Abstract
Background
To describe the prevalence of mammography use, and to estimate its association with sociodemographics.
Methods
A sample of 6207 women aged 60 and older from the first interview of Health, Well-Being and Aging in Latin America and the Caribbean Study (SABE) in seven cities (Buenos Aires, Bridgetown, Havana, Mexico, Montevideo, Santiago, and Sao Paulo). The outcome was reporting a mammogram within the last 2 years.
Results
Prevalence of mammography use ranged from 9.8% in Havana to 34.4% in Sao Paulo. Independent predictors of mammography use across cities were older age (lowest odds ratio [OR] = 0.92, 95% confidence interval [CI] 0.89–0.95), higher education (highest OR = 1.14, 95% CI 1.08–1.20), public health insurance (lowest OR = 0.28, 95% CI 0.11–0.76), or no insurance (lowest OR = 0.08, 95% CI 0.02–0.34) compared with private insurance. In a combined sample of six cities, higher education was associated with higher mammography use, but older age and insurance (public: OR = 0.54, 95% CI 0.45–0.65; no insurance: OR = 0.30, 95% CI 0.23–0.40; compared with private insurance) were associated with lower mammography use.
Conclusions
Prevalence of mammography use across cities was lower than that reported for Hispanic populations in the US. In the overall sample, mammography use was increased in highly educated people and decreased in people without insurance.
Keywords: Mammography, SABE study, Older women, Latin America and Caribbean, Health insurance, Cancer screening
Introduction
Incidence and mortality rates from breast cancer have been increasing in the last two decades in women from many Latin American and Caribbean countries. Socioeconomic development and consequent changes in life-style and reproductive behaviors are thought to have contributed to the increased risk of breast cancer in Latin America. Lack of early detection programs with consequent more advanced stages of breast cancer at diagnosis (Lannin et al., 1998; Bradley et al., 2001), which are usually found in Latin America, may explain the relatively high overall mortality from breast cancer in these countries compared to the US (Robles and Galanis, 2002; Miranda-Hernandez and Rivera-Magana, 2001).
Screening mammography, as part of an integral breast cancer prevention and control program, reduces mortality from breast cancer (Urban et al., 1994). However, there is growing concern that its cost may limit its broad use in many countries where public health expenditures are already restricted (Robles and Galanis, 2002).
Factors related to mammography use in Hispanic US women include younger age, high education or income, having health care insurance, high number of medical conditions, high number of doctor visits, physician's recommendation – that is considered a crucial factor – and having had a clinical breast examination and Pap smear (Coughlin et al., 2003; Wu et al., 2001; Zambrana et al., 1999).
There are several studies on barriers to breast cancer detection practices (Pierart and Pierart, 2001; Sanchez-Ayendez et al., 2001; Godinho and Koch, 2002; Modeste et al., 1999), but no population-based studies on prevalence and predictors of mammography use among older women in Latin America and the Caribbean. The objective of this study is to describe the prevalence of mammography use, and to estimate its association with sociodemographic factors in older women from seven cities using data from the Health, Well-Being and Aging in Latin America and the Caribbean Study (SABE).
Methods
Study population
Women who reported diagnosis of cancer were excluded. The study population was a total number of 6207 women aged 60 years or older living in Buenos Aires (Argentina, n = 603), Bridgetown (Barbados, n = 993), Sao Paulo (Brazil, n = 1167), Santiago (Chile, n = 777), Havana (Cuba, n = 1143), Mexico City (Mexico, n = 690), and Montevideo (Uruguay, n = 834).
