhinal cortex in frontotemporal dementia and Alzheimer’s disease: a morphometric MRI study. Biol Psychiatr. 2000;47:1056–63. doi: 10.1016/s0006-3223(99)00306-6. [DOI] [PubMed] [Google Scholar]
- Lerch JP, Pruessner JC, Zijdenbos A, Hampel H, Teipel SJ, Evans AC. Focal decline of cortical thickness in Alzheimer’s disease identified by computational neuroanatomy. Cereb Cortex. 2005;15:995–1001. doi: 10.1093/cercor/bhh200. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Campbell MJ, Terry RD, Morrison JH. Laminar and regional distributions of neurofibrillary tangles and neuritic plaques in Alzheimer’s disease: a quantitative study of visual and auditory cortices. J Neurosci. 1987;7:1799–808. doi: 10.1523/JNEUROSCI.07-06-01799.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likeman M, Anderson VM, Stevens JM, et al. Visual assessment of atrophy on magnetic resonance imaging in the diagnosis of pathologically confirmed young-onset dementias. Arch Neurol. 2005;62:1410–5. doi: 10.1001/archneur.62.9.1410. [DOI] [PubMed] [Google Scholar]
- Lipton AM, Benavides R, Hynan LS, et al. Lateralization on neuroimaging does not differentiate frontotemporal lobar degeneration from Alzheimer’s disease. Dement Geriatr Cogn Disord. 2004;17:324–7. doi: 10.1159/000077164. [DOI] [PubMed] [Google Scholar]
- Mann DM, South PW. The topographic distribution of brain atrophy in frontal lobe dementia. Acta Neuropathol (Berl) 1993;85:334–40. doi: 10.1007/BF00227731. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Pearson RC, Esiri MM, Hiorns RW, Wilcock GK, Powell TP. Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer disease. Proc Natl Acad Sci USA. 1985;82:4531–4. doi: 10.1073/pnas.82.13.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Gorno-Tempini ML, Goldman WP, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58:198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Narvaez JM, Hallam B, et al. Neuropsychological and functional measures of severity in Alzheimer disease, frontotemporal dementia, and semantic dementia. Alzheimer Dis Assoc Disord. 2004;18:202–7. [PubMed] [Google Scholar]
- Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128:2612–25. doi: 10.1093/brain/awh628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield E, Kersaitis C, Shepherd CE, Kril JJ, Halliday GM. Severity of gliosis in Pick’s disease and frontotemporal lobar degeneration: tau-positive glia differentiate these disorders. Brain. 2003;126:827–40. doi: 10.1093/brain/awg085. [DOI] [PubMed] [Google Scholar]
- Siri S, Benaglio I, Frigerio A, Binetti G, Cappa SF. A brief neuropsychological assessment for the differential diagnosis between frontotemporal dementia and Alzheimer’s disease. Eur J Neurol. 2001;8:125–32. doi: 10.1046/j.1468-1331.2001.00179.x. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Josephs KA, Rossor MN, et al. Magnetic resonance imaging signatures of tissue pathology in frontotemporal dementia. Arch Neurol. 2005;62:1402–8. doi: 10.1001/archneur.62.9.1402. [DOI] [PubMed] [Google Scholar]
- Williams GB, Nestor PJ, Hodges JR. Neural correlates of semantic and behavioural deficits in frontotemporal dementia. Neuroimage. 2005;24:1042–51. doi: 10.1016/j.neuroimage.2004.10.023. [DOI] [PubMed] [Google Scholar]


