Abstract
Mycoplasma alligatoris causes acute lethal cardiopulmonary disease of susceptible hosts. A survey of its genome implicated sialidase and hyaluronidase, synergistic regulators of hyaluronan receptor CD44-mediated signal transduction leading to apoptotic cell death, as virulence factors of M. alligatoris. In this study, after the existence of a CD44 homolog in alligators was established by immunolabeling primary pulmonary fibroblasts with monoclonal antibody IM7 against murine CD44, the sialidase inhibitor 2,3-didehydro-2-deoxy-N-acetylneuraminic acid (DANA) was used to examine the effects of sialidase on fibroblast apoptosis following in vitro infection with M. alligatoris. While their CD44 expression remained constant, infected cells exhibited morphologic changes characteristic of apoptosis including decreased size, rounding, disordered a-tubulin, and nuclear disintegration compared to untreated controls. DANA was a potent, non-toxic inhibitor of the sialidase activity, equivalent to about 1 mU of Clostridium perfringens Type VI sialidase, expressed by M. alligatoris in the inoculum. Although DANA did not measurably reduce the proportion of infected fibroblasts labeled by a specific ligand of activated caspases, co-incubation with DANA protected (P < 0.01) fibroblasts in a concentration-dependent fashion from the M. alligatoris-induced trends toward increased apoptosis receptor CD95 expression, and increased 5-bromo-2′-deoxyuridine incorporation measured in a terminal dUTP nick end-labeling apoptosis assay. In contrast, incubation with 200-fold excess purified C. perfringens sialidase alone did not affect CD95 expression or chromatin integrity, or induce fibroblast apoptosis. From those observations we conclude that interaction of its sialidase with hyaluronidase or another virulence factor(s) is necessary to elicit the pro-apoptotic effects of M. alligatoris infection.
Keywords: 2,3-didehydro-2-deoxy-N-acetylneuraminic acid; apoptosis; caspases; Fas receptor CD95; hyaluronan receptor CD44; sialidase
1. Introduction
Respiratory mycoplasmosis is often subclinical or chronic, but Mycoplasma alligatoris causes acute lethal cardiopulmonary disease of susceptible hosts (Brown et al., 2001a). M. alligatoris can be cultured in high numbers from the lower respiratory tract of infected individuals, where pathologic changes include fibrinous pleuritis, pulmonary edema and congestion, and diffuse interstitial to fibronecrotic pneumonia as early as 1 wk after exposure (Brown et al., 2001b; Brown et al., 2001c; Pye et al., 2001). A genome survey revealed that M. alligatoris strain A21JP2T notably possesses the “spreading factors” sialidase and hyaluronidase, a combination unprecedented among mycoplasmas but common among other invasive pathogens, which could degrade host extracellular matrix (ECM) glycans during nutrient scavenging to contribute to disease (Brown et al., 2004). Its comparatively attenuated sibling species Mycoplasma crocodyli also expresses hyaluronidase, so mechanical ECM damage alone seems insufficient to explain the particular severity of M. alligatoris infection. For example, intrapleural inoculation with a dose of M. crocodyli strain MP145T which was 3 log10 greater than the LD50 of M. alligatoris strain A21JP2T caused only mild respiratory disease (Mohan et al., 2001; Mohan et al., 1995). However, M. crocodyli strain MP145T does not possess sialidase (Brown et al., 2004).
In other diseases, the host hyaluronan (HA) receptor CD44 is uniquely modulated by a specific synergy of sialidase and hyaluronidase (Lesley et al., 1997). Sialidase can unmask the CD44 molecules which are present on the surface of most host cells, but prohibited from binding HA by sialylation (Bartolazzi et al., 1996; Gee et al., 2004; Katoh et al., 1995). Following CD44 desialylation, binding of low molecular weight HA fragments from ECM degraded by hyaluronidase (Gee et al., 2004; Lesley et al., 1997) initiates intracellular signaling by CD44 through networks leading to production of pro-inflammatory cytokines (Sague et al., 2004), synthesis of cytotoxic nitric oxide (Iacob and Knudson, 2005), and inappropriate apoptosis involving the “fibroblast-associated” (Fas) receptor CD95-mediated signal transduction pathway (Fujii et al., 2001; Menaker and Jones, 2003). Those observations formed our hypothesis that synergy of bacterial sialidase and hyaluronidase co-localized directly at the host-pathogen interface during infection might potentiate CD44-transduced signals leading to inflammation and host cell death in some niches, consistent with the fulminant necrotizing disease caused by M. alligatoris. In this report we describe the first direct evidence of a CD44 homolog in the class Reptilia, and an in vitro model to establish the role of sialidase in M. alligatoris-induced pulmonary fibroblast apoptosis.
