Abstract
Malaria transmission occurs during a blood-meal of an infected Anopheles mosquito. Visualization and quantification of sporozoites along the journey from the mosquito midgut, where they develop, to the vertebrate liver, their final target organ, is important for understanding many aspects of sporozoite biology. Here we describe the generation of Plasmodium berghei parasites that express the reporter gene lacZ as a stable transgene, under the control of the sporozoite-specific CSP promoter. Transgenic sporozoites expressing β-galactosidase can be simply visualized and quantified in an enzymatic assay. In addition, these sporozoites can be used to quantify sporozoites deposited in subcutaneous tissue during natural infection.
Keywords: Plasmodium, Sporozoite, LacZ, β-Galactosidase, Reporter gene, Quantitative assay
1. Introduction
Plasmodium sporozoites are tailor-made for transmission by infected Anopheles mosquitoes and display vigorous gliding locomotion, fast breaching of biological barriers, and efficient invasion of the final target cell [1,2]. Sporozoites are formed in a complex process, termed sporogony, that represents the longest developmental phase of the Plasmodium life cycle [3]. Although sporozoites are likely passively transported throughout the hemocoel, there is evidence that they specifically recognize the salivary glands through a receptor-mediated process [4–7]. Sporozoites breach the basal lamina and acinar gland cells and accumulate in the salivary duct [8]. When the infected mosquito takes another blood-meal, salivary gland sporozoites are injected into the avascular portion of the skin [9,10] where they start vigorous gliding locomotion in search of a capillary [11,12]. Once in the circulation they reach the liver and eventually invade its final target cell, a suitable hepatocyte [1,2,13].
To better understand the molecular events involved in this complex journey within the invertebrate and vertebrate hosts transgenic parasites that express GFP or a red fluorescent protein, RedStar, under the control of the sporozoite-specific cir-cumsporozoite protein (CSP) or the constitutive elongation factor 1α (EF1α) promoter have been generated [14–16]. These transgenic parasites were a prerequisite for the application of intravital microscopy to describe the Plasmodium life cycle in whole animals [9,11,12,16]. Fluorescent sporozoites can be monitored over long time intervals leading to novel insights into the roles of locomotion, migration through cells, and cell invasion in the establishment of infection in the vertebrate host. For quantification of parasite populations in different tissues of mosquito and vertebrate hosts, an enzymatic reporter assay is desirable. For these types of experiments, alternative reporter genes such as firefly luciferase, chloramphenicol acetyltransferase, and E. coli β-galactosidase are particularly suitable. The ability of β-galactosidase to hydrolyze β-D-galactopyranoside analogs, such as X-Gal, CPRG, or MUG, allows reliable quantification of reporter enzyme expression over a wide range using simple colorimetric assays. Parasites that stably express β-galactosidase have been successfully introduced to the related apicomplexan parasite Toxoplasma gondii [17]. T. gondii tachyzoites express high intracellular levels of the reporter enzyme and have been productively used for quantification of tachyzoites [17], assessment of drug activity [18], detection of rare life cycle stages [19], and as a marker for cell lysis in tachyzoite secretion experiments [20].
In this study we aimed to generate a transgenic Plasmodium berghei sporozoite that expresses high levels of β-galactosidase. We placed the reporter gene under the control of the CSP promoter, the strongest sporozoite promoter known to date. Using these transgenic parasites we quantified sporozoite loads en route from oocysts to the skin.
2. Materials and methods
2.1. Plasmodium berghei cycle
Anopheles stephensi were maintained at 21 °C, 80% humidity and fed daily on 10% sucrose. For mosquito infection experiments 4–5-day-old A. stephensi were fed for 10 min on anesthetized NMRI mice infected with either wild-type (WT) or transgenic Plasmodium berghei parasites (strain NK65). Infected mice were checked by blood smear for the abundance of gametocyte-stage parasites and their capacity to exflagellate. At day 10 post-infection, midguts were checked for infection. To isolate oocyst sporozoites midguts were dissected at day 14 post-infection, gently ground and harvested from the supernatant after a brief low-speed centrifugation to remove mosquito debris. Similarly, salivary gland sporozoites were harvested at day 17 post-infection or as indicated from dissected salivary glands. Sporozoite gliding motility was visualized as described previously [21].
2.2. Construction of the CSP-lacZ targeting vector
For reporter gene expression a targeting vector (pSE05) was assembled that contained 1.5 kb of the 5′UTR of CSP including the CSP promoter region [22], the reporter gene lacZ and 1.2 kb of the 3′UTR of the P. berghei dihydrofolate reductase/thymidylate synthase (DHFR/TS). For positive selection with the antifolate pyrimethamine the targeting plasmid contains the Toxoplasma gondii DHFR/TS open reading frame under the control of the P. berghei DHFR/TS regulatory sequences. For the CSP 5′UTR the primers p5′CSfor (5′-CCGGAATTCACATAAAAGGGAATATGGAATATACTAGC-3′; EcoRI site is underlined) and p5′CSrev (5′-CGCGGATCC-AAATATATGCGTGTATATATAGATTTTG-3′, BamHI site is underlined) were used. The 3′UTR of P. berghei DHFR/TS was amplified from the transfection vector pExpEF (kindly provided by Drs. Chris J. Janse and Andrew P. Waters, Leiden) using primers SEb3Dfor (5′-TGCTCTAGACGTTTTTCTTA-CTTATATAT-3′, XbaI site is underlined) and SEb3Drev (5′-TCCCCGCGGCGGTGTGAAATACCGCACAGA-3′, SacII site is underlined). The reporter gene lacZ was amplified from a vector containing the lacZ gene (pNeoβGal, Stratagene) using primers LacZfor (5′-CGCGGATCCAAAATGGCA-CTGGCCGTCGTTTTACAACGTCG-3′, BamHI site is underlined) and LacZrev (5′-GGAAGATCTCCCCTGCCCGGTT-ATTATTATTTTTGAC-3′, BglII site is underlined).
