Abstract
OBJECTIVES
To determine the association between socioeconomic status (SES) and survival in older patients with melanoma.
DESIGN
Retrospective cohort study.
SETTING
Surveillance, Epidemiology and End Results (SEER): a population-based cancer registry covering 14% of the U.S. population.
PARTICIPANTS
Twenty-three thousand sixty-eight patients aged 65 and older with melanoma between 1988 and 1999.
MEASUREMENTS
Outcome was melanoma-specific survival. Main independent variable was SES (measured as census tract median household income) taken from the SEER-Medicare linked data.
RESULTS
Subjects residing in lower-income areas (≤$30,000/y) had lower 5-year survival rates (88.5% vs 91.1%, P<.001) than subjects residing in higher-income areas (>$30,000/y). In Cox proportional hazard models, higher income was associated with lower risk of death from melanoma (hazard ratio = 0.88, 95% confidence interval = 0.79–0.98, P = .02) after adjusting for sociodemographics, stage at diagnosis, thickness, histology, anatomic site, and comorbidity index. There was an interaction effect between SES and ethnicity and survival from melanoma. For whites and nonwhites (all other ethnic groups), 5-year survival rates increased as income increased, although the effect was greater for nonwhites (77.6% to 90.1%, 1st to 5th quintiles, P = .01) than for whites (89.0% to 91.9%, 1st to 5th quintiles, P<.001).
CONCLUSION
Older subjects covered by Medicare residing in lower-SES areas had poorer melanoma survival than those residing in higher-SES areas. Further research is needed to determine whether low SES is associated with late-stage disease biology and poorer early detection of melanoma.
Keywords: melanoma, socioeconomic status, ethnicity, survival, elderly
White older men have had a sustained increase in melanoma incidence and mortality in the last 2 decades in the United States. From 1969 through 1999, it has been reported that mortality rates from melanoma increased 157% in men aged 65 and older, more than three times greater than the increase for women of the same age.1 Similarly, the incidence of melanoma increased nearly five times for older men; the incidence also increased for older women, but the increase was less than in older men. Another study2 found that the rate of thick melanomas (≥4 mm) has increased significantly in men aged 60 and older but not in men of other ages or in women.
Socioeconomic status (SES) influences cancer survival. Two English studies reported that patients from affluent areas had better survival rates than patients from deprived areas for lung, breast, colorectal, bladder, prostate, uterine, and cervical cancer.3,4 Disparities in cancer survival have been also reported in the United States and Canada between socioeconomic and ethnic groups.5,6
Population-based, cohort, and hospital-based registry studies have showed lower survival from melanoma in low-SES populations.7 In those studies, SES was measured at individual (occupation) or aggregate levels (median household income, education, socioeconomic deprivation, or poverty percentage). SES may interact with other sociodemographic factors and affect living conditions and availability of health care, and by affecting health status or by determining stage at diagnosis, may affect survival from melanoma.4,7
The Surveillance Epidemiology and End Results (SEER) tumor registry, which has been linked to Medicare claims data at the National Cancer Institute, was used in this study.8 Few studies on melanoma have been done using the SEER-Medicare linked database and none on the association between SES and melanoma.9 The objective of this study was to examine the relationship between SES and survival in patients with melanoma in the older Medicare population. The hypothesis is that low SES is associated with poorer survival in patients with melanoma.
METHODS
Data Source
Data for the study were obtained from a database of linked tumor registry records from the SEER program and Medicare claims from the Health Care Financing Administration (now called the Centers for Medicare and Medicaid Services). Cases reported by the SEER registries from 1988 to 1999 were matched against Medicare’s master enrollment file.9,10 The method of linking these data has been described previously.10
Study Sample
Because of an interest in disparities, subjects with unknown ethnic group were excluded from this study. The sample included 23,068 subjects aged 65 and older who were Medicare beneficiaries, resided in a SEER area (11 areas), and received a new diagnosis of melanoma in 1988 through 1999.
Measures
The outcome was melanoma-specific survival. Survival is the period of time between the date of diagnosis and the date of death from melanoma, measured in months.
SES was the main independent variable. SES was measured according to median annual household income per year collected at the census tract level. Median annual household income was used as a categorical and a continuous variable. For bivariate comparisons, income was used in quintiles and dichotomized as $30,000 or less and more than $30,000; the cutoff point corresponds to the median household income in the year before the decennial census for the United States in 1989.11 For multivariate comparisons, it was used as a continuous variable (natural logarithm (ln) of (income/1,000)). The use of census-derived measures of SES has been validated in previous studies.11,12
Age was categorized into four groups (65–69, 70–74, 75–79, and ≥80). Other variables were sex (male, female), marital status (married, unmarried, unknown), and ethnicity, which was used in two ways: first, in nine categories (white, black, Hispanic, Chinese, Japanese, Filipino, Hawaiian, American Indian, and other) and second, in two categories (white, nonwhite (all other ethnic groups)). Cohort effect was assessed by period 1988 to 1993 (1) versus period 1994 to 1999 (0).
