Abstract
Familial hypercholesterolaemia (FH), defined as the heritable occurrence of severe hypercholesterolaemia with cholesterol deposits in tendons and premature heart disease, is caused by at least four genes in sterol and lipoprotein pathways and displays varying gene-dose effects. The genes are the low-density lipoprotein (LDL) receptor, apolipoprotein (apo) B, proprotein convertase subtilisin/kexin 9, and the autosomal recessive hypercholesterolaemia (ARH) adaptor protein. All of these disorders have in common defective clearance of LDL within a complex system of lipid and lipoprotein metabolism and regulation. Normal cellular cholesterol and lipoprotein metabolism is reviewed before describing the disorders, their metabolic derangements and their clinical effects. FH is classified as two simplified phenotypes of disease according to the severity of the metabolic derangement. The dominantly inherited heterozygous phenotype comprises defects in the LDL receptor, apoB100, and neural apoptosis regulatory cleavage protein. The homozygous phenotype is co-dominant in defects of the LDL receptor, and occurs also as the ARH of adapter protein mutations. Defective binding of apoB100 does not result in a significant gene dose effect, but enhances the severity of heterozygotes for LDL receptor mutations. The genetic diagnosis of FH has provided greater accuracy in definition and detection of disease and exposes information about migration of populations. All of these disorders pose a high risk of atherosclerosis, especially in the homozygous phenotype. Studies of influences on the phenotype and responses to treatment are also discussed in the context of the metabolic derangements.
Introduction
FH is an interesting disease to which attention was drawn by a pathologist, Harbitz, by virtue of the coincidence of lipid deposits (xanthomata) in tendons and sudden death, before being described by Muller as a clinical entity in the first half of the previous century.1 Studies in patients and cultured cells revealed a defect in the receptor for LDL and culminated in the Nobel prize for Brown and Goldstein who also investigated the regulation of the cholesterol synthetic pathway.2,3 The world-wide prevalence of FH is about 1 in 500 people. This prevalence is sufficiently high to warrant the diagnosis and treatment of FH at primary health care level by the World Health Organisation.4 More recently, FH has been shown to be due to other molecular mechanisms in lipoprotein metabolism.5
In the past decade alone, there have been more than 2000 publications on FH. This article will provide a brief review of cholesterol metabolism in cells and circulatory lipoproteins to provide the context in which FH occurs. The genetic disorders are discussed, with an emphasis on the more common disorders. Recent investigations that give insight into the pathophysiology of disease and factors modulating the phenotype are discussed and referenced. Although this review focuses on the role of the LDL receptor in cholesterol metabolism, other roles for this receptor and related receptors are emerging in endocytosis and signalling.6
Definition of FH
FH is best defined as an entity in clinical practice that is recognisable from features in the patient’s family and personal history and physical examination, as well as readily available chemical analysis of the blood. The diagnosis of FH is important not only for the prognosis of the patient, but also has implications for the family members who may have inherited the same disorder. The clinical pattern is also an important starting point of research for the biochemist, clinical pathologist, epidemiologist, geneticist or healthcare provider.
FH may be defined as an heritable disorder, involving a single gene, that produces a clinically recognisable pattern (in the majority of subjects) that consists of severe hypercholesterolaemia due to the accumulation of LDL in the plasma, cholesterol deposition in tendons and occasionally in skin, and a high risk of atherosclerosis manifesting almost exclusively as coronary artery disease (CAD). These disorders all share the mechanism of decreased clearance of LDL, but their inheritance may be autosomal dominant or recessive and not all dominant disorders have a gene-dose effect.
FH may be loosely classified into an “heterozygous” and an “homozygous” clinical phenotype (Table 1). The heterozygous phenotype is inherited in an autosomal dominant manner and has a LDL-cholesterol concentration between 5 and 12 mmol/L, tendon xanthomata are common and CAD before 55 years of age is also common. The leading cause of the heterozygous phenotype is a mutation in the LDL receptor. Occasionally a mutation disrupting the binding of apoB to the LDL receptor is encountered. Recently a newly discovered protein, neural apoptosis regulated convertase 1 (NARC1), the product of the gene proprotein convertase subtilisin/kexin type 9 (PCSK9) has been referred to as autosomal dominant FH 3.
Table 1.
The homozygous phenotype has more severe LDL hypercholesterolaemia (>12 mmol/L) and typically there are also xanthomata in the skin and tendons. On westernised diets, the LDL-cholesterol concentration usually exceeds 15 mmol/L. Included in this phenotype of cutaneous and tendinous xanthomata are other sterol disorders that do not result in the same degree of hypercholesterolaemia: phytosterolaemia and cerebrotendinous xanthomatosis (CTX). The genetic causes of the homozygous phenotype include the concurrence of two LDL receptor defects, but the same phenotype is not seen with concurrence of binding defective apoB for reasons that will be discussed below. The homozygous phenotype is recessively inherited when mutations disrupt the function of an adaptor protein, now known by its property to confer ARH. Phytosterolaemia is inherited in an autosomal recessive manner as a result of mutations in adenosine binding cassette transporter proteins G5 and G8. Whilst phytosterolaemia can have significant hypercholesterolaemia, it seldom exceeds the heterozygous FH range. CTX, also autosomal recessively inherited, is due to mutations in 27-hydroxylase (CYP27). CTX is not associated severe hypercholesterolaemia.
The term FH is thus restricted to specific disorders and does not simply mean a heritable hypercholesterolaemia. Whilst some lipidologists may still retain the term FH specifically for LDL receptor defects, the heterozygous phenotype was and still is used to identify subjects who were inferred to have LDL receptor defects but who may well have had some of the other defects outlined in this review. It is conceivable that more defects for these phenotypes will still be discovered. In this review, FH will refer to the broader context outlined in Table 1 and will generally refer to the heterozygous pattern.
Cholesterol Metabolism and Transport
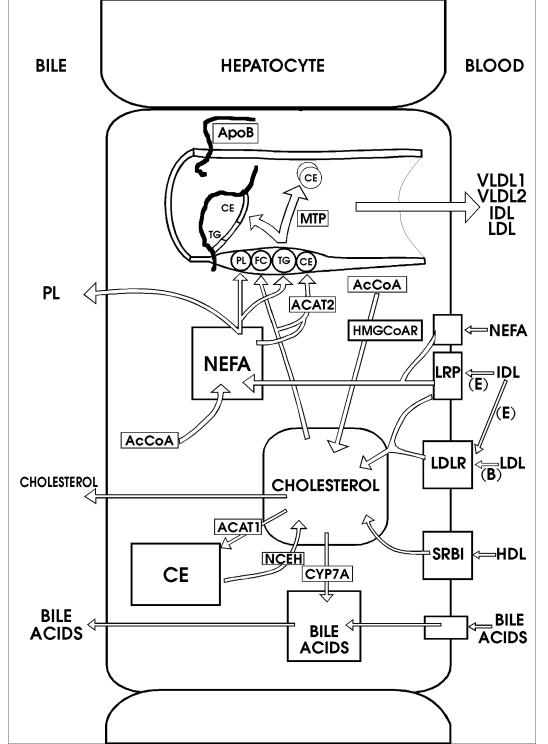
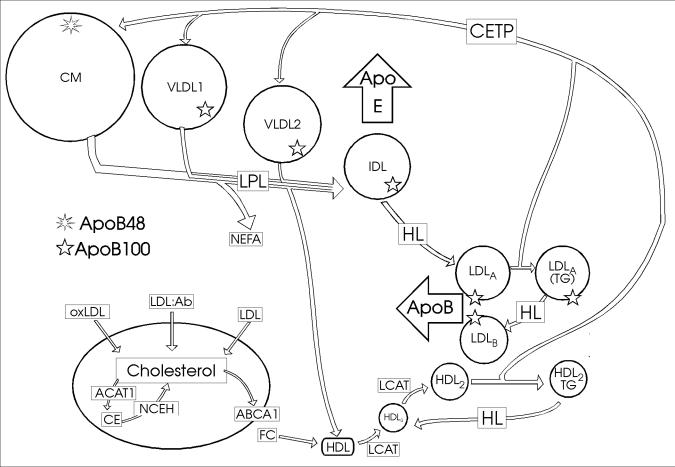
In this section cholesterol synthesis in the cell and its regulation will be discussed. The course of cholesterol can be traced within the cell, to its incorporation into lipoproteins and delivery to cells, recycling, conversion to bile acids and excretion. Figure 1 summarises cholesterol metabolism in the hepatocyte, the most active cell in cholesterol metabolism and homeostasis of the human body. Figure 2 depicts the general scheme of lipoprotein metabolism.
Figure 1.
Cholesterol metabolism and pathways in the hepatocyte. The cholesterol pool receives cholesterol from the blood through the LDL receptor (LDLR), LRP and SRB1. Additional inputs to the cholesterol pool are from CE by NCEH and de novo synthesis from AcCoA involving HMG-CoA reductase. Outputs of cholesterol from the pool are to lipoproteins as free cholesterol (FC), and CE by the reaction of cholesterol with non-esterified fatty acid (NEFA) catalysed by ACAT2. ACAT1 catalyses esterification of cholesterol for intracellular storage and cytochrome P4507A (CYP7A) initiates bile acid synthesis. Lipoprotein assembly in the lumen of the ER involves (PL) and TG, apoB and MTP. The lipoproteins synthesised by the liver include VLDL1 and VLDL2, IDL and LDL. The ligand for internalisation of IDL is apoE (E) and for internalisation of LDL is apoB100 (B).
Figure 2. Lipoprotein metabolism in plasma and RCT.
Predominantly TG-rich lipoproteins are secreted into the plasma: CMs containing apoB48 from the intestine and VLDL1 and VLDL2 from the liver, containing apoB100. The TG in these lipoproteins is hydrolysed by LPL on vascular endothelium to release non-esterified fatty acids (NEFA) and redundant phospholipid, free cholesterol and apoA-I bud off to form HDL. IDL constituting remnants of the TG-rich lipoproteins leaves the plasma by virtue of the ligand, apoE. HL acts on IDL to produce LDL. Large LDL (LDLA) becomes enriched with TG by exchange between the TG-rich lipoproteins and cholesterol-rich LDL by the action of CETP. HL converts the TG-rich LDLA to a smaller LDL species (LDLB). LDL enters the liver as a result of apoB100 being a ligand for the LDL receptor, but oxidatively modified LDL (oxLDL) as well as LDL complexed with antibody (LDL:Ab) can be internalised by macrophages depicted in the lower left hand corner. The macrophage cholesterol pool is regulated by esterification of cholesterol to CE by ACAT1 and export via the adenosine binding cassette transporter A1 (ABCA1) as free cholesterol (FC) to HDL. LCAT esterifies cholesterol and thus increases the size of smaller HDL into the density range of HDL3 and subsequently into more buoyant HDL2. NCEH catalyses the production of cholesterol from CE.
Cellular Cholesterol Metabolism
Cholesterol is an essential component of cell membranes but is also used by hepatocytes and enterocytes for the production of lipoproteins. The hepatocyte also secretes bile that contains cholesterol and bile acids that are derived from cholesterol. Adrenal and gonadal cells synthesise corticosteroid hormones from cholesterol. All nucleated cells can synthesise the enzymes to produce cholesterol. This synthetic pathway produces cholesterol as well as other isoprene products involved in the prenylation of proteins and the synthesis of ubiquinone. The rate-limiting enzyme in this pathway is 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase. The product of HMG-CoA reductase, mevalonic acid, appears in plasma and urine and may be used as a guide to cholesterol synthetic acitivity. The plasma mevalonate concentration varies in a diurnal rhythm with the highest synthetic rate in the early hours of the morning.
