Abstract
The increase in extracellular dopamine (DA) following cocaine administration plays a major role in cocaine abuse. In vitro, cocaine binds to DA transporters (DAT) and blocks DA uptake. Moreover, cocaine can increase extracellular DA concentration as measured by in vivo neurochemical methods. The present study examined the effects of cocaine and other drugs on DA, NE and 5-HT uptake using an ex vivo assay. Rats were injected i.v. with saline or drug and sacrificed at various time points after injections. Brains were dissected for regional monoamine uptake studies ex vivo. In most brain regions, cocaine given in vivo blocked monoamine uptake as expected. [3H]DA uptake in nucleus accumbens was inhibited with an ED50 = 22.3 μmol/kg. Cocaine fully inhibited [3H]NE uptake (ED50 = 4.58 μmol/kg) in the occipital cortex and partially inhibited [3H]5-HT uptake (33% at 30 μmol/kg) in the midbrain. However, under the same conditions [3H]DA uptake in the striatum was not inhibited after injections of cocaine up to 56 μmol/kg.. Although the mechanism for this discrepancy is unclear, DA binding and uptake sites may be distinct and/or there may be regional differences in DA transporters.
Keywords: Cocaine, Monoamine uptake, Ex vivo, Striatum, Nucleus accumbens
Cocaine blocks the uptake of monoamine neurotransmitters in vitro, with about equal potency for the three major monoamines, dopamine (DA), norepinephrine (NE), and serotonin (5-HT) [22]. Further, it is widely held that the behavioral effects of cocaine are related to the increase in extraneuronal levels of monoamine neurotransmitters in the CNS [2, 18]. A substantial body of data implicates blockade of CNS dopamine (DA) reuptake by dopamine transporters (DAT) as a primary mechanism in the abuse-related effects of cocaine [34, 35].
In vivo assays also indicate that cocaine can increase the extracellular levels of monoamines. Research with in vivo microdialysis has demonstrated an increase in monoamine concentrations in rat striatum and nucleus accumbens following cocaine administration [19], and in monkey striatum as well [29]. Experiments utilizing in vivo electrochemistry have reported that systemic administration of cocaine can decrease the clearance of locally-applied dopamine in rat striatum and accumbens [4, 38], suggesting that blockade of uptake underlies the monoamine elevations.
Although these studies are consistent with the hypothesis that blockade of DA uptake by the DAT is necessary to the behavioral effects of cocaine, other recent data challenge this notion. Self-administration of cocaine is maintained in mice lacking the DAT (DAT knockout mice) [21]. Cocaine-induced place preference was unaffected in mice lacking either the DAT or the serotonin transporter (SERT) [25], but eliminated in mice lacking both DAT and SERT [26]. The present study was designed as an additional test of the hypothesis that cocaine blocks the uptake of monoamine neurotransmitters in vivo. Various monoamine uptake blockers were administered i.v. to rats, after which the rats were sacrificed, brains were dissected, and monoamine uptake assays were performed ex vivo. The assay has previously been used to study the blockade of DA uptake by cocaine [15] and other drugs [9, 31].
All animal use procedures were approved by the University of Mississippi Medical Center’s Animal Care and Use Committee and in accordance with National Institutes of Health guidelines.
Male Sprague-Dawley rats (Harlan Sprague-Dawley, Inc., Indianapolis, IN) weighing between 250 and 300 g were used. Rats were initially housed in groups of 3 in plastic cages and with a 12:12 light/dark cycle. Food and water were available ad libitum. Rats were anesthetized with pentobarbital (50 mg/kg, i.p.) and a femoral catheter was implanted. After surgery, they were housed individually for 72 h then used experimentally.
For ex vivo monoamine uptake studies, the method was similar to that published by others [9, 15]. Briefly, catheterized rats were injected i.v. with saline or test drugs via the catheter. At designated time points they were sacrificed by decapitation. Striatum, occipital cortex, midbrain (approximately 60 mg tissue/rat) and accumbens (14 mg tissue/rat) were dissected chopped into slices and incubated (37 °C) for 5 min in 1.0 ml of buffer containing [3H]DA (5.0 nM), [3H]NE (5.0 nM), or [3H]5-HT (5.0 nM), respectively. Non-specific uptake was measured under identical conditions but at 4 °C. Other details of the assay have been published [33].
