Abstract
Condition-specific competition, wherein competitive superiority varies with the abiotic environment, can determine species’ distributions in a spatially heterogeneous environment. We investigated this phenomenon with two competing container-dwelling mosquitoes. We tested the hypothesis that habitat drying alters the outcome of interspecific competition, predicting that the competitive impact of Aedes albopictus on Aedes aegypti would be severe in wetter environments, but greatly reduced in drier environments. We tested these predictions in a laboratory experiment within cages, with aquatic larvae residing in water-filled cups within the cage, and adults emerging within the cage and ovipositing on the cups. We raised each species alone or with the competitor. Environmental treatments were fluctuating (water in cups evaporated to 50% of the original volume and then cups were refilled), and drying (water in cups evaporated completely and cups remained dry for two weeks before refilling). There was a significant interaction between treatment and species combination for adult populations of both species. Interspecific competition was highly asymmetrical. In the wetter fluctuating environment, interspecific competition had a large negative effect on A. aegypti, but in the drying environment, interspecific competition had a large negative effect on A. albopictus, and relatively little impact on A. aegypti. The main cause of the shift in competitive advantage appeared to be a greater increase in egg mortality for A. albopictus under dry conditions, compared to A. aegypti. Thus, mortality impinging on noncompeting life cycle stages can alter the population level impact of interspecific competition. The hypothesis that dry conditions shift competitive advantage away from A. albopictus is supported in this experiment.
Keywords: abiotic factors, Aedes aegypti, Aedes albopictus, environmental tolerances, invasive species
Introduction
Understanding mechanisms governing species distributions is an important goal of ecology. Theoretical and empirical work indicates that with one limiting factor in a constant environment, interspecific competition should result in competitive exclusion (Tilman 1982, Chase and Leibold 2003). However, competitors can escape local extinction via a number of mechanisms, including differential resource use (e.g., Tilman 1982), trade-offs between competitive ability and susceptibility to enemies (Chase and Leibold 2003), or between competitive ability and environmental tolerances (Chesson 1986, 2000, Dunson and Travis 1991, Hemphill 1991, Chesson and Huntly 1997). Although tolerances of the abiotic environment can shape species distributions across varying environments, it is the role of the abiotic environment in modifying biotic interactions such as competition that can have the most important effects on species distributions and co-occurrence. Condition-specific competition, wherein competitive superiority is altered or reversed in different abiotic environments, can foster coexistence between competing species when there is temporal or spatial variation in abiotic conditions (Tilman 1982, Chesson 2000, Taniguchi and Nakano 2000). Abiotic factors and physiological responses to those factors can be primary determinants of the community composition when interspecific differences in physiology are functionally linked to condition-specific competition (Dunson and Travis 1991). Empirical studies documenting condition-specific competition (e.g., Persson 1986, Hemphill 1991, Warner et al. 1993, Barata et al. 1996, Taniguchi and Nakano 2000, Holway et al. 2002) suggest that this phenomenon may be widespread in natural communities.
The invasion of North America by Aedes albopictus (Asian tiger mosquito; see Plate 1) and its interactions with resident species provide an ideal opportunity for investigations of condition-specific competition. Aedes albopictus was introduced in the 1980s through tire shipments from Asia (Hawley 1988). A closely related, ecologically similar species, Aedes aegypti (yellow fever mosquito), first invaded the New World from Africa in the 15th–16th centuries and was well established and common in South and Central America and the southern United States by the time A. albopictus arrived (Tabachnick 1991). Aquatic larvae and pupae of both species occur in man-made and natural water-filled containers such as tires, vases, and tree holes. Larvae feed on microorganisms in the water column and on surfaces (Christophers 1960, Hawley 1988).
In the southeastern United States (Hobbs et al. 1991) and in Florida in particular (Hornby et al. 1994, O’Meara et al. 1995), there was a rapid decline in the abundance, sometimes to local extinction, of A. aegypti following the invasion of A. albopictus. Field (Juliano 1998, Braks et al. 2004) and laboratory (Barrera 1996, Daugherty et al. 2000) competition experiments with larvae indicate that A. albopictus is superior to A. aegypti in resource competition when leaf detritus is the nutrient base, maintaining greater population growth at high combined densities (Juliano 1998, Daugherty et al. 2000, Braks et al. 2004), and producing greater survivorship at lower food availability (Barrera 1996). Field manipulations indicate that interspecific competition is common, and indeed, strong, in artificial containers in Florida (Juliano et al. 2004).