There is a demand for cholesterol during cell growth and in cells that use cholesterol for specialised purposes. To meet such demands, the cell can synthesise cholesterol de novo, can import it through specialised receptors such as the LDL and related receptors, and can use cholesterol after cleavage of stored cholesterol ester (CE) by the action of neutral cholesterol ester hydrolase (NCEH). The regulation of both cholesterol synthesis and importation by LDL receptors occurs by sensing the amount of cholesterol in the membranes. A depletion of membrane cholesterol will up-regulate transcription of both HMG-CoA reductase and the LDL receptor through a complex mechanism.7
The upregulation of cholesterol synthesis and importation in the time of need is made possible by an interaction of a regulatory protein with the promoters of the genes for the LDL receptor, HMG-CoA reductase and HMG-CoA synthase. The LDL receptor promoter has three imperfect repeats. Repeat 2, consisting of 16 base pairs, forms a sterol regulatory element (SRE) that interacts with the SRE binding protein (SREBP) under cholesterol deprivation. SREBP has three isoforms in humans, each comprising approximately 1150 amino acids. SREBP has three domains: the amino-terminal domain contains a basic helix-loop-helix leucine zipper region that enables it to bind to DNA, a carboxy-terminal domain binding to sterol cleavage activating protein (SCAP) at the cell membrane, and an intermediate domain that contains two transmembrane portions linked by a loop of about 30 amino acids into the lumen of the endoplasmic reticulum (ER). SCAP is associated with SREBP in the membrane where the complex is stationary until cholesterol depletion permits its migration to the Golgi apparatus. In the Golgi apparatus two membrane-bound proteases process the SREBP:SCAP complex to create a cleavage product that migrates to the nucleus. Site 1 protease is a serine protease that cleaves the loop in the central domain of SREBP. Site 2 protease is a metalloprotease that cleaves the amino-terminal portion of SREBP that translocates to the nucleus. Additional complexity is introduced into the regulation of lipid metabolism by the three isoforms of SREBP. Alternative transcription start sites on chromosome 17 create differences in exon 1 of SREBP to produce SREBP1a and SREBP1c. SREBP2 is coded on chromosome 22. Whilst SREBP1a has broad specificity for all genes with SREBP response elements, SREBP1c favours promoters of genes for fatty acid synthesis and SREBP2 enhances cholesterol synthesis and importation into the cell.
Cholesterol importation into cells typically involves a high affinity, saturable process by means of the LDL receptor and a low affinity process of passive association with the cell membrane and internalisation. Additional receptors, such as the LDL receptor-related protein (LRP), may import lipoproteins in certain cells.8 Whilst the low affinity process accounts for very little uptake at low concentrations of LDL, higher concentrations of LDL may result in the internalisation of sufficient cholesterol to result in storage of cholesterol as its ester, to regulate cholesterol synthesis and possibly also LDL receptor expression. At least some contribution to low affinity clearance may be attributable to an interaction of fatty acids with cell membranes at physiologically appropriate concentration9 and some clearance may also take place by means of the scavenger receptor B1 (SRB1) even though this receptor has conventionally been viewed as a receptor for other lipoproteins.10
The LDL Receptor
The LDL receptor is a well-characterised transmembrane protein encoded on chromosome 19 and comprises 18 exons in 48 kb.2 The 843 amino acids can be placed in the following domains: ligand-binding domain (about 292 amino acids, exons 2 to 6), epidermal growth factor precursor homologous domain (about 400 amino acids, exons 7 to 14), a carbohydrate-rich domain (about 58 amino acids, exon 15), a transmembrane domain (about 22 amino acids, exon 16) and a cytoplasmic tail (about 50 amino acids). The protein is translated to form a 120 kDa precursor that matures in the Golgi apparatus to 160 kDa before being transported to the cell surface. On the cell surface it finds its way to the clathrin-coated pits.
The amino-terminus of the LDL receptor is situated extracellularly and has 7 repeats of about 40 amino acids in the binding domain. Cysteine is prominent in these “LDL-A” modules that mediate binding to lipoproteins.11 Binding requires calcium and involves interaction with negatively charged amino acids. The fifth repeat is essential for binding apoE whilst repeats 2 to 7 are necessary to bind the larger ligand, apoB. After synthesis and maturation, the LDL receptor is sorted and trafficks to the basolateral surface of the cell. The bound complex of lipoprotein and receptor is internalised through the clathrin-coated pit with assistance by adapter proteins. The cytoplasmic tail has an important function in signalling internalisation. Once in the endosomal trafficking system, the LDL receptor dissociates from the lipoprotein during acidification; the epidermal growth factor-like domain is critical for this function. The epidermal growth factor-like domain has repeats A and B, a β-propeller structure and a repeat C. The β-propeller region competes for binding to the LDL-A repeats 4 and 5 and the lipoprotein leaves the LDL receptor in the endosome.12 Hereafter, the lipoprotein proceeds to the lysosome for enzymic degradation into its constituent cholesterol, fatty acids and amino acids. The receptor recycles approximately 150 times.
Since excess cholesterol in cell membranes imparts unfavourable properties, excess cholesterol is metabolised within cells to CE by the action of acylcoenzyme A:cholesterol acyltransferase (ACAT). This enzyme is embedded in the membrane and is allosterically activated by cholesterol. ACAT1 is a transmembrane protein with its catalytic site orientated towards the cytosol which houses the cholesterol ester formed under overloading conditions. Under conditions of cholesterol overload, HMG-CoA reductase is subject to instant control through phosphorylation. Furthermore, excess cholesterol will also result in down-regulation of HMG-CoA reductase and LDL receptors by limiting the migration of the SREBP-SCAP complex. Cellular cholesterol balance has been reviewed in detail recently.13 Adenosine binding cassette (ABC) protein A1 facilitates transfer of intracellular cholesterol to apoprotein or lipoprotein acceptors to rid cells of excess free cholesterol.14
Lipoprotein Synthesis
Lipoproteins, composed of lipid and apolipoproteins, are a means of transporting insoluble lipids, chiefly triacylglycerol (TG), CE and cholesterol. Different mechanisms exist to target TG and cholesterol within lipoproteins to cells that require fatty acids and/or cholesterol. Lipoproteins generally have a spherical shape but vary significantly in size, density, and constituent lipids and apoproteins. Greater lipid content makes for larger diameters and lower density, chiefly by TG content. The shell contains phospholipid (PL), cholesterol and apoproteins while the core contains TG and CE.
The enterocyte and hepatocyte are the sites of assembly for TG-rich lipoproteins, using apoB for assembly of the lipoproteins. The TG-rich lipoproteins secreted by the enterocyte are termed chylomicrons (CM) and those secreted by the hepatocytes are termed very low-density lipoproteins (VLDL). The enterocyte uses an edited mRNA of apoB100 to synthesise apoB48.15 CMs contain one copy of apoB48 and VLDL contains one copy of apoB100.
Almost half of the cholesterol in the gut is absorbed but this efficiency varies amongst individuals. Enterocytes, like hepatocytes, express ACAT2 that produces CE but in contrast to ACAT1, its catalytic site is on the lumenal aspect of the ER. The CE globules as well as those of TG in the lumen of the ER, are used in lipoprotein assembly. Both enterocytes and hepatocytes initiate lipoprotein assembly by adding a critical initial amount of neutral lipid to apoB so that this constitutively expressed protein is protected from ubiquitinylation and degradation. The critical protein responsible for this is microsomal TG transfer protein (MTP). Further remodelling of the lipoprotein takes place in the Golgi region before secretion.
As may be expected, there is in reality a range in size and content of lipoproteins secreted by lipoprotein-producing cells, especially by hepatocytes. These include: TG-rich VLDL with flotation rates of Sf 60–400 termed VLDL1, smaller CE-rich VLDL (Sf 20–60) termed VLDL2, intermediate-density lipoproteins (IDL, Sf 12–20) and LDL (Sf 0–12).
Lipoprotein Metabolism
A general scheme of lipoprotein metabolism is presented in Figure 2. CMs enter the venous circulation from the thoracic duct and lipoproteins made by the liver enter the hepatic venous system. Redistribution of apoproteins from especially high-density lipoprotein (HDL) supplies the bulk of the apoproteins on these TG-rich lipoproteins.
The circulating TG-rich lipoproteins attach briefly to proteoglycans in the vascular beds of muscle and adipose tissue. Lipoprotein lipase (LPL) also resident on the proteoglycans, is activated by apoC-II and inhibited by apoC-III. TG is hydrolysed by activated LPL to release fatty acids that will diffuse into the tissue or be sequestered on albumin. The lipolytic process results in a smaller core and the redundant shell around the core buds off with apoA-I to form HDL.
The remnants of CM and VLDL return to the liver, being cleared by virtue of apoE that serves as the ligand for the LDL receptor, as well as the LRP. VLDL remnants, entering the IDL flotation range, are further hydrolysed by hepatic lipase (HL) to produce LDL. ApoB is the only apoprotein on LDL. Only on LDL will apoB serve as the ligand for the LDL receptor.
Whilst it is known that the liver can secrete a range of apoB-containing lipoproteins, the classical pathway is the production of mainly VLDL1 with subsequent conversion of this lipoprotein through VLDL2 to IDL before being removed into the liver from the circulation; but some IDL is metabolised to LDL. The half-lives of the lipoproteins increase down the lipolytic cascade, with LDL being the longest resident in plasma.
The larger lipoproteins are remodelled in the circulation by LPL as mentioned above. Smaller lipoproteins like IDL, LDL and HDL are modified by HL. An additional complexity is brought about by CE transfer protein (CETP) which exchanges neutral lipids between lipoproteins; TG being moved towards cholesterol-rich LDL and HDL, and CE being moved from these lipoproteins to CM and VLDL. The latter two apoB-containing lipoproteins can thus participate in the reverse cholesterol transport (RCT) of cholesterol from HDL to the liver. TG-rich LDL and HDL are substrates for HL, the action of which will decrease their size by hydrolysis of TG.
The lipoproteins that lack apoB constitute mainly HDL. This class of lipoproteins is important for RCT. The other functions of HDL include being a reservoir for apopoproteins A, C and E, CETP and lecithin:cholesterol acyltransferase (LCAT), the provision of anti-oxidant activity by paraoxonase, and modulation of endothelial function and immunity. Having accepted cholesterol from a cell, a small HDL particle will enlarge as a result of esterification of cholesterol by LCAT as CE migrates to the core and additional cholesterol that is now accommodated in the shell, also becomes esterified. Not only can HDL transfer CE to the TG-rich lipoproteins, but it can deliver it directly to the liver through SRB1.
Clinical Aspects of FH
In clinical practice, the heterozygous FH phenotype (see Table 1) is encountered most often whilst the rarely encountered homozygous phenotype has much worse sequelae. Patients with FH typically presented with premature CAD in the past but today with greater awareness, simpler chemical assays that are widely available, the diagnosis is made earlier. FH management with modern drug treatment has improved the prognosis.16
The family history of premature ischaemic heart disease is very helpful to identify an autosomal dominant mode of inheritance and the patient’s ancestry may suggest the molecular defect if there is a link to a population with a founder effect. Despite the high risk of heart disease in heterozygous FH, the risk is significantly modified by other genes and environmental factors, so that the family history may occasionally be misleadingly benign. In such a setting, a recessive disorder should be considered, or a new mutation giving rise to a dominantly inherited pattern should be considered.17,18
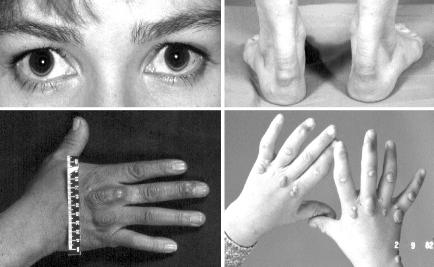
Physical signs due to lipid deposition in the eye, the skin, and the tendons are valuable, but not invariant clues. The physical signs are depicted in Figure 3. The heterozygous phenotype generally has tendinous manifestations alone and the homozygous phenotype tends to have both tendinous and cutaneous manifestations. In the heterozygous phenotype the manifestations are usually not readily apparent until about the age of 25 to 30 years, whereas in the homozygous phenotype they are usually evident before the age of 10 years.
Figure 3. Clinical manifestations of FH.
Top left panel: arcus cornealis is a thin white crescentic line due to cholesterol deposition, typically on the lower aspect of the cornea. Top right panel: thickening of the Achilles tendons above the heels due to xanthoma formation. Bottom left panel: the tendons of the middle and ring fingers of this hand have visible thickening due to xanthoma formation adjacent to the knuckles and there are two small xanthomas in the skin. Bottom right panel: skin xanthomata on the hands of a child with homozygous FH, the planar xanthomata on the webs between the fingers being highly specific for this condition. Tendon xanthomata appear later.