To verify the effects of cocaine in a different tissue preparation, ex vivo uptake experiments were conducted using whole homogenized tissue. Rats were sacrificed five min after cocaine injections. Rather than chopping, brain tissue was homogenized using 10 strokes with a Teflon pestle homogenizer (Glas-Col, Terre Hante, IN) at 1000 rpm.
To verify the presence of cocaine in tissues, we also studied ex vivo [3H]cocaine binding and, in other rats, measured the concentration of [3H]cocaine in striatum. Catheterized rats were injected i.v. with [3H]cocaine (20 μCi/rat) and sacrificed by decapitation at various time points up to 10 min after injection. Striatum and cerebellum were dissected, put into separate 10 ml glass vials and 10 μl/mg tissue Solvable® was added. After 24 hours, 1 μl/mg tissue glacial acetic acid was added to neutralize Solvable. Radioactivity was counted using a scintillation counter (Top Count®, Packard Instruments, Downers Grove, IL). The concentration of cocaine achieved in striatum was estimated by multiplying total injected cocaine (30 μmol/kg) by the proportion of [3H]cocaine bound in striatum relative to the total injected.
All uptake data from drug-pretreated rats were converted to percentages of control, with data from rats pretreated with saline on the same experimental day serving as control values. ED50 values were calculated using nonlinear regression assuming sigmoidal dose-responses with variable slopes (Prism 4.0, Graphpad, San Diego, CA). For [3H]cocaine binding, striatum/cerebellum ratios of binding were calculated. One-way ANOVA with Bonferroni’s multiple comparison was used with p < 0.05 considered statistically significant.
There was a dose-related inhibition of uptake of [3H]DA in accumbens (Figure 1, circles; ED50 =1.0 μmol/kg) and striatum (Figure 1, squares; ED50 = 5.45 μmol/kg) of rats given GBR 12909. Similarly, dose-dependent and complete inhibitions of [3H]5-HT uptake in the midbrain and [3H]NE uptake in the occipital cortex were seen in rats given citalopram and DMI, respectively (Figure 1, triangles: ED50 citalopram = 3.26 μmol/kg; inverted triangles: ED50 DMI = 0.50 μmol/kg). DMI (10 μmol/kg) failed to block [3H]NE and [3H]DA uptake (105 ± 4 % and 98.9 ± 18 % of control respectively) and citalopram (30 μmol/kg) failed to block [3H]DA uptake (120 ± 17 % of control) in accumbens.
Figure 1.
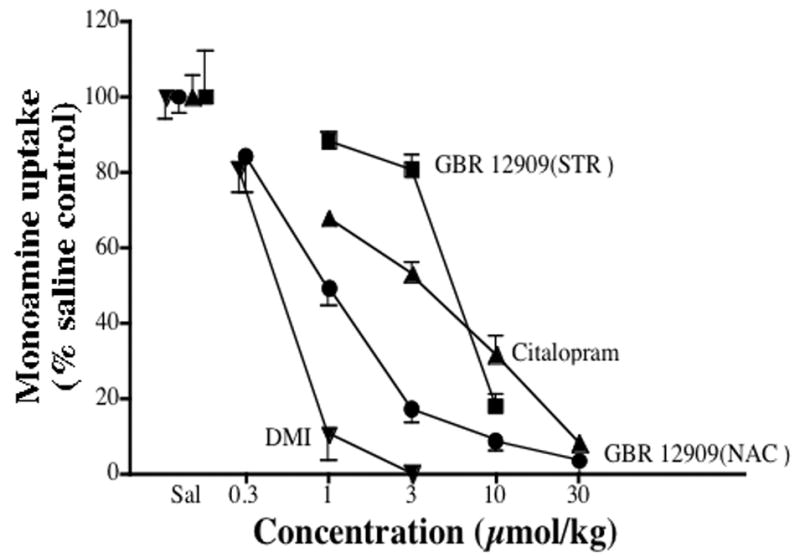
Effects of selective monoamine uptake blockers on monoamine uptake in various brain regions. Rats (n=3–4/group) were injected with saline or the indicated drug doses 5 min before sacrifice. Points are mean values calculated as percent of the values for rats injected with saline on same day and vertical lines are the S.E.M.
