Abstract
Dyslipoproteinaemia is a cardinal feature of the metabolic syndrome that accelerates atherosclerosis. It is usually characterised by high plasma concentrations of triglyceride-rich and apolipoprotein (apo) B-containing lipoproteins, with depressed concentrations of high-density lipoprotein (HDL). Dysregulation of lipoprotein metabolism in these subjects may be due to a combination of overproduction of very-low-density lipoprotein (VLDL) apoB-100, decreased catabolism of apoB-containing particles, and increased catabolism of HDL apoA-I particles. These abnormalities may be consequent on a global metabolic effect of insulin resistance that increases the flux of fatty acids from adipose tissue to the liver, the accumulation of fat in the liver, the increased hepatic secretion of VLDL-triglycerides and the remodelling of both low-density lipoprotein (LDL) and HDL particles in the circulation; perturbations in lipolytic enzymes and lipid transfer proteins contribute to the dyslipidaemia. Our in vivo understanding of the kinetic defects in lipoprotein metabolism in the metabolic syndrome has been chiefly achieved by ongoing developments in the use of stable isotope tracers and mathematical modelling. Knowledge of the pathophysiology of lipoprotein metabolism in the metabolic syndrome is well complemented by extensive cell biological data. Nutritional modifications and increased physical exercise may favourably alter lipoprotein transport in the metabolic syndrome by collectively decreasing the hepatic secretion of VLDL-apoB and the catabolism of HDL apoA-I, as well as by increasing the clearance of LDL-apoB. Pharmacological treatments, such as statins, fibrates or fish oils, can also correct the dyslipidaemia by several mechanisms of action including decreased secretion and increased catabolism of apoB, as well as increased secretion and decreased catabolism of apoA-I. The complementary mechanisms of action of lifestyle and drug therapies support the use of combination regimens to treat dyslipidaemia in the metabolic syndrome.
Introduction
Visceral obesity, insulin resistance, dyslipidaemia, hypertension and a pro-inflammatory/thrombotic state collectively define the metabolic syndrome.1,2 Individuals with themetabolic syndrome have a significant increase in cardiovascular morbidity and mortality.3–5 A recent estimate of the prevalence of the metabolic syndrome suggests that 25% of adults in the United States have this condition.6,7 Recognising its clinical importance, the National Cholesterol Education Program (NCEP) Adult Treatment Panel III has recently identified the metabolic syndrome as a secondary target of therapy for the management of cardiovascular disease (CVD) beyond LDL-cholesterol lowering.1
Dyslipidaemia is probably the major mediator of atherogenicity in the metabolic syndrome.8,9 This review focuses on the dysregulation and therapeutic regulation of lipoprotein transport in non-diabetic subjects with the metabolic syndrome from studies chiefly carried out in vivo with stable isotopes. We also place the human work within the context of contemporary molecular and cell biological studies that have contributed to knowledge in this field.
Defining the Metabolic Syndrome: Importance of Dyslipidaemia
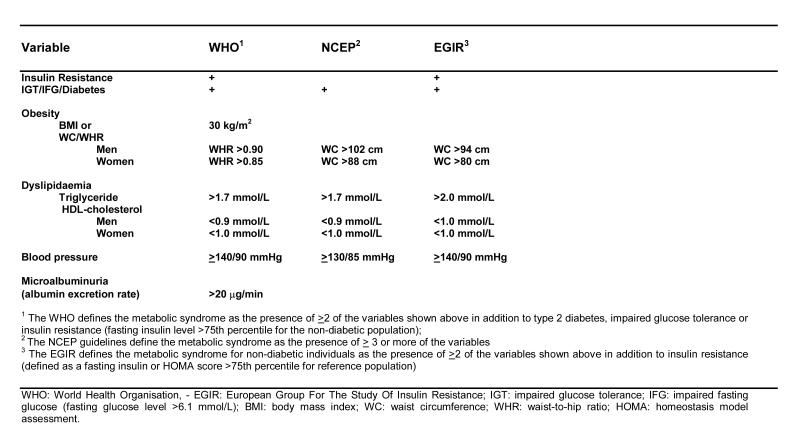
To aid research and clinical practice, several definitions of the metabolic syndrome have been proposed by various expert groups.1,10,11 The individual definitions are given in Table 1. Despite the different definitions, dyslipidaemia (specifically, high plasma triglycerides and low HDL-cholesterol concentrations) is a common feature of all the definitions given in Table 1. The prevalence of the metabolic syndrome including dyslipidaemia increases from normal, through IGT to diabetes. However, within populations most subjects with the metabolic syndrome do not have diabetes mellitus.3 The importance of dyslipidaemia is evidenced by prospective epidemiological data showing that it is a major, independent risk factor for coronary heart disease (CHD) within the metabolic syndrome.9 Dyslipidaemia also incrementally increases the risk of CHD in diabetes.12
Table 1.
Tracer Studies of Lipoprotein Metabolism
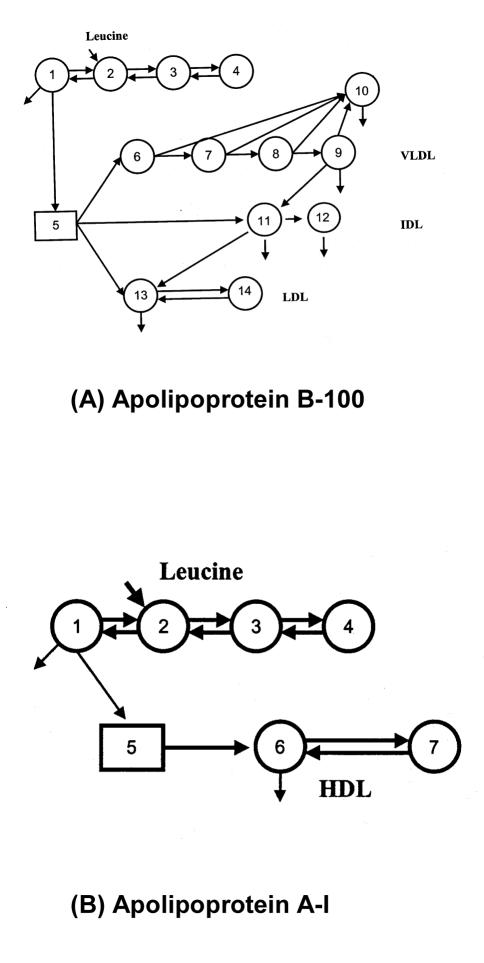
Measurements of plasma lipid and lipoprotein concentrations are static estimates traditionally employed to characterise disorders of lipoprotein metabolism. However, lipoprotein metabolism is complex and abnormal plasma concentrations can result from alterations in the rates of production and/or catabolism of the various lipoprotein particles.13 Tracer studies, whether utilising radioactive or stable isotopes, provide data from which mechanistic, kinetic models can be developed and tested against experimental data.14 Such models can provide novel insight to further understanding of metabolic disorders and effects of treatments. Radioisotope tracers were previously employed to study lipoprotein kinetics,15–17 but this approach had several methodological drawbacks and was potentially bio-hazardous.18 Advances in gas chromatography-mass spectrometry (GCMS) technology and widespread availability of inexpensive, stable isotopes have seen the increasing use of endogenous labelling of apoproteins with amino acid precursor molecules to study lipoprotein kinetics in humans.18–22 Stable isotopically-labelled amino acids (typically 13C-leucine or D3-leucine) can be administered intravenously as a bolus or primed infusion with serial blood sampling over several hours/days to study the turnover of VLDL, intermediate- density lipoprotein (IDL) and LDL-apoB, as well as HDL apoA-I and A-II. Enrichment data are generated by GCMS analysis after separation of the relevant apoproteins. The data are then subjected to multicompartmental analysis to assess the fractional turnover and conversion rates of lipoproteins, from which absolute transport rates are calculated. Typical models to assess apoB and apoA kinetics are shown in Figure 1. Full methodological details of the aforementioned techniques are provided in several of our publications.19,23–26 Stable isotope and multicompartmental analysis may also be used to study the turnover of chylomicron remnants, triglycerides, cholesterol and fatty acids.27–30
Figure 1.
Compartment model describing apoB-100 (a), apoA-I (b). For further explanations see References 25, 26.
Stable Isotopic Studies in the Metabolic Syndrome
1. Observational Studies
I. VLDL
With stable isotopes, we have consistently demonstrated that centrally obese subjects have elevated hepatic VLDL-apoB secretion compared with non-obese individuals.26,31,32 This abnormality is also associated with delayed clearance of IDL, LDL and chylomicron remnant particles.32,33 We also showed that changes in the hepatic secretion of VLDL-apoB are positively correlated with changes in visceral adipose tissue area measured by magnetic resonance imaging.34 This correlation may in part reflect the impact of visceral fat on hepatic fat content, an increasingly recognised major determinant of hepatic insulin resistance and lipoprotein metabolism.35,36
Dysregulation of VLDL metabolism in the metabolic syndrome is a critical event that has a major qualitative and quantitative impact on the metabolism of LDL and HDL. The mechanisms whereby visceral obesity increases plasma VLDL concentration are complex, but can be best understood in relation to increased hepatic lipid supply and availability,37–39 and the intrinsic effects of insulin resistance on hepatic output of VLDL and catabolism of VLDL in peripheral tissue.40–42 The findings from stable isotope studies to date can be well reconciled with cell and molecular biological data.40,43,44 Since intra-abdominal adipocytes are very lipolytically active,45 visceral fat accumulation in the metabolic syndrome results in markedly increased flux of free fatty acids in the portal vein to the liver. The increased portal flux of fatty acids to the liver stimulates hepatic secretion of apoB by increasing synthesis of cholesterol esters and triglycerides.38,39 The net effect of these processes is increased production of larger, triglyceride-rich VLDL1 particles rather than smaller VLDL2 particles, as shown by studies from Malmstrom et al.46 and recently reviewed by Taskinen.47
Consistent with this notion, type 2 diabetics were shown to have a specific increase in VLDL1 production that was not suppressible with an acute insulin infusion.47,48 Coupling of both triglyceride and apoB production in the setting of increased VLDL1 particle secretion has recently been reported by Taskinen’s group employing dual tracer methodology.47,49 These data suggest increased VLDL1 particle secretion and not increased VLDL particle size related to increased packaging of triglycerides onto VLDL-apoB. Increased hepatic secretion of VLDL1 particles may have particularly important consequences for post-prandial dyslipidaemia, as well as for the generation of small dense LDL particles and increased catabolism of HDL apoA-I.47
Clinical and cell biological studies have clearly shown that insulin resistance increases hepatic VLDL secretion by multiple mechanisms:42,50–52 These mechanisms include: increased fatty acid flux to the liver, resistance to a direct inhibitory effect of insulin on apoB secretion, decreased post-translational degradation of apoB, increased expression of microsomal triglyceride transfer protein (MTP), increased de novo lipogenesis related to increased expression of sterol regulatory element-binding protein-1c (SREBP-1c), and decreased expression of peroxisome proliferator-activated receptors (PPARs). Hepatic insulin resistance may increase VLDL particle production at the first step of assembly in the smooth endoplasmic reticulum by upregulating MTP, and at the second step of assembly in the rough endoplasmic reticulum by activating phospholipase D and ADP-ribosylation factor.47,53 Chronic hyperinsulinaemia also increases expression of SREBP-1c, thereby activating hepatic lipogenic enzymes (fatty acid synthase and acetyl CoA carboxylase) and channelling hepatic fatty acids from storage triglyceride pools into a secretory pool.35 Increased de novo lipogenesis also increases the availability of triglycerides for assembly and secretion of VLDL particles. This mechanism is compounded by increased fatty acid flux to the liver that contributes chronically to expansion in the triglyceride storage pool and, hence, liver fat content. Independent of the effects of hyperinsulinaemia on SREBP-1c expression, an insulin signalling defect involving decreased activation of the insulin receptor substrate-1/ phosphatidylinositide-3-kinase pathway results in increased hepatic secretion of triglyceride-rich VLDL.41 In skeletal muscle and adipose tissue, insulin resistance operating by a similar signalling pathway also impairs triglyceride-rich lipoprotein catabolism by decreasing lipoprotein lipase (LPL) activity.54,55 Defective clearance of exogenously-derived lipoproteins in insulin resistance also exacerbates hypertriglyceridaemia by increasing the competition between chylomicrons and VLDL for lipolysis by LPL, on the one hand, and between chylomicron remnants and VLDL remnants for LDL receptor-mediated clearance, on the other.42 Hence, both hepatic over-secretion of VLDL particles and triglycerides, as well as intrinsic and competitive clearance defects account for hypertriglyceridaemia in the metabolic syndrome. These abnormalities are predominantly a consequence of insulin resistance and are seen in dyslipidaemic subjects with the metabolic syndrome irrespective of the presence of diabetes.