2.3. P. berghei transfection and genotyping
P. berghei transfection was performed essentially as described [23]. Sixty microgram Acc65I-linearized plasmid pSE-05 was included in the electroporation of gradient-purified P. berghei schizonts. Positive selection for the integration of the targeting plasmid was by daily treatment with 25 mg kg−1 pyrimethamine. Transfer of the emerging P. berghei population into näive animals confirmed pyrimethamine resistance. Selected parasite populations were genotyped by integration specific PCR using primers Tgfor (5′-CCCGCACGGACGAAT-CCAGATGG-3′) and CStest (5′-GCAAGTAATCTGTTGA-CTGTATTTCG-3′). Three clonal transgenic parasite populations, termed PbBlue, were obtained by limiting dilution into 15 naive NMRI mice.
2.4. Western blot analysis
For immunoblotting, extracts from 100,000 PbBlue or WT sporozoites isolated from midguts or salivary glands of infected mosquitoes were separated on 8% polyacrylamide gels and transferred to nitrocellulose membranes by electroblotting. lacZ and CSP expression was detected by incubation of membranes with a monoclonal anti-β-Galactosidase antibody (Promega) and a monoclonal anti-CSP antibody [24], respectively, followed by incubation with horseradish-peroxidase-conjugated anti-mouse antibody (Sigma). Immunostained proteins were visualized by enhanced chemiluminescence (Amersham).
2.5. X-Gal staining of sporozoites
Parasites were immobilised and permeabilized for 10 min at room temperature with 2% formaldehyde, 0.2% glutaraldehyde, 0.02% Triton-X 100 in phosphate buffered saline (PBS). After washing with PBS the parasites were incubated with staining solution (5 mM potassium ferricyanid, 5 mM potassium ferrcyanid, 0.04% deoxycholate, 2 mM magnesium chloride in phosphate buffered saline) containing 1 mg ml−1 X-Gal until appearance of staining.
2.6. X-Gal staining of the skin
This experiment was performed with mosquitoes infected with the PbBlue parasites and uninfected mosquitoes that had taken a blood-meal 2 weeks prior to the experiment. Swiss Webster mice were anesthetized with ketamine/xylazine until deep anesthesia was attained (as assessed by lack of response to painful stimuli) and maintained at 37 °C on a slide warmer. Five to eight mosquitoes were placed in a small feeding cage made of Plexiglas tubing (2 cm diameter) with fine mesh over one end and sealed with a rubber dam on the other end. The mosquito cage was placed mesh-side down on the ventral side of the mouse’s ear which was taped so that the mosquitoes could probe only on a restricted portion. After 5 min this group of mosquitoes was removed and another group of five mosquitoes was placed on the mouse’s ear. After the second group probed for 5 min, the mouse’s ear was removed and the anesthetized mouse was sacrificed by cervical dislocation in accordance with institutional guidelines. The two leaflets of the ear were separated from one another under a dissecting microscope using fine forceps. Ear leaflets were fixed in 4% paraformaldehyde/0.5% glutaraldehyde in phosphate buffered saline (PBS) supplemented with 2 mM MgCl2, 0.02% Triton X-100 and 0.04% deoxycholate for 45 min at room temperature. The ears were briefly washed in PBS and then incubated in PBS containing 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 2 mM MgCl2, 0.02% Triton X-100, 0.04% deoxycholate and 0.3 mg ml−1 X-Gal overnight at 37 °C. Ear leaflets were then rinsed in PBS and mounted in 50% glycerol/50% PBS for observation using brightfield microscopy.
2.7. Quantitative β-Gal assays
Quantification with the β-D-galactopyranoside analog chlorophenol red-β-D-galactopyranoside (CPRG; Roche) was performed with known numbers of sporozoites lysed in DMEM (without phenol red) and 0.2% Triton-X-100. Fifteen microlitres of sporozoite lysate was added to 135 μl CPRG-solution (1 mM CPRG, 1 mM magnesium chloride, 45 mM β-mercaptoethanol, 0.1% Triton-X-100, in 0.1 M sodium phosphate; pH 7.5) and incubated for 1.5 h at 37 °C. Samples were measured in 96-well plates at 570 nm using an absorbance reader (Fluostar, BMG Laboratories).