Data
Data were from the SABE study that consisted of a round of cross-sectional surveys from cities of seven Latin American and Caribbean countries during 1999–2000 (Albala et al., 2005; Peláez et al., 2003). The Pan American Health Organization (PAHO) coordinates the overall SABE study and the Center for Demography and Ecology, University of Wisconsin-Madison who, jointly with PAHO, designed the study and directed its logistics. Local, country-based teams constituted by a principal investigator and his/her associates trained the interviewers in each city. The universe of the study was the urban population aged 60 and older residing in each of the cities; the response rates were 60% in Buenos Aires, 85% in Bridgetown, 85% in Sao Paulo, 84% in Santiago, 95% in Havana, 85% in Mexico City, and 66% in Montevideo; percent of interviews by proxy were 3.7% in Buenos Aires, 3.9% in Bridgetown, 13.1% in Sao Paulo, 7.9% in Santiago, 9.7% in Havana, 8.2% in Mexico City, and 1.4% in Montevideo. A classical multistage clustered sampling with stratification of the units at the highest levels of aggregation was used: the primary sampling unit was a cluster of independent households within predetermined geographic areas, grouped into socioeconomic strata, and divided into secondary sampling units, each containing a smaller number of households. Finally, the household and target individuals, person 60 years and older, were randomly selected. Then, the potential women were contacted to set an interview at home. The interviews were conducted in English for Bridgetown (Barbados) and in Spanish for all other cities, using the same validated questionnaires. Oral and written consent forms –approved by the Human Subjects Committee at each city with the corresponding affiliated Medical Research Institution or University – were obtained from all subjects, and personal identifiers were deleted. If a person having accepted to be interviewed failed the cognitive test, a proxy was selected to respond some parts of the questionnaire (Albala et al., 2005; Peláez et al., 2003).
Variables
The dependent variable – mammography use – was assessed in each city by this question: “In the last 2 years did you have a mammogram of your breasts?” Mammography use was dichotomized as user (response: yes, code = 1) and nonuser (response: no, code = 0).
Potential predictors of mammography use include sociodemographics, medical conditions, and functional status. Sociodemographic variables include age (years), marital status (unmarried, code = 0, and married, code = 1), education (years of schooling), and health care insurance (private insurance, code = 1, public or military insurance, code = 2, and no insurance, code = 3).
Medical conditions were assessed with a series of questions asking the respondent if she had been ever told by a doctor or other health care provider that she had arthritis, diabetes mellitus, hypertension, heart disease, or stroke (each coded 1 for yes and 0 for no). A summary score for medical conditions was constructed, from 0 to 5, and used as continuous variable. Functional status was assessed by eight Instrumental Activities of Daily Living items (IADL) (Fillenbaum, 1985). IADL included using the telephone, traveling alone, going shopping for groceries, preparing own meals, doing light housework, taking own medicine, handling own money, and doing heavy housework. Subjects were asked if they had difficulty performing the activity at the time of the interview. IADL was used as continuous variable, with a possible score from 0 to 8.
Statistical analysis
Descriptive statistics were used to report prevalence of mammography use and to describe characteristics of the study population. Multivariate logistic regression analyses were used to estimate the odds of having a mammogram in each survey and in the combined sample of surveys from Buenos Aires, Bridgetown, Mexico, Montevideo, Santiago, and Sao Paulo (n = 5064, excluding Havana where health insurance does not apply), where dummy variables for city were included. We did not use sampling weights because we wanted to estimate the odds of mammography use in the combined sample of the cities, and the data for Bridgetown did not include sampling rates. All analyses were performed using the SAS System for Windows, version 9.1 (SAS Institute, Inc., Cary, NC); significant level was considered P < 0.05.
Results
Table 1 shows sociodemographics and health characteristics of Latin American and Caribbean older women (aged ≥60) from the SABE study. Mexico City has a relatively younger population and Sao Paulo and Havana have a relatively older population compared with other cities. About a third of subjects were married (ranged from 21.7% in Havana to 39.3% in Mexico City). Most subjects had health insurance (ranged from 72.3% in Mexico City to 98.6% in Montevideo), except in Bridgetown where most subjects were uninsured (86.6%), and does not apply for Havana. There was a great variation on the percentages of private (ranged from 2.7% in Mexico City to 66.9% in Montevideo) and public health insurance and/or military (ranged from 4.2% in Bridgetown to 85.6% in Santiago). Education level was highest in Bridgetown (6.7 ± 3.4) and the lowest in Sao Paulo (3.9 ± 3.0). The highest number of medical conditions was reported in Havana (1.7 ± 1.2) and the lowest in Mexico City (1.2 ± 1.0). The highest number of IADL difficulties was reported in Sao Paulo (1.1 ± 1.7) and the lowest in Montevideo (0.4 ± 1.0).
Table 1.