Genetic factors evidently also determine the kinetics of VLDL-apoB and triglyceride metabolism in obesity.56,57 These include genetic polymorphisms of transport and/or enzymic proteins involved in the regulation of lipid substrate availability and the processing of apoB in the liver. Accordingly, we have previously reported that in subjects with visceral obesity the hepatic secretion of apoB is dependent on apoB signal peptide, apoE, cholesteryl ester transfer protein (CETP) and MTP gene polymorphisms.
II. IDL and Chylomicron Remnants
Using a bolus injection of D3-leucine and compartment modelling, we have found that in the post-absorptive state obese men with the metabolic syndrome have 30% lower fractional catabolic rate (FCR) of IDL-apoB compared with non-obese controls.32 This is supported by our chylomicron remnant studies in similar subjects and in obese, post-menopausal women with type 2 diabetes.58 These data were obtained using an intravenous injection of a stable isotope labelled (13C-cholesteryl oleate) chylomicron remnant-like emulsion that may be cleared by the same receptor as IDL-apoB. In both groups of subjects, we found that the FCR of the chylomicron remnant-like emulsion was 40% lower compared with control subjects. We also recently reported that viscerally obese men had a four-fold increase in plasma concentrations of remnant-like particle (RLP)-cholesterol and a two-fold increase in apoB-48 concentrations, both recognised markers of triglyceride-rich lipoproteins of intestinal origin.33 In the study of obese diabetic women, we found a significant positive association between delayed fractional clearance of the chylomicron remnant-like emulsion, measured as rate of appearance of breath 13CO2, and plasma apoB48 concentrations.58 Kinetic abnormalities in remnant lipoproteins reflect delayed clearance of triglyceride-rich lipoproteins related to an effect of insulin resistance that decreases LPL activity, hepatic remnant receptor and the synthesis of heparan sulphate proteoglycans (HSPG).54,59–61 In the same study, we also found that plasma concentration of apoC-III was significantly higher in the centrally obese men compared with non-obese controls (162 ± 34 mg/L vs. 118 ± 24 mg/L, p<0.01).32 More importantly, plasma apoC-III concentration was positively associated with plasma triglyceride, RLP-cholesterol, apoB-48 concentrations, and inversely associated with the rate of conversion of VLDL to IDL particles.32 This metabolic abnormality may relate to the inhibitory effect of apoC-III on lipolysis and hepatic clearance of triglyceride-rich lipoproteins.62 Enhanced synthesis of apoC-III in the metabolic syndrome may be due to an effect of insulin resistance that decreases the expression of PPAR-α in the liver,63 However, this requires confirmation in vivo since a delay in VLDL clearance per se could also elevate plasma apoC-III levels.
The aforementioned stable isotope studies of lipoprotein remnant kinetics have been carried out in the post-absorptive state, but the findings are compatible with the consistent demonstration, based on retinol labelling and time-dependent apoB-48 responses, of excessive post-prandial lipaemia in both obese and diabetic subjects.64–66 The removal of chylomicron remnants and IDL by the liver involves receptors, such as LDL and LDL receptor-related protein, which bind the particles and interact with HSPG, as well as the enzymes LPL and hepatic lipase (HL).67
Our observation that LDL receptor activity is decreased in obesity supports our isotopic findings of delayed fractional catabolism of IDL-apoB and chylomicron remnant-like emulsion.32,33,66 That synthesis of perlecan (a core protein of HSPG) is decreased in experimental diabetes also points to an additional mechanism for delayed remnant clearance by the liver involving decreased binding of this lipoprotein to proteoglycans.61 HL is also involved in hepatic uptake of triglyceride-rich lipoproteins.68 Since the activity of this enzyme is increased in insulin resistance,69 an alternative mechanism must account for delayed clearance of remnants by the liver. Whether increased production of apoB-48 occurs in human insulin resistance and type 2 diabetes is unclear. As with VLDL-apoB kinetics, the turnover of remnant lipoproteins (in both post-absorptive and post-prandial states) is also critically dependent on genetic factors,70 but present evidence based on several genetic polymorphisms suggest that these only account for a small proportion of the variance in plasma triglyceride levels.
III. LDL
In a recent stable isotope study, we found that the fractional catabolism of LDL-apoB is 40% lower in men with the metabolic syndrome compared with non-obese controls (0.35 ± 0.02 vs. 0.56 ± 0.10 pools/day, p<0.05).32 This in fact explains the elevated plasma LDL-apoB concentration in the metabolic syndrome. Using plasma ratios of lathosterol: cholesterol and campesterol:cholesterol, as markers of cholesterol synthesis and absorption, respectively, we have also suggested that subjects with the metabolic syndrome have an increased rate of de novo cholesterol synthesis and depressed fractional rate of cholesterol absorption.71 These changes in cholesterol homeostasis may be causally related to reduced FCR of LDL-apoB. As suggested by radiokinetic studies, the increase in pool size of VLDL in insulin resistance results in increased production of small dense LDL particles that are catabolised more slowly by the liver.47,72,73 This mechanism has not, however, been formally demonstrated in the metabolic syndrome with stable isotopes.
Insulin resistance down-regulates LDL receptor expression and activity via a direct mechanism74 and indirectly by altering hepatic cholesterol metabolism. SREBP-2 has recently been shown to regulate cholesterol metabolism by activating the expression of genes required for synthesis and transport of cholesterol.52 Insulin stimulates the liver X receptor (LXR) and this could enhance hepatic cholesterogenesis via SREBP-2.75 We hypothesise that an expansion in a hepatic cholesterol pool resulting from an absolute increase in cholesterol availability in the liver could down-regulate the activity of SREBP-2 and diminish LDL receptor activity. This suggests that in the metabolic syndrome delayed clearance of LDL particles by the liver may be partly governed by an increase in the pool size of free cholesterol in the liver. Delayed catabolism of LDL in insulin resistance may also reflect re-modelling of LDL particles as a consequence of hypertriglyceridaemia, increased lipid transfer via CETP and increased lipolysis by HL.69,76
Plasma concentrations of LDL-cholesterol are, however, usually normal or marginally elevated in individuals with visceral obesity and insulin resistance.77,78 This is because the LDL particles have been remodelled to the small, dense LDL subclass. These LDL particles are rich in apoB relative to cholesterol and are generated by the concerted action of CETP and HL. The primary metabolic event that allows the remodelling of LDL is considered to be increased hepatic secretion of VLDL1.47 Hypertriglyceridaemia, which in insulin resistance and diabetes chiefly reflects increased plasma VLDL1-triglyceride concentrations, enhances the CETP-mediated exchange of VLDL-triglycerides for LDL cholesteryl esters. Under the action of HL, which is upregulated in insulin resistance, the triglyceride-rich LDL particles are hydrolysed into small, dense LDL. Hence, the accumulation of small, dense LDL in the metabolic syndrome involves re-modelling of LDL particles as a consequence of hypertriglyceridaemia.47,73 As reviewed later, the same metabolic process is involved in remodeling of HDL particles. The accumulation of small, dense LDL in the metabolic syndrome is particularly significant, since these particles are catabolised slowly by the liver, interact avidly with arterial wall matrix proteoglycans, and are highly susceptible to chemical modification such as oxidation, all of which critically increase their atherogenicity.79,80 The importance of these LDL particles for progression of coronary artery disease in the metabolic syndrome has been underscored recently in an angiographic analysis of the Diabetes Atherosclerosis Intervenetion Study (DAIS) trial.81
IV. HDL
The kinetics of HDL apoA-I and apoA-II has been studied with stable isotopes in both insulin resistant and diabetic patients. Pietzsch et al 82 and Pont et al 83 independently found that in overweight/obese subjects with IGT and insulin resistance, low plasma levels of HDL-cholesterol and apoA-I were associated with enhanced HDL apoA-I catabolism, but no relationship was found with HDL apoA-II kinetics that was reported as being normal.79,80 Similar findings were reported in type 2 diabetes mellitus by Duvillard et al.84 Frenais et al suggested that in type 2 diabetes mellitus increased HDL apoA-I catabolism was probably a consequence of increased HDL-triglyceride concentration, insulin resistance and a decreased ratio of LPL to HL activities,85 consistent with experimental work of Lewis’ group.86,87 In a study of dyslipidaemic subjects with the metabolic syndrome, we have confirmed the foregoing observations that low plasma levels of HDL apoA-I are strongly related to an increase in HDL apoA-I catabolism (0.30 ± 0.01 vs. 0.20 ± 0.03 pools/day, p<0.05) compared with controls.26 However, we also found that subjects with the metabolic syndrome had an increased rate of HDL apoA-I production and a trend to a significant increase in the catabolism of apoA-II (0.23 ± 0.02 vs. 0.17 ± 0.03 pools/day, p=0.089). We have also recently investigated the kinetics of LpA-I and LpA-I:A-II particles in similar subjects. Compared with non-obese controls, we found that the metabolic syndrome subjects had significantly lower LpA-I and LpA-I:A-II concentrations in plasma related to an increase in the catabolism of both of these particles (Chan & Watts, unpublished data). These findings require confirmation, but suggest that in the metabolic syndrome the catabolism of both LpA-I and LpA-I:A-II particles is increased.
The above findings with stable isotopes in the metabolic syndrome are compatible with earlier radiokinetic data. Brinton et al showed that subjects with hypertriglyceridaemia and low HDL-cholesterol, who did not necessarily have the metabolic syndrome, had higher fractional catabolism of both HDL apoA-I and apoA-II.88 Smaller studies, however, suggested that in hypertriglyceridaemic subjects the transport rate of HDL apoA-I and the fractional catabolism of HDL apoA-II were both increased.89
Experimental radiokinetic studies in the New Zealand white rabbit clearly show that enhanced HDL apoA-I clearance is directly dependent on the triglyceride enrichment of HDL and on the activity of HL.86 In healthy individuals, an intra-lipid infusion that increased the triglyceride content of the HDL resulted in a 26% increase in the FCR of apoA-I, chiefly due to enhanced clearance of LpA-I (HDL2 subpopulation) but not LpA-I:A-II (HDL3 subpopulation) particles.87 Several sources of experimental and clinical evidence show that HL activity is increased in insulin resistance and correlates with central adiposity.90–92 Despres et al, for example, showed positive correlations of intra-abdominal fat with post-HL activities which in turn correlated inversely with HDL2-cholesterol.92 Triglyceride enrichment of HDL results in a particle that is the preferred substrate for HL, which is increased in insulin resistance, and this per se contributes significantly to the accelerated catabolism of HDL apoA-I. The apparent increase in HDL apoA-I catabolism from in vivo studies concurs with in vitro data showing that A-I enhances HL-mediated phospholipid hydrolysis of reconstituted HDL particles whereas HDL A-II inhibits this process.93–95 The precise mechanism of increased HL in insulin resistance is unknown, but is considered to be due to a chronic effect of hyperinsulinaemia.96 Up to 30% of individual variation in HL can be accounted for by a common single nucleotide polymorphism in the promoter region of the HL gene, suggesting that the impact of insulin resistance on the catabolism of HDL also depends on genetic factors.97 In summary, compelling data suggest that hepatic oversecretion of VLDL-triglyceride and increased HL activity in insulin resistance is critical not only to the increased production of small, dense LDL, but also to an increased catabolism of HDL2 particles.