The palpation of the Achilles tendon requires some experience, but is the best clinical sign for diagnosing FH. Ultrasound examination can establish more accurate dimensions. Thickening of the tendons by cholesterol deposition may lead to inflammation and pain and eventually the deposition of lipid leads to an irregular surface with nodules and sometimes visibly enlarged tendons. The high tension transmitted through the relatively avascular tendon to its septae containing the blood vessels is presumed to cause exudation or bleeds into the tissue. The connective tissue appears to bind lipoproteins and cholesterol accumulates. RCT is not effective as the high plasma LDL concentration and the relatively poor blood supply favour the accumulation of tissue lipid. Uncommonly, xanthomata also form in the extensor tendons to the fingers and rarely elsewhere in the body. The detection of xanthomata at clinical examination not only has diagnostic implications, but may also indicate risk, as FH subjects with xanthomata were found to have more heart disease.19
Deposition of lipid on the cornea of the eye results in a crescentic white line referred to as an arcus cornealis. The deposition of lipid in the eyelids as flat yellow plaques, referred to as xanthelasma, is not specific for FH and may even be observed in individuals without significant disturbances of the plasma lipoproteins.
Vascular Disease in FH
Subclinical atherosclerosis is evident in young persons with FH when sought by vascular imaging techniques. In homozygous FH patients, aortic stenosis is a well-established complication as a result of lipid deposition in the aortic valve and root. Other vessels may also have turbulent flow in homozygous FH, especially the femoral arteries.
Atherosclerosis is imperfectly understood, but is clearly a multifactorial disease in which especially in FH, LDL hypercholesterolaemia plays a strong role. In FH, the disease is more severe and results in clinical complications earlier. High concentrations of LDL, resident in the circulation for prolonged periods, permit more oxidative modification in the circulation and tissues. These oxidised lipoproteins influence vascular endothelium adversely, promoting the attraction of monocytes that penetrate into or remain in the sub-endothelial space, where an indolent form of chronic inflammation takes place.20 Monocytes mature into macrophages that take up the lipid to become foam cells when ACAT1 esterifies cholesterol. Whilst the proportion of apoB that is glycated is normal in FH, the absolute concentration is increased.21 This may contribute to atherosclerosis by the adverse effects of advanced glycation endproducts. Under relatively low rates of entrance of post-prandial and endogenous lipoproteins into the arterial wall and low oxidative stress on these lipoproteins, RCT may counter atherogenesis and its complications that include inflammation, plaque rupture and thrombosis. The pathological outcome in a high risk condition such as FH, may thus still be modulated by many additional factors to apoB-containing lipoproteins, including RCT and factors governing tissue responses suggested above.
FH can thus serve as a model for investigating the utility of other strategies for prevention of atherosclerosis, even if the contribution from other risk factors may be greater in less severe dyslipidaemia. Modern non-invasive vascular imaging with ultrasound has also shown that the intima-media thickness of the carotid increases more rapidly in adolescents with FH, when compared with their peers.22 Vascular endothelial function is impaired in children with FH.23 Aggressive lipid-lowering therapy can improve the vascular abnormality in FH.24
Pathophysiology of FH
The genetic reasons for the heterozygous phenotype are mutations in the LDL receptor, apoB100 and NARC1. All 3 are characterised by an increase in LDL-cholesterol and normal VLDL concentrations. There is controversy about the metabolism of remnants of TG-rich lipoproteins, especially with LDL receptor defects. Differences in biochemical derangement and atherogenic impact LDL receptor and apoB defects are also controversial, along with responses to treatment.
LDL Receptor Defects
The discovery of the LDL receptor and its defective function led to a great advance in the understanding of the pathophysiology of FH.2 Although importation rates of cholesterol into growing cells would theoretically be impaired and plasma concentration approximately doubled in heterozygous FH, cells can compensate by synthesising more cholesterol and can also obtain more cholesterol by low affinity processes as a result of a higher concentration in their surrounds. Assuming for the moment that hepatocytes export lipoproteins at a normal rate in FH, hyperlipidaemia will result from reduced clearance of IDL and LDL. A reduction in the clearance of IDL would promote conversion of IDL to LDL, compounding the increase in LDL already induced by the decreased clearance of LDL. The clinical homozygous FH phenotype may be due to a true genetic homozygote which has the same mutation on both alleles, or may be due to a genetically compound heterozygote in which a different mutation is found at each allele. The impact on LDL hypercholesterolaemia is much more severe, with more striking clinical features and vascular disease.
The hepatocyte has significant demands for cholesterol. De novo synthesis of cholesterol and some input from LDL receptor activity may be regulatable, but the higher plasma LDL-cholesterol concentration results in greater uptake of cholesterol and storage as CE. An increase in CE may be postulated to cause greater lipoprotein production that compounds the LDL hypercholesterolaemia. When both genes for the LDL receptor are dysfunctional, the impact would be worse because clearance of LDL is negligible or absent. Depending on the dietary cholesterol intake and absorption, biliary secretion and bile acids synthesis and losses, de novo synthesis of cholesterol will vary in the maintenance of the steady state conditions in FH.
Initially, LDL receptor activity was studied in the homozygous phenotype. The LDL receptor activity was declared “defective” if LDL handling by the cultured fibroblasts was low (<30%), and “negative” if the handling was <2% when compared with normal fibroblasts. Typically radio-iodinated LDL and a monoclonal antibody, IgGC7 are used to describe LDL handling in fibroblasts which have undergone incubation in a lipoprotein deficient medium to maximally upregulate LDL receptors. At 4°C, LDL receptor trafficking is disrupted and binding to the cell surface can be studied, the number of receptors can be determined using the antibody and their binding activity by using LDL. Immunoprecipitation of radio-isotopically labelled LDL receptors can be used to examine their expression and maturation in cells.
Defects in the LDL receptor are functionally divided into six classes depending on the impact of the mutation on the presence of mRNA, receptor maturation in cells, disparity between LDL and immunoglobulin binding on the cell surface, LDL receptor degradation and trafficking. Class 1 defects, mainly due to promoter mutations, leave no detectable mRNA and no detectable LDL receptor. Class 2 defects are due to mutations that retard the maturation of the LDL receptor by glycosylation, from 120 to 160 kDa; either in a limited fashion (class 2B) or completely (class 2A). Mutations such as D206E in exon 4 and involving binding modules, have persistent folding defects demonstrable on nuclear magnetic resonance spectroscopy that will influence maturation.25 In Class 3 the defective LDL receptors mature normally but have defects in binding the ligand. In Class 4, mutated LDL receptors mature normally to occupy the cell membrane but are not internalised, typically due to mutations in the cytoplasmic tail. Class 5 mutations relate to LDL receptor degradation that prevents receptors from reaching the cell surface and from recycling to the cell surface. Class 6 mutations are due to a failure of directing LDL receptors to the basolateral surface of polarised cells.26
The determination of the amino acid sequence of the LDL receptor and the impressive developments in molecular biology over the past two decades have permitted the characterisation of related receptors to the LDL receptor.27 Numerous defects in the LDL receptor have been catalogued and gave an understanding into their functional impact.28 A register of LDL receptor mutations is available at a website (http//:www.ucl.uk/fh).29
Familial Binding Defective apoB100
In contrast to the numerous mutations in the LDL receptor disrupting the binding of apoE and apoB, very few mutations have been reported in apoB100 that will disrupt binding to the LDL receptor.
ApoB100, a large protein of 550 kDa, is encoded on chromosome 2 and has 26 exons. The binding region is rich in positively charged amino aids and interacts with the binding domains of the LDL receptor when the conformation is appropriate on LDL. As a result of impaired clearance relative to normal apoB, the defective apoB100 becomes enriched in LDL. In contrast to subjects with LDL receptor mutations in whom IDL clearance will be impaired, apoE remains a normally functioning ligand on these particles in familial binding defective apoB100 (FDB). The functional LDL receptor in these subjects will permit normal entry of cholesterol into the liver from remnants of CM and VLDL. This was confirmed by a study in which there was decreased production of LDL.30 A compensatory upregulation of LDL receptors can be expected to ameliorate the biochemical abnormality in heterozygotes for FDB and especially in homozygotes.
The clinical phenotypes of LDL receptor mutations and FDB are very similar, but FDB does seem to be biochemically less severe in children.31 Increased LDL receptor activity during growth may be responsible for this finding. In a comparison of the impacts of LDL receptor and apoB mutations on atherosclerosis, it was found that LDL receptor mutations rendered the plasma lipoprotein concentration higher and was associated with higher incidences of coronary artery complications, as well as carotid plaques.32 There is evidence that there is a more benign phenotype for FDB when compared with LDL receptor defects in adults,33 but the clinical features and complications are so similar for the heterozygous phenotype that they could be viewed as identical.34–37
In homozygous FDB, there appears to be no significant gene dose effect.38 Two homozygotes for FDB were reported to have cholesterol concentrations in the range for heterozygotes for LDL receptor mutations.39 The binding affinity for LDL receptors of VLDL was shown to be normal in these subjects whilst LDL affinity was 10% of normal. In homozygous FDB, the longer plasma residence time of the LDL particle renders it smaller, presumably from modulation by CETP and HL.40 FDB has been attributed to recurrent mutations. A Chinese subject was shown to have the R3500Q mutation on a different haplotype of apoB100 to Caucasian subjects with the same condition.41 There appears to be a higher prevalence of FDB in subjects of Celtic origin and even a founder effect in central Europe for FDB that is very rare in some populations, including Finland.42,43
ARH
The homozygous FH phenotype in subjects whose parents had normal plasma lipoprotein concentrations was found to relate to a problem of LDL receptor internalisation.44 Endocytosis was disrupted less in up-regulated cultured fibroblasts than in lymphoblasts. The disorder is due to mutations in the adaptor protein for endocytosis of the LDL receptor.45 A very specific interaction between the cytoplasmic tail of the LDL receptor (NPVY) with the phosphotyrosine binding domain of ARH is defective, failing to link the LDL receptor to clathrin and the adapter protein 2.46 The impact on the clearance of LDL into the liver is such that a homozygous FH phenotype results. This disorder seems to be more prevalent in Sardinia.47
Other FH Phenotypes
Autosomal dominant FH3 refers to a heterozygous FH phenotype due to mutations in NARC1, a protein of 692 amino acids coded for by the gene PCSK9 on chromosome 1p32.48 This protein is part of the subtilase family of proteases that includes subtilisin kexin iso-enzyme 1/site-1-protease that has an important role in sterol regulatory element binding protein metabolism.
Phytosterolaemia is due to an overabsorption of plant sterols from enterocytes, as well as an underexcretion from hepatocytes into the bile as a result of disruptions of ABC transporters G5 and G8 and has variable LDL hypercholes-terolaemia and high plasma plant sterol concentrations. CTX is due to mutations in cholesterol 27 hydroxylase, an essential step in cholesterol oxidation for bile acid synthesis.49 Although LDL-cholesterol is not raised, the xanthomata resemble the homozygous FH phenotype, but in addition the nervous system and lenses of the eye are affected.
Lipoprotein Metabolism in FH
A detailed description of serum apoproteins in FH found apoB concentration to be 2.5 times normal, consistent with the elevation in LDL-cholesterol.50 ApoC-I, C-II and E were significantly increased whilst apoA-I and apoA-II concentrations were not significantly different.
Turnover studies done with radio-iodinated ultracentrifugally isolated apoB-containing lipoproteins were traditionally employed to study lipoprotein metabolism, but currently synthetic rates and clearance rates for apoB-containing lipoproteins can be worked out more directly with stable isotope studies.51 Non-steady state kinetic analyses may also be done, but are subject to several assumptions, the most important of which are the occurrences of compensatory changes in production or clearance as a result of interventions (plasmapheresis). A direct comparison of steady state kinetics with radio-iodine studies and plasma exchange was done by Eriksson et al. who found that radioiodination studies yielded higher synthetic rates in normal and heterozygous FH states.52 Maugeais et al. also found no statistically significant differences between modelling with stable isotopes and non-steady state kinetics after apheresis in heterozygous FH, although there was a trend for increasing the clearance of LDL that may be attributable to an upregulation of LDL receptors.53 In homozygous FH, non-steady state turnover data may be more reliable because of the very low LDL receptor activity.