As seen with GBR 12909, there was a dose-related inhibition of [3H]DA uptake in accumbens (Figure 2, circles; ED50 = 22.3 μmol/kg) of rats given cocaine (3–30 μmol/kg, 1–10 mg/kg, i.v.). However, cocaine had no effect on [3H]DA uptake in the striatum over this same dose range. A higher dose (56 μmol/kg, 19 mg/kg) was lethal in all rats tested (n = 3). Rats pretreated with diazepam (2.0 mg/kg, i.v.) survived an injection of 56 μmol/kg cocaine, but with no evidence of blockade of [3H]DA uptake in the striatum (Figure 2, squares). Blockade of striatal [3H]DA uptake was also not evident when the time between cocaine (30 μmol/kg) injection and sacrifice was varied (Table). Indeed, [3H]DA uptake increased in the striatum when rats were sacrificed 15 min after the injection [F (9,20) = 4.8; P < 0.001]. Cocaine completely blocked uptake of [3H]NE in the occipital cortex (Figure 2, inverted triangles; ED50 = 4.58 μmol/kg). Uptake of [3H]5HT in the midbrain was decreased to 67 % of control at 30 μmol/kg cocaine [Figure 2, triangles; F (3,8) = 9.9; P < 0.005].
Figure 2.
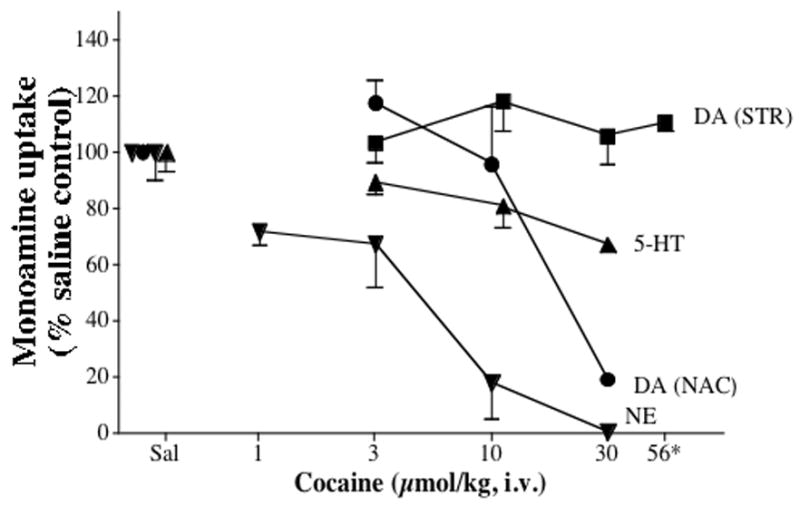
Effects of cocaine on monoamine uptake in various brain regions. Rats (n=3–4/group) were injected with saline or cocaine 5 min before sacrifice. Vertical lines are S.E.M. *For 56 μmol/kg, rats were injected with diazepam (2 mg/kg, i.v., over 50 sec) 5 min before the cocaine injection, administered over 2 min.
Table.
Time course of cocaine effects on [3H]dopamine uptake in striatum.
| Sacrifice Time (min after cocaine) | 1 | 2 | 5 | 15 | 30 |
|---|---|---|---|---|---|
| Uptake (% saline control) | 113.2 | 131.1 | 105.8 | 160.8* | 120.9 |
Rats were injected with cocaine (30 μmol/kg, i.v.) and sacrificed at designated time points.
Significant increase (P < 0.05)
In experiments using homogenized tissue, [3H]DA uptake in striatum was increased to 121 ± 3 % of saline control after injections of cocaine (30 μmol/kg, P < 0.005). [3H]DA uptake in accumbens was reduced to 74 ± 2.5 % of saline control, [3H]NE uptake in occipital cortex was reduced to 8 ± 4 % of saline control, and [3H]5-HT uptake in mid-brain was reduced to 42 ± 5 % of saline control (all comparisons P < 0.001).
