Abstract
During development of the visual system, retinal ganglion cells (RGCs) require cell-cell adhesion molecules and extracellular matrix proteins for axon growth. In this study, we demonstrate that the classical cadherin, E-cadherin, is expressed in RGCs from E6 to E12 and promotes neurite outgrowth from all regions of the chick retina at E6, E8 and E10. E-cadherin is also expressed in the optic tectum. E-cadherin adhesion blocking antibodies specifically inhibit neurite outgrowth on an E-cadherin substrate. The receptor-type protein tyrosine phosphatase, PTPμ, associates with E-cadherin. In this manuscript, we demonstrate that antisense-mediated down regulation of PTPμ, overexpression of catalytically inactive PTPμ, and perturbation of endogenous PTPμ using a specific PTPμ inhibitor peptide results in a substantial reduction in neurite outgrowth on E-cadherin. Taken together these findings demonstrate that E-cadherin is an important adhesion molecule for chick RGC neurite outgrowth and suggest that PTPμ expression and catalytic activity are required for outgrowth on an E-cadherin substrate.
Keywords: neurite outgrowth, receptor protein tyrosine phosphatase (PTPμ), cadherin, retina, tectum, axon guidance
Introduction
The chick visual system serves as a well-established model to investigate the molecular mechanisms involved in axon growth and guidance. Retinal ganglion cells (RGCs) are the first cells to differentiate within the retina at embryonic (E) day 4 (reviewed in Mey and Thanos, 2000, 2001). Development within the retina proceeds in a central-to-peripheral gradient, with cells in the temporal region of the retina being the most differentiated. RGCs first extend an axon toward the optic fissure, and then travel out of the eye along the optic nerve to the chiasm where they cross and continue on the retinofugal pathway to their target, the optic tectum. Retinal axons reach the anterior portion of the tectum by E6 and extend along the tectal surface to form the stratum opticum (SO). Temporal axons innervate the anterior surface while nasal axons extend to the posterior tectum at E10. RGCs extend axons toward the optic tectum in response to various molecular cues on the surface of other cells or in the extracellular environment (Mey and Thanos, 2000).
Cell adhesion molecules are important for the formation of the visual system (Hirano et al., 2003; Thiery, 2003; Kiryushko et al., 2004). Classical cadherins are cell surface integral membrane glycoproteins that mediate cell-cell adhesion, cell migration and cell sorting via calcium-dependent, homophilic interactions (Gumbiner, 2005). Cadherins are tethered to the actin cytoskeleton by their association with the catenins, α-catenin, β-catenin, plakoglobin and p120 (Lilien and Balsamo, 2005). N-cadherin is predominantly expressed in the developing nervous system and mediates axon guidance and synapse formation (Redies, 2000; Kiryushko et al., 2004; Takeichi and Abe, 2005). Previous studies have demonstrated that N-cadherin promotes neurite outgrowth in vitro and in vivo (Bixby and Zhang, 1990; Riehl et al., 1996). Within the chick retina, N-cadherin has been shown to be regulated by tyrosine phosphorylation (Lilien and Balsamo, 2002, 2005).
Receptor protein tyrosine phosphatases (RPTPs) are expressed in the developing chick visual system and a subset of RPTPs have been suggested to play a role in retinotectal pathfinding (Brady-Kalnay, 2001; Ensslen-Craig and Brady-Kalnay, 2004; Johnson and Van Vactor, 2003). RPTPmu (PTPμ) is comprised of CAM-like extracellular domains that mediate cell-cell adhesion and associates with E-, N-, R- and VE-cadherin and the catenins, α-catenin, β-catenin and p120 (Brady-Kalnay et al., 1995, 1998; Hiscox and Jiang, 1998, 1999; Zondag et al., 2000; Sui et al., 2005).
Another classical cadherin, E-cadherin is expressed by mouse RGCs (Faulkner-Jones et al., 1999; Xu et al., 2002). However, a role for E-cadherin in neurite outgrowth has not been examined. In this study, we used a retinal explant model system to demonstrate that E-cadherin promotes neurite outgrowth of RGCs when used as a culture substrate in vitro. E-cadherin is expressed in the chick retina from E6 to E12 and promotes neurite outgrowth from all regions of the retina. Neurite outgrowth is specific to E-cadherin since outgrowth on an E-cadherin substrate is inhibited by addition of E-cadherin adhesion blocking antibodies. We have shown previously that PTPμ is present in a complex with E-cadherin in other systems (Brady-Kalnay et al., 1995, 1998). In order to determine the physiological significance of an association between PTPμ and E-cadherin in neurite outgrowth, the expression level of PTPμ was perturbed in retinal explants. The phosphatase activity of PTPμ was also perturbed in retinal explants. Down-regulation of PTPμ expression through antisense techniques and overexpression of catalytically inactive PTPμ resulted in a substantial reduction in neurite outgrowth on an E-cadherin substrate. In adddition, perturbation of endogenous PTPμ in retnal explants using a specific PTPμ inhibitor peptide also resulted in a decrease in both N-cadherin and E-cadherin-mediated neurite outgrowth. These findings indicate that PTPμ expression and catalytic activity are required for neurite outgrowth by RGCs on an E-cadherin substrate.
Results
Expression of E-cadherin in the visual system
Molecules that regulate axon outgrowth can be expressed in a gradient within the chick visual system. Since RGCs from nasal versus temporal regions of the retina extend axons to distinct locations in the tectum, we examined nasal versus temporal E-cadherin expression at several developmental time points corresponding to peak RGC axon growth in the retina and tectum (Mey and Thanos, 2000). Lysates were made, separated by SDS-PAGE and immunoblotted for E-cadherin (Fig. 1). E-cadherin is expressed during development from E6 to E12, the earliest and latest time-points examined (Fig. 1), and is expressed in the nasal and temporal regions of the retina. N-cadherin is expressed in the retina from E8 to E10 as tested by immunoblot analysis (Matsunaga et al., 1988; Lagunowich and Grunwald, 1989; Burden-Gulley and Brady-Kalnay, 1999). PTPμ is also expressed in the retina (Burden-Gulley and Brady-Kalnay 1999, 2002). Full length PTPμ migrates at ~200 kDa whereas the proteolytically processed form of PTPμ that contains the cytoplasmic domain migrates at 100 kDa (Brady-Kalnay and Tonks, 1994). In retinal lysates, an additional 95 kDa immunoreactive band is also present (Burden-Gulley and Brady-Kalnay, 1999; Burden-Gulley et. al., 2002). Full length PTPμ increases in size, possibly due to glycosylation or alternative splicing. To ensure equal protein loading immunoblots were stripped and reprobed with antibodies to vinculin (Fig. 1).
Figure 1.
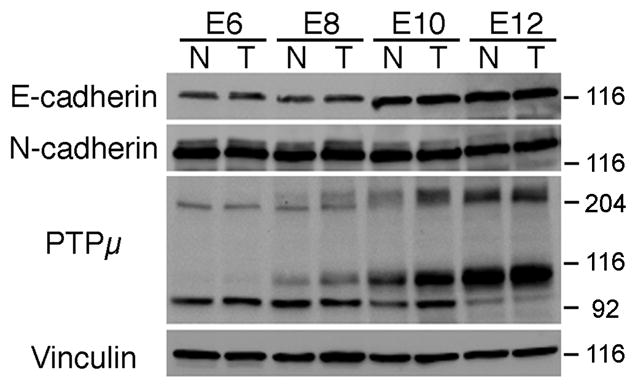
Immunoblot of E-cadherin, N-cadherin and PTPμ in the developing chick retina. Lysates from nasal or temporal retina were prepared from E6, E8, E10 and E12 chicks, separated by SDS-PAGE, transferred to nitrocellulose membrane, and probed with an antibody to E-cadherin, N-cadherin or PTPμ (SK18). E-cadherin protein migrates at ~120 kDa, while N-cadherin migrates at ~130 kDa. Full length PTPμ is ~200 kDa whereas the proteolyticaly processed form of PTPμ containing the cytoplasmic domain migrates at ~100 kDa (Brady-Kalnay and Tonks, 1994). A 95 kDa immunoreactive band is also present. Each immunoblot was stripped and reprobed with antibodies against vinculin to verify equal protein load.
To further characterize the expression of E-cadherin in the developing retina, E8 retinas (stage 32) were sectioned and immunohistochemically labeled with an anti-E-cadherin antibody (Fig. 2A). E8 retinas were used since this time point in development coincides with peak RGC axon extension (Mey and Thanos, 2001). Coronal sections of the retina were taken in order to view both the dorsal and ventral region of the retina. E-cadherin is expressed in the retinal ganglion cells and optic fiber layer (Fig. 2A, B). Serial sections of retina were stained with Hematoxylin to indicate the nuclear location of the RGC cell bodies (Fig. 2C, D), or incubated in the absence of primary antibody (Fig. 2E, F) as a control.
Figure 2.