Quantification with the β-D-galactopyranoside analog MUG was performed as described previously [25]. Briefly, PbBlue sporozoites were lysed in lysis buffer (100 mM Hepes pH 8.0, 1 mM MgSO4, 1% Triton X-100 and 5 mM DTT), incubated at 50 °C for 1 h and spun at 14,000 rpm in a microfuge for 15 min at room temperature. Fifty microlitres supernatant was added to 50 μl LacZ substrate in lysis buffer (final concentration, 0.85 mM 4-methylumbelliferyl-β-D-galactoside) and incubated at 37 °C for 2 h. One hundred microlitres stop buffer (25 mM EDTA, 500 mM glycine; pH 11.2) was added and the sample was read in a Fluoroskan II Plate Reader (Labsystems, Helsinki, Finland) with emission filter 355 nm and excitation filter 460 nm.
Quantification of liver stages with a commercial β-galactosidase ELISA (Roche) was performed according to the manufacturer’s procedure. Briefly, infected hepatoma cells were thoroughly washed with PBS, removed from the chamber slide with lysis buffer, and stored until measurement at −20 °C. Total extracts were measured in 96-well plates at room temperature and 405 nm absorbance (reference wavelength 490 nm) using an absorbance reader (Fluostar).
2.8. Quantification of sporozoite transmission
A Swiss Webster mouse was anesthetized, placed on a slide warmer set to 37 °C, ventral side upwards. One ear was secured onto the warmer with tape and a plexi-glass cage covered with mosquito netting on one side, containing five to eight mosquitoes infected with PbBlue sporozoites was placed on the ear and the mosquitoes were allowed to probe and/or feed for 4 min. This was repeated with another cage of 5–8 mosquitoes so that a total of about 10–15 mosquitoes probed on each ear. The ear was then cut off, snap-frozen in liquid nitrogen and stored at −80 °C until homogenization. The anesthetized mouse was immediately sacrificed by cervical dislocation. Each ear was homogenized in 750 μl lysis buffer (without Triton X-100) using a PowerGen 125 tissue homogenizer (Fisher Scientific). Triton X-100 was added to the homogenate (final concentration 1%) and then incubated for 1 h at 50 °C. The lysate was vortexed and then spun in a microfuge at 14,000 rpm for 15 min at room temperature. The top layer of lipid and hair was removed with a pasteur pipet and the middle aqueous phase was assayed for β-galactosidase activity using MUG as outlined above. Only 50 μl of the total lysate was analyzed.
3. Results and discussion
3.1. Generation of a transgenic β-galactosidase expressing P. berghei parasite
In order to obtain sporozoites that express high intracellular levels of the reporter gene β-galactosidase we employed a transfection strategy that allowed stable integration of a reporter cassette into the P. berghei CSP locus (Fig. 1A). The reporter cassette contains 1.5 kb of the CSP 5′UTR including the CSP promoter [22], the 3.1 kb open reading frame of E. coli β-galactosidase, and 1.2 kb of the DHFR/TS 3′UTR. Similar to the strategy employed by Natarajan et al. [14] the reporter cassette is integrated into the CSP 5′UTR via a single cross-over event upon linearization at the endogenous Acc65I site (Fig. 1A). Genotyping of the resulting clonal parasite lines confirmed integration at the desired locus (Fig. 1B). To control for β-galactosidase expression levels in the recombinant parasites we performed Western blot analysis (Fig. 2). We readily detected a specific ~116 kDa protein in immunoblots with extracts from transgenic oocyst and salivary gland sporozoites that was absent in WT sporozoites. Notably, CSP expression was not influenced by co-expression of the CSP promoter, which appears to be constitutively active throughout sporozoite maturation from immature oocyst sporozoites to infectious salivary gland sporozoites [26]. These data demonstrate that we generated a transgenic P. berghei parasite line that expresses the reporter enzyme β-galactosidase under regulation of the CSP promoter.
Fig. 1.
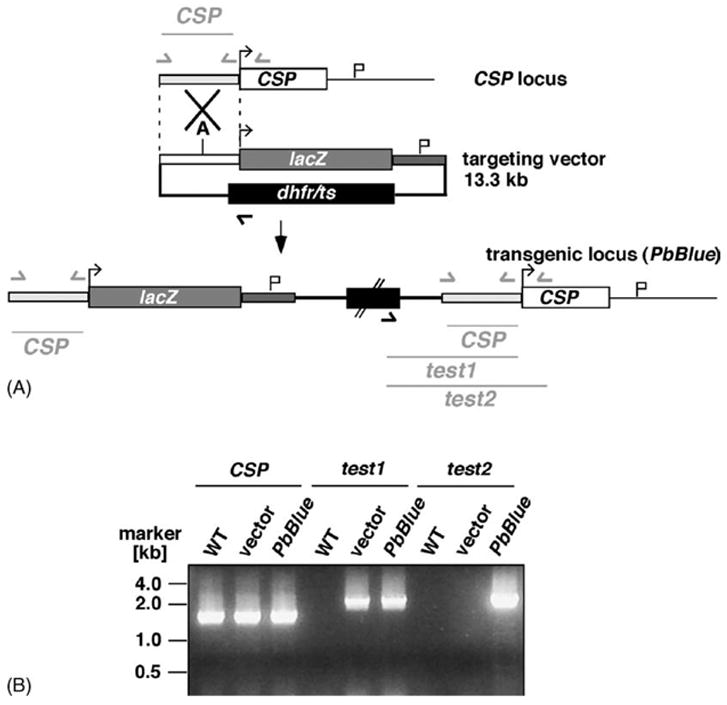
Generation of a transgenic β-galactosidase-expressing Plasmodium berghei parasite. (A) Insertion strategy to place lacZ expression under control of the CSP promoter. The targeting vector contains the CSP promoter region (pale grey bar), the lacZ coding region (grey box) and the 3′UTR of the DHFR/TS gene (dark grey bar) to allow for proper transcript processing, together with the positive selectable marker dhfr/ts (black box). The targeting vector was linearized within the CSP 5′UTR by Acc65I [A]. Test primers are indicated by arrows and expected fragments are shown as lines. (B) Integration-specific PCR confirms the predicted transgenic locus. Note that the primer combination ‘test2’ is indicative of an integration event.