2. Materials and methods
2.1 CD44 and CD95
Primary pulmonary fibroblast cultures were established from distal lung parenchyma obtained at necropsy of two alligators that were culture-, PCR-, and seronegative for M. alligatoris. The cells were cultured as described (Hunt and Brown, 2005) and passaged without attempts to transform or immortalize them. The cells fully maintain their differentiated state when cultured in vitro (Bayreuther et al., 1991; Bradley et al., 1980). CD44 expression was detected with a FITC-conjugated IgG2b (ab19622 [IM7]; Abcam, Cambridge MA) monoclonal antibody (mAb) against a conserved epitope occurring between residues 115 and 127 of mouse CD44 (Mikecz et al., 1995; Tanaka et al., 2002). IM7 recognizes the most immunogenic epitope and all isoforms (Mackay et al., 1994) of CD44. IgG1 (AHS4411 [SFF-2]; BioSource International, Camarillo CA) and IgG2a (ab6124 [F10-44-2]; Abcam) mAbs against the standard isoform of human CD44, and monoclonal (HB-258 [FW11-24-17-36]; American Type Culture Collection) and polyclonal IgG1 (AHS4441; BioSource International) antibodies which recognize epitopes encoded by exons v3-v10 of human variant CD44 isoforms, were also tested. CD95 expression was detected by fluorescence imaging and flow cytometry as described (Hunt and Brown, 2005), using polyclonal IgG antibodies which recognize the N-terminus of CD95 (ab13550; Abcam). Primary antibodies were detected using Alexa Fluor 488- or TRITC-conjugated secondary antibodies (Molecular Probes, Eugene OR). An IgG2a mAb against a synthetic peptide mapping at residues 196–220 of the CD95-agonistic ligand FasL (CD178) of mouse origin (ab16104 [H11]; Abcam), predicted to crossreact with CD178 of multiple species, was also tested by fluorescence imaging and Western blot.
2.2 Sialidase and sialidase inhibitor assays
M. alligatoris strain A21JP2T was cultured as described (Hunt and Brown, 2005). Sialidase activity was assayed quantitatively in 96-well plates (FluoroNunc 437112; Nalge Nunc International, Rochester NY) using the fluorogenic substrate 2′-(4-methylumbelliferyl)-α-D-N-acetylneuraminic acid (MUAN; Sigma-Aldrich, St. Louis MO) and a spectrofluorometer (SpectraMax M5 with SoftMax Pro 5 software; Molecular Devices, Sunnyvale CA). The negative controls were M. crocodyli strain MP145T and fresh culture medium. The positive control was Type VI Clostridium perfringens sialidase (Sigma-Aldrich). The intensity of cyan fluorescence at 450 nm, excited at 365 nm with a cutoff filter at 420 nm, was proportional to sialidase activity (Ying and Gervay-Hague, 2005). To rule out potential antibiotic effects of the sialidase inhibitor 2,3-didehydro-2-deoxy-N-acetylneuraminic acid (DANA; Sigma-Aldrich), M. alligatoris was cultured in broth supplemented with 500 μg/ml (1.6 mM) DANA (Usuki et al., 1988). The rate of acidification of the broth was compared to untreated cultures. For perspective, the IC50 of DANA for 1 U of C. perfringens sialidase is 1.25 μg/ml (4 μM). To quantitate sialidase inhibition, 104 to 108 CFU of M. alligatoris were incubated with 0.3 to 3 mg/ml DANA and 40 μg/ml MUAN, then measured by spectrofluorometry. The positive controls were 0.05 to 5 mU of C. perfringens sialidase.
2.3 Infection studies
Subconfluent (50–70%) fibroblasts were inoculated with approximately 109 CFU of M. alligatoris (initial multiplicity of infection approximately 104) and incubated as described (Hunt and Brown, 2005). To test the effect of sialidase inhibition, infected cultures were co-incubated with 50 or 500 μg/ml DANA. Mycoplasmal viability was confirmed by culture on agar at the end of the incubation period, and the identity of the mycoplasmas recovered was confirmed by 16S rDNA PCR-RFLP. Uninfected control cultures were incubated with 100 mU/ml C. perfringens sialidase (Katoh et al., 1995; Lesley et al., 1997), or with DANA, then analyzed by fluorescence imaging and flow cytometry as described below.
2.4 Caspases and apoptosis
During controlled apoptosis, oligomerized CD95 activates an intracellular cysteine-aspartic acid protease (caspase) cascade leading to appropriate cell death. Caspase expression by alligator fibroblasts was detected by Western blot and immunofluorescence imaging using a panel of polyclonal antibodies which recognize the active site of mammalian initiator caspase 9 and effector caspases 3 and 7 (9502, 9662, and 9492, respectively; Cell Signaling Technology, Danvers MA). Those antibodies detect both the respective full-length procaspase zymogens, and the large fragment of each that remains after activation through proteolysis. A separate panel of antibodies which exclusively recognize the small cleavage fragments generated by activation through proteolysis, but not the caspase 3, 7, or 9 zymogens (9661, 9491, and 9501, respectively; Cell Signaling Technology), was tested similarly. Activated caspases were detected in fixed cells by fluorescence imaging and a species-independent fluorochrome inhibitor of caspases (FLICA) assay employing a carboxyfluorescein-labeled valylalanylaspartic acid fluoromethylketone oligopeptide ligand (Immunochemistry Technologies, Bloomington MN). The fluorescence intensity, measured by flow cytometry, was proportional to the total number of all activated caspase 1, 3, 4, 5, 6, 7, 8, and 9 molecules. Apoptosis was detected by immunofluorescence staining and a species-independent terminal deoxynucleotide transferase dUTP nick end-labeling (TUNEL) assay (APO-BrdU TUNEL Assay Kit; Molecular Probes) as described (Hunt and Brown, 2005). Apoptosis was induced in positive controls by activation with an agonistic IgM mAb against the extracellular domain of CD95 (CH11; Upstate, Lake Placid NY) as described for alligator cardiac fibroblasts (Hunt and Brown, 2005). Negative controls were untreated or sham induced either with an irrelevant IgM mAb (sc-7293; Santa Cruz Biotechnology, Santa Cruz CA) as an isotype control for CH11, or with an irrelevant IgG1 mAb. Antibodies to uncleaved and cleaved mammalian poly(ADP-ribose) polymerase (9541 and 9542, respectively; Cell Signaling Technology), an enzyme inhibited by activated caspase 7 to prevent DNA damage repair in nuclei during controlled apoptosis, were also tested by fluorescence imaging and Western blot.