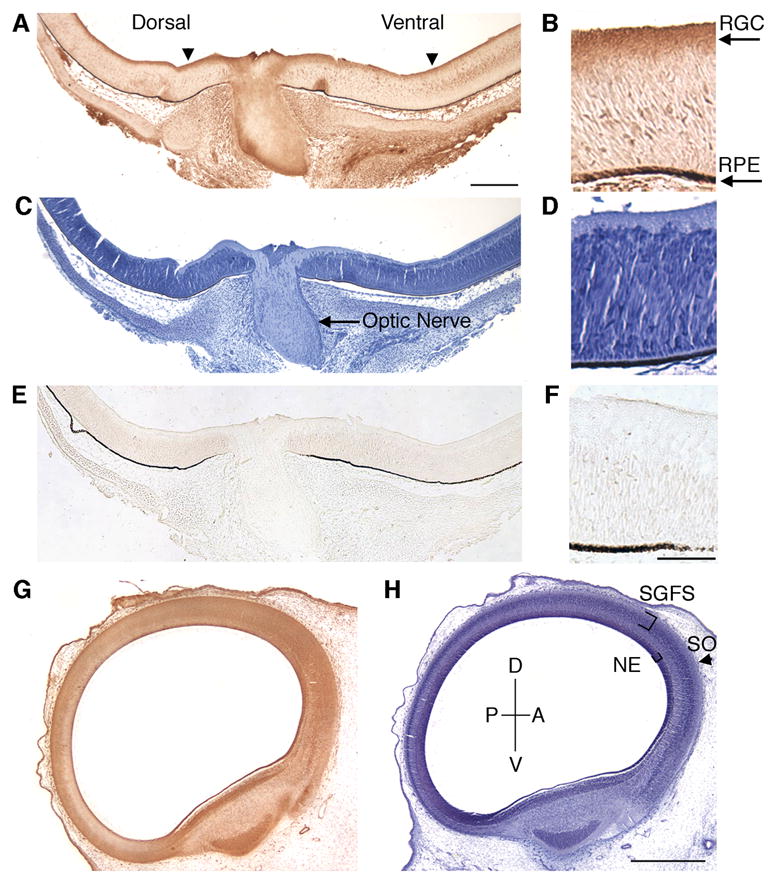
Expression of E-cadherin in chick retina and optic tectum at E8. Coronal sections of E8 chick retina (A–F) and sagittal sections of tectum (G, H) were immunohistochemicaly labeled with antibodies against E-cadherin (A, B, G). The nuclei of each serial section were stained with Hematoxylin (C, D, H). A no primary antibody control is shown (E, F) as an indicator of background staining. E-cadherin expression is present in the retinal ganglion cells (A, arrowheads). In the optic tectum, E-cadherin is also expressed (G). RGC, retinal ganglion cell layer; RPE, retinal pigmented epithelium; P, posterior; D, dorsal; A, anterior; V, ventral; SGFS, stratum grisium et fibrosum; SO, stratum opticum; NE, neuroepithelium. Scale bar (A) 200 μm, (F) 50 μm, (H) 500 μm.
We then examined the expression of E-cadherin in the optic tectum. By E8, RGC axons have migrated out of the retina, across the optic chiasm and are innervating the anterior region of the tectum (Mey and Thanos, 2001). Retinal axons extend along the tectal surface to form the stratum opticum (SO). Temporal axons innervate the anterior surface while nasal axons extend to the posterior tectum at E10. E-cadherin is expressed in E8 optic tectum in the stratum opticum (SO), the outermost layer of the tectum, and the stratum griseum et fibrosum superficiale (SGFS), where RGC axons innervate (Fig. 2G). E-cadherin was also expressed in the neuroepithelium of the tectum (Fig. 2G). At E8, undifferentiated neuroepithelium is most prominent in the anterior portion of the tectum and gives rise to differentiating cells which migrate to the pial surface (LaVail and Cowan, 1971).
E-cadherin promotes neurite outgrowth
Early in embryogenesis, one or two leading RGC axons migrate along the optic stalk toward the optic tectum (Mey and Thanos, 2001). As development continues, successive waves of axons project along the neuronal and glial cells within the optic nerve (Mey and Thanos, 2001). Thus, cadherins expressed on the surface of these cells can serve as a “substrate” for axonal migration. To determine whether E-cadherin promotes neurite outgrowth, we used a well-established in vitro model lab to investigate neurite outgrowth (Lagenaur and Lemmon, 1987; Burden-Gulley and Brady-Kalnay, 1999). Purified recombinant E-cadherin-Fc chimera was coated on tissue culture dishes and used as a substrate to culture chick retinal explants. Neurite outgrowth on an E-cadherin substrate was observed from retinal explants taken at E6, E8 and E10, after 20 hours in culture (Fig. 3A, B, C). Neurite length and density was similar between all time points examined, suggesting that E-cadherin is equally effective at promoting neurite outgrowth at these ages. Neurite outgrowth on E-cadherin was similar in length and density to that observed on N-cadherin (Fig. 6D, G).
Figure 3.
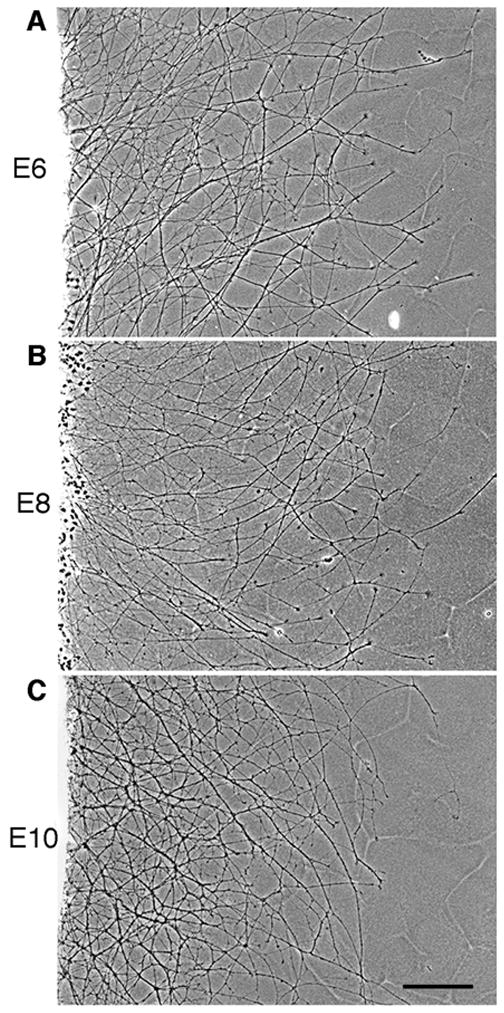
E-cadherin promotes RGC neurite outgrowth at various stages of development. E6 (A), E8 (B) and E10 (C) chick retinal explants were isolated and cultured on an E-cadherin substrate for 20 hours. Scale bar, 200 μm.
Figure 6.
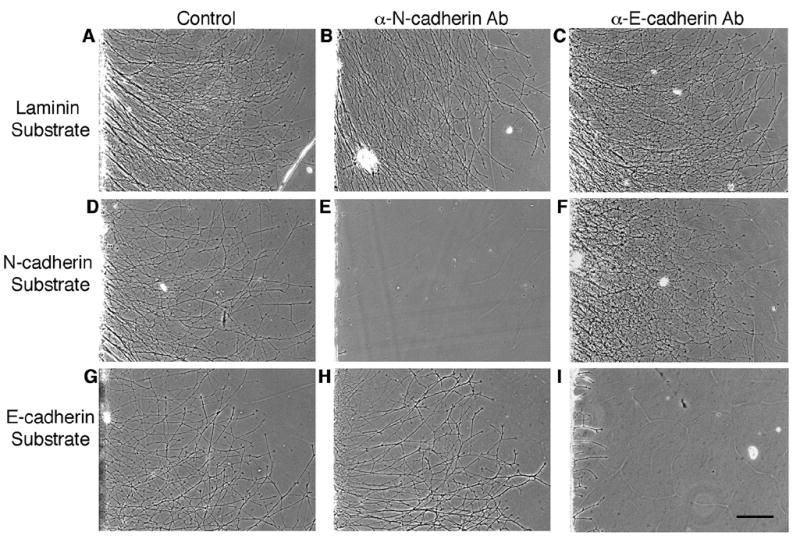
E-cadherin-mediated neurite outgrowth is specifically blocked by E-cadherin adhesion blocking antibodies. Retinal explants from E8 chick embryos were cultured on a laminin (A, B, C), N-cadherin (D, E, F) or E-cadherin (G, H, I) substrate in the presence of adhesion blocking antibodies to N-cadherin (B, E, H) or E-cadherin (C, F, I). Antibodies against N-cadherin inhibited neurite outgrowth on an N-cadherin (E) substrate, whereas they had no effect on neurite outgrowth on laminin (B) or E-cadherin (H) substrates. Similarly, antibodies against E-cadherin inhibited neurite outgrowth on an E-cadherin (I) substrate, while they had no effect on neurite outgrowth on laminin (C) or N-cadherin (F) substrates. Scale bar, 200μm.