Fig. 2.
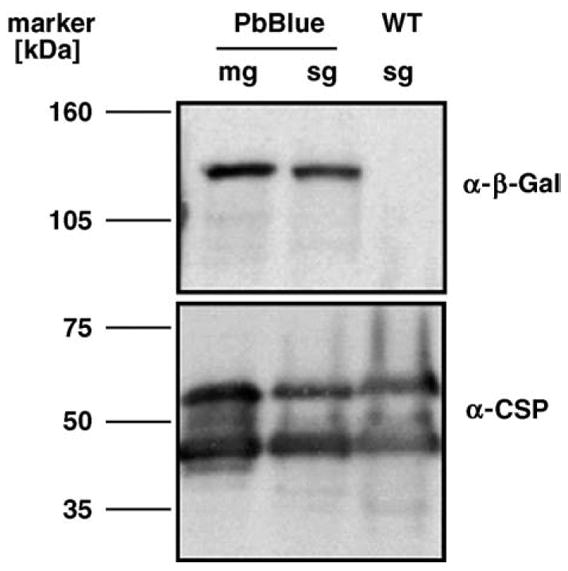
Western blot analysis of the PbBlue sporozoites. Shown are extracts of 100,000 sporozoites isolated from either midgut oocysts (mg) or salivary glands (sg). Antibody specificity is verified by the absence of the β-galactosidase signal in WT sporozoites. As a loading control the blot was re-probed with an anti-CSP monoclonal antibody [24].
3.2. Visualization of transgenic sporozoites
We next asked whether the expressed enzyme is catalytically active and, hence, can be used to stain sporozoites in situ. As expected, the clonal transgenic parasites displayed normal development of sexual and asexual stages (data not shown). In these stages, reporter gene expression could neither be detected by immuno-blotting nor by immunofluorescence with an anti-β-galactosidase antibody (data not shown). When Anopheles mosquitoes were infected with the transgenic parasites we observed mature oocysts adjacent to the midgut epithelium in numbers comparable to the WT parasite. These oocysts were readily visualized by X-Gal staining (Fig. 3A). Staining of per-meabilized infected salivary glands revealed an accumulation of sporozoites in one or both lateral lobes (Fig. 3B). These parasites should permit qualitative assessment of vector transmission capacities in the absence of anti-parasite antisera and expensive equipment. Similarly, individual sporozoites can be simply stained with X-Gal (Fig. 3C). Because the transgenic sporozoites express active β-galactosidase we termed these parasites PbBlue. Notably, sporozoite functions, such as gliding motility (Fig. 3C), are not affected by the high intracellular expression levels of the heterologous reporter enzyme. Sporozoite invasion into hepatocytes and liver stage development is also indistinguishable from WT parasites (data not shown). Intracellular liver stages can be stained with X-Gal (Fig. 3D), presumably because β-galactosidase typically displays a relatively long half-life [27], together with the fact that the CSP promoter is active in the initial hours after sporozoite transformation [28]. Importantly, these recombinant parasites permit simple detection and staining of pre-erythrocytic Plasmodium stages.
Fig. 3.
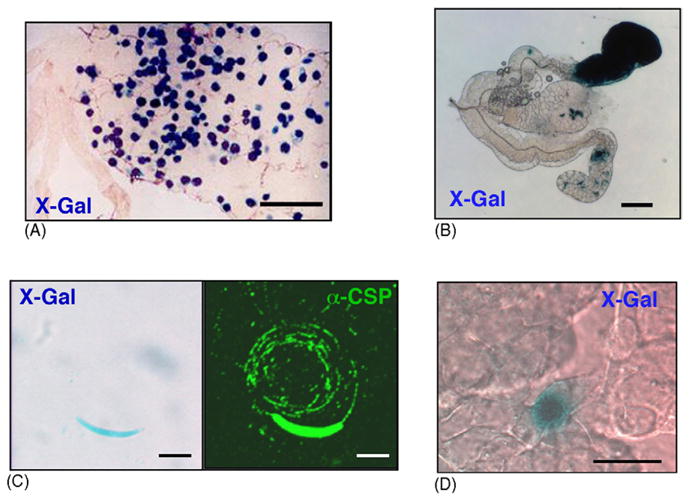
Detection of transgenic sporogonic and pre-erythrocytic Plasmodium stages expressing β-galactosidase. (A) Midgut oocysts of infected Anopheles mosquitoes at day 13 post feeding. All oocysts can be stained with X-Gal after fixation and permeabilization. Scale bar is 200 μm. (B) X-Gal staining of an infected salivary gland after permeabilization. Scale bar is 100 μm. (C) Transgenic sporozoites express β-galactosidase (left panel) and perform gliding locomotion indistinguishable from WT sporozoites (right panel). Scale bar is 5 μm. (D) Infected heptocytes can be stained with X-Gal after fixation and permeabilization. Shown is a 48 h liver stage. Scale bar is 20 μm.