Despite the competitive superiority of A. albopictus, A. aegypti persists in urban and southern regions of the Florida peninsula (Hornby et al. 1994, O’Meara et al. 1995). Competition experiments at sites of coexistence or exclusion of these species do not indicate any site-specific differences in competitive interaction among larvae (Juliano et al. 2004), which suggests that non-aquatic life cycle stages (eggs, adults) may play important roles in determining variation in population level competitive outcomes. In Florida, the distribution of A. aegypti populations is associated with a hot, seasonally dry climate, and frequency of containers occupied by A. albopictus is negatively associated with these conditions (Juliano et al. 2002). Further, the percentage of cemetery vases occupied by A. albopictus is lower following a dry period than at the peak of the wet season; in contrast, the proportion of vases occupied by A. aegypti is independent of season (Juliano et al. 2002). Both species oviposit on the sides of containers, and their desiccation-tolerant eggs can endure dry periods (Christophers 1960, Hawley 1988). Eggs of A. aegypti are more resistant to death by desiccation under controlled laboratory conditions than are eggs of A. albopictus (Sota and Mogi 1992, Juliano et al. 2002).
The geographic pattern of persistent A. aegypti suggests that temperature, humidity, and seasonal drought may modify the outcome of interspecific competition. Periods of low precipitation cause water levels in containers to fluctuate, and may cause containers to dry out completely (Lounibos 1985, Bradshaw and Holzapfel 1988). If a container dries out completely, mosquito populations are directly affected by increased egg (Sota and Mogi 1992) and larval (Bradshaw and Holzapfel 1988) mortality due to desiccation. This suggests that mortality of a noncompeting stage (eggs) due to abiotic conditions could affect the population level outcome of competition among larvae (Juliano et al. 2002). Falling water levels also affect development time (Juliano and Stoffregen 1994) and resource quality (Aspbury and Juliano 1998).
In this paper we test experimentally whether habitat drying produces a condition-specific advantage in interspecific competition between these species. Such a condition-specific advantage, together with persistent, environmentally resistant eggs, could be sufficient for coexistence of these species in the seasonal climate of south Florida despite the disadvantage of A. aegypti larvae in resource competition (Barrera 1996, Juliano 1998, Braks et al. 2004). Specifically, we tested the hypothesis that habitat drying shifts the competitive outcome between A. albopictus and A. aegypti. From this hypothesis we derived the following predictions: (1) Aedes albopictus will have a greater competitive impact on A. aegypti in a wetter environment; and (2) Aedes aegypti will have a greater competitive impact on A. albopictus in a drier environment. We tested these predictions in a multigeneration laboratory competition experiment.
Materials and Methods
Competing populations were housed throughout their entire life cycle in 20-L nylon screen cages with a sleeve for access. Our experimental procedures were similar to those used successfully to test the effects of temperature and pattern of water input to containers on populations of A. albopictus (Alto and Juliano 2001a, b).
Aedes used in the experiment were offspring of field-collected individuals from cemetery vases in Florida. The experiment began with cohorts of larvae in three combinations (A. aegypti: A. albopictus): 60:0; 60:60; 0:60. Each cage contained three 175-mL cups, each filled with 160 mL of deionized (DI) water, providing suitable habitat for larvae and pupae and for oviposition by adults. Each cup held one-third of the initial cohort of larvae. Senescent live oak (Quercus virginiana) leaves and foxtail grass (Setaria faberi) leaves and stems (1.0 and 0.3 g dry mass, respectively, per cup) provided detritus substrates for microorganisms that are food for larvae.
Three days prior to addition of larvae we added detritus to the cups along with 1 mL of field-collected water from water-filled tires to provide microbial inoculum. Larvae were synchronously hatched (Novak and Shroyer 1978) and added to cups as ~24-h-old first-instar larvae. Cages were randomly assigned one of two simulated precipitation regimes: fluctuating, wherein water in the cups evaporated to 50% of the initial volume, and cups were then refilled with DI water; and drying, wherein water in cups evaporated completely and cups remained dry for two weeks before being refilled with DI water.