Characteristics of the study population, women aged 60 years and older in Latin America and the Caribbean, 1999–2000
| Buenos Aires
n = 603 |
Bridgetown
n = 993 |
Sao Paulo
n = 1167 |
Santiago
n = 777 |
Havana
n = 1143 |
Mexico City
n = 690 |
Montevideo
n = 834 |
|
|---|---|---|---|---|---|---|---|
| Prevalence of mammography use, n (%) | 166 (27.5) | 184 (18.5) | 401 (34.4) | 155 (20.0) | 112 (9.8) | 91 (13.2) | 226 (27.1) |
| Age (years), mean ± SD | 71.4 ± 7.4 | 72.2 ± 8.3 | 72.8 ± 8.4 | 72.1 ± 8.3 | 72.7 ± 9.0 | 69.9 ± 7.7 | 71.1 ± 7.7 |
| Married, n (% ) | 181 (30.0) | 340 (34.2) | 435 (37.3) | 227 (29.2) | 248 (21.7) | 271 (39.3) | 302 (36.2) |
| Education (years), mean ± SD | 5.8 ± 3.7 | 6.7 ± 3.4 | 3.9 ± 3.0 | 6.0 ± 5.4 | 6.7 ± 3.9 | 4.1 ± 4.0 | 6.3 ± 4.5 |
| Health insurance, n (%) | |||||||
| Private | 55 (9.1) | 91 (9.2) | 422 (36.1) | 34 (4.4) | – | 19 (2.7) | 558 (66.9) |
| Public or military | 458 (76.0) | 42 (4.2) | 714 (61.2) | 665 (85.6) | – | 480 (69.6) | 264 (31.7) |
| None | 90 (14.9) | 860 (86.6) | 31 (2.7) | 78 (10.0) | – | 191 (27.7) | 12 (1.4) |
| Number of medical conditions, mean ± SD | 1.5 ± 1.0 | 1.5 ± 1.1 | 1.5 ± 1.1 | 1.5 ± 1.1 | 1.7 ± 1.2 | 1.2 ± 1.0 | 1.4 ± 1.1 |
| Number of IADL difficulties, mean ± SD | 0.5 ± 1.0 | 0.5 ± 1.1 | 1.1 ± 1.7 | 1.0 ± 1.8 | 0.9 ± 1.7 | 0.9 ± 1.6 | 0.4 ± 1.0 |
Medical conditions include arthritis, diabetes, hypertension, heart attack, and stroke; SD = standard deviation; IADL = instrumental activities of daily living.
Table 2 shows the results of the multiple logistic regression analyses predicting odds of mammography use in the previous 2 years. Factors associated with increased odds of having a mammogram were higher education (Buenos Aires, Santiago, and Mexico City), being married (Bridgetown, Santiago and Montevideo), and high number of medical conditions (Havana). Factors associated with decreased odds of having a mammogram were older age (Buenos Aires, Bridgetown, Sao Paulo, Santiago, Havana, and Montevideo), public insurance (Buenos Aires, Bridgetown, Sao Paulo, and Mexico City), or no insurance (Buenos Aires, Bridgetown, Sao Paulo, Santiago, and Mexico City) – compared with private insurance– being married (Mexico City), and increased number of IADL difficulties (Sao Paulo). Additional analyses excluding proxies' interviews did not affect the above associations.
Table 2.