There are several new directions for research into the effect of the metabolic syndrome on HDL metabolism. In vitro studies have recently pointed to key roles of several new proteins in reverse cholesterol transport (RCT), including ATP-binding cassette A1 (ABCA1) transporters, scavenger receptor class B (SRB1) and phospholipid transfer protein (PLTP).98–101 Hyperglycaemia and insulin resistance may down-regulate ABCA1 and increase PLTP activity,102,103 but the precise significance of this for HDL transport in vivo remains unclear. Recent evidence also supports a pivotal role for pre-β HDL particles in HDL metabolism.104 Hence, further studies should also examine the effect of insulin resistance on pre-β HDL kinetics and the relationship with cellular cholesterol efflux and RCT. The effect of insulin resistance on the transport of LpA-I and LpA-I:A-II particles also needs further investigation in vivo. A key methodological objective of future tracer studies is to establish a reliable, reproducible and practical method for examining cholesterol efflux from peripheral tissue as well as its transport/exchange among plasma lipoproteins and excretion in bile.
2. Interventional Studies
I. Lifestyle Modifications
Weight Loss
In a randomised controlled dietary trial, we investigated the effect of weight loss, and the reduction in adipose tissue compartments, on apoB kinetics using a primed infusion of 13C-leucine and magnetic resonance imaging in viscerally obese men.34 Weight reduction of approximately 10 kg decreased hepatic apoB secretion and reciprocally up-regulated LDL catabolism by 50% and 125%, respectively. These changes were chiefly related to loss of intraperitoneal adipose tissue mass, by contrast to changes in subcutaneous and retroperitoneal fat and to improvements in hepatic insulin sensitivity. Changes in visceral adipose tissue mass remained a significant predictor of the change in VLDL-apoB secretion after adjusting for changes in insulin sensitivity and dietary fat intake. The increase in LDL-apoB clearance following weight loss may be due to increased LDL receptor activity,105 and is likely to be accompanied by increased chylomicron remnant clearance.67 HDL kinetic changes in response to weight loss have not yet been reported in the literature.
Dietary Factors
We have shown in a meta-analysis of stable isotope studies that total dietary fat (chiefly saturated fatty acids) exerts a significant effect in increasing hepatic secretion of VLDL-apoB.106 Decreasing total fat is therefore likely to have a significant impact in decreasing hepatic apoB secretion in visceral obesity. However, it appears that total daily energy and carbohydrate intake may be a more important consideration in managing these subjects. A low-fat, high-carbohydrate, energy-deficient diet has conventionally been used for weight reduction in obese subjects.1,107 However, a low-carbohydrate, high-protein, high-fat diet (i.e the Atkins diet) has become popular of late.108 However, the precise mechanism of action of a low-carbohydrate diet on the kinetics of apoB and apoA-containing lipoproteins in subjects with the metabolic syndrome is unknown. The atherogenic properties and long-term safety of the Atkins diet warrant further investigation.
Other dietary factors, such as fatty acid composition, alcohol intake, plant sterols and fish consumption can also play an important role in regulating lipoprotein metabolism,109,110 but their effects in the metabolic syndrome have not been adequately studied.
Physical Activity
The effect of increased physical activity on lipoprotein kinetics has not been fully investigated with stable isotopes in metabolic syndrome. A preliminary study in type 2 diabetes has suggested that prolonged, aerobic exercise can decrease hepatic secretion of VLDL-apoB.111 In an early radiokinetic report, Thompson et al demonstrated that prolonged exercise raised HDL-cholesterol and apoA-I concentrations via a dual mechanism involving decreased apoA-I catabolism and increased HDL apoA-I production, but the study was uncontrolled.112 Sviridov et al have shown that in diabetes, a single session of cycling exercise lasting 25 min increases the plasma concentration of pre-βHDL particles, suggesting increased secretion of lipid-free apoA-I.113 Further stable isotope studies of the effect of exercise (and different forms of exercise) on lipoprotein kinetics are required in the metabolic syndrome.
II. Pharmacotherapies
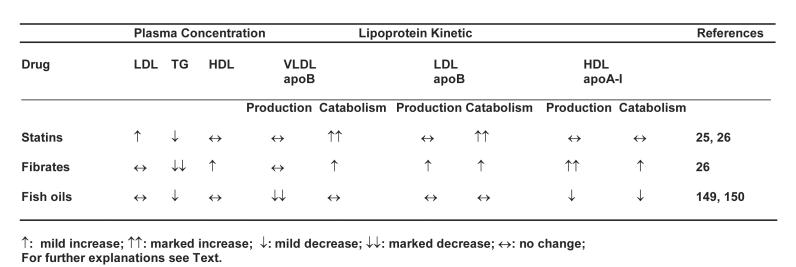
Several lipid-regulating agents may be used to improve dyslipidaemia in the metabolic syndrome.114,115 The current commonly used lipid-regulating agents include 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, fibric acid derivatives, niacin, and fish oil supplementation. The putative mechanisms of action of these agents on lipoprotein metabolism as applied to the metabolic syndrome are summarised in Table 2. Only the kinetic effects of statins, fibrates and fish oils on lipoprotein metabolism in metabolic syndrome will be reviewed.
Table 2.
Kinetic effects of the major lipid-regulating agents on lipoprotein metabolism in the metabolic syndrome.
HMG-CoA Reductase Inhibitors
The fundamental mechanism of action of statins on cholesterol metabolism involves decreased conversion of HMG-CoA to mevalonic acid by competitive inhibition of the rate-limiting enzyme HMG-CoA reductase.116 This decreases the flux of mevalonate to sterol precursors and cholesterol. The reduction in intracellular cholesterol content increases the expression and synthesis of LDL receptors. The up-regulation of LDL receptor may operate via sterol-independent and sterol-dependent pathways possibly involving increased expression of SREBP-2 and SREBP-1a.52 In vivo this in turn increases the clearance of LDL and chylomicron remnants from plasma. In addition to an inhibitory effect of de novo cholesterol synthesis, statins may also decrease cholesterol esterification rates, triglyceride substrate availability, the expression of apoB, and possibly other related gene products such as MTP.117–119 The kinetic effects of statins in lipoprotein transport may partly account for their efficacy in decreasing cardiovascular events in subjects with IGT and type 2 diabetes.
Consistent with the aforementioned mechanisms, we have consistently demonstrated that in centrally obese subjects atorvastatin (40mg daily for 6 weeks) significantly increases the catabolism of all apoB-100 containing lipoproteins, including VLDL, IDL and LDL-apoB.120 However, in these subjects atorvastatin did not decrease VLDL-apoB secretion. We have interpreted this as being partly due to direct or indirect effect of persistent insulin resistance on the hepatic processing of lipids and apoB. Forster et al and Bilz et al have also independently reported that in combined hyperlipidaemia atorvastatin increases the conversion rate of VLDL to LDL and the catabolic rate of LDL without altering apoB production,121,122 but their subjects did not formally have the metabolic syndrome. In a recent kinetic study by Caslake et al in subjects with moderate hypercholesterolaemia, rosuvastatin (40 mg daily for 8 weeks) was shown to improve dyslipidaemia chiefly by increasing the clearance of LDL with a small effect in decreasing the synthesis of VLDL1.123 These kinetic studies clearly question whether persistent insulin resistance plays a major role in blunting the effect of statins on hepatic secretion of apoB in the metabolic syndrome. However, the level of insulin resistance of the subjects employed in these studies was not documented. We have also reported that atorvastatin can improve triglyceride-rich lipoprotein metabolism in insulin resistance by decreasing plasma concentrations of apoB-48, apoC-III and RLP-cholesterol, as well as by increasing the FCR of IDL-apoB and chylomicron remnants.120,124,125 The efficacy of statins in improving chylomicron remnant metabolism clearance may depend on their potency in inhibiting HMG-CoA reductase, for we have found that pravastatin, a weaker statin than atorvastatin, does not significantly alter plasma apoB-48 levels or the FCR of chylomicron remnant-like emulsion in type 2 diabetes (Watts GF et al. 2003, unpublished data). In another study, we reported that inhibition of HMG-CoA reductase with atorvastatin promoted intestinal absorption of dietary cholesterol, as measured by plasma campesterol:cholesterol ratio.126 This counter-regulatory mechanism could increase lipid substrate availability in the liver and stimulate apoB secretion, thereby diminishing the effect of statins on hepatic secretion of apoB and the triglyceride-lowering potency of these drugs. For this reason, consideration should be given to combining a statin with agents that improve triglyceride metabolism and/or cholesterol absorption in subjects with the metabolic syndrome.
Generally, statins do not appreciably raise plasma HDL-cholesterol concentrations. The significant increases reported are probably consequent on a triglyceride-lowering effect. Asztalos et al have also suggested that statins that are more potent in inhibiting CETP activity, such as atorvastatin, are more likely to increase the plasma concentrations of α1 and pre-α1 HDL subspecies.127In vitro data suggest that inhibition of cholesterol biosynthesis with statins increases the production of apoA-I by decreasing the Rho signalling pathway and activating PPAR-α.128 Whether this translates into an appreciable effect on HDL apoA-I transport in vivo remains to be demonstrated. In a recent isotope study from our group, however, atorvastatin did not alter HDL apoA-I production or catabolism in the metabolic syndrome.26 Recent clinical data findings suggest that high dose of atorvastatin lowers HDL-cholesterol concentrations,129,130 but this is not seen with simvastatin, pravastatin or rosuvastatin. An animal study suggests that this effect of atorvastatin results from enhanced HDL apoA-I catabolism, without a concurrent increase in apoA-I production.131 This observation is, however, incompatible with human data showing that atorvastatin increases the plasma concentrations of large cholesterol-rich α1 HDL particles,127 since this should slow the FCR of HDL apoA-I.