When [3H]cocaine was injected, the striatum/cerebellum ratio of binding was 1.6 within one min after injection. This ratio ranged between 1.6 and 1.8 for at least 10 min after the injection [F (17,64) = 15.7; P < 0.0001). Five minutes after injection, a dose of 30 μmol/kg of cocaine was estimated to achieve 1200 nM of cocaine in the ex vivo uptake incubation buffer.
Together with previously published results [9, 31], the present results confirm the utility of this ex vivo assay for the study of in vivo monoamine uptake blockade. When administered i.v., GBR 12909 caused a dose-related blockade of the uptake of [3H]DA in striatum and accumbens. This result is consistent with results of both in vitro and in vivo studies with GBR 12909 [12, 3]. Similarly, our results with DMI and citalopram are consistent with existing in vitro [11, 24] and in vivo [19, 17] results.
By-and-large, the effects of i.v. cocaine were as expected. Cocaine caused a dose-related blockade of the uptake of [3H]DA in accumbens. This result is consistent with both in vitro and in vivo studies with cocaine and DA in the accumbens [22, 38]. The failure of DMI or citalopram to block [3H]NE or [3H]DA uptake in the accumbens argues that blockade of [3H]DA uptake involved a DAT action of cocaine. The effective dose was 3-30 μmol/kg (approximately 1–10 mg/kg), appropriately lower than the i.p. doses of 20 and 30 mg/kg reported to increase extracellular DA in vivo [38], though somewhat higher than the 0.25–1.25 mg/kg i.v. dose range reported to increase extracellular DA [18]. The present results also support, at least qualitatively, the conclusion that blockade of DA uptake in the accumbens is involved in the behavioral effects of i.v. cocaine. Quantitatively, however, the range of effective doses in the present study was higher than the range of 0.25–1.25 mg/kg/injection of cocaine reported to maintain i.v. self-administration and increase extracelluar DA in accumbens [18]. However, animals can accumulate injections in a typical self-administration study. Moreover, for in vivo microdialysis neurotransmitter concentrations are measured continuously over several minutes rather than precisely at five min post-injection. Therefore, the present data do not seriously challenge the hypothesis that blockade of DA uptake in the accumbens plays a central role in the reinforcing effect of cocaine.
Potency relationships between cocaine and GBR 12909 with regard to DA uptake were consistent with the literature. In vitro, GBR 12909 has approximately 10-fold higher DAT binding affinity than cocaine [30]. As noted, cocaine doses of 20–30 mg/kg, i.p. increase extracellular DA in vivo while the effective dose of GBR 12909 for in vivo microdialysis was approximately 10-fold lower [2.5–5 mg/kg, i.p.; 27]. Similarly, GBR 12909 was 10-fold more potent than cocaine in the present study. These comparisons strengthen the validity of the present assay. Interestingly, doses of GBR 12909 that are self-administered by rats [0.5–1.5 mg/kg/inj; 28] are not notably different from cocaine doses that are self-administered by rats. The reason(s) for this discrepancy is not clear, though it also may relate to the availability of multiple doses and longer duration of action of GBR 12909 relative to cocaine.
As with DMI, [3H]NE uptake was decreased in occipital cortex following i.v. cocaine. This is consistent with studies using in vivo microdialysis [19] and supports an in vivo mechanism of blockade of NE uptake by cocaine. It has also well established that cocaine can block the uptake of 5-HT in vitro [22]. An increase in extracellular 5-HT following cocaine has also been demonstrated in vivo [16]. Unexpectedly, cocaine only partially blocked [3H]5-HT uptake in the present study, up to lethal doses. Several investigators have proposed an involvement of 5-HT in the behavioral effects of cocaine [32]. Taken at face value, the present results argue that the effects of cocaine that involve increased 5-HT neurotransmission are a consequence of only partial blockade of 5-HT uptake.
