Abstract
Selective inhibition of the localized excess production of NO by neuronal nitric oxide synthase (nNOS) has been targeted as a potential means of treating various neurological disorders. Based on observations from the X-ray crystal structures of complexes of nNOS with two nNOS-selective inhibitors, (4S)-N-{4-amino-5-[(2-aminoethylamino]pentyl}-N′-nitroguanidine (L-Arg(NO2)-L-Dbu-NH2 (1) and 4-N-(Nω-nitro-L-argininyl)-trans-4-amino-Lproline amide (2), a series of descarboxamide analogues was designed and synthesized (3-7). The most potent compound was aminopyrrolidine analogue 3, which exhibited better potency and selectivity for nNOS than parent compound 2. In addition, 3 provided higher lipophilicity and a lower molecular weight than 2, therefore having better physicochemical properties. Nα-Methylated analogues (8-11) also were prepared for increased lipophilicity of the inhibitors, but they had 4-5 fold weaker binding affinity compared to their parent compounds.
Keywords: Neuronal nitric oxide synthase inhibitor, Isoform selective inhibition, Descarboxamide, N-Methylation
1. Introduction
Nitric oxide (NO),1 an essential cellular signaling molecule, is produced in mammalian cells by a family of enzymes called nitric oxide synthases (NOS, EC 1.14.13.39).2 Three isoforms of NOS have been isolated so far: neuronal NOS (nNOS) in the central nervous system, endothelial NOS (eNOS) in the cardiovascular system, and inducible NOS (iNOS) in the immune system.3 Each isozyme catalyzes the conversion of L-arginine to NO and L-citrulline. NO is involved with diverse physiological events, which include neurotransmission (nNOS),4 vasodilation (eNOS),5 and cytoprotection (iNOS).6 Despite the importance of this molecule, uncontrolled production of NO is associated with potentially harmful effects; an overproduction of NO in neuronal cells has been implicated in strokes,7 migraine headaches,8 and various neurodegenerative diseases.9 Because NO generated from eNOS is crucial to vascular regulation, selective inhibition of nNOS is a prerequisite for the inhibitor to be therapeutically useful.10,11
Previously, Nω-nitro-L-arginine-containing dipeptide amide inhibitors 1 12 and 2 13 (Figure 1) were found to be highly selective for neuronal nitric oxide synthase. To further the understanding of the inhibitor-active site interactions, Flinspach et al. Resolved the X-ray crystal structures for nNOS complexed with 1 and 2 (Supporting Information).14 Compound 2 is a conformationally restricted analogue of 1, but the binding modes for both inhibitors in the nNOS active site are somewhat different. The C-terminal carboxamide of 1 faces toward the ceiling of the active site and is surrounded by a hydrogen bond network of Arg481, Gln478, and Ser477; it also appears to have a weak interaction with Asn569. The carboxamide of 2, however, is placed in the substrate access channel and does not form hydrogen bonds with Arg481, Gln478, and Ser477. Instead, the carboxamide appears to have a weak hydrogen bond interaction with the heme propionate. The strength of each hydrogen bond cannot be quantitatively evaluated, but it is presumed that the carboxamide of 1 contributes more significantly to the enzyme-inhibitor binding than does the carboxamide of 2 based on the number and the distances of the hydrogen bonds. Indeed, Huang et al. demonstrated the importance of the carboxamide moiety in 1 by evaluating a series of descarboxamide analogues.15 These descarboxamide derivatives showed 2.5 to 4 times lower nNOS binding affinity than 1 depending on the side chain carbon length. However, as observed in the X-ray crystal structures, the carboxamide moiety of 2 may not be as important as that in 1. Based on this hypothesis, we designed a series of descarboxamide analogues of 2 with different ring sizes and stereochemistry (3-7, Figure 1). These aminopyrrolidine and aminopiperidine analogues represent three possible advantages: first, removal of the polar carboxamide functionality from 2 should afford a less polar compound for potential improved pharmacokinetic properties; second, the lower molecular weight is a desirable property; and third, there is one less stereogenic center.
Figure 1.
Structures of Neuronal Nitric Oxide Synthase-Selective Inhibitors
In a continued effort to modify inhibitors for enhanced lipophilicity, we also designed simple Nα-methylated nitro-L-argininamide analogues (8-11, Figure 1). Recently, we reported analogues of 2 in which the pyrrolidine ring nitrogen was alkylated; this led to a decrease in both potency and selectivity.16 The Nα-methylated analogues described here will be assessed for effective hydrogen bonding interactions between active site residues and the α-amino moiety as either a primary amine or a secondary amine. Provided that the additional methyl group does not disrupt existing hydrogen bonding interactions, it is expected that the Nα-methylated derivatives would have more favorable physiocochemical properties.17
2. Chemistry
2.1. Descarboxamide inhibitors
Five descarboxamide analogues were designed using 3-aminopyrrolidines (3 and 4) and 3 or 4-aminopiperidines (5, 6, and 7 in Figure 1). Compounds 3-7 were synthesized by a peptide coupling reaction using HBTU/HOBt/DIEA and subsequent deprotection (Scheme 1).
Scheme 1.
2.2. Nα-Methylated inhibitors
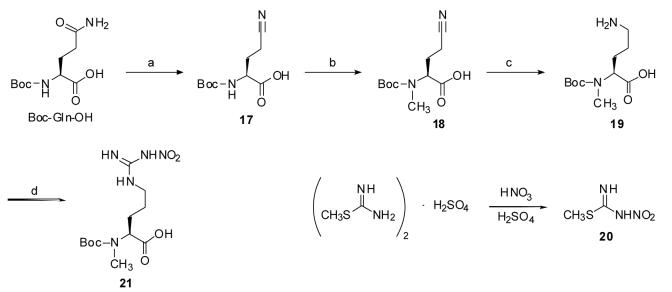
For the synthesis of 8-11, Nα-Boc-Nα-methyl-L-nitroarginine (21) was prepared as shown in Scheme 2. Nα-Boc-Nα-Methyl-L-ornithine (19) was synthesized according to a literature procedure18 using a modification of the nitrile reduction method. The synthesis started with Boc-L-Gln-OH; the γ-carboxamide of Boc-L-Gln-OH was dehydrated to a nitrile using acetic anhydride and pyridine. The resulting nitrile-containing compound was selectively alkylated with iodomethane, providing Nα-Boc-2-methylamino-4-cyanobutyric acid (18). As originally reported, the reduction of the nitrile to an amine was first tried with PtO2-catalyzed hydrogenation in isopropanol in the presence of HCl.19 Later we found that the nitrile reduction could be performed in a more facile and consistent manner by hydrogenation at 1 atmosphere pressure with Pd(OH)2/C as the catalyst in methanol/acetic acid (1:1) solution. The Pd(OH)2 catalyst also is much cheaper than PtO2, propitious for a large scale reaction. After filtration of the catalyst and evaporation of the solvent, Boc-L-MeOrn-OH (19) directly underwent nitroguanidination using 2-methyl-1-nitro-2-thiopseudourea (20), which was prepared by nitration of commercially available S-methylisothiourea hemisulfate salt (Scheme 2).19
Scheme 2a.
a Reagents and conditions: (a) Acetic anhydride, pyridine, 60%; (b) CH3I, NaH, THF, 83%; (c) H2/Pd(OH)2, MeOH/AcOH (1:1), quantitative; (d) 20, Et3N, EtOH, 40°C, 43%
Boc-L-MeArg(NO2)-OH (21) was then coupled to (R)-(+)-1-Boc-3-aminopyrrolidine, (R)-(+)-1-Boc-3-aminopiperidine, and 4-amino-1-Boc-piperidine, and the resulting Nα-methylated L-nitroargininamides were deprotected with TFA, purified by preparative HPLC, and lyophilized (9-11, Scheme 1).
Compound 8 was prepared by the synthetic route shown in Scheme 3. Key intermediate 30 was synthesized from the starting material, N-Boc-L-trans-4-hydroxyproline. Methyl ester protection and hydroxyl group inversion were achieved by an intramolecular Mitsunobu reaction 20 and a sodium azide catalyzed methanolysis 21 to provide 26. As precursor of a primary amine, an azide was incorporated by another Mitsunobu reaction using diphenylphosphoryl azide (DPPA). Methyl ester 27 was then hydrolyzed and converted to amide 29 by a mixed anhydride coupling reaction using 29% aqueous ammonia. Palladium on carbon catalyzed hydrogenation provided 30. Peptide coupling of 21 and 30 followed by Boc deprotection provided 8.
