Abstract
Increasing numbers of cochlear implant subjects have some level of residual hearing at the time of implantation. The present study examined whether (i) hair cells that have survived one pathological insult (aminoglycoside deafening), can survive and function following long-term cochlear implantation and electrical stimulation (ES); and (ii) chronic ES in these cochleae results in greater trophic support of spiral ganglion neurons (SGNs) compared with cochleae devoid of hair cells. Eight cats, with either partial (n=4) or severe (n=4) sensorineural hearing loss, were bilaterally implanted with scala tympani electrode arrays 2 months after deafening, and received unilateral ES using charge balanced biphasic current pulses for periods of up to 235 days. Frequency-specific compound action potentials and click-evoked auditory brainstem responses (ABRs) were recorded periodically to monitor the residual acoustic hearing. Electrically-evoked ABRs (EABRs) were recorded to confirm the stimulus levels were 3-6 dB above the EABR threshold. On completion of the ES program the cochleae were examined histologically. Partially deafened animals showed no significant increase in acoustic thresholds over the implantation period. Moreover, chronic ES of an electrode array located in the base of the cochlea did not adversely affect hair cells in the middle or apical turns. There was evidence of a small but statistically significant rescue of SGNs in the middle and apical turns of stimulated cochleae in animals with partial hearing. Chronic ES did not, however, prevent a reduction in SGN density for the severely deaf cohort, although SGNs adjacent to the stimulating electrodes did exhibit a significant increase in soma area (p<0.01). In sum, chronic ES in partial hearing animals does not adversely affect functioning residual hair cells apical to the electrode array. Moreover, while there is an increase in the soma area of SGNs close to the stimulating electrodes in severely deaf cochleae, this trophic effect does not result in increased SGN survival.
Keywords: neural degeneration, auditory nerve, deafness, electrical stimulation, cochlear implant, electric-acoustic stimulation
1. Introduction
The development and maintenance of the primary afferent neurons of the cochlea - spiral ganglion neurons (SGNs) - appears to be regulated by a combination of neural activity (Hegarty et al., 1997; Hansen et al., 2001) and neurotrophic support from hair cells (Fritzsch et al., 1999). The loss of hair cells produces a dramatic reduction in both the driven and spontaneous activity of SGNs (Liberman and Kiang, 1978; Hartmann and Klinke, 1984; Shepherd and Javel, 1997) and also removes a key source of neurotrophic support (Ylikoski et al., 1993; Fritzsch et al., 1999; Schimmang et al., 2003; Tan and Shepherd, 2006), resulting in SGN degeneration. The degeneration of SGNs is a gradual process initially involving the loss of the peripheral process, a significant reduction in soma area and partial demyelination; ultimately resulting in cell death (Terayama et al., 1977; Spoendlin, 1984; Leake and Hradek, 1988; Spoendlin and Schrott, 1989; Shepherd and Javel, 1997; Hardie and Shepherd, 1999).
SGNs are the target neurons for cochlear implants, therefore therapies designed to prevent their degeneration would be expected to result in improved clinical performance among cochlear implant patients (Nadol et al., 1989; Gantz et al., 1993). Although depolarization has been shown to provide a trophic influence on early postnatal SGNs in vitro (Hegarty et al., 1997; Hansen et al., 2001), chronic electrical stimulation (ES) of SGNs in ototoxically deafened animals has not consistently demonstrated that depolarization rescues SGNs in vivo. Several studies have reported an increase in SGN survival in deafened, chronically stimulated cochleae (Lousteau, 1987; Hartshorn et al., 1991; Hultcrantz et al., 1991; Leake et al., 1992; Leake et al., 1995; Mitchell et al., 1997; Leake et al., 1999), however other studies – including those from our laboratory - have shown no evidence of stimulus-induced trophic support of SGNs (Shepherd et al., 1994; Araki et al., 1998; Li et al., 1999; Shepherd et al., 2005).
Any attempt to establish key differences between studies that may reveal the mechanism(s) responsible for depolarization-induced trophic support of SGNs in vivo, is complicated by the many different variables across these studies (Miller, 2001). Factors including species, electrode geometry, age at stimulation, stimulus intensity, rate and duration, and the charge recovery technique, do not appear to be significant variables (review: Shepherd et al., 2006).
Since hair cells and supporting cells of the organ of Corti provide endogenous neurotrophic support to SGNs (Ylikoski et al., 1993; Stankovic et al., 2004; Tan and Shepherd, 2006), and hair cells can be readily stimulated electrically (Moxon, 1971; van den Honert and Stypulkowski, 1984; Javel and Shepherd, 2000), one significant factor affecting SGN survival could be the extent of hair cell survival in deafened cochleae. Indeed, it is possible that residual hair cells chronically stimulated via ES may provide a global trophic influence that extends to SGNs devoid of hair cells.
The survival of residual hair cells following long-term cochlear implantation also has important implications for speech perception in implant subjects with residual hearing. Improved speech perception, particularly in the presence of background noise, has been reported using combined electric and acoustic hearing over ES alone (Gantz and Turner, 2003; Kiefer et al., 2005). Using normal hearing animals, we have previously demonstrated that hair cells apical to an electrode array can survive chronic cochlear implantation in the absence of electrode insertion trauma or severe inflammation (Shepherd et al., 1983; Ni et al., 1992; Xu et al., 1997). However, it is also important to examine hair cell survival post cochlear implantation using partially deafened animal models, as hair cells that survive deafening may be more susceptible to a second insult from electrode insertion and chronic ES.
The present study therefore extends our previous work by investigating (i) the survival and long-term functional response of residual hair cells following cochlear implantation and chronic ES; and (ii) the potential trophic effects of ES on SGNs in partially versus severely deafened cochleae. We systemically deafened animals to produce a bilaterally symmetrical sensorineural hearing loss (SNHL; Shepherd and Martin, 1995), and used bilateral cochlear implantation with unilateral ES to determine the effects of ES per se on residual hair cells and SGNs.
2. Materials and methods
2.1 Experimental Groups
Eight healthy cats with otoscopically normal tympanic membranes were used in this study. They were divided into two experimental groups; one cohort had a severe hearing loss (n=4) while the second had a partial hearing loss (n=4). All animals received unilateral ES via (i) a narrow region of stimulation using one section of the array (electrodes 1-3; 6-8 mm from the round window; n=3); or (ii) a broad region of stimulation using two sections of the array (electrodes 1-3; 6-8 mm and electrodes 6-8; 2.5-4 mm from the round window; n=5; Table 1).
Table 1.
Description of subjects in the study
| Animal I.D.1 |
Hearing Status2 | Mean Acoustic Threshold (dB p.e. SPL) |
Stimulus regime group3 |
Duration of Deafness (days) |
Duration of implantation (days) |
Duration of Stimulation (hours) |
|---|---|---|---|---|---|---|
| I1s | Severe | > 93 | Narrow | 325 | 252 | 1093 |
| I5s | Severe | > 93 | Narrow | 264 | 195 | 833 |
| I2s | Severe | > 93 | Broad | 289 | 220 | 993 |
| I3s | Severe | > 93 | Broad | 179 | 111 | 357 |
| I4p | Partial | 68 | Narrow | 318 | 238 | 930 |
| I6p | Partial | 78 | Broad | 266 | 195 | 830 |
| I7p | Partial | 58 | Broad | 280 | 207 | 901 |
| I8p | Partial | 78 | Broad | 329 | 251 | 1127 |
Notes:
I, bilateral cochlear implant; unilateral chronic ES; s, severe; p, partial hearing loss;
Severe, bilateral click thresholds ≥ 93 dB p.e. SPL; Partial, bilateral click thresholds >48 - <93 dB p.e. SPL
Narrow, chronic ES restricted to upper basal turn (electrodes 1-3); Broad, chronic ES delivered over a broad region of the basal turn (electrodes 1-3 and 6-8);
2.2 Deafening procedure
At 14 days of age each animal was anaesthetized (halothane/oxygen) and deafened using kanamycin (330 mg/kg; subcutaneously [s.c.]) and ethacrynic acid (27.5 mg/kg; intravenously [i.v.]; Shepherd and Martin, 1995). Based on their hearing status, the animals were categorized as either having a severe SNHL (i.e. no click-evoked auditory brainstem response (ABR) at 93 dB peak equivalent (p.e.) sound pressure level (SPL) in both ears), or a partial SNHL (ABR threshold >48 and <93 dB p.e. SPL).