Growth cones located at the distal tip of the axon allow neurons to interact with the extracellular environment. Each growth cone recognizes cues in the extracellular environment and on the surface of adjacent cells via membrane-associated proteins such as the cadherins (Hirano et al., 2003; Kiryushko et al., 2004). These interactions lead to intracellular signaling events, which induce cytoskeletal rearrangements that ultimately regulate axon guidance. DiI labeling of RGCs illustrates that the morphology of the growth cones present on an E-cadherin substrate consists of large, broad lamellipodia with a few short filopodia (Fig. 4C). In contrast, growth cones on N-cadherin had smaller lamellipodia with several short filopodial processes (Fig. 4B), which is consistent with previous published work (Bixby and Zhang, 1990; Payne et al., 1992). Growth cones with small lamellipodia were observed on laminin (Fig. 4A). The differences in growth cone morphology observed on each cadherin substrate suggest that distinct signaling mechanisms may be involved in E-cadherin versus N-cadherin-dependent neurite outgrowth.
Figure 4.
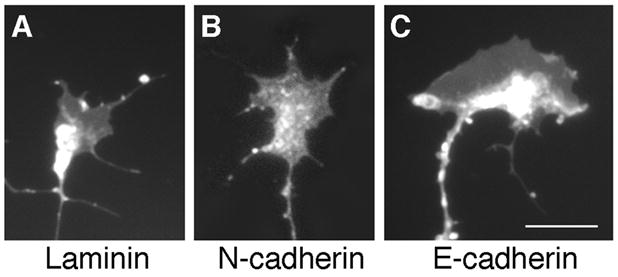
Growth Cone Morphology. DiI labeling demonstrates that growth cones on a laminin substrate (A) appear to have small lamellipodia with few, short filopodia. Growth cones on an N-cadherin substrate (B) have larger lamellipodia in addition to short filopodial processes. On an E-cadherin substrate (C), growth cones have very large, broad lamellipodia with short filopodial processes. Scale bar, 10 μm.
The 3-dimensional position of the RGC cell body within the retina determines which positional cues the RGC cell body and therefore its axon will respond to. Previous studies have shown that at E8, N-cadherin-mediated neurite outgrowth predominantly occurs from RGCs originating from the ventral-nasal, ventral-temporal and dorsal-temporal retina while little to no growth occurs from RGCs from the dorsal-nasal region (Burden-Gulley et al., 2002). In order to identify which regions of the retina promote neurite outgrowth on an E-cadherin substrate, explants from distinct regions of the retina were isolated and cultured in vitro. In contrast to N-cadherin, robust neurite outgrowth on E-cadherin was observed from all regions of the retina (Fig. 5B). Laminin, which has been shown to promote robust neurite outgrowth from all regions of the retina (Burden-Gulley et al., 2002), was used as a control (Fig. 5A).
Figure 5.
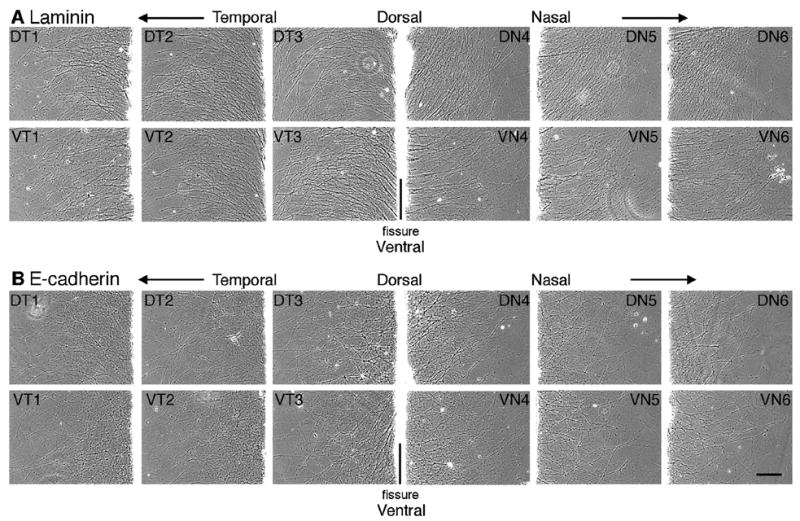
Neurite outgrowth on E-cadherin and laminin is independent of RGC cell body origin. Explants from E8 chick retina were cut parallel to the optic fissure and explants from retina were cultured on E-cadherin (B) or laminin (A) substrates. Images were acquired after 20 hours in culture from a location corresponding to the outer third of each explant. Each number indicates the explant number (e.g. 1 and 6 are most peripheral). Dorsal (D), ventral (V), nasal (N), temporal (T). Scale bar, 200 μm.
Classical cadherins are predominantly homophilic binding proteins (Ivanov et al., 2001; Gooding et al., 2004). To confirm that neurite outgrowth on E- or N-cadherin substrates is specific, E8 retinal explants were cultured on an E-cadherin, N-cadherin or laminin substrate in the presence or absence of adhesion-blocking antibodies. Neurite outgrowth on an E-cadherin substrate was blocked when cultured in the presence of antibodies against the extracellular domain of chick E-cadherin (Fig. 6I). These E-cadherin blocking antibodies had no effect on N-cadherin-mediated outgrowth (Fig. 6F). Antibodies against the extracellular domain of chick N-cadherin (Hatta and Takeichi, 1986) had no effect on E-cadherin-mediated neurite outgrowth (Fig. 6H). However, N-cadherin adhesion blocking antibodies did block neurite outgrowth on an N-cadherin substrate (Fig. 6E). Neurite outgrowth on laminin was unaffected by E- and N-cadherin adhesion blocking antibodies (Fig. 6B, C). Taken together, these data suggest that neurite outgrowth on an E-cadherin substrate is due to specific E-cadherin binding.
PTPμ expression and catalytic activity are required for E-cadherin-mediated neurite outgrowth
Previously our laboratory has demonstrated that PTPμ is expressed in the retina (Fig. 1), interacts with N-cadherin, and is required for N-cadherin-mediated neurite outgrowth (Burden-Gulley and Brady-Kalnay, 1999). We have also shown that PTPμ interacts directly with E-cadherin in other cell types (Brady-Kalnay et al., 1995, 1998). We therefore hypothesized that PTPμ expression might be required for E-cadherin-mediated neurite outgrowth. In order to test this, we infected E8 retinal explants with herpes simplex virus (HSV) encoding antisense PTPμ (AS) (Ensslen et al., 2003). Previous studies have demonstrated that infection of cultured retinal neuroepithelial cells (RNE) with PTPμ AS HSV reduces expression of full length PTPμ by 60% (Ensslen et al., 2003). We confirm that PTPμ AS HSV decreased full length PTPμ by 64%, cleaved PTPμ (100 kDa) decreased by 59% and the 95 kDa band decreased by 51% when normalized to vinculin. In addition, infection of RNE with PTPμ AS HSV had no significant effect on E- or N-cadherin expression (Fig. 7). Retinal explants infected with PTPμ AS HSV were cultured on either an E-cadherin, N-cadherin or laminin substrate. Neurite outgrowth was observed after 20 hours of incubation in the presence of the virus (Fig. 8). Neurite length decreased by 63% and density decreased by 77% when retinal explants were cultured on an E-cadherin substrate in the presence of PTPμ AS HSV (Fig. 8K, Fig. 9). Similar to previously reported data using PTPμ antisense retrovirus (Burden-Gulley and Brady-Kalnay, 1999), neurite length and density of retinal explants grown on an N-cadherin substrate in the presence of PTPμ AS HSV decreased by 51% and 76% respectively (Fig. 8G, Fig. 9). PTPμ AS HSV had no effect on neurite outgrowth of retinal explants grown on a laminin substrate (Fig. 8C, Fig. 9), indicating that the amount of virus used is not toxic and does not exhibit nonspecific effects on neurite outgrowth. These results suggest that PTPμ expression is required for E-cadherin to mediate neurite outgrowth.
Figure 7.
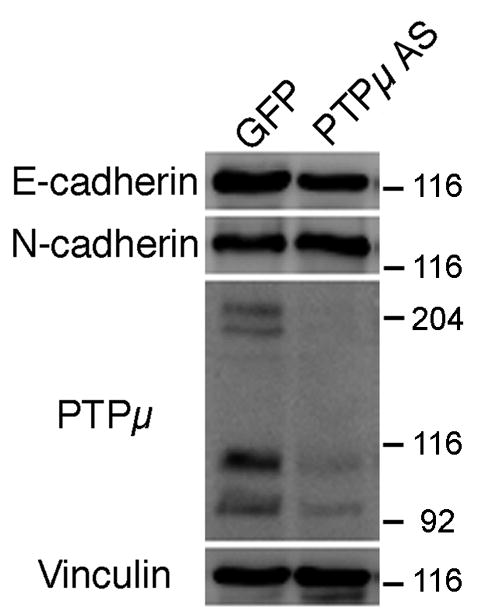
PTPμ antisense HSV infection does not alter cadherin expression. E6 retinal neuroepithelial cells were infected with IRES-GFP HSV or PTPμ AS HSV for 20 hours. Lysates from infected RNE cells were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and probed with an antibody to E-cadherin, N-cadherin or PTPμ (SK15). Full-length (200-kDa) PTPμ expression decreased by 64%, cleaved PTPμ (100 kDa) decreased by 59% and the 95 kDa band decreased by 51% in the presence of PTPμ AS HSV, while there was no significant effect on E- or N-cadherin expression as measured by densitometry. Each immunoblot was stripped, reprobed with antibodies against vinculin to verify equal protein load, normalized to vinculin and quantitated by densitometry.
