Abstract
PURPOSE
The purpose of this study was to characterize temporally stromal growth and transparency in lumican-deficient and normal neonatal mice.
METHODS
Lumican-deficient mice and CD1 wild-type mice were evaluated by in vivo confocal microscopy through-focusing (CMTF) to quantify stromal and epithelial thickness and corneal light-scattering and by laser scanning CM to determine density of keratocytes from 1 day to 12 weeks after birth.
RESULTS
CD1 corneas showed a rapid loss of light-scattering, decreasing by 50% from day 1 to day 12, that paralleled a 60% decrease in density of keratocytes. By contrast, the stroma demonstrated a marked swelling from day 8 to day 12, followed by thinning at day 14. Compared to corneas from CD1 mice, lumican-deficient corneas showed significantly increased (P < 0.05) light-scattering beginning at week 3 that remained elevated above wild-type levels for the duration of the study. Stromal development was also markedly altered, with thinning detected at week 3, followed by no detectable stromal growth for the duration of the study. Density of keratocytes was significantly increased, but the total cell number was similar compared with that in the wild-type cornea, suggesting no effect on keratocyte differentiation.
CONCLUSIONS
Development of normal neonatal corneal transparency appears related to changes in density of keratocytes. The stroma, however, undergoes a marked swelling and thinning at the time of eyelid opening (days 8-14). In the lumican-deficient mouse, stromal swelling is abolished, indicating that this critical phase in stromal development is lumican dependent and essential for normal stromal growth and maintenance of stromal transparency.
The development of corneal opacification in the lumicandeficient mouse supports the hypothesis that lumican, a major keratan sulfate proteoglycan (KSPG) of the cornea, plays a critical role in the development and maintenance of corneal transparency in the neonatal and the adult mouse.1,2 Lumican is a member of the small leucine-rich proteoglycan (SLRP) family that includes decorin, biglycan, fibromodulin, epiphycan, osteoglycin/mimecan, and keratocan.3-5 Three SLRP-KSPGs have been identified in the cornea: lumican, keratocan, and mimecan.3,5,6 Structural and biophysical studies implicate these three KSPGs in the regulation and control of corneal collagen fibril diameter and spacing, essential for the development and maintenance of corneal transparency. Specifically, the presence of KSPG and PG core proteins during polymerization of collagen has been shown to delay the formation of fibrils and result in thin collagen fibrils.7,8 In addition, the model structure of SLRPs, based on homology to the leucinerich repeats of the porcine RNase inhibitor, predicts that the core protein assumes a horseshoe shape, with the outer arch having protruding glycosaminoglycan (GAG) side chains and an inner arch lined with charged residues that surround the collagen triple helix, providing regulation of both fibril size and spacing.9 The keratan sulfate GAG side chains extending from the KSPG core protein are also uniquely expressed during embryonic and neonatal development and temporally coincide with the appearance of corneal transparency in the chick, mouse, and rabbit.6,10,11 Loss of KSPG expression during corneal scaring and replacement with larger chondroitin-sulfate-containing proteoglycans is associated with the slightly larger collagen fibril size and increased fibril spacing.12
In the lumican-deficient mouse, corneal opacification is clinically detectable by 4 to 5 weeks of age,1 and corneas show altered collagen organization within the posterior cornea with increased mean fiber diameter, suggestive of fusion of collagen fibers.2 In vivo confocal microscopy (CM) has also shown that lumican-deficient corneas are significantly thinner and show prominent diffuse light-scattering at the posterior region of the stroma, consistent with the ultrastructural findings. Scattering of light from the anterior region has also been detected. The localization of collagen fibril abnormalities is also consistent with the high expression of lumican within the posterior stroma in wild-type mice.2 X-ray diffraction studies show a small increase in average collagen fiber spacing with considerable fibrillar disorganization, as well as a broader range of fiber diameters throughout the cornea in lumican-deficient corneas compared with normal ones.13
Recently, in vivo CM has been used to evaluate corneal transparency after injury and repair, by measuring the intensity of the backscattered light as a function of corneal depth, using a novel confocal microscopy through-focusing (CMTF) approach.14,15 More recent studies have used CMTF to measure light scattering in the lumican-deficient compared with the TGFβ-overexpressing transgenic mouse and have identified respectively a diffuse versus cell-based light-scattering profile suggestive of two uniquely different mechanisms underlying corneal opacification that may involve extracellular versus cellular protein organization.16 Because x-ray diffraction studies of 2- and 6-month-old mice show similar collagen disarray indicating that lumican deficiency affects collagen organization earlier in life,13 in the present study, for the first time we measured by CMTF the light-scattering properties of the cornea during neo-natal development of transparency in the wild-type compared with the lumican-deficient mouse and correlated changes with stromal and epithelial thickness and density of keratocytes.
This article reports the finding that neonatal stromal development undergoes a critical phase of swelling and thinning before and after eyelid opening that is absent in the lumican-deficient cornea. Although development of transparency and reduced light-scattering in the wild-type mouse directly coincided with the progressive decline in density of keratocytes, the lumican-deficient mouse showed marked thinning and diffuse light-scattering, most prominent in the posterior stroma, which was first detected after eyelid opening when the wildtype stroma swelled maximally.
METHODS AND MATERIALS
Animals
Gene-targeted mice homozygous for a null mutation in lumican lumtm1sc/lumtm1sc in the CD-1 genetic background, and wild-type mice (CD1) were bred and housed at the Animal Resource Center, University of Texas Southwestern Medical Center (Dallas, TX). Handling of all animals conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The study was divided into two separate experiments. In experiment 1 (normal corneal development), a total of 39 wild-type mice were killed at 1, 4, 8, 10, 12, 14, 20, and 30 days after birth to evaluate the normal development of the corneal stroma. In experiment 2 (stromal development in the lumican-deficient cornea) a total of 29 lumican-deficient mice and 33 age-matched wild-type mice were killed at 1, 2, 3, 4, 8, and 12 weeks after birth to evaluate the development of the corneal stroma in the lumican-deficient compared with the wild-type mouse. For both experiments, adult female animals were bred and dates of delivery recorded. At the specific times noted, neonatal mice were anesthetized with 100 mg/kg ketamine (Dodge Animal Health, Fort Dodge, IA) and 10 mg/kg xylazine (Akorn, Inc., Decatur, IL) by intraperitoneal injection. After induction of anesthesia, one eye of each mouse in experiment 1 and both eyes of each mouse in experiment 2 were evaluated by in vivo CM, as described in the next section, to measure epithelial and stromal thickness and quantify light-scattering. After in vivo CM, mice were humanely killed by cervical neck dislocation of the anesthetized animal. Eyes were then collected for laser scanning confocal analysis to measure density of keratocytes, using techniques to be described.