The traditional view that a defect in the LDL receptor brings about an increase of LDL only as a result of decreased clearance, explains most, but not all of the dyslipoproteinaemia in FH. The overproduction of apoB-containing lipoproteins, the suppression of HMG-CoA reductase and the increased hepatic content of cholesterol in FH were indicators of a need to review the classic paradigm.54 Barrett and Watts more recently reviewed the possibility of increased production of apoB-containing lipoproteins and concluded that there is increased production of apoB-containing lipoproteins in FH.55
A comparison of the turnover of apoB-containing lipoproteins in normal and FH subjects, revealed that in normal subjects almost all the LDL is formed from VLDL while in FH almost a third of LDL is formed by the secretion of IDL-LDL particles.56 Additionally, there was almost complete conversion of VLDL to LDL in FH, whereas in normal subjects there is considerable efflux of TG-rich lipoprotein remnants. Investigations of heterozygous FH and normal subjects found increased LDL production in heterozygous FH as well as a reduced fractional catabolic rate.52 A study undertaken by Zulewski et al. found increased production of apoB containing lipoproteins in subjects with heterozygous FH phenotype, including FDB.57 A study examined the impact of high carbohydrate low fat diets that are generally accepted as lowering plasma cholesterol in normal subjects and FH.58 As expected for such diets, plasma TG was raised. Kinetic modelling of apoB revealed increased production of more TG-rich VLDL and a greater delay in its release. Only normal subjects showed increased clearance of the precursors for LDL. Both normal and heterozygous FH subjects had increased clearance of LDL. It can be deduced that dietary cholesterol restriction increased LDL receptor expression but not cholesterol synthesis as faecal sterol balance studies showed a decrease in cholesterol synthesis.
There is no final explanation for the overproduction (over-secretion) of smaller, cholesterol-rich lipoproteins in FH. It is possible that the higher influx of cholesterol through low-affinity processes, compounded by a lack of a mechanism to regulate this influx, leads to increased cholesterol esterification, at least in part by ACAT2, and becomes available in the lumen of the ER where this neutral lipid can promote the commitment of apoB to synthesis of smaller cholesterol-rich lipoproteins. Another attractive hypothesis is that functional LDL receptors could retain newly synthesised lipoproteins and possibly target them for degradation during vesicular trafficking in the cell. Evidence for this came from the observation that a LDL receptor mutation construct that retains the receptors in the ER, practically abolished the secretion of apoB-containing lipoproteins.59 This effect was examined quantitatively in cell culture experiments: LDL receptor-negative cells degraded less than 20% of apoB whereas wildtype cells degraded about 55%.60 LDL receptors may thus modulate pre-secretory degradation of apoB and/or mediate immediate re-uptake and degradation of secreted lipoproteins. The overproduction of apoB-containing lipoproteins in FDB could be as a result of the same phenomenon when this ligand does not bind the LDL receptor.57
A turnover study of apoB in normal, LDL receptor deficient and FDB subjects revealed similarly decreased FCR for LDL in the LDL receptor deficient and FDB subjects but striking differences in the conversion of VLDL to LDL: this step was 44% less in FDB when compared to the other two states.61
HDL metabolism is also of interest in FH. Whilst the contention that HDL cholesterol is generally low in FH may be true, in clinical practice there is significant variation. A preponderance of smaller HDL species was linked to increased HL activity in FH.62 A detailed study found that HDL cholesterol, but not apoA-I concentration, was lower in FH when compared to normal subjects.63 This was also found previously.50 The production and clearance rates of apoA-I were both increased. To establish whether LDL concentration influences HDL metabolism directly, subjects with FH who had undergone plasmapheresis to reduce LDL concentration, were studied.64 The HDL measurements were stable. The calculated fractional catabolic rate for apoA-I was not statistically significantly higher, but the production rate increased.
Biliary cholesterol excretion may be increased in FH. A decrease in the cholesterol saturation index of fasting gall bladder bile was noted during treatment with pravastatin,65 and another study confirmed supersaturation of bile with cholesterol.66
Lipoprotein(a) [Lp(a)] is formed by the linking of a LDL-like lipoprotein by a disulphide bridge to apo(a). The apo(a) consists of kringle-like repeats probably duplicated from plasminogen and apo(a) also has an inactive protease domain of plasminogen. The number of kringle repeats determines the molecular mass of apo(a). The plasma concentration of Lp(a) is determined by the molecular mass of apo(a), in an inverse fashion. It appears that the production rate of Lp(a) is the main determinant of its concentration,67 and its clearance is little affected by homozygous FH.68 Nevertheless, heterozygous FH is associated with a higher plasma concentration of Lp(a) and this appears to affect prognosis.69
Repeated elevations of serum creatine kinase of skeletal muscle origin in about 40% of heterozygous FH subjects who were untreated and in whom exercise was not contributing to such elevations, suggests that there may be secondary abnormalities in muscle function.70 Heterozygous FH subjects had greater exercise-related release of creatine kinase and myoglobin, but was not aggravated by simvastatin.71
Genotype-Phenotype Interactions
The heterozygous and homozygous FH phenotypes are subject to considerable variation in their degree of metabolic derangement, as well as clinical and therapeutic outcome. Modulation of the phenotype may not only relate to the gene responsible for the disease, but also may relate to the impact of the mutation on this molecule. Additionally, other proteins that modulate lipoprotein assembly, metabolism and clearance can influence the phenotype.
Residual LDL receptor activity influences LDL concentration in heterozygous FH. A LDL receptor mutation that confers a very mild phenotype lacks exon 15 which is thought to act only as a stalk for the receptor.72 A comparison of plasma LDL concentration in Afrikaners with a defective mutation (D206E) and a negative mutation (V408M) in the LDL receptor demonstrated significant differences in LDL-cholesterol concentration.73 Amongst French Canadian heterozygous FH subjects, receptor-negative patients had worse hypercholesterolaemia and more vascular disease.74 In the homozygous phenotype, LDL receptor activity also influenced the LDL-cholesterol concentration, with almost no overlap between negative and defective LDL receptor status in French Canadians.75
Since remnants of the TG-rich lipoproteins are cleared by the binding of apoE to the LDL receptor and LRP, it is of interest to examine the clearance of remnants in FH. CM can be labelled with orally administered retinyl esters and the plasma excursion of retinyl palmitate can be followed in whole plasma or in different density fractions for analysis. An alternative strategy to examine CM clearance is to measure plasma apoB48 concentration. Cabezas et al. examined retinyl palmitate clearance in untreated heterozygous FH and normal subjects.76 Whilst TG clearance (lipolysis) displayed no difference, retinyl palmitate clearance was delayed significantly in FH. ApoB48 concentration in heterozygous FH was more than double that of normal persons.77,78 This abnormality was also reported in homozygous FH.79 Retinyl palmitate clearance was not abnormal in the early part of an oral fat loading test in homozygous FH.80 The findings by Watts et al. in a novel breath test that examined oxidation of C13-labelled acyl-cholesterol in subjects with proven LDL receptor defects in the homozygous phenotype, also suggested that remnant-like particle clearance is not impaired.81 Since apoB48 is a specific and sensitive marker for chylomicron remnant clearance and increased CM production is unlikely in FH, its elevation should be taken as proof of impaired clearance of remnants of TG-rich lipoproteins.
ApoE influences the LDL-cholesterol concentration: the E2 isoform binds poorly to the LDL receptor and results in delayed clearance of remnants and consequently in increased clearance of LDL after up-regulation of the LDL receptor. In the setting of normal production rates of VLDL and limited dietary fat intake, apoE2 homozygosity is thus associated with a lower LDL concentration, whilst apoE concentration is increased. ApoE4 homozygosity is associated with higher LDL-cholesterol concentration, that is attributable to increased absorption. The impact of apoE on the FH phenotype has not been consistent. ApoE isoforms were not found to influence LDL concentration in several studies.82–84
Cholesterol esterase is an enzyme of pancreatic origin that requires bile acids for full activation. Unexpectedly, it appears to influence plasma LDL concentration significantly in normal individuals and to promote the formation of smaller LDL particles. In FH, serum cholesterol esterase activity is not increased, but its impact on LDL concentration is not known.85
The possible influence of the TaqIB polymorphism in CETP on the heterozygous FH phenotype was examined by Carmena-Ramon et al.86 The B2 allele was associated with higher HDL-cholesterol as well as apoA-I concentrations and lower LDL-cholesterol. This seemed to translate to a more benign clinical phenotype.
ApoH, modulating lipolysis, was found to influence the TG concentration by the V247L mutation.87 A functional polymorphism of MTP was found to influence the lipoprotein phenotype of FH differently to normal subjects.88 The -439G/G status in the promoter for MTP was responsible for practically a doubling of serum TG concentration when compared with the T/T status. In contrast to healthy individuals, there appeared to be no effect on LDL concentration in FH. This supports the notion that lipoprotein clearance and not production, is the major problem in FH.
The ApoA-I promoter polymorphism (-75 A/G) that may influence metabolism of HDL and indirectly also LDL, had an impact on LDL and apoB concentrations with G/A subjects having lower values.89 A normal variant of apoA-IV that may influence fat absorption, apoA-IV-2, was responsible for a mild lowering effect on LDL cholesterol in heterozygous FH, but less dietary responsiveness of LDL cholesterol concentration.90
A curious variant form of FH has corneal opacification, and polyneuropathy, as well as stroke due to carotid disease.91 The index patient had a mosaicism of 46XX/45XO, defective monocyte function and elevations of serum lipid hydroperoxides. This is presumed to be due to additional genetic abnormalities to the causes of FH.
A genetic disorder, unrelated to lipoprotein metabolism, may affect the phenotype of FH as well. In Sardinia, subjects with proven mutations of the LDL receptor had an average lowering of LDL cholesterol by 2.49 mmol/L when the subject also inherited β-thalassaemia.92 The mechanism may relate to consumption of LDL by erythroid hyperplasia.
Coexistent Dyslipidaemias with FH
LDL particle size and density vary according to the properties of the secreted form of the lipoprotein and the modulation of LDL particle size by hypertriglyceridaemia, CETP and HL. The metabolic syndrome (atherogenic lipoprotein phenotype) in subjects with insulin resistance is characterised by mild hypertriglyceridaemia, low HDL-cholesterol and small LDL particles. This lipoprotein phenotype is due to environmental and likely multiple genetic factors. In diabetes, the lack of suppression of hormone-sensitive lipase in adipocytes leads to increased release of fatty acids. After re-assembly of fatty acids into TG in the liver, the increased secretion of VLDL, as well as a modest reduction in the expression of LPL in the diabetic state, leads to hypertriglyceridaemia, low HDL-cholesterol concentrations and small dense LDL, as well as increased glycation of plasma proteins. To date, there has been little information on the interaction of diabetes and FH, although it is known that remnants are increased even further in diabetics or in subjects who have impaired glucose tolerance.93 LDL particle size was described as being large in 16 of 26 FH subjects and was influenced by TG concentration.94 More studies are required to establish whether LDL in FH has the same sensitivity to modulation and whether small LDL has a significant impact on the pathogenesis of atherosclerosis.
The co-existence of FH and FDB has been described.95–97 The clinical features of the complex heterozygote for LDL receptor and apoB mutations was no different to heterozygous FH in one case, and intermediate between heterozygous and homozygous FH in the other 2 cases.95
Until there is a specific marker for familial combined hyperlipidaemia (FCH), its co-existence with FH cannot be studied properly. Since FCH is believed to be due to an overproduction of VLDL, it is possible that it may account for hypertriglyceridaemia occasionally seen in FH. More severe hypertriglyceridaemia may also be seen with concurrent dysbetalipoproteinaemia and FH.
ApoE plays an important role in the clearance of apoB-containing lipoproteins larger than LDL. The apoE2 isoform is homozygous in approximately 1% of the population. Under conditions that increase lipoprotein production and/or decrease clearance, remnants of TG-rich lipoproteins accumulate to produce a mixed hyperlipidaemia (dysbetal-ipoproteinaemia). The phenotype in FH subjects also homozygous for apoE2 could be similarly affected to normal subjects. An initial amelioration in childhood could reverse after changes in metabolism with age and disease to produce significant hypertriglyceridaemia and cutaneous xanthomata. The presence of significant concentrations of LDL in subjects with dysbetalipoproteinaemia should suggest the co-existence of FH. A study of a relatively small cohort of co-existent dysbetalipoproteinaemia and FH revealed no aggravation of the atherosclerosis complications.98 Cutaneous xanthomata, rare in heterozygous FH may be prominent in coexisting dysbetalipoproteinaemia and FH.99
FH subjects with LPL deficiency have no LDL.100 The co-inheritance of hypobetalipoproteinaemia and a heterozygous FH condition would render the LDL cholesterol low.
Clinical Application of Biochemical Insights
The initial clinical observations led to biochemical investigations that provided insight into the causes and pathophysiology of FH. The advances in biochemistry and genetics now allow better research into and understanding of observations of clinical phenotypes and therapeutic manipulation and its variability.