Stage of melanoma at diagnosis is divided into five categories: (1) in situ, (2) local (invasive cancer confined to the organ of origin), (3) regional (spread to adjacent organs by direct extension or to regional lymph nodes), (4) distant (extension to organs other than those covered in the regional category or metastases to distant organs or distant lymph nodes), and (5) unstaged or unknown stage. Tumor thickness (Breslow’s depth, in mm) was treated as a categorical variable, according to the American Joint Committee on Cancer categories (≤1.00 mm, 1.01–2.00 mm, 2.01–4.00 mm, >4.00 mm, and unknown).13 Histology was categorized into nodular, lentigo maligna, superficial spreading, acral lentiginous, and other. Site of the tumor was categorized as trunk, face, upper limb, lower limb, and nonspecified. Comorbidity was ascertained from Medicare inpatient and outpatient claims through diagnoses or procedures made 1 year before the diagnosis of melanoma. The comorbidity index created earlier14 and later validated15 was used, using the International Classification of Diseases, Ninth Revision, Clinical Modification, diagnosis and procedure codes. Comorbidity index was categorized into none, one, and two or more.
Analyses
For descriptive analyses, frequencies and percentages were used to describe the study population. Pearson chi-square (χ2) analysis was used to test bivariate differences across categories of variables. Survival analysis techniques were used to estimate the risk of death from melanoma after diagnosis. The date of censoring was at death from other causes or on December 31, 2000, whichever came first. Kaplan-Meier product limit was used to estimate the event-free duration for SES and ethnic categories. The log-rank test was used to compare survival curves across SES and ethnic variable categories.
Adjusted Cox regression proportional hazard regression models (PHREG procedure) were used to predict the risk of death from melanoma at follow-up and to determine the independent influence of SES and other potential predictors on the event (death from melanoma). Assumptions underlying the model, such as proportionality and an absence of colinearity, were tested and found to be met. For analyses of interaction between income and ethnicity, ethnicity was dichotomized because of the small number of other ethnic groups different from whites. The PHREG procedure and the contrast statement for calculations related to interactions were used. For all analyses, the significance level (two tailed) was set at P<.05. All computer programming and analyses were completed using SAS for Windows version 9.1 (SAS Institute, Inc., Cary, NC).
RESULTS
Table 1 shows that 60% of the study subjects were men, 45.2% were aged 75 and older, 51.2% were married, and 96.3% were white. In addition, 58.3% of cases corresponded to the period 1994 to 1999; 32.8% and 45.7% had in situ and localized stages at diagnosis, respectively; 30.7% had lesion thickness of less than 1 mm; 7.1% were nodular, 30.4% were lentigo maligna, and 21.6% were superficial spreading melanomas; and 37.4% of lesions were located on the face and 22.8% on the trunk. Residing in low-income areas (≤$30,000/y) was associated with older age (especially ≥80), female sex, being unmarried, being black or Hispanic, being diagnosed between 1988 and 1993, having regional and distant stages at diagnosis, having thicker lesions (2.01–4.00 mm, >4.00 mm), having nodular or acral lentiginous melanoma, and having two or more comorbid conditions. Whether a cohort effect influence the data was also analyzed. Subjects who were diagnosed during the later period (1994–99) were more likely to have an in situ diagnosis (37.2% vs 26.7%); and less likely to have unstaged melanoma (2.9% v 20%, a potential underascertainment bias of cases) than those diagnosed during the earlier period (1988–93) (P<.001).
Table 1.