In Vivo Confocal Microscopy
Thickness measurements for the epithelium and stroma, along with the assessment of light-scattering from the stroma were determined as previously described for CMTF, by using in vivo CM.16-18 Briefly, examinations were performed on anesthetized mice with the eyelids held open by tape or the eyelids surgically removed before eyelid opening at approximately neonatal day 12. Eyes were examined with a scanning confocal microscope (Tandem Scanning Corp., Reston, VA) with a 24× surface-contact objective (numerical aperture, 0.6, working distance, 1.5 mm). During observations, both eyes were kept moist with topically applied artificial tear solution (Celluvisc; Allergan, Inc., Irvine, CA), to avoid corneal desiccation, and a drop of artificial tears was placed on the tip of the objective to serve as an immersion fluid. CMTF collects a series of digital, two-dimensional images through the cornea at known intervals that can later be reconstructed to generate a three-dimensional image of the cornea.
Two to three CMTF scans in the forward anterior-to-posterior direction were performed in the central cornea of each eye with each data set saved online for later analysis. Depth-intensity profiles were then generated from each data set and the z-axis position for the surface epithelium, epithelial-stromal interface, and corneal endothelium marked with an interactive cursor. Thickness measurements for the epithelium and stroma were then obtained by using previously published formulas.17,18 To assess light-scattering, the area under the pixel intensity curve (Uauc) corresponding to the corneal stroma was integrated to provide an estimate of backscattering light using previously published techniques.17,19 Average epithelial and stromal thicknesses and average light-scattering were then calculated and recorded for each eye.
Laser Scanning CM and Determination of Density of Keratocytes
Eyes were first enucleated and fixed with 1% paraformaldehyde (Electron Microscopy Grade; Polysciences, Warrington, PA) in phosphate-buffered saline. Corneas were then removed, bisected, washed in PBS and stained with 0.2 mg/mL propidium iodide (Sigma, St. Louis, MO). Tissues were washed and mounted onto glass slides with a 1:1 solution of glycerol/PBS. Intact tissue samples were imaged with a confocal microscope (model SP2) equipped with argon/krypton, HeNe, and GrNe lasers and three photograph-multiplier detectors to provide single-line excitation at 488 nm, 543 nm, and 633 nm, respectively (Leica Microsystems, Heidelberg, Germany).
To determine density of keratocytes, tissues were microscopically examined with a 63 × 1.2-numerical aperture, water-immersion objective (Leica). Using excitation for propidium iodide (543 nm), a three-dimensional stack of images extending from the corneal endothelium through the corneal epithelium was collected at 2-μm intervals. The stack of images was then analyzed on computer (MetaMorph, ver. 4.6 image-analysis software; Universal Imaging Corp., Downingtown, PA) and the number of keratocyte nuclei counted within the entire stack, by using a modified counting program similar to that used by Petroll et al.20 for counting rabbit and human keratocytes in intact tissues by laser scanning CM. For each corneal sample three, randomly selected areas within the central corneal region were counted. Density of keratocytes for each region was determined by dividing the total number of cells counted by area of the laser scanning confocal microscopic section (119 × 119 μm) and the stromal thickness measured by in vivo CM. Using the in vivo measurement of stromal thickness provided an adjustment for shrinkage or swelling of the sample in the z-axis due to tissue staining. The average density of keratocytes was calculated from the three central regions and recorded for each cornea.
Confocal images of keratocyte nuclear staining were individually selected from three-dimensional data sets and the images imported into an image-analysis program (Photoshop; Adobe, San Jose, CA), contrast adjusted, and exported to a printer (8650 PS Color Printer; Eastman Kodak, Rochester, NY).
Statistics
All statistical comparison between wild-type and lumican-deficient mouse corneas were made using SigmaStat 1.0 (SPSS Sciences, Chicago, IL). Comparison between groups and time points were evaluated by analysis of variance and a Tukey pair-wise multiple comparison procedure. All data are reported as the mean ± SD.
RESULTS
Normal Corneal Development
The CD1 wild-type mice were born with a thin corneal epithelium of one to two cell layers, averaging 12.7 ± 1.8 μm thick at day 1 (Fig. 1A). The corneal stroma was also thin at birth, averaging 62.9 ± 4.9 μm, and translucent, with marked backscattering of light as measured by CMTF, averaging 5198 ± 350 Uauc (Figs. 1B, 1C). During the early neonatal period from day 1 to day 30 after birth, there was a gradual and uniform increase in the corneal epithelial thickness, achieving adult thickness by day 30 that averaged 49.3 ± 5.7 μm (Fig. 1A). By contrast, neonatal corneal stromal development was characterized by a marked increase in stromal thickness, starting at day 8 and peaking at day 12, with the stroma increasing in thickness from 55.2 ± 6.3 μm to 89.8 ± 11.6 μm, or approximately 60% compared with the thickness 4 days immediately before eyelid opening (Fig. 1B). After eyelid opening on day 12, the stroma gradually decreased in thickness to 66.9 ± 7.5 μm at day 20 but then increased to adult levels by day 30, averaging 81.5 ± 10.7 μm (Fig. 1B).
FIGURE 1.