There were a total of six treatment and species combinations; due to a shortage of A. albopictus eggs there were only five replicates of all combinations with A. albopictus and eight replicates of combinations with A. aegypti alone, yielding 36 cages (experimental units). The experiment was executed in three identical environmental chambers (Percival I-35VL, Percival Scientific, Perry, Iowa, USA) with a 14:10 L:D photo-period, and a 30°C:25°C thermocycle (comparable to field conditions in Florida). Positions of cages within each chamber were systematically rotated each day to overcome any heterogeneity in temperature, relative humidity, or air-flow within the chambers. The experiment lasted 120 d, providing ample time for production of 4–6 generations of mosquitoes. Adults had continuous access to a 10% sugar solution and were offered weekly blood meals from anesthetized mice. To maintain resource levels, we added 0.3 g and 0.1 g dried live oak leaves and foxtail grass leaves and stems, respectively, on days 30, 60, and 90.
For both species, oviposition usually takes place above the water line on the container wall, and hatching is induced when the container is filled and the eggs are immersed (Christophers 1960, Hawley 1988). Although larvae cannot survive complete drying, eggs are desiccation resistant. Eggs on the cup walls in the fluctuating treatment remained within millimeters of water and endured relatively short periods before being immersed. In contrast, eggs in the drying treatment were exposed to totally dry conditions and endured relatively long periods of time in dry containers before being immersed. We expected drying to affect primarily the egg stage, resulting in high egg mortality that would affect the competitive outcome between these two species. Because A. albopictus eggs are more susceptible to death by desiccation (Juliano et al. 2002), we expected that during the 2-wk dry period there would be greater desiccation-induced mortality of A. albopictus eggs than of A. aegypti eggs. We predicted this effect would reduce population growth of A. albopictus, and reduce or eliminate its competitive impact on A. aegypti. In contrast, in the fluctuating treatment, we expected egg mortality due to desiccation to be low for both species as eggs were always in relatively humid conditions.
Data collection
At the end of the experiment, remaining adults were killed, counted, and identified, as were living larvae and pupae. We determined if there were any apparent extinctions, defined as the absence of adults, larvae, and pupae of a particular species in a given cage at the end of the experiment. Although it is possible to identify eggs of these two species via scanning electron microscopy (Linley 1989), the large number of eggs in cups made this counting and identification impractical. To determine the effects of the drying treatments on mortality of eggs, we subjected the eggs from each cup in the single-species cages to a hatching stimulus, and then counted the number of larvae produced. All the eggs on the cup were then bleached (Trpis 1970) and examined microscopically to determine the number of infertile, hatched, and dead eggs, and to calculate egg mortality.
Analyses
At the end of the experiment, we determined the number of adults of each species (adult population), and number of larvae and pupae of each species found in all the cups of a given cage (larval population). We only included larval counts from cups that were not completely dry at the time the experiment ended. All data were transformed, if necessary, to meet the assumptions of normality and homogeneity of variances. For all analyses we used pairwise comparisons with Bonferroni correction and experimentwise α = 0.05. We used univariate ANOVA (SAS Institute 1989) to test for effects of simulated precipitation (fluctuating, drying), species combination (alone, with competitor), and interaction of simulated precipitation and competition, with environmental chamber as a block variable.
For single-species cages, we calculated proportion of egg mortality for each cage by dividing the total number of dead eggs in all cups within a cage by the sum of dead and hatched eggs for all cups within a cage (Juliano et al. 2002). We analyzed this variable by ANOVA to detect treatment, species, or interaction effects.
Results
Adults
For A. aegypti, there were significant effects of species combination and interaction but no main effect of treatment (Table 1). Within the fluctuating treatment, A. aegypti’s mean adult populations were significantly lower in the presence of A. albopictus than in the absence of A. albopictus. However, in the drying treatment, there were no differences in the number of A. aegypti adults in cages with or without A. albopictus (Fig. 1A).
Table 1.
Two-way ANOVA results for mean adult and larval populations of Aedes aegypti and A. albopictus.
| Aedes aegypti
|
Aedes albopictus
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Adults
|
Larvae
|
Adults
|
Larvae
|
|||||
| Source | F1,20 | P | F1,20 | P | F1,14 | P | F1,14 | P |
| Treatment | 1.91 | 0.1822 | 0.83 | 0.3732 | 21.25 | 0.0004 | 7.12 | 0.0184 |
| Species combination | 5.01 | 0.0367 | 7.56 | 0.0124 | 18.63 | 0.0007 | 14.19 | 0.0021 |
| Treatment × Species combination | 5.44 | 0.0302 | 4.19 | 0.0541 | 6.57 | 0.0225 | 0.24 | 0.6349 |
Fig. 1.