Diagnostic Work-up of FH
A thorough clinical assessment and the results from routine chemical assays deriving LDL-cholesterol concentration by the Friedewald calculation [LDL-cholesterol = total cholesterol − HDL-cholesterol − (TG/2.2) in mmol/L] or directly, can identify the heterozygous phenotype, and could even discriminate the rare sterol defects within the homozygous phenotype.
The clinical diagnosis of the heterozygous FH phenotype is adequate for risk stratification and appropriate pharmaceutical management by general practitioners and specialists. Precision in the diagnosis of FH could be of value for discriminating between polygenic or secondary dyslipidaemias and FH in family studies. Genetic counselling is similar as all three disorders are inherited in an autosomal dominant fashion, but for affected couples accurate diagnosis may be of value in counselling about disease in offspring. When both parents are heterozygous for LDL receptor defects, there is a 25% chance of producing a homozygote. However, in counselling the inheritance of dyslipidaemia in couples who have different genes for the heterozygous phenotype, it is known that co-inheritance of LDL receptor mutation and FDB results in an intermediate phenotype. The co-inheritance of LDL receptor defects and NARC1 has not yet been reported, but is likely similar to FDB and LDL receptor co-inheritance.
Although investigations with radio-iodinated LDL can demonstrate deficient clearance of normal LDL in FH subjects with LDL receptor defects, and abnormal clearance of binding defective LDL in normal subjects, these procedures are labour intensive, expensive and not used in practice. Analysis of peripheral blood mononuclear cells101 or cultured cells after up-regulation of LDL receptors with lipoprotein deficient media by means of radio-iodinated or fluorescent LDL or by fluorescently-tagged antibodies in flow cytometry may discriminate normal from heterozygous FH subjects, but remains experimental.
The genetic diagnosis by polymerase chain reaction for LDL and apoB defects is now available in many laboratories. Only a few mutations are known in apoB, but there are more than 750 LDL receptor mutations. In populations with founder effects, genetic diagnosis is easier. In other populations, where research centres can sequence the genes in affected families, molecular diagnosis can be made in family members. In the future, micro-array chips may provide simultaneous evaluation for many mutations.
Patients with a homozygous FH phenotype should be referred to specialised centres for diagnostic work-up and management. Cell culture is more reliable to identify LDL receptor defects. In fibroblast cultures, LDL receptor mutations will result in low binding, whereas adaptor protein mutations will not. It is worth considering measurement of plasma phytosterols for phytosterolaemia and cholestanol for CTX and investigating the genes influencing the concentration of these products.
Founder Effects in FH
The frequency of FH has been established at approximately 0.002 in the general population at large. There is a very low mutation rate in genes that result in FH and heterozygous FH seldom if ever results in death before reproduction, so that the mutated genes are retained. A variety of mutations in the LDL receptor can be demonstrated in populations that have been isolated for long periods of time and have grown to large numbers.
The founder effect refers to the occurrence of FH at a higher frequency than 0.002 and/or the predominance of one or a few mutations in a population. This phenomenon is typically found in more recently established populations that have migrated in small groups and expanded into a geographically and/or culturally isolated setting. Examples are the Afrikaners in South Africa, French Canadians, Christian Lebanese and the Finns. Such a founder effect may undergo genetic drift according to selection or may become diluted by admixture.
South Africa is well known for its high prevalence of FH in Afrikaners, due to three founder mutations.102 The prevalence of FH was estimated at 1 in 83 in a rural community.103 Although it is generally true that three founder mutations explain 95% of FH,104 the extent to which these three founder mutations account for FH in the Afrikaner varies. A lower proportion of FH is attributable to the founder mutations in the Cape, the portal of entry into South Africa.105 The V408M mutation that ranks second in frequency in the Afrikaner has been described from the Netherlands and there was evidence that it came from northern Germany.106 Ancestral linkage of these populations was indicated by the presence of a specific haplotype, which included the Dutch immigrants to Canada. A recurrent mutation of V408M has been reported in a different population group in South Africa and Cuba.107,108 The prevalence of FH is estimated to be high in the South African Jews, with the common mutation being the deletion of codon 197 that is believed to be from Lithuania and also accounts for about a third of FH in St Petersburg and appears limited to Jews in this region.109 The occurrence of the same haplotype on samples from Jews with FH in many countries also indicated an ancestral link.110 In South Africa, FH in the Jewish community is also accounted for by local mutations.111
In French Canadians, 11 mutations in the LDL receptor describe approximately 90% of subjects with FH,112 and regional variation in the prevalence of different mutations is striking.113 A recently identified founder effect has been described from Tunisia which, at 1 in 165, is less common than in French Canada.114 In most countries, a wider range of mutations has been reported in the LDL receptor. In the Danish population about 5 of the 29 documented mutations occurred in 50% of the population.115 Germany also lacks a founder effect: 37 mutations have been described in 56 individuals with FH.116 At least 29 mutations have been identified in the Chinese, with no apparent founder effect.117
Therapeutic Strategies in FH
Modern pharmacotherapy can achieve desirable concentrations of LDL in heterozygotes, but treatment for homozygotes remains problematic. Heterozygous FH responds like normal subjects to lipid-modifying treatment, as there is a functional LDL receptor that will clear LDL from plasma. Dietary cholesterol and saturated fat restriction will have a modest LDL-lowering effect owing to an up-regulation of LDL receptors when the liver receives less cholesterol through CM remnants. The reduction achieved in plasma LDL-cholesterol is approximately 10%, but is variable. A low-fat and low-cholesterol diet may not only up-regulate LDL receptor activity, but may also decrease the endogenous synthesis of cholesterol as suggested by decreased lathosterol concentration in a study of high- and low- fat diets together with statin treatment.118
Bile acid sequestrants that waste more bile acids for the patient result in a reduction of the cholesterol pool in cells, and there is a consequent increase in de novo cholesterol synthesis, as well as in LDL receptor synthesis. LDL-cholesterol concentration decreases by 25% at maximal doses. Cholesterol absorption is reduced by phytosterols and phytostanols. LDL concentrations decrease by about 6 to 10%.
The most powerful cholesterol-lowering agents for FH are the statins. By inhibiting de novo cholesterol synthesis at HMG-CoA reductase, compensatory upregulation of LDL receptors lowers plasma LDL concentration. FH subjects who achieved steady states of sterol and lipoprotein metabolism with higher de novo cholesterol synthetic rates have higher fasting early morning plasma mevalonic acid concentration and respond better to statins than those with lower plasma mevalonic acid concentration.119
There is no clarity about residual activity of LDL receptors and response to treatment in heterozygous FH. A greater percentage decrease of LDL-cholesterol was reported in receptor-negative subjects.120,121 One study that examined LDL responses in heterozygous FH subjects treated with pravastatin, found some correlation (r = 0.68) between maximal fibroblast LDL receptor activity and LDL reduction that ranged from 30 to 70%.122 A comparison of responses of cohorts with different mutations revealed that the LDL receptor mutation explained 41% of the response in LDL-cholesterol to fluvastatin.123 Two mutations with detectable differences in their phenotype responded similarly to statins.124 FDB responds well to statins125 as the LDL receptor up-regulation increases the clearance of both remnants and LDL. It is also likely that statins reduce the production of lipoproteins.
If up-regulation of LDL receptors were the only mechanism by which statins lowered LDL, subjects with homozygous FH due to complete absence of LDL receptor activity will not respond. A decreased production rate of LDL was described in a small cohort of homozygous FH subjects on statins whose rebounds in plasma LDL were analysed.126 Clearance constants for LDL did not change significantly. One subject had receptor-negative status. These findings indicated that lipoprotein production must be limited, but it is not clear how this happens. De novo synthesised cholesterol or a regulatory response to cholesterol depletion may play an important role in lipoprotein assembly and secretion. It appeared unlikely that delivery of lipoproteins to the liver plays a strong modulatory role in production and clearance rates for apoB-containing lipoproteins in the plasma as regular apheresis resulted in no change of these parameters.127
Ezetimibe, a recently developed drug that limits cholesterol absorption, lowers LDL cholesterol by about 15%. Interestingly, the impact is similar in heterozygous and homozygous FH.128 The mechanism of its action is presumably by limiting lipoprotein production through a poorly understood disruption of cholesterol supply to the liver by lipoprotein remnants.
The inhibition of MTP disrupts lipoprotein assembly and is highly successful at controlling hypercholesterolaemia in the Watanabe rabbit, an animal model of homozygous FH.129 However, the accumulation of fat in the liver as a result of decreased export may be detrimental.
Plasmapheresis is a well-established procedure that, together with statin and ezetimibe therapy is the treatment of choice for homozygous FH. The original procedure of plasma exchange in which albumin in sterile physiologic saline is used, has been replaced by more specific adsorption of lipoproteins onto dextran sulphate or anti-apoB immunoglobulin columns, extracorporeal precipitation with heparin, or double filtration. Liver transplantation, by correcting the function of the most important organ in cholesterol homeostasis, is probably reserved for subjects requiring heart transplant. Gene therapy will hopefully permit effective treatment of this severe disorder in the future.
Conclusions
FH is a serious and common disorder that results in premature atherosclerosis, but recently, with the advent of statin treatment, has become amenable to treatment. This review has assigned a broad definition to FH and has categorised the disorder as it may present to medical practitioners as a heterozygous, homozygous and intermediate phenotype. Molecular and metabolic differences in disorders producing the phenotypes have been outlined. The LDL receptor has been characterised in detail and the abundance of mutations in this receptor has given insight into the cell biology of receptor maturation, localisation, endocytosis and recycling. Genetic tests offer specific aetiologic diagnosis, but are currently not practicable in countries which do not have founder effects. FDB is amenable to genetic diagnosis by screening for a few mutations. The prevalence and metabolic implications of the more recently discovered disorders still need more study. Other monogenic disorders causing hypercholesterolaemia may still be uncovered in the future.
The complexity of lipoprotein metabolism has been described to provide insight into the multiple genes that may affect the clinical and biochemical manifestations of the disease. These genes may account for variability in the biochemical phenotype, atherosclerosis and factors that influence drug response but probably act in concert, rather than singly, to modify the high risk of atherosclerosis in FH.
Acknowledgments
The author expresses his gratitude to the staff in Lipidology in the Cape Heart Centre, especially Dr D J Blom and Mrs S Jones, for their assistance and support in making this review possible.