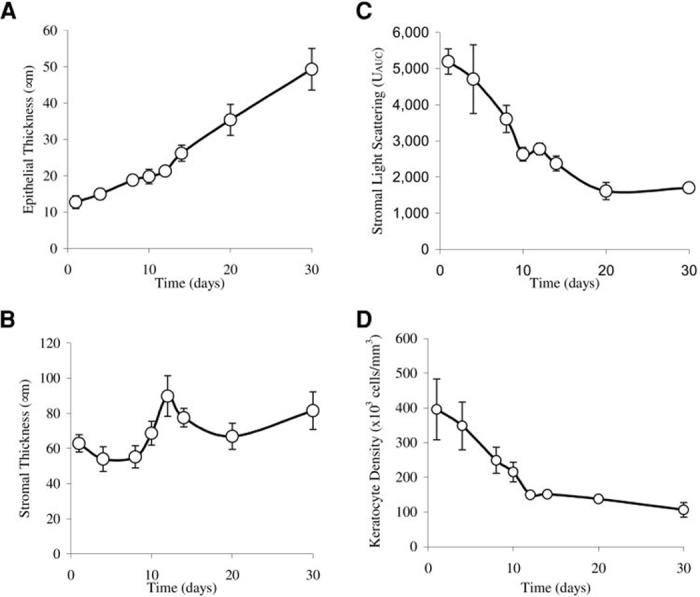
Neonatal corneal development in the wild-type mouse from day 1 to day 30. (A) During early neonatal development there was a gradual and uniform increase in thickness of the corneal epithelium. (B) The developing stroma showed a marked increase in thickness that peaked at day 12, increasing approximately 60% before eyelid opening. (C) There was a rapid decrease in light-scattering before eyelid opening at day 12. (D) Quantitative measurement of cell density showed that the number of stromal cells rapidly decreased from day 1 to day 12.
The development of transparency, in contrast, was characterized by a rapid decrease in light-scattering from day 1 to eyelid opening at day 12, leading to an average light-scattering of 2779 ± 160 Uauc, or approximately 50% of the light-scattering detected at day 1 (Fig. 1C). Images from the in vivo CM examination showed that light-scattering at day 1 had a finely but irregular granular appearance, organized into larger aggregates suggestive of densely packed ovoid or elliptical cell bodies (Fig. 2, day 1, arrow). This general pattern of stromal light-scattering persisted, except for a slow increase in spacing between light-scattering, ovoid structures up to day 12. At day 14 there was a marked decrease in the granular appearance, as smoother contours of individual cells were detected (Fig. 2, day 14). Light-scattering continued to diminish up to day 30, when the faint outlines of cell bodies could barely be detected surrounding more reflective central cell nuclei (Fig. 2, day 30, arrow).
FIGURE 2.
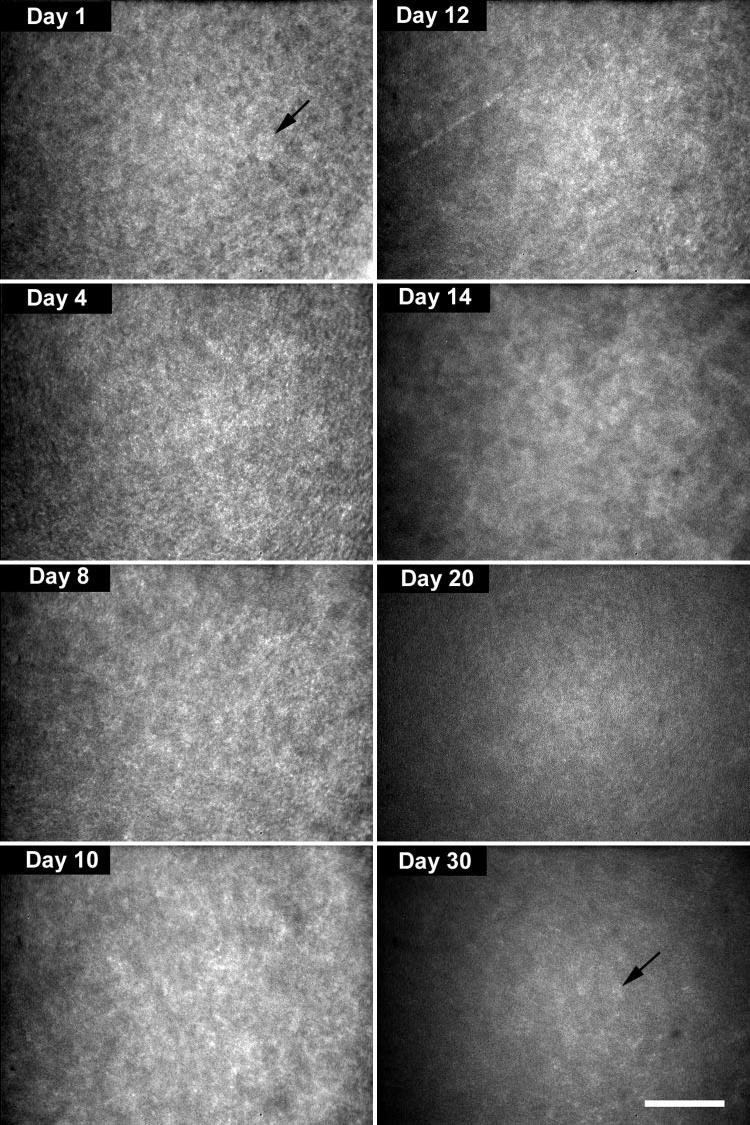
Light-scattering detected by in vivo CM suggested the presence of densely packed ovoid or elliptical cell bodies (day 1, arrow). At day 14, smoother contours of individual cells could be detected (day 14). At day 30, faint outlines of more sparsely packed cells were detected (day 30, arrow). Bar, 100 μm.