Fibric-acid Derivatives
The mechanisms of action of fibrates, such as gemfibrozil, fenofibrate, bezafibrate and ciprofibrate, on lipoprotein metabolism and atherogenesis have been consistently elucidated in animal and in vitro studies.132–136 Fibrates are ligands that bind to and activate PPAR-α, chiefly in the liver. This results in the formation of a heterodimer with the 9-cis retinoic acid receptor (RXR).137 The PPAR/RXR heterodimer binds to specific peroxisome proliferator response elements (PPRE), located in the promoter region of several target genes.138 Via this mechanism PPAR-α activation regulates a variety of genes involved in lipid metabolism, thrombosis and inflammation. 132,133
In a recent stable isotope study from our group, fenofibrate significantly increased the catabolism of VLDL-apoB (3.77 ± 0.30 vs. 5.00 ± 0.49 pool/day, p<0.01), IDL-apoB (2.86 ± 0.21 vs. 3.84 ± 0.40 pools/day, p<0.01) and LDL-apoB (0.35 ± 0.02 vs. 0.44 ± 0.02 pools/day, p<0.01) in subjects with the metabolic syndrome.26 The latter is consistent with the work of Caslake et al showing that in mixed hyperlipidaemia fenofibrate increases the production of large LDL particles that have a higher affinity for and are catabolised more rapidly by hepatic LDL receptors.139 Relative to atorvastatin, we also found that fenofibrate also increased the production of apoA-I (15.4 ± 0.70 vs. 18.1 ± 1.6 mg/kg/day, p<0.01), despite the fact that CETP activity was inhibited less with fenofibrate than with atorvastatin.26 These kinetic changes with fenofibrate treatment were coupled with a significant increase in plasma concentrations of apoA-I and apoA-II, as well as a decrease in plasma apoB, apoC-III and lathosterol concentrations. Fenofibrate treatment in this study did not decrease the hepatic secretion of VLDL apoB, and this again could be owing to an effect of persistent insulin resistance. Our kinetic findings of the effects of fenofibrate are similar to those recently reported in subjects with frank mixed hyperlipidaemia in the absence of apparent insulin resistance.122 These results are also compatible with an earlier radiokinetic study.140
The aforementioned kinetic data are congruent with our current understanding of mechanism of action of fibrates on lipid and lipoprotein metabolism as a consequence of PPAR-α activation.132,133 PPAR-α agonists reduce triglyceride substrate availability in the liver by stimulation of peroxisomal and mitochrondrial β-oxidation, thereby decreasing hepatic secretion of VLDL. The stimulation of fatty acid catabolism results from an effect of PPAR-α activation that increases the expression of key proteins, including fatty acid binding protein, acyl-CoA synthase and carnitine palmitoyl transferase-I.141 Fibrates also promote intravascular VLDL lipolysis by inducing and repressing the genetic expression of LPL and apoC-III, respectively, via the corresponding PPREs.142,143 Fibrates also increase the expression of apoA-I, apoA-II, ABCA1 transporters and SRB1 by activating the LXR-α pathway, thereby promoting cholesterol transport from the periphery to the liver via HDL.102,136,144,145 In our experience, these mechanisms are more important in explaining the improvement in HDL apoA-I transport than a selective inhibition of plasma CETP activity. This evidence also suggests that fibrates stimulate the receptor-mediated uptake of LDL by the liver by inhibiting hepatic cholesterol synthesis via regulation of SREBPs.145,146 The kinetic effects of fibrates on lipid and lipoprotein metabolism, in particular HDL metabolism, potentially provide a major mechanism for the benefit of fibrates in clinical endpoint trials. The increased production of HDL apoA-I may well translate into a stimulation of RCT and negative corporeal cholesterol balance, as suggested in a recent study following the infusion of recombinant HDL apoA-I.147 The newly proposed concept of selective PPAR-α modulation suggests that some fibrates, such as fenofibrate, may be more potent stimulators of apoA-I and A-II expression than others, such as gemfibrozil.148 The lack of therapeutic effect of fibrates on hepatic oversecretion and hypercatabolism of apoA-I in subjects with the metabolic syndrome supports the use of additional agents to optimally regulate the dyslipoproteinaemia of insulin resistance. The effect of fibrates on LpA-I, LpA-I:A-II, apoA-V and corporeal cholesterol transport requires further examination.
Fish Oil Supplementation
We have reported that in subjects with metabolic syndrome, supplementation with fish oils, rich in docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), diminishes VLDL-apoB production (14.8 ± 2.3 vs. 10.1 ± 1.2 mg/kg/day, p<0.05) and enhances conversion of VLDL to LDL-apoB (22.7 ± 3.9 vs. 39.2 ± 4.5 %, p<0.05).149 In this study, fish oil supplementation did not alter the FCR of apoB in VLDL, IDL and LDL, nor the catabolism of chylomicron remnants. The enhanced conversion of VLDL to LDL without a change in VLDL FCR suggests decreased direct clearance of VLDL by the liver.49In an uncontrolled study, Frenais et al found that in five diabetic patients the FCR and absolute production rate of HDL apoA-I were significantly decreased after treatment with fish oils.150 The authors concluded that this is probably linked to a decrease in plasma triglyceride level which in turn enhances the stabilisation of HDL particles in plasma. In another study of overweight or type 2 diabetic patients by our group, fish oils also significantly increased plasma concentrations of HDL-cholesterol, in particular HDL2-cholesterol, consistent with a decrease in catabolism of HDL apoA-I.151 Moreover, fish oil supplementation has the potential for reducing post-prandial triglyceride-rich lipoprotein concentrations in both lean and obese individuals.152,153 The precise mechanism of action of fish oils on plasma triglyceride levels is not fully understood. Fish oils decrease hepatic triglyceride synthesis via inhibition of diacylglycerol acyltransferase, fatty acid synthase and acetyl-CoA carboxylase enzyme activities.154–156 Fish oils also enhance fatty acid β-oxidation by stimulating PPAR-α, although their effects on this transcription factor are much weaker than fibrates.157 Fish oils additionally decrease hepatic pool of triglycerides by suppressing the expression of SREBP-1c gene, thereby inhibiting de novo synthesis of both fatty acids and triglyceride. ω-3 fatty acids apparently decrease the expression of the SREBP-1 gene by accelerating the catabolic rate of SREBP-1 mRNA.158,159 In a recent study of obese subjects, we also found that EPA and DHA have differential effects on plasma lipid and lipoproteins. EPA significantly decreased HDL3-cholesterol by 7% whereas DHA increased HDL2-cholesterol and LDL-cholesterol by 29% and 8%, respectively.160 Further investigations should also explore the effects of fish oils, as well as the differential effects of EPA and DHA, on free fatty acid, triglyceride, apoB and apoA-I kinetics in the metabolic syndrome.
Combination Therapy
In many patients with the metabolic syndrome, lipid-regulating monotherapy (e.g statins or fibrates) may not adequately improve dyslipidaemia. More aggressive treatment strategies may involve adjunctive use of more than one lipid-regulating agent by harnessing the complementary mechanism of action of the different agents.161 Several possible combinations include statin-fibrate, statin-niacin and statin-fish oils regimens. A number of studies have demonstrated that these regimens can markedly improve mixed hyperlipidaemia with significant elevation of HDL-cholesterol concentration.125,165–166 However, kinetic data on the efficacy of combination therapy on lipoprotein metabolism are limited.
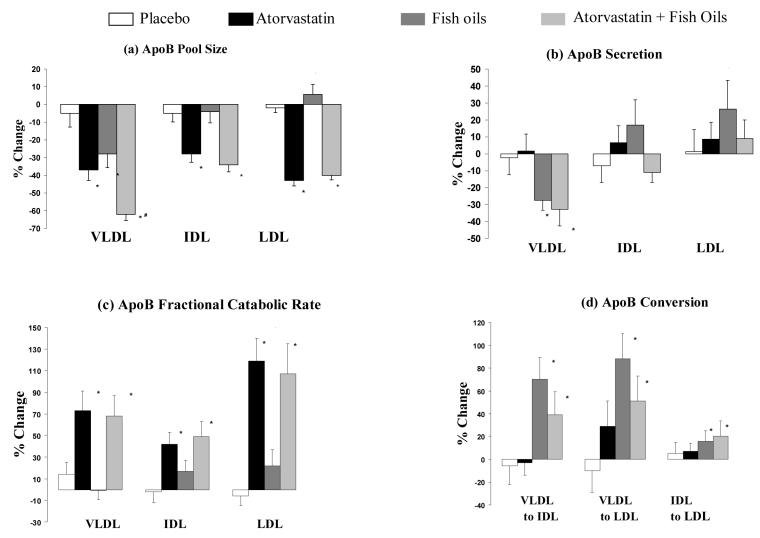
Recently, we reported that in insulin-resistant obese men combination treatment with atorvastatin and fish oils had an additive effect in decreasing plasma triglyceride (−40%) and elevating HDL-cholesterol (+15%).125 The kinetic data revealed that atorvastatin plus fish oils decreased VLDL-apoB secretion and increased the FCRs of VLDL, IDL and LDL-apoB and the percent conversion of VLDL to LDL (Figure 2).25 Despite increased conversion of VLDL to IDL or LDL with combination treatment, the pool sizes of IDL and LDL-apoB fell significantly by 35% and 40%, respectively. This suggests that the increased flux of apoB from VLDL to IDL or LDL was offset by enhanced hepatic clearance of these lipoproteins due to atorvastatin. The precise mechanism of action of this combination of agents on HDL metabolism is unclear. Since atorvastatin did not apparently have a significant impact on HDL metabolism, the HDL-raising effect of this combination treatment may be chiefly due to a decreased fractional catabolism of HDL apoA-I with fish oil treatment, as suggested elsewhere.150
Fig 2.
Percentage change in apoB (a) pool sizes, (b) secretion rate, (c) FCR and (d) conversion in VLDL, IDL and LDL on placebo, atorvastatin, fish oils and atorvastatin plus fish oils, respectively. Full details see Reference 25.
As reviewed earlier, dyslipidaemia in the metabolic syndrome is due to increased hepatic supply and availability of lipid substrates, as well as the intrinsic effects of insulin resistance on hepatic output of VLDL and catabolism of VLDL in peripheral tissue.42 Statins inhibit de novo cholesterol synthesis whereas fish oils decrease hepatic triglyceride synthesis. Clearly, the complementary mechanisms of action of statin and fish oils on lipid and lipoprotein metabolism support their use as a combination therapy in the metabolic syndrome
When accompanied by hypertriglyceridaemia and low HDL-cholesterol, plasma LDL-cholesterol elevations may be managed using a statin in combination with either niacin, a fibrate or fish oils. Statins are first-line agents to lower LDL-cholesterol and non-HDL-cholesterol.1,107 Fibrates, niacins, and fish oils are generally more effective than statins in lowering plasma VLDL-triglyceride levels, as well as in increasing HDL-cholesterol and reducing the formation of atherogenic small, dense LDL particles. As reviewed earlier, the mechanisms of action of all these agents on lipoprotein kinetics are different. These complementary mechanisms provide the rationale for combination therapy. In respect of the metabolic syndrome, we have previously reported that atorvastatin and fenofibrate monotherapy have differential corrective effects on plasma apoB and apoA-I kinetics, which underscores the dual use of these drugs to optimise the dyslipidaemia in this disorder.26 Future kinetic studies should investigate the mechanisms of action on lipoprotein metabolism of new drug regimens, such as fibrate or statins in combination with glitazones, metformin, ezetimibe or CETP inhibitors.
Conclusions
The metabolic syndrome is an escalating public health problem related to adverse gene-environment interactions that result in progressive increase in body weight and phenotypic expression of a spectrum of metabolic and cardiovascular risk factors. The metabolic syndrome is frequently associated with visceral obesity, insulin resistance and dyslipidaemia, that in turn is causally related to an increased risk of type 2 diabetes and CVD. Dyslipidaemia, or dyslipoproteinaemia, is a cardinal feature of the metabolic syndrome characterised by elevated plasma triglycerides, reduced HDL-cholesterol, elevated apoB concentrations and a predominance of small, dense LDL particles. The mechanisms for dyslipidaemia in the metabolic syndrome have recently been elucidated by stable isotope tracer kinetic studies. The abnormalities in lipoprotein metabolism arise from dysregulation of both apoB and apoA metabolism, including overproduction of VLDL-apoB and decreased catabolism of chylomicron remnants, VLDL, IDL and LDL-apoB, as well as an increased catabolism of HDL apoA-I particles. These mechanisms are compatible with a wide body of experimental and cell biological studies indicating that increased lipid substrate availability in the liver is a critical abnormality that dysregulates lipoprotein transport in plasma. Stable isotope kinetic studies have also elucidated the mechanism of action of several therapeutic approaches for regulating dyslipidaemia in metabolic syndrome, including weight loss, statins, fibrates and fish oils. The differential effects of many of these therapies on lipoprotein kinetics support the use of complementary treatments, a good example of this is statin-fibrate combination that enhances the catabolism of apoB-containing lipoproteins and increases the production of apoA-I.