2.5 Fluorescence imaging and flow cytometry
Immunolabeled fibroblasts were imaged as described (Hunt and Brown, 2005). To quantitate receptor expression, fibroblast monolayers were incubated with IM7 or ab13550, followed by secondary antibody, then analyzed by flow cytometry. The cytometer gate was set to exclude approximately 99.5% of untreated secondary antibody control fibroblasts processed similarly. For FLICA cytometry, fibroblast populations were displayed as histograms of cell number versus log10 fluorescence intensity. The cytometer gate was set to exclude the major subpopulation of uninfected cells. Induction of caspase activity by infection created a shoulder or a second peak having increased fluorescence intensity. A minimum of 104 cells was counted for each sample. The effect of treatment on the proportion of gated cells was analyzed by using the Kruskal-Wallis nonparametric equivalent of a one-way analysis of variance by ranks, and by Fisher’s Protected Least Significant Difference test for posthoc pairwise comparisons of ranked means when the effect of treatment was significant (Hunt and Brown, 2005). A P value < 0.05 was considered significant.
3. Results
Sialidase and hyaluronidase, potential synergistic promotors of HA receptor CD44-mediated eukaryotic cell death, were implicated by comparative genomics as virulence factors of M. alligatoris. This study first confirmed the existence of a CD44 homolog in alligators, then examined the effects of in vitro infection with M. alligatoris on expression of CD44, CD95, and downstream elements of the Fas apoptosis signal transduction pathway of pulmonary fibroblasts. Those are the major cell type of a target organ of M. alligatoris infection in vivo. Treatments with purified sialidase and the sialidase inhibitor DANA were used to explore the role of sialidase in M. alligatoris-induced fibroblast apoptosis.
3.1 Mycoplasma alligatoris infection promotes CD95 expression and apoptosis of pulmonary fibroblasts
Untreated pulmonary fibroblasts had an approximate doubling time of 6 to 10 d. The cells could be subcultivated at a ratio of 1:2 for up to 10 passages. A uniform distribution of CD44 on fixed fibroblasts was demonstrated by immunolabeling with mAb IM7 against murine CD44 (Fig. 1a). The mAb IM7 is not recommended by the supplier for application in Western blot, and no specific binding to an antigen corresponding to the predicted molecular mass of CD44 could be detected by probing Western blots of alligator or control mouse embryo 3T3 fibroblast lysates with IM7. The mAbs SFF-2 and F10-44-2 against the standard isoform of human CD44, and mAb FW11-24-17-36 and polyclonal antibodies against human variant CD44 isoforms, did not bind to alligator fibroblasts, helping to rule out non-specific binding of mAb IM7. CD95 expression on fixed fibroblasts was demonstrated using polyclonal antibody ab13550 against murine CD95 (Fig. 1b). Fas ligand CD178 could be detected by fluorescence imaging, but not by Western blot, using mAb H11 against murine CD178 (Fig. 1c).
Fig. 1.
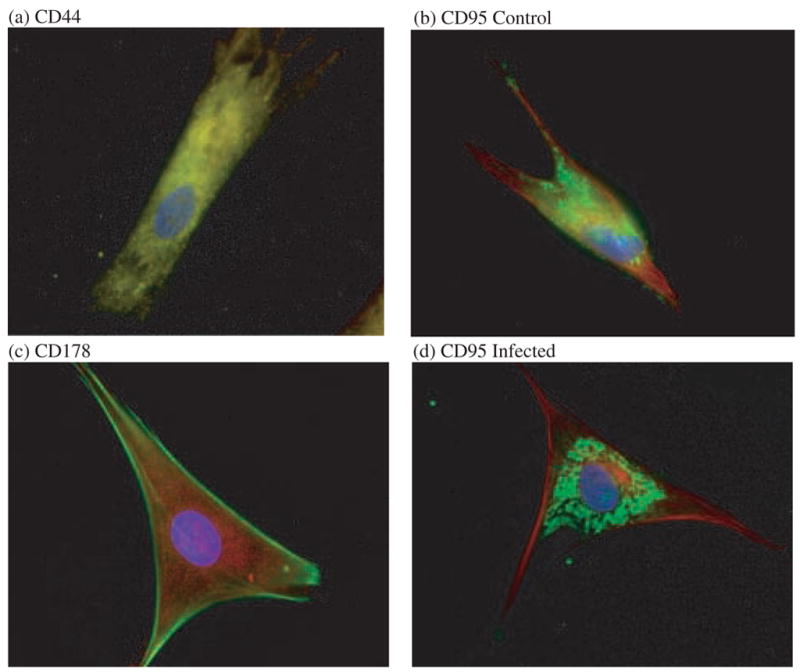
Receptor and ligand expression by primary alligator pulmonary fibroblasts. Nuclei were stained with 4′,6′-diamidino-2-phenylindole hydrochloride (blue) and cytoskeletal proteins were immunolabeled with complementary fluorophores. (a) Hyaluronan receptor CD44 (yellow double-tagged with fluorescein and tetramethylrhodamine) immunolabeled with mAb IM7; (b) Fas receptor CD95 (green) immunolabeled with polyclonal IgG ab13550, and a-tubulin (red) immunolabeled with mAb DM1A (Sigma-Aldrich); (c) Fas ligand CD178 (red) immunolabeled with mAb H11, and β-actin (green) immunolabeled with mAb 13E5 (Cell Signaling Technology); (d) CD95 (green) upregulation and redistribution following incubation with Mycoplasma alligatoris.