2.3 Auditory Brainstem Response and Compound Action Potential recordings
ABRs were recorded at 1 month of age (2 weeks post deafening) to confirm the status of each animal's hearing; at this age ABR thresholds in cats are essentially adult-like (Walsh et al., 1986). ABRs were recorded in an electrically screened and sound attenuated room. The animals were anaesthetized (xylazine, 4 mg/kg; s.c.; ketamine, 20 mg/kg: intra-muscular [i.m.]) and their temperature maintained at 37±1°C. Computer generated 100 μs rarefaction clicks were presented from a loudspeaker placed 10 cm from the pinna. The contralateral external ear canal was plugged with an ear mould compound (Otoplastik®). ABRs were recorded differentially using subcutaneous stainless steel electrodes (vertex positive; neck negative and thorax ground). Five hundred stimuli were presented at a rate of 33 per second and responses amplified by 105, filtered (high pass: 150 Hz, 24 dB/octave; low pass: 3 kHz, 6 dB/octave) and averaged. The threshold was defined as the minimum stimulus required to produce an amplitude of ≥0.2 μV for wave IV (4.0-4.5 ms following stimulus onset) in 2 repeated recordings.
In addition, frequency specific compound action potentials (CAPs) were periodically recorded throughout the chronic stimulation program from the partially-deafened chronically stimulated cohort (CAPs could not be recorded from severely-deafened chronically stimulated animals). CAPs were evoked using computer generated tone-pips (1 ms rise/fall, 3 ms plateau) at frequencies of 0.5, 1, 2 and 4 kHz. Although higher frequencies were tested, no animals exhibited CAP responses above 4 kHz. The responses were recorded differentially using the most apical electrode on the scala tympani array (+ve) against a subcutaneous stainless steel electrode (neck –ve; thorax ground). The responses were amplified by 104; transducer and filtering were the same as that used for ABRs. CAP threshold for each frequency tested was taken as the lowest stimulus level in which the response was visually detected using a cathode ray oscilloscope (Rajan et al., 1991). The ABR and CAP thresholds were normalized to the first ABR (recorded pre-operatively) or CAP (recorded post-operatively) threshold determined for each animal.
2.4 Scala tympani electrode array
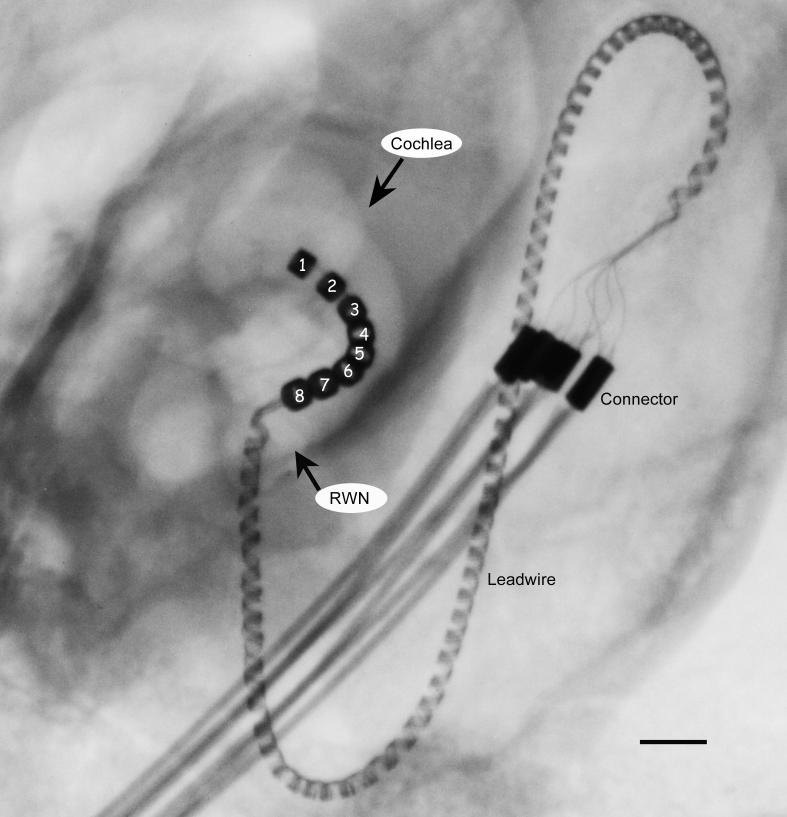
The electrode arrays consisted of 8 Platinum (Pt) electrodes located on a silastic carrier (Shepherd et al., 1983). The electrodes were numbered 1 through 8, with electrode 1 - the most apical - located 8 mm from the round window (Fig. 1). Each Pt electrode was 0.3 mm wide and the inter-electrode separation was 0.45 mm. An insulated platinum/iridium wire was welded to each electrode, which in turn was connected to a stainless steel leadwire assembly (Fig. 1).
Figure 1.
X-ray image of a cat cochlea showing the electrode array within the scala tympani. The helical platinum-iridium leadwire system is connected to a stainless steel leadwire system at the connector which is located within the animal's bulla. The stainless steel leadwire exits the skin at the nape of the neck and is directly connected to a portable programmable stimulator worn in a back pack (not illustrated). Electrodes are numbered 1-8 (apical to basal); round window niche; RWN. Scale bar = 1 mm
2.5 Surgery
Animals were bilaterally implanted at eight weeks of age. The left cochlea was implanted with a scala tympani electrode array-leadwire assembly (stimulated); while the right cochlea received a scala tympani electrode array with no external leadwire (non-stimulated control). The surgery was performed under sterile conditions. Each animal was premedicated (acepromazine maleate/atropine sulphate, 0.05 ml/kg s.c.) with anesthesia maintained at a surgical level using a closed circuit anesthetic machine (halothane/oxygen). The detailed surgical procedure has been described previously (Shepherd et al., 1983; Xu et al., 1997). The bulla cavity was opened, flushed with amoxicillin (10 mg/ml) and the round window membrane incised. The array was inserted 8 mm into the scala tympani from the round window (electrodes 1-3: 6.2-8mm from the round window; electrodes 6-8: 2.45-4.25mm from the round window). This insertion depth placed electrode 3 at ∼12kHz place and electrode 8 at ∼26kHz place (Brown et al., 1992). The round window was sealed with crushed muscle to prevent perilymph leakage and the leadwire was fixed with Lars Mesh® ties at the bulla and on the dorso-lateral part of the skull. The leadwire passed subcutaneously and then exited the body through an incision at the nape of the neck and connected to a portable stimulator. During surgery the animal's respiratory rate, expired CO2 and body temperature were monitored and maintained within normal levels (respiration rate 10-20; expired CO2 3-5%; body temperature 37±1°C). Analgesic (Carprofen 4 mg/kg s.c.), systemic antibiotic (Noroclav® 0.05 ml/kg s.c.) and compound sodium lactate fluids (10ml s.c.) were administered following surgery.
2.6 Electrically-evoked auditory brainstem responses
Electrically-evoked ABRs (EABRs) were periodically recorded from the stimulated cochleae throughout the chronic stimulation program. Biphasic current pulses (100 μs per phase; 10 μs interphase gap) were generated under optically-isolated computer control and delivered to a pair of electrodes on the intracochlear electrode array. Responses were recorded differentially as described for the ABR except for the inclusion of a sample-and-hold circuit to remove electrical artifact (Black et al., 1983). Two recordings were made at each current level and current amplitude was reduced to levels below threshold. EABR input output (IO) functions (peak-trough amplitude of wave IV versus stimulus current) were plotted for each stimulated electrode pair. EABR thresholds and IO functions recorded over time were normalized to the first post-operative EABR recorded.