Figure 8.
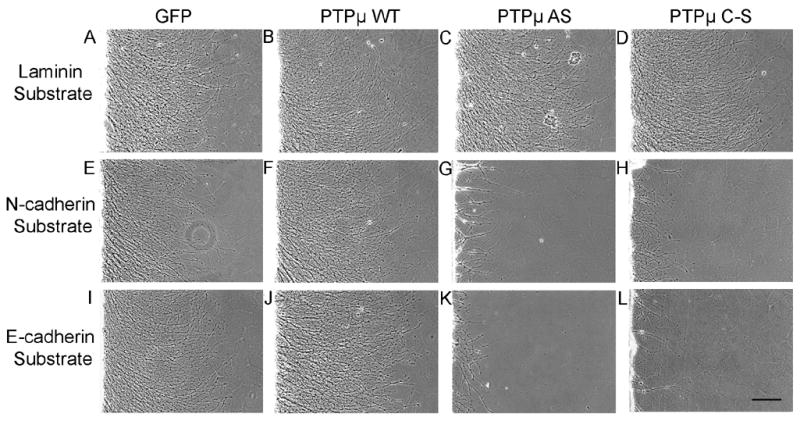
PTPμ expression and catalytic activity is required for neurite outgrowth on N-cadherin and E-cadherin. E8 chick retinal explants were infected with HSV encoding GFP control (A, E, I), PTPμ WT (B, F, J), PTPμ AS (C, G, K) or PTPμ C–S (D, H, L) and cultured on laminin (A, B, C, D), N-cadherin (E, F, G, H) or E-cadherin (I, J, K,L) substrates for 20 hours. No difference in neurite length or density was observed in cultures infected with GFP, PTPμ WT, PTPμ AS, or PTPμ C–S when cultured on laminin (A, B, C, D). Overexpression of PTPμ WT also had no effect on laminin (B), N-cadherin (F) or E-cadherin (J) substrates. Infection with PTPμ AS or PTPμ C–S resulted in a dramatic decrease in neurite outgrowth when cultured on N-cadherin (G, H) or E-cadherin (K, L) substrates. Scale bar, 200 μm.
Figure 9.
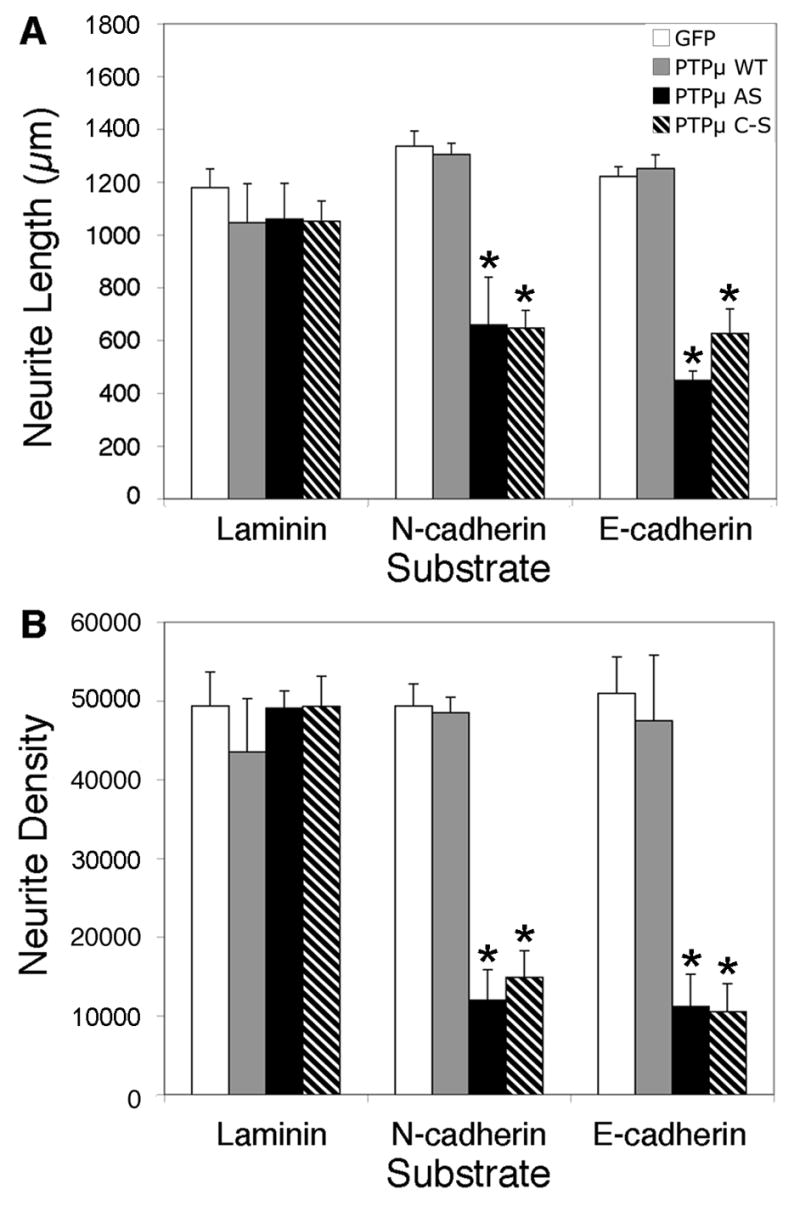
Quantitation of PTPμ perturbation on neurite outgrowth. Infection of E8 chick retinal explants with HSV encoding GFP control (white bars) or PTPμ WT (gray bars), had no effect on laminin, N-cadherin or E-cadherin-dependent neurite outgrowth. PTPμ AS HSV (black bars) infection decreased neurite length (A) by 51% on N-cadherin and by 63% on E-cadherin substrates while neurite density (B) decreased by 76% on N-cadherin and by 77% on E-cadherin. PTPμ C–S HSV (hatched bars) infection also decreased neurite length (A) by 52% on N-cadherin and by 49% on E-cadherin substrates, while neurite density (B) decreased by 70% on N-cadherin and by 79% on E-cadherin. PTPμ AS and PTPμ C–S HSV infection had no effect on laminin-dependent neurite outgrowth. Asterisk denotes statistically significant changes in neurite length or density compared to control. n = 5 for laminin, n = 5 for N-cadherin, n = 6 for E-cadherin.
In addition to PTPμ expression, PTPμ tyrosine phosphatase activity is required for N-cadherin-mediated neurite outgrowth (Burden-Gulley and Brady-Kalnay, 1999). Since infection with HSV encoding PTPμ AS does not tell us whether PTPμ adhesion or phosphatase activity is regulating E-cadherin-mediated neurite outgrowth we ivestigated the requirement for PTPμ catalytic activity in E-cadherin-mediated neurite outgrowth. In order to test this, E8 retinal explants were infected with HSV encoding either wild-type PTPμ (WT) or full length catalytically inactive PTPμ (C–S) (Ensslen et al., 2003) and cultured on either an E-cadherin, N-cadherin or laminin substrate (Fig. 8). Overexpression of PTPμ WT also had no effect on laminin (Fig. 8B) and N-cadherin-mediated neurite outgrowth (Fig. 8F) as previously published (Burden-Gulley and Brady-Kalnay, 1999). We also demonstrate that PTPμ WT had no effect on E-cadherin-mediated neurite outgrowth (Fig. 8J, Fig. 9). After 20 hours of incubation in the presence of PTPμ C–S HSV, neurite length decreased by 49% and density decreased by 79% when cultured on an E-cadherin substrate (Fig. 8L, Fig. 9). Neurite length and density of retinal explants grown on an N-cadherin substrate in the presence of PTPμ C–S HSV decreased by 52% and 70% respectively (Fig. 8H, Fig. 9). PTPμ C–S HSV had no effect on neurite outgrowth of retinal explants grown on a laminin substrate (Fig. 8D, Fig. 9). Taken together, we demonstrate that PTPμ catalytic activity is also required for E-cadherin-mediated neurite outgrowth.
To test whether endogenous PTPμ function is required for E-cadherin and N-cadherin-mediated neurite outgrowth, a PTPμ specific peptide inhibitor was used. The peptide resembles the HLH wedge-shaped sequence (Hoffmann et al., 1997), located in the juxtamembrane domain near the D1 PTPμ catalytic domain (Xie, et. al., 2006). The peptide utilized mimics inter/intramolecular interactions and is proposed to regulate catalytic activity of the phosphatase (for reviews see Bixby, 2001, Brady-Kalnay et al., 2001; Ensslen-Craig and Brady-Kalnay, 2004). The PTPμ wedge peptide (WPTPμ-Tat) binds to itself in a bead binding assay but not to the wedge peptide LAR (WLAR-Tat), another member of the type II RPTP subfamily (Xie, et. al., 2006), demonstrating that the WPTPμ-Tat is specific and does not interact with other RPTP family members. In addition, WPTPμ-Tat but not WLAR-Tat was shown to perturb PTPμ-mediated neurite outgrowth (Xie, et. al., 2006) which requires PTPμ catalytic activity (Ensslen-Craig and Brady-Kalnay, 2005).