M. alligatoris readily persisted in co-culture with the fibroblasts. No increase in surface CD44 expression was detectable by flow cytometry after 24-hr infection with M. alligatoris or incubation with CD95-agonistic mAb CH11. Compared to uninfected controls, the proportion of CD95-gated fibroblasts increased approximately twofold after infection, and the proportion of BrdU-gated fibroblasts increased approximately fivefold, although the effects did not reach statistical significance (Fig. 2). As we observed previously for alligator cardiac, skeletal muscle, and embryonic fibroblasts, the window of opportunity for measurement was narrow, accounting for some of the variability observed, because often far fewer than the necessary minimum of 104 fibroblasts remained beyond 24-hr incubation with M. alligatoris. Induction by incubation with mAb CH11 tended to have similar effects on the proportion of CD95- and BrdU-gated cells, but the increase from untreated controls was proportionately less than previously observed for M. alligatoris-infected cardiac fibroblasts. Sham induction with irrelevant mAbs did not influence CD95 expression or apoptosis, helping to rule out an increase in non-specific IgG binding by M. alligatoris-infected or IgM-induced fibroblasts when measured using ab13550 as the primary antibody in flow cytometry. In addition, a-tubulin immunolabeling was stringently specific for the cytoskeleton, and there was no non-specific binding of seven different secondary antibodies used for fluorescence imaging.
Fig. 2.
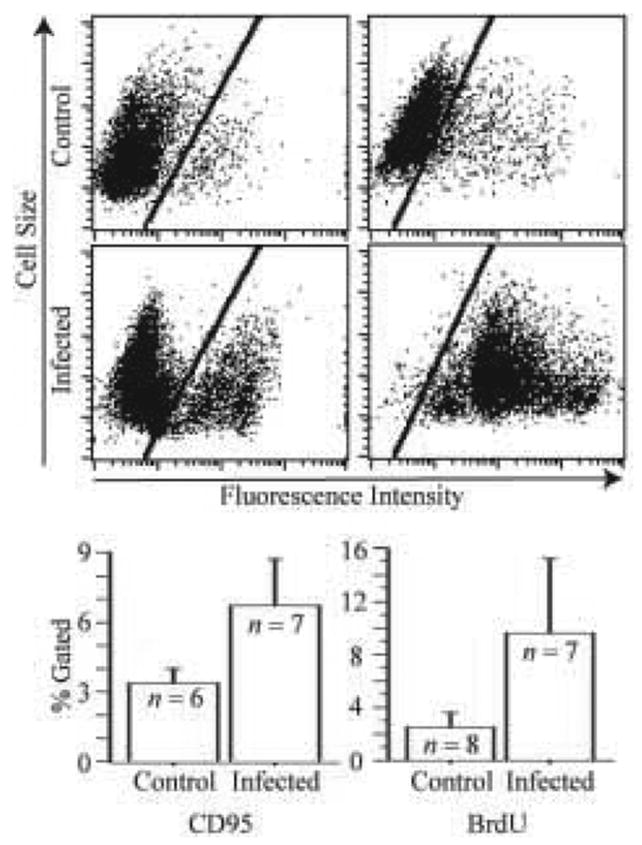
Mycoplasma alligatoris infection promotes surface CD95 expression and apoptosis of primary pulmonary fibroblasts. Subconfluent fibroblasts were infected 24 hr with 109 CFU M. alligatoris, then CD95 expression was measured with primary antibody ab13550, and 5-bromo-2′deoxyuridine (BrdU) DNA nick end-labeling was measured with primary antibody PRB-1 (Molecular Probes), by flow cytometry. Control fibroblasts were incubated with medium only. Gate = 0.5% of secondary antibody controls. n = number of replicate wells tested. Bars = 1 standard error.
Morphologic changes characteristic of apoptosis, including decreased cell size, rounding, disordered a-tubulin, and nuclear disintegration were apparent in the M. alligatoris-infected cells. Less severe changes were evident in positive controls activated by the CD95-agonistic mAb CH11, which was consistent with the proportions of CD95- and BrdU-gated positive control lung cells and previous results with CH11-induced cardiac cells (Hunt and Brown, 2005). Caspase 3, 7, and 9 expression was detected by Western blot and fluorescence imaging with antibodies against the full-length zymogens (Fig. 3). However, even though binding of the ligand of activated caspases was readily visualized in both control and infected fibroblasts, a predicted change in the cumulative fluorescence intensity of infected fibroblasts was not consistently demonstrable by flow cytometry. Antibodies to the small cleavage fragments of activated mammalian caspases and to mammalian poly(ADP-ribose) polymerase did not bind to fixed alligator fibroblasts, and yielded equivocal results in Western blot analyses.
Fig. 3.