The most surprising result of the present study was that under conditions that were effective for inhibiting [3H]DA uptake in the accumbens, cocaine failed to block [3H]DA uptake in striatum, even when the dose was increased to the lethal range. No such difference has been seen in vitro [1, 7]. This finding is even more surprising given the blockade of [3H]DA uptake in the striatum after GBR 12909. Differences in the effects of cocaine on extracellular DA in accumbens and striatum have been reported previously in vivo [4, 13], though these have been potency differences as opposed to the efficacy difference reported herein. The present results demonstrating increased potency of GBR 12909 in the accumbens relative to the striatum extend those observations. While the affinity of the transporter for DA in the two areas appears to be the same, the DAT density in the accumbens and the amount of DA are lower than in the striatum. Cass et al. [4] proposed that the increased sensitivity of the accumbens derives from the fact that there are fewer DATs to inhibit. Others have argued that a lower density of uptake sites should reduce the effect of an uptake inhibitor on neurotransmitter levels [23]. Wu et al. [37] have proposed that lower rates of DA release and uptake in the accumbens underlie regional differences in the effects of cocaine. If DA is released at higher rates resulting in higher concentrations DA at the DAT in the striatum as compared to the accumbens, then it would seem possible that cocaine binding in vivo would be displaced more effectively in the striatum than accumbens. Such an effect would contribute to the diminished potency of GBR 12909 in the striatum relative to accumbens observed in the present study.
The possibility that pharmacokinetics may contribute to the present results should also be considered. The estimated concentration of cocaine achieved in the striatum was clearly adequate to block DA uptake. Perhaps cocaine’s penetration to the DAT is slower in the striatum than in accumbens. This seems untenable considering data demonstrating competitive in vivo DAT binding by cocaine in striatum 2–10 min after i.v. injection [37]. The in vivo [3H]cocaine binding results in the present paper also showed [3H]cocaine binding in striatum at 1–10 min after i.v. injection. Others have reported striatal binding of [3H]cocaine over 1–10 min after i.v. injection [8]. It is also conceivable that some or all of the [3H]cocaine binding in those studies was to inactive transporter sites.
Cocaine was not, however, without effect in striatum. Striatal DA uptake was actually increased at 15 min after injection. Using a similar ex vivo assay, Daws et al. also found that 30 min after an i.p. injection of 30 mg/kg cocaine, [3H]DA uptake into synaptosomes was increased in accumbens [6]. Although we did not see increased uptake in accumbens, we did not vary the time of sacrifice in our experiments with accumbens. Additionally, we used slice and whole homogenized tissue while Daws et al. [6] used P2 synaptosomes. Daws et al. also reported that cocaine can upregulate the surface expression of DAT in HEK 293 cells incubated for 10 min with 10 μM cocaine [6], and that this could account for increased uptake. In their voltammetry study [6], a low dose of cocaine increased DA clearance in rat striatum. David et al. found similar result in the shell of rat nucleus accumbens (5). In another study [10], DA uptake by human DAT-transfected mouse neuroblastoma cells was increased after 24 of exposure to 1 μM of cocaine. A similar mechanism could be operative in the present experiment. Together, these results support that suggestion that cocaine has effects in vivo that are not predicted in vitro.
In summary, the present study demonstrates several effects of cocaine in an ex vivo assay that are consistent with existing data regarding mechanism of action of cocaine. However, although cocaine is generally found in vitro to be equipotent as an uptake blocker across the monoamines [22], the present results suggest that this may not be the case in vivo. Moreover, cocaine failed to block DA uptake in the striatum. Because the assay behaved as expected in all other respects, including evident blockade of DA uptake in the accumbens, the mechanism for this discrepancy remains unclear. It is conceivable that the DAT that is expressed in the striatum has different properties from the DAT in the accumbens. For example, the DAT in striatum and accumbens have different apparent molecular weights [14]. It is also interesting that the predominant cellular location of DAT in the striatum is on DA terminals originating in the nigra, whereas the predominant cellular location of DAT in the accumbens is on terminals originating in the ventral tegmental area [20]. Hence, the possibility exists that these different cell groups may process the DAT protein differently resulting in distinct pharmacodynamic profiles. Also the effects of cocaine in ex vivo DAT binding and ex vivo DA uptake are apparently disassociated in striatum, suggesting that DA binding sites and uptake sites may differ. It is also possible that cocaine’s binding to the striatal DAT does not necessarily result in DA uptake inhibition.