3.3. Quantification of PbBlue sporozoites
Next, we established quantitative assays to take full advantage of the reporter enzyme. First we quantified sporozoites isolated from Anopheles midguts and salivary glands. Quantification of oocyst sporozoites was readily achieved using the β-D-galactopyranoside analog CPRG as a substrate (Fig. 4A). As expected, increasing sporozoite numbers resulted in a proportional increase of the absorption at 570 nm. However, absolute values at any given sporozoite number varied between experiments. This variation likely represents batch-to-batch differences in sporozoite quality that are typically seen in multiple feeding experiments. Therefore, a titration curve is required for every mosquito batch and only rough estimates of sporozoite numbers may be possible when different mosquito batches are compared directly. Similarly, quantification of salivary gland sporozoites was done using a simple CPRG assay (Fig. 4B). In an attempt to analyze the variation between mosquito batches we compared the amount of reporter protein, as measured in the CPRG assay, with the sporozoite load in the glands. In good agreement with previous observations by Beier et al. [29] we detected a strict inverse correlation, i.e. reduced amounts of reporter gene whenever sporozoite numbers per gland exceeded 10,000 (data not shown). These findings together may indicate that expression at the CSP locus may be regulated in response to extracellular abundance of CSP.
Fig. 4.
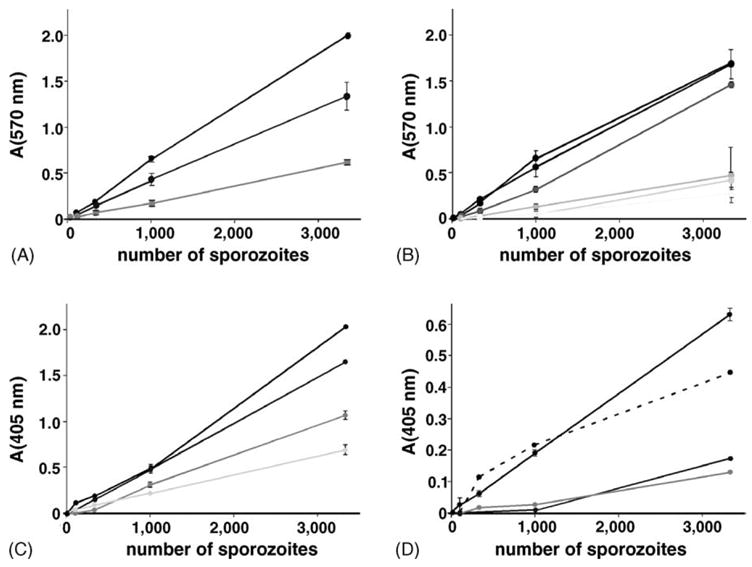
Quantification of transgenic sporogonic and pre-erythrocytic stages. (A) Quantification of isolated oocyst sporozoites with the β-D-galactopyranoside analog CPRG (absorption at 570 nm). Shown are titration curves from three independent feeding experiments (black, dark grey and light grey). (B) Quantification of salivary gland sporozoites. Shown are mean values with standard deviations from duplicate measurements of six independent feeding experiments (black to light grey). (C and D) β-galactosidase ELISA to quantify infected hepatocytes. Shown are β-galactosidase levels (absorption at 405 nm) of infected hepatocytes 15 h (C) or 48 h (D) after addition of PbBlue sporozoites. Sporozoites were dissected at day 17 (lines) or day 19 (dashed line) post-infection. Liver stage development was done from at least three independent feeding experiments (black to light grey). Shown is the mean with standard deviations of duplicates.
Since we could stain intracellular liver stages with X-Gal, we asked whether we can measure β-galactosidase activity of infected hepatocytes by the same methodology. Sporozoite invasion and subsequent liver stage development in vitro is relatively inefficient. Typically, addition of 10,000 P. berghei sporozoites to subconfluent hepatoma cells result in ~200 liver stages. Using the CPRG-based colorimetric assay we could not detect substantial activity above background which is generally high in hepatocytes (data not shown). In order to increase the sensitivity of our assay we next tested a commercial β-galactosidase-ELISA (Fig. 4C and D). When we measured β-galactosidase levels of extracts generated from infected hepatocytes 15 h after invasion we observed substantial reporter protein levels in hepatocytes incubated with 10,000 sporozoites or more (Fig. 4C). The titration curves of liver stages from independent sporozoite batches were linear but showed different slopes. After 48 h, the time that corresponds to full maturation of liver stages in vitro, β-galactosidase levels of infected hepatocytes dropped (Fig. 4D). Hence, this sensitive ELISA permits reliable quantification only of early Plasmodium liver stages in cultured cells.
3.4. Visualization of sporozoite transmission
We next asked whether we can visualize low numbers of transgenic sporozoites in subcutaneous tissue after natural mosquito transmission. We allowed infected mosquitoes to probe on the ear of mice for up to ten minutes. Subsequent fixation of the dermis and incubation with the β-D-galactopyranoside analog X-Gal revealed specific staining of deposited sporozoites (Fig. 5). Intriguingly, the majority of the sporozoites are concentrated to a small region that likely represents the site of mosquito probing. Some sporozoites, however are observed as far as 100 μm from the area of highest sporozoite density. This could reflect the path of the mosquito proboscis as it probes for blood or migration of the sporozoites from the site of deposition. This type of assay will be particularly informative for determination of vector capacities and studies on immunized animals.