Odds ratios (95% confidence intervals) for mammography use in the prior 2 years for women aged 60 years or older, by city, 1999–2000
| Buenos Aires
n = 603 |
Bridgetown
n = 993 |
Sao Paulo
n = 1167 |
Santiago
n = 777 |
Havana
n = 1143 |
Mexico City
n = 690 |
Montevideo
n = 834 |
|
|---|---|---|---|---|---|---|---|
| Age, years | 0.92 (0.89–0.95) | 0.95 (0.93–0.97) | 0.95 (0.93–0.97) | 0.93 (0.90–0.96) | 0.94 (0.91–0.97) | 1.01 (0.98–1.04) | 0.97 (0.94–0.98) |
| Married | 1.41 (0.92–2.14) | 1.51 (1.07–2.13) | 1.28 (0.98–1.68) | 2.19 (1.48–3.23) | 1.13 (0.72–1.78) | 0.58 (0.35–0.97) | 2.40 (1.72–3.35) |
| Education, years | 1.14 (1.08–1.20) | 1.05 (0.99–1.10) | 1.04 (0.99–1.09) | 1.06 (1.03–1.10) | 1.01 (0.96–1.07) | 1.10 (1.05–1.16) | 1.05 (1.01–1.09) |
| Public/military insurance a | 0.40 (0.21–0.76) | 0.40 (0.16–0.99) | 0.47 (0.36–0.61) | 0.49 (0.23–1.06) | – | 0.28 (0.11–0.76) | 0.96 (0.66–1.40) |
| None | 0.22 (0.09–0.51) | 0.31 (0.19–0.50) | 0.08 (0.02–0.34) | 0.16 (0.06–0.45) | – | 0.30 (0.10–0.85) | 0.26 (0.03–2.10) |
| Number of medical conditions | 1.13 (0.92–1.39) | 0.93 (0.79–1.09) | 1.11 (0.98–1.25) | 1.11 (0.93–1.33) | 1.35 (1.13–1.60) | 1.08 (0.86–1.37) | 1.00 (0.86–1.17) |
| Number of IADL difficulties | 0.90 (0.72–1.12) | 0.87 (0.69–1.09) | 0.88 (0.80–0.96) | 1.00 (0.89–1.13) | 0.90 (0.76–1.07) | 0.91 (0.76–1.07) | 0.98 (0.81–1.19) |
Odds ratios were adjusted for all variables in the table using logistic regression; medical conditions include arthritis, diabetes, hypertension, heart attack, and stroke.
The comparison group is private insurance; IADL = instrumental activities of daily living.
Table 3 shows the results of predictors of mammography use in the previous 2 years using the combined sample of six cities. Older age, higher education, and health insurance status remain as strong predictors of mammography use in this analysis. Indeed, women without insurance and with public insurance have 70% and 46%, respectively, lower odds for having a mammogram than women with private insurance. With the exception of Montevideo, women living in other cities had increased odds of having a mammogram compared with those in Mexico City.
Table 3.
Predictors of mammogram use in the prior 2 years for women aged 60 years or older, six cities combined (n = 5064) a
| Variable | Odds ratios b (95% confidence intervals) | Wald chi-square | P value |
|---|---|---|---|
| Age, years | 0.95 (0.94–0.96) | 91.4 | <0.0001 |
| Unmarried | 1.00 | ||
| Married | 1.49 (1.29–1.72) | 29.4 | <0.0001 |
| Education, years | 1.06 (1.05–1.08) | 49.2 | <0.0001 |
| Health insurance | |||
| Private | 1.00 | ||
| Public/military | 0.54 (0.45–0.65) | 42.9 | <0.0001 |
| None | 0.30 (0.23–0.40) | 68.6 | <0.0001 |
| Number of medical conditions c | 1.06 (0.99–1.13) | 2.5 | 0.1143 |
| Number of IADL difficulties | 0.92 (0.87–0.97) | 8.3 | 0.0039 |
| Mexico City (Mexico) | 1.00 | ||
| Buenos Aires (Argentina) | 2.34 (1.74–3.15) | 31.8 | <0.0001 |
| Sao Paulo (Brazil) | 3.13 (2.39–4.11) | 67.6 | <0.0001 |
| Santiago (Chile) | 1.54 (1.14–2.07) | 8.13 | 0.0043 |
| Montevideo (Uruguay) | 1.33 (0.98–1.81) | 3.40 | 0.0652 |
| Bridgetown (Barbados) | 1.85 (1.32–2.58) | 13.00 | 0.0003 |
Include these cities: Buenos Aires, Bridgetown, Sao Paulo, Santiago, Mexico City, and Montevideo (corresponding countries in parenthesis).
Odds ratios were adjusted for all variables in the table.
Medical conditions include arthritis, diabetes, hypertension, heart attack, and stroke; IADL = instrumental activities of daily living.
Discussion
This is the first comparative analysis of mammography use among older urban women in Latin American and Caribbean capitals. Overall, we found that Latin American and Caribbean older women have a low prevalence of mammography use, and that sociodemographic factors are key determinants of mammography use in these populations.