Characteristics of the Study Population of Subjects with Cutaneous Melanoma, 1988–1999, 11 Surveillance, Epidemiology and End Results Areas in the United States
| Overall Sample (N = 23,068) | Subjects Residing in Areas with Annual Income ≤$30,000 (n = 5,442) | Subjects Residing in Areas with Annual Income >$30,000 (n = 17,108) | |
|---|---|---|---|
| Characteristic | n (%) | ||
| Age | * | ||
| 65–69 | 6,954 (28.0) | 1,376 (25.3) | 4,935 (28.8) |
| 70–74 | 6,191 (26.8) | 1,357 (24.9) | 4,686 (27.4) |
| 75–79 | 4,896 (21.0) | 1,114 (20.5) | 3,627 (21.2) |
| ≥80 | 5,577 (24.2) | 1,595 (29.3) | 3,860 (22.6) |
| Sex | * | ||
| Male | 13,843 (60.0) | 3,018 (55.5) | 10,494 (61.3) |
| Female | 9,225 (40.0) | 2,424 (44.5) | 6,614 (38.7) |
| Marital status | * | ||
| Married | 11,817 (51.2) | 2,504 (46.0) | 9,105 (53.2) |
| Unmarried | 5,905 (25.6) | 1725 (31.7) | 4,070 (23.8) |
| Unknown | 5,346 (23.2) | 1213 (22.3) | 3,933 (23.0) |
| Race/ethnicity | * | ||
| White | 22,227 (96.3) | 5,128 (94.2) | 16,620 (97.1) |
| Black | 148 (0.6) | 88 (1.7) | 59 (0.3) |
| Hispanic | 459 (2.0) | 175 (3.2) | 266 (1.5) |
| Chinese | 36 (0.2) | 7 (0.1) | 26 (0.2) |
| Japanese | 75 (0.3) | 18 (0.3) | 51 (0.3) |
| Filipino | 36 (0.2) | 8 (0.2) | 24 (0.1) |
| Hawaiian | 15 (0.1) | 0 (0.0) | 11 (0.1) |
| American Indian | 17 (0.1) | 7 (0.1) | 9 (0.1) |
| Other | 55 (0.2) | 11 (0.2) | 42 (0.3) |
| Period | * | ||
| 1988–1993 | 9,621 (41.7) | 2,425 (55.4) | 6,972 (40.7) |
| 1994–1999 | 13,447 (58.3) | 3,017 (44.6) | 10,136 (59.3) |
| Stage at diagnosis | * | ||
| In situ | 7,570 (32.8) | 1,714 (31.5) | 5,677 (33.2) |
| Localized | 10,543 (45.7) | 2,506 (46.0) | 7,821 (45.7) |
| Regional | 1,966 (8.5) | 546 (10.0) | 1,383 (8.1) |
| Distant | 675 (3.0) | 216 (4.0) | 450 (2.6) |
| Unknown or unstaged | 2,314 (10.0) | 460 (8.5) | 1,777 (10.4) |
| Tumor thickness, mm (Breslow depth) | * | ||
| ≤1.00 | 7,082 (30.7) | 1,599 (29.4) | 5,139 (30.0) |
| 1.01–2.00 | 2,042 (8.8) | 526 (9.7) | 1,482 (8.7) |
| 2.01–4.00 | 1,511 (6.6) | 427 (7.8) | 1,062 (6.2) |
| >4.00 | 898 (3.9) | 268 (4.9) | 609 (3.6) |
| Unknown or unmeasured | 11,535 (50.0) | 2,622 (48.2) | 8,816 (51.3) |
| Histology | * | ||
| Nodular | 1,647 (7.1) | 447 (8.2) | 1,169 (6.8) |
| Lentigo maligna | 7,003 (30.4) | 1,662 (30.6) | 5,168 (30.2) |
| Superficial spreading | 4,982 (21.6) | 1,142 (21.0) | 3,758 (22.0) |
| Acral lentiginous | 316 (1.4) | 105 (1.9) | 205 (1.2) |
| Other | 9,120 (39.5) | 2,086 (38.3) | 6,808 (39.8) |
| Site | * | ||
| Trunk | 5,262 (22.8) | 1,032 (18.9) | 4,114 (24.0) |
| Face | 8,640 (37.4) | 2,230 (41.0) | 6,205 (36.3) |
| Upper limb | 5,121 (22.2) | 1,186 (21.8) | 3,821 (22.3) |
| Lower limb | 3,063 (13.3) | 739 (13.6) | 2,272 (13.3) |
| Nonspecified | 982 (4.3) | 255 (4.7) | 696 (4.1) |
| Comorbidities | * | ||
| 0 | 18,944 (82.1) | 4,303 (79.1) | 14,218 (83.1) |
| 1 | 2,705 (11.7) | 712 (13.1) | 1,932 (11.3) |
| ≥2 | 1,419 (6.2) | 427 (7.8) | 958 (5.6) |
Note: Data on income were missing for 518 subjects; data from U.S. Census 1990.
P<.001 (calculated using Pearson chi-square comparing ≤$30,000 with >$30,000).