Fig. 5.
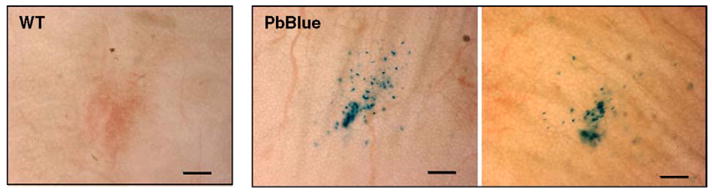
Visualization of PbBlue sporozoites injected into a live mouse by infected mosquitoes. Shown is a X-Gal stain of infected mouse ears that were bitten by Anopheles mosquitoes infected with either WT (left panel) or PbBlue parasites (right panels). Scale bar is 100 μm.
3.5. Quantification of sporozoite transmission
We finally wanted to test whether the PbBlue sporozoites can be used to quantify the sporozoite inoculum. Because of the detection limit of the CPRG assay observed in liver stages we tested the more sensitive β-D-galactopyranoside analog MUG. Indeed, using the fluorescent substrates we observed a linear dose curve over a range from 100 to 1000 sporozoites (Fig. 6A). According to a previous study in which RT-PCR was used to quantify the sporozoite innoculum [10], the mean number of sporozoites injected by a single mosquito is 123 sporozoites with a median of 18 and a range of 0–1297. We therefore estimated that the sporozoite inocula of 10–15 infected mosquitoes would be in the range detectable by this assay. When we quantified the β-galactosidase activity of homogenized skin tissue that had been fed-upon by infected mosquitoes, we could determine the inoculum of the mosquito population (Fig. 6B). Subfractions of the homogenized tissue equivalent to the inoculum of one infected mosquito gave values between 120 and 280 sporozoites. These values are in excellent agreement with the mean inoculum of 123 sporozoites, quantified by real-time RT-PCR analysis [10].
Fig. 6.
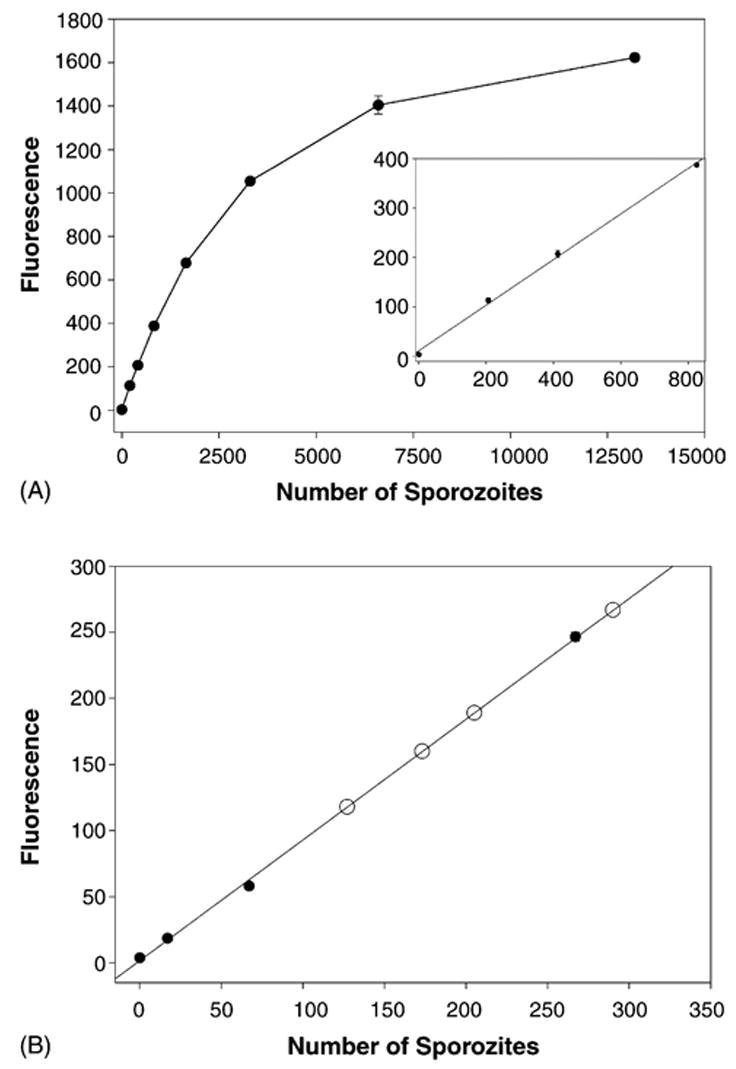
Quantification of PbBlue sporozoites injected into a live mouse by infected mosquitoes. (A) The fluorescent substrate 4-methylumbelliferyl-β-D-galactoside was used to detect PbBlue sporozoites dissected from salivary glands of infected mosquitoes. The inset shows the lower end of the curve in more detail. Shown is the mean with standard deviations of duplicates. (B) Different numbers of PbBlue sporozoites were added to homogenized mouse skin and a standard curve was generated (black circles). Infected mosquitoes (10–15) were allowed to probe on a mouse’s ear for 5 min, the ear was removed and processed as outlined in Section 2. Shown are the results from four ears (open circles) which are plotted on the standard curve generated above. The number shown is 1/15th of the total number deposited by the mosquitoes since only this fraction of the lysate was analyzed.