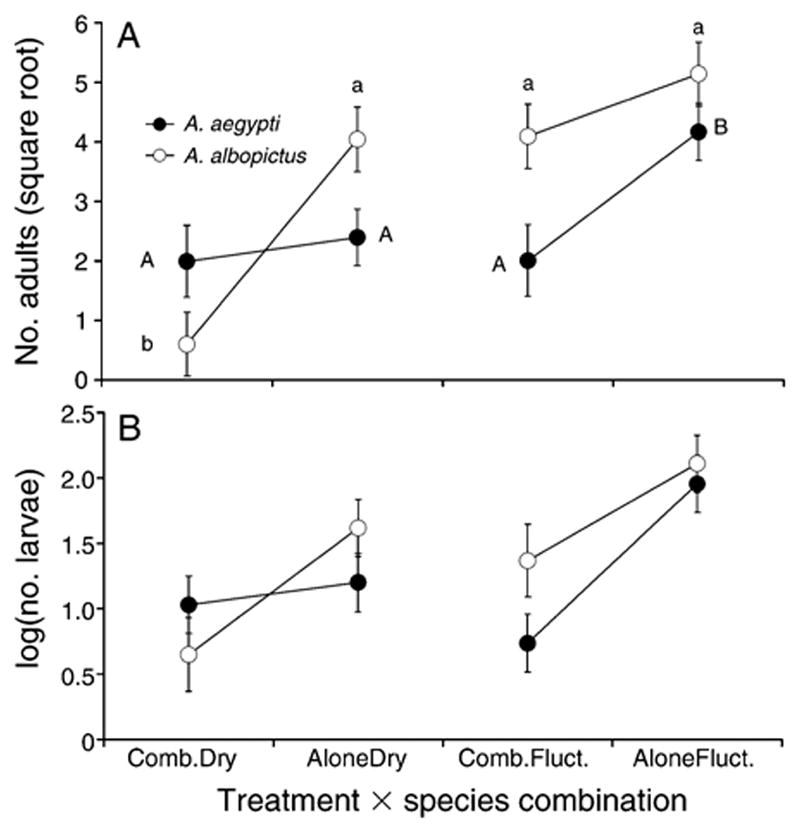
(A) Number of adults (mean ± SE) of Aedes aegypti and A. albopictus across species × treatment combinations. (B) Log-transformed number of larvae (mean ± SE) of A. aegypti and A. albopictus across species × treatment combinations. Combination cages where both species were present are indicated by “Comb.,” and cages where species were alone are indicated with “Alone.” “Dry” indicates drying treatments and “Fluct.” indicates fluctuating treatments. Significant differences (P < 0.05) in pairwise comparisons are indicated by uppercase letters for A. aegypti and lowercase letters for A. albopictus.
For A. albopictus, treatment, species combination, and interaction were all significant (Table 1). Within the drying treatment, A. albopictus mean adult populations were significantly lower in the presence of A. aegypti than in the absence of A. aegypti (Fig. 1A). In the fluctuating treatment, there was no significant difference in the number of A. albopictus adults in cages with or without A. aegypti (Fig. 1A). The numbers of adult A. albopictus were similar in the fluctuating and drying treatment in the absence of A. aegypti. It was only in the drying treatment with A. aegypti that production of A. albopictus adults was lower (Fig. 1A).
Larvae
There was a significant effect of species combination, no significant effect of treatment, and a marginally significant interaction of treatment and species combination for A. aegypti larvae (Table 1). The addition of A. albopictus in both the fluctuating and drying treatments yielded a significantly lower number of A. aegypti larvae at the end of the experiment (Fig. 1B).
For A. albopictus larvae, there were significant effects of treatment and species combination, but no significant interaction (Table 1). Pairwise comparisons indicated A. albopictus larvae were significantly less abundant in drying cages than in fluctuating cages. At the end of the experiment there were significantly fewer A. albopictus larvae in cages with A. aegypti than in cages without A. aegypti. The addition of A. aegypti in the drying treatment resulted in a lower number of A. albopictus larvae, which was not the case in the fluctuating treatments (Fig. 1B).
Egg mortality
There were significant effects of treatment (F1,18 = 25.26, P < 0.0001), species (F1,18 = 12.23, P = 0.0026), and interaction (F1,18 = 5.86, P = 0.0263) on egg mortality. Proportion of eggs dying was significantly greater for A. albopictus in the drying treatment than for A. albopictus in the fluctuating treatment or for A. aegypti in the drying treatment (Fig. 2). Egg mortality was not different between treatments for A. aegypti (Fig. 2).