The use of stable isotopes, mass spectrometry and mathematical modelling methods has played a fundamental role in advancing our knowledge of this important metabolism disorder. However, this approach is time-consuming, expensive and requires a high level of expertise. Its success relies on a multi-disciplinary team involving biochemists, physicians and biostatisticians, so that these studies are best carried out in specialised research centres. These types of investigations not only provide a rationale for how to optimally employ existing therapies for dyslipidaemia in the metabolic syndrome, but also assist in drug discovery and development programs. Relevant pipeline agents that will need investigating in the future include CETP inhibitors, LXR/farnesoid X receptor (FXR) agonists, PPAR agonists, specific ABCA1 agonists and cholesterol absorption inhibitors. These studies will require the development of new tracer methodologies for investigating in vivo cholesterol efflux from cells, cholesterol transport in plasma, corporeal cholesterol balance, and the turnover of several subpopulations of HDL particles.
Acknowledgements
This work was supported by grants from the National Health and Medical Research Council, the National Heart Foundation and the National Institutes of Health (NIBIB P41 EB-001975). Support was also provided by the Raine Medical Research Foundation, the Royal Perth Hospital Medical Research Foundation, and Pfizer. PHRB is a Career Development Fellow of the National Heart Foundation. DCC is a postdoctorate research fellow of Raine-National Heart Foundation.
References
- 1.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 2.Reaven GM. The insulin resistance syndrome. Curr Atheroscler Rep. 2003;5:364–71. doi: 10.1007/s11883-003-0007-0. [DOI] [PubMed] [Google Scholar]
- 3.Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–9. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 4.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–16. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 5.Turner RC, Millns H, Neil HAW, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom prospective diabetes study (UKPDS: 23) BMJ. 1998;316:823–8. doi: 10.1136/bmj.316.7134.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 7.Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163:427–36. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ginsberg HN, Stalenhoef AF. The metabolic syndrome: targeting dyslipidaemia to reduce coronary risk. J Cardiovasc Risk. 2003;10:121–8. doi: 10.1097/01.hjr.0000060840.46106.ea. [DOI] [PubMed] [Google Scholar]
- 9.Alexander CM, Landsman PB, Teutsch SM, Haffner SM. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52:1210–4. doi: 10.2337/diabetes.52.5.1210. [DOI] [PubMed] [Google Scholar]
- 10.Definition, diagnosis and classification of diabetes mellitus and its nomplications: Report of a WHO Consultation. Geneva, Switzerland: Department of Noncommunicable Disease Surveillance. World Health Organization; 1999.
- 11.Balkau B, Charles MA, Drivsholm T, et al. The European Group for the study of Insulin Resistance (EGIR). Frequency of the WHO metabolic syndrome in European cohorts, and an alternative definition of an insulin resistance syndrome. Diabetes Metab. 2002;28:364–76. [PubMed] [Google Scholar]
- 12.Taskinen MR. Diabetic dyslipidemia. Atherosclerosis. 2002;3:S47–51. doi: 10.1016/s1567-5688(01)00006-x. [DOI] [PubMed] [Google Scholar]
- 13.Burnett JR, Barrett PHR. Apolipoprotein B metabolism: tracer kinetics, models, and metabolic studies. Crit Rev Clin Lab Sci. 2002;39:89–137. doi: 10.1080/10408360208951113. [DOI] [PubMed] [Google Scholar]
- 14.Berman M. Kinetic analysis and modeling: theory and application to lipoproteins. In Berman M, Grundy SM and Howard BV, eds. Lipoprotein kinetics and modeling. New York: Academic Press, 1982, 3–36.
- 15.Osborne JC, Jr, Schaefer EJ, Powell GM, Lee NS, Zech LA. Molecular properties of radioiodinated apolipoprotein A-I. J Biol Chem. 1984;259:347–53. [PubMed] [Google Scholar]
- 16.Beltz WF, Kesaniemi YA, Howard BV, Grundy SM. Development of an integrated model for analysis of the kinetics of apolipoprotein B in plasma very low density lipoproteins, intermediate density lipoproteins, and low density lipoproteins. J Clin Invest. 1985;76:575–85. doi: 10.1172/JCI112009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramakrishnan R, Arad Y, Wong S, Ginsberg HN. Nonuniform radiolabeling of VLDL apolipoprotein B: implications for the analysis of studies of the kinetics of the metabolism of lipoproteins containing apolipoprotein B. J Lipid Res. 1990;31:1031–42. [PMC free article] [PubMed] [Google Scholar]
- 18.Packard CJ. The role of stable isotopes in the investigation of plasma lipoprotein metabolism. Baillieres Clin Endocrinol Metab. 1995;9:755–72. doi: 10.1016/s0950-351x(95)80129-4. [DOI] [PubMed] [Google Scholar]
- 19.Cummings MH, Watts GF. Stable isotopes in lipoprotein research: kinetic studies of very low density lipoprotein apolipoprotein B-100 metabolism in human subjects. Endocrinol Metab. 1996;3:73–89. [Google Scholar]
- 20.Demant T, Packard CJ, Demmelmair H, et al. Sensitive methods to study human apolipoprotein B metabolism using isotope-labeled amino acids. Am J Physiol. 1996;270:E1022–36. doi: 10.1152/ajpendo.1996.270.6.E1022. [DOI] [PubMed] [Google Scholar]
- 21.Patterson BW. Use of stable isotopically labeled tracers for studies of metabolic kinetics: an overview. Metabolism. 1997;46:322–9. doi: 10.1016/s0026-0495(97)90260-2. [DOI] [PubMed] [Google Scholar]
- 22.Maugeais C, Ougerram K, Krempf M, Magot P. Kinetic study of apo B100-containing lipoprotein metabolism using amino acids labeled with stable isotopes: methodological aspects. Clin Chem Lab Med. 1998;36:739–45. doi: 10.1515/CCLM.1998.131. [DOI] [PubMed] [Google Scholar]
- 23.Barrett PHR, Foster DM. Design and analysis of lipid tracer kinetic studies. Curr Opin Lipidol. 1996;7:143–8. doi: 10.1097/00041433-199606000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Dwyer KP, Barrett PHR, Chan DC, Foo JI, Watts GF, Croft KD. Oxazolinone derivative of leucine for GC-MS: a sensitive and robust method for stable isotope kinetic studies of lipoproteins. J Lipid Res. 2002;43:344–9. [PubMed] [Google Scholar]
- 25.Chan DC, Watts GF, Barrett PHR, Beilin LJ, Redgrave TG, Mori TA. Regulatory effects of HMGCoA reductase inhibitor and fish oils on apolipoprotein B100 kinetics with insulin resistant obese male subjects with dyslipidaemia. Diabetes. 2002;51:2377–86. doi: 10.2337/diabetes.51.8.2377. [DOI] [PubMed] [Google Scholar]
- 26.Watts GF, Barrett PHR, Ji J, et al. Differential regulation of lipoprotein kinetics by atorvastatin and fenofibrate in subjects with the metabolic syndrome. Diabetes. 2003;52:803–11. doi: 10.2337/diabetes.52.3.803. [DOI] [PubMed] [Google Scholar]
- 27.Redgrave TG, Watts GF, Martins IJ, et al. Chylomicron remnant metabolism in familial dyslipidemias studied with a remnant-like emulsion breath test. J Lipid Res. 2001;42:710–5. [PubMed] [Google Scholar]
- 28.Mittendorfer B, Liem O, Patterson BW, Miles JM, Klein S. What does the measurement of whole-body fatty acid rate of appearance in plasma by using a fatty acid tracer really mean? Diabetes. 2003;52:1641–8. doi: 10.2337/diabetes.52.7.1641. [DOI] [PubMed] [Google Scholar]
- 29.Patterson BW, Mittendorfer B, Elias N, Satyanarayana R, Klein S. Use of stable isotopically labeled tracers to measure very low density lipoprotein-triglyceride turnover. J Lipid Res. 2002;43:223–33. [PubMed] [Google Scholar]
- 30.Ostlund RE. A minimal model for human whole body cholesterol metabolism. Am J Physiol. 1993;265:513–20. doi: 10.1152/ajpendo.1993.265.3.E513. [DOI] [PubMed] [Google Scholar]
- 31.Riches FM, Watts GF, Naoumova RP, Kelly JM, Croft KD, Thompson GR. Hepatic secretion of very-low-density lipoprotein apolipoprotein B-100 studied with a stable isotope technique in men with visceral obesity. Int J Obes Relat Metab Disord. 1998;22:414–23. doi: 10.1038/sj.ijo.0800602. [DOI] [PubMed] [Google Scholar]
- 32.Chan DC, Watts GF, Redgrave TG, Mori TA, Barrett PHR. Apolipoprotein B-100 kinetics in visceral obesity: associations with plasma apolipoprotein C-III concentration. Metabolism. 2002;29:1041–6. doi: 10.1053/meta.2002.33339. [DOI] [PubMed] [Google Scholar]
- 33.Chan DC, Watts GF, Barrett PHR, Mamo CL, Redgrave TG. Markers of triglyceride-rich lipoprotein remnant metabolism in visceral obesity. Clin Chem. 2002;48:278–83. [PubMed] [Google Scholar]
- 34.Riches FM, Watts GF, Hua J, Stewart GR, Naoumova RP, Barrett PHR. Reduction in visceral adipose tissue is associated with improvement in apolipoprotein B-100 metabolism in obese men. J Clin Endocrinol Metab. 1999;84:2854–61. doi: 10.1210/jcem.84.8.5925. [DOI] [PubMed] [Google Scholar]
- 35.Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 2002;23:201–29. doi: 10.1210/edrv.23.2.0461. [DOI] [PubMed] [Google Scholar]
- 36.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–81. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis GF. Fatty acid regulation of very low density lipoprotein production. Curr Opin Lipidol. 1997;8:146–53. doi: 10.1097/00041433-199706000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Sniderman AD, Cianflone K. Substrate delivery as a determinant of hepatic apoB secretion. Arterioscler Thromb Vasc Biol. 1993;13:629–36. doi: 10.1161/01.atv.13.5.629. [DOI] [PubMed] [Google Scholar]
- 39.Thompson GR, Naoumova R, Watts GF. Role of cholesterol in regulating apolipoprotien B secretion by the liver. J Lipid Res. 1996;37:439–47. [PubMed] [Google Scholar]
- 40.Adeli K, Taghibiglou C, Van Iderstine SC, Lewis GF. Mechanisms of hepatic very low-density lipoprotein overproduction in insulin resistance. Trends Cardiovasc Med. 2001;11:170–6. doi: 10.1016/s1050-1738(01)00084-6. [DOI] [PubMed] [Google Scholar]