E8 retinal explants were cultured in the presence the PTPμ wedge peptide (WPTPμ-Tat), which includes a Tat-derived domain linked to the C terminus for uptake of the peptide into the cell, or scrambled control (SPTPμ-Tat) and cultured on either an E-cadherin, N-cadherin or laminin substrate for 20 hours (Fig. 10). Incubation with WPTPμ-Tat had no effect on laminin-dependent neurite outgrowth (Fig. 10B) when compared to SPTPμ-Tat control (Fig. 10A). Neurite length (Fig. 10G) decreased by 46% on N-cadherin and by 80% on E-cadherin substrates, while neurite density (Fig. 10H) decreased by 84% on N-cadherin and by 90% on E-cadherin in the presence of WPTPμ-Tat when compared to SPTPμ-Tat control. Perturbation of E-cadherin and N-cadherin-mediated neurite outgrowth using the PTPμ specific wedge peptide inhibitor, confirms the importance of PTPμ catalytic activity in cadherin-dependent neurite outgrowth.
Figure 10.
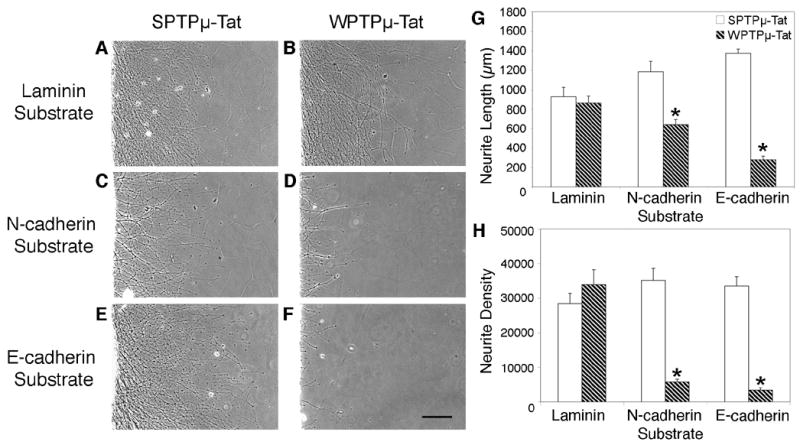
PTPμ-specific inhibitor peptide blocks neurite outgrowth on E-cadherin and N-cadherin. E8 chick retinal explants were cultured on laminin (A, B), N-cadherin (C, D), or E-cadherin (E, F) substrates for 20 hours in the presence of either scrambled control (SPTPμ-Tat) (A, C, E) or the PTPμ wedge peptide (WPTPμ-Tat) (B, D, F) at a final concentration of 5.5 μM. Cultures incubated with WPTPμ-Tat had no effect on laminin-dependent neurite outgrowth (A) when compared to SPTPμ-Tat control (B). Neurite length (G) decreased by 46% on N-cadherin and by 80% on E-cadherin substrates, while neurite density (H) decreased by 84% on N-cadherin and by 90% on E-cadherin in the presence of WPTPμ-Tat (hatched bars) when compared to SPTPμ-Tat control (white bars). Asterisk denotes statistically significant changes in neurite length or density compared to control. n = 4 for laminin, n = 5 for N-cadherin n =6 for E-cadherin. Scale bar, 200 μm.
Discussion
Although many cell adhesion molecules are expressed within the nervous system, only a subset of these molecules have been shown to be permissive to axon outgrowth in vivo. In order to address the functional role of CAMs in axon extension, an in vitro RGC neurite outgrowth assay using various CAMs as substrates is utilized. Integrins and their ligands the extracellular matrix (ECM) molecules, the immunoglobulin superfamily of cell adhesion molecules (CAMs) and cadherins comprise the three primary classes of proteins known to mediate neurite outgrowth (Kiryushko et al., 2004). Integrin receptors are present on the surface of RGCs and signal to the cell to extend neurites onto certain ECM molecules including fibronectin and laminin (Kiryushko et al., 2004). L1, an Ig superfamily CAM, is known to promote neurite outgrowth from RGCs (Burden-Gulley et al., 1995; Kamiguchi, 2003; Skaper, 2005). Within the cadherin superfamily only two classical cadherins, N-cadherin and R-cadherin have been shown to promote neurite outgrowth (Bixby and Zhang, 1990; Redies and Takeichi, 1993). Previous studies have shown that E-cadherin is expressed in mouse RGCs (Faulkner-Jones et al., 1999; Xu et al., 2002). However, the role of E-cadherin in neurite outgrowth is unknown. In this study we show that E-cadherin is expressed within the chick visual system and identified a functional role for E-cadherin in promoting neurite outgrowth from RGCs.
In this manuscript, we demonstrate that E-cadherin is expressed in the retina from E6 to E12. At E8, E-cadherin is expressed by the RGCs of the retina and is also present in the chick tectum. In order to stimulate the elongation of retinal axons, RGCs require molecules with growth permissive properties (Hirano et al., 2003; Thiery, 2003; Kiryushko et al., 2004). We demonstrate that RGC neurons extended neurites onto an E-cadherin substrate early in retinal development at E6, throughout peak axon extension at E8 to E10. E-cadherin is a homophilic binding protein, meaning E-cadherin on the surface of one cell has the ability to interact in trans with an E-cadherin molecule on the surface of another cell (Ivanov et al., 2001; Gooding et al., 2004). We show that neurite outgrowth on an E-cadherin substrate was blocked by the addition of an E-cadherin function blocking antibody. These data suggest that E-cadherin-mediated neurite outgrowth is specific to E-cadherin.
Distinct differences in neurite outgrowth were observed on an E-cadherin substrate versus N-cadherin. Neurite outgrowth on an E-cadherin substrate was robust from all regions of the retina at E8, whereas little to no neurite outgrowth is observed from the dorsal-nasal region of the retina on N-cadherin (Burden-Gulley et al., 2002). Growth cones on an E-cadherin substrate had large, broad lamellipodia with very few short filopodia in contrast to growth cones on N-cadherin with smaller lamellipodia and several short filopodia, indicating that different downstream signaling molecules may be regulating E-cadherin versus N-cadherin-mediated neurite outgrowth.
Expression of E-cadherin during embryonic development is classically associated with epithelial cell organization and maintenance of stable cell-cell adhesion (Thiery, 2003). Epithelial cells express E-cadherin, however down-regulation of E-cadherin or loss of E-cadherin function occurs during epithelial-mesenchymal transition (EMT) (Thiery, 2003; Larue and Bellacosa, 2005). In contrast to the role of E-cadherin in maintaining cell-cell adhesion in epithelial cells, recent findings in D. melanogaster have indicated a role for E-cadherin in axon growth and cell migration. Drosophila epithelial (DE) cadherin is expressed in postembryonic neuroblasts which form the Drosophila brain and is required for proper axon tract formation (Dumstrei et al., 2003a; 2003b). Border cells also express DE-cadherin and require DE-cadherin for migration during oogenesis (Niewiadomska et al., 1999). Lack of DE-cadherin in border cells blocks cell migration, and expression of extracellular DE-cadherin alone is unable to rescue border cell migration (Pacquelet and Rorth, 2005). These data highlight the importance of the DE-cadherin cytoplasmic domain in DE-cadherin-mediated cell migration.
Intracellular tyrosine phosphorylation of cadherins is associated with a loss of cadherin-mediated adhesion and destabilization of adherens junctions (Brunton et al., 2004; Andl and Rustgi, 2005; Erez et al., 2005). In addition, dephosphorylation of E-cadherin or E-cadherin associated proteins may be required for proper cell adhesion (Brady-Kalnay, 2001; Beltran and Bixby, 2003; Lilien and Balsamo, 2005). In the retina, PTPμ is primarily expressed on RGCs and is developmentally regulated (Burden-Gulley and Brady-Kalnay, 1999; Ensslen et al., 2003). PTPμ interacts with the E-cadherin/catenin complex in many cell types (Brady-Kalnay et al., 1995, 1998). Our laboratory has previously reported that expression and catalytic activity of PTPμ are required for neurite outgrowth on an N-cadherin substrate (Burden-Gulley and Brady-Kalnay, 1999). In this study, we report that PTPμ expression and catalytic activity is also required for neurite outgrowth on an E-cadherin substrate. Although distinct downstream signaling pathways between E-cadherin and N-cadherin-mediated neurite outgrowth may be involved, it is clear that PTPμ expression and catalytic activity are required for both E-cadherin and N-cadherin-mediated neurite outgrowth.