3.6. Concluding remarks
In this study we generated transgenic reporter sporozoites that can be visualized and quantified using simple enzymatic assays. Within the invertebrate vector, sporozoite loads can be quantified using the β-D-galactopyranoside analog CPRG. Upon transmission to the vertebrate host sporozoite numbers drop dramatically. Therefore, detection and quantification of pre-erythrocytic stages are difficult, particularly when adapted to high throughput approaches. By using two sensitive alternative methods, the substrate MUG and a β-galactosidase ELISA, we could demonstrate reliable quantification of rare parasite stages. Quantification of sporozoite inocula and early liver stages are important for many aspects of vector, host, and Plasmodium biology. Due to the simple read-out, the PbBlue sporozoite is a versatile tool for large-scale processes, such as selection of refractory and high-transmission mosquito lines and screening of transmission to immunized animals. A long-term goal of many laboratories worldwide is to establish simple screens for inhibitors of Plasmodium sporozoite transmission, locomotion, liver invasion and initiation of liver stage development. Towards these aims, quantitative assays, such as the ones described in this study, avoid the need for microscopy-based screens. Additional applications for the PbBlue sporozoite, currently under investigation in our laboratories, include, but are not limited to, quantification of sporozoite clearance from the peripheral blood, detection of transient sporozoite locations in host organs, secretion assays for sporozoite-specific micronemal proteins, and determination of promoter activities.
Acknowledgments
This work was supported by a National Institutes of Health grant RO1 AI56840-01 (P.S.) and a grant from the Deutsche Forschungsgemeinschaft (CRC544, #B10) (K.M.).
Abbreviations
- β-Gal
β-galactosidase
- CPRG
chlorophenol red-β-D-galactopyranoside
- CSP
circumsporozoite protein
- GFP
green fluorescent protein
- MUG
4-methyl-umbelliferyl-β-D-galactopyranoside
- X-Gal
5-bromo-4-chloro-3-indoyl-β-D-galactopyranoside
Contributor Information
Photini Sinnis, Email: Photini.Sinnis@med.nyu.edu.
Kai Matuschewski, Email: Matuschewski@med.uni-heidelberg.de.
References
- 1.Kappe SHI, Kaiser K, Matuschewski K. The Plasmodium sporozoite journey: a rite of passage. Trends Parasitol. 2003;19:135–43. doi: 10.1016/s1471-4922(03)00007-2. [DOI] [PubMed] [Google Scholar]
- 2.Mota MM, Giordano S, Rodriguez A. Targeting Plasmodium host cells: survival within hepatocytes. Trends Mol Med. 2004;10:487–92. doi: 10.1016/j.molmed.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Sinden RE. Molecular interactions between Plasmodium and its insect vectors. Cell Microbiol. 2002;4:713–24. doi: 10.1046/j.1462-5822.2002.00229.x. [DOI] [PubMed] [Google Scholar]
- 4.Barreau C, Touray M, Pimenta PF, Miller LH, Vernick KD. Plasmodium gallinaceum: sporozoite invasion of Aedes aegyptii salivary glands is inhibited by anti-gland antibodies and by lectins. Exp Parasitol. 1995;81:332–43. doi: 10.1006/expr.1995.1124. [DOI] [PubMed] [Google Scholar]
- 5.Sidjanski SP, Vanderberg J, Sinnis P. Anopheles stephensi salivary glands bear receptors for region I of the circumsporozoite protein of Plasmodium falciparum. Mol Biochem Parasitol. 1997;90:33–41. doi: 10.1016/s0166-6851(97)00124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan JDG, Kent M, Dhar R, Fujioka H, Kumar N. Anopheles gambiae salivary gland proteins as putative targets for blocking transmission of malaria parasites. Proc Natl Acad Sci USA. 2000;97:13859–64. doi: 10.1073/pnas.250472597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mo Myung J, Marshall P, Sinnis P. The Plasmodium circumsporozoite protein is involved in mosquito salivary gland invasion by sporozoites. Mol Biochem Parasitol. 2004;133:53–9. doi: 10.1016/j.molbiopara.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Pimenta PF, Touray M, Miller L. The journey of malaria sporozoites in the mosquito salivary gland. J Eukaryot Microbiol. 1994;41:608–24. doi: 10.1111/j.1550-7408.1994.tb01523.x. [DOI] [PubMed] [Google Scholar]