The overall 5-year Kaplan-Meier survival rate was 90.5% (95% confidence interval (CI) = 90.0–90.9). When subjects were stratified by income (≤$30,000/y vs >$30,000/y), there was a significant difference on survival rates between low-income (88.5%, 95% CI = 87.5–89.4) and high-income areas (91.1%, 95% CI = 90.6–91.5). Subjects aged 80 and over had significantly lower survival rates (87.4%, 95% CI = 86.2–88.4) than subjects of other age categories (65–69: 91.7%, 95% CI = 90.9–92.4; 70–74: 91.4%, 95% CI = 90.7–92.2; 75–79: 90.6%, 95% CI = 89.6–91.5). In addition, men had significantly lower survival rates (89.7%, 95% CI = 89.1–90.3) than women (91.5%, 95% CI = 90.9–92.1).
In the adjusted Cox proportional regression analyses (Table 2), as income increased, there was a significant decrease in the risk of death from melanoma. In Model 1, adjusting for other sociodemographic variables, the hazard ratio (HR) for income was 0.79 (95% CI = 0.71–0.88, P<.001). Blacks, Hispanics, Chinese, Filipinos, and Hawaiians had a greater risk of death from melanoma than whites. In the fully adjusted Model 2, although the effect decreased, higher income was also associated with lower risk of death from melanoma (HR = 0.88, 95% CI = 0.79–0.98, P = .02). The effect of race disappeared and ethnic groups were no longer significant after adjusting for stage, tumor characteristics, and comorbidities. Other factors independently associated with greater risk of death from melanoma were older age, male sex, being unmarried, and being diagnosed between 1988 and 1993. Advanced stage at diagnosis of melanoma (localized, regional, distant vs in situ) and thicker lesions (1.01–2.00 mm, 2.01–4.00 mm, >4.00 mm vs ≤1.00 mm) were associated with greater risk of death. Lentigo maligna, superficial spreading, acral lentiginous, and other histological subtypes were associated with a lower risk of death than nodular melanoma. Melanomas located on the face and upper limbs were associated with a lower risk of death, but melanomas in a nonspecified site were associated with a higher risk of death than melanomas located on the trunk. Finally, subjects with two or more comorbid conditions had a higher risk of death from melanoma than subjects without comorbidities.
Table 2.
Survival of Patients with Cutaneous Melanoma: Multivariate Analyses Using Cox Proportional Hazard Models, 1988–1999
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Patient and Tumor Characteristics | Hazard Ratio (95% Confidence Interval) P-value | |||
| Census tract median income* | 0.79 (0.71–0.88) | <.001 | 0.88 (0.79–0.98) | .02 |
| Race/ethnicity | ||||
| White | 1.00 | 1.00 | ||
| Black | 1.82 (1.22–2.70) | .003 | 1.11 (0.74–1.67) | .61 |
| Hispanic | 1.51 (1.16–1.98) | .002 | 1.08 (0.83–1.42) | .55 |
| Chinese | 2.54 (1.21–5.33) | .014 | 1.90 (0.90–4.00) | .09 |
| Japanese | 1.56 (0.78–3.12) | .21 | 1.76 (0.87–3.53) | .11 |
| Filipino | 3.26 (1.63–6.53) | <.001 | 1.48 (0.73–2.98) | .27 |
| Hawaiian | 3.42 (1.10–10.6) | .03 | 2.08 (0.67–6.48) | .20 |
| American Indian | 1.75 (0.44–7.01) | .43 | 1.45 (0.36–5.90) | .59 |
| Other | 0.65 (0.16–2.62) | .54 | 0.76 (0.19–3.06) | .70 |
| Age | ||||
| 65–69 | 1.00 | 1.00 | ||
| 70–74 | 1.10 (0.97–1.24) | .15 | 1.15 (1.01–1.30) | .04 |
| 75–79 | 1.17 (1.02–1.33) | .02 | 1.24 (1.08–1.42) | .001 |
| ≥80 | 1.56 (1.37–1.77) | <.001 | 1.48 (1.30–1.68) | <.001 |
| Sex | ||||
| Male | 1.00 | 1.00 | ||
| Female | 0.68 (0.62–0.76) | <.001 | 0.70 (0.62–0.77) | <.001 |
| Marital status | ||||
| Married | 1.00 | 1.00 | ||