Scheme 3a.
a Reagents and conditions: (a) DIAD, PPh3, THF, 97%; (b)MeOH, NaN3, 40 °C, 98%; (c) DPPA, DEAD, PPh3, THF, 94%; (d) 5% LiOH, THF, 91%; (e) iButylchloroformate, NMM, NH4OH, THF, 83%; (f) H2, Pd/C, MeOH, 94%; (g) Boc-MeArg(NO2)-OH, HBTU, HOBt, DCM/DMF, 30%; (h) 50% TFA, CH2Cl2, quant.
3. Results and Discussion
Physicochemical properties (MW, logP, and logD7.4) for the inhibitors were calculated using Advanced Chemistry Development, Inc. software (Toronto, Canada), and the results are summarized in Table 1. Descarboxamide compounds 3, 5, and 7, although still poorly lipophilic, have increased lipophilicity compared with parent compound 2, by approximately two orders of magnitude, and the molecular weights are lower. Nα-Methylation (8-11) also resulted in increased lipophilicity of the compounds.
Table 1.
Physicochemical properties of nNOS inhibitors.
| Compound | MW | logPa,b | logD7.4a |
|---|---|---|---|
| 2 | 330.34 | −3.81 | −6.57 |
| 3 | 287.32 | −2.22 | −5.20 |
| 5 | 301.35 | −1.79 | −4.77 |
| 7 | 301.34 | −1.84 | −4.83 |
| 8 | 344.37 | −3.58 | −6.33 |
| 9 | 301.35 | −1.99 | −4.96 |
| 10 | 315.37 | −1.56 | −4.52 |
| 11 | 315.37 | −1.60 | −4.58 |
Lipophilicity data were calculated with ACD/LogD version 7.0, Advanced Chemistry Development, Inc, Toronto, Canada.
Octanol-water partition coefficient calculated for neutral compounds.
The in vitro enzyme inhibitory activity was evaluated using a hemoglobin capture assay with descarboxamides 3-7 as well as parent compound 2; the assay results are summarized in Table 2. These results indicate that 3 is a more potent inhibitor of nNOS than is 2.
Table 2.
In vitro NOS inhibition by descarboxamide and Nα-methylated analogues.a
| compound |
Ki (μM)b |
selectivityd |
|||
|---|---|---|---|---|---|
| nNOSc | iNOS | eNOS | n/i | n/e | |
| L-NNA | 0.61 ± 0.005 | 4.28 | 0.72 | 7 | 1.2 |
| 2 | 0.33 ± 0.019 | 17 | 72 | 52 | 218 |
| 3 | 0.22 ± 0.021 | 55 | 68 | 250 | 309 |
| 4 | 1.76 ± 0.065 | 57 | 77 | 32 | 44 |
| 5 | 0.48 ± 0.018 | 90 | 225 | 188 | 469 |
| 6 | 7.62 ± 0.587 | 535 | 221 | 70 | 29 |
| 7 | 0.47 ± 0.011 | 202 | 262 | 430 | 557 |
| 8 | 1.09 ± 0.106 | 65 | 290 | 60 | 266 |
| 9 | 1.08 ± 0.025 | 124 | 160 | 115 | 148 |
| 10 | 2.26 ± 0.038 | 242 | 174 | 107 | 77 |
| 11 | 2.38 ± 0.110 | 515 | 114 | 216 | 48 |
The enzyme used for the Ki determinations are rat brain nNOS, recombinant murine iNOS, and recombinant bovine eNOS. See Experimental Section.
In most cases, the Ki values represent single measurements with 5-6 data points and correlation coefficients 0.948-0.996.
Ki ± SEM. Results are given as a mean of two or more independent experiments.
The ratio of Ki (iNOS or eNOS) to Ki (nNOS).
Two inferences can be made from this result: 1) the carboxamide moiety of 2 is not crucial for enzyme binding provided that other changes resulting from the removal of the carboxamide moiety, such as solubility or conformation, have minimal effects; 2) removal of the carboxamide moiety affords better interactions between the α-amino group of 3 and the active site Glu592 (Figure 2 and 3). From computer modeling, the excision of the carboxamide group results in the loss of a weak interaction between the heme propionate and the carboxamide -NH, but the loss of this interaction allows the α-amino group of 3 to move slightly closer to Glu592 and results in a stronger hydrogen bond. The slightly higher nNOS potency of 3 relative to 2 indicates that the latter effect probably outweighs the former. The key hydrogen bond between the secondary amine of the pyrrolidine ring and the heme propionate group through structural water molecule 2 remains similar (Figure 3B). Compound 3 also shows enhanced selectivity for nNOS over the other isoforms. The opposite stereochemistry at the 3-position of the pyrrolidine ring (S-stereochemistry) has little effect on the affinity for iNOS and eNOS, but is detrimental to nNOS binding (compare 3 and 4).
Figure 2.

Two perspectives of X-ray crystal structure of 2 bound in nNOS active site. The distances of the hydrogen bonds are marked in Å.
Figure 3.

Superimposition of the crystallographic conformation of 2 (gray) and the docked conformation of 3 (orange) in the nNOS active site. Distances of the hydrogen bonds are marked in Å.
Piperidine-containing compounds 5 and 7 exhibited a two-fold reduced potency for nNOS relative to 3, indicating that a 5-membered ring fits better than a 6-membered ring at that position in the nNOS active site. As in the case of the pyrrolidine-containing compounds, the S-stereochemistry at the piperidine ring (6) was disadvantageous, relative to R-stereochemistry (5), for binding with the neuronal and inducible isoforms. The binding affinity for eNOS is highly dependent on the size of the ring. Piperidines (5-7) have about three times lower activity against eNOS than do the pyrrolidines (2-4). This reflects the difference between the active site sizes of nNOS and eNOS and is consistent with the fact that nNOS has a slightly larger active site than eNOS.22 In this series of descarboxamide derivatives, 3 showed the best potency for nNOS, and 7 showed the best selectivity for nNOS over iNOS and eNOS.
An additional structure-activity relationship study was carried out by Nα-methylation of 2, 3, 5, and 7 (Figure 1). We rationalized that if methylation did not disrupt the hydrogen bonds of the α-amino group, we could generate inhibitors with higher lipophilicities. In addition, the effect of Nα-methylation could be evaluated for future amine-prodrug design, because a promoiety on a secondary amine produces a more stable prodrug than that on a primary amine.23
Entries 8-11 in Table 2 give the in vitro NOS inhibitory activities of the Nα-methylated derivatives. All of the methylated compounds had approximately 4-5 fold decreased nNOS binding affinity, which suggests that the methyl moiety on the α-amino group causes an unfavorable effect on the hydrogen bond network. Apparently, the methyl group cannot be accommodated tightly in the space around Glu592 and water molecule 3 (Figure 2); rather, the methyl group might cause the inhibitor to move away from Glu592, water molecule 2, and water molecule 3, weakening the hydrogen bonding interactions with them. This shift of the inhibitor (moving toward the top in Figure 2A or the right in Figure 2B) does not seem to be beneficial. The assay results for the Nα-methylated derivatives support the significance of the interaction between the α-amino group and Glu592/water molecule 3 and the interaction between the secondary amino group on the pyrrolidine ring and water molecule 2 (Figure 2).
4. Conclusion
We designed and synthesized Nω-nitro-L-argininamides containing aminopyrrolidine or aminopiperidine substituents as descarboxamide analogues of 2. In accordance with our hypothesis, the carboxamide group of 2 is not essential for enzyme-inhibitor binding; 3 provided higher in vitro potency and selectivity with nNOS than did 2. The descarboxamides also offer the potential benefit of lower polarity. We also corroborated the importance of the hydrogen bonding interaction between the α-amino group on the nitroargininamide and Glu592/water 3 by preparing and testing Nα-methylated analogues 8-11, which exhibited lower affinities for nNOS than 2. These results broaden our understanding of nNOS-inhibitor interactions and provide strategies for the design of more effective nNOS-selective inhibitors.