Fig. 2.
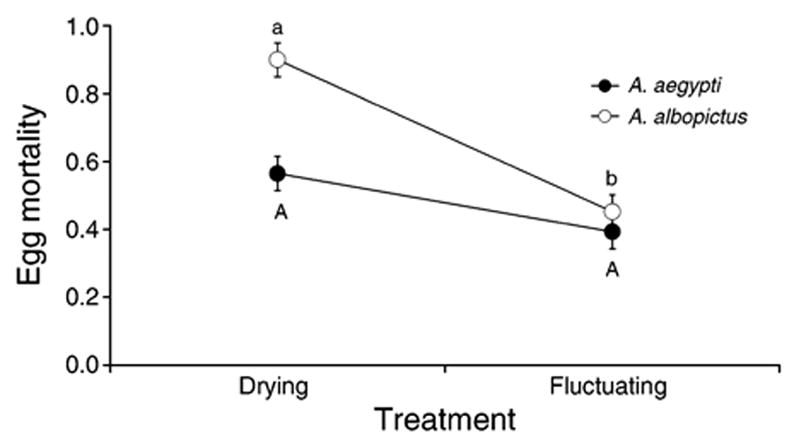
Proportion of dead eggs (mean ± SE) for Aedes albopictus and A. aegypti across treatments. Significant differences in pairwise comparisons are indicated by uppercase letters for A. aegypti and lowercase letters for A. albopictus.
Apparent extinctions
Across both treatments, there were no apparent extinctions in single-species cages. In the fluctuating treatment, two of five two-species cages had no surviving A. aegypti larvae or adults at the end of the experiment. In the drying treatment, two of five two-species cages had no surviving A. albopictus larvae or adults at the end of the experiment.
Discussion
Our results indicate that competition between A. albopictus and A. aegypti is condition dependent with respect to drying. This study illustrates the interaction of the abiotic environment and interspecific competition, and shows how abiotic effects on noncompeting stages can alter the population level outcome of competition. Although the drying environment resulted in lower adult populations for both species when compared to the fluctuating environment, drying of containers was more detrimental to A. albopictus and appeared to result in a reduction or reversal of the competitive advantage of this species over A. aegypti. Dry conditions have multiple detrimental effects on container-dwelling mosquitoes (Bradshaw and Holzapfel 1988, Sota and Mogi 1992, Juliano and Stoffregen 1994, Aspbury and Juliano 1998), but this is the first study to demonstrate that these detrimental effects interact with interspecific competition in ways that could affect competitive outcomes.
Our predictions regarding competitive impacts were supported. Interspecific competition most strongly affected A. albopictus in the drying environment and A. aegypti in the fluctuating environment, and this effect is clearest for the adult stage. This difference in the success of the two species in the two environments was probably a result of effects of drying on eggs. Egg mortality for A. albopictus was significantly greater in the drying environment. The difference in inter-flooding intervals is expected to accentuate interspecific differences in desiccation-induced mortality of eggs. This interspecific difference in egg mortality is consistent with previous laboratory observations indicating that under controlled temperature and humidity conditions, A. albopictus eggs suffer greater mortality than do A. aegypti eggs (Juliano et al. 2002).
In our study, drier conditions cause differential egg mortality, but are unlikely to directly alter the effects of interspecific competition on larvae. Simple models of competition for a single resource show that R*, the level of resources (in this case, for larvae) necessary to produce zero net population growth determines competitive advantage, and that ability to harvest resources and physiological efficiency affect R* (Tilman 1982, Grover 1997). Ability to harvest resources and physiological efficiency of larvae are unlikely to be altered by drying. However, these simple models also show that R* increases as mortality rate increases (Tilman 1982, Grover 1997), and this effect occurs at the population level, so that mortality of noncompeting stages (in this case eggs) also determines R*. For A. albopictus to maintain a stable population in the dry environment, the greater egg mortality must be balanced by greater production of adults, which raises the value of R*. If this increase is sufficient, it can reverse the outcome of competition, even though the interaction among larvae considered in isolation is unchanged. Thus, in our system, the differential impact of drying on egg mortality of the two species appears to have been sufficiently large to raise A. albopictus’ R* in the drying treatment above that for A. aegypti, and thus to shift the population level competitive advantage in dry environment.