- 9.Vanderberg JP, Frevert U. Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int J Parasitol. 2004;34:991–6. doi: 10.1016/j.ijpara.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Medica DL, Sinnis P. Quantitative dynamics of Plasmodium yoelii sporozoite transmission by infected anopheline mosquitoes. Infect Immun. 2005;73:4363–569. doi: 10.1128/IAI.73.7.4363-4369.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frischknecht F, Baldacci P, Martin B, et al. Imaging movement of malaria parasites during transmission by Anopheles mosquitoes. Cell Microbiol. 2004;6:687–94. doi: 10.1111/j.1462-5822.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- 12.Amino R, Ménard R, Frischknecht F. In vivo imaging of malaria parasites-recent advances and future directions. Curr Opin Microbiol. 2005;8:407–14. doi: 10.1016/j.mib.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 13.Sinnis P, Nardin E. Sporozoite antigens: biology and immunology of the circumsporozoite protein and thrombospondin-related anonymous protein. In: Perlmann P, Troye-Blomberg M, editors. Malaria immunology. Basel Switzerland: S. Karger Press; 2002. pp. 70–96. [DOI] [PubMed] [Google Scholar]
- 14.Natarajan R, Thathy V, Mota MM, Hafalla JCR, Menard R, Vernick KD. Fluorescent Plasmodium berghei sporozoites and pre-erythrocytic stages: a new tool to study mosquito and mammalian host interactions with malaria parasites. Cell Microbiol. 2001;3:371–9. doi: 10.1046/j.1462-5822.2001.00117.x. [DOI] [PubMed] [Google Scholar]
- 15.Franke-Fayard B, Trueman H, Ramesar J, et al. A Plasmodium berghei reference line that constitutively expresses GFP at high levels throughout the complete life cycle. Mol Biochem Parasitol. 2004;137:23–33. doi: 10.1016/j.molbiopara.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Frevert U, Engelmann S, Zoubede S, et al. Intravital observation of Plasmodium berghei sporozoite infection of the liver. PLoS Biol. 2005;3:e192. doi: 10.1371/journal.pbio.0030192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seeber F, Boothroyd JC. Escherichia coli β-galactosidase as an in vitro and in vivo reporter enzyme and stable transfection marker in the intra-cellular protozoan parasite Toxoplasma gondii. Gene. 1996;169:39–45. doi: 10.1016/0378-1119(95)00786-5. [DOI] [PubMed] [Google Scholar]
- 18.McFadden DC, Seeber F, Boothroyd JC. Use of Toxoplasma gondii expressing β-galactosidase for colorimetric assessment of drug activity in vitro. Antimicrob Agents Chemother. 1997;41:1849–53. doi: 10.1128/aac.41.9.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dao A, Soete M, Sergent V, Deslee D, Fortier B, Dubremetz JF. Potential of β-galactosidase-expressing Toxoplasma gondii for in situ localization and observation of rare stages of the parasite life cycle. Parasitol Res. 2002;88:69–72. doi: 10.1007/s004360100499. [DOI] [PubMed] [Google Scholar]
- 20.Carruthers VB, Sibley LD. Mobilization of intracellular calcium stimulates microneme discharge in Toxoplasma gondii. Mol Microbiol. 1999;31:421–8. doi: 10.1046/j.1365-2958.1999.01174.x. [DOI] [PubMed] [Google Scholar]
- 21.Stewart MJ, Vanderberg JP. Malaria sporozoites leave behind trails of circumsporozoite protein during gliding motility. J Protozool. 1988;35:389–93. doi: 10.1111/j.1550-7408.1988.tb04115.x. [DOI] [PubMed] [Google Scholar]
- 22.Thathy V, Fujioka H, Gantt S, Nussenzweig R, Nussenzweig V, Ménard R. Levels of circumsporozoite protein in the Plasmodium oocyst determine sporozoite morphology. EMBO J. 2002;21:1586–96. doi: 10.1093/emboj/21.7.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thathy V, Ménard R. Gene targeting in Plasmodium berghei. Methods Mol Med. 2002;72:317–31. doi: 10.1385/1-59259-271-6:317. [DOI] [PubMed] [Google Scholar]
- 24.Potocnjak P, Yoshida N, Nussenzweig RS, Nussenzweig V. Monovalent fragments (Fab) of monoclonal antibodies to a sporozoite surface antigen (Pb44) protect mice against malarial infection. J Exp Med. 1980;151:1504–13. doi: 10.1084/jem.151.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young DC, Kingsley SD, Ryan KA, Dutko FJ. Selective inactivation of eukaryotic β-galactosidase in assays for inhibitors of HIV-1 TAT using bacterial β-galactosidase as a reporter enzyme. Anal Biochem. 1993;215:24–30. doi: 10.1006/abio.1993.1549. [DOI] [PubMed] [Google Scholar]
- 26.Matuschewski K, Ross J, Brown SM, Kaiser K, Nussenzweig V, Kappe SHI. Infectivity-associated changes in the transcriptional repertoire of the malaria parasite sporozoite stage. J Biol Chem. 2002;277:41948–53. doi: 10.1074/jbc.M207315200. [DOI] [PubMed] [Google Scholar]
- 27.Bachmair A, Finley D, Varshavsky A. In vivo half-life of a protein is a function of its amino-terminal residue. Science. 1986;234:179–86. doi: 10.1126/science.3018930. [DOI] [PubMed] [Google Scholar]
- 28.Wang Q, Brown S, Roos DS, Nussenzweig V, Bhanot P. Transcriptome of axenic liver stages of Plasmodium yoelii. Mol Biochem Parasitol. 2004;137:161–8. doi: 10.1016/j.molbiopara.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Beier JC, Vaughan JA, Madani A, Noden BH. Plasmodium falciparum: release of circumsporozoite protein by sporozoites in the mosquito vector. Exp Parasitol. 1992;75:248–56. doi: 10.1016/0014-4894(92)90185-d. [DOI] [PubMed] [Google Scholar]