The prevalence of mammography use across the seven cities (ranged from 8.2% in Havana to 30.2% in Sao Paulo) among women aged 65 or older was much lower than that reported in studies of US Hispanics (Fig. 1). Using data from the Behavioral Risk Factors Surveillance System (1999–2000), Coughlin et al. (2003) reported a prevalence of mammography use in the previous 2 years of 59.2% for Hispanics women aged 65 and over in US–Mexico border counties. Using data from the National Health Interview Survey (2000), Swan et al. (2003) reported a prevalence of mammography use in the previous 2 years of 68.2% for Hispanic women aged 65 and over in the US. Wu et al. (2001) reported a prevalence of 41.2% among Mexican American women aged 67 and older residing in the Southwestern United States. A great heterogeneity also exists among Latinas in the US (Garbers and Chiasson, 2004). Foreign-born Latinas have higher mammography rates than US whites but have no differences from US-born Latinas after adjusting for socioeconomic factors (Rodríguez et al., 2005; Valdez et al., 2001); Mexican American women have lower mammography rates than Puerto Rican and Cuban American women (Suarez et al., 2000), and Mexican women have lower mammography rates than Mexican American women (Zambrana et al., 1999).
Fig. 1.
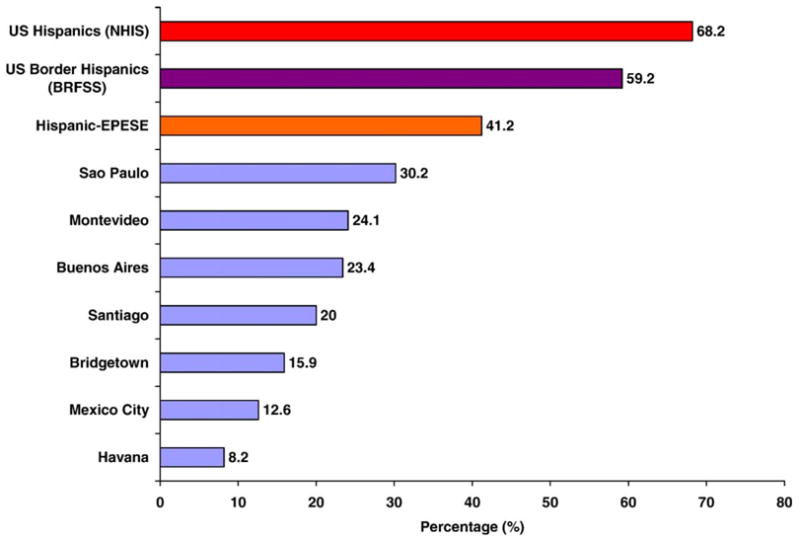
Prevalence of having a mammogram within the last 2 years among women 65 years and older in Latin America, the Caribbean (1999–2000) and the US Hispanic populations. Data from 2000 US National Health Interview Survey (Swan et al., 2003), the US Border Counties (1999–2000 Behavioral Risk Factors Surveillance System; (Coughlin et al., 2003), and the Hispanic EPESE (1995–1996) (Wu et al., 2001).
The low prevalence rates of mammography use in Latin America and the Caribbean might be partially explained by the high cost of mammograms. For example, according to the 2000 Chilean health system budget, the projected cost for screening mammograms would be approximately four times higher than the cost for the national immunization program (Pierart and Pierart, 2001). Differences in the prevalence of mammography use across cities may also be related to differences in screening recommendations. For example, Chile only recently added mammography to its screening program; prior to this change, only breast self-exam and clinical breast examination were universally recommended (Robles and Galanis, 2002).
Four of the countries included in the SABE study –Argentina, Barbados, Cuba and Uruguay – are in very advanced stages of the aging process whereas the other three – Chile, Brazil and Mexico – are in intermediate stages of the aging process. The demographic transition of Latin American and Caribbean countries along with increased poverty, economic inequality, unemployment rates, and deficits in public health budgets translates into strong effects at the individual level. Older persons experience a sustained decrease in real income, poorer access to health services, and erosion of their capacity to claim public sources for retirement and welfare (Palloni et al., 2002). This aging panorama along with the increased breast cancer incidence rates in women aged 65 years and older from Latin American and Caribbean countries (Ferlay et al., 2001) and our data creates a great concern.