The apparent extinctions in our experiment emphasize the importance of the interaction of the abiotic and biotic processes. Apparent extinctions occurred only in cages with interspecific competition, never in single-species cages. Extinctions of adult and larval stages occurred only in drying cages for A. albopictus, and only in the fluctuating cages for A. aegypti. Even if these species persisted as unsampled egg populations, these results strongly suggest that the two simulated precipitation regimes were sufficiently different to shift competitive exclusion from one species to the other.
Distributions of these species in Florida are strongly related to mean temperature, seasonality of precipitation, and total precipitation (Juliano et al. 2002). Because A. aegypti’s eggs are less susceptible to death by desiccation than are those of A. albopictus, Juliano et al. (2002) hypothesized that persistence of A. aegypti in seasonally dry regions resulted from its ability to withstand dry seasons as dormant eggs. In the northern Florida peninsula and panhandle there is considerable winter rain and no long periods of drought (Fernald and Patton 1985). Containers in these regions in Florida are occupied almost exclusively by A. albopictus (O’Meara et al. 1995); in many locations A. aegypti was formerly present but is now locally extinct. In contrast, in the southern part of the peninsula, where A. aegypti persists and coexists with A. albopictus (Hornby et al. 1994, O’Meara et al. 1995), the climate is subtropical, with high seasonality in precipitation, leaving many dry winter months (Bradshaw and Holzapfel 1984, Fernald and Patton 1985). The results of our laboratory experiments are entirely consistent with Juliano et al.’s (2002) hypothesis. Condition-specific competition between these species in response to seasonal drought has the potential to provide a refuge for A. aegypti and to shift the competitive outcome. To evaluate whether seasonal drought and associated container drying and egg mortality at any location are sufficient to produce competitive reversal, we need to know how frequently containers are dry for periods similar to the 14 days used in our experiment.
Temporal and spatial environmental variation may disrupt competitive equilibrium, prevent a consistent outcome of competition, and may result in a nonequilibrium community higher in richness than a community at competitive equilibrium (Wiens 1977, Huston 1979). Recent theoretical work (e.g., Chesson 1986, 2000, Chesson and Huntly 1997) shows the insufficiency of simple environmental harshness, and associated population reductions, as a mechanism yielding coexistence, and emphasizes the importance of differential responses of competitors to environmental variation for coexistence (Chesson and Huntly 1997, Chesson 2000). Thus, condition-specific competition may be one of the critical features necessary for coexistence of competitors in a variable environment. Only when there is environmental fluctuation, such that each species has an advantage during a different period, along with persistent stages (i.e., adults, eggs) that carry the population through unfavorable periods, is coexistence possible (Cáceres 1997, Chesson and Huntly 1997, Chesson 2000). For these container mosquitoes, which have desiccation resistant eggs and condition-specific competitive outcomes, seasonal drought is a potential mechanism of coexistence that merits field investigation. Along with earlier work on this system (Juliano 1998, Lounibos et al. 2001, 2002, Juliano et al. 2002, 2004), this study shows how biotic and abiotic effects combine to influence community composition.
Plate 1.
Nonnative, container-dwelling mosquitoes of the southeastern United States. (Left) Fourth-instar larvae of Aedes albopictus (on right) and Aedes aegypti. These species co-occur in man made containers in South Florida, and interspecific competition among larvae can be intense. (Right) An adult female Aedes albopictus taking a blood meal. Photo credits: (left) S. A. Juliano, (right) B. Kesavaraju.
Acknowledgments
We thank K. Damal, K. Kotas, C. Lackey, M. H. Lee, K. Mormann, D. Ramoutar, S. L. Rokosik, S. Rowe, D. K. Tomevi, and D. A. Yee for aid in the laboratory, and V. A. Borowicz, L. P. Lounibos, C. Cáceres, R. C. Anderson, W. L. Perry, K. Damal, D. A. Yee, A. Ives, and two anonymous referees for useful comments on the manuscript. We were supported by grants from the National Institute of Allergy and Infectious Disease (R01 AI-44793) and Illinois State University to S. A. Juliano, and by a Mockford Summer Fellowship (Phi Sigma Biological Honor Society, Illinois State University) to K. S. Costanzo.