Overall, the evaluation of the in vivo CM images suggested the presence of densely packed, highly light-scattering cell bodies at birth that showed a decreasing density with little change in light-scattering from day 1 to day 12. Laser scanning CM of neonatal corneas stained with propidium iodide to localize cell nuclei confirmed this observation (Fig. 3). At day 1, stromal cells appeared densely packed both in frontal (left) and sagittal/anterior-posterior (right) optical sections. Quantitative measurement of the cell density showed that the number of stromal cells rapidly decreased from day 1 to day 12 averaging 395,568 ± 87,654 cells/mm3 at day 1 and 149,499 ± 6,865 cells/mm3 at day 12 (Figs. 1D, 3, day 12). The cell density continued to decrease, albeit more slowly, from day 14 to day 30, averaging 150,843 ± 3,672 cells/mm3 at day 14 and 106,103 ± 20,750 cells/mm3 at day 30.
FIGURE 3.
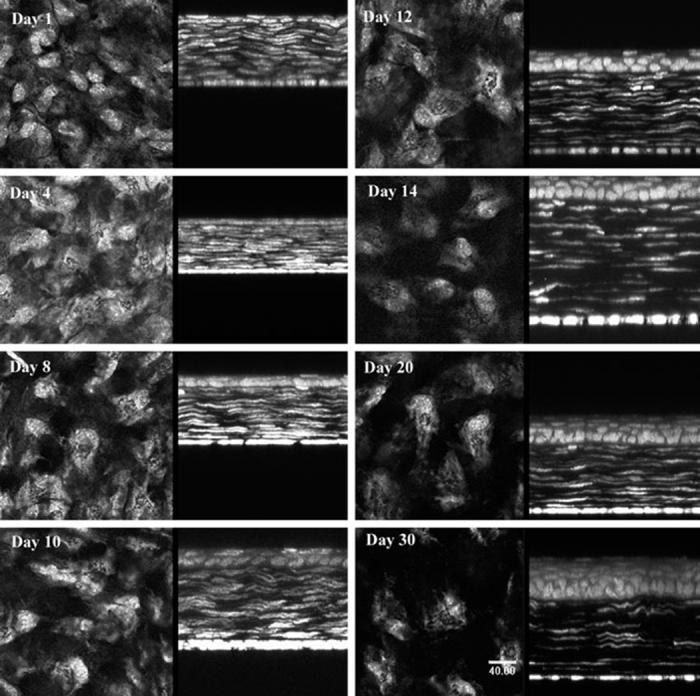
Laser scanning confocal micrographs of neonatal corneas stained with propidium iodide to localize cell nuclei confirmed the in vivo confocal microscopic observation of decreasing cell density after birth. At day 1, stromal cells appeared densely packed, both in frontal (left) and sagittal/anterior-posterior (right) optical sections. Bar, 40 μm.
Stromal Development in the Lumican-Deficient Cornea
In the second study, stromal development in the wild-type mouse was similar to that already established for study 1, particularly regarding stromal swelling at week 2 and the rapid loss of stromal light-scattering. Differences in corneal neonatal development between lumican-deficient and wild-type mice are shown in Table 1 and Figure 4. Although significant differences in the corneal epithelial thickness were noted at weeks 2, 4, and 12 (Fig. 4A, P < 0.01), these differences were variable from week to week and did not differ by more than 6 μm at any time point. Changes in the stromal thickness, however, showed a consistent significant difference (Fig. 4B, P < 0.001). In the normal mouse, the stroma was thin at week 1 (64.9 ± 6.2 μm), increased abruptly to 86.7 ± 10.2 μm at week 2, decreased to 75.8 ± 9.9 μm by week 3, and slowly reached adult thickness by week 12 (87.7 ± 4.3 μm). Unlike the normal mouse, the lumican-deficient stroma never increased in thickness from week 1 to 2, remaining significantly thinner (P < 0.001) than normal at 60.0 ± 4.9 μm 2 weeks after birth. The lumican-deficient stroma became significantly thinner from week 2 to week 3 (P < 0.05) after eyelid opening, showing a parallel change with the normal mouse stroma that left the stroma very thin at 50.7 ± 8.4 μm. The stroma did not significantly change in thickness after 3 weeks and remained at 50.4 ± 1.6 μm by the end of the study.
TABLE 1.
Corneal Development in the Lumican-Deficient Mouse
| Postnatal Age (wk) | Sample Size (n) | Epithelial Thickness (μm) | P* | Stromal Thickness (μm) | p | Corneal Haze(U) | p | Keratocyte Density (cells/mm3) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n) | Cell Count | P | ||||||||||||
| Wild Type | ||||||||||||||
| 1 | 10 | 11.0 | 1.8 | 64.9 | 6.2 | 3,761 | 383 | 5 | 163,639 | 12,265 | ||||
| 2 | 10 | 25.5 | 3.5 | 86.7 | 10.2 | 2,591 | 268 | 5 | 92,063 | 11,671 | ||||
| 3 | 16 | 34.3 | 2.9 | 75.8 | 9.9 | 2,225 | 236 | 5 | 75,868 | 9,228 | ||||
| 4 | 10 | 42.9 | 2.5 | 79.2 | 6.3 | 1,932 | 151 | 5 | 65,967 | 12,032 | ||||
| 8 | 10 | 42.3 | 3.6 | 87.7 | 4.3 | 1,393 | 162 | 5 | 64,496 | 7,371 | ||||
| 12 | 10 | 41.3 | 3.4 | 81.6 | 5.1 | 1,211 | 160 | 5 | 48,382 | 16,574 | ||||
| Lumican-/- | ||||||||||||||
| 1 | 10 | 12.8 | 1.3 | 60.4 | 4.6 | 4,020 | 327 | 5 | 211,764 | 188,738 | ||||
| 2 | 10 | 30.9 | 2.7 | <0.01 | 60.0 | 4.9 | <0.001 | 2,793 | 475 | 5 | 119,752 | 27,309 | <0.05 | |
| 3 | 8 | 33.1 | 2.8 | 50.7 | 8.4 | <0.001 | 3,167 | 871 | <0.001 | 5 | 116,031 | 18,110 | <0.001 | |
| 4 | 10 | 39.0 | 2.8 | <0.01 | 53.9 | 4.8 | <0.001 | 2,400 | 309 | <0.01 | 7 | 92,879 | 22,149 | |
| 8 | 10 | 43.4 | 3.5 | 50.7 | 5.5 | <0.001 | 1,908 | 640 | <0.001 | 6 | 82,320 | 20,522 | ||
| 12 | 10 | 47.2 | 4.4 | <0.01 | 50.4 | 1.6 | <0.001 | 1,990 | 300 | <0.001 | 5 | 69,360 | 9,106 | <0.05 |
Data are expressed as the mean ± SD.