2.7 Chronic ES Program
A chronic ES program commenced 14 days following surgery using programmable stimulators designed to have electrical parameters similar to the Cochlear® CI24 cochlear implant. The stimulator delivered 100 μs per phase charge-balanced biphasic current pulses with a 40 μs interphase gap to bipolar electrodes at a stimulus rate of 1200 pulses per second. The stimulus waveform was amplitude modulated to a depth of 50% at 30 Hz to mimic temporally challenging stimuli used in cochlear implants. Electrode shorting and capacitive coupling were used to ensure complete charge recovery (Huang et al., 1999). The amplitude of the stimulus waveform was set so that the minimum current level of the modulation was equal to the post-operative EABR threshold. With 50% modulation this resulted in a maximum current level 6 dB above EABR threshold. The stimulus level was then confirmed to be behaviorally acceptable to the animal. Maximum stimulus current amplitudes used in this study were 0.25-1.75 mA at 100 μs/phase, which produced charge densities within safe levels for use with Pt electrodes (Shepherd et al., 1983; Xu et al., 1997). The portable stimulator was carried in a harness worn by the animal; each animal received 6 hours of stimulation per day, 5 days per week for implant periods of up to 252 days (Table 1). Stimulus current and electrode voltage waveforms were monitored twice daily to ensure that appropriate stimulation levels were set for each animal (Xu et al., 1997).
2.8 Histology
On completion of the stimulation program each animal was euthanized with sodium pentobarbitone (150 mg/kg; i.p.) and perfused intra-arterially with heparinized normal saline at 37°C followed by 4% paraformaldehyde solution at 4°C; both solutions were buffered to a pH of 7.35 with 0.1 M phosphate buffer.
The electrode arrays were removed and both cochleae were harvested and processed for histological analysis. Following overnight fixation in 4% paraformaldehyde, the cochleae were decalcified (10% EDTA in 10% neutral buffered formalin), dehydrated, embedded in resin (Spurr) and serially sectioned at a thickness of 2 μm. Sections every 126 μm were stained with haematoxylin and eosin (H&E).
Cochleae were graphically reconstructed using analysis procedures based on Schuknecht (1953) in order to identify the basal (0-50% of the length of the basilar membrane [BM]); middle (50-75%) and apical turns (75-100%). The presence of inner (IHC) and outer hair cells (OHC), and electrode insertion trauma was determined, and if present the location along the BM was recorded. The extent of new bone growth and inflammation within the scala tympani was expressed as a proportion of the cross sectional area.
SGN density was measured by a single observer using techniques published previously (Shepherd et al., 1983; Xu et al., 1997). In each section, the cochlear turns were identified (basal, middle and apical) and the cross-sectional area of Rosenthal's canal was measured using NIH Image (http://rsb.info.nih.gov/nih-image/). All neurons with a visible nucleus were then counted and SGN density calculated as cells per square millimeter.
SGN soma area was measured by a single observer using NIH Image. Sections were selected randomly so that a maximum of 40 neurons were measured in each cochlear region (basal, middle and apical turns). Only soma area neurons with a visible nucleolus were measured.
This study was conducted under the approval of the Animal Research Ethics Committee, Royal Victorian Eye and Ear Hospital, Melbourne, Australia.
3. Results
3.1 Cochlear Physiology
3.1.1 ABRs and CAPs
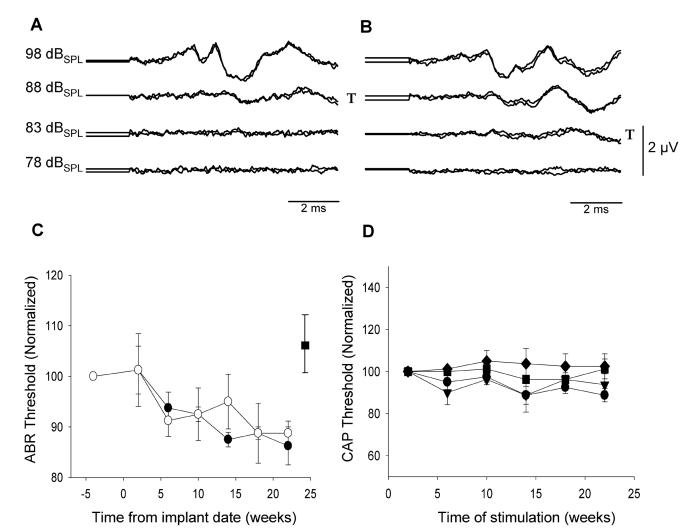
Representative ABRs from a partial hearing animal recorded prior to implantation and at the end of the ES period 195 days later are illustrated in Fig. 2. There was no difference in the click-evoked ABR threshold recorded immediately after cochlear implantation when compared with those recorded prior to surgery for either ear (paired t-test; left side n=4, p=0.39; right side n=4, p=0.85). Furthermore, thresholds were not adversely affected by chronic implantation. Indeed, there was a trend for click-evoked ABR thresholds to decrease slightly over the implant period (Fig. 2C; 2-way ANOVA; n=4, p<0.01). This decrease was not influenced by chronic ES (i.e. stimulated and non stimulated cochleae exhibited a similar shift). Finally, CAP thresholds, recorded from the stimulated ear at frequencies of 0.5, 1.0, 2.0, and 4.0 KHz, showed no significant change over time at each of these frequencies (Fig. 2D; 2-way ANOVA; n=4, p=0.71).
Figure 2.
- A and B: Representative examples of click-evoked ABRs recorded prior to implantation (A) and at the end of ES, 195 days post implant surgery (B; I6p). T= denotes threshold.
- C: Normalized ABR thresholds as a function of implantation time for ES (●) and non-stimulated (○) cochleae of partially deaf animals (n= 4; mean ± sem). Click thresholds exhibited a statistically significant reduction as a function of implantation time. This reduction was not influenced by chronic ES.
- D: CAP thresholds recorded from the stimulated cochleae as a function of implantation time: 0.5 KHz ●; 1.0 KHz▼; 2.0 KHz■ ; 4.0 KHz◆. (n= 4; normalized mean ± sem). CAP thresholds showed no significant change over time.