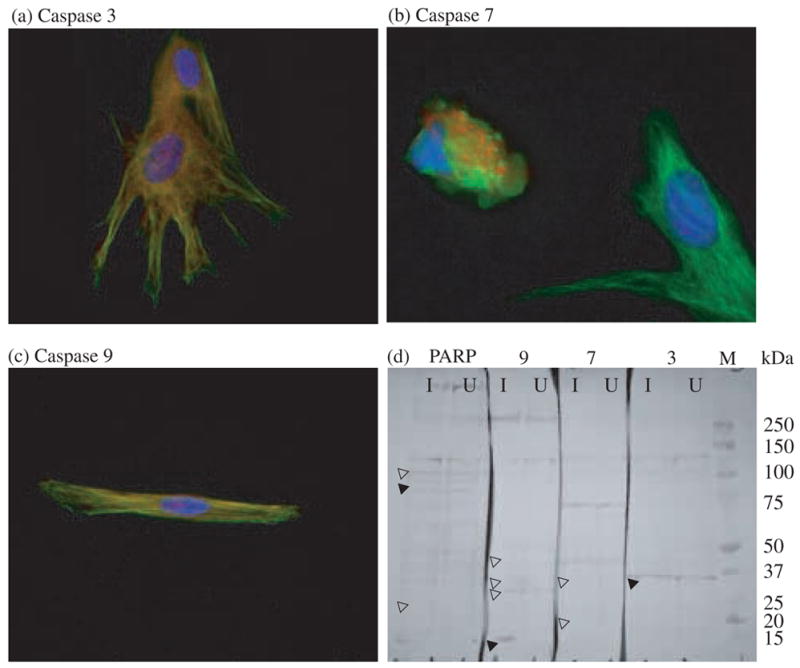
Caspase expression in primary alligator pulmonary fibroblasts. Nuclei were stained with 4′,6′-diamidino-2-phenylindole hydrochloride (blue) and a-tubulin (green) was immunolabeled with mAb DM1A. (a) Caspase 3 (red) immunolabeled with polyclonal antibody 9662; (b) Caspase 7 (red) immunolabeled with polyclonal antibody 9492; (c) Caspase 9 (red) immunolabeled with polyclonal antibody 9502; (d) Western blots of M. alligatoris-infected (I) and control (U) fibroblast lysates probed with primary antibodies against uncleaved poly(ADP-ribose) polymerase (PARP; 9542) and caspases 3, 7, and 9. Alkaline phosphatase-conjugated secondary antibodies were detected with 5-bromo-4-chloro-3-indolyl phosphate p-toluidine salt plus nitroblue tetrazolium. Blots were digitized by using an AlphaImager (Alpha Innotech, San Leandro, CA). Contrast was normalized for better clarity by using the Auto Contrast feature after cropping and alignment with Adobe Photoshop CS 8.0 (Adobe Systems, San Jose, CA). Filled arrowheads indicate bands consistent with the predicted sizes of uncleaved and cleaved mammalian caspases and PARP. Unfilled arrowheads indicate absence of predicted bands.
3.2 DANA inhibits Mycoplasma alligatoris sialidase and protects pulmonary fibroblasts from M. alligatoris-induced apoptosis
The inoculum of 109 CFU of M. alligatoris was estimated by MUAN assay to deliver the equivalent of about 1 mU of purified C. perfringens sialidase, therefore the concentrations of DANA tested in the fibroblast infection studies were 104 to 105 fold in excess of the IC50 for the equivalent amount of C. perfringens sialidase (Ying and Gervay-Hague, 2005). The DANA, a competitive transition state analog, was a potent inhibitor also of M. alligatoris sialidase, although cumulative MUAN degradation could be detected following prolonged incubation of purified C. perfringens sialidase or M. alligatoris with as much as 3 mg/ml DANA. Culture densities and the rates of acidification of broth cultures of M. alligatoris plus 500 μg/ml DANA indicated that DANA was not antibiotic to M. alligatoris and that there were no detrimental consequences associated with inhibited sialidase activity to growth of the mycoplasma. No changes in morphology or the proportion of CD95- or BrdU-gated uninfected fibroblasts were detected following incubation with 500 μg/ml DANA, indicating that the DANA was not toxic to alligator fibroblasts and did not influence CD95 expression or apoptosis. Further, co-incubation with up to 500 μg/ml DANA did not affect binding of the oligopeptide ligand of activated caspases by control or infected fibroblasts.
Most importantly, co-incubation with DANA protected (P < 0.01) infected fibroblasts from M. alligatoris-induced increases in surface CD95 expression and BrdU incorporation in a concentration-dependent fashion (Fig. 4), indications that sialidase is necessary for M. alligatoris to induce apoptosis of pulmonary fibroblasts. In contrast, no morphologic changes or trend toward increases in the proportion of CD95- or BrdU-gated cells were detected following 24-hr incubation with 100 mU/ml purified C. perfringens sialidase, which was about 200 fold more than the activity present in the M. alligatoris inoculum, indicating that sialidase alone was not sufficient to induce apoptosis of pulmonary fibroblasts.
Fig. 4.
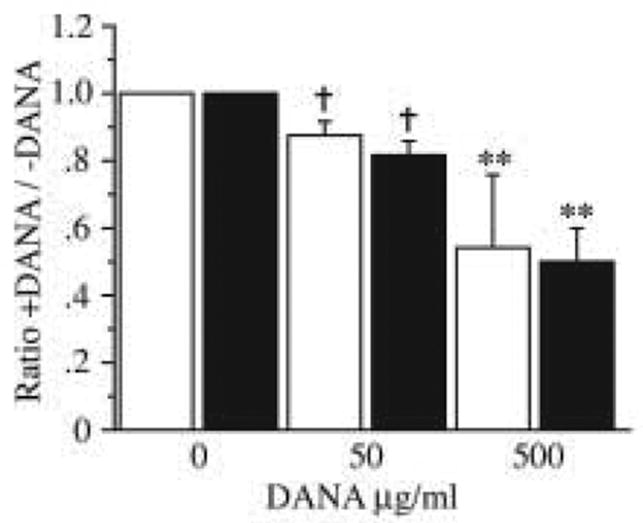
DANA protects pulmonary fibroblasts from Mycoplasma alligatoris-induced increases in surface CD95 expression and apoptosis in a dose-dependent fashion. Subconfluent fibroblasts were infected 24 hr with 109 CFU M. alligatoris, then CD95 expression (open bars, n = 2 replicates) was measured with primary antibody ab13550, and 5-bromo-2′deoxyuridine (BrdU) DNA nick end-labeling (filled bars, n = 3 replicates) was measured with primary antibody PRB-1, by flow cytometry. Error bars = 1 standard error, † = P< 0.10, ** = P< 0.01 by Fisher’s Protected Least Significant Difference test.