Comparison between lumican deficient versus wild-type mice.
FIGURE 4.
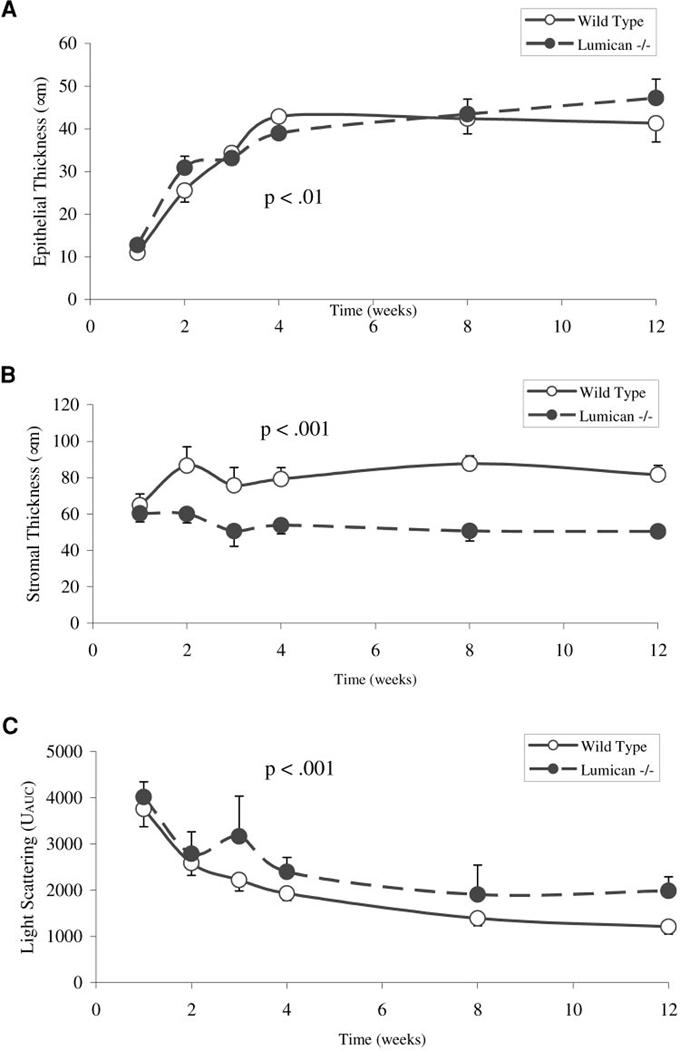
Neonatal stromal development in the wild-type and lumican-deficient mouse cornea. (A) The differences in epithelial thickness between the wild-type and lumican-deficient mouse was minimal. (B) The differences in stromal thickness were marked, with a very thin stroma at week 3. In addition, the lumican-deficient mouse showed no increase in stromal thickness at day 12. (C) Light-scattering was significantly increased in the lumican-deficient mouse compared with the wild-type, beginning at week 3 and remaining so until 3 months after birth.
Scattering of light from the lumican-deficient mouse also differed significantly from the normal progressive development of transparency observed in the wild-type mouse (Fig. 4C, Table 1). Although light-scattering was not significantly different at week 1 and 2 and showed some decreased scattering, there was a significant (P < 0.001) increase in scattered light detected at week 3, increasing from 2793 ± 475 to 3167 ± 871 Uauc. The increased scattering occurred at the time that the stroma thinned significantly. Light-scattering decreased over time from 3 weeks to 12 weeks, but always remained significantly greater than that detected in the wild-type mouse.
In addition to increased light-scattering, the pattern of backscattered light also showed distinct differences (Fig. 5). Whereas light-scattering in the wild-type mouse appeared granular, light-scattering in the lumican-deficient mouse appeared more diffuse and indistinct. Furthermore, lumican-deficient mice never showed the nuclear pattern of light-scattering that was detected in the wild-type mice at 3 weeks; the pattern remained diffuse and indistinct up to 12 weeks after birth (compare Fig. 2, day 30, with Fig. 5, weeks 3-12). As reported earlier,2 the location of backscattered light was predominantly in the posterior stroma from 2 weeks to 12 weeks after birth(Fig. 6). This was in contrast to the light-scattering from the wild-type mouse that showed uniform light-scattering from anterior to posterior stroma (Fig. 6, Wild Type). As light-scattering diminished in the wild-type mouse, the stroma became barely detectable in the x-z slices from the CMTF data sets, with only slight scattering of light detected from the epithelial stromal interface (Fig. 6, Wild Type, arrow). In contrast, lumican-deficient mice always showed pronounced light-scattering starting at the epithelia-stroma interface (Fig. 6, Lumican-/-, arrow), which became more intense within the posterior cornea (arrowhead). Taken together, these findings suggest that lumican deficiency affects both anterior and posterior cornea, with the posterior cornea being more severely affected.
FIGURE 5.
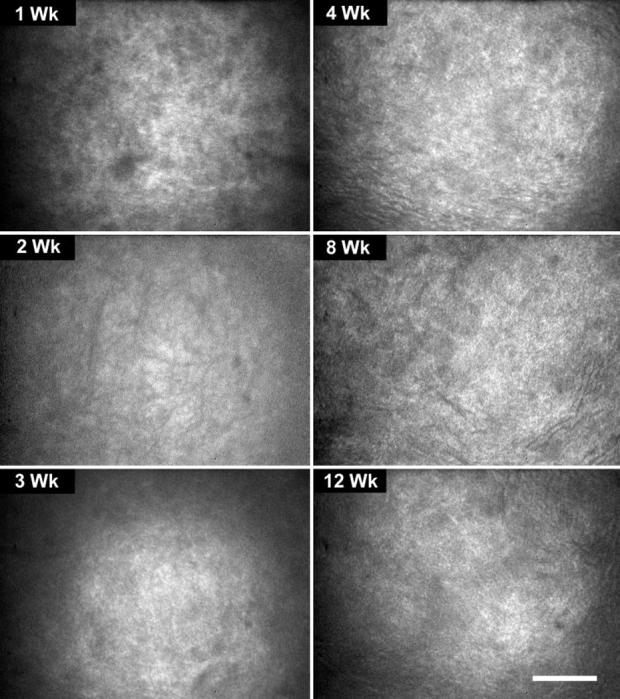
In vivo confocal microscopic images from the lumican-deficient mouse from 1 to 12 weeks of age showing a diffuse rather than granular light-scattering pattern, compared with the wild-type mouse. Bar, 100 μm.