3.1.2 EABRs
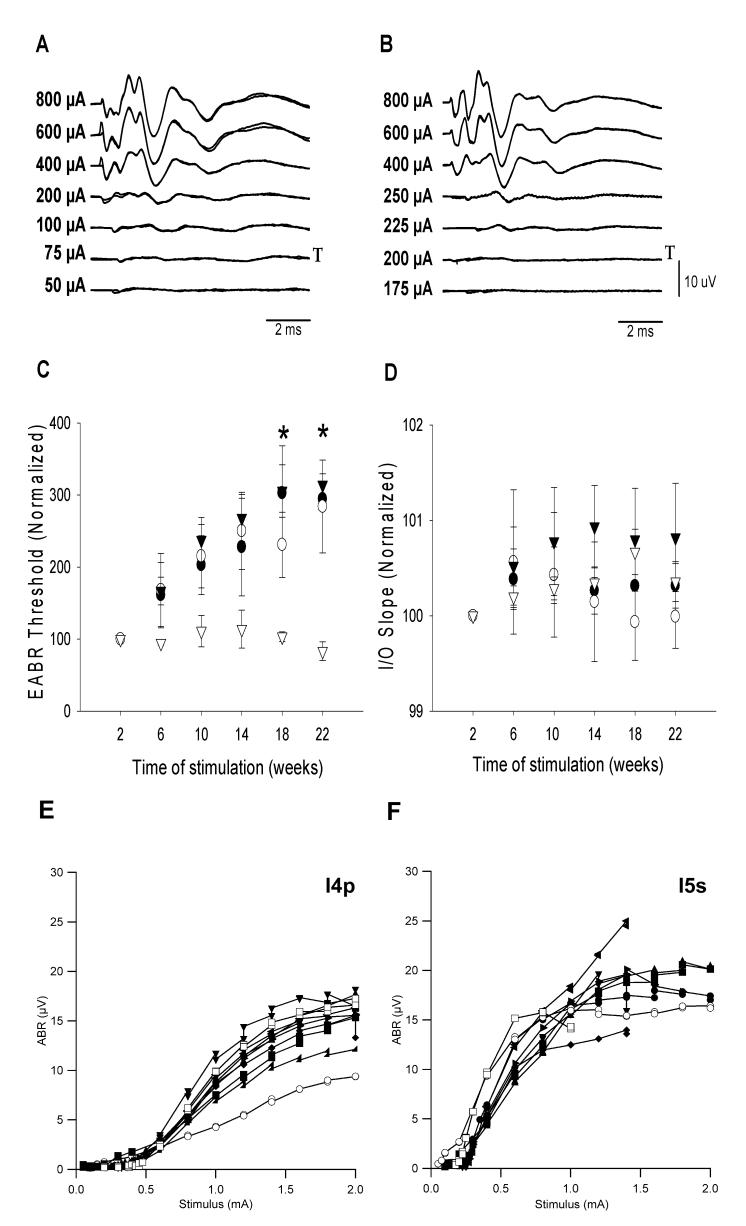
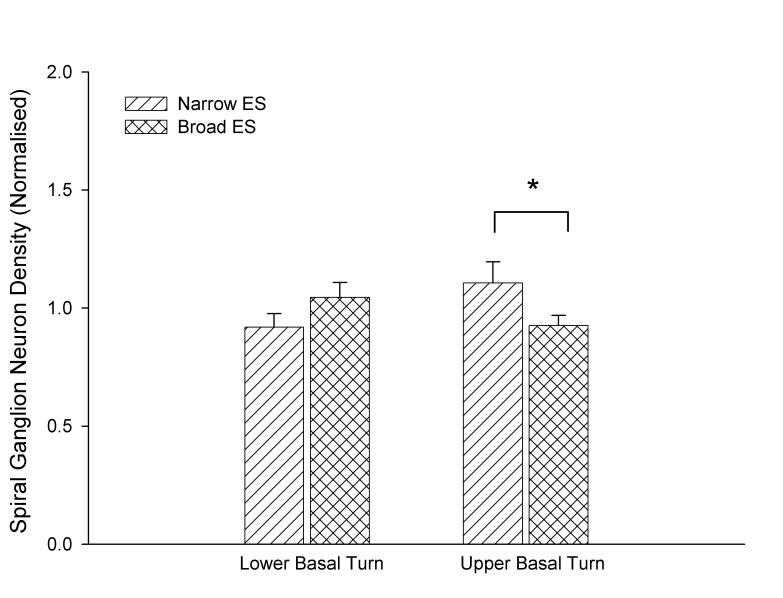
Representative EABRs recorded post-operatively and at the completion of the stimulation period 195 days later are illustrated in Fig 3. The effects of chronic ES were analyzed according to their stimulus regime. In the narrow ES cohort, EABR thresholds recorded from pair 6-8 (the lower basal electrodes), which were not chronically stimulated, remained unchanged over time. However, EABR thresholds recorded from all other electrode combinations in both the narrow and broad ES cohorts groups increased over time (Fig. 3C; 2-way ANOVA; n=6, p< 0.01). Within both ES groups, there was no statistically significant difference between EABR thresholds of severe and partially deaf animals (2-way ANOVA n=6; narrow ES: p=0.10 and broad ES: p=0.48).
Figure 3.
- A & B: Representative EABRs recorded post operatively (A) and at the completion of ES, 195 days later (B; I5s). T= threshold.
- C: Change in EABR threshold normalized to the first post-operative response as a function of implant time (n=6; mean ± sem; * = p<0.05).
- D: Change in the gradient of the normalized input-output function (I/O) curve as a function of stimulation time (n=6; mean ± sem). ● Recorded from apical electrodes of broad ES group: (electrodes 1-3); ○ Recorded from basal electrodes of broad ES group (electrodes 6-8); ▼Recorded from apical electrodes of narrow ES group (electrodes 1-3); ▽ Recorded from basal electrodes of narrow ES group (electrodes 6-8).
- E (I4p) & F (I5s): Two representative EABR I/O functions plotted periodically over the course of their chronic stimulation program. Open symbols are used to identify the first and last I/O recordings for each animal.
○ 2 weeks ; ■ 6 weeks; ▲10 weeks; ● 14 weeks; ▶18 weeks; ▼ 22 weeks; ◀ 26 weeks; ◆ 30 weeks; ◢ 34 weeks; □ 36 weeks post implantation
The gradient of EABR IO functions recorded periodically over the course of the chronic ES program (e.g. Fig. 3 E & F) showed evidence of a slight increase over time, however this was not statistically significant (Fig. 3D; 2-way ANOVA; n=6, p=0.29).
3.2 Cochlear Pathology
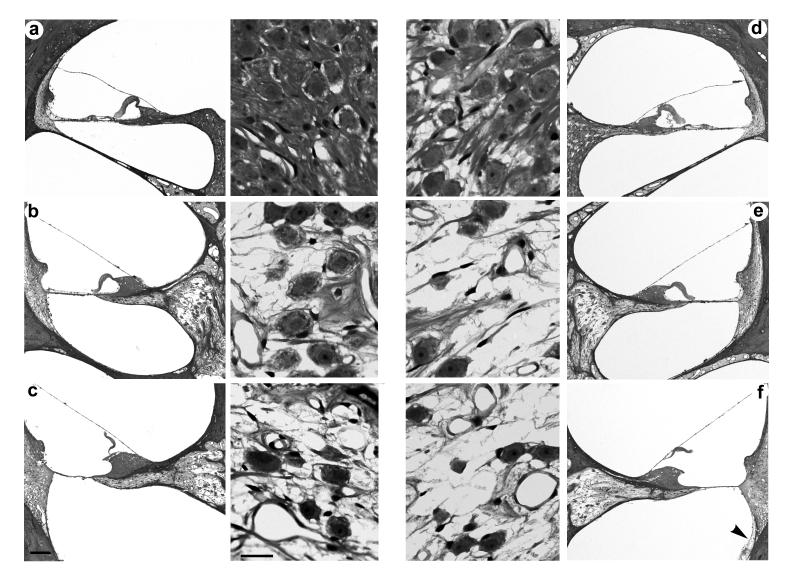
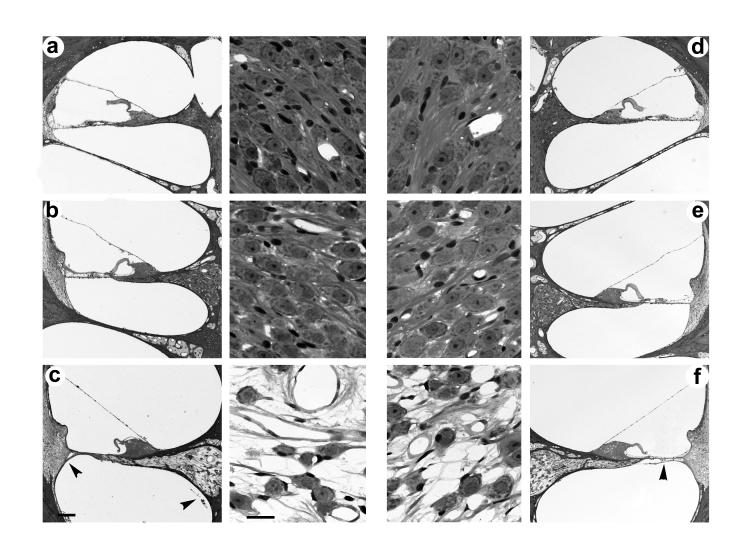
Representative photomicrographs of both severe and partially deafened cochleae are illustrated in Figs. 4 and 5 respectively. In each example both the left (stimulated) and right (non-stimulated) cochleae had been implanted for periods of more than 200 days and the left cochleae were chronically stimulated for >900 hours. Both figures also show examples of a minimal fibrous tissue response associated with the electrode array (arrowheads).