5. Experimental Section
5.1. General methods
Fisher silica gel 60 (230-400 mesh) was used for flash column chromatography with >10% methanol eluent systems; otherwise Sorbent Technologies silica gel 60 (200-400 mesh) was used. Thin-layer chromatography was carried out on E. Merck precoated silica gel 60 F254 plates. Compounds were visualized with a ninhydrin spray reagent or a UV/vis lamp. Combustion analyses were performed by Atlantic Microlab, Inc., Norcross, Georgia, USA. Electrospray mass spectra were obtained on a VG70-250SE mass spectrometer in the Analytical Service Laboratory at Northwestern University. High resolution mass spectrometric analyses were carried out at the University of Illinois, Urbana-Champaign using a Micromass Quattro II spectrometer. 1H NMR spectra were recorded on a Varian Mercury 400 or Varian Inova 500 NMR spectrometer. Chemical shifts are reported as δ values in parts per million downfield from TMS (δ 0.0) as the internal standard in CDCl3. Melting points were measured on a Buchi melting point B-540 apparatus and are uncorrected. An Orion Research model 701 pH meter with a general combination electrode was used for pH measurements.
Final compounds (1.0 mL injection of a solution of 8 mg/mL sample) were purified by preparative HPLC (Waters Prep LC 4000 system) using a Phenomenex Gemini 5 [.proportional] C18 (250 X 21.20 mm) column. Samples were eluted using a mobile phase of 4% solvent A (60% CH3CN + 40% water + 0.08% TFA) and 96% solvent B (water + 0.1% TFA) for 30 min isocratically at a flow rate of 18 mL/min. Product peaks were detected by absorbance at 254 nm with a Waters 2487 Dual lambda absorbance detector. Generally, product peaks appeared at 10-15 min elution times. Fractions containing the pure products were concentrated in vacuo, and the residues were lyophilized.
All reagents were purchased from Aldrich, Advanced ChemTech, and Nova Biochem and were used without further purification unless stated otherwise. NADPH, calmodulin, and human ferrous hemoglobin were obtained from Sigma-Aldrich Co. Tetrahydrobiopterin (H4B) was purchased from Alexis Biochemicals Co., San Diego, CA, USA. HEPES, DTT, and conventional organic solvents were purchased from Fisher Scientific. Tetrahydrofuran was distilled under nitrogen from sodium/benzophenone. Diethyl azodicarboxylate (DEAD) was purchased from Lancaster Synthesis, Inc., Pelham, NH, USA. (R) and (S) isomers of both 1-Boc-3-aminopyrrolidine and 1-Boc-3-aminopiperidine were purchased from CNH Technologies, Inc., Woburn, MA, USA.
5.2. General method for the syntheses of 3-7
To a solution of Boc-L-Arg(NO2)-OH (2 mmol), HOBt (2 mmol), and HBTU (2 mmol) in dry DMF (3 mL) and dry CH2Cl2 (6 mL) was added the appropriate amine (1 mmol) in dry DMF (3 mL) dropwise at 0 °C. DIEA (2 mmol) in dry CH2Cl2 (4 mL) was added slowly, and stirring continued overnight at room temperature under argon. The solvent was removed under reduced pressure, and the residue was treated with EtOAc (10 mL). The organic mixture was washed successively with 5% NaHCO3 (10 mL), water (10 mL), 0.5N HCl (10 mL), and brine (10 mL), and dried over Na2SO4. The solution was concentrated in vacuo, and the residue was purified by flash column chromatography (EtOAc/MeOH = 19:1, Rf = 0.20) to afford the product as a white foam. The Boc protected analogues were treated with trifluoroacetic acid/CH2Cl2 (1:1) at 0 °C under argon, the reaction temperature was allowed to rise to room temperature, and stirring was continued for 1 h. Excess TFA and solvent were removed by evaporation in vacuo. The residue was repeatedly dissolved in CH2Cl2 and the solvents evaporated to remove traces of TFA. The residue was dissolved in a small amount of water, and the solution was washed with CH2Cl2 and lyophilized to give a white solid in a quantitative yield.
5.2.1. (3R)-3-((2S)-2-tert-Butoxycarbonylamino-5-nitroguanidinopentanoylamino)-pyrrolidine-1-carboxylic acid tert-butyl ester (12)
Boc-L-Arg(NO2)-OH (342 mg, 1.07 mmol), HOBt (145 mg, 1.07 mmol), HBTU (406 mg, 1.07 mmol), DIEA (138 mg, 1.07 mmol), and (R)-(+)-1-Boc-3-aminopyrrolidine (100 mg, 0.54 mmol) were used as described above to provide 12 (240 mg, 0.49 mmol, 91%) as a white solid; mp 102.9-103.7 °C. 1H NMR (500 MHz, CDCl3) δ 8.70 (s, 1H), 7.50 and 7.44 (2 × s, 1H), 5.76 and 5.67 (2 × s, 1H), 4.38 (m, 1H), 4.26 (m, 1H), 3.56 (dd, J = 6.0 Hz, 4.5 Hz, 1H), 3.29 - 3.39 (m, 4H), 3.17 (m, 1H), 2.06 (m, 2H), 1.82 (s, 1H), 1.75 (s, 1H), 1.67 (s, 2H), 1.41 (s, 9H), 1.39 (s, 9H), rotamers; 13C NMR (125 MHz, CDCl3) δ 172.2, 159.6, 156.6, 154.8, 80.4, 79.9, 60.7, 51.6, 50.8, 49.7, 48.9, 44.5, 44.0, 40.7, 38.9, 31.7, 31.2, 30.9, 28.7, 28.6, 24.8, 21.3, 14.4, rotamers; HRMS (ES) (m/z): M + H+ calcd for C20H38N7O7 488.2833, found 488.2841.
5.2.2. (2S)-2-Amino-5-nitroguanidinopentanoic acid ((3R)-pyrrolidin-3-yl)amide (3)
A white solid was obtained after Boc deprotection; mp 58.0-59.8 °C. 1H NMR (400 MHz, D2O) δ 4.13 (t, J = 6.0 Hz, 1H), 3.82 (t, J = 6.0 Hz, 1H), 3.43 (dd, J = 8.0 Hz, 6.5 Hz, 1H), 3.26 (m, 2H), 3.15 (s, 2H), 3.11 (m, 1H), 2.19 (m, 1H), 1.85 (m, 1H), 1.76 (s, 2H), 1.49 (s, 2H); 13C NMR (100 MHz, D2O) δ 169.6, 158.9, 114.9, 52.9, 49.5, 49.1, 44.6, 40.3, 29.6, 28.0; Anal. Calcd for C10H21N7O3·2.5TFA: C, 31.48, H, 4.14, N, 17.13; Found: C, 31.11, H, 4.35, N, 17.22.
5.2.3. (3S)-3-((2S)-2-tert-Butoxycarbonylamino-5-nitroguanidinopentanoylamino)-pyrrolidine-1-carboxylic acid tert-butyl ester (13)
Boc-L-Arg(NO2)-OH (342 mg, 1.07 mmol), HOBt (145 mg, 1.07 mmol), HBTU (406 mg, 1.07 mmol), DIEA (138 mg, 1.07 mmol), and (S)-(−)-1-Boc-3-aminopyrrolidine (100 mg, 0.54 mmol) were used as described above to provide 13 (261 mg, 5.35 mmol, 99%) as a white solid; mp 100.9-101.7 °C. 1H NMR (500 MHz, CDCl3) δ 8.61 (s, 1H), 7.47 (s, 1H), 5.73 (s, 1H), 4.34 (s, 1H), 4.24 (s, 1H), 3.53 (s, 1H), 3.20 - 3.36 (m, 4H), 3.12 (m, 1H), 2.05 (s, 2H), 1.82 (s, 1H), 1.63 (m, 3H), 1.39 (s, 9H), 1.36 (s, 9H), rotamers; 13C NMR (125 MHz, CDCl3) δ 172.4, 159.6, 156.4, 154.8, 80.2, 79.9, 60.6, 51.6, 50.9, 49.6, 49.0, 44.4, 44.0, 40.7, 38.8, 31.6, 31.2, 30.7, 28.7, 28.5, 24.8, 21.3, 14.4, rotamers; HRMS (ES) (m/z): M + H+ calcd for C20H38N7O7 488.2833, found 488.2847.