One possible mechanism for the regulation of E-cadherin-mediated neurite outgrowth by PTPμ is through the recruitment of other regulatory proteins to the cadherin/catenin complex. The protein kinase C (PKC) family of serine/threonine kinases has been implicated in the regulation of E-cadherin-mediated adhesion and formation of adherens junctions (Lewis et al., 1994; Skoudy et al., 1995; Hellberg et al., 2002). PKC is able to bind the receptor for activated protein kinase C 1 (RACK1) (Ron et al., 1999), a scaffolding protein known to regulate signaling pathways in the central nervous system (Sklan et al., 2006). Within the chick retina, RACK1, PKCδ and PTPμ are found in complex together (Rosdahl et al., 2002). RACK1 and PTPμ have also been found in complex in epithelial cells and regulate E-cadherin dependent adhesion (Chattopadhyay et al., 2003). Regulation of PKCδ activity is required for restoration of E-cadherin-mediated adhesion in LNCaP cells (Hellberg et al., 2002). It is possible that PTPμ recruits RACK1/PKCδ to the cadherin/catenin complex at the cell surface where PKCδ may regulate E-cadherin-mediated cell adhesion. Future studies will investigate the PTPμ signaling pathways required for E-cadherin dependent neurite outgrowth.
Experimental Methods
Immunoblot analysis
Tissue lysates were prepared by dissecting nasal retina from temporal retina at various developmental stages in ice-cold calcium-magnesium-free Hank’s buffered saline (CMF) and transferred to cold lysis buffer (20mM Tris pH 7.6, 1% Triton X-100, 1mM benzamidine, 1mM sodium orthovanadate, 0.1 mM ammonium molybdate, 0.2 mM phenyl arsine oxide, 0.3% protease inhibitor cocktail (P8340; Sigma). The tissue was lysed by vigorous trituration and incubated on ice for 20 minutes. The triton insoluble material was removed by centrifugation (5,000 rpm for 5 min in an Eppendorf Microcentrifuge), and the protein concentration of the supernatant was determined by the Bradford method (Bradford, 1976). Equal amounts of protein were loaded per lane and separated by SDS-PAGE (6% gels). Proteins were transferred to nitrocellulose membrane (Schleicher and Schuell, Keene, NH) and immunoblotted as described previously using an antibody generated against PTPμ (SK18 or SK15) (Brady-Kalnay et al., 1993; Brady-Kalnay and Tonks, 1994), E-cadherin (610182; BD Biosciences, San Diego, CA) or N-cadherin (610920; BD Biosciences). To verify equal protein load per lane, the immunoblots were stripped and reprobed (Reblot Plus; Chemicon International, Temecula, CA) with a monoclonal antibody generated against vinculin (V9131; Sigma, St. Louis, MO). All immunoblot data were acquired on a Bio-Rad Fluor-S Max MultiImager system (Bio-Rad, Hercules, CA), using the Quantity One (Bio-Rad) image processing software. For quantitation, bands were normalized to vinculin.
Immunohistochemistry
Retina and brain were dissected out in ice-cold CMF. Tissue was fixed in 3.7% formaldehyde for 30–45 minutes at room temperature followed by a PBS rinse. Tissue was taken through alcohol dehydration and then embedded in paraffin wax. Coronal sections were taken of the retina in that the blade cut across the eye, parallel to the optic fissure. Sections were cut on a microtome at 12μm intervals. Next, sections were dried for 1 hour, cleared with xylene and taken through alcohol rehydration. After rinsing in PBS, sections were heated at 37°C in 10mM sodium citrate, pH 6, 3 times for 6 minutes each to unmask antigenic sites. Sections were allowed to cool for 20 min before incubating in 3% H2O2 for 20 min to block endogenous peroxidase activity. Sections were blocked with 1.5% horse serum/PBS. In order to block endogenous avidin/biotin activity, sections were incubated with avidin D followed by biotin (Avidin/Biotin Blocking Kit; Vector Laboratories, Burlingame, CA). Sections were then incubated in monoclonal anti-E-cadherin antibody (BD Biosciences) in blocking buffer overnight at 4°C. After rinsing in PBS, sections were incubated in biotinylated secondary antibody (Vectastain Elite avidin-biotin complex (ABC) kit; Vector Laboratories) in blocking buffer for 25 min at room temperature. Sections were rinsed and then incubated in ABC reagent in PBS for 45 min at room temperature. After PBS rinses, sections were incubated with diaminobenzidine (DAB) solution (Vector Laboratories) for 5–10 min and then rinsed with PBS. DAB produces a brown precipitate, making protein expression in the retinal pigmented epithelium (RPE) indistinguishable from the brown melanin found in the RPE. Sections were dehydrated through a graded ethanol series and then coverslipped using Permount mounting medium (Fisher Scientific, Hampton, NH). All images were collected using an RT Slider digital camera (Diagnostic Instruments, Sterling Heights, MI) mounted on an Olympus BX 60 Upright Microscope (Tokyo, Japan).
Neurite outgrowth assays
Human E-cadherin-Fc and N-cadherin-Fc were obtained from R&D Systems (Minneapolis, MN). Laminin was obtained from Sigma. Briefly, 35mm tissue culture dishes were coated with nitrocellulose in methanol (Lagenaur and Lemmon, 1987) and allowed to dry. Several different lots of substrate were used over the course of the experiments resulting in variablitity in the concentration of substrate used. 0.25–0.50 μg of E-cadherin-Fc, 0.06–0.15 μg of N-cadherin-Fc or 2.50–4.00 μg of laminin was spread across the center of each dish and incubated for 20 minutes at room temperature. Remaining binding sites on the nitrocellulose were blocked with 2% BSA in CMF, and the dishes were rinsed with RPMI 1640 medium (Hyclone, Logan, UT).
Embryonic day 8 (stage 32–33 according to Hamburger and Hamilton, 1951) chick eyes were dissected in cold CMF and the retinal explants were prepared as described (Halfter et al., 1983; Drazba and Lemmon, 1990; Burden-Gulley and Brady-Kalnay, 1999). Briefly, neural retinas were flattened on concavalin-coated nitrocellulose filters and cut into 350μm-wide explants. Explants were placed retinal ganglion side down onto substrate coated dishes and cultured in RPMI-1640, 10% fetal bovine serum (Hyclone), 2% chick serum (Invitrogen, Carlsbad, CA), 100 U/ml penicillin, 0.1mg/ml streptomycin, 0.025 μg/ml amphotericin (Sigma).
For growth cone visualization, Lab-TekII Chamber Slides (Fisher Scientific) were coated with 0.01% poly-L-lysine overnight, rinsed 5 x with distilled H20 and allowed to dry overnight. The slides were then coated with E-cadherin, N-cadherin or laminin substrate as described above. Retinas were prepared as described above. Before placing the explant retinal ganglion side down onto the substrate coated slide, DiI crystals (Invitrogen) were placed on the tissue. Culture medium containing serum was then added and explants were incubated for 20 hours.
For antibody inhibition studies, N-cadherin blocking antibody, NCD2 (Hatta and Takeichi, 1986) at a final concentration of 11 μg/ml, or E-Cadherin blocking antibody, goat anti-L-CAM (chick E-cadherin) (Renaud-Young and Gallin, 2002) at a final concentration of 1 mg/ml. The goat anti-L-CAM (chick E-cadherin) antibody was a kind gift from Drs. Bruce Cunningham and Warren Gallin. The antibodies were added to the culture media in each substrate-coated culture dish and incubated at room temperature for 30 minutes prior to addition of the explant. Explants were incubated for 20 hours in the presence of the blocking antibody.
For viral perturbation studies, 7.5μl of replication-defective herpes simplex virus (HSV) encoding green fluorescent protein (IRES-GFP), wildtype PTPμ (WT), antisense PTPμ (AS) or catalytically inactive PTPμ (C–S), as previously described (Ensslen et al., 2003), in RPMI-1640 alone was added at the time of explanting. The virus was allowed to incubate at 37°C for 2 hours. Culture media containing serum was then added. All explants were incubated at 37°C for 20 hours, fixed in 4% paraformaldehyde, 0.1% glutaraldehyde and imaged.
For PTPμ inhibitor peptide studies, a PTPμ wedge peptide (WPTPμ-Tat) or scrambled control (SPTPμ-Tat), was added as previously described (Xie et. al., 2006). A final concentration of 5.5 μM peptide was added at the time of explanting. Both peptides include a membrane-penetrant Tat-derived sequence at the C terminus, which promotes cellular uptake of the peptide (Wadia and Dowdy, 2002). All explants were incubated at 37°C for 20 hours, fixed in 4% paraformaldehyde, 0.1% glutaraldehyde and imaged.