4. Discussion and conclusion
The lung is a target organ of M. alligatoris in vivo, and acute interstitial or fibronecrotic pneumonia is the likely proximate cause of death of individuals that succumb to M. alligatoris infection (Brown et al., 2001b; Pye et al., 2001). Alveolar fibroblasts are the major cell type comprising the gas-exchanging structures of the lung. They are developmentally and functionally distinct from fibroblasts in other organs and tissues (Bradley et al., 1980; Brown et al., 2005). Because the fibroblasts used in the present study were derived from distal lung parenchyma, which is dominated by alveoli rather than arteries, veins, or the bronchial tree, they were probably of alveolar origin (Bradley et al., 1980). During infection and other lung injury, alveolar fibroblasts normally defend the lung by secreting upstream elements of the classical complement activation pathway, ingesting foreign particles by pinocytosis, and secreting prostaglandins and other factors to promote inflammation and phagocyte recruitment (Bradley et al., 1980). Apoptosis is involved in the pathogenesis of several forms of acute lung injury, during which CD95 is upregulated in alveolar cells while its agonist CD178 is upregulated in infiltrating inflammatory cells (Dockrell, 2003), leading to excessive alveolar cell death (Behnia et al., 2000). Loss of fibroblasts exacerbates disease by preventing alveolar repair and clearance of fibrin debris dependent on plasminogen activator and growth factors produced by the fibroblasts as part of normal tissue healing (Behnia et al., 2000). Inappropriate apoptosis of alveolar fibroblasts is thus a significant mechanism of lung impairment.
Pro-apoptotic effects on various cell types have been attributed to DNA damage by mycoplasmal endonucleases or CD95-independent caspase activation by membrane lipoproteins of cytopathic mycoplasmas, but M. alligatoris was the first mycoplasma shown to have direct effects on an upstream component of the CD95 signal transduction pathway (Hunt and Brown, 2005). The present work confirms that observation using another type of differentiated cell, and extends it with the first evidence of CD178 and caspase 3, 7, and 9 homologs in the class Reptilia, further proof that apoptosis signaling pathways are evolutionarily conserved across vertebrate species. Although the observed CD95 upregulation could not be correlated with polycaspase activation by FLICA assay, only subtle shifts in the balances especially of activated initiator caspases may be required to promote the increased DNA fragmentation, nuclear disintegration, and other distinctive morphological changes characteristic of apoptosis consistently observed following M. alligatoris infection.
The ability of the sialidase inhibitor DANA to protect against M. alligatoris-induced morphological changes and increases in fibroblast CD95 expression and BrdU incorporation indicates an essential role for the mycoplasmal sialidase in induction of apoptosis. However, incubation with a high concentration of purified C. perfringens sialidase alone was not sufficient to induce apoptosis. Interaction of sialidase with another virulence factor(s) seems necessary to cause the pro-apoptotic effects of M. alligatoris infection. A prominent candidate that emerged from our M. alligatoris genome survey is hyaluronidase (Brown et al., 2004), because in other diseases the host cell HA receptor CD44 is modulated by the specific combination of sialidase and hyaluronidase to transduce signals leading to inflammation, CD95 upregulation, and excessive cell death (Bartolazzi et al., 1996; Fujii et al., 2001; Gee et al., 2004; Hauptschein et al., 2005; Katoh et al., 1995; Lesley et al., 1997; Pure and Cuff, 2001). Although there is no consensus on the molecular mechanisms by which CD44 is linked to apoptosis (Hauptschein et al., 2005), HA fragments generated from the ECM by hyaluronidases stimulate HER2 and c-Src tyrosine kinase phosphorylation of cytoskeletal proteins, and Rho GTPase signaling, through desialylated CD44 receptor-specific networks (Ponta et al., 2003; Turley et al., 2002). As we observed in the present study, expression of CD44 itself may not change following ligand binding, depending on the cell type or basal level of CD44 expression (Ohno-Nakahara et al., 2004), but the observed increase in CD95 expression is consistent with transcription factor upregulation following HA fragment binding by desialylated CD44 (Fieber et al., 2004; Ohno et al., 2005). CD44 genes are annotated in fish, amphibian, and avian genomes, but our data establish for the first time that reptile cells also express a CD44 homolog, and further suggest that CD44-specific tyrosine kinase signaling networks are also conserved across vertebrate species.
CD44 expression is ubiquitous among mammalian cells, but CD44 receptors exist as alternatively-spliced isoforms and may be alternatively glycosylated in various differentiated cell types, a possible clue to one reason why certain cell types or organs are more severely damaged by in vivo infection with M. alligatoris (Mackay et al., 1994; Ponta et al., 2003). For example, the target tissues of M. alligatoris infection may be determined in part by substrate specificity of its sialidase, possibly influenced by both the isoform of the CD44 aglycone and the types of glycosidic linkages present (Corfield, 1992). Although potential substrate specificity of the M. alligatoris sialidase remains uncharacterized, most bacterial sialidases preferentially cleave α(2–3)-linked sialic acids. The M. alligatoris sialidase is a homolog of the large exo-α-sialidase (GenBank protein NP_561641) secreted by C. perfringens, which cleaves α(2–3)-, α(2–6)-, and α(2–8)- glycosidic linkages, but even that sialidase with very broad substrate specificity was not sufficient alone to influence CD95 expression or induce apoptosis of pulmonary fibroblasts.