With the exception of Mexico, older age was associated with low mammography use in our study, which is consistent with previous studies (Sanchez-Ayendez et al., 2001; Burack et al., 1998). Physicians may perceive that older women have limited life expectancies and therefore do not recommend cancer screening (Fox et al., 1991). Also, breast cancer screening policies in many Latin American countries have upper age limits (e.g., Chile: 50–64 years, Argentina: 50–70, Cuba: 50–65, Uruguay: 50–64), but Mexico has no age limit (Robles and Galanis, 2002).
The lower likelihoods of mammography use among older women with public sources of health insurance and no having insurance (Buenos Aires, Bridgetown, Sao Paulo, Santiago, and Mexico City), compared with private sources of health insurance, are consistent with other reports (Hsia et al., 2000; Coughlin et al., 2003; Selvin and Brett, 2003; Rodríguez et al., 2005). Lack of insurance or public insurance, which are markers of poverty, might limit access to preventive care and ability to afford a mammogram (e.g., due to its high cost) in these cities.
Higher education (Buenos Aires, Santiago, and Mexico City) was associated with increased mammography use and consistent with other reports (Swan et al., 2003; Peek and Han, 2004; Levy-Storms et al., 2004). Education is closely linked to income and health care access. The association between being married and higher mammography use in Bridgetown, Santiago, and Montevideo, but in the opposite direction in Mexico City (lower use), suggests that family and social support may play a role in screening practices (Hsia et al., 2000). Increased number of chronic conditions that was associated with higher mammography use in Havana and increased IADL difficulty that was associated with lower mammography use in Sao Paulo were consistent with other studies (Bostick et al., 1994; Chao et al., 1987; Blustein and Weiss, 1998).
This study has some limitations. Data on mammography use were self-reported. However, self-reports of mammography use have been found to be accurate in several studies (Etzi et al., 1994; Zapka et al., 1996). Our reliance in cross-sectional data precludes establishing causal order between certain variables and mammography use. Also, we could not distinguish between screening and diagnostic mammogram. However, approximately 90% of mammograms in the community are for screening purposes and only 10% are diagnostic (Breen et al., 2001); therefore, we consider that using a mix of both screening and diagnostic procedures would not affect much our conclusions based on the assumptions that most mammograms where for screening purposes. Also, income and occupation information was often incomplete, so we could not use them as measures of socioeconomic resources. Finally, the SABE survey does not represent the diversity of the elderly population that exists in the selected countries because the information was collected only in large cities.
On the other hand, our results may aid the development of specific health policies for breast cancer screening. For cities with lower prevalence of mammography, education campaigns, insurance coverage, and lower cost mammograms would be priority policies. An extension of the age limit for mammography screening will be necessary in most countries. Older women who go regularly to the doctor for chronic conditions should get information on breast cancer screening options.
In conclusion, prevalence of mammography use among older women in Latin American and Caribbean cities is lower than rates reported for Hispanic and non-Hispanic populations in the United States. Older age is a consistent risk factor for not having a mammogram in most cities. In the overall combined sample, mammography use was increased in high educated women but decreased in women having public insurance or without insurance compared with women having private insurance.
Acknowledgments
This study was supported in part by Grant P50 CA105631 (UTMB Center for Population Health and Health Disparities) funded by the National Institute of Health and National Cancer Institute, and by Grant number 5 R24 HS011618 from the Agency for Healthcare Research and Quality. The project was initiated and analyzed by the investigators.