5.2.4. (2S)-2-Amino-5-nitroguanidinopentanoic acid ((3S)-pyrrolidin-3-yl)amide (4)
A white solid was obtained after Boc deprotection; mp 63.6-65.0 °C. 1H NMR (500 MHz, D2O) δ 4.29 (t, J = 6.0 Hz, 1H), 3.79 (t, J = 6.5 Hz, 1H), 3.41 (dd, J = 7.5 Hz, 5.0 Hz, 1H), 3.27 (m, 1H), 3.19 (m, 1H), 3.10 (s, 2H), 3.01 (dd, J = 7.5 Hz, 5.0 Hz, 1H), 2.15 (q, J = 7.0 Hz, 1H), 1.84 (q, J = 7.0 Hz, 1H), 1.72 (s, 2H), 1.47 (s, 2H); 13C NMR (100 MHz, D2O) δ 169.7, 158.9, 114.8, 52.8, 49.4, 49.0, 44.6, 40.3, 29.6, 28.0. HRMS (ES) (m/z): M + H+ calcd for C10H22N7O3 288.1784, found 288.1783; Anal. Calcd for C10H21N7O3·3TFA·0.75H2O: C, 29.89, H, 4.00, N, 15.25; Found: C, 29.89, H, 4.13, N, 15.35.
5.2.5. (3R)-3-((2S)-2-tert-Butoxycarbonylamino-5-nitroguanidinopentanoylamino)-piperidine-1-carboxylic acid tert-butyl ester (14)
Boc-L-Arg(NO2)-OH (224 mg, 0.70 mmol), HOBt (95 mg, 0.70 mmol), HBTU (265 mg, 0.70 mmol), DIEA (90 mg, 0.70 mmol), and (R)-1-Boc-3-aminopiperidine (70 mg, 0.35 mmol) were used as described above to provide 14 (162 mg, 0.32 mmol, 92%) as a white solid; mp 111.6-113.0 °C. 1H NMR (500 MHz, CDCl3) δ 8.69 (s, 1H), 7.10 (s, 1H), 5.70 (s, 1H), 3.78 (s, 2H), 3.54 (s, 1H), 3.28 - 3.42 (m, 3H), 3.03 (s, 2H), 1.83 (s, 1H), 1.75 (s, 1H), 1.63 - 1.66 (m, 6H), 1.40 (s, 9H), 1.38 (s, 9H), rotamers; 13C NMR (125 MHz, CDCl3) δ 171.8, 159.6, 156.5, 155.2, 80.4, 80.1, 60.6, 54.1, 45.8, 40.8, 38.9, 31.2, 30.1, 28.6, 28.5, rotamers; HRMS (ES) (m/z): M + H+ calcd for C21H40N7O7 502.2989, found 502.2994.
5.2.6. (2S)-2-Amino-5-nitroguanidinopentanoic acid ((3R)-piperidin-3-yl)amide (5)
A white solid was obtained after Boc deprotection; mp 58.5-59.5 °C. 1H NMR (400 MHz, D2O) δ 3.88 (t, J = 10.4 Hz, 1H), 3.80 (t, J = 6.0 Hz, 1H), 3.30 (m, 1H), 3.12 - 3.19 (m, 3H), 2.78 (t, J = 12.0 Hz, 1H), 2.70 (t, J = 11.6 Hz, 1H), 1.83 (d, J = 11.6 Hz, 2H), 1.75 (s, 2H), 1.61 (m, 1H), 1.49 (s, 2H), 1.40 (m, 1H); 13C NMR (100 MHz, D2O) δ 169.1, 158.9, 114.9, 53.0, 46.1, 44.2, 43.6, 40.3, 28.0, 27.5, 20.7; Anal. Calcd for C11H23N7O3·2.5TFA·H2O: C, 31.79, H, 4.59, N, 16.22; Found: C, 31.79, H, 4.36, N, 16.39.
5.2.7. (3S)-3-((2S)-2-tert-Butoxycarbonylamino-5-nitroguanidinopentanoylamino)-piperidine-1-carboxylic acid tert-butyl ester (15)
Boc-L-Arg(NO2)-OH (224 mg, 0.70 mmol), HOBt (95 mg, 0.70 mmol), HBTU (265 mg, 0.70 mmol), DIEA (90 mg, 0.70 mmol), and (S)-1-Boc-3-aminopiperidine (70 mg, 0.35 mmol) were used as described above to provide 15 (152 mg, 0.30 mmol, 87%) as a white solid; mp 107.2-108.0 °C. 1H NMR (500 MHz, CDCl3) δ 8.60 (s, 1H), 6.86 (s, 1H), 5.62 and 5.61 (2 × s, 1H), 4.28 (s, 1H), 3.90 (s, 1H), 3.75 (t, J = 6.0 Hz, 1H), 3.60 (s, 1H), 3.33 (m, 3H), 3.25 (s, 1H), 1.85 (s, 1H), 1.79 (m, 1H), 1.68 (br s, 4H), 1.53 (m, 2H), 1.45 (s, 9H), 1.42 (s, 9H), rotamers; 13C NMR (125 MHz, CDCl3) δ 171.4, 159.7, 156.5, 155.3, 80.3, 68.2, 60.6, 45.7, 40.8, 28.6, 28.5, 25.8, 21.3, 14.4, rotamers; HRMS (ES) (m/z): M + H+ calcd for C21H40N7O7 502.2989, found 502.2987.
5.2.8. (2S)-2-Amino-5-nitroguanidinopentanoic acid ((3S)-piperidin-3-yl)amide (6)
A white solid was obtained after Boc deprotection; mp 89.5-91.3 °C. 1H NMR (400 MHz, D2O) δ 3.87 (t, J = 10.0 Hz, 1H), 3.80 (t, J = 5.6 Hz, 1H), 3.27 (d, J = 11.6 Hz, 1H), 3.16 (m, 1H), 3.11 (s, 2H), 2.75 (t, J = 12.8 Hz, 1H), 2.64 (t, J = 11.6 Hz, 1H), 1.85 (m, 2H), 1.73 (s, 2H), 1.61 (m, 1H), 1.47 (s, 2H), 1,41 (m, 1H); 13C NMR (100 MHz, D2O) δ 169.1, 158.9, 114.9, 52.9, 46.1, 44.3, 43.7, 40.3, 28.1, 27.4, 20.8. HRMS (ES) (m/z): M + H+ calcd for C11H24N7O3 302.1941, found 302.1940; Anal. Calcd for C11H23N7O3·2.5TFA·H2O: C, 31.79, H, 4.59, N, 16.22; Found: C, 31.64, H, 4.54, N, 16.10.
5.2.9. 4-((2S)-2-tert-Butoxycarbonylamino-5-nitroguanidinopentanoylamino)-piperidine-1-carboxylic acid tert-butyl ester (16)
Boc-L-Arg(NO2)-OH (319 mg, 1.0 mmol), HOBt (135 mg, 1.0 mmol), HBTU (379 mg, 1.0 mmol), DIEA (129 mg, 1.0 mmol), and 1-Boc-4-aminopiperidine (100 mg, 0.50 mmol) were used as described above to provide 16 (219 mg, 0.44 mmol, 87%) as a white solid; mp 119.4-120.1 °C. 1H NMR (500 MHz, CDCl3) δ 8.83 (s, 1H), 7.25 (s, 1H), 5.68 (s, 1H), 4.31 (s, 1H), 3.97 (s, 2H), 3.86 (t, J = 3.5 Hz, 1H), 3.50 (s, 1H), 3.24 (s, 1H), 2.84 (m, 2H), 1.82 (m, 3H), 1.70 (br s, 5H), 1.43 (s, 9H), 1.41 (s, 9H), rotamers; 13C NMR (125 MHz, CDCl3) δ 171.5, 159.6, 156.8, 155.0, 80.5, 80.0, 60.7, 47.0, 40.7, 31.8, 28.7, 28.6, 21.3, 14.4, rotamers; HRMS (ES) (m/z): M + H+ calcd for C21H40N7O7 502.2989, found 502.2978.