- 41.Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest. 2000;106:453–8. doi: 10.1172/JCI10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ginsberg HN, Huang LS. The insulin resistance syndrome: impact on lipoprotein metabolism and atherothrombosis. J Cardiovasc Risk. 2000;7:325–31. doi: 10.1177/204748730000700505. [DOI] [PubMed] [Google Scholar]
- 43.Dashti N. The effect of low density lipoproteins, cholesterol, and 25-hydroxycholesterol on apolipoprotein B gene expression in HepG2 cells. J Biol Chem. 1992;267:7160–9. [PubMed] [Google Scholar]
- 44.Cianflone K, Zhang Z, Vu H, Kohen-Avramoglu R, Kalant D, Sniderman AD. The effect of individual amino acids on ApoB100 and Lp(a) secretion by HepG2 cells. J Biol Chem. 1996;271:29136–45. doi: 10.1074/jbc.271.46.29136. [DOI] [PubMed] [Google Scholar]
- 45.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 46.Malmstrom R, Packard CJ, Caslake M, et al. Effects of insulin and acipimox on VLDL1 and VLDL2 apolipoprotein B production in normal subjects. Diabetes. 1998;47:779–87. doi: 10.2337/diabetes.47.5.779. [DOI] [PubMed] [Google Scholar]
- 47.Taskinen MR. Diabetic dyslipidaemia: from basic research to clinical practice. Diabetologia. 2003;46:733–49. doi: 10.1007/s00125-003-1111-y. [DOI] [PubMed] [Google Scholar]
- 48.Taskinen MR, Packard CJ, Shepherd J. Effect of insulin therapy on metabolic fate of apolipoprotein B-containing lipoproteins in NIDDM. Diabetes. 1990;39:1017–27. doi: 10.2337/diab.39.9.1017. [DOI] [PubMed] [Google Scholar]
- 49.Taskinen MR. Is liver the culprit of diabetic dyslipidemia? Atherosclerosis (Suppl) 2003;4:190. [Google Scholar]
- 50.Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3–10. [PubMed] [Google Scholar]
- 51.Roith D, Zick Y. Recent advances in our understanding of insulin action and insulin resistance. Diabetes Care. 2001;24:588–97. doi: 10.2337/diacare.24.3.588. [DOI] [PubMed] [Google Scholar]
- 52.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–31. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carpentier A, Taghibiglou C, Leung N, et al. Ameliorated hepatic insulin resistance is Associated with normalization of microsomal triglyceride transfer protein expression and reduction in very low density lipoprotein assembly and secretion in the fructose-fed hamster. J Biol Chem. 2002;277:28795–802. doi: 10.1074/jbc.M204568200. [DOI] [PubMed] [Google Scholar]
- 54.Panarotto D, Remillard P, Bouffard L, Maheuz P. Insulin resistance affects the regulation of lipoprotein lipase in the postprandial period and in an adipose tissue-specific manner. Eur J Clin Invest. 2003;32:84–92. doi: 10.1046/j.1365-2362.2002.00945.x. [DOI] [PubMed] [Google Scholar]
- 55.Lewis GF, O'Meara NM, Soltys PA, et al. Fasting hypertriglyceridemia in noninsulin-dependent diabetes mellitus is an important predictor of postprandial lipid and lipoprotein abnormalities. J Clin Endocrinol Metab. 1991;72:934–44. doi: 10.1210/jcem-72-4-934. [DOI] [PubMed] [Google Scholar]
- 56.Riches FM, Watts GF, van Bockxmeer FM, et al. Apolipoprotein B signal peptide and apolipoprotein E genotypes as determinants of the hepatic secretion of VLDL apoB in obese men. J Lipid Res. 1998;39:1752–8. [PubMed] [Google Scholar]
- 57.Watts GF, Riches FM, Humphries SE, Talmud PJ, van Bockxmeer FM. Genotypic associations of the hepatic secretion of VLDL apolipoprotein B-100 in obesity. J Lipid Res. 2000;41:481–8. [PubMed] [Google Scholar]
- 58.Dane-Stewart CA, Watts GF, Barrett PHR, et al. Chylomicron remnant metabolism studied with a new breath test in postmenopausal women with and without type 2 diabetes mellitus. Clin Endocrinol. 2003;58:415–20. doi: 10.1046/j.1365-2265.2003.01731.x. [DOI] [PubMed] [Google Scholar]
- 59.Eckel RH. Lipoprotein lipase. A multifunctional enzyme relevant to common metabolic diseases. N Engl J Med. 1989;320:1060–8. doi: 10.1056/NEJM198904203201607. [DOI] [PubMed] [Google Scholar]
- 60.Wade DP, Knight BL, Soutar AK. Hormonal regulation of low-density lipoprotein (LDL) receptor activity in human hepatoma Hep G2 cells. Insulin increases LDL receptor activity and diminishes its suppression by exogenous LDL. Eur J Biochem. 1988;174:213–8. doi: 10.1111/j.1432-1033.1988.tb14084.x. [DOI] [PubMed] [Google Scholar]
- 61.Ebara T, Conde K, Kako Y, et al. Delayed catabolism of apoB-48 lipoproteins due to decreased heparan sulphate proteoglycan production in diabetic mice. J Clin Invest. 2000;105:1807–18. doi: 10.1172/JCI8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shachter NS. Apolipoproteins C-I and C-III as important modulators of lipoprotein metabolism. Curr Opin Lipidol. 2001;12:297–304. doi: 10.1097/00041433-200106000-00009. [DOI] [PubMed] [Google Scholar]
- 63.Torra IP, Chinetti G, Duval C, et al. Peroxisome proliferator-activated receptors:From transcriptional control to clinical practice. Curr Opin Lipidol. 2001;12:245–54. doi: 10.1097/00041433-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 64.Mekki N, Christofilis MA, Charbonnier M, et al. Influence of obesity and body fat distribution on postprandial lipemia and triglyceride-rich lipoproteins in adult women. J Clin Endocrinol Metab. 1999;84:184–91. doi: 10.1210/jcem.84.1.5397. [DOI] [PubMed] [Google Scholar]
- 65.Lewis GF, O'Meara NM, Soltys PA, et al. Fasting hypertriglyceridemia in noninsulin-dependent diabetes mellitus is an important predictor of postprandial lipid and lipoprotein abnormalities. J Clin Endocrinol Metab. 1991;72:934–44. doi: 10.1210/jcem-72-4-934. [DOI] [PubMed] [Google Scholar]
- 66.Mamo JC, Watts GF, Barrett PHR, Smith D, James AP, Pal S. Postprandial dyslipidemia in men with visceral obesity: an effect of reduced LDL receptor expression? Am J Physiol. 2001;81:E626–32. doi: 10.1152/ajpendo.2001.281.3.E626. [DOI] [PubMed] [Google Scholar]
- 67.Cooper AD. Hepatic uptake of chylomicron remnants. J Lipid Res. 1997;38:2173–92. [PubMed] [Google Scholar]
- 68.Heeren J, Beisiegel U. Intracellular metabolism of triglyceride-rich lipoproteins. Curr Opin Lipidol. 2001;12:255–60. doi: 10.1097/00041433-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 69.Baynes C, Henderson AD, Anyaoku V, et al. The role of insulin insensitivity and hepatic lipase in the dyslipidaemia of type 2 diabetes. Diabetic Medicine. 1991;8:560–6. doi: 10.1111/j.1464-5491.1991.tb01652.x. [DOI] [PubMed] [Google Scholar]
- 70.Talmud PJ. Genetic determinants of plasma triglycerides: impact of rare and common mutations. Curr Atheroscler Rep. 2001;3:191–9. doi: 10.1007/s11883-001-0061-4. [DOI] [PubMed] [Google Scholar]
- 71.Chan DC, Watts GF, Barrett PHR, O'Neill FH, Redgrave TG, Thompson GR. Relationship between cholesterol homeostasis and triacylglycerol-rich lipoprotein remnant metabolism in the metabolic syndrome. Clin Sci. 2003;104:383–8. doi: 10.1042/cs1040383. [DOI] [PubMed] [Google Scholar]
- 72.Kesaniemi YA, Vega GL, Grundy SM. Kinetics of apolipoprotein B in normal and hyperlipidaemic man: review of current data. In: Berman M, Grundy SM, Howard BC, eds. Lipoprotein Kinetics and Modeling. New York: Academic Press, 1982:181–205.
- 73.Packard CJ, Shepherd J. Lipoprotein Heterogeneity and Apolipoprotein B Metabolism. Arterioscler Thromb Vasc Biol. 1997;17:3542–56. doi: 10.1161/01.atv.17.12.3542. [DOI] [PubMed] [Google Scholar]
- 74.Mazzone T, Foster D, Chait A. In vivo stimulation of low-density lipoprotein degradation by insulin. Diabetes. 1984;33:333–8. doi: 10.2337/diab.33.4.333. [DOI] [PubMed] [Google Scholar]
- 75.Tobin KA, Ulven SM, Schuster GU, et al. Liver X Receptors as Insulin-mediating Factors in Fatty Acid and Cholesterol Biosynthesis. J Biol Chem. 2002;277:10691–7. doi: 10.1074/jbc.M109771200. [DOI] [PubMed] [Google Scholar]
- 76.Bagdade JD, Ritter MC, Subbaiah PV. Accelerated cholesteryl ester transfer in patients with insulin-dependent diabetes mellitus. Eur J Clin Invest. 1991;21:161–7. doi: 10.1111/j.1365-2362.1991.tb01805.x. [DOI] [PubMed] [Google Scholar]
- 77.Terry RB, Wood PD, Haskell WL, Stefanick ML, Krauss RM. Regional adiposity patterns in relation to lipids, lipoprotein cholesterol, and lipoprotein subfraction mass in men. J Clin Endocrinol Metab. 1989;68:191–9. doi: 10.1210/jcem-68-1-191. [DOI] [PubMed] [Google Scholar]
- 78.Peeples LH, Carpenter JW, Israel RG, Barakat HA. Alterations in low-density lipoproteins in subjects with abdominal adiposity. Metabolism. 1989;38:1029–36. doi: 10.1016/0026-0495(89)90017-6. [DOI] [PubMed] [Google Scholar]
- 79.Austin MA. Triglyceride, small, dense low-density lipoprotein, and the atherogenic lipoprotein phenotype. Curr Atheroscler Rep. 2000;2:200–7. doi: 10.1007/s11883-000-0021-4. [DOI] [PubMed] [Google Scholar]
- 80.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320:915–24. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 81.Vakkilainen J, Steiner G, Ansquer JC, et al. Relationships between low-density lipoprotein particle size, plasma lipoproteins, and progression of coronary artery disease: The Diabetes Atherosclerosis Intervention Study (DAIS) Circulation. 2003;107:1733–7. doi: 10.1161/01.CIR.0000057982.50167.6E. [DOI] [PubMed] [Google Scholar]
- 82.Pietzsch J, Julius U, Nitzsche S, Hanefeld M. In vivo evidence for increased apolipoprotein A-I catabolism in subjects with impaired glucose tolerance. Diabetes. 1998;47:1928–34. doi: 10.2337/diabetes.47.12.1928. [DOI] [PubMed] [Google Scholar]
- 83.Pont F, Duvillard L, Florentin E, Gambert P, Verges B. High-density lipoprotein apolipoprotein A-I kinetics in obese insulin resistant patients. An in vivo stable isotope study. Int J Obes. 2002;26:1151–8. doi: 10.1038/sj.ijo.0802070. [DOI] [PubMed] [Google Scholar]
- 84.Duvillard L, Pont F, Florentin E, Gambert P, Verges B. Inefficiency of insulin therapy to correct apolipoprotein A-I metabolic abnormalities in non-insulin-dependent diabetes mellitus. Atherosclerosis. 2000;152:229–37. doi: 10.1016/s0021-9150(99)00473-6. [DOI] [PubMed] [Google Scholar]