Retinal neuroepithelial cell infection
Embryonic day 6 RNE cultures were prepared as previously described (Burden-Gulley and Brady-Kalnay, 1999). Briefly, E6 chick retinas were dissected in cold CMF and dissociated in 0.25% Trypsin, 4Na EDTA (Invitrogen) for 20 minutes at 37° shaking, followed by vigorous trituration. Cells were resuspended, plated at a concentration of 5 x 105 and allowed to attach overnight at 37° in RPMI-1640, 10% fetal bovine serum, 2% chick serum, 100 U/ml penicillin, 0.1mg/ml streptomycin, 0.025 μg/ml amphotericin. RNE cells were then infected with 2μl PTPμ AS HSV or IRES-GFP HSV for 2 hours in RPMI-1640 alone, followed by 18 hours of incubation in RPMI-1640, 10% fetal bovine serum, 2% chick serum, 100 U/ml penicillin, 0.1mg/ml streptomycin, 0.025 μg/ml amphotericin in the presence of HSV.
Quantitation of neurite outgrowth
Neurite outgrowth from specific regions of the retina was analyzed using a SPOT RT digital camera and image acquisition software (Diagnostic Instruments, Inc., Sterling Heights, MI). In short, the length of the five longest neurites per given area of the explant were measured perpendicular to the explant tissue. To calculate neurite density, images were analyzed using Metamorph software version 6.3r4 (Universal Imaging, Downington, PA). The data from all similar experiments were combined, analyzed by Student’s t test and graphed (Microsoft Excel, 10.0.0 2001).
Acknowledgments
This study was supported by the National Institute of Health Grant RO1-EY12251 to S.B.K., and a predoctoral fellowship to S.A.O. from Visual Sciences Training Grant T32-EY07157. Additional support was provided by the Visual Sciences Research Center Core Grant (PO-EY11373) from the National Eye Institute.
The goat anti-L-CAM (chick E-cadherin) antibody was a kind gift from Drs. Bruce Cunningham and Warren Gallin. We also thank Denise Hatala and Catherine Doller for immunohistochemistry, Scott Howell for densitometry analysis, Carol Luckey for generation of HSV plasmids, Scott Becka for technical assistance and all of the members of the Brady-Kalnay lab for their insightful discussions, especially Susan Burden-Gulley.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andl CD, Rustgi AK. No one-way street: cross-talk between E-cadherin and receptor tyrosine kinase (RTK) signaling: a mechanism to regulate RTK activity. Cancer Biol Ther. 2005;4:28–31. doi: 10.4161/cbt.4.1.1431. [DOI] [PubMed] [Google Scholar]
- Beltran PJ, Bixby JL. Receptor protein tyrosine phosphatases as mediators of cellular adhesion. Front Biosci. 2003;8:d87–99. doi: 10.2741/941. [DOI] [PubMed] [Google Scholar]
- Bixby JL, Zhang R. Purified N-cadherin is a potent substrate for the rapid induction of neurite outgrowth. J Cell Biol. 1990;110:1253–1260. doi: 10.1083/jcb.110.4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixby JL. Ligands and signaling through receptor-type tyrosine phosphatases. IUBMB Life. 2001;51:157–63. doi: 10.1080/152165401753544223. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brady-Kalnay SM, Flint AJ, Tonks NK. Homophilic binding of PTPμ, a receptor-type protein tyrosine phosphatase, can mediate cell-cell aggregation. J Cell Biol. 1993;122:961–972. doi: 10.1083/jcb.122.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady-Kalnay SM, Tonks NK. Identification of the homophilic binding site of the receptor protein tyrosine phosphatase PTPμ. J Biol Chem. 1994;269:28472–284727. [PubMed] [Google Scholar]
- Brady-Kalnay SM, Rimm DL, Tonks NK. Receptor protein tyrosine phosphatase PTPμ associates with cadherins and catenins in vivo. J Cell Biol. 1995;130:977–986. doi: 10.1083/jcb.130.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady-Kalnay SM, Mourton T, Nixon JP, Pietz GE, Kinch M, Chen H, Brackenbury R, Rimm DL, Del Vecchio RL, Tonks NK. Dynamic interaction of PTPμ with multiple cadherins in vivo. J Cell Biol. 1998;141:287–296. doi: 10.1083/jcb.141.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady-Kalnay SM. Protein tyrosine phosphatases. In: Beckerle M, editor. Cell Adhesion: Frontiers in Molecular Biology. Vol. 39. Oxford Univ. Press; Oxford UK: 2001. pp. 217–258. [Google Scholar]
- Brunton VG, MacPherson IR, Frame MC. Cell adhesion receptors, tyrosine kinases and actin modulators: a complex three-way circuitry. Biochim Biophys Act. 2004;1692:122–144. doi: 10.1016/j.bbamcr.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Burden-Gulley SM, Payne HR, Lemmon V. Growth cones are actively influenced by substrate-bound adhesion molecules. J Neurosci. 1995;15:4370–4381. doi: 10.1523/JNEUROSCI.15-06-04370.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden-Gulley SM, Brady-Kalnay SM. PTPmu regulates N-cadherin-dependent neurite outgrowth. J Cell Biol. 1999;144:1323–1336. doi: 10.1083/jcb.144.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden-Gulley SM, Ensslen SE, Brady-Kalnay SM. Protein tyrosine phosphatase-mu differentially regulates neurite outgrowth of nasal and temporal neurons in the retina. J Neurosci. 2002;22:3615–3627. doi: 10.1523/JNEUROSCI.22-09-03615.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay N, Wang Z, Ashman LK, Brady-Kalnay SM, Kreidberg JA. alpha3beta1 integrin-CD151, a component of the cadherin-catenin complex, regulates PTPμ expression and cell-cell adhesion. J Cell Biol. 2003;163:1351–1362. doi: 10.1083/jcb.200306067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drazba J, Lemmon V. The role of cell adhesion molecules in neurite outgrowth on Muller cells. Dev Biol. 1990;138:82–93. doi: 10.1016/0012-1606(90)90178-l. [DOI] [PubMed] [Google Scholar]
- Dumstrei K, Wang F, Nassif C, Hartenstein V. Early development of the Drosophila brain: V. Pattern of postembryonic neuronal lineages expressing DE-cadherin. J Comp Neurol. 2003a;455:451–462. doi: 10.1002/cne.10484. [DOI] [PubMed] [Google Scholar]
- Dumstrei K, Wang F, Hartenstein V. Role of DE-Cadherin in Neuroblast Proliferation, neural morphogenesis and axon tract formation in Drosophila larval brain development. J Neurosci. 2003b;23:3325–3335. doi: 10.1523/JNEUROSCI.23-08-03325.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensslen SE, Rosdahl JA, Brady-Kalnay SM. The receptor protein tyrosine phosphatase-mu, PTPμ, regulates histogenesis of the chick retina. Dev Biol. 2003;264:106–118. doi: 10.1016/j.ydbio.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Ensslen SE, Brady-Kalnay SM. PTPμ signaling via PKCδ is instructive for retinal ganglion cell guidance. Mol Cell Neurosci. 2004;25:558–571. doi: 10.1016/j.mcn.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Ensslen-Craig SE, Brady-Kalnay SM. Receptor protein tyrosine phosphatases regulate neural development and axon guidance. Dev Biol. 2004;275:12–22. doi: 10.1016/j.ydbio.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Ensslen-Craig SE, Brady-Kalnay SM. PTP mu expression and catalytic activity are required for PTP mu-mediated neurite outgrowth and repulsion. Mol Cell Neurosci. 2005;28:177–88. doi: 10.1016/j.mcn.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Erez N, Bershadsky A, Geiger B. Signaling from adherens-type junctions. Eur J Cell Biol. 2005;84:235–244. doi: 10.1016/j.ejcb.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Faulkner-Jones BE, Godinho LN, Tan SS. Multiple cadherin mRNA expression and developmental regulation of a novel cadherin in the developing mouse eye. Exp Neurol. 1999;156:316–325. doi: 10.1006/exnr.1999.7026. [DOI] [PubMed] [Google Scholar]
- Gooding JM, Yap KL, Ikura M. The cadherin-catenin complex as a focal point of cell adhesion and signaling: new insights from three-dimensional structures. Bioessays. 2004;26:497–511. doi: 10.1002/bies.20033. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- Halfter W, Newgreen DF, Sauter J, Schwarz U. Oriented axon outgrowth from avian embryonic retina in culture. Dev Biol. 1983;95:56–64. doi: 10.1016/0012-1606(83)90006-4. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Hatta K, Takeichi M. Expression of N-cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature. 1986;320:447–449. doi: 10.1038/320447a0. [DOI] [PubMed] [Google Scholar]