Figure 4.
Representative photomicrographs illustrating a low power of each cochlear turn and a higher power photomicrograph of the adjacent Rosenthal's canal from the ES (a-c; left panel) and non-stimulated cochleae (d-f; right panel) of a severely deafened animal (I1s). The cochleae in this example were implanted for a period of 252 days and the left cochlea received in excess of 1000 h of ES. Apical turn 4a and 4d; middle turn 4b and 4e; basal turn 4c and 4f. Scale bar: low power=100μm; high power=20μm.
Figure 5.
Representative photomicrographs illustrating a low power of each cochlear turn and a higher power photomicrograph of the adjacent Rosenthal's canal from the ES (a-c; left panel) and non-stimulated cochleae (d-f; right panel) of a partially deafened animal (I7p). The cochleae in this example were implanted for a period of 207 days and the left cochlea received 900 h of ES. Apical turn 5a and 5d; middle turn 5b and 5e; basal turn 5c and 5f. Scale bar: low power=100μm; high power=20μm.
There was no evidence of hair cell survival in the basal turn of any cochlea. The severely deafened cohort occasionally exhibited small numbers of IHCs in the apical turn. In contrast, hair cells were usually present in the middle and apical turns of the partially deafened cohort (Fig. 6). Importantly, there was no significant difference in hair cell survival between the stimulated and non-stimulated groups in both the middle (Mann-Whitney Rank Sum test; n=4, p=0.89) and apical turns (Mann-Whitney Rank Sum test; n=4, p=0.34).
Figure 6.
Mean (± sem) hair cell survival in the partial hearing group expressed as a percentage hair cell survival. ■ non-stimulated (n=4 cochleae); □ ES (n=4).
Electrode insertion trauma was minimal in this study. No trauma was associated with the osseous spiral lamina, Reissner's membrane, stria vascularis or spiral ligament in all 16 cochleae. However, two of the 16 cochleae exhibited a localized rupture of the BM. In one instance (I2s non-stimulated side), rupture of the BM (∼0.45 mm in length) was observed adjacent to the tip of the array and was associated with a small fibrous tissue reaction. The second cochlea (I5s stimulated side) exhibited evidence of a rupture of the BM (∼0.45 mm) in the lower basal region resulting in a localized inflammatory response and new bone growth.
Fibrous tissue growth was apparent in 13 of the 16 cochleae (Table 2) and was typically restricted to ≤10% of the cross sectional area of the basal turn scala tympani (e.g. Figs. 4 & 5). Only one cochlea exhibited a fibrous tissue reaction extending beyond the basal turn (I3s non-stimulated side). There was no difference in the extent of fibrous tissue between stimulated and non-stimulated cochleae (Kruskal-Wallis 1-way ANOVA on ranks; n=8, p=0.96). Fibrous tissue growth in excess of 20% of the basal turn scala tympani was always associated with new bone growth.
Table 2.
Fibrous tissue and new bone growth expressed as a mean percentage of the basal turn in all implanted animals
| Soft Tissue Growth (mean % of cross sectional area of scala tympani) |
New Bone (mean % of cross sectional area of scala tympani) |
|||
|---|---|---|---|---|
| Animal I.D. | ES | Control | ES | Control |
| I1s | 8 | 1 | - | - |
| I2s | 1 | 28* | - | 8* |
| I3s | 54 | 42 | 2 | 2 |
| I4p | - | 8 | - | 6 |
| I5s | 37* | 53 | 5* | 7 |
| I6p | 10 | 1 | 10 | - |
| I7p | 1 | 1 | - | - |
| I8p | - | - | - | - |
Notes:
electrode insertion trauma
New bone formation was observed in the scala tympani of the basal turn in three of eight stimulated, and four of eight non-stimulated cochleae (Table 2). There was no significant difference between these two groups (Kruskal-Wallis 1-way ANOVA on ranks; n=8, p=0.65). If present, new bone typically occupied ≤ 5% of the cross sectional area of the basal turn scala tympani; only in restricted regions of a few animals were there greater amounts of new bone (Table 2).
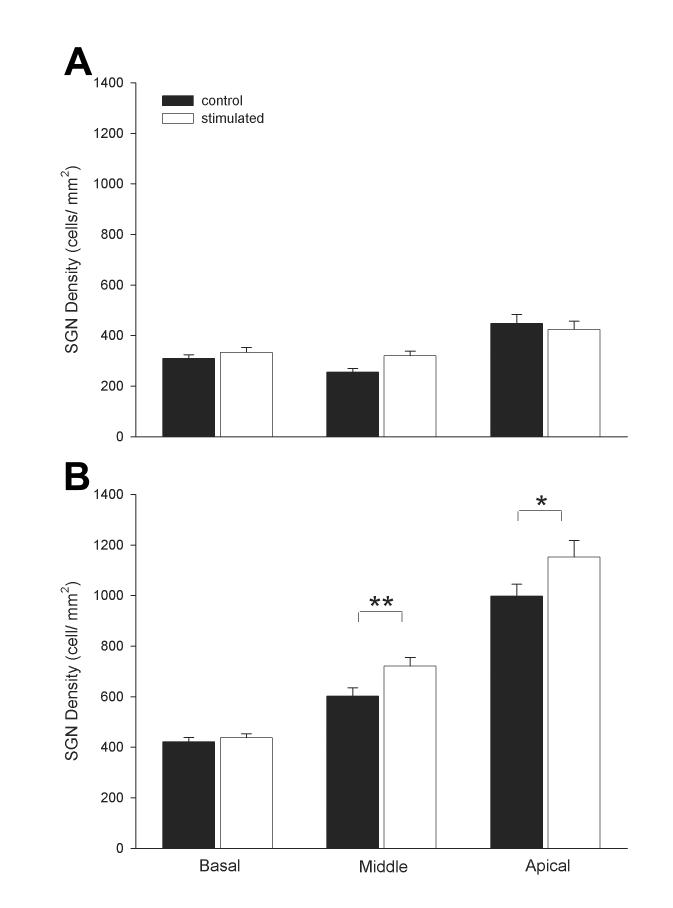
There was no statistically significant difference in SGN density between the stimulated and the non-stimulated cochleae for all turns of the severely deaf cohort (Fig. 7A; 2-way ANOVA; n=8, p=0.22). In contrast, the stimulated cochleae in the partially deafened cohort exhibited a small but statistically significant increase in SGN density in the middle and apical turns (i.e. distal to the electrode array) compared with the non-stimulated cochleae (Fig. 7B; 2-way ANOVA; n=8, p=0.002 and p=0.021 respectively). However, there was no statistically significant difference in SGN density in the basal turn.
Figure 7.
A. Mean SGN density for the basal, middle and apical turns of the ■ non-stimulated (n=4 cochleae) and □ ES (n=4) of the severely deaf group. B. Mean SGN density for the basal, middle and apical turns of ■ non-stimulated (n=4) and □ ES (n=4) of the partial hearing group (mean ± sem).