5.2.10. (2S)-2-Amino-5-nitroguanidinopentanoic acid piperidin-4-ylamide (7)
A white solid was obtained after Boc deprotection; mp 78.5-81.0 °C. 1H NMR (400 MHz, D2O) δ 3.78 (t, J = 5.6 Hz, 2H), 3.25 (s, 2H), 3.11 (s, 2H), 2.92 (t, J = 12.0 Hz, 2H), 1.93 (t, J = 14.0 Hz, 2H), 1.73 (s, 2H), 1.52 (m, 4H); 13C NMR (100 MHz, D2O) δ 168.8, 158.8, 114.8, 52.9, 44.8, 42.9, 40.3, 28.1, 27.8, 27.7; Anal. Calcd for C11H23N7O3·2.5TFA: C, 32.77, H, 4.38, N, 16.72; Found: C, 32.63, H, 4.60, N, 17.00.
5.2.11. N-Boc-4-hydroxy-L-proline lactone (25)
To a stirred ice-cold solution of Boc-Hyp-OH (4.48 g, 19.4 mmol) and triphenylphosphine (6.26 g, 23.2 mmol) in freshly distilled THF (100 mL) under nitrogen was added DIAD (4.70 g, 23.2 mmol) dropwise. The reaction temperature was allowed to rise to room temperature, and stirring continued for 20 h. The solvent was evaporated under reduced pressure, and the residue was directly purified by flash column chromatography (EtOAc/hexane = 1 : 1, Rf = 0.40), to give 4.05 g (97%) of 25 as a white foamy solid; mp 89.9-91.2 °C. 1H NMR (400 MHz, CDCl3) δ 4.95 (s, 1H), 4.38 (br s, 1H), 3.37 (d, J = 10.4 Hz, 1H), 3.24 (d, J = 10.4 Hz, 1H), 2.04 (d, J = 10.8 Hz, 1H), 1.87 (d, J = 6.4 Hz, 1H), 1.28 (s, 9H), rotamers.
5.2.12. N-Boc-cis-4-hydroxy-L-proline methyl ester (26)
A solution of 25 (0.11 g, 0.50 mmol) and sodium azide (0.06 g, 1.00 mmol) in dry MeOH (15 mL) was stirred for 5 h at 40 °C under argon. The solvent was evaporated under reduced pressure, and the residue was partitioned between water and EtOAc. The organic layer was washed with brine, dried over Na2SO4, and concentrated. The residue was purified by flash column chromatography (EtOAc/hexane = 2 : 1, Rf = 0.3) to give 26 (0.12 g, 98%) as a white foamy solid; 81.6-82.0 °C. 1H NMR (500 MHz, CDCl3) δ 4.30 (d, J = 7.0 Hz, 1H), 3.71 and 3.70 (2 × s, 3H), 3.66 (m, 1H), 3.47 - 3.56 (m, 2H), 2.25 - 2.33 (m, 1H), 2.03 (t, J = 6.5 Hz, 1H), 1.35 and 1.39 (2 × s, 9H), rotamers; 13C NMR (125 MHz, CDCl3) δ 175.4, 175.1, 154.7, 154.0, 80.5, 71.1, 70.0, 58.1, 57.8, 55.8, 55.2, 52.9, 52.6, 38.7, 38.0, 28.5, 28.4, rotamers.
5.2.13. N-Boc-trans-4-azido-L-proline methyl ester (27)
DEAD (0.09 g, 0.53 mmol) was slowly added dropwise via syringe to an ice-cold stirred solution of 26 and triphenylphosphine (0.13 g, 0.51 mmol) in dry THF (5 mL) under nitrogen. DPPA (0.14 g, 0.51 mmol) was then added dropwise via syringe to the solution, and the temperature was allowed to rise to room temperature. After 21 h of stirring, the solvent was evaporated under reduced pressure, and the residue was purified by flash column chromatography (EtOAc/hexane = 1 : 4, Rf = 0.30), to give 0.10 g (94%) of 27 as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 4.33 and 4.26 (2 × t, J = 6.0 Hz and J = 7.0 Hz, 1H), 4.14 (s, 1H), 3.67 (s, 3H), 3.61 - 3.65 (m, 1H), 3.51 and 3.41 (2 × d, J = 11.5 Hz and J = 11.0 Hz, 1H), 2.23 - 2.28 (m, 1H), 2.08 - 2.13 (m, 1H), 1.34 and 1.40 (2 × s, 9H), rotamers; 13C NMR (125 MHz, CDCl3) δ 173.1, 172.9, 154.1, 153.5, 80.7, 59.4, 58.9, 57.9, 57.5, 52.5, 52.4, 51.6, 51.4, 36.4, 35.5, 28.5, 28.3, rotamers; MS (ESI, CH3OH) [M+Na+] = 293.3.
5.2.14. N-Boc-trans-4-azido-L-proline (28)
A mixture of 27 (0.10 g, 0.37 mmol) in THF (3 mL) and 5% aq. LiOH (3 mL) was stirred at 0 °C. The reaction was allowed to rise to room temperature, and stirring continued for 17 h. After evaporation of THF under reduced pressure, the aqueous solution was washed twice with EtOAc (5 mL), acidified with 1N HCl to pH 3, and extracted twice with EtOAc (10 mL). The organic extract was washed with brine, dried over Na2SO4, filtered, and evaporated. The residue was further dried under reduced pressure and used in the next reaction without further purification (28, 0.86 mg, 91%). 1H NMR (500 MHz, CDCl3) δ 10.4 (s, 1H), 4.40 and 4.30 (2 × t, J = 6.5 Hz and J = 7.0 Hz, 1H), 4.18 (s, 1H), 3.61 - 3.66 (m, 1H), 3.55 and 3.44 (2 × d, J = 12.0 Hz, 1H), 2.18 - 2.36 (m, 2H), 1.38 and 1.43 (2 × s, 9H), rotamers; 13C NMR (125 MHz, CDCl3) δ 177.1, 175.9, 171.9, 155.1, 153.9, 81.7, 81.5, 59.2, 58.9, 57.9, 57.7, 51.7, 51.5, 36.4, 35.2, 28.5, 28.3, 21.2, 14.3, rotamers; MS (ESI, CH3CN) [3M+Na+] = 790.5.
5.2.15. N-Boc-trans-4-azido-L-prolinamide (29)
Compound 28 (0.14 g, 0.55 mmol) was dissolved in THF (5 mL) at ambient temperature, and the solution was cooled to −10 °C. To this solution were added N-methylmorpholine (0.09 g, 0.83 mmol) and ibutyl chloroformate (0.11 g, 0.83 mmol) successively, and stirring continued for 30 min at −10 °C. Aqueous ammonia (29%, 0.15 mL) was added, and the mixture was stirred at −10 °C for 30 min then at room temperature for 2.5 h. After addition of water (10 mL), the solution was extracted twice with EtOAc (10 mL), washed with brine, dried over Na2SO4, filtered, and evaporated. The residue was purified by flash column chromatography (EtOAc, 100%, Rf = 0.30) to afford 29 (0.12 g, 83%) as an oil. 1H NMR (500 MHz, CDCl3) δ 6.99 and 6.54 (2 × s, 1H), 6.38 and 6.28 (2 × s, 1H), 4.36 and 4.21 (2 × s, 1H), 4.16 (d, J = 4.5 Hz, 1H), 3.41 - 3.57 (m, 2H), 2.43 and 2.30 (2 × m, 1H), 2.21 and 2.07 (2 × m, 1H), 1.41 (s, 9H), rotamers; 13C NMR (125 MHz, CDCl3) δ 175.3, 174.2, 171.4, 155.4, 154.3, 81.3, 60.6, 59.5, 59.2, 58.3, 51.8, 36.9, 34.3, 28.5, 21.2, 14.4, rotamers; MS (ESI, CH3CN/H2O) [M+H+] = 256.0.