| Unmarried | 1.36 (1.22–1.51) | <.001 | 1.15 (1.04–1.28) | .003 |
| Unknown | 0.30 (0.25–0.36) | <.001 | 0.61 (0.51–0.73) | <.001 |
| Period (years of diagnosis) | ||||
| 1988–1993 | 2.03 (1.84–2.23) | <.001 | 1.68 (1.52–1.87) | <.001 |
| 1994–1999 | 1.00 | 1.00 | ||
| Stage at diagnosis | ||||
| In situ | 1.00 | |||
| Localized | 8.83 (6.01–12.9) | <.001 | ||
| Regional | 23.2 (15.7–34.3) | <.001 | ||
| Distant | 94.0 (63.3–139.5) | <.001 | ||
| Unknown or unstaged | 19.1 (13.1–27.8) | <.001 | ||
| Tumor thickness, mm (Breslow depth) | ||||
| ≤1.00 | 1.00 | |||
| 1.01–2.00 | 2.06 (1.69–2.50) | <.001 | ||
| 2.01–4.00 | 3.11 (2.57–3.76) | <.001 | ||
| >4.00 | 3.17 (2.56–3.92) | <.001 | ||
| Unknown or unmeasured | 2.05 (1.70–2.47) | <.001 | ||
| Histology | ||||
| Nodular | 1.00 | |||
| Lentigo maligna | 0.18 (0.14–0.24) | <.001 | ||
| Superficial spreading | 0.48 (0.41–0.57) | <.001 | ||
| Acral lentiginous | 0.61 (0.43–0.87) | .003 | ||
| Other | 0.78 (0.68–0.89) | <.001 | ||
| Site | ||||
| Trunk | 1.00 | |||
| Face | 0.81 (0.71–0.93) | .003 | ||
| Upper limb | 0.69 (0.60–0.81) | <.001 | ||
| Lower limb | 1.02 (0.87–1.19) | .81 | ||
| Unspecified | 1.93 (1.61–2.30) | <.001 | ||
| Comorbidity | ||||
| 0 | 1.00 | |||
| 1 | 1.06 (0.92–1.23) | .41 | ||
| ≥2 | 1.29 (1.07–1.56) | .008 | ||
US $/year, ln [income/1,000], continuous.
Figure 1 presents the 5-year survival rates (%) by income quintiles (<$28,089, $28,089–35,440, $35,441–42,924, $42,925–55,025, >$55,025) and two ethnic groups. Both groups, whites and nonwhites, tended to have greater survival rates as income increased (nonwhites, from 77.6 to 90.1, log-rank χ2 = 12.2, P = .01; whites, from 89.0 to 91.9, log-rank χ2 = 19.5, P<.001), but there are wide confidence intervals for nonwhites because of their small number. Nonwhites had lower survival rates in the first two quintiles (77.6, 95% CI = 71.4–82.6; 82.8, 95% CI = 74.2–88.7) than whites (89.0, 95% CI = 87.8–90.0; 90.2, 95% CI = 89.1–91.2), but in the third quintile and over, the confidence intervals overlapped. Two-way interaction effects were tested to assess whether the risk of death from melanoma varied by census tract median income for ethnicity (P = .02). For whites and nonwhites, the risk of death decreased as income increased, although their effects differ in magnitude; for example, the HR for income in whites was 0.76 (95% CI = 0.68–0.85) and in nonwhites was 0.47 (95% CI = 0.32–0.70). When adjusting for all other variables, the interaction was no longer significant; the HR for income in whites was 0.89 (95% CI = 0.79–0.99) and in nonwhites was 0.79 (95% CI = 0.54–1.17) (interaction term P = .56).
Figure 1.
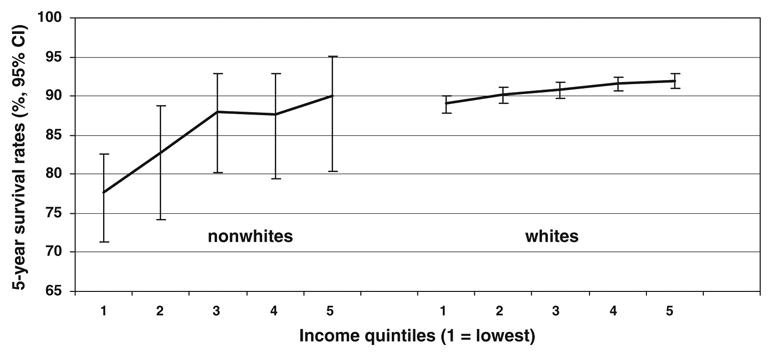
Five-year Kaplan-Meier survival rates by income quintiles and ethnic groups. CI = confidence interval.
Additional analyses show that nonwhites were more likely to be in advanced stages at diagnosis (regional or distant) and have thicker lesions (>2 mm) than white people when they were stratified by income (≤$30,000 vs >$30,000). Percentages for advanced stages were 22.9% (nonwhite and ≤$30,000), 17.2% (nonwhite and >$30,000), 13.5% (white and ≤$30,000), and 10.5% (white and >$30,000) (P<.001). Percentages for thicker lesions (>2 mm) were 15.9% (nonwhite and ≤$30,000), 13.1% (nonwhite and >$30,000), 12.6% (white and ≤$30,000), and 9.7% (white and >$30,000) (P<.001). This may explain in part why the interaction effect between income and ethnicity and survival disappears after adjusting for stage, thickness, and other tumor characteristics.