Acknowledgments
Supported by NIDA grant DA-10352. W.L.W. is the recipient of NIDA Senior Scientist Award K05-DA-15343. G.A.O. is the recipient of NIMH Independent Scientist Award K02-MH-02031. We gratefully acknowledge the technical assistance of Christine Tiep.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boja JW, Kuhar MJ. [3H]cocaine binding and inhibition of [3H]dopamine uptake is similar in both the rat striatum and nucleus accumbens. Eur J Pharmacol. 1989;173:215–217. doi: 10.1016/0014-2999(89)90524-4. [DOI] [PubMed] [Google Scholar]
- 2.Broderick PA, Rahni DN, Zhou Y. Acute and subacute effects of risperdone and cocaine on accumbens dopamine and serotonin release using in vivo microvoltammetry on line with open-field behavior. Prog Neuro-Psychopharmacol Biol Psychiatry. 2003;27:1037–1054. doi: 10.1016/S0278-5846(03)00176-3. [DOI] [PubMed] [Google Scholar]
- 3.Budygin EA, Kilpatrick MR, Gainetdinov RR, Wightman RM. Correlation between behavior and extracellular dopamine levels in rat striatum: comparison of microdialysis and fast-scan cyclic voltammetry. Neurosci Lett. 2000;281:9–12. doi: 10.1016/s0304-3940(00)00813-2. [DOI] [PubMed] [Google Scholar]
- 4.Cass WA, Gerhardt GA, Mayfield RD, Curella P, Zahniser NR. Differences in dopamine clearance and diffusion in rat striatum and nucleus accumbens following systemic cocaine administration. J Neurochem. 1992;59:259–266. doi: 10.1111/j.1471-4159.1992.tb08899.x. [DOI] [PubMed] [Google Scholar]
- 5.David DJ, Zahniser NR, Hoffer BJ, Gerhardt GA. In vivo electrochemical studies of dopamine clearance in subregions of rat nucleus accumbens: differential properties of the core and shell. Experimental Neurology. 1998;153:277–286. doi: 10.1006/exnr.1998.6898. [DOI] [PubMed] [Google Scholar]
- 6.Daws LC, Callaghan PD, Moron JA, Kahlig KM, Shippenberg TS, Javitch JA, Galli A. Cocaine increases dopamine uptake and cell surface expression of dopamine transporters. Biochem Biophys Res Commun. 2002;290:1545–1550. doi: 10.1006/bbrc.2002.6384. [DOI] [PubMed] [Google Scholar]
- 7.Elsworth JD, Taylor JR, Berger P, Roth RH. Cocaine-sensitive and -insensitive dopamine uptake in prefrontal cortex, nucleus accumbens and striatum, [erratum appears in Neurochem Int 1995 Apr;26(4):I] Neurochem Int. 1993;23:61–9. doi: 10.1016/0197-0186(93)90144-t. [DOI] [PubMed] [Google Scholar]
- 8.Fowler JS, Volkow ND, Logan J, Gatley SJ, Pappas N, King P, Ding YS, Wang GJ. Measuring dopamine transporter occupancy by cocaine in vivo: radiotracer considerations. Synapse. 1998;28:111–6. doi: 10.1002/(SICI)1098-2396(199802)28:2<111::AID-SYN1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 9.Gatley SJ, Gifford AN, Carroll FI, Volkow ND. Sensitivity of binding of high-affinity dopamine receptor radioligands to increased synaptic dopamine. Synapse. 2000;38:483–488. doi: 10.1002/1098-2396(20001215)38:4<483::AID-SYN14>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 10.Ho M, Segre M. Individual and combined effects of ethanol and cocaine on the human dopamine transporter in neuronal cell lines. Neurosci Lett. 2001;299:229–33. doi: 10.1016/s0304-3940(01)01526-9. [DOI] [PubMed] [Google Scholar]
- 11.Hyttel J. Effect of a specific 5-HT uptake inhibitor, citalopram (Lu 10-171), on 3H-5-HT uptake in rat brain synaptosomes in vitro. Psychopharmacology. 1978;60:13–18. doi: 10.1007/BF00429172. [DOI] [PubMed] [Google Scholar]