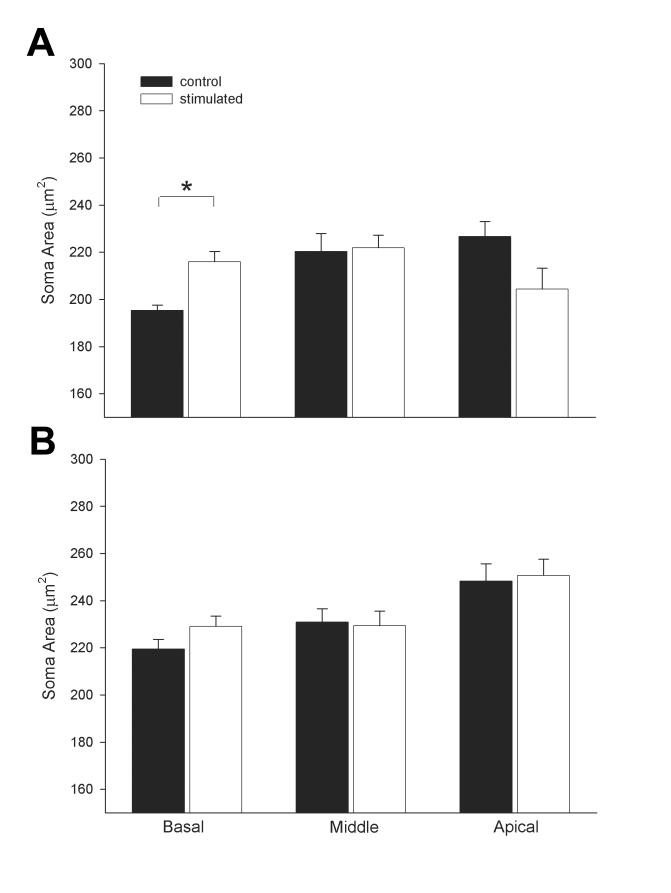
Finally, SGN soma from the severely deaf cohort exhibited greater shrinkage compared with similar cochlear regions in the partial hearing cohort (Fig. 8). Importantly, in the severe deaf cohort, ES produced a significant increase in the soma area of SGNs in the basal turn of the stimulated ear compared to SGNs in the basal turn of the non-stimulated ear (Fig. 8A; 2-way ANOVA; n=8, p<0.0001). There was no statistically significant difference in soma area between ES and non-stimulated cochleae in the partial deaf group (Fig. 9B).
Figure 8.
A. Mean SGN soma area in the basal, middle and apical turns of the ■ non-stimulated (n=4 cochleae) and □ ES (n=4) of the severely deaf group. B. Mean SGN soma area in basal, middle and apical turns of the ■ non-stimulated control (n=4) and □ ES (n=4) of the partial hearing group (mean ± sem).
4. Discussion
Using both histological and electrophysiological measures, the present study has shown that hair cells apical to a scala tympani electrode array can survive chronic cochlear implantation and ES. In addition, while observing a significant increase in the soma area of SGNs adjacent to the stimulating electrodes in the severely deaf cohort, this trophic effect did not result in a significant increase in SGN survival.
Significant levels of SGN rescue associated with ES only occurred in the middle and apical turns of animals with a partial hearing loss; i.e. in regions of cochleae distal to the electrode array but adjacent to a normal or near normal organ of Corti. This finding is consistent with that of Lousteau (1987), who reported significant preservation of SGNs only in cochlear regions containing a remnant of the organ of Corti. While the mechanism(s) underlying this trophic effect remains unclear and requires further investigation, it is possible that residual hair cells excited by acoustic and electrophonic activation, provide trophic support via endogenous neurotrophin release to proximal SGNs.
The lack of a trophic influence of ES on proximal SGNs in the severely deaf cohort is at odds with previous studies (reviewed in the Introduction). It is possible that the status of the SGNs following deafening might play a critical role in determining the efficacy of subsequent ES initiating SGN rescue. While the SGN population adjacent to the electrode array in the severely deaf cohort was very low (∼25% of normal), we note that other studies have reported a significant trophic advantage associated with ES in cochlear regions with similar levels of SGN survival (e.g. Leake et al., 1999). It is possible, however, that the status of the SGNs differed at the time of cochlear implantation and commencement of ES due to the use of different deafening techniques (single application of an aminoglycoside and loop diuretic used in the present study versus daily application of an aminoglycoside used by Leake and colleagues). For example, one technique may have produced a more rapid initial loss of SGNs, or specifically targeted cells within the cochlea in addition to hair cells, that may have affected the response of SGNs to ES. Additional experiments using the same deafening technique would be required to test this hypothesis.
The reduction in soma area of residual SGNs following deafness is a common finding in the mammalian cochlea (Elverland and Mair, 1980; Leake and Hradek, 1988; Nadol et al., 1989; Shepherd and Javel, 1997; Araki et al., 1998; White et al., 2000). The present study supports this result and also demonstrates that greater SGN shrinkage was evident in the severe versus the partially deaf cohort (Fig. 8). Neuronal shrinkage presumably reflects a down regulation in biosynthetic activity within the SGN following a dramatic reduction in neural activity associated with the loss of hair cells. The increased SGN soma area evident in the partially deaf cohort supports such a hypothesis, as this cohort would have large numbers of SGNs still innervating hair cells in the middle and apical turns. Furthermore, the partial recovery of soma area evident in the basal turn of the severely deaf cohort following ES is also consistent with a presumed increase in biosynthesis of SGNs. Previous studies have described a similar effect (Araki et al., 1998; Leake et al., 1999).
The EABR data demonstrated that chronically stimulated SGNs from long-term deafened cochleae remained physiologically viable throughout the ES period. However, significant increases in EABR threshold were observed over time for all chronically stimulated electrodes. This finding is consistent with previous chronic ES studies performed in both the cat and guinea pig (Xu et al., 1997; Shepherd et al., 2005). While an elevation in EABR threshold may reflect an ongoing degeneration of SGNs, other potential mechanisms must also be considered. First, an increase in the thickness of the tissue capsule surrounding the electrode array could alter the proportion of current being shunted directly between bipolar stimulating electrodes, thereby elevating thresholds. Second, changes in SGN size can influence EABR thresholds as electrical thresholds are inversely related to nerve fiber diameter (McNeal, 1976). SGN shrinkage associated with deafness (Leake and Hradek, 1988; Nadol et al., 1989; Shepherd and Javel, 1997; Shepherd et al., 2005) could at least partially contribute to the increases in EABR thresholds observed in deafened cochleae.
The present study also demonstrated that chronic ES, using charge-balanced biphasic current pulses delivered to electrodes located in the 10-26 kHz region of the cochlea, showed no effect on residual low-frequency hearing (frequencies ≤ 4 kHz). These data extend our previous experimental results using normal hearing animals (Shepherd et al., 1983; Ni et al, 1992; Xu et al., 1997), by demonstrating that hair cells that survive aminoglycoside deafening are not more susceptible to a second insult from cochlear implantation and chronic ES. Furthermore, maintenance of both ABR and CAP thresholds over long-term cochlear implantation suggests that these hair cells not only survive but are physiologically functional.
This work supports clinical research investigating improved speech perception in severely deaf patients using combined electric and acoustic stimulation (von Ilberg et al., 1999; Gantz and Turner, 2003; Kiefer et al., 2005). While the implantation of a scala tympani electrode array has been associated with loss of residual hearing (Rizer et al., 1988; Boggess et al., 1989), the development of electrode arrays and surgical techniques designed to minimize insertion trauma have led to the preservation of residual hearing in some patients (Keifer et al., 2005; Adunka et al., 2004; Gantz and Turner, 2003). The lack of significant insertion trauma observed in the present study supports these clinical findings and indicates that long-term preservation of low-frequency hearing is possible in implant subjects.