5.2.16. N-Boc-trans-4-amino-L-prolinamide (30)
Compound 29 (33 mg, 0.13 mmol) was dissolved in MeOH (3 mL) and subjected to catalytic hydrogenation with 10% Pd/C (8 mg) at 1 atm. After 2 h the reaction mixture was filtered on Celite, and the filtrate was concentrated to dryness to afford an oily residue 30 (28 mg, 94%). Rf = 0.40 (EtOAc/MeOH/Et3N = 1 : 1 : 0.1); 1H NMR (500 MHz, acetone-d6) δ 7.21 and 7.16 (2 × s, 1H), 6.82 and 6.76 (2 × s, 1H), 4.35 and 4.32 (2 × s, 1H), 4.20 (s, 1H), 3.65 (m, 2H), 3.26 (m, 1H), 3.10 (br s, 2H), 2.07 - 2.14 (m, 2H), 1.47 and 1.43 (2 × s, 9H), rotamers; 13C NMR (125 MHz, acetone-d6) δ 175.8, 175.1, 167.9, 154.9, 154.1, 79.3, 79.2, 60.1, 59.8, 58.0, 57.3, 53.1, 38.5, 36.7, 28.1, 28.0, rotamers; MS (ESI, CH3OH) [M+H+] = 230.1.
5.2.17. N-Boc-4-trans-[(2S)-5-Nitroguanidino-2-(tert-butoxycarbonylmethylamino)-pentanoylamino]-L-prolinamide (31)
To a solution of 21 (124 mg, 0.37 mmol), HOBt (62 mg, 0.46 mmol), and HBTU (174 mg, 0.46 mmol) in dry DMF (1 mL) and dry CH2Cl2 (4 mL), was added 30 (70 mg, 0.31 mmol) in dry DMF (3 mL) dropwise at 0 °C. DIEA (80 mg, 0.62 mmol) in dry CH2Cl2 (1 mL) was added slowly, and stirring continued overnight at room temperature under argon. The solvent was removed under reduced pressure, and the residue was treated with EtOAc (10 mL). The organic mixture was washed successively with 5% NaHCO3, water, 0.5N HCl, and brine, and dried over Na2SO4. The solution was concentrated in vacuo, and the residue was purified by flash column chromatography (CH2Cl2/MeOH = 10:1, Rf = 0.15) to afford 31 (46 mg, 30%) as a white foam. 1H NMR (500 MHz, acetone-d6) δ 7.43 (s, 1H), 7.11 and 7.04 (2 × s, 1H), 6.55 (s, 1H), 4.64 and 4.50 (2 × s, 1H), 4.31 and 4.28 (2 × s, 1H), 3.72 (s, 1H), 3.40 (s, 2H), 3.29 (m, 2H), 2.97 and 2.81 (2 × s, 3H), 2.26 (s, 1H), 2.21 (m, 1H), 1.96 - 2.03 (m, 2H), 1.54 - 1.77 (m, 4H), 1.45 (s, 9H), 1.41 (s, 9H), rotamers; HRMS (ES) (m/z): M + H+ calcd for C22H41N8O8 545.3047, found 545.3046.
5.2.18. 4-trans-((2S)-5-Nitroguanidino-2-methylaminopentanoylamino)-L-prolinamide (8)
Compound 31 (33 mg, 0.066 mmol) was treated with TFA/CH2Cl2 (3 mL/3 mL) at 0 °C under nitrogen. The reaction was then allowed to rise to room temperature, and stirring continued for 1 h. Excess TFA and solvent were removed by evaporation in vacuo. The residue was repeatedly dissolved in CH2Cl2 (10 mL) and the solvents evaporated to remove traces of TFA. The residue was dissolved in a small amount of water, and the solution was washed with CH2Cl2 and lyophilized to give 8 as a white solid (47 mg, 96 %); mp 108.7-110.0 °C. 1H NMR (500 MHz, D2O) δ 4.48 (t, J = 8.0 Hz, 1H), 4.43 (t, J = 6.0 Hz, 1H), 3.75 (t, J = 6.5 Hz, 1H), 3.67 (dd, J = 7.0 Hz and J = 5.5 Hz, 1H), 3.25 (dd, J = 7.5 Hz and J = 5.5 Hz, 1H), 3.17 (s, 2H), 2.55 (s, 3H), 2.35 (m, 2H), 1.83 (s, 2H), 1.51 (s, 2H); Anal. Calcd for C12H24N8O4·2.75TFA·1.5H2O: C, 30.69, H, 4.38, N, 16.36; Found: C, 30.49, H, 4.27, N, 16.58.
5.2.19. (2S)-2-tert-Butoxycarbonylamino-4-cyanobutyric acid (17)
A solution of Boc-LGln-OH (100 mg, 0.41 mmol) in dry pyridine (3 mL) and acetic anhydride (50 mg, 0.49 mmol) was stirred at room temperature for 6 h and concentrated. The residue was taken up in EtOAc, and the solution was washed three times with 5% citric acid (10 mL) and brine (10 mL) and dried over Na2SO4. The solution was concentrated in vacuo, and the residue was purified by flash column chromatography (CH2Cl2/MeOH = 10:1, Rf = 0.15) to afford 17 (56 mg, 60%) as a syrup. 1H NMR (500 MHz, acetone-d6) δ 6.33 (d, J = 7.0 Hz, 1H), 4.27 (d, J = 4.0 Hz, 1H), 2.62 (t, J = 7.0 Hz, 2H), 2.25 (m, 1H), 2.05 (m, 1H), 1.42 (s, 9H); 13C NMR (125 MHz, acetone-d6) δ 172.6, 156.0, 119.3, 79.0, 52.7, 28.8, 27.9, 13.8; MS (ESI, CH3OH) [2M+H+] = 457.3.
5.2.20. (2S)-2-(tert-Butoxycarbonylmethylamino)-4-cyanobutyric acid (18)
To an ice-cold solution of 17 (4.15 g, 18.2 mmol) in THF (40 mL) was added iodomethane (20.6 g, 145.4 mmol) and NaH (1.31 g, 54.5 mmol), and the mixture was stirred at room temperature for 3 days. EtOAc (10 mL) was added followed by water (20 mL). The pH of the solution was adjusted to 9 by addition of 0.1 N HCl, and the solution was concentrated to a small volume. Sodium thiosulfate (0.1 N, 5 mL) was added followed by water (20 mL). The solution was extracted twice with ether, and the water layer was acidified to pH 3 with 5% citric acid with stirring. The solution was extracted three times with EtOAc, and the combined ethyl acetate solution was washed with brine four times, dried over Na2SO4, and concentrated. The residue was purified by flash column chromatography (CH2Cl2/MeOH/AcOH = 20:1:0.1, Rf = 0.25) to afford 18 (3.66 g, 83%) as a yellowish crystalline solid; mp 86.2-87.6 °C. 1H NMR (500 MHz, CD3OD) δ 4.63 and 4.43 (2 × m, 1H), 2.87 and 2.85 (2 × s, 3H), 2.47-2.59 (m, 2H), 2.30 (m, 1H), 2.16 (m, 1H), 1.47 and 1.49 (2 × s, 9H), cis & trans; 13C NMR (125 MHz, CD3OD) δ 172.3, 156.8, 156.1, 122.3, 119.5, 81.2, 80.6, 59.2, 58.2, 32.3, 31.5, 27.6, 27.5, 25.5, 24.8, 13.8, cis & trans; MS (ESI, CH2Cl2) [M + Na+] = 265.1.
5.2.21. (2S)-2-(tert-Butoxycarbonylmethylamino)-5-nitroguanidinopentanoic acid (21)
Compound 18 (0.69 g, 2.83 mmol) was dissolved in a mixture of MeOH/AcOH (10 mL/10 mL) and subjected to catalytic hydrogenation with Pd(OH)2 on carbon (20 wt %, 93 mg) at 1 atm. After overnight stirring, the reaction mixture was filtered through Celite, and the filtrate was concentrated to dryness to afford 19 (0.69 g, 99%) as a solid. Without further purification, 19 (0.69 g, 2.83 mmol) was dissolved in absolute EtOH (20 mL). To this stirred solution was added 2-methyl-1-nitro-2-thiopseudourea (1.9 g, 14.2 mmol)16 and triethylamine (2.9 g, 28.3 mmol), successively. The reaction temperature was then raised to 40 °C, and stirring continued overnight under nitrogen. The solvent was evaporated by reduced pressure, and the residue was purified by flash column chromatography (CH2Cl2/MeOH/AcOH = 10:1:0.1, Rf = 0.25), to give 21 (0.41 g, 43%) as a clear syrup. 1H NMR (500 MHz, D2O) δ 4.42 and 4.12 (m, 1H), 3.20 (m, 2H), 2.74 and 2.68 (2 × s, 3H), 1.82 (s, 2H), 1.51 (s, 2H), 1.30 and 1.24 (2 × s, 9H), rotamers; MS (ESI, CH3OH) [2M+H+] = 666.9.