- 12.Jacocks HM, Izenwasser S, Werling LL, Cox BM. Comparison of dopamine uptake and release in vitro in sheep and rat striatum. Life Sci. 1991;49:481–8. doi: 10.1016/0024-3205(91)90064-i. [DOI] [PubMed] [Google Scholar]
- 13.Kuczenski R, Segal DS. Differential effects of amphetamine and dopamine uptake blockers (cocaine, nomifensine) on caudate and accumbens dialysate dopamine and 3-methoxytyramine. J Pharmacol Exp Ther. 1992;262:1085–1094. [PubMed] [Google Scholar]
- 14.Lew R, Vaughan R, Simantov R, Wilson A, Kuhar MJ. Dopamine transporters in the nucleus accumbens and the striatum have different apparent molecular weights. Synapse. 1991;8:152–3. doi: 10.1002/syn.890080209. [DOI] [PubMed] [Google Scholar]
- 15.Missale C, Castelletti L, Govoni S, Spano PF, Trabucchi M, Hanbauer I. Dopamine uptake is differentially regulated in rat striatum and nucleus accumbens. J Neurochem. 1985;45:51–56. doi: 10.1111/j.1471-4159.1985.tb05473.x. [DOI] [PubMed] [Google Scholar]
- 16.Parsons LH, Justice JB., Jr Serotonin and dopamine sensitization in the nucleus accumbens, ventral tegmental area, and dorsal raphe nucleus following repeated cocaine administration. J Neurochem. 1993;61:1611–1619. doi: 10.1111/j.1471-4159.1993.tb09794.x. [DOI] [PubMed] [Google Scholar]
- 17.Pawlowski L, Nowak G, Gorka Z, Mazela H. Ro 11-2465 (cyan-imipramine), citalopram and their N-desmethyl metabolites: effects on the uptake of 5-hydroxytryptamine and noradrenaline in vivo and related pharmacological activities. Psychopharmacology. 1985;86:156–63. doi: 10.1007/BF00431702. [DOI] [PubMed] [Google Scholar]
- 18.Pettit HO, Justice JB., Jr Dopamine in the nucleus accumbens during cocaine self-administration as studied by in vivo microdialysis. Pharmacol Biochem Behav. 1989;34:899–904. doi: 10.1016/0091-3057(89)90291-8. [DOI] [PubMed] [Google Scholar]
- 19.Reith ME, Li MY, Yan QS. Extracellular dopamine, norepinephrine, and serotonin in the ventral tegmental area and nucleus accumbens of freely moving rats during intracerebral dialysis following systemic administration of cocaine and other uptake blockers. Psychopharmacology. 1997;134:309–317. doi: 10.1007/s002130050454. [DOI] [PubMed] [Google Scholar]
- 20.Reith ME. Neurotransmitter Transporters. Totowa; New Jersey: 1997. p. 322. [Google Scholar]
- 21.Rocha BA, Fumagalli F, Gainetdinov RR, Jones SR, Ator R, Giros B, Miller GW, Caron MG. Cocaine self-administration in dopamine-transporter knockout mice, [see comment][erratum appears in Nat Neurosci 1998 Aug;1(4):330] Nat Neurosci. 1998;1:132–7. doi: 10.1038/381. [DOI] [PubMed] [Google Scholar]
- 22.Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Sabeti J, Gerhardt GA, Zahniser NR. Acute cocaine differentially alters accumbens and striatal dopamine clearance in low and high cocaine locomotor responders: behavioral and electrochemical recording in freely moving rats. J Pharmacol Exp Ther. 2002;302:1201–1211. doi: 10.1124/jpet.102.035816. [DOI] [PubMed] [Google Scholar]
- 24.Seiden LS, Dykstra LA. Psychopharmacology, A Biochemical and Behavioral Approach. Van Nostrand Reinhold Company; NY: 1977. [Google Scholar]