FIGURE 6.
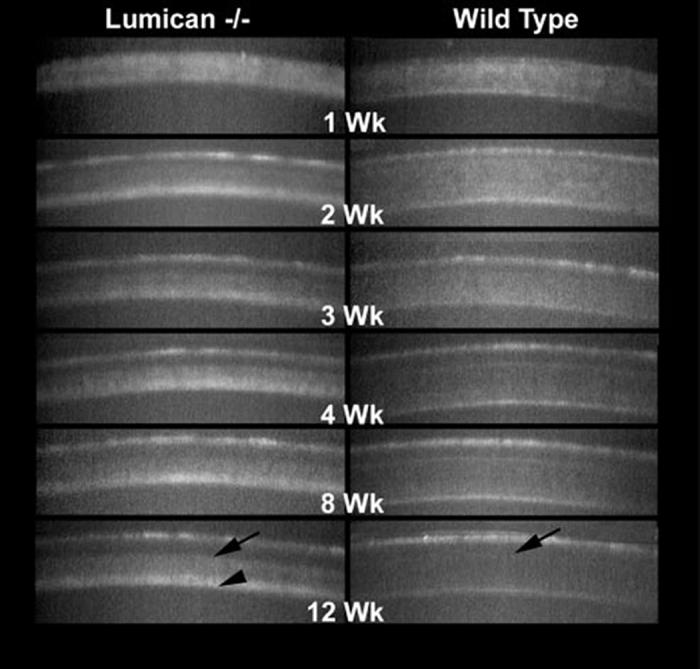
X-Z CMTF scans from lumican-deficient and wild-type mice from 1 to 12 weeks of age. Note that light-scattering in the lumican-deficient mouse occurred throughout the stroma compared to wild-type (arrows) but was more prominent in the posterior cornea from 2 to 12 weeks after birth (arrowhead).
Measurement of density of keratocytes in the lumican-deficient mouse also paralleled the changes in the density from birth to week 12 in the wild-type mouse; decreasing from week 1 to week 4 and remaining stable out to week 12 (Fig. 7A). However, density of keratocytes remained consistently elevated above that of the wild-type mouse and was significantly elevated at 2, 3, and 12 weeks (Table 1). Nevertheless, the number of keratocytes counted within an optical volume for the determination of density of keratocytes were similar between wild-type and lumican-deficient mice (Fig. 7B). This finding therefore indicates that the differences in density of keratocytes were due to the changes in corneal thickness rather than to any effect on keratocyte proliferation or differentiation.
FIGURE 7.
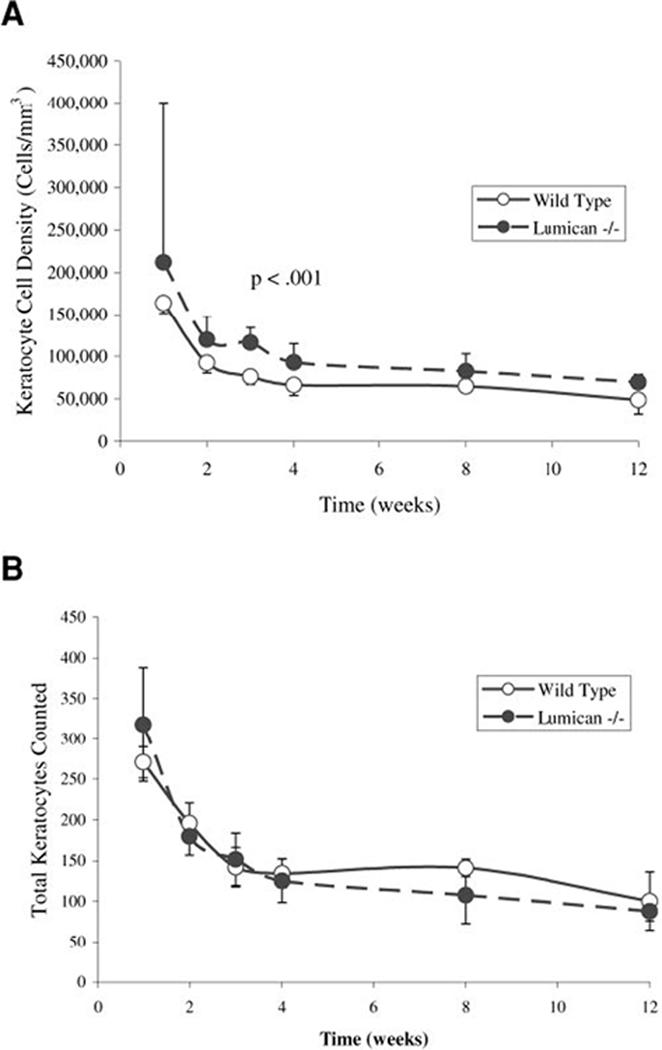
Density of keratocytes in the wild-type and lumican-deficient mouse stroma. (A) The density of keratocytes in the lumican-deficient mouse remained significantly elevated, compared with that in the wild-type cornea (P < 0.001). (B) Comparison of the total number of keratocytes counted, however, showed similar numbers between wild-type and lumican-deficient corneas within the optical volume counted, unadjusted for stromal thickness.