DISCUSSION
The findings indicate that SES, measured according to census tract median household income, is significantly associated with survival from melanoma, even after adjusting for other sociodemographic variables, stage at diagnosis, co-morbidity index, and tumor characteristics such as thickness, histology, and location of the lesion in the body. This suggests that residing in low-income areas is an independent poor prognostic factor for melanoma survival.
This association between SES and melanoma survival is in accordance with other reports in the literature in the United States and other countries.7,16–21 One population-based study in the United States16 reported that men and women of all ages with invasive melanoma living in poor areas had lower survival rates than those living in wealthier areas. A study using hospital-based cancer registries data17 reported that patients living in low-income areas (<$20,000, by ZIP code) had lower survival rates from melanoma than patients living in high-income areas (>$47,000) (83.2% vs 90.9%). Finally, a population-based study in Massachusetts18 reported that advanced stage of melanoma was associated with lower census tract or ZIP code income (lowest vs highest tertiles rate ratio = 1.6, 95% CI = 1.2–2.3). In studies in other countries, lower melanoma survival rates were reported for patients from lower-SES groups or residing in lower-SES areas than for patients from higher-SES groups or residing in higher-SES areas.19–21
SES may affect melanoma survival through at least four types of factors: biological characteristics of the tumor, stage or thickness at diagnosis, host factors, and treatment.22 In the current study, nodular melanoma was more frequent in subjects residing in low SES areas (Table 1) and was associated with a higher risk of dying from melanoma (Table 2). In other studies the nodular type of melanoma was reported as a poor prognostic factor for melanoma survival.13,23,24 In addition, in the current study, advanced stage at diagnosis and thicker lesions were associated with residing in low-SES areas (Table 1) and with a higher risk of death from melanoma (Table 2). Other studies have been also reported the association between SES and stage or thickness of melanoma at diagnosis and between SES and melanoma survival, adjusting for stage or thickness.17–19,25,26 Furthermore, in the current study, a higher number of comorbid conditions were associated with a higher risk of death from melanoma. Indeed, host factors such as comorbidities, nutritional status, and immune functions are usually associated with SES.4–6,22 Comorbidities may influence the progression of the tumor after diagnosis and the ability to tolerate the treatment, which in turn could affect survival from melanoma. Finally, treatment may affect survival from melanoma.22,27 Although data on treatment were not available for this analysis, earlier detection and local resection of melanoma, which would potentially enhance survival, remain as the main strategies for treating melanoma. Indeed, adjuvant therapy (e.g., interferon alpha) and treatment of metastatic disease have appreciable toxicity and unclear survival influence and would not likely influence results in older adults.28
The second major finding from this study was the interaction effect between income and ethnicity and survival of subjects with melanoma. Indeed, nonwhites exhibited poorer survival rates than whites, especially if they resided in low-income areas (≤$30,000/y), although the interaction effect disappeared after adjusting for stage and tumor characteristics, probably because it was demonstrated that nonwhites were more likely to advanced stage and thicker lesions than whites even when stratified by income categories (see Results).
The findings of the current study that older age, male sex, and being unmarried were associated with greater risk of death from melanoma are in accordance with some previous studies. Recent studies have showed that older age has been associated with lower survival in patients with melanoma.13,28 Older patients are usually screened less than younger patients, have later stages of melanoma when diagnosed, and have more nodular melanomas (high potentiality for metastases).17,28,29 Sex may influence the clinical course of melanoma, which has a worse prognosis in men than women.19,25 Women may have a distribution of melanoma lesions localized to more-favorable anatomic sites than men; women are also more likely to seek screening. Furthermore, men also present with a higher proportion of thicker lesions.13,28 Married people usually have more social ties and support that may affect their response to the disease or cause them to look for early diagnosis of melanoma.30
This study showed that there was a survival advantage during 1994 to 1999 over the earlier period. There are two possible explanations for this phenomenon. First is its association with low poverty; in the later period, a lower percentage of subjects resided in low-income areas. Second is the presence of lead-time bias; in the later period, there was a higher percentage of in situ stage, probably because of increased skin screening.