Our ability to study the interaction of sialidase and hyaluronidase by further in vitro infection and inhibitor experiments is restricted by the lack of potent bacterial hyaluronidase inhibitors. One candidate is 6-O-palmitoyl-L-ascorbic acid (AP) (Tabak et al., 2003), but in a series of similar preliminary experiments co-incubation with 100 μg/ml AP, the highest concentration without cytotoxic effects, failed to protect against M. alligatoris-induced primary fibroblast apoptosis measured as described above (our unpublished data). In addition, even potent competitive inhibitors like DANA probably cannot completely extinguish bacterial surface-associated sialidase activity directly at the intimate host-pathogen interface during infection. Because its small genome includes only single copies of sialidase and hyaluronidase genes (Brown et al., 2004), M. alligatoris should be readily amenable to gene inactivation and complementation approaches to model the effects of bacterial sialidase and hyaluronidase on host CD44 receptor and apoptosis signal transduction pathways potentially modulated by many important pathogens.
Acknowledgments
Tissues were harvested with University of Florida Institutional Animal Care and Use Committee approval. The mtDNA genotyping, spectrofluorometry, and flow cytometry were performed through the Interdisciplinary Center for Biotechnology Research at the University of Florida. This work was supported by Public Health Service grants 1R15HG02389-01A1 from the National Human Genome Research Institute and 1R01GM076584-01A1 from the National Institute of General Medical Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartolazzi A, Nocks A, Aruffo A, Spring F, Stamenkovic I. Glycosylation of CD44 is implicated in CD44-mediated cell adhesion to hyaluronan. J Cell Biol. 1996;132:1199–1208. doi: 10.1083/jcb.132.6.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayreuther K, Francz PI, Gogol J, Hapke C, Maier M, Meinrath HG. Differentiation of primary and secondary fibroblasts in cell culture systems. Mut Res. 1991;256:233–242. doi: 10.1016/0921-8734(91)90014-3. [DOI] [PubMed] [Google Scholar]
- Behnia M, Robertson KA, Martin WJ., 2nd Lung infections: role of apoptosis in host defense and pathogenesis of disease. Chest. 2000;117:1771–1777. doi: 10.1378/chest.117.6.1771. [DOI] [PubMed] [Google Scholar]
- Bradley KH, Kawanami O, Ferrans VJ, Crystal RG. The fibroblast of human lung alveolar structures: a differentiated cell with a major role in lung structure and function. Meth Cell Biol. 1980;21A:37–64. doi: 10.1016/s0091-679x(08)60757-8. [DOI] [PubMed] [Google Scholar]
- Brown DR, Farley JM, Zacher LA, Carlton JMR, Clippinger TL, Tully JG, Brown MB. Mycoplasma alligatoris sp nov, from American alligators. Int J Syst Evol Microbiol. 2001a;51:419–424. doi: 10.1099/00207713-51-2-419. [DOI] [PubMed] [Google Scholar]
- Brown DR, Nogueira MF, Schoeb TR, Vliet KA, Bennett RA, Pye GW, Jacobson ER. Pathology of experimental mycoplasmosis in American alligators. J Wildl Dis. 2001b;37:671–679. doi: 10.7589/0090-3558-37.4.671. [DOI] [PubMed] [Google Scholar]
- Brown DR, Schumacher IM, Nogueira MF, Richey LJ, Zacher LA, Schoeb TR, Vliet KA, Bennett RA, Jacobson ER, Brown MB. Detection of antibodies to a pathogenic mycoplasma in American alligators (Alligator mississippiensis), broad-nosed Caimans (Caiman latirostris), and Siamese crocodiles (Crocodylus siamensis) J Clin Microbiol. 2001c;39:285–292. doi: 10.1128/JCM.39.1.285-292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DR, Zacher LA, Farmerie WG. Spreading factors of Mycoplasma alligatoris, a flesh-eating mycoplasma. J Bacteriol. 2004;186:3922–3927. doi: 10.1128/JB.186.12.3922-3927.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Ann Rev Pharmacol Toxicol. 2005;45:657–687. doi: 10.1146/annurev.pharmtox.45.120403.095802. [DOI] [PubMed] [Google Scholar]
- Corfield T. Bacterial sialidases--roles in pathogenicity and nutrition. Glycobiology. 1992;2:509–521. doi: 10.1093/glycob/2.6.509. [DOI] [PubMed] [Google Scholar]
- Dockrell DH. The multiple roles of Fas ligand in the pathogenesis of infectious diseases. Clin Microbiol Infect. 2003;9:766–779. doi: 10.1046/j.1469-0691.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- Fieber C, Baumann P, Vallon R, Termeer C, Simon JC, Hofmann M, Angel P, Herrlich P, Sleeman JP. Hyaluronan-oligosaccharide-induced transcription of metalloproteases. J Cell Sci. 2004;117:359–367. doi: 10.1242/jcs.00831. [DOI] [PubMed] [Google Scholar]
- Fujii K, Fujii Y, Hubscher S, Tanaka Y. CD44 is the physiological trigger of Fas up-regulation on rheumatoid synovial cells. J Immunol. 2001;167:1198–1203. doi: 10.4049/jimmunol.167.3.1198. [DOI] [PubMed] [Google Scholar]
- Gee K, Kryworuchko M, Kumar A. Recent advances in the regulation of CD44 expression and its role in inflammation and autoimmune diseases. Arch Immunol Ther Exp (Warsz) 2004;52:13–26. [PubMed] [Google Scholar]