Footnotes
Available online 23 March 2006
References
- Albala C, Lebrao ML, Leon Diaz EM, Ham-Chande R, Hennis AJ, Palloni A, Pelaez M, Prats O. Encuesta Salud, Bienestar y Envejecimiento (SABE): metodologia de la encuesta y perfil de la poblacion estudiada. Rev Panam Salud Publica. 2005;17 (5):307–322. doi: 10.1590/s1020-49892005000500003. [DOI] [PubMed] [Google Scholar]
- Blustein J, Weiss LJ. The use of mammography by women aged 85 and older: factors related to health, functioning, and age. J Am Geriatr Soc. 1998;46 (8):941–946. doi: 10.1111/j.1532-5415.1998.tb02746.x. [DOI] [PubMed] [Google Scholar]
- Bostick RM, Sprafka JM, Virnig BA, Potter JD. Predictors of cancer prevention attitudes and participation in cancer screening examinations. Prev Med. 1994;23 (6):816–826. doi: 10.1006/pmed.1994.1139. [DOI] [PubMed] [Google Scholar]
- Bradley CJ, Given CW, Roberts C. Disparities in cancer diagnosis and survival. Cancer. 2001;91 (1):178–188. doi: 10.1002/1097-0142(20010101)91:1<178::aid-cncr23>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Breen N, Wagener DK, Brown ML, Davis WW, Ballard-Barbash R. Progress in cancer screening over a decade: results of cancer screening from the 1987, 1992, and 1998 National Health Interview Surveys. J Natl Cancer Inst. 2001;93 (22):1704–1713. doi: 10.1093/jnci/93.22.1704. [DOI] [PubMed] [Google Scholar]
- Burack RC, Gurney JG, McDaniel AM. Health status and mammography use among older women. J Gen Intern Med. 1998;13 (6):366–372. doi: 10.1046/j.1525-1497.1998.00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao A, Paganini-Hill A, Ross RK, Henderson BE. Use of preventive care by the elderly. Prev Med. 1987;16 (5):710–722. doi: 10.1016/0091-7435(87)90053-3. [DOI] [PubMed] [Google Scholar]
- Coughlin SS, Uhler RJ, Richards T, Wilson KM. Breast and cervical cancer screening practices among Hispanic and non-Hispanic women residing near the United States–Mexico border, 1999–2000. Fam Commun Health. 2003;26 (2):130–139. doi: 10.1097/00003727-200304000-00006. [DOI] [PubMed] [Google Scholar]
- Etzi S, Lane DS, Grimson R. The use of mammography vans by low-income women. The accuracy of self-reports. Am J Public Health. 1994;84 (1):107–109. doi: 10.2105/ajph.84.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Bray F, Pisani P. Globocan 2000: Cancer Incidence, Mortality and Prevalence Worldwide, Version 1.0. Lyon: IARC Press; 2001. IARC Cancer Base No. 5. [Google Scholar]
- Fillenbaum GG. Screening the elderly: a brief instrumental activities of daily living measure. J Am Geriatr Soc. 1985;33 (10):698–706. doi: 10.1111/j.1532-5415.1985.tb01779.x. [DOI] [PubMed] [Google Scholar]
- Fox SA, Murata PJ, Stein JA. The impact of physician compliance on screening mammography for older women. Arch Intern Med. 1991;151 (1):50–56. [PubMed] [Google Scholar]
- Garbers S, Chiasson MA. Patterns of agreement on breast cancer screening knowledge and practices among women in Dominican and Mexican families in New York City. Med Sci Monit. 2004;10(11):CR628–CR634. [PubMed] [Google Scholar]
- Godinho ER, Koch HA. O perfil da mulher que se submete a mamografia em Goiania-uma contribuicao a bases para um programa deteccao precoce do cancer de mama. Radiol Bras. 2002;35 (3):139–145. [Google Scholar]
- Hsia J, Kemper E, Kiefe C, Zapka J, Sofaer S, Pettinger M, Bowen D, Limacher M, Lillington L, Mason E. The importance of health insurance as a determinant of cancer screening: evidence from the Women's Health Initiative. Prev Med. 2000;31 (3):261–270. doi: 10.1006/pmed.2000.0697. [DOI] [PubMed] [Google Scholar]
- Lannin DR, Mathews HF, Mitchell J, Swanson MS, Swanson FH, Edwards MS. Influence of socioeconomic and cultural factors on racial differences in late-stage presentation of breast cancer. JAMA. 1998;279 (22):1801–1807. doi: 10.1001/jama.279.22.1801. [DOI] [PubMed] [Google Scholar]