5.3. General method for the syntheses of 9-11
Compounds 9-11 were prepared by the same procedure described above for the syntheses of 3-7 except Boc-L-MeArg(NO2)-OH (21) was used instead of Boc-L-Arg(NO2)-OH.
5.3.1. (3R)-3-[(2S)-2-(tert-Butoxycarbonylmethylamino)-5-nitroguanidinopentanoylamino]-pyrrolidine-1-carboxylic acid tert-butyl ester (22)
Boc-L-MeArg(NO2)-OH (100 mg, 0.30 mmol), HOBt (45 mg, 0.33 mmol), HBTU (125 mg, 0.33 mmol), DIEA (49 mg, 0.38 mmol), and (R)-(+)-1-Boc-3-aminopyrrolidine (47 mg, 0.25 mmol) were used as described above to provide 22 (85 mg, 0.17 mmol, 68%). 1H NMR (500 MHz, CDCl3) δ 4.59 (s, 1H), 4.37 (t, J = 5.0 Hz, 1H), 3.57 (d, J = 5.5 Hz, 1H), 3.38 (m, 4H), 3.13 (m, 1H), 2.74 (s, 3H), 2.12 (m, 1H), 1.93 (m, 1H), 1.81 (s, 1H), 1.66 - 1.74 (m, 3H), 1.45 (s, 9H), 1.43 (s, 9H), rotamers; HRMS (ES) (m/z): M + H+ calcd for C21H40N7O7 502.2989, found 502.2972.
5.3.2. (2S)-2-Methylamino-5-nitroguanidino-pentanoic acid ((3R)-pyrrolidin-3-yl)amide (9)
A white foamy solid was obtained after deprotection; mp 59.6-59.9 °C. 1H NMR (500 MHz, D2O) δ 4.31 (t, J = 5.0 Hz, 1H), 3.67 (t, J = 6.5 Hz, 1H), 3.43 (dd, J = 7.5 Hz and J = 5.0 Hz, 1H), 3.24 (m, 2H), 3.08 (m, 3H), 2.47 (s, 3H), 2.17 (m, 1H), 1.76 - 1.81 (m, 3H), 1.43 (s, 2H); Anal. Calcd for C11H23N7O3·3TFA·H2O: C, 30.87, H, 4.27, N, 14.82; Found: C, 30.71, H, 4.20, N, 15.01.
5.3.3. (3R)-3-[(2S)-2-(tert-Butoxycarbonyl-methyl-amino)-5-nitroguanidinopentanoyl-amino]-piperidine-1-carboxylic acid tert-butyl ester (23)
Boc-L-MeArg(NO2)-OH (120 mg, 0.36 mmol), HOBt (49 mg, 0.36 mmol), HBTU (137 mg, 0.36 mmol), DIEA (70 mg, 0.54 mmol) and (R)-1-Boc-3-aminopiperidine (34 mg, 0.18 mmol) were used as described above to provide 23 (67 mg, 0.13 mmol, 71%). 1H NMR (500 MHz, CDCl3) δ 4.52 (s, 1H), 3.88 (s, 1H), 3.57 (s, 1H), 3.36 (m, 3H), 3.27 (s, 1H), 3.15 (s, 1H), 2.76 (s, 3H), 2.00 (m, 1H), 1.84 (s, 1H), 1.70 (s, 2H), 1.62 (m, 2H), 1.55 (m, 2H), 1.48 (s, 9H), 1.46 (s, 9H), rotamers; HRMS (ES) (m/z): M + H+ calcd for C22H42N7O7 516.3146, found 516.3137.
5.3.4. (2S)-2-Methylamino-5-nitroguanidino-pentanoic acid ((3R)-piperidin-3-yl)amide (10)
A white foamy solid was obtained after deprotection; mp 61.0-61.6 °C. 1H NMR (500 MHz, D2O) δ 3.94 (s, 1H), 3.71 (s, 1H), 3.36 (d, J = 12.0 Hz, 1H), 3.21 (m, 1H), 3.16 (s, 2H), 2.78 (dt, J = 12.0 Hz, 6.0 Hz, 2H), 2.54 (s, 3H), 1.82 - 1.88 (m, 4H), 1.66 (d, J = 12.0 Hz, 1H), 1.40 - 1.49 (m, 3H); Anal. Calcd for C12H25N7O3·3TFA: C, 32.88, H, 4.29, N, 14.91; Found: C, 32.58, H, 4.60, N, 14.96.
5.3.5. 4-[(2S)-2-(tert-Butoxycarbonyl-methyl-amino)-5-nitroguanidino-pentanoylamino]-piperidine-1-carboxylic acid tert-butyl ester (24)
Boc-L-MeArg(NO2)-OH (56 mg, 0.17 mmol), HOBt (24 mg, 0.18 mmol), HBTU (68 mg, 0.18 mmol), DIEA (27 mg, 0.21 mmol) and 1-Boc-4-aminopiperidine (28 mg, 0.14 mmol) were used as described above to provide 24 (63 mg, 0.12 mmol, 87%). 1H NMR (500 MHz, CDCl3) δ 4.57 (s, 1H), 3.97 (s, 2H), 3.87 (m, 1H), 3.34 (s, 2H), 2.86 (m, 2H), 2.74 (s, 3H), 1.94 (m, 1H), 1.83 (m, 2H), 1.71 (m, 2H), 1.44 (m, 18H), 1.31 (m, 3H), rotamers; HRMS (ES) (m/z): M + H+ calcd for C22H42N7O7 516.3146, found 516.3151.
5.3.6. (2S)-2-Methylamino-5-nitroguanidino-pentanoic acid piperidin-4-ylamide (11)
A white foamy solid was obtained after deprotection; mp 58.9-59.3 °C.1H NMR (500 MHz, D2O) δ 3.85 (m, 1H), 3.68 (t, J = 6.5 Hz, 1H), 3.30 (m, 2H), 3.14 (s, 2H), 2.96 (m, 2H), 2.53 (s, 3H), 1.94 - 2.02 (m, 2H), 1.79 (s, 2H), 1.55 - 1.63 (m, 2H), 1.47 (m, 2H); Anal. Calcd for C12H25N7O3·3TFA·H2O: C, 32.01, H, 4.48, N, 14.52; Found: C, 31.96, H, 4.37, N, 14.20.
5.4. Enzyme and Assay
All of the NOS isoforms used were recombinant enzymes overexpressed in E. coli from different sources. Murine macrophage iNOS,24 rat nNOS,25 and bovine eNOS26 were expressed and isolated as reported. Nitric oxide formation from NOS was monitored by the hemoglobin capture assay at 30 °C as described previously.27 Briefly, nitric oxide transforms oxyhemoglobin (Fe2+) to methemoglobin (Fe3+), which is monitored at the maximum absorbance (401 nm) upon the addition of the enzyme into a mixture of substrate L-Arg, inhibitor, cofactors, and Hepes buffer. A solution of nNOS or eNOS contained 10 μM L-arginine, 1.6 mM CaCl2, 11.6 μg/mL calmodulin, 100 μM DTT, 100 μM NADPH, 6.5 μM H4B, 6 μM oxyhemoglobin, and specified inhibitor concentrations in 100 mM Hepes (pH 7.5) in 600 μL total volume; iNOS contained the same concentrations of cofactors, except CaCl2 and calmodulin were not added. The assays were initiated by addition of enzyme and recorded on a Perkin-Elmer Lambda 10 UV/vis spectrophotometer.