Literature Cited
- Alto BW, Juliano SA. Precipitation and temperature effects on populations of Aedes albopictus (Diptera: Culicidae): implications for range expansion. Journal of Medical Entomology. 2001a;38:646–656. doi: 10.1603/0022-2585-38.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto BW, Juliano SA. Temperature and population dynamics of Aedes albopictus (Diptera: Culicidae) Journal of Medical Entomology. 2001b;38:548–556. doi: 10.1603/0022-2585-38.4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspbury AS, Juliano SA. Negative effects of habitat drying and prior exploitation on the detritus resource in an ephemeral aquatic habitat. Oecologia. 1998;115:137–148. doi: 10.1007/s004420050500. [DOI] [PubMed] [Google Scholar]
- Barata C, Hontoria F, Amat F, Browne R. Competition between sexual and parthenogenetic Artemia: temperature and strain effects. Journal of Experimental Marine Biology and Ecology. 1996;196:313–328. [Google Scholar]
- Barrera R. Competition and resistance to starvation in larvae of container-inhabiting Aedes mosquitoes. Ecological Entomology. 1996;21:117–127. [Google Scholar]
- Bradshaw WE, Holzapfel CM. Seasonal development of tree-hole mosquitoes (Diptera:Culicidae) and Chaoborids in relation to weather and predation. Journal of Medical Entomology. 1984;21:366–378. [Google Scholar]
- Bradshaw WE, Holzapfel CM. Drought and the organization of tree-hole mosquito communities. Oecologia. 1988;74:507–514. doi: 10.1007/BF00380047. [DOI] [PubMed] [Google Scholar]
- Braks MAH, Honório NA, Lounibos LP, Lourençode-Oliveira R, Juliano SA. Interspecific competition between two invasive species of container mosquitoes in Brazil. Annals of the Entomological Society of America. 2004;97:130–139. [Google Scholar]
- Cáceres C. Temporal variation, dormancy, and coexistence: a field test of the storage effect. Proceedings of the National Academy of Sciences (USA) 1997;94:9171–9175. doi: 10.1073/pnas.94.17.9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase JM, Leibold MA. Ecological niches: linking classical and contemporary approaches. University of Chicago Press; Chicago, Illinois, USA: 2003. [Google Scholar]
- Chesson PL. Environmental variation and the coexistence of species. In: Diamond J, Case TJ, editors. Community ecology. Harper and Row; New York, New York, USA: 1986. pp. 240–256. [Google Scholar]
- Chesson P. Mechanisms of maintenance of species diversity. Annual Review of Ecology and Systematics. 2000;31:343–366. [Google Scholar]
- Chesson P, Huntly N. The roles of harsh and fluctuating conditions in the dynamics of ecological communities. American Naturalist. 1997;150:519–553. doi: 10.1086/286080. [DOI] [PubMed] [Google Scholar]
- Christophers R. Cambridge University Press; London, UK: 1960 . Aëdes aegypti (L.) the yellow fever mosquito. Its life history, bionomics and structure. [Google Scholar]
- Daugherty MP, Alto BW, Juliano SA. Invertebrate carcasses as a resource for competing Aedes albopictus and Aedes aegypti (Diptera: Culicidae) Journal of Medical Entomology. 2000;37:364–372. doi: 10.1093/jmedent/37.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunson WA, Travis J. The role of abiotic factors in community organization. American Naturalist. 1991;138:1067–1091. [Google Scholar]
- Fernald EA, Patton DJ, editors. Water resources atlas of Florida. Florida State University; Tallahassee, Florida, USA: 1985. [Google Scholar]
- Grover JP. Resource competition. Chapman and Hall; London, UK: 1997. [Google Scholar]
- Hawley WA. The biology of Aedes albopictus. Journal of the American Mosquito Control Association. 1988;4(Supplement):1–40. [PubMed] [Google Scholar]
- Hemphill N. Disturbance and variation in competition between two stream insects. Ecology. 1991;72:864–872. [Google Scholar]
- Hobbs JH, Hughes EA, Eichold BH., II Replacement of Aedes aegypti by Aedes albopictus in Mobile, Alabama. Journal of the American Mosquito Control Association. 1991;7:488–489. [PubMed] [Google Scholar]
- Holway DA, Suarez AV, Case TJ. Role of abiotic factors in governing susceptibility to invasion: a test with Argentine ants. Ecology. 2002;83:1610–1619. [Google Scholar]