- 85.Frenais R, Ouguerram K, Maugeais C, et al. High density lipoprotein apolipoprotein AI kinetics in NIDDM: a stable isotope study. Diabetologia. 1997;40:578–83. doi: 10.1007/s001250050718. [DOI] [PubMed] [Google Scholar]
- 86.Rashid S, Barrett PHR, Uffelman KD, Watanabe T, Adeli K, Lewis GF. Lipolytically modified triglyceride-enriched HDLs are rapidly cleared from the circulation. Arterioscler Thromb Vasc Biol. 2002;22:483–7. doi: 10.1161/hq0302.105374. [DOI] [PubMed] [Google Scholar]
- 87.Lamarche B, Uffelman KD, Carpentier A, et al. Triglyceride enrichment of HDL enhances in vivo metabolic clearance of HDL apo A-I in healthy men. J Clin Invest. 1999;103:1191–9. doi: 10.1172/JCI5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brinton EA, Eisenberg S, Breslow JL. Increased apo A-I and apo A-II fractional catabolic rate in patients with low high density lipoprotein-cholesterol levels with or without hypertriglyceridemia. J Clin Invest. 1991;87:536–44. doi: 10.1172/JCI115028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fidge N, Nestel P, Ishikawa T, Reardon M, Billington T. Turnover of apoproteins A-I and A-II of high density lipoprotein and the relationship to other lipoproteins in normal and hyperlipidemic individuals. Metabolism. 1980;29:643–53. doi: 10.1016/0026-0495(80)90109-2. [DOI] [PubMed] [Google Scholar]
- 90.Brinton EA, Eisenberg S, Breslow JL. Human HDL cholesterol levels are determined by apoA-I fractional catabolic rate, which correlates inversely with estimates of HDL particle size. Effects of gender, hepatic and lipoprotein lipases, triglyceride and insulin levels, and body fat distribution. Arterioscler Thromb. 1994;14:707–20. doi: 10.1161/01.atv.14.5.707. [DOI] [PubMed] [Google Scholar]
- 91.Deeb SS, Zambon A, Carr MC, Ayyobi AF, Brunzell JD. Hepatic lipase and dyslipidemia: interactions among genetic variants, obesity, gender, and diet. J Lipid Res. 2003;44:1279–86. doi: 10.1194/jlr.R200017-JLR200. [DOI] [PubMed] [Google Scholar]
- 92.Despres JP, Ferland M, Moorjani S, et al. Role of hepatic-triglyceride lipase activity in the association between intra-abdominal fat and plasma HDL cholesterol in obese women. Arterioscler Thromb Vasc Biol. 1989;9:485–92. doi: 10.1161/01.atv.9.4.485. [DOI] [PubMed] [Google Scholar]
- 93.Hime NJ, Barter PJ, Rye KA. Evidence that apolipoprotein A-I facilitates hepatic lipase-mediated phospholipid hydrolysis in reconstituted HDL containing apolipoprotein A-II. Biochemistry. 2001;8:5496–505. doi: 10.1021/bi0016671. [DOI] [PubMed] [Google Scholar]
- 94.Weng W, Brandenburg NA, Zhong S, et al. ApoA-II maintains HDL levels in part by inhibition of hepatic lipase: studies in apoA-II and hepatic lipase double knockout mice. J Lipid Res. 1999;40:1064–70. [PubMed] [Google Scholar]
- 95.Mowri HO, Patsch JR, Gotto AM, Patsch W. Apolipoprotein A-II influences the substrate properties of human HDL2 and HDL3 for hepatic lipase. Arterioscler Thromb Vasc Biol. 1996;16:755–62. doi: 10.1161/01.atv.16.6.755. [DOI] [PubMed] [Google Scholar]
- 96.Knauer TE, Woods JA, Lamb RG, Fallon HJ. Hepatic triacylglycerol lipase activities after induction of diabetes and administration of insulin or glucagon. J Lipid Res. 1982;23:631–7. [PubMed] [Google Scholar]
- 97.Zambon A, Deeb SS, Brown BG, Hokanson JE, Brunzell JD. Common hepatic lipase gene promoter variant determines clinical response to intensive lipid-lowering treatment. Circulation. 2001;103:792–8. doi: 10.1161/01.cir.103.6.792. [DOI] [PubMed] [Google Scholar]
- 98.Rader DJ. Regulation of reverse cholesterol transport and clinical implications. Am J Cardiol. 2003;92:J42–9. doi: 10.1016/s0002-9149(03)00615-5. [DOI] [PubMed] [Google Scholar]
- 99.Attie AD, Kastelein JP, Hayden MR. Pivotal role of ABCA1 in reverse cholesterol transport influencing HDL levels and susceptibility to atherosclerosis. J Lipid Res. 2001;42:1717–26. [PubMed] [Google Scholar]
- 100.Rigotti A, Miettinen HE, Krieger M. The Role of the High-Density Lipoprotein Receptor SR-BI in the Lipid Metabolism of Endocrine and Other Tissues. Endocr Rev. 2003;24:357–87. doi: 10.1210/er.2001-0037. [DOI] [PubMed] [Google Scholar]
- 101.Huuskonen J, Olkkonen VM, Jauhiainen M, Ehnholm C. The impact of phospholipid transfer protein (PLTP) on HDL metabolism. Atherosclerosis. 2001;155:269–81. doi: 10.1016/s0021-9150(01)00447-6. [DOI] [PubMed] [Google Scholar]
- 102.Forcheron F, Cachefo A, Thevenon S, Pinteur C, Beylot M. Mechanisms of the triglyceride- and cholesterol-lowering effect of fenofibrate in hyperlipidemic type 2 diabetic patients. Diabetes. 2002;51:3486–91. doi: 10.2337/diabetes.51.12.3486. [DOI] [PubMed] [Google Scholar]
- 103.Tan KC, Shiu SW, Wong Y. Plasma phospholipid transfer protein activity and small, dense LDL in type 2 diabetes mellitus. Eur J Clin Invest. 2003;33:301–6. doi: 10.1046/j.1365-2362.2003.01132.x. [DOI] [PubMed] [Google Scholar]
- 104.Rye KA, Barter PJ. Formation and metabolism of prebeta-migrating, lipid-poor apolipoprotein A-I. Arterioscler Thromb Vasc Biol. 2004 doi: 10.1161/01.ATV.0000104029.74961.f5. (in press) [DOI] [PubMed] [Google Scholar]
- 105.James AP, Watts GF, Barrett PHR, et al. Effect of weight loss on postprandial lipemia and low-density lipoprotein receptor binding in overweight men. Metabolism. 2003;52:136–41. doi: 10.1053/meta.2003.50032. [DOI] [PubMed] [Google Scholar]
- 106.Watts GF, Moroz P, Barrett PHR. Kinetics of very-low-density lipoprotein apolipoprotein B-100 in normolipidemic subjects: pooled analysis of stable-isotope studies. Metabolism. 2000;49:1204–10. doi: 10.1053/meta.2000.8621. [DOI] [PubMed] [Google Scholar]
- 107.Haffner SM American Diabetes Association. Management of dyslipidaemia in adults with diabetes. Diabetes Care. 2003;26:S83–6. doi: 10.2337/diacare.26.2007.s83. [DOI] [PubMed] [Google Scholar]
- 108.Atkins RC. Dr Atkins' new diet revolution. Rev ed. New York: Avon Books. 1998.
- 109.Schaefer EJ. Lipoproteins, nutrition, and heart disease. Am J Clin Nutr. 2002;75:191–212. doi: 10.1093/ajcn/75.2.191. [DOI] [PubMed] [Google Scholar]
- 110.Barrett PHR, Watts GF. Kinetic studies of lipoprotein metabolism in the metabolic syndrome including effects of nutritional interventions. Curr Opin Lipidol. 2003;14:61–8. doi: 10.1097/00041433-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 111.Alam S, Stolinski M, Pentecost C, et al. The effect of a 6-month exrcise programme on VLDL apolipoprotein B secretion in type 2 diabetes. J Clin Endocrinol Metab. 2004 doi: 10.1210/jc.2003-031036. (in press). [DOI] [PubMed] [Google Scholar]
- 112.Thompson PD, Yurgalevitch SM, Flynn MM, et al. Effect of prolonged exercise training without weight loss on high-density lipoprotein metabolism in overweight men. Metabolism. 1997;46:217–23. doi: 10.1016/s0026-0495(97)90305-x. [DOI] [PubMed] [Google Scholar]
- 113.Sviridov D, Kingwell B, Hoang A, Dart A, Nestel P. Single session exercise stimulates formation of pre beta 1-HDL in leg muscle. J Lipid Res. 2003;44:522–6. doi: 10.1194/jlr.M200436-JLR200. [DOI] [PubMed] [Google Scholar]
- 114.Scheen AJ. Current management strategies for coexisting diabetes mellitus and obesity. Drugs. 2003;63:1165–84. doi: 10.2165/00003495-200363120-00001. [DOI] [PubMed] [Google Scholar]
- 115.Ginsberg HN. Treatment for patients with the metabolic syndrome. Am J Cardiol. 2003;91:E29–9. doi: 10.1016/s0002-9149(02)03386-6. [DOI] [PubMed] [Google Scholar]
- 116.Goldstein JL, Brown MS. Regulation of low-density lipoprotein receptors: implications for pathogenesis and therapy of hypercholesterolemia and atherosclerosis. Circulation. 1987;76:504–7. doi: 10.1161/01.cir.76.3.504. [DOI] [PubMed] [Google Scholar]
- 117.Kempen HJ, Vermeer M, de Wit E, Havekes LM. Vastatins inhibit cholesterol ester accumulation in human monocyte-derived macrophages. Arterioscler Thromb. 1991;11:146–53. doi: 10.1161/01.atv.11.1.146. [DOI] [PubMed] [Google Scholar]
- 118.Cook GA, Khan B, Heimberg M. Feeding of lovastatin to rats increases the activity of the hepatic mitochondrial outer carnitine palmitoyltransferase. Biochem Biophys Res Commun. 1998;150:1077–82. doi: 10.1016/0006-291x(88)90739-5. [DOI] [PubMed] [Google Scholar]
- 119.Wilcox LJ, Barrett PHR, Huff MW. Differential regulation of apolipoprotein B secretion from HepG2 cells by two HMG-CoA reductase inhibitors, atorvastatin and simvastatin. J Lipid Res. 1999;40:1078–89. [PubMed] [Google Scholar]
- 120.Chan DC, Watts GF, Barrett PHR, Mori TA, Redgrave TG, Beilin LJ. Mechanism of action of an HMGCoA reductase inhibitor on apolipoprotein B-100 kinetics in visceral obesity. J Clin Endocrinol Metab. 2002;87:2283–9. doi: 10.1210/jcem.87.5.8455. [DOI] [PubMed] [Google Scholar]
- 121.Forster LF, Stewart G, Bedford D, et al. Influence of atorvastatin and simvastatin on apolipoprotein B metabolism in moderate combined hyperlipidemic subjects with low VLDL and LDL fractional clearance rates. Atherosclerosis. 2002;164:129–45. doi: 10.1016/s0021-9150(02)00052-7. [DOI] [PubMed] [Google Scholar]
- 122.Bilz S, Wagner S, Schmitz M, Bedynek A, Keller U, Demant T. Effects of atorvastatin versus fenofibrate on apolipoprotein B-100 and apolipoprotein A-I kinetics in mixed hyperlipidemia. J Lipid Res. 2004 doi: 10.1194/jlr.M300309-JLR200. (in press). [DOI] [PubMed] [Google Scholar]
- 123.Caslake MJ, Bedford D, Stewart G, et al. Rouvastatin kinetics of apoB-containing lipoproteins in moderate hypercholesterolaemia. Atherosclerosis (suppl) 2003;4:234. [Google Scholar]
- 124.Chan DC, Watts GF, Barrett PHR, et al. Effect of atorvastatin on chylomicron remnant metabolism in visceral obesity: a study employing a new stable isotope breath test. J Lipid Res. 2002;43:706–12. [PubMed] [Google Scholar]
- 125.Chan DC, Watts GF, Mori TA, Barrett PHR, Beilin LJ, Redgrave TG. Factorial study of the effects of atorvastatin and fish oil on dyslipidemia in visceral obesity. Eur J Clin Invest. 2002;32:429–36. doi: 10.1046/j.1365-2362.2002.01001.x. [DOI] [PubMed] [Google Scholar]