5.5. Determination of Ki Values
The reversible inhibition of NOS by the L-nitroargininamide compounds was studied under initial rate conditions with the hemoglobin assay as described above. The parameters of the following inhibition equation28 were fitted to the initial velocity data: % inhibition = 100[I]/{[I] + Ki (1 +[S]/Km)}. Km values for L-arginine were 1.3 μM (nNOS), 8.3 μM (iNOS), and 1.7 μM (eNOS). The selectivity of an inhibitor was defined as the ratio of the respective Ki values.
5.6. Docking Analysis
Molecular modeling calculations were performed using the software packages SYBYL 6.8 and AutoDock 3.0.5 running on a Silicon Graphics Octane 2 workstation. The protein structure used in the docking study was prepared as described previously.29 The 3-D structure of the ligand was built in SYBYL 6.8 by modifying the molecular structure of 2, which was extracted from the crystal structure (pdb id: 1p6j). Using the SYBYL program, correct atom types were assigned assuming physiological pH. Energy minimizations were performed following both the addition of polar hydrogen atoms and partial atom charge calculations by the the Gasteiger-Marsilli method.30 The torsion and rotatable bonds in the ligand were defined by AutoTors, an auxiliary program of AutoDock 3.0.5, which also united the nonpolar hydrogens and partial atomic charges to the bonded carbon atoms. Parameters for the docking experiment were used as described in detail previously.27 A flexible docking calculation yielded 100 docked conformations, and appropriate conformations were chosen by visual comparison of the superimposition of the guanidino moiety of the predicted conformations and that of 2.
Supplementary Material
Acknowledgements
We are grateful to the National Institutes of Health (GM 49725 to R.B.S. and GM52419 and HL30050 to Prof. Bettie Sue Masters, in whose laboratory P.M. and L.J.R. work) for financial support of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.(a) Kerwin JF, Jr., Lancaster JR., Jr. Med. Res. Rev. 1994;14:23–74. doi: 10.1002/med.2610140103. [DOI] [PubMed] [Google Scholar]; (b) Stuehr DJ. J. Nutr. 2004;134(10 Suppl):2748S–2751S. doi: 10.1093/jn/134.10.2748S. [DOI] [PubMed] [Google Scholar]
- 2.Moncada S, Higgs A, Furchgott R. Pharmacol. Rev. 1997;49:137–142. [PubMed] [Google Scholar]
- 3.Griffith OW, Stuehr DJ. Annu. Rev. Physiol. 1995;57:707–736. doi: 10.1146/annurev.ph.57.030195.003423. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt HHHW, Walter U. Cell. 1994;78:919–925. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 5.Forstermann U, Pollock JS, Schmidt HHHW, Heller M, Murad F. Proc. Natl. Acad. Sci. U.S.A. 1991;88:1788–1792. doi: 10.1073/pnas.88.5.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacMicking J, Xie QW, Nathan C. Annu. Rev. Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 7.Choi DW, Rothman SM. Annu. Rev. Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- 8.Thomson LL, Iversen HK, Lassen LH, Olesen J. CNS Drugs. 1994;2:417–422. [Google Scholar]
- 9.(a) Mayer B, Hemmens B. Trends Biochem. Sci. 1997;22:477–481. doi: 10.1016/s0968-0004(97)01147-x. [DOI] [PubMed] [Google Scholar]; (b) Dawson VL, Dawson TM, London ED, Bredt DS, Snyder SH. Proc. Natl. Acad. Sci. U.S.A. 1991;88:6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marletta MA. J. Med. Chem. 1994;37:1899–1907. doi: 10.1021/jm00039a001. [DOI] [PubMed] [Google Scholar]
- 11.Erdal EP, Litzinger EA, Seo J, Zhu Y, Ji H, Silverman RB. Curr. Top. Med. Chem. 2005;5:603–624. doi: 10.2174/1568026054679317. [DOI] [PubMed] [Google Scholar]
- 12.Huang H, Martásek P, Roman LJ, Masters BSS, Silverman RB. J. Med. Chem. 1999;42:3147–3153. doi: 10.1021/jm990111c. [DOI] [PubMed] [Google Scholar]
- 13.(a) Ji H, Gómez-Vidal JA, Martásek P, Roman LJ, Silverman RB. J. Med. Chem. 2006;49:6254–6263. doi: 10.1021/jm0604124. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Gómez-Vidal JA, Martásek P, Roman LJ, Silverman RB. J. Med. Chem. 2004;47:703–710. doi: 10.1021/jm030297m. [DOI] [PubMed] [Google Scholar]
- 14.Flinspach ML, Li H, Jamal J, Yang W, Huang H, Hah J, Gómez-Vidal JA, Litzinger EA, Silverman RB, Poulos TL. Nat. Struc. Mol. Biol. 2004;11:54–59. doi: 10.1038/nsmb704. [DOI] [PubMed] [Google Scholar]
- 15.Huang H, Martásek P, Roman LJ, Silverman RB. J. Med. Chem. 2000;43:2938–2945. doi: 10.1021/jm000127z. [DOI] [PubMed] [Google Scholar]
- 16.Ji H, Gómez-Vidal JA, Martásek P;J, Roman LJ, Silverman RB. J. Med. Chem. 2006;49:6254–6263. doi: 10.1021/jm0604124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodgers JD, Johnson BL, Wang H, Erickson-Viitanen S, Klabe RM, Bacheler L, Cordova BC, Chang C-H. Bioorg. Med. Chem. Lett. 1998;8:715–720. doi: 10.1016/s0960-894x(98)00118-8. [DOI] [PubMed] [Google Scholar]
- 18.Xue C, DeGrado WF. Tet. Lett. 1995;36:55–58. [Google Scholar]
- 19.Fishbein L, Gallaghan JA. J. Am. Chem. Soc. 1954;76:1877–1879. [Google Scholar]
- 20.Gómez-Vidal JA, Silverman RB. Org. Lett. 2001;3:2481–2484. doi: 10.1021/ol0161054. [DOI] [PubMed] [Google Scholar]
- 21.Gómez-Vidal JA, Forrester MT, Silverman RB. Org. Lett. 2001;3:2477–2479. doi: 10.1021/ol016104b. [DOI] [PubMed] [Google Scholar]
- 22.(a) Gerber NC, Rodriguez-Crespo I, Nishida CR, Ortiz de Montellano PR. J. Biol. Chem. 1997;272:6285–6290. doi: 10.1074/jbc.272.10.6285. [DOI] [PubMed] [Google Scholar]; (b) Erdal EP, Litzinger EA, Seo J, Zhu Y, Ji H, Silverman RB. Curr. Top. Med. Chem. 2005;5:603–624. doi: 10.2174/1568026054679317. [DOI] [PubMed] [Google Scholar]
- 23.Krogsgaard-Larsen P, Bundgarrd H. A Textbook of Drug Design and Development. Taylor & Francis; New York: 2002. [Google Scholar]
- 24.Roman LJ, Sheta EA, Martasek P, Gross SS, Liu Q, Masters BSS. Proc. Natl. Acad. Sci. U.S.A. 1995;92:8428–8432. doi: 10.1073/pnas.92.18.8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerber NC, Montellano PR. J. Biol. Chem. 1995;270:17991–17996. [Google Scholar]
- 26.Martasek P, Liu Q, Roman LJ, Gross SS, Sessa WC, Masters BSS. Biochem. Biophys. Res. Commun. 1996;219:359–365. doi: 10.1006/bbrc.1996.0238. [DOI] [PubMed] [Google Scholar]
- 27.Hevel JM, Marletta M. Methods Enzymol. 1994;133:250–258. doi: 10.1016/s0076-6879(94)33028-x. [DOI] [PubMed] [Google Scholar]
- 28.Segel IH. Enzyme Kinetics. John Wiley and Sons; New York: 1975. p. 105. [Google Scholar]
- 29.Ji H, Li H, Flinspach M, Poulos TL, Silverman RB. J. Med. Chem. 2003;46:5700–5711. doi: 10.1021/jm030301u. [DOI] [PubMed] [Google Scholar]
- 30.Gasteiger J, Marsilli M. Tetrahedron. 1980;36:3219–3228. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.