- Hauptschein RS, Sloan KE, Torella C, Moezzifard R, Giel-Moloney M, Zehetmeier C, Unger C, Ilag LL, Jay DG. Functional proteomic screen identifies a modulating role for CD44 in death receptor-mediated apoptosis. Cancer Res. 2005;65:1887–1896. doi: 10.1158/0008-5472.CAN-04-3571. [DOI] [PubMed] [Google Scholar]
- Hunt ME, Brown DR. Mycoplasma alligatoris infection promotes CD95 (FasR) expression and apoptosis of primary cardiac fibroblasts. Clin Diagnostic Lab Immunol. 2005;12:1370–1377. doi: 10.1128/CDLI.12.12.1370-1377.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacob S, Knudson CB. Hyaluronan fragments activate nitric oxide synthase and the production of nitric oxide by articular chondrocytes. Int J Biochem Cell Biol. 2005;38:123–133. doi: 10.1016/j.biocel.2005.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh S, Zheng Z, Oritani K, Shimozato T, Kincade PW. Glycosylation of CD44 negatively regulates its recognition of hyaluronan. J Exp Med. 1995;182:419–429. doi: 10.1084/jem.182.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesley J, Hyman R, English N, Catterall JB, Turner GA. CD44 in inflammation and metastasis. Glycoconj J. 1997;14:611–622. doi: 10.1023/a:1018540610858. [DOI] [PubMed] [Google Scholar]
- Mackay CR, Terpe HJ, Stauder R, Marston WL, Stark H, Gunthert U. Expression and modulation of CD44 variant isoforms in humans. J Cell Biol. 1994;124:71–82. doi: 10.1083/jcb.124.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menaker RJ, Jones NL. Fascination with bacteria-triggered cell death: the significance of Fas-mediated apoptosis during bacterial infection in vivo. Microbes Infect. 2003;5:1149–1158. doi: 10.1016/j.micinf.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Mikecz K, Brennan FR, Kim JH, Glant TT. Anti-CD44 treatment abrogates tissue oedema and leukocyte infiltration in murine arthritis. Nat Med. 1995;1:558–563. doi: 10.1038/nm0695-558. [DOI] [PubMed] [Google Scholar]
- Mohan K, Foggin CM, Dziva F, Muvavarirwa P. Vaccination to control an outbreak of Mycoplasma crocodyli infection. Onderstepoort J Vet Res. 2001;68:149–150. [PubMed] [Google Scholar]
- Mohan K, Foggin CM, Muvavarirwa P, Honywill J, Pawandiwa A. Mycoplasma-associated polyarthritis in farmed crocodiles (Crocodylus niloticus) in Zimbabwe. Onderstepoort J Vet Res. 1995;62:45–49. [PubMed] [Google Scholar]
- Ohno S, Im HJ, Knudson CB, Knudson W. Hyaluronan oligosaccharide-induced activation of transcription factors in bovine articular chondrocytes. Arthritis Rheum. 2005;52:800–809. doi: 10.1002/art.20937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Nakahara M, Honda K, Tanimoto K, Tanaka N, Doi T, Suzuki A, Yoneno K, Nakatani Y, Ueki M, Ohno S, Knudson W, Knudson CB, Tanne K. Induction of CD44 and MMP expression by hyaluronidase treatment of articular chondrocytes. J Biochem (Tokyo) 2004;135:567–575. doi: 10.1093/jb/mvh069. [DOI] [PubMed] [Google Scholar]
- Ponta H, Sherman L, Herrlich PA. CD44: From adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- Pure E, Cuff CA. A crucial role for CD44 in inflammation. Trends Mol Med. 2001;7:213–221. doi: 10.1016/s1471-4914(01)01963-3. [DOI] [PubMed] [Google Scholar]
- Pye GW, Brown DR, Nogueira MF, Vliet KA, Schoeb TR, Jacobson ER, Bennett RA. Experimental inoculation of broad-nosed caimans (Caiman latirostris) and Siamese crocodiles (Crocodylus siamensis) with Mycoplasma alligatoris. J Zoo Wildl Med. 2001;32:196–201. doi: 10.1638/1042-7260(2001)032[0196:EIOBNC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Sague SL, Tato C, Pure E, Hunter CA. The regulation and activation of CD44 by natural killer (NK) cells and its role in the production of IFN-gamma. J Interferon Cytokine Res. 2004;24:301–309. doi: 10.1089/107999004323065093. [DOI] [PubMed] [Google Scholar]
- Tabak M, Armon R, Rosenblat G, Stermer E, Neeman I. Diverse effects of ascorbic acid and palmitoyl ascorbate on Helicobacter pylori survival and growth. FEMS Microbiol Lett. 2003;224:247–253. doi: 10.1016/S0378-1097(03)00439-7. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Makiyama Y, Mitsui Y. Anti-CD44 monoclonal antibody (IM7) induces murine systemic shock mediated by platelet activating factor. J Autoimmun. 2002;18:9–15. doi: 10.1006/jaut.2001.0559. [DOI] [PubMed] [Google Scholar]
- Turley EA, Noble PW, Bourguignon LY. Signaling properties of hyaluronan receptors. J Biol Chem. 2002;277:4589–4592. doi: 10.1074/jbc.R100038200. [DOI] [PubMed] [Google Scholar]
- Usuki S, Hoops P, Sweeley CC. Growth control of human foreskin fibroblasts and inhibition of extracellular sialidase activity by 2-deoxy-2,3-dehydro-N-acetylneuraminic acid. J Biol Chem. 1988;263:10595–10599. [PubMed] [Google Scholar]
- Ying L, Gervay-Hague J. One-bead-one-inhibitor-one-substrate screening of neuraminidase activity. Chembiochem. 2005;6:1857–1865. doi: 10.1002/cbic.200500006. [DOI] [PubMed] [Google Scholar]