- 126.Watts GF, Chan DC, Barrett PHR, O'Neill FH, Thompson GR. Effect of a statin on hepatic apolipoprotein B-100 secretion and plasma campesterol levels in the metabolic syndrome. Int J Obes. 2003;27:862–5. doi: 10.1038/sj.ijo.0802287. [DOI] [PubMed] [Google Scholar]
- 127.Asztalos BF, Horvath KV, McNamara JR, Roheim PS, Rubinstein JJ, Schaefer EJ. Comparing the effects of five different statins on the HDL subpopulation profiles of coronary heart disease patients. Atherosclerosis. 2002;164:361–9. doi: 10.1016/s0021-9150(02)00149-1. [DOI] [PubMed] [Google Scholar]
- 128.Martin G, Duez H, Blanquart C, et al. Statin-induced inhibition of the Rho-signaling pathway activates PPAR and induces HDL apoA-I. J Clin Invest. 2001;107:1423–32. doi: 10.1172/JCI10852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jones P, Kafonek S, Laurora I, Hunninghake D. Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hypercholesterolemia (The CURVES study) Am J Cardiol. 1998;81:582–7. doi: 10.1016/s0002-9149(97)00965-x. [DOI] [PubMed] [Google Scholar]
- 130.Wierzbicki AS, Mikhailidis DP. Dose-response effects of atorvastatin and simvastatin on high-density lipoprotein cholesterol in hypercholesterolaemic patients: A review of five comparative studies. Int J Cardiol. 2002;84:53–7. doi: 10.1016/s0167-5273(02)00118-3. [DOI] [PubMed] [Google Scholar]
- 131.Rashid S, Uffelman KD, Barrett PHR, Lewis GF. Effect of Atorvastatin on High-Density Lipoprotein Apolipoprotein A-I Production and Clearance in the New Zealand White Rabbit. Circulation. 2002;106:2955–60. doi: 10.1161/01.cir.0000038303.84249.4a. [DOI] [PubMed] [Google Scholar]
- 132.Berge J, Moller DE. The mechanisms of action of PPARs. Ann Rev Med. 2002;53:409–35. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 133.Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998;98:2088–93. doi: 10.1161/01.cir.98.19.2088. [DOI] [PubMed] [Google Scholar]
- 134.Duez H, Chao YS, Hernandez M, et al. Reduction of atherosclerosis by the peroxisome proliferator-activated receptor alpha agonist fenofibrate in mice. J Biol Chem. 2002;277:48051–7. doi: 10.1074/jbc.M206966200. [DOI] [PubMed] [Google Scholar]
- 135.Martin G, Poirier H, Hennuyer N, et al. Induction of the fatty acid transport protein 1 and acyl-CoA synthase genes by dimer-selective rexinoids suggests that the peroxisome proliferator-activated receptor-retinoid X receptor heterodimer is their molecular target. J Biol Chem. 2000;275:12612–8. doi: 10.1074/jbc.275.17.12612. [DOI] [PubMed] [Google Scholar]
- 136.Vu-Dac N, Chopin-Delannoy S, Gervois P, et al. The nuclear receptors peroxisome proliferator-activated receptor alpha and Rev-erbalpha mediate the species-specific regulation of apolipoprotein A-I expression by fibrates. J Biol Chem. 1998;273:25713–20. doi: 10.1074/jbc.273.40.25713. [DOI] [PubMed] [Google Scholar]
- 137.Keller H, Dreyer C, Medin J, Mahfoudi A, Ozato K, Wahli W. Fatty acids and retinoids control lipid metabolism through activation of peroxisome proliferator-activated receptor-retinoid X receptor heterodimers. Proc Natl Acad Sci USA. 1993;90:2160–4. doi: 10.1073/pnas.90.6.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schoonjans K, Peinado-Onsurbe J, Lefebvre AM, et al. PPARalpha and PPARgamma activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J. 1996;15:5336–48. [PMC free article] [PubMed] [Google Scholar]
- 139.Caslake MJ, Packard CJ, Gaw A, et al. Fenofibrate and LDL metabolic heterogeneity in hypercholesterolemia. Arterioscle Thromb. 1993;13:702–11. doi: 10.1161/01.atv.13.5.702. [DOI] [PubMed] [Google Scholar]
- 140.Saku K, Gartside PS, Hynd BA, Kashyap ML. Mechanism of action of gemfibrozil on lipoprotein metabolism. J Clin Invest. 1985;75:1702–12. doi: 10.1172/JCI111879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Martin G, Poirier H, Hennuyer N, et al. Induction of the fatty acid transport protein 1 and acyl-CoA synthase genes by dimer-selective rexinoids suggests that the peroxisome proliferator-activated receptor-retinoid X receptor heterodimer is their molecular target. J Biol Chem. 2000;275:12612–8. doi: 10.1074/jbc.275.17.12612. [DOI] [PubMed] [Google Scholar]
- 142.Zhang Y, Repa JJ, Gauthier K, Mangelsdorf DJ. Regulation of lipoprotein lipase by the oxysterol receptors, LXRalpha and LXRbeta. J Biol Chem. 2001;276:43018–24. doi: 10.1074/jbc.M107823200. [DOI] [PubMed] [Google Scholar]
- 143.Clavey V, Copin C, Mariotte MC, et al. Cell culture conditions determine apolipoprotein CIII secretion and regulation by fibrates in human hepatoma HepG2 cells. Cellular Physiol Biochem. 1999;9:139–49. doi: 10.1159/000016311. [DOI] [PubMed] [Google Scholar]
- 144.Vu-Dac N, Schoonjans K, Kosykh V, et al. Fibrates increase human apolipoprotein A-II expression through activation of the peroxisome proliferator-activated receptor. J Clin Invest. 1995;96:741–50. doi: 10.1172/JCI118118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Repa JJ, Liang G, Ou J, et al. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000;14:2819–30. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schneider A, Stange EF, Ditschuneit HH, Ditschuneit H. Fenofibrate treatment inhibits HMG-CoA reductase activity in mononuclear cells from hyperlipoproteinemic patients. Atherosclerosis. 1985;56:257–62. doi: 10.1016/0021-9150(85)90001-2. [DOI] [PubMed] [Google Scholar]
- 147.Nissen SE, Tsunoda T, Tuzcu EM, et al. Effect of recombinant apoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290:2292–300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 148.Wang M, Tafuri S. Modulation of PPARgamma activity with pharmaceutical agents: treatment of insulin resistance and atherosclerosis. J Cell Biochem. 2003;89:38–47. doi: 10.1002/jcb.10492. [DOI] [PubMed] [Google Scholar]
- 149.Chan DC, Watts GF, Mori TA, Barrett PHR, Redgrave TG, Beilin LJ. Randomized controlled trial of the effect of n-3 fatty acids supplementation on apolipoprotein B-100 and chylomicron remnant metabolism in visceral obesity. Am J Clin Nutr. 2003;77:300–9. doi: 10.1093/ajcn/77.2.300. [DOI] [PubMed] [Google Scholar]
- 150.Frenais R, Ouguerram K, Maugeais C, et al. Effect of dietary omega-3 fatty acids on high-density lipoprotein apolipoprotein AI kinetics in type II diabetes mellitus. Atherosclerosis. 2001;157:131–5. doi: 10.1016/s0021-9150(00)00723-1. [DOI] [PubMed] [Google Scholar]
- 151.Dunstan DW, Mori TA, Puddey IB, et al. The independent and combined effects of aerobic exercise and dietary fish intake or serum lipids and glycemic control in NIDDM. A randomized controlled study. Diabetes Care. 1997;20:913–21. doi: 10.2337/diacare.20.6.913. [DOI] [PubMed] [Google Scholar]
- 152.Harris WS, Muzio F. Fish oil reduces postprandial triglyceride concentrations without accelerating lipid-emulsion removal rates. Am J Clin Nutr. 1993;58:68–74. doi: 10.1093/ajcn/58.1.68. [DOI] [PubMed] [Google Scholar]
- 153.Westphal S, Orth M, Ambrosch A, Osmundsen K, Luley C. Postprandial chylomicrons and VLDLs in severe hypertriacylglycerolemia are lowered more effectively than are chylomicron remnants after treatment with n-3 fatty acids. Am J Clin Nutr. 2000;71:914–20. doi: 10.1093/ajcn/71.4.914. [DOI] [PubMed] [Google Scholar]
- 154.Clark SD. Polyunsaturated fatty acid regulation of gene transcription: a molecular mechanism to improve the metabolic syndrome. J Nutr. 2001;131:1129–32. doi: 10.1093/jn/131.4.1129. [DOI] [PubMed] [Google Scholar]
- 155.Baillie RA, Takada R, Nakamura M, Clarke SD. Coordinate induction of peroxisomal acyl-CoA oxidase and UCP-3 by dietary fish oil: a mechanism for decreased body fat deposition. Prostaglandins Leukotrienes. Essent Fatty Acids. 1999;60:351–6. doi: 10.1016/s0952-3278(99)80011-8. [DOI] [PubMed] [Google Scholar]
- 156.Jump DB. The biochemistry of n-3 polyunsaturated fatty acids. J Biol Chem. 2002;277:8755–8. doi: 10.1074/jbc.R100062200. [DOI] [PubMed] [Google Scholar]
- 157.Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci USA. 1997;94:4312–7. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Xu J, Nakamura MT, Cho HP, Clarke SD. Sterol regulatory element binding protein-1 expression is suppressed by dietary polyunsaturated fatty acids. A mechanism for the coordinate suppression of lipogenic genes by polyunsaturated fats. J Biol Chem. 1999;274:23577–83. doi: 10.1074/jbc.274.33.23577. [DOI] [PubMed] [Google Scholar]
- 159.Price PT, Nelson CM, Clarke SD. Omega-3 polyunsaturated fatty acid regulation of gene expression. Curr Opin Lipidol. 2000;11:3–7. doi: 10.1097/00041433-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 160.Mori TA, Burke V, Puddey IB, et al. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am J Clin Nutr. 2000;71:1085–94. doi: 10.1093/ajcn/71.5.1085. [DOI] [PubMed] [Google Scholar]
- 161.Jacobson TA. Combination lipid-altering therapy: an emerging treatment paradigm for 21st century. Curr Atheroscler Rep. 2001;3:373–82. doi: 10.1007/s11883-001-0075-y. [DOI] [PubMed] [Google Scholar]
- 162.Athyros VG, Papageorgiou AA, Hatzikonstandinou HA, et al. Safety and efficacy of long-term statin-fibrate combinations in patients with refractory familial combined hyperlipidemia. Am J Cardiol. 1997;80:608–13. doi: 10.1016/s0002-9149(97)00430-x. [DOI] [PubMed] [Google Scholar]
- 163.Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. New Engl J Med. 2001;345:1583–92. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 164.Bhatnagar D, Mackness MI, Durrinton PN. Treatment of mixed hyperlipidaemia using a combination of omega-3 fatty acids and HMG CoA reductase inhibitor. Eur Heart J. 2001;3:D53–8. [Google Scholar]
- 165.Bays HE, Dujovne CA, McGovern ME, et al. ADvicor Versus Other Cholesterol-Modulating Agents Trial Evaluation. Comparison of once-daily, niacin extended-release/lovastatin with standard doses of atorvastatin and simvastatin (the ADvicor Versus Other Cholesterol-Modulating Agents Trial Evaluation [ADVOCATE]) Am J Cardiol. 2003;91:667–72. doi: 10.1016/s0002-9149(03)00007-9. [DOI] [PubMed] [Google Scholar]