This study has some limitations. First, the study findings applied only to older patients enrolled in Medicare. Second, census tract level socioeconomic data are imperfect proxy measures of individual level SES. Third, data were not available for other factors (e.g., nutritional status) or treatment that could affect survival in people with melanoma.22 Fourth, a small number of minority patients were included, which could cause an underestimation of the association between race and survival. Fifth, tumor ulceration and lymph node involvement are important prognostic factors for melanoma not captured by the database.13 Nevertheless, this study has some strengths. First, no detailed report related to the interaction between SES and ethnicity and the risk of death from melanoma was found in the literature. Second, the effect of SES on survival from melanoma was demonstrated, adjusting for other variables that are well known to be major prognostic factors for death from melanoma. Third, the SEER-Medicare linked database used in the study is considered to be a complete database and may capture 88% of melanoma cases.9 Fourth, the finding that there was a greater percentage of nodular melanoma in older adults residing in low-SES areas is important, because nodular melanoma often eludes early detection and presents at a more-advanced stage, especially in older adults.17,28,29
In conclusion, older subjects covered by Medicare residing in low-SES areas had a greater risk for death from melanoma. For whites and nonwhites, the risk of death decreased as income increased, although the effect was greater for nonwhites than whites. Further research is needed to determine whether low SES leads to later diagnosis, worst biological characteristics of the tumor (e.g., ulceration), or poorer early detection or resection of melanoma in older adults.
Acknowledgments
Financial Disclosure: None of the authors have any conflict of interest related to this work. This study was supported by Grant R24 HS011618-04 from the Agency of Healthcare Research and Quality. It was also supported, in part, by the University of Texas Medical Branch, Center on Population Health and Health Disparities, National Institutes of Health Grant P50CA105631. We are indebted to the Applied Research Program, National Cancer Institute; to the Office of Research, Development, and Information, Centers for Medicare and Medicaid Services; and to the SEER Program for the creation of the SEER-Medicare database.
Author Contributions: Carlos A. Reyes-Ortiz: content, literature review, concept and design, acquisition of data, analysis and interpretation of the data, preparation of the manuscript, statistical expertise, supervision. James S. Goodwin: content, concept and design, analysis and interpretation of the data, editing of the manuscript, obtaining funding, supervision. Jean L. Freeman: content, concept and design, acquisition of data, analysis and interpretation of the data, and obtaining funding. Yong-Fang Kuo, PhD: concept and design, statistical expertise, analysis and interpretation of the data.
Sponsor’s Role: The sponsors had no role in the design, methods, data collection, analysis, or manuscript preparation. The interpretation and reporting of these data are the sole responsibilities of the authors.
Footnotes
An earlier version of this work was presented as a poster at the 2006 American Geriatrics Society meeting, Chicago, Illinois.
References
- 1.Geller AC, Miller DR, Annas GD, et al. Melanoma incidence and mortality among US whites, 1969–99. JAMA. 2002;288:1719–1720. doi: 10.1001/jama.288.14.1719. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Devesa SS, Hartge P, et al. Recent trends in cutaneous melanoma incidence among whites in the United States. J Natl Cancer Inst. 2001;93:678–683. doi: 10.1093/jnci/93.9.678. [DOI] [PubMed] [Google Scholar]
- 3.Schrijvers CT, Mackenbach JP, Lutz JM, et al. Deprivation, stage at diagnosis and cancer survival. Int J Cancer. 1995;63:324–329. doi: 10.1002/ijc.2910630303. [DOI] [PubMed] [Google Scholar]
- 4.Kogevinas M, Marmot MG, Fox AJ, et al. Socioeconomic differences in cancer survival. J Epidemiol Community Health. 1991;45:216–219. doi: 10.1136/jech.45.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 6.Mackillop WJ, Zhang-Salomons J, Boyd CJ, et al. Associations between community income and cancer incidence in Canada and the United States. Cancer. 2000;89:901–912. doi: 10.1002/1097-0142(20000815)89:4<901::aid-cncr25>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 7.Reyes-Ortiz CA, Goodwin JS, Freeman JL. The effect of socioeconomic factors on incidence, stage at diagnosis and survival of cutaneous melanoma. Med Sci Monit. 2005;11:RA163–RA172. [PubMed] [Google Scholar]