- Hornby JA, Moore DE, Miller TW., Jr Aedes albopictus distribution, abundance, and colonization in Lee County, Florida, and its effect on Aedes aegypti. Journal of American Mosquito Control Association. 1994;10:397–402. [PubMed] [Google Scholar]
- Huston M. A general hypothesis of species diversity. American Naturalist. 1979;113:81–101. [Google Scholar]
- Juliano SA. Species introduction and replacement among mosquitoes: Interspecific resource competition or apparent competition? Ecology. 1998;79:255–268. [Google Scholar]
- Juliano SA, Lounibos LP, O’Meara GF. A field test for competitive effects of Aedes albopictus on Aedes aegypti in South Florida: Differences between sites of coexistence and exclusion? Oecologia. 2004;139:583–593. doi: 10.1007/s00442-004-1532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, O’Meara GF, Morrill JR, Cutwa MM. Desiccation and thermal tolerance of eggs and the coexistence of competing mosquitoes. Oecologia. 2002;130:458–459. doi: 10.1007/s004420100811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, Stoffregen TL. Effects of habitat drying on size at and time to metamorphosis in the tree hole mosquito Aedes triseriatus. Oecologia. 1994;97:369–376. doi: 10.1007/BF00317327. [DOI] [PubMed] [Google Scholar]
- Linley JR. Structure of eggs of Aedes albopictus, A. aegypti, and A. bahamensis (Diptera: Culicidae) Journal of Medical Entomology. 1989;26:510–521. doi: 10.1093/jmedent/26.6.510. [DOI] [PubMed] [Google Scholar]
- Lounibos LP. Interactions influencing production of treehole mosquitoes in south Florida. In: Lounibos LP, Rey JR, Frank JH, editors. Ecology of mosquitoes: Proceedings of a Workshop. Florida Medical Entomology Laboratory; Vero Beach, Florida, USA: 1985. pp. 65–78 . [Google Scholar]
- Lounibos LP, O’Meara GF, Escher RL, Nishimura N, Cutwa M, Nelson T, Campos RE, Juliano SA. Testing predictions of displacement of native Aedes by the invasive Asian tiger mosquito Aedes albopictus in Florida, USA. Biological Invasions. 2001;3:151–166. [Google Scholar]
- Lounibos LP, Suárez S, Menéndez Z, Nishimura N, Escher RL, O’Connell SM, Rey JR. Does temperature affect the outcome of larval competition between Aedes aegypti and Aedes albopictus? Journal of Vector Ecology. 2002;27:86–95. [PubMed] [Google Scholar]
- Novak RJ, Shroyer DA. Eggs of Aedes triseriatus and A. hendersoni: a method to stimulate optimal hatch. Mosquito News. 1978;38:515–521. [Google Scholar]
- O’Meara GF, Evans LF, Getman AD, Jr, Cuda JP. Spread of Aedes albopictus and decline of A. aegypti (Diptera: Culicidae) in Florida. Journal of Medical Entomology. 1995;2:554–562. doi: 10.1093/jmedent/32.4.554. [DOI] [PubMed] [Google Scholar]
- Persson L. Temperature-induced shift in foraging ability in two fish species, roach (Rutilus rutilus) and perch (Perca fluviatilis): implications for coexistence between poikilotherms. Journal of Animal Ecology. 1986;55:829–839. [Google Scholar]
- SAS Institute. SAS/STAT user’s guide. 4. Vol. 2. SAS Institute; Cary, North Carolina, USA: 1989. Version 6. [Google Scholar]
- Sota T, Mogi M. Interspecific variation in desiccation survival time of Aedes (Stegomyia) mosquito eggs is correlated with habitat and egg size. Oecologia. 1992;90:353–358. doi: 10.1007/BF00317691. [DOI] [PubMed] [Google Scholar]
- Tabachnick W. Evolutionary genetics and arthropod-borne disease. Yellow fever mosquito. American Entomologist. 1991;37:14–24. [Google Scholar]
- Taniguchi Y, Nakano S. Condition-dependent competition: implications for the distributions of stream fishes. Ecology. 2000;81:2027–2039. [Google Scholar]
- Tilman D. Resource competition and community structure. Princeton University Press; Princeton, New Jersey, USA: 1982. [PubMed] [Google Scholar]
- Trpis M. A new bleaching and decalcifying method for general use in zoology. Canadian Journal of Zoology. 1970;48:892–893. [Google Scholar]
- Warner SC, Travis J, Dunson WA. Effect of pH variation on interspecific competition between two species of hylid tadpoles. Ecology. 1993;74:183–194. [Google Scholar]
- Wiens JA. On competition and variable environments. American Scientist. 1977;65:590–597. [Google Scholar]