DISCUSSION
This study has identified two aspects of normal neonatal stromal development that have gone unrecognized. First, stromal development in the neonatal mouse appears to undergo a critical phase of swelling before eyelid opening that is immediately followed by stromal thinning and the resumption of slow continued growth to adult thickness. Second, light-scattering from the stroma seems to show a progressive decline with increasing transparency from birth to adulthood that directly parallels changes in stromal density of keratocytes while appearing unrelated to developmental changes in stromal thickness. Although the mechanisms underlying these developmental events require further study, these findings have important implications for our overall understanding of stromal transparency.
First, marked stromal swelling suggests a major change in corneal hydration before eyelid opening that may be precipitated by the deposition of KSPG. Others have noted that sulfation of KSPG occurs initially at around the time of eyelid opening in the mouse.11 In addition, incorporation of radiolabeled sulfate in the neonatal rabbit cornea achieves adult levels only after eyelid opening.10,21 These observations suggest that an important event before eyelid opening is the synthesis of KS that may lead to an increase in water absorption, explaining the marked increase in stromal thickness before eyelid opening in the mouse. The complete abolition of stromal swelling before eyelid opening in the lumican-deficient mouse also suggests that sulfation may be critical to this process. In this regard, it should be noted that lumican-deficient mice have a 25% reduction of KS content in the whole eye.2
Second, corneal thinning after eyelid opening suggests dehydration of the cornea, perhaps precipitated by exposure to air or the initiation of corneal endothelial pump function. Thinning of the cornea, or corneal compaction, has been noted to be essential in the development of transparency in the chick and in early studies by Coulombre and Coulombre22 suggest that transparency is inversely related to corneal hydration, because hydration decreases from 96% to 75% from embryonic day 14 to day 19, the time when transparency develops. More recent studies using x-ray diffraction analysis indicate that corneal dehydration is a two-stage process that may involve the corneal endothelial pump function as well as other molecular factors, including the synthesis of different collagens and proteoglycans.23 Nevertheless, major changes in hydration are associated with major changes in the interfibrillar spacing, because collagen fibers are packed more closely together. Control of corneal hydration is a recognized function of the corneal endothelium. In the rat, corneal endothelium begins to develop adult organization of apical junctional proteins necessary in establishing pump function around neonatal day 13 to day 20.24 Similarly, measurements of paracellular permeability in the rabbit show increasing corneal endothelial pump function after birth, with the greatest change occurring after eyelid opening.25 Therefore thinning of the stroma after eyelid opening in mammals and during compaction in the chick is most likely related to the establishment of normal endothelial function.
Sulfation of lumican in the chick also parallels the development of transparency beginning at embryonic day 15 during the early phase of dehydration and tissue compaction.21 Although mRNA for lumican and decorin have been shown to increase from embryonic day 9 to day 18 in the chick, with lumican synthesis increasing dramatically between days 7 and 9, this increased synthesis is not associated with the deposition of sulfated proteoglycan. Rather, lumican synthesized before day 15 contains a non-sulfated polylactosamine that is replaced with KS at the time transparency begins to develop at embryonic day 15. Therefore, deposition of lumican at the time of corneal dehydration appears to occur in both mammals and chicks.24,25
The lumican-deficient mouse also showed stromal thinning after eyelid opening, suggesting that endothelial development and function may be normal in these mice. More important, however, stromal thinning in the lumican-deficient mouse led to increased light-scattering and the development of corneal opacification. These findings, taken together with what is known about the development of transparency in the chick and mammal, suggest that the synthesis and deposition of KSPG may play an important protective role in counterbalancing the effects of stromal dehydration and that the lateral fusion of collagen fibers in the posterior stroma of the lumicandeficient mouse may occur during stromal compaction when collagen fibers are brought into closer proximity than normally occurs in the presence of KS.
In contrast, the increase in stromal thickness before eyelid opening may be related to enhanced deposition of stromal matrix. Lumican-deficient mice show an almost complete lack of stromal growth after eyelid opening, suggesting a major defect in collagen deposition. Corneal dry weight measurements indicate that lumican-deficient corneas have significantly decreased stromal mass, also supporting a role for lumican-controlling collagen synthesis and deposition (Chakravarti S, unpublished data, 1998). Because the stromal thickness at day 7 before eyelid opening was similar between wild-type and lumican-deficient eyes, lumican’s role in this process may be exerted during later stages of neonatal development.
Concerning the role of keratocytes in the development of corneal transparency, both the wild-type and lumican-deficient corneal stroma showed prominent light-scattering at birth that progressively declined up to eyelid opening.2 The scattering of light at birth appeared to originate from cellular structures within the cornea. The cellular origin is supported both by the in vivo CM images and the laser scanning CM images used for measuring density of keratocytes. Furthermore, the intensity of light-scattering appeared to decrease as a function of density of keratocytes, in that the rapid decline in light-scattering from day 1 to day 12 paralleled the rapid decrease in density of keratocytes over this time. After eyelid opening, the more gradual decrease in light-scattering was associated with an apparent decrease in scattering from the keratocyte cell body, with only the cell nuclei scattering light in the adult cornea. By contrast, in the lumican-deficient cornea, light-scattering after eyelid opening was more diffuse and indistinct, particularly in the posterior cornea. The diffuse nature of the light-scattering may suggest that the diameter of the scattering structures within the cornea are below the limits of microscopic resolution and are consistent with an extracellular matrix origin. Taken together, these findings suggest that light-scattering in the normal neonatal stroma may have a cellular component rather than an exclusive extracellular basis, as in the lumican-deficient cornea.
Such a hypothesis has recently been reported to explain light-scattering from the cornea after injury.14 Studies of rabbit corneas have identified the abundant expression of a few water-soluble proteins in corneal keratocytes that have been proposed to regulate the transparent and light-scattering properties similar to lens crystallin proteins. The expression of these corneal crystallin proteins have been previously identified in the corneal epithelium and are known to be developmentally and environmentally regulated, with expression dramatically increased after eyelid opening.26,27 Although further study is needed to identify the expression of keratocyte crystallin proteins in the mouse, these finding support a role for keratocytes and crystallin proteins in the development of neonatal corneal transparency.