- 8.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 9.Barzilai DA, Koroukian SM, Neuhauser D, et al. The sensitivity of Medicare data for identifying incident cases of invasive melanoma (United States) Cancer Causes Control. 2004;15:179–184. doi: 10.1023/B:CACO.0000019504.74553.32. [DOI] [PubMed] [Google Scholar]
- 10.Potosky AL, Riley GF, Lubitz JD, et al. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31:732–748. [PubMed] [Google Scholar]
- 11.Krieger N, Chen JT, Waterman PD, et al. Choosing area based socioeconomic measures to monitor social inequalities in low birth weight and childhood lead poisoning: The Public Health Disparities Geocoding Project (US) J Epidemiol Community Health. 2003;57:186–199. doi: 10.1136/jech.57.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofer T, Wolfe R, Tedeschi P, et al. Use of community versus individual socioeconomic data predicting variation in hospital use. Health Serv Res. 1998;33:243–259. [PMC free article] [PubMed] [Google Scholar]
- 13.Balch CM, Soong S-J, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: Validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622–3634. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: Differing perspectives. J Clin Epidemiol. 1993;46:1075–1079. doi: 10.1016/0895-4356(93)90103-8. [DOI] [PubMed] [Google Scholar]
- 16.Singh GK, Miller BA, Hankey BF, et al., editors. NCI Cancer Surveillance Monograph Series, Number 4 (NIH Publication no. 03–5417) Bethesda, MD: National Cancer Institute; 2003. Area Socioeconomic Variations in U.S. Cancer Incidence, Mortality, Stage, Treatment, and Survival, 1975–1999. [Google Scholar]
- 17.Chang AE, Kamell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma. A summary of 84,836 cases from the past decade. Cancer. 1998;83:1664–1678. doi: 10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 18.Geller AC, Miller DR, Lew RA, et al. Cutaneous melanoma mortality among the socioeconomically disadvantaged in Massachusetts. Am J Public Health. 1996;86:538–544. doi: 10.2105/ajph.86.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackie RM, Hole DJ. Incidence and thickness of primary tumors and survival of patients with cutaneous malignant melanoma in relation to socioeconomic status. BMJ. 1996;312:1125–1128. doi: 10.1136/bmj.312.7039.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luke CG, Coventry BJ, Foster-Smith EJ, et al. A critical analysis of reasons for improved survival from invasive cutaneous melanoma. Cancer Causes Control. 2003;14:871–878. doi: 10.1023/b:caco.0000003838.48507.8b. [DOI] [PubMed] [Google Scholar]
- 21.Coleman NP, Rachet N, Woods LM. Trends and socioeconomic inequalities in cancer survival in England and Wales up to 2001. Br J Cancer. 2004;90:1367–1373. doi: 10.1038/sj.bjc.6601696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Auvinen A, Karjalainen S. Possible explanations for social class differences in cancer patient survival. In: Kogevinas M, Pearce N, Susser M, et al., editors. Social Inequalities and Cancer (IARC Scientific Publications no. 138) Lyon, France: International Agency for Research on Cancer; 1997. pp. 377–397. [PubMed] [Google Scholar]
- 23.Chamberlain AJ, Fritschi L, Giles GG, et al. Nodular type and older age as the most significant associations of thick melanoma in Victoria, Australia. Arch Dermatol. 2002;138:609–614. doi: 10.1001/archderm.138.5.609. [DOI] [PubMed] [Google Scholar]
- 24.Demierre M-F, Chung C, Miller DR, et al. Early detection of thick melanomas in the United States. Beware of the nodular type. Arch Dermatol. 2005;141:745–750. doi: 10.1001/archderm.141.6.745. [DOI] [PubMed] [Google Scholar]
- 25.Van Durme DJ, Ferrante JM, Pal N, et al. Demographic predictors of melanoma stage at diagnosis. Arch Fam Med. 2000;9:606–611. doi: 10.1001/archfami.9.7.606. [DOI] [PubMed] [Google Scholar]
- 26.Carli P, De Giorgi V, Palli D, et al. Patterns of detection of superficial spreading and nodular-type melanoma: A multicenter Italian study. Dermatol Surg. 2004;30:1371–1376. doi: 10.1111/j.1524-4725.2004.30434.x. [DOI] [PubMed] [Google Scholar]
- 27.McMullen EA, Kee F, Patterson CC, et al. Improved survival for melanoma in Northern Ireland: A comparison of two 5-year periods (1984–88 and 1994–98) Br J Dermatol. 2004;151:587–593. doi: 10.1111/j.1365-2133.2004.06071.x. [DOI] [PubMed] [Google Scholar]
- 28.Swetter SM, Geller AC, Kirkwood JM. Melanoma in the older person. Oncology. 2004;18:1187–1196. [PubMed] [Google Scholar]
- 29.Hanrahan PF, Hersey P, D’Este CA. Factors involved in presentation of older people with thick melanoma. Med J Aust. 1998;169:410–414. doi: 10.5694/j.1326-5377.1998.tb126830.x. [DOI] [PubMed] [Google Scholar]
- 30.Goodwin JS, Hunt WC. Samet Relationship of marital status to stage at diagnosis, choice of treatment and survival in individuals with cancer. JAMA. 1987;258:3125–3130. [PubMed] [Google Scholar]