References
- 1.Ose L. Muller-Harbitz disease--familial hypercholesterolemia. Tidsskr Nor Laegeforen. 2002;122:924–5. [PubMed] [Google Scholar]
- 2.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 4.Defesche J. World Health Organisation report on familial hypercholesterolemia. Atherosclerosis. 2001;154:242. doi: 10.1016/s0021-9150(00)00646-8. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein JL, Brown MS. Molecular medicine. The cholesterol quartet. Science. 2001;292:1310–2. doi: 10.1126/science.1061815. [DOI] [PubMed] [Google Scholar]
- 6.Schneider WJ, Nimpf J. LDL receptor relatives at the crossroad of endocytosis and signaling. Cell Mol Life Sci. 2003;60:892–903. doi: 10.1007/s00018-003-2183-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–31. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu KCW, Chen W, Cooper AD. LDL receptor-related protein mediates cell-surface clustering and hepatic sequestration of chylomiron remnants in LDLR-deficient mice. J Clin Invest. 2001;107:1387–94. doi: 10.1172/JCI11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bihain BE, Yen FT. Free fatty acids activate a high-affinity saturable pathway for degradation of low-density lipoproteins in fibroblasts from a subject homozygous for familial hypercholesterolemia. Biochemistry. 1992;31:4628–36. doi: 10.1021/bi00134a013. [DOI] [PubMed] [Google Scholar]
- 10.Tai ES, Adiconis X, Ordovas JM, et al. Polymorphisms at the SRBI locus are associated with lipoprotein levels in subjects with heterozygous familial hypercholesterolemia. Clin Genet. 2003;63:53–8. doi: 10.1034/j.1399-0004.2003.630108.x. [DOI] [PubMed] [Google Scholar]
- 11.Fass D, Blacklow S, Kim PS, Berger JM. Molecular basis of familial hypercholesterolaemia from structure of LDL receptor module. Nature. 1997;388:691–3. doi: 10.1038/41798. [DOI] [PubMed] [Google Scholar]
- 12.Innerarity TL. LDL receptor's β propeller displaces LDL. Science. 2003;298:2337–9. doi: 10.1126/science.1080669. [DOI] [PubMed] [Google Scholar]
- 13.Simons K, Ikonen E. How cells handle cholesterol. Science. 2000;12:1721–6. doi: 10.1126/science.290.5497.1721. [DOI] [PubMed] [Google Scholar]
- 14.Schmitz G, Langmann T. Structure, funtion and regulation of the ABC1 gene product. Curr Opin Lipidol. 2001;12:129–40. doi: 10.1097/00041433-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Powell LM, Wallis SC, Pease RJ, Edwards YH, Knott TJ, Scott J. A novel form of tissue-specific RNA processing produces apolipoprotein-B48 in intestine. Cell. 1987;50:831–40. doi: 10.1016/0092-8674(87)90510-1. [DOI] [PubMed] [Google Scholar]
- 16.Marks D, Thorogood M, Neil HA, Humphries SE. A review on the diagnosis, natural history, and treatment of familial hypercholesterolaemia. Atherosclerosis. 2003;168:1–14. doi: 10.1016/s0021-9150(02)00330-1. [DOI] [PubMed] [Google Scholar]
- 17.Cassanelli S, Bertolini S, Rolleri M, et al. A 'de novo' point mutation of the low-density lipoprotein receptor gene in an Italian subject with primary hypercholesterolemia. Clin Genet. 1998;53:391–5. doi: 10.1111/j.1399-0004.1998.tb02752.x. [DOI] [PubMed] [Google Scholar]
- 18.Pisciotta L, Cantafora A, De Stefano F, Langheim S, Calandra S, Bertolini S. A "de novo" mutation of the LDL-receptor gene as the cause of familial hypercholesterolemia. Biochim Biophys Acta. 2002;1587:7–11. doi: 10.1016/s0925-4439(02)00047-9. [DOI] [PubMed] [Google Scholar]
- 19.Neil HA, Huxley RR, Hawkins MM, Durrington PN, Betteridge DJ, Humphries SE. Comparison of the risk of fatal coronary heart disease in treated xanthomatous and non-xanthomatous heterozygous familial hypercholesterolaemia: a prospective registry study. Atherosclerosis. 2003;170:73–8. doi: 10.1016/s0021-9150(03)00233-8. [DOI] [PubMed] [Google Scholar]
- 20.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 21.Tames FJ, Mackness MI, Arrol S, Laing I, Durrington PN. Non-enzymatic glycation of apolipoprotein B in the sera of diabetic and non-diabetic subjects. Atherosclerosis. 1992;93:237–44. doi: 10.1016/0021-9150(92)90260-n. [DOI] [PubMed] [Google Scholar]
- 22.Lavrencic A, Kosmina B, Keber I, Videcnik V, Keber D. Carotid intima-media thickness in young patients with familial hypercholesterolaemia. Heart. 1996;76:321–5. doi: 10.1136/hrt.76.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–5. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 24.Smilde TJ, van Wissen S, Wollersheim H, Trip MD, Kastelein JJP, Stalenhoef AFH. Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolaemia (ASAP): a prospective, randomised, double blind trial. Lancet. 2001;357:577–81. doi: 10.1016/s0140-6736(00)04053-8. [DOI] [PubMed] [Google Scholar]
- 25.North CL, Blacklow SC. Solution structure of the sixth LDL-A module of the LDL receptor. Biochemistry. 2000;39:2564–71. doi: 10.1021/bi992087a. [DOI] [PubMed] [Google Scholar]
- 26.Koivisto UM, Hubbard AL, Mellman I. A novel cellular phenotype for familial hypercholesterolemia due to a defect in polarized targeting of LDL receptor. Cell. 2001;105:575–85. doi: 10.1016/s0092-8674(01)00371-3. [DOI] [PubMed] [Google Scholar]
- 27.Hussain MM, Strickland DK, Bakillah A. The mammalian low-density lipoprotein receptor family. Annu Rev Nutr. 1999;19:141–72. doi: 10.1146/annurev.nutr.19.1.141. [DOI] [PubMed] [Google Scholar]
- 28.Hobbs HH, Russell DW, Brown MS, Goldstein JL. The LDL receptor locus in familial hypercholesterolemia: mutational analysis of a membrane protein. Annu Rev Genet. 1990;24:133–70. doi: 10.1146/annurev.ge.24.120190.001025. [DOI] [PubMed] [Google Scholar]
- 29.Heath KE, Gahan M, Whitall RA, Humphries SE. Low-density lipoprotein receptor gene (LDLR) world-wide website in familial hypercholesterolaemia: update, new features and mutational analysis. Atherosclerosis. 2000;154:243–6. doi: 10.1016/s0021-9150(00)00647-x. [DOI] [PubMed] [Google Scholar]
- 30.Schaefer JR, Scharnagl H, Baumstark MW, et al. Homozygous familial defective apolipoprotein B-100. Enhanced removal of apolipoprotein E-containing VLDLs and decreased production of LDLs. Arterioscler Thromb Vasc Biol. 1997;17:348–53. doi: 10.1161/01.atv.17.2.348. [DOI] [PubMed] [Google Scholar]
- 31.Pimstone SN, Defesche JC, Clee SM, Bakker HD, Hayden MR, Kastelein JJ. Differences in the phenotype between children with familial defective apolipoprotein B-100 and familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1997;17:826–33. doi: 10.1161/01.atv.17.5.826. [DOI] [PubMed] [Google Scholar]
- 32.Brugger D, Schuster H, Zollner N. Familial hypercholesterolemia and familial defective apolipoprotein B-100: comparison of the phenotypic expression in 116 cases. Eur J Med Res. 1996;1:383–6. [PubMed] [Google Scholar]
- 33.Miserez AR, Keller U. Differences in the phenotypic characteristics of subjects with familial defective apolipoprotein B-100 and familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1995;15:1719–29. doi: 10.1161/01.atv.15.10.1719. [DOI] [PubMed] [Google Scholar]
- 34.Defesche J, Pricker KL, Hayden MR, van der Ende BE, Kastelein JJ. Familial defective apolipoprotein B-100 is clinically indistinguishable from familial hypercholesterolemia. Arch Int Med. 1993;153:2349–56. [PubMed] [Google Scholar]
- 35.Maher VM, Gallagher JJ, Thompson GR, Myant NB. Does the presence of the 3500 mutant apolipoprotein B-100 in low density lipoprotein particles affect their atherogenicity? Atherosclerosis. 1995;118:105–10. doi: 10.1016/0021-9150(95)05598-q. [DOI] [PubMed] [Google Scholar]
- 36.Myant NB. Familial defective apolipoprotein B-100: a review, including some comparisons with familial hypercholesterolaemia. Atherosclerosis. 1993;104:1–18. doi: 10.1016/0021-9150(93)90171-p. [DOI] [PubMed] [Google Scholar]
- 37.Schuster H, Rauh G, Kormann B, et al. Familial defective apolipoprotein B-100. Comparison with familial hypercholesterolemia in 18 cases detected in Munich. Arteriosclerosis. 1990;10:577–81. doi: 10.1161/01.atv.10.4.577. [DOI] [PubMed] [Google Scholar]
- 38.Marz W, Ruzicka C, Pohl T, Usadel KH, Gross W. Familial defective apolipoprotein B-100: mild hypercholesterolaemia without atherosclerosis in a homozygous patient. Lancet. 1992;340:1362. doi: 10.1016/0140-6736(92)92554-s. [DOI] [PubMed] [Google Scholar]
- 39.Gallagher JJ, Myant NB. The affinity of low-density lipoproteins and of very-low-density lipoprotein remnants for the low-density lipoprotein receptor in homozygous familial defective apolipoprotein B-100. Atherosclerosis. 1995;115:263–72. doi: 10.1016/0021-9150(94)05528-q. [DOI] [PubMed] [Google Scholar]
- 40.Marz W, Baumstark MW, Scharnagl H, et al. Accumulation of "small dense" low density lipoproteins (LDL) in a homozygous patients with familial defective apolipoprotein B-100 results from heterogenous interaction of LDL subfractions with the LDL receptor. J Clin Invest. 1993;92:2922–33. doi: 10.1172/JCI116915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bersot TP, Russell SJ, Thatcher SR, et al. A unique haplotype of the apolipoprotein B-100 allele associated with familial defective apolipoprotein B-100 in a Chinese man discovered during a study of the prevalence of this disorder. J Lipid Res. 1993;34:1149–54. [PubMed] [Google Scholar]
- 42.Miserez AR, Muller PY. Familial defective apolipoprotein B-100: a mutation emerged in the mesolithic ancestors of Celtic peoples? Atherosclerosis. 2000;148:433–6. doi: 10.1016/s0021-9150(99)00470-0. [DOI] [PubMed] [Google Scholar]
- 43.Hamalainen T, Palotie A, Aalto-Setala K, Kontula K, Tikkanen MJ. Absence of familial defective apolipoprotein B-100 in Finnish patients with elevated serum cholesterol. Atherosclerosis. 1990;82:177–83. doi: 10.1016/0021-9150(90)90038-k. [DOI] [PubMed] [Google Scholar]
- 44.Norman D, Sun XM, Bourbon M, Knight BL, Naoumova RP, Soutar AK. Characterization of a novel cellular defect in patients with phenotypic homozygous familial hypercholesterolemia. J Clin Invest. 1999;104:619–28. doi: 10.1172/JCI6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He G, Gupta S, Yi M, Michaely P, Hobbs HH, Cohen JC. ARH is a modular adaptor protein that interacts with the LDL receptor, clathrin, and AP-2. J Biol Chem. 2002;277:44044–9. doi: 10.1074/jbc.M208539200. [DOI] [PubMed] [Google Scholar]
- 46.Mishra SK, Watkins SC, Traub L. The autosomal recessive hypercholesterolemia (ARH) protein interfaces directly with the clathrin-coat machinery. Proc Natl Acad Sci USA. 2002;99:16099–104. doi: 10.1073/pnas.252630799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arca M, Zuliani G, Wilund K, et al. Autosomal recessive hypercholesterolaemia in Sardinia, Italy, and mutations in the ARH: a clinical and molecular genetic analysis. Lancet. 2002;359:841–7. doi: 10.1016/S0140-6736(02)07955-2. [DOI] [PubMed] [Google Scholar]
- 48.Abifadel M, Varret M, Rabes JP, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–6. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 49.Moghadasian MH, Salen G, Frohlich JJ, Scudamore CH. Cerebrotendinous xanthomatosis: a rare disease with diverse manifestations. Arch Neurol. 2002;59:527–9. doi: 10.1001/archneur.59.4.527. [DOI] [PubMed] [Google Scholar]
- 50.Kajinami K, Mabuchi H, Koizumi J, Takeda R. Serum apolipoproteins in heterozygous familial hypercholesterolemia. Clin Chim Acta. 1992;211:93–9. doi: 10.1016/0009-8981(92)90108-3. [DOI] [PubMed] [Google Scholar]