Finally, general cochlear histopathology was not influenced by chronic ES. Similar levels of fibrous tissue growth were observed in both stimulated and non-stimulated cochleae, and were due likely to the presence of the electrode array in the scala tympani. Moreover, the nature and severity of the tissue response observed in the present study was consistent with our previous cochlear implantation and ES studies in normal hearing cochleae (Shepherd et al., 1983; Ni et al., 1992; Xu et al., 1997). In contrast, we have previously reported an increased fibrous tissue reaction following chronic ES in neonatally deafened cochleae (Shepherd et al., 1994), suggesting that the young deafened cochleae may be predisposed to a more vigorous tissue reaction in response to cochlear implantation and ES. New bone formation, observed in 7 of the 16 chronically implanted cochleae in the present study, was limited to a small region of the basal turn scala tympani. We have reported a similar incidence and extent of new bone in a previous study using normal hearing animals (Xu et al., 1997). Although osteoneogenesis was typically associated with more extensive fibrous tissue growth (Table 2), it was not associated with ES per se; a finding consistent with previous chronic ES studies using charge balanced biphasic current pulses (Shepherd et al., 1983; Ni et al., 1992; Shepherd et al., 1994; Clark et al., 1995; Xu et al., 1997).
Conclusions
The present results indicate that chronic ES using an electrode array located in the cochlear base and stimulated at up to 6 dB above EABR threshold does not adversely affect low frequency hearing thresholds in partially deaf cochleae and suggests that hair cells that have survived one pathological insult (aminoglycoside deafening) can survive cochlear implantation. Moreover, while chronic ES results in an increase in the soma area of SGNs close to the stimulating electrodes in severely deaf cochleae this trophic effect does not result in increased levels of SGN survival.
Acknowledgements
We are grateful to Ms Helen Feng for electrode manufacture, Ms Maria Clarke and Ms Prue Nielsen for histology, Dr Sue Pierce for veterinary advice, Ms Elisa Borg for animal husbandry and to Ms Jacqueline Andrew for comments on earlier versions of the manuscript.
Footnotes
Supporting grants: This work was funded by the National Institutes of Health NIDCD (NO1-DC-3-1005), The Bionic Ear Institute and a Wagstaff Fellowship from the Royal Victorian Eye & Ear Hospital.
References
- Araki S, Kawano A, Seldon L, Shepherd RK, Funasaka S, Clark GM. Effects of chronic electrical stimulation on spiral ganglion neuron survival and size in deafened kittens. Laryngoscope. 1998;108:687–95. doi: 10.1097/00005537-199805000-00012. [DOI] [PubMed] [Google Scholar]
- Black RC, Clark GM, O'Leary SJ, Walters C. Intracochlear electrical stimulation of normal and deaf cats investigated using brainstem response audiometry. Acta Oto-Laryngologica. 1983;(Supplement 399):5–17. doi: 10.3109/00016488309105588. [DOI] [PubMed] [Google Scholar]
- Boggess WJ, Baker JE, Balkany TJ. Loss of residual hearing after cochlear implantation. Laryngoscope. 1989;99:1002–5. doi: 10.1288/00005537-198210000-00005. [DOI] [PubMed] [Google Scholar]
- Brown M, Shepherd RK, Webster WR, Martin RL, Clark GM. Cochleotopic selectivity of a multichannel scala tympani electrode array using the 2-deoxyglucose technique. Hear Res. 1992;59:224–40. doi: 10.1016/0378-5955(92)90119-8. [DOI] [PubMed] [Google Scholar]
- Clark GM, Shute SA, Shepherd RK, Carter TD. Cochlear implantation: osteoneogenesis, electrode-tissue impedance, and residual hearing. Ann Otol Rhinol Laryngol Suppl. 1995;166:40–2. [PubMed] [Google Scholar]
- Elverland HH, Mair IW. Hereditary deafness in the cat. An electron microscopic study of the spiral ganglion. Acta Otolaryngol. 1980;90:360–9. doi: 10.3109/00016488009131737. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Pirvola U, Ylikoski J. Making and breaking the innervation of the ear: neurotrophic support during ear development and its clinical implications. Cell & Tissue Research. 1999;295:369–82. doi: 10.1007/s004410051244. [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Turner CW. Combining acoustic and electrical hearing. Laryngoscope. 2003;113:1726–30. doi: 10.1097/00005537-200310000-00012. [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Woodworth GG, Knutson JF, Abbas PJ, Tyler RS. Multivariate predictors of audiological success with multichannel cochlear implants. Ann Otol Rhinol Laryngol. 1993;102:909–16. doi: 10.1177/000348949310201201. [DOI] [PubMed] [Google Scholar]
- Hansen MR, Zha XM, Bok J, Green SH. Multiple distinct signal pathways, including an autocrine neurotrophic mechanism, contribute to the survival-promoting effect of depolarization on spiral ganglion neurons in vitro. J Neurosci. 2001;21:2256–67. doi: 10.1523/JNEUROSCI.21-07-02256.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie NA, Shepherd RK. Sensorineural hearing loss during development: morphological and physiological response of the cochlea and auditory brainstem. Hear Res. 1999;128:147–65. doi: 10.1016/s0378-5955(98)00209-3. [DOI] [PubMed] [Google Scholar]
- Hartmann R, Klinke R. Electrical stimulation of the cat cochlea - discharge pattern of sinle auditory fibres. Adv Audiol. 1984;1:18–29. [Google Scholar]
- Hartshorn DO, Miller JM, Altschuler RA. Protective effect of electrical stimulation in the deafened guinea pig cochlea. Archives of Otolaryngology - Head and Neck Surgery. 1991;104:311–319. doi: 10.1177/019459989110400305. [DOI] [PubMed] [Google Scholar]
- Hegarty JL, Kay AR, Green SH. Trophic support of cultured spiral ganglion neurons by depolarization exceeds and is additive with that by neurotrophins or cAMP and requires elevation of [Ca2+]i within a set range. J Neurosci. 1997;17:1959–70. doi: 10.1523/JNEUROSCI.17-06-01959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CQ, Shepherd RK, Carter PM, Seligman PM, Tabor B. Electrical stimulation of the auditory nerve: direct current measurement in vivo. IEEE Trans Biomed Eng. 1999;46:461–70. doi: 10.1109/10.752943. [DOI] [PubMed] [Google Scholar]
- Hultcrantz M, Snyder R, Rebscher S, Leake P. Effects of neonatal deafening and chronic intracochlear electrical stimulation on the cochlear nucleus in cats. Hear Res. 1991;54:272–80. doi: 10.1016/0378-5955(91)90121-o. [DOI] [PubMed] [Google Scholar]
- Javel E, Shepherd RK. Electrical stimulation of the auditory nerve. III. Response initiation sites and temporal fine structure. Hear Res. 2000;140:45–76. doi: 10.1016/s0378-5955(99)00186-0. [DOI] [PubMed] [Google Scholar]
- Kiefer J, Pok M, Adunka O, Sturzebecher E, Baumgartner W, Schmidt M, Tillein J, Ye Q, Gstoettner W. Combined electric and acoustic stimulation of the auditory system: results of a clinical study. Audiol Neurootol. 2005;10:134–44. doi: 10.1159/000084023. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT. Cochlear pathology of long term neomycin induced deafness in cats. Hear Res. 1988;33:11–33. doi: 10.1016/0378-5955(88)90018-4. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Snyder RL. Chronic electrical stimulation by a cochlear implant promotes survival of spiral ganglion neurons after neonatal deafness. J Comp Neurol. 1999;412:543–62. doi: 10.1002/(sici)1096-9861(19991004)412:4<543::aid-cne1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Leake PA, Snyder RL, Hradek GT, Rebscher SJ. Chronic intracochlear electrical stimulation in neonatally deafened cats: effects of intensity and stimulating electrode location. Hear. Res. 1992;64:99–117. doi: 10.1016/0378-5955(92)90172-j. [DOI] [PubMed] [Google Scholar]