- 25.Sora I, Wichems C, Takahashi N, Li XF, Zeng Z, Revay R, Lesch KP, Murphy DL, Uhl GR. Cocaine reward models: conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proc Natl Acad Sci U S A. 1998;95:7699–704. doi: 10.1073/pnas.95.13.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB, Wichems C, Lesch KP, Murphy DL, Uhl GR. Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc Natl Acad Sci U S A. 2001;98:5300–5. doi: 10.1073/pnas.091039298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanda G, Pontieri FE, Frau R, Di Chiara G. Contribution of blockade of the noradrenaline carrier to the increase of extracellular dopamine in the rat prefrontal cortex by amphetamine and cocaine. Eur J Neurosci. 1997;9:2077–85. doi: 10.1111/j.1460-9568.1997.tb01375.x. [DOI] [PubMed] [Google Scholar]
- 28.Tella SR, Ladenheim B, Andrews AM, Goldberg SR, Cadet JL. Differential reinforcing effects of cocaine and GBR-12909: biochemical evidence for divergent neuroadaptive changes in the mesolimbic dopaminergic system. J Neurosci. 1996;16:7416–27. doi: 10.1523/JNEUROSCI.16-23-07416.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsukada H, Harada N, Nishiyama S, Ohba H, Kakiuchi T. Dose- response and duration effects of acute administrations of cocaine and GBR 12909 on dopamine synthesis and transporter in the conscious monkey brain: PET studies combined with microdialysis. Brain Res. 2000;860:141–148. doi: 10.1016/s0006-8993(00)02057-6. [DOI] [PubMed] [Google Scholar]
- 30.Valchar M, Hanbauer I. Comparison of [3H]WIN 35,428 binding, a marker for dopamine transporter, in embryonic mesencephalic neuronal cultures with striatal membranes of adult rats. J Neurochem. 1993;60:469–76. doi: 10.1111/j.1471-4159.1993.tb03174.x. [DOI] [PubMed] [Google Scholar]
- 31.Vickroy TW, Johnson KM. In vivo administration of phencyclidine inhibits 3H-dopamine accumulation in rat brain striatal slices. Subst Alcohol Actions Misuse. 1980;1:351–354. [PubMed] [Google Scholar]
- 32.Walsh SL, Cunningham KA. Serotonergic mechanisms involved in the discriminative stimulus, reinforcing and subjective effects of cocaine. [Review] Psychopharmacology. 1997;130:41–58. doi: 10.1007/s002130050210. [DOI] [PubMed] [Google Scholar]
- 33.Wilcox KM, Paul IA, Ordway GA, Woolverton WL. Role of the dopamine transporter and the sodium channel in the cocaine-like discriminative stimulus effects of local anesthetics in rats. Psychopharmacology. 2001;157:260–268. doi: 10.1007/s002130100796. [DOI] [PubMed] [Google Scholar]
- 34.Wise RA. Neural mechansims of the reinforcing actions of cocaine. In: Grabowski J, editor. Cocaine: Pharmacologoy, Effects and Treatment of Abuse. NIDA research Monograph 50. US gov't. Printing Office; Washington DC: 1984. [Google Scholar]
- 35.Woolverton WL, Johnson KM. Neurobiology of cocaine abuse. Trends Pharmacol Sci. 1992;13:193–200. doi: 10.1016/0165-6147(92)90063-c. [DOI] [PubMed] [Google Scholar]
- 36.Woolverton WL, Ranaldi R, Wang Z, Ordway GA, Paul IA, Petukhov P, Kozikowski A. Reinforcing strength of a novel dopamine transporter ligand: pharmacodynamic and pharmacokinetic mechanisms. J Pharmacol Exp Ther. 2002;303:211–217. doi: 10.1124/jpet.102.037812. [DOI] [PubMed] [Google Scholar]
- 37.Wu Q, Reith ME, Kuhar MJ, Carroll FI, Garris PA. Preferential increases in nucleus accumbens dopamine after systemic cocaine administration are caused by unique characteristics of dopamine neurotransmission. J Neurosci. 2001;21:6338–6347. doi: 10.1523/JNEUROSCI.21-16-06338.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zahniser NR, Larson GA, Gerhardt GA. In vivo dopamine clearance rate in rat striatum: regulation by extracellular dopamine concentration and dopamine transporter inhibitors. J Pharmacol Exp Ther. 1999;289:266–277. [PubMed] [Google Scholar]