- 51.Demant T, Packard CJ, Demmelmair H, et al. Sensitive methods to study human apolipoprotein B metabolism using stable isotope-labeled amino acids. Am J Physiol. 1996;270:E1022–E1036. doi: 10.1152/ajpendo.1996.270.6.E1022. [DOI] [PubMed] [Google Scholar]
- 52.Eriksson M, Berglund L, Gabrielsson J, Lantz B, Angelin B. Non-steady-state kinetics of low density lipoproteins in man: studies after plasma exchange in healthy subjects and patients with familial hypercholesterolaemia. Eur J Clin Invest. 1993;23:746–52. doi: 10.1111/j.1365-2362.1993.tb01295.x. [DOI] [PubMed] [Google Scholar]
- 53.Maugeais C, Ouguerram K, Frenais R, et al. Effect of low-density lipoprotein apheresis on kinetics of apolipoprotein B in heterozygous familial hypercholesterolemia. J Clin Endocrinol Metab. 2001;86:1679–86. doi: 10.1210/jcem.86.4.7428. [DOI] [PubMed] [Google Scholar]
- 54.Fisher WR, Zech LA, Stacpoole PW. ApoB metabolism in familial hypercholesterolemia. Inconsistencies with the LDL receptor paradigm. Arterioscler Thromb. 1994;14:501–10. doi: 10.1161/01.atv.14.4.501. [DOI] [PubMed] [Google Scholar]
- 55.Barrett PHR, Watts GF. Shifting the LDL-receptor paradigm in familial hypercholesterolemia: novel insights from recent kinetic studies of apolipoprotein B-100 metabolism. Atherosclerosis Suppl. 2002;2:1–4. doi: 10.1016/s1567-5688(01)00012-5. [DOI] [PubMed] [Google Scholar]
- 56.Fisher WR, Zech LA, Kilgore LL, Stacpoole PW. Metabolic pathways of apolipoprotein B in heterozygous familial hypercholesterolemia: studies with a [3H]leucine tracer. J Lipid Res. 1991;32:1823–36. [PubMed] [Google Scholar]
- 57.Zulewski H, Ninnis R, Miserez AR, Baumstark MW, Keller U. VLDL and IDL apolipoprotein B-100 kinetics in familial hypercholesterolemia due to impaired LDL receptor function or to defective apolipoprotein B-100. J Lipid Res. 1998;39:380–7. [PubMed] [Google Scholar]
- 58.Stacpoole PW, Von Bergmann K, Kilgore LL, Zech LA, Fisher WR. Nutritional regulation of cholesterol synthesis and apolipoprotein B kinetics: studies in patients with familial hypercholesterolemia and normal subjects treated with a high carbohydrate, low fat diet. J Lipid Res. 1991;32:1837–48. [PubMed] [Google Scholar]
- 59.Gillian-Daniel DL, Bates PW, Tebon A, Attie AD. Endoplasmic reticulum localization of the low density lipoprotein receptor mediates presecretory degradation of apolipoprotein B. Proc Natl Acad Sci USA. 2002;99:4337–42. doi: 10.1073/pnas.072557199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Twisk J, Gillian-Daniel DL, Tebon A, Wang L, Barrett PH, Attie AD. The role of the LDL receptor in apolipoprotein B secretion. J Clin Invest. 2000;105:521–32. doi: 10.1172/JCI8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gaffney D, Forster L, Caslake MJ, et al. Comparison of apolipoprotein B metabolism in familial defective apolipoprotein B and heterogeneous familial hypercholesterolemia. Atherosclerosis. 2002;162:33–43. doi: 10.1016/s0021-9150(01)00679-7. [DOI] [PubMed] [Google Scholar]
- 62.Moriguchi EH, Tamachi H, Goto Y. Hepatic lipase activity and high density lipoproteins in familial hypercholesterolemia: adaptational mechanisms for LDL-receptor deficient state. Tokai J Exp Clin Med. 1990;15:401–6. [PubMed] [Google Scholar]
- 63.Frenais R, Ouguerram K, Maugeais C, et al. Apolipoprotein A-I kinetics in heterozygous familial hypercholesterolemia: a stable isotope study. J Lipid Res. 1999;40:1506–11. [PubMed] [Google Scholar]
- 64.Frenais R, Maugeais C, Ouguerram K, et al. Effect of low-density lipoproteins on apolipoprotein AI kinetics in heterozygous familial hypercholesterolemia. Metabolism. 2001;50:635–9. doi: 10.1053/meta.2001.23285. [DOI] [PubMed] [Google Scholar]
- 65.Hoogerbrugge-vd LN, de Rooy FW, Jansen H, van Blankenstein M. Effect of pravastatin on biliary lipid composition and bile acid synthesis in familial hypercholesterolaemia. Gut. 1990;31:348–50. doi: 10.1136/gut.31.3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tanno N, Oikawa S, Koizumi M, et al. Biliary lipid composition in heterozygous familial hypercholesterolemia and influence of treatment with probucol. Dig Dis Sci. 1994;39:1586–91. doi: 10.1007/BF02088069. [DOI] [PubMed] [Google Scholar]
- 67.Parhofer KG, Demant T, Ritter MM, Geiss HC, Donner M, Schwandt P. Lipoprotein (a) metabolism estimated by nonsteady-state kinetics. Lipids. 1999;34:325–35. doi: 10.1007/s11745-999-0370-z. [DOI] [PubMed] [Google Scholar]
- 68.Knight BL, Perombelon N, Soutar AK, Wade DP, Seed M. Catabolism of lipoprotein(a) in familial hypercholesterolaemic subjects. Atherosclerosis. 1991;87:227–37. doi: 10.1016/0021-9150(91)90025-x. [DOI] [PubMed] [Google Scholar]
- 69.Seed M, Hoppichler F, Reaveley D, et al. Relation of serum lipoprotein(a) concentration and apolipoprotein(a) phenotype to coronary heart disease in patients with familial hypercholesterolemia. N Engl J Med. 1990;322:1494–9. doi: 10.1056/NEJM199005243222104. [DOI] [PubMed] [Google Scholar]
- 70.Bhatnagar D, Durrington PN, Neary R, Miller JP. Elevation of skeletal muscle isoform of serum creatine kinase in heterozygous familial hypercholesterolaemia. J Intern Med. 1990;228:493–5. doi: 10.1111/j.1365-2796.1990.tb00268.x. [DOI] [PubMed] [Google Scholar]
- 71.Smit JW, Bar PR, Geerdink RA, Erkelens DW. Heterozygous familial hypercholesterolaemia is associated with pathological exercise-induced leakage of muscle proteins, which is not aggravated by simvastatin therapy. Eur J Clin Invest. 1995;25:79–84. doi: 10.1111/j.1365-2362.1995.tb01530.x. [DOI] [PubMed] [Google Scholar]
- 72.Koivisto PV, Koivisto UM, Kovanen PT, Gylling H, Miettinen TA, Kontula K. Deletion of exon 15 of the LDL receptor gene is associated with a mild form of familial hypercholesterolemia. FH-Espoo. Arterioscler Thromb. 1993;13:1680–8. doi: 10.1161/01.atv.13.11.1680. [DOI] [PubMed] [Google Scholar]
- 73.Kotze MJ, De Villiers WJ, Steyn K, et al. Phenotypic variation among familial hypercholesterolemics heterozygous for either one of two Afrikaner founder LDL receptor mutations. Arterioscler Thromb. 1993;13:1460–8. doi: 10.1161/01.atv.13.10.1460. [DOI] [PubMed] [Google Scholar]
- 74.Gaudet D, Vohl MC, Couture P, et al. Contribution of receptor negative versus receptor defective mutations in the LDL-receptor gene to angiographically assessed coronary artery disease among young (25–49 years) versus middle-aged (50–64 years) men. Atherosclerosis. 1999;143:153–61. doi: 10.1016/s0021-9150(98)00268-8. [DOI] [PubMed] [Google Scholar]
- 75.Moorjani S, Roy M, Torres A, Betard C, Gagne C, Lambert M, Brun D, Davignon J, Lupien P. Mutations of low-density-lipoprotein-receptor gene, variation in plasma cholesterol, and expression of coronary heart disease in homozygous familial hypercholesterolaemia. Lancet. 1993;341:1303–6. doi: 10.1016/0140-6736(93)90815-x. [DOI] [PubMed] [Google Scholar]
- 76.Cabezas MC, de Bruin TW, Westerveld HE, Meier E, Erkelens TW. Delayed chylomicron remnant clearance in subjects with heterozygous familial hypercholes-terolaemia. J Intern Med. 1998;244:299–307. doi: 10.1046/j.1365-2796.1998.00367.x. [DOI] [PubMed] [Google Scholar]
- 77.Dane-Stewart CA, Watts GF, Mamo JC, Dimmitt SB, Barrett PHR, Redgrave TG. Elevated apolipoprotein B-48 and remnant-like particle-cholesterol in heterozygous familial hypercholesterolaemia. Eur J Clin Invest. 2001;31:113–7. doi: 10.1046/j.1365-2362.2001.00785.x. [DOI] [PubMed] [Google Scholar]
- 78.Sauvage Nolting PR, Twickler MB, Dallinga-Thie GM, Buirma RJ, Hutten BA, Kastelein JJ. Elevated remnant-like particles in heterozygous familial hypercholesterolemia and response to statin therapy. Circulation. 2002;106:788–92. doi: 10.1161/01.cir.0000025586.89221.4b. [DOI] [PubMed] [Google Scholar]
- 79.Mamo JC, Smith D, Yu KC, et al. Accumulation of chylomicron remnants in homozygous subjects with familial hypercholesterolaemia. Eur J Clin Invest. 1998;28:379–84. doi: 10.1046/j.1365-2362.1998.00284.x. [DOI] [PubMed] [Google Scholar]
- 80.Rubinsztein DC, Cohen JC, Berger GM, van der Westhuyzen DR, Coetzee GA, Gevers W. Chylomicron remnant clearance from the plasma is normal in familial hypercholesterolemic homozygotes with defined receptor defects. J Clin Invest. 1990;86:1306–12. doi: 10.1172/JCI114839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Watts GF, Barrett PHR, Marais AD, et al. Chylomicron remnant metabolism in familial hypercholesterolaemia studied with a stable isotope breath test. Atherosclerosis. 2001;157:519–23. doi: 10.1016/s0021-9150(01)00534-2. [DOI] [PubMed] [Google Scholar]
- 82.Berglund L, Wiklund O, Eggertsen G, et al. Apolipoprotein E phenotypes in familial hypercholesterolaemia: importance for expression of disease and response to therapy. J Intern Med. 1993;233:173–8. doi: 10.1111/j.1365-2796.1993.tb00670.x. [DOI] [PubMed] [Google Scholar]
- 83.Carmena-Ramon R, Real JT, Ascaso JF, Ordovas JM, Carmena R. Effect of apolipoprotein E genotype on lipid levels and response to diet in familial hypercholesterolemia. Nutr Metab Cardiovasc Dis. 2000;10:7–13. [PubMed] [Google Scholar]
- 84.O'Malley JP, Illingworth DR. The influence of apolipoprotein E phenotype on the response to lovastatin therapy in patients with heterozygous familial hypercholesterolemia. Metabolism. 1990;39:150–4. doi: 10.1016/0026-0495(90)90068-n. [DOI] [PubMed] [Google Scholar]
- 85.Brodt-Eppley J, White P, Jenkins S, Hui DY. Plasma cholesterol esterase level is a determinant for an atherogenic lipoprotein profile in normolipidemic human subjects. Biochim Biophys Acta. 1995;1272:69–72. doi: 10.1016/0925-4439(95)00083-g. [DOI] [PubMed] [Google Scholar]
- 86.Carmena-Ramon R, Ascaso JF, Real JT, Najera G, Ordovas JM, Carmena R. Association between the TaqIB polymorphism in the cholesteryl ester transfer protein gene locus and plasma lipoprotein levels in familial hypercholesterolemia. Metabolism. 2001;50:651–6. doi: 10.1053/meta.2001.23289. [DOI] [PubMed] [Google Scholar]
- 87.Takada D, Ezura Y, Ono S, et al. Apolipoprotein H variant modifies plasma triglyceride phenotype in familial hypercholesterolemia: a molecular study in an eight-generation hyperlipidemic family. J Atheroscler Thromb. 2003;10:79–84. doi: 10.5551/jat.10.79. [DOI] [PubMed] [Google Scholar]
- 88.Bjorn L, Leren TP, Ose L, Hamsten A, Karpe F. A functional polymorphism in the promoter region of the microsomal triglyceride transfer protein (MTP -493G/T) influences lipoprotein phenotype in familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2000;20:1784–8. doi: 10.1161/01.atv.20.7.1784. [DOI] [PubMed] [Google Scholar]
- 89.Carmena-Ramon RF, Ordovas JM, Ascaso JF, Real J, Priego MA, Carmena R. Influence of genetic variation at the apo A-I gene locus on lipid levels and response to diet in familial hypercholesterolemia. Atherosclerosis. 1998;139:107–13. doi: 10.1016/s0021-9150(98)00064-1. [DOI] [PubMed] [Google Scholar]
- 90.Carmena-Ramon R, Ascaso JF, Real JT, Ordovas JM, Carmena R. Genetic variation at the apoA-IV gene locus and response to diet in familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1998;18:1266–74. doi: 10.1161/01.atv.18.8.1266. [DOI] [PubMed] [Google Scholar]