- Leake PA, Snyder RL, Hradek GT, Rebscher SJ. Consequences of chronic extracochlear electrical stimulation in neonatally deafened cats. Hear. Res. 1995;82:65–80. doi: 10.1016/0378-5955(94)00167-o. [DOI] [PubMed] [Google Scholar]
- Li L, Parkins CW, Webster DB. Does electrical stimulation of deaf cochleae prevent spiral ganglion degeneration? Hear Res. 1999;133:27–39. doi: 10.1016/s0378-5955(99)00043-x. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Kiang NY. Acoustic trauma in cats. Cochlear pathology and auditory-nerve activity. Acta Otolaryngol Suppl. 1978;358:1–63. [PubMed] [Google Scholar]
- Lousteau RJ. Increased spiral ganglion cell survival in electrically stimulated, deafened guinea pig cochleae. Laryngoscope. 1987;97:836–842. [PubMed] [Google Scholar]
- McNeal DR. Analysis of a model for excitation of myelinated nerve. IEEE Trans Biomed Eng. 1976;23:329–37. doi: 10.1109/tbme.1976.324593. [DOI] [PubMed] [Google Scholar]
- Miller AL. Effects of chronic stimulation on auditory nerve survival in ototoxically deafened animals. Hear Res. 2001;151:1–14. doi: 10.1016/s0378-5955(00)00226-4. [DOI] [PubMed] [Google Scholar]
- Mitchell A, Miller JM, Finger PA, Heller JW, Raphael Y, Altschuler RA. Effects of chronic high-rate electrical stimulation on the cochlea and eighth nerve in the deafened guinea pig. Hear Res. 1997;105:30–43. doi: 10.1016/s0378-5955(96)00202-x. [DOI] [PubMed] [Google Scholar]
- Moxon EL. Neural and Mechanical Responses to Electrical Stimulation of the Cat's Inner Ear. Cambridge, MA: 1971. [Google Scholar]
- Nadol JB, Jr., Young YS, Glynn RJ. Survival of spiral ganglion cells in profound sensorineural hearing loss: implications for cochlear implantation. Ann Otol Rhinol Laryngol. 1989;98:411–6. doi: 10.1177/000348948909800602. [DOI] [PubMed] [Google Scholar]
- Ni D, Shepherd RK, Seldon HL, Xu S-A, Clark GM, Millard RE. Cochlear pathology following chronic electrical stimulation of the auditory nerve. I: normal hearing kittens. Hear. Res. 1992;62:63–81. doi: 10.1016/0378-5955(92)90203-y. [DOI] [PubMed] [Google Scholar]
- Rajan R, Irvine DR, Cassell JF. Normative N1 audiogram data for the barbiturate-anaesthetised domestic cat. Hear Res. 1991;53:153–8. doi: 10.1016/0378-5955(91)90222-u. [DOI] [PubMed] [Google Scholar]
- Rizer FM, Arkis PN, Lippy WH, Schuring AG. A postoperative audiometric evaluation of cochlear implant patients. Otolaryngol Head Neck Surg. 1988;98:203–6. doi: 10.1177/019459988809800304. [DOI] [PubMed] [Google Scholar]
- Schimmang T, Tan J, Muller M, Zimmermann U, Rohbock K, Kopschall I, Limberger A, Minichiello L, Knipper M. Lack of Bdnf and TrkB signalling in the postnatal cochlea leads to a spatial reshaping of innervation along the tonotopic axis and hearing loss. Development. 2003;130:4741–50. doi: 10.1242/dev.00676. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF. Techniques for study of cochlear function and pathology in experimental animals. Archives of Otolaryngology. 1953;58:377–400. doi: 10.1001/archotol.1953.00710040399001. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Martin RL. Onset of ototoxicity in the cat is related to onset of auditory function. Hear. Res. 1995;92:131–142. doi: 10.1016/0378-5955(95)00211-1. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Javel E. Electrical stimulation of the auditory nerve. I. Correlation of physiological responses with cochlear status. Hear. Res. 1997;108:112–144. doi: 10.1016/s0378-5955(97)00046-4. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Clark GM, Black RC. Chronic electrical stimulation of the auditory nerve in cats. Physiological and histopathological results. Acta Oto-Laryngologica. 1983;(Supplement 399):19–31. doi: 10.3109/00016488309105589. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Matsushima J, Martin RL, Clark GM. Cochlear pathology following chronic electrical stimulation of the auditory nerve: II Deafened kittens. Hear. Res. 1994;81:150–166. doi: 10.1016/0378-5955(94)90162-7. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Coco A, Epp SB, Crook JM. Chronic depolarization enhances the trophic effects of brain-derived neurotrophic factor in rescuing auditory neurons following a sensorineural hearing loss. J Comp Neurol. 2005;486:145–158. doi: 10.1002/cne.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RK, Meltzer NE, Fallon JB, Ryugo DK. Consequences of deafness and electrical stimulation on the peripheral and central auditory system. In: Waltzman SB, Roland TJ, editors. Cochlear Implants. 2nd ed. Thieme Medical Publishers, Inc; New York: 2006. pp. 25–39. [Google Scholar]
- Spoendlin H. Factors inducing retrograde degeneration of the cochlear nerve. Ann Otol Rhinol Laryngol Suppl. 1984;112:76–82. doi: 10.1177/00034894840930s415. [DOI] [PubMed] [Google Scholar]
- Spoendlin H, Schrott A. Analysis of the human auditory nerve. Hear Res. 1989;43:25–38. doi: 10.1016/0378-5955(89)90056-7. [DOI] [PubMed] [Google Scholar]
- Stankovic K, Rio C, Xia A, Sugawara M, Adams JC, Liberman MC, Corfas G. Survival of adult spiral ganglion neurons requires erbB receptor signaling in the inner ear. J Neurosci. 2004;24:8651–61. doi: 10.1523/JNEUROSCI.0733-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Shepherd RK. Aminoglycoside-induced degeneration of adult spiral ganglion neurons involves differential modulation of tyrosine kinase B and p75 neurotrophin receptor signaling. Am J Pathol. 2006;169:528–43. doi: 10.2353/ajpath.2006.060122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terayama Y, Kaneko Y, Kawamoto K, Sakai N. Ultrastructural changes of the nerve elements following disruption of the organ of Corti. I. Nerve elements in the organ of Corti. Acta Otolaryngol. 1977;83:291–302. doi: 10.3109/00016487709128848. [DOI] [PubMed] [Google Scholar]
- van den Honert C, Stypulkowski PH. Physiological properties of the electrically stimulated auditory nerve. II. Single fiber recordings. Hear. Res. 1984;14:225–243. doi: 10.1016/0378-5955(84)90052-2. [DOI] [PubMed] [Google Scholar]
- von Ilberg C, Kiefer J, Tillein J, Pfenningdorff T, Hartmann R, Sturzebecher E, Klinke R. Electric-acoustic stimulation of the auditory system. New technology for severe hearing loss. ORL J Otorhinolaryngol Relat Spec. 1999;61:334–40. doi: 10.1159/000027695. [DOI] [PubMed] [Google Scholar]
- Walsh EJ, McGee J, Javel E. Development of auditory-evoked potentials in the cat. II. Wave latencies. Journal of the Acoustical Society of America. 1986;79:725–744. doi: 10.1121/1.393462. [DOI] [PubMed] [Google Scholar]
- White JA, Burgess BJ, Hall RD, Nadol JB. Pattern of degeneration of the spiral ganglion cell and its processes in the C57BL/6J mouse. Hear Res. 2000;141:12–8. doi: 10.1016/s0378-5955(99)00204-x. [DOI] [PubMed] [Google Scholar]
- Xu J, Shepherd RK, Millard RE, Clark GM. Chronic electrical stimulation of the auditory nerve at high stimulus rates: a physiological and histopathological study. Hear. Res. 1997;105:1–29. doi: 10.1016/s0378-5955(96)00193-1. [DOI] [PubMed] [Google Scholar]
- Ylikoski J, Pirvola U, Moshnyakov M, Palgi J, Arumae U, Saarma M. Expression patterns of neurotrophin and their receptor mRNAs in the rat inner ear. Hear Res. 1993;65:69–78. doi: 10.1016/0378-5955(93)90202-c. [DOI] [PubMed] [Google Scholar]