- Hellberg CB, Burden-Gulley SM, Pietz GE, Brady-Kalnay SM. Expression of the receptor protein-tyrosine phosphatase, PTPμ, restores E-cadherin-dependent adhesion in human prostate carcinoma cells. J Biol Chem. 2002;277:11165–11173. doi: 10.1074/jbc.M112157200. [DOI] [PubMed] [Google Scholar]
- Hirano S, Suzuki ST, Redies C. The cadherin superfamily in neural development: diversity, function and interaction with other molecules. Front Biosci. 2003;8:d306–355. doi: 10.2741/972. [DOI] [PubMed] [Google Scholar]
- Hiscox S, Jiang WG. Association of PTPμ with catenins in cancer cells: a possible role for E-cadherin. Int J Oncol. 1998;13:1077–1080. doi: 10.3892/ijo.13.5.1077. [DOI] [PubMed] [Google Scholar]
- Hiscox S, Jiang WG. Association of the HGF/SF receptor, c-met, with the cell-surface adhesion molecule, E-cadherin, and catenins in human tumor cells. Biochem Biophys Res Commun. 1999;261:406–11. doi: 10.1006/bbrc.1999.1002. [DOI] [PubMed] [Google Scholar]
- Ivanov DB, Philippova MP, Tkachuk VA. Structure and functions of classical cadherins. Biochemistry (Mosc) 2001;66:1174–1186. doi: 10.1023/a:1012445316415. [DOI] [PubMed] [Google Scholar]
- Hoffmann KM, Tonks NK, Barford D. The crystal structure of domain 1 of receptor protein-tyrosine phosphatase mu. J Biol Chem. 1997;272:27505–8. doi: 10.1074/jbc.272.44.27505. [DOI] [PubMed] [Google Scholar]
- Johnson KG, Van Vactor D. Receptor protein tyrosine phosphatases in nervous system development. Physiol Rev. 2003;83:1–24. doi: 10.1152/physrev.00016.2002. [DOI] [PubMed] [Google Scholar]
- Kamiguchi H. The mechanism of axon growth: what we have learned from the cell adhesion molecule L1. Mol Neurobiol. 2003;28:219–228. doi: 10.1385/MN:28:3:219. [DOI] [PubMed] [Google Scholar]
- Kiryushko D, Berezin V, Bock E. Regulators of neurite outgrowth: role of cell adhesion molecules. Ann N Y Acad Sci. 2004;1014:140–154. doi: 10.1196/annals.1294.015. [DOI] [PubMed] [Google Scholar]
- Lagenaur C, Lemmon V. An L1-like molecule, the 8D9 antigen, is a potent substrate for neurite extension. Proc Natl Acad Sci U S A. 1987;84:7753–7757. doi: 10.1073/pnas.84.21.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagunowich LA, Grunwald GB. Expression of calcium-dependent cell adhesion during ocular development: a biochemical, histochemical and functional analysis. Dev Biol. 1989;135:158–171. doi: 10.1016/0012-1606(89)90166-8. [DOI] [PubMed] [Google Scholar]
- Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3' kinase/AKT pathways. Oncogene. 2005;24:7443–7454. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- LaVail JH, Cowan WM. The development of the chick optic tectum. II. Autoradiographic studies. Brain Res. 1971;28:421–441. [PubMed] [Google Scholar]
- Lewis JE, Jensen PJ, Johnson KR, Wheelock MJ. E-cadherin mediates adherens junction organization through protein kinase C. J Cell Sci. 1994;107:3615–3621. doi: 10.1242/jcs.107.12.3615. [DOI] [PubMed] [Google Scholar]
- Lilien J, Balsamo J, Arregui C, Xu G. Turn-off, drop-out: functional state switching of cadherins. Dev Dyn. 2002;224:18–29. doi: 10.1002/dvdy.10087. [DOI] [PubMed] [Google Scholar]
- Lilien J, Balsamo J. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of beta-catenin. Curr Opin Cell Biol. 2005;17:459–65. doi: 10.1016/j.ceb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Matsunaga M, Hatta K, Takeichi M. Role of N-cadherin cell adhesion molecules in the histogenesis of neural retina. Neuron. 1988;1:289–295. doi: 10.1016/0896-6273(88)90077-3. [DOI] [PubMed] [Google Scholar]
- Mey J, Thanos S. Development of the visual system of the chick. I. Cell differentiation and histogenesis. Brain Res Brain Res Rev. 2000;32:343–379. doi: 10.1016/s0165-0173(99)00022-3. [DOI] [PubMed] [Google Scholar]
- Mey J, Thanos S. Development of the visual system of the chick. II. Mechanisms of axonal guidance. Brain Res Brain Res Rev. 2001;35:205–245. doi: 10.1016/s0165-0173(01)00049-2. [DOI] [PubMed] [Google Scholar]
- Niewiadomska P, Godt D, Tepass U. DE-Cadherin is required for intercellular motility during Drosophila oogenesis. J Cell Biol. 1999;144:533–547. doi: 10.1083/jcb.144.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacquelet A, Rorth P. Regulatory mechanisms required for DE-cadherin function in cell migration and other types of adhesion. J Cell Biol. 2005;170:803–812. doi: 10.1083/jcb.200506131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne HR, Burden SM, Lemmon V. Modulation of growth cone morphology by substrate-bound adhesion molecules. Cell Motil Cytoskeleton. 1992;21:65–73. doi: 10.1002/cm.970210108. [DOI] [PubMed] [Google Scholar]
- Redies C, Takeichi M. N- and R-cadherin expression in the optic nerve of the chicken embryo. Glia. 1993;8:161–171. doi: 10.1002/glia.440080304. [DOI] [PubMed] [Google Scholar]
- Redies C. Cadherins in the central nervous system. Prog Neurobiol. 2000;61:611–648. doi: 10.1016/s0301-0082(99)00070-2. [DOI] [PubMed] [Google Scholar]
- Renaud-Young M, Gallin WJ. In the first extracellular domain of E-cadherin, heterophilic interactions, but nit the conserved His-Ala-Val motif, are required for adhesion. J Biol Chem. 2002;277:39609–39616. doi: 10.1074/jbc.M201256200. [DOI] [PubMed] [Google Scholar]
- Riehl R, Johnson K, Bradley R, Grunwald GB, Cornel E, Lilienbaum A, Holt CE. Cadherin function is required for axon outgrowth in retinal ganglion cells in vivo. Neuron. 1996;17:837–848. doi: 10.1016/s0896-6273(00)80216-0. [DOI] [PubMed] [Google Scholar]
- Ron D, Jiang Z, Yao L, Vagts A, Diamond I, Gordon A. Coordinated movement of RACK1 with activated betaIIPKC. J Biol Chem. 1999;274:27039–27046. doi: 10.1074/jbc.274.38.27039. [DOI] [PubMed] [Google Scholar]
- Rosdahl JA, Mourton TL, Brady-Kalnay SM. Protein kinase C delta (PKCdelta) is required for protein tyrosine phosphatase mu (PTPμ)-dependent neurite outgrowth. Mol Cell Neurosci. 2002;19:292–306. doi: 10.1006/mcne.2001.1071. [DOI] [PubMed] [Google Scholar]
- Skaper SD. Neuronal growth-promoting and inhibitory cues in neuroprotection and neuroregeneration. Ann N Y Acad Sci. 2005;1053:376–385. doi: 10.1196/annals.1344.032. [DOI] [PubMed] [Google Scholar]
- Sklan EH, Podoly E, Soreq H. RACK1 has the nerve to act: Structure meets function in the nervous system. Pro Neurobiol. 2006;78:117–134. doi: 10.1016/j.pneurobio.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Skoudy A, Garcia de Herreros A. The protein kinase C activator TPA modulates cellular levels and distribution of E-cadherin in HT-29 human intestinal epithelial cells. FEBS Lett. 1995;374:415–418. doi: 10.1016/0014-5793(95)01167-d. [DOI] [PubMed] [Google Scholar]
- Sui FX, Kiser TD, Hyun SW, Angelini DJ, Del Vecchio RL, Young BA, Hasday JD, Romer LH, Passaniti A, Tonks NK, Goldblum SE. Receptor protein tyrosine phosphatase micro regulates the paracellular pathway in human lung microvascular endothelia. Am J Pathol. 2005;166:1247–1258. doi: 10.1016/s0002-9440(10)62343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M, Abe K. Synaptic contact dynamics controlled by cadherin and catenins. Trends Cell Biol. 2005;15:216–221. doi: 10.1016/j.tcb.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Wadia JS, Dowdy SF. Protein transduction technology. Curr Opin Biotechnol. 2002;13:52–6. doi: 10.1016/s0958-1669(02)00284-7. [DOI] [PubMed] [Google Scholar]
- Xie Y, Massa SM, Ensslen-Craig SE, Major DL, Yang T, Tisi MA, Derevyanny VD, Runge WO, Mehta BP, Moore LA, Brady-Kalnay SM, Longo FM. Protein-tyrosine phosphatase (PTP) wedge domain peptides: a novel approach for inhibition of PTP function and augmentation of protein-tyrosine kinase function. J Biol Chem. 2006;281:16482–92. doi: 10.1074/jbc.M603131200. [DOI] [PubMed] [Google Scholar]
- Xu L, Overbeek PA, Reneker LW. Systematic analysis of E-, N- and P-cadherin expression in mouse eye development. Exp Eye Res. 2002;74:753–760. doi: 10.1006/exer.2002.1175. [DOI] [PubMed] [Google Scholar]
- Zondag GC, Reynolds AB, Moolenaar WH. Receptor protein-tyrosine phosphatase RPTPμ binds to and dephosphorylates the catenin p120 (ctn) J Biol Chem. 2000;275:11264–11269. doi: 10.1074/jbc.275.15.11264. [DOI] [PubMed] [Google Scholar]