- 91.Ihara Y, Nobukuni K, Namba R, et al. A family of familial hypercholesterolemia with cerebral infarction and without coronary heart disease. An unusual case with corneal opacity, polyneuropathy and carpal tunnel syndrome in the family: therapy with probucol and tocopherol nicotinate. J Neurol Sci. 1991;106:10–8. doi: 10.1016/0022-510x(91)90187-c. [DOI] [PubMed] [Google Scholar]
- 92.Deiana L, Garuti R, Pes GM, et al. Influence of beta-thalassemia on the phenotypic expression of heterozygous familial hypercholesterolemia : a study of patients with familial hypercholesterolemia from Sardinia. Arterioscler Thromb Vasc Biol. 2000;20:236–43. doi: 10.1161/01.atv.20.1.236. [DOI] [PubMed] [Google Scholar]
- 93.Yanagi K, Yamashita S, Kihara S, et al. Characteristics of coronary artery disease and lipoprotein abnormalities in patients with heterozygous familial hypercholesterolemia associated with diabetes mellitus or impaired glucose tolerance. Atherosclerosis. 1997;132:43–51. doi: 10.1016/s0021-9150(97)00076-2. [DOI] [PubMed] [Google Scholar]
- 94.De Graaf J, Demacker PN, Stalenhoef AF. The effect of simvastatin treatment on the low-density lipoprotein subfraction profile and composition in familial hypercholesterolaemia. Neth J Med. 1993;43:254–61. [PubMed] [Google Scholar]
- 95.Rauh G, Schuster H, Fischer J, Keller C, Wolfram G, Zollner N. Identification of a heterozygous compound individual with familial hypercholesterolemia and familial defective apolipoprotein B-100. Klin Wochenschr. 1991;69:320–4. doi: 10.1007/BF01644767. [DOI] [PubMed] [Google Scholar]
- 96.Rubinsztein DC, Raal FJ, Seftel HC, Pilcher G, Coetzee GA, van der Westhuyzen DR. Characterization of six patients who are double heterozygotes for familial hypercholesterolemia and familial defective apo B-100. Arterioscler Thromb. 1993;13:1076–81. doi: 10.1161/01.atv.13.7.1076. [DOI] [PubMed] [Google Scholar]
- 97.Tai ES, Koay ES, Chan E, et al. Compound heterozygous familial hypercholesterolemia and familial defective apolipoprotein B-100 produce exaggerated hypercholesterolemia. Clin Chem. 2001;47:438–43. [PubMed] [Google Scholar]
- 98.Carmena R, Roy M, Roederer G, Minnich A, Davignon J. Coexisting dysbetalipoproteinemia and familial hypercholesterolemia. Clinical and laboratory observations. Atherosclerosis. 2000;148:113–24. doi: 10.1016/s0021-9150(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 99.Sakuma N, Iwata S, Ikeuchi R, et al. Coexisting type III hyperlipoproteinemia and familial hypercholesterolemia: a case report. Metabolism. 1995;44:460–5. doi: 10.1016/0026-0495(95)90052-7. [DOI] [PubMed] [Google Scholar]
- 100.Zambon A, Torres A, Bijvoet S, et al. Prevention of raised low-density lipoprotein cholesterol in a patient with familial hypercholesterolaemia and lipoprotein lipase deficiency. Lancet. 1993;341:1119–21. doi: 10.1016/0140-6736(93)93129-o. [DOI] [PubMed] [Google Scholar]
- 101.Banyai M, Lupattelli G, Li SR, et al. Comparison of iodine-123 low-density lipoprotein (LDL) and indium-111 LDL binding to mononuclear cells of healthy normolipaemic controls and patients with heterozygous familial hypercholesterolaemia. Eur J Nucl Med. 1994;21:634–9. doi: 10.1007/BF00285585. [DOI] [PubMed] [Google Scholar]
- 102.Gevers W. Three mutations that cause familial hypercholesterolaemia in Afrikaners identified - a milestone in South African medicine. S Afr Med J. 1989;76:393–4. [PubMed] [Google Scholar]
- 103.Steyn K, Goldberg YP, Kotze MJ, et al. Estimation of the prevalence of familial hypercholesterolaemia in a rural Afrikaner community by direct screening for three Afrikaner founder low density lipoprotein receptor gene mutations. Hum Genet. 1996;98:479–84. doi: 10.1007/s004390050243. [DOI] [PubMed] [Google Scholar]
- 104.Kotze MJ, Langenhoven E, Warnich L, du Plessis L, Retief AE. The molecular basis and diagnosis of familial hypercholesterolaemia in South African Afrikaners. Ann Hum Genet. 1991;55:115–21. doi: 10.1111/j.1469-1809.1991.tb00404.x. [DOI] [PubMed] [Google Scholar]
- 105.Graadt van Roggen JF, van der Westhuyzen DR, Coetzee GA, et al. FH Afrikaner-3 LDL receptor mutation results in defective LDL receptors and causes a mild form of familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1995;15:765–72. doi: 10.1161/01.atv.15.6.765. [DOI] [PubMed] [Google Scholar]
- 106.Defesche JC, Lansberg PJ, Reymer PW, Lamping RJ, Kastelein JJ. Analysis of the Afrikaner mutation in exon 9 of the low-density lipoprotein receptor gene in a large Dutch kindred suffering from familial hypercholesterolaemia. Neth J Med. 1993;42:53–60. [PubMed] [Google Scholar]
- 107.Kotze MJ, Langenhoven E, Theart L, Loubser O, Micklem A, Oosthuizen CJ. Recurrent LDL-receptor mutation causes familial hypercholesterolaemia in South African coloureds and Afrikaners. S Afr Med J. 1995;85:357–61. [PubMed] [Google Scholar]
- 108.Pereira E, Ferreira R, Hermelin B, et al. Recurrent and novel LDL receptor gene mutations causing heterozygous familial hypercholesterolemia in La Habana. Hum Genet. 1995;96:319–22. doi: 10.1007/BF00210415. [DOI] [PubMed] [Google Scholar]
- 109.Mandelshtam M, Chakir K, Shevtsov S, et al. Prevalence of Lithuanian mutation among St. Petersburg Jews with familial hypercholesterolemia. Hum Mutat. 1998;12:255–8. doi: 10.1002/(SICI)1098-1004(1998)12:4<255::AID-HUMU6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 110.Durst R, Colombo R, Shpitzen S, et al. Recent origin and spread of a common Lithuanian mutation, G197del LDLR, causing familial hypercholesterolemia: positive selection is not always necessary to account for disease incidence among Ashkenazi Jews. Am J Hum Genet. 2001;68:1172–88. doi: 10.1086/320123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vergotine J, Thiart R, Kotze MJ. Clinical versus molecular diagnosis of heterozygous familial hypercholesterolaemia in the diverse South African population. S Afr Med J. 2001;91:1053–9. [PubMed] [Google Scholar]
- 112.Couture P, Morrisette J, Gaudet D. Fine mapping of low-density-lipoprotein receptor gene by genetic linkage on chromosome 19p13.1–p13.3 and study of founder effect of four French Canadian low-density -lipoprotein receptor gene mutations. Atherosclerosis. 1999;143:145–51. doi: 10.1016/s0021-9150(98)00267-6. [DOI] [PubMed] [Google Scholar]
- 113.Vohl MC, Moorjani S, Roy M. Geographic distribution of French-Canadian low-density-lipoprotein receptor gene mutations in the province of Quebec. Clin Genet. 1997;52:1–6. doi: 10.1111/j.1399-0004.1997.tb02506.x. [DOI] [PubMed] [Google Scholar]
- 114.Slimane MN, Pousse H, Maatoug F, Hammami M, Ben Farhat MH. Phenotypic expression of familial hypercholesterolaemia in central and southern Tunisia. Atherosclerosis. 1993;104:153–8. doi: 10.1016/0021-9150(93)90186-x. [DOI] [PubMed] [Google Scholar]
- 115.Jensen HK, Jensen LG, Meinertz H, Hansen PS, Gregersen N, Faergeman O. Spectrum of LDL receptor gene mutations in Denmark: implications for molecular diagnostic strategy in heterozygous familial hypercholesterolemia. Atherosclerosis. 1999;146:337–44. doi: 10.1016/s0021-9150(99)00158-6. [DOI] [PubMed] [Google Scholar]
- 116.Mak YT, Pang CP, Tomlinson B, et al. Mutations in the low-density lipoprotein receptor gene in Chinese familial hypercholesterolemia patients. Arterioscler Thromb Vasc Biol. 1998;18:1600–5. doi: 10.1161/01.atv.18.10.1600. [DOI] [PubMed] [Google Scholar]
- 117.Nauck MS, Koster W, Dorfer K, et al. Identification of recurrent and novel mutations in the LDL receptor gene in German patients with familial hypercholesterolemia. Hum Mutat. 2001;18:165–6. doi: 10.1002/humu.1171. [DOI] [PubMed] [Google Scholar]
- 118.Chisholm A, Sutherland W, Ball M. The effect of dietary fat content on plasma noncholesterol sterol concentrations in patients with familial hypercholesterolemia treated with simvastatin. Metabolism. 1994;43:310–4. doi: 10.1016/0026-0495(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 119.Naoumova RP, Marais AD, Mountney J, et al. Plasma mevalonic acid, an index of cholesterol synthesis in vivo, and responsiveness to HMG-CoA reductase inhibitors in familial hypercholesterolaemia. Atherosclerosis. 1996;119:203–13. doi: 10.1016/0021-9150(95)05649-1. [DOI] [PubMed] [Google Scholar]
- 120.Jeenah M, September W, Graadt vR, de Villiers W, Seftel H, Marais D. Influence of specific mutations at the LDL-receptor gene locus on the response to simvastatin therapy in Afrikaner patients with heterozygous familial hypercholesterolaemia. Atherosclerosis. 1993;98:51–8. doi: 10.1016/0021-9150(93)90222-g. [DOI] [PubMed] [Google Scholar]
- 121.Couture P, Brun LD, Szots F, et al. Association of specific LDL receptor gene mutations with differential plasma lipoprotein response to simvastatin in young French Canadians with heterozygous familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1998;18:1007–12. doi: 10.1161/01.atv.18.6.1007. [DOI] [PubMed] [Google Scholar]
- 122.Gaddi A, Arca M, Ciarrocchi A, Fazio S, D'Alo G, Tiozzo R, Descovich GC, Calandra S. Pravastatin in heterozygous familial hypercholesterolemia: low-density lipoprotein (LDL) cholesterol-lowering effect and LDL receptor activity on skin fibroblasts. Metabolism. 1991;40:1074–8. doi: 10.1016/0026-0495(91)90132-g. [DOI] [PubMed] [Google Scholar]
- 123.Leitersdorf E, Eisenberg S, Eliav O, et al. Genetic determinants of responsiveness to the HMG-CoA reductase inhibitor fluvastatin in patients with molecularly defined heterozygous familial hypercholesterolemia. Circulation. 1993;87(4 Suppl):III35–III44. [PubMed] [Google Scholar]
- 124.Vuorio AF, Ojala JP, Sarna S, Turtola H, Tikkanen MJ, Kontula K. Heterozygous familial hypercholesterolaemia: the influence of the mutation type of the low-density-lipoprotein receptor gene and PvuII polymorphism of the normal allele on serum lipid levels and response to lovastatin treatment. J Intern Med. 1995;237:43–48. doi: 10.1111/j.1365-2796.1995.tb01138.x. [DOI] [PubMed] [Google Scholar]
- 125.Illingworth DR, Vakar F, Mahley RW, Weisgraber KH. Hypocholesterolaemic effects of lovastatin in familial defective apolipoprotein B-100. Lancet. 1992;339:598–600. doi: 10.1016/0140-6736(92)90875-4. [DOI] [PubMed] [Google Scholar]
- 126.Marais AD, Naoumova RP, Firth JC, Penny C, Neuwirth CK, Thompson GR. Decreased production of low density lipoprotein by atorvastatin after apheresis in homozygous familial hypercholesterolemia. J Lipid Res. 1997;38:2071–8. [PubMed] [Google Scholar]
- 127.Parhofer KG, Barrett PHR, Demant T, Richter WO, Schwandt P. Effects of weekly LDL-apheresis on metabolic parameters of apolipoprotein B in heterozygous familial hypercholesterolaemia. J Lipid Res. 1996;37:2383–93. [PubMed] [Google Scholar]
- 128.Gagne C, Gaudet D, Bruckert E. Efficacy and safety of ezetimibe coadministered with atorvastatin or simvastatin in patients with homozygous familial hypercholesterolemia. Circulation. 2002;105:2469–75. doi: 10.1161/01.cir.0000018744.58460.62. [DOI] [PubMed] [Google Scholar]
- 129.Wetterau JR, Gregg RE, Harrity TW, et al. An MTP inhibitor that normalizes atherogenic lipoprotein levels in WHHL rabbits. Science. 1998;282:751–4. doi: 10.1126/science.282.5389.751. [DOI] [PubMed] [Google Scholar]