- Levy-Storms L, Bastani R, Reuben DB. Predictors of varying levels of nonadherence to mammography screening in older women. J Am Geriatr Soc. 2004;52 (5):768–773. doi: 10.1111/j.1532-5415.2004.52216.x. [DOI] [PubMed] [Google Scholar]
- Miranda-Hernandez H, Rivera-Magana NO. Cancer de mama en la senectud. Rev Med Hosp Gen Mex. 2001;64 (1):21–26. [Google Scholar]
- Modeste NN, Caleb-Drayton VL, Montgomery S. Barriers to early detection of breast cancer among women in a Caribbean population. Pan Am J Public Health. 1999;5 (3):152–156. doi: 10.1590/s1020-49891999000300003. [DOI] [PubMed] [Google Scholar]
- Palloni A, Pinto-Aguirre G, Pelaez M. Demographic and health conditions of ageing in Latin America and the Caribbean. Int J Epidemiol. 2002;31 (4):762–771. doi: 10.1093/ije/31.4.762. [DOI] [PubMed] [Google Scholar]
- Peek ME, Han JH. Disparities in screening mammography. J Gen Intern Med. 2004;19 (2):184–194. doi: 10.1111/j.1525-1497.2004.30254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peláez M, Palloni A, Albala C, Alfonso JC, Ham-Chande R, Hennis A, Lebrao ML, Leon-Diaz E, Pantelides A, Pratts O. Survey on Aging, Health and Wellbeing, 2000. Pan American Health Organization (PAHO/WHO); Washington, DC: 2003. [Google Scholar]
- Pierart J, Pierart C. Analisis predictivo del impacto de un programa de tamizaje mamografico en Chile a partir de los resultados de una muestra piloto. Rev Chil Cir. 2001;53 (5):473–477. [Google Scholar]
- Robles SC, Galanis E. Breast cancer in Latin America and the Caribbean. Pan Am J Public Health. 2002;11 (3):178–185. doi: 10.1590/s1020-49892002000300007. [DOI] [PubMed] [Google Scholar]
- Rodríguez MA, Ward LM, Perez-Stable EJ. Breast and cervical cancer screening: impact of health insurance status, ethnicity, and nativity of Latinas. Ann Fam Med. 2005;3 (3):235–241. doi: 10.1370/afm.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Ayendez M, Suarez-Perez E, Oliver-Vazquez O, Velez-Almodovar H, Nazario CM. Knowledge and beliefs of breast cancer among elderly women in Puerto Rico. Puerto Rico Health Sci J. 2001;20 (4):351–359. [PubMed] [Google Scholar]
- Selvin E, Brett KM. Breast and cervical cancer screening: socio-demographic predictors among White, Black, and Hispanic women. Am J Public Health. 2003;93 (4):618–623. doi: 10.2105/ajph.93.4.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez L, Ramirez AG, Villarreal R, Marti J, McAlister A, Talavera GA, Trapido E, Perez-Stable EJ. Social networks and cancer screening in four U.S. Hispanic groups. Am J Prev Med. 2000;19 (1):47–52. doi: 10.1016/s0749-3797(00)00155-0. [DOI] [PubMed] [Google Scholar]
- Swan J, Breen N, Coates RJ, Rimer BK, Lee NC. Progress in cancer screening practices in the United States: results from the 2000 National Health Interview Survey. Cancer. 2003;97 (6):1528–1540. doi: 10.1002/cncr.11208. [DOI] [PubMed] [Google Scholar]
- Urban N, Anderson GL, Peacock S. Mammography screening: how important is cost as a barrier to use? Am J Public Health. 1994;84 (1):50–55. doi: 10.2105/ajph.84.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez A, Banerjee K, Ackerson L, Fernandez M, Otero-Sabogal R, Somkin CP. Correlates of breast cancer screening among low-income, low-education Latinas. Prev Med. 2001;33 (5):495–502. doi: 10.1006/pmed.2001.0913. [DOI] [PubMed] [Google Scholar]
- Wu HZ, Black SA, Freeman JL, Markides KS. Older Mexican American women and cancer screening: progress toward targets for Healthy People 2000. Ethn Dis. 2001;11 (4):645–651. [PubMed] [Google Scholar]
- Zambrana RE, Breen N, Fox SA, Gutierrez-Mohamed ML. Use of cancer screening practices by Hispanic women: analyses by subgroup. Prev Med. 1999;29 (6 Pt 1):466–477. doi: 10.1006/pmed.1999.0566. [DOI] [PubMed] [Google Scholar]
- Zapka JG, Bigelow C, Hurley T, Ford LD, Egelhofer J, Cloud WM, Sachsse E. Mammography use among sociodemographically diverse women: the accuracy of self-report. Am J Public Health. 1996;86 (7):1016–1021. doi: 10.2105/ajph.86.7.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]