Furthermore, neither stromal swelling before nor thinning after eyelid opening produced any marked change in light-scattering from the normal stroma. Because these changes in thickness most likely underlie major changes in stromal organization, the absence of any clear effect on stromal light-scattering is perplexing. One possible explanation is that the collagen organization as laid down during embryologic development may optimally transmit light within the range of collagen fibril spacing that develops in the stroma during the neonatal stage. Such a possibility would be consistent with the hypothesis that the differentiation of the cellular elements moving from biosynthetically active to quiescent is primarily responsible for the development of neonatal transparency. Further work is necessary to establish more clearly the separate contributions of cellular and extracellular light-scattering in the development of neonatal transparency.
Overall, this mouse model has several important clinical implications for understanding corneal transparency in corneal dystrophies and in corneal scarring. Of particular interest is macular corneal dystrophy, which demonstrates a reduction in corneal thickness and collagen interfibrillar spacing.28 A deficiency in keratan sulfate is thought to be responsible for macular corneal dystrophy.29,30 Studies have detected a glycoprotein with oligosaccharide side chains that were larger than other oligosaccharides but smaller than normal keratan sulfate side chains.31 Thus, the disorder may be due to an abnormality in glycoprotein processing of lumican side chains. Corneal scars have also been shown to have decreased lumican, which may explain the increased collagen fibril size and spacing in this tissue.32
In conclusion, this study is the first to demonstrate that neonatal stromal development involves a marked thickening of the stroma before eyelid opening, suggesting that the increase in synthesis of KSPG noted by others may play an important role in hydration of the cornea in preparation for corneal desiccation resulting from exposure to air or endothelial pump function. The absence of stromal swelling in the lumican-deficient mouse and the consequent development of stromal opacification suggests that KSPG and lumican in particular may be critical to the separation of collagen fibers without which stromal compaction after desiccation may precipitate lateral collagen fiber association, increased fibril diameter, and marked scattering of light. This study has also identified for the first time that density of keratocytes appears to be directly and inversely correlated with the development of neonatal corneal transparency supporting the novel hypothesis that light transmission through the cornea has both a cellular and an extracellular basis.
Footnotes
Supported by National Eye Institute Grants EY13215 (JVJ) and EY11654 (SC) and an unrestricted grant from Research to Prevent Blindness.
Disclosure: J. Song, None; Y.-G. Lee, None; J. Houston, None; W.M. Petroll, None; S. Chakravarti, None; H.D. Cavanagh, None; J.V. Jester, None
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be marked “advertisement” in accordance with 18 U.S.C. §1734 solely to indicate this fact.
References
- 1.Chakravarti S, Magnuson T, Lass JH, Jepsen KJ, LaMantia C, Carroll H. Lumican regulates collagen fibril assembly: skin fragility and corneal opacity in the absence of lumican. J Cell Biol. 1998;141:1277–1286. doi: 10.1083/jcb.141.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakravarti S, Petroll WM, Hassell JR, et al. Corneal opacity in lumican-null mice: defect in collagen fibril structure and packing in the posterior stroma. Invest Ophthalmol Vis Sci. 2000;41:3365–3373. [PMC free article] [PubMed] [Google Scholar]
- 3.Funderburgh JL, Corpuz LM, Roth MR. Fundergurgh ML, Tasheva ES, Conrad GW. Mimecan, the 25-kDa corneal keratan sulfate proteoglycan, is a product of the gene producing osteoglycin. J Biol Chem. 1997;272:28089–28095. doi: 10.1074/jbc.272.44.28089. [DOI] [PubMed] [Google Scholar]
- 4.Iozzo RV, Murdoch AD. Proteoglycans of the extracellular environment: clues from the gene and protein side offer novel perspectives in molecular diversity and function. FASEB J. 1996;10:598–614. [PubMed] [Google Scholar]
- 5.Corpuz LM, Funderburgh JL, Funderburgh ML, Bottomley GS, Prakash S, Conrad GW. Molecular cloning and tissue distribution of keratocan. J Biol Chem. 1996;271:9759–9763. doi: 10.1074/jbc.271.16.9759. [DOI] [PubMed] [Google Scholar]
- 6.Blochberger TC, Vergnes JP, Hempel J, Hassell JR. cDNA to chick lumican (corneal keratan sulfate proteoglycan) reveals homology to the small interstitial proteoglycan gene family and expression in muscle and intestine. J Biol Chem. 1992;267:347–352. [PubMed] [Google Scholar]
- 7.Birk DE, Lande MA. Corneal and scleral collagen fiber formation in vitro. Biochim Biophys Acta. 1981;670:362–369. doi: 10.1016/0005-2795(81)90108-2. [DOI] [PubMed] [Google Scholar]
- 8.Rada JA, Cornuet PK, Hassell JR. Regulation of corneal collagen fibrillogenesis in vitro by corneal proteoglycan (lumican and decorin) core proteins. Exp Eye Res. 1993;56:635–648. doi: 10.1006/exer.1993.1081. [DOI] [PubMed] [Google Scholar]
- 9.Weber IT, Harrison RW, Iozzo RV. Model structure of decorin and implications for collagen fibrillogenesis. J Biol Chem. 1996;271:31767–31770. doi: 10.1074/jbc.271.50.31767. [DOI] [PubMed] [Google Scholar]
- 10.Smelser GK, Ozanics V. Distribution of radioactive sulfate in the developing eye. Am J Ophthalmol. 1957;44:102–109. doi: 10.1016/0002-9394(57)90437-3. [DOI] [PubMed] [Google Scholar]
- 11.Ying S, Shiraishi A, Cao CW-C, et al. Characterization and expression of the mouse lumican gene. J Biol Chem. 1997;272:30306–30313. doi: 10.1074/jbc.272.48.30306. [DOI] [PubMed] [Google Scholar]
- 12.Hassell JR, Cintron C, Kublin C, Newsome DA. Proteoglycan change during restoration of corneal transparency in corneal scars. Arch Biochem Biophys. 1983;222:363–369. doi: 10.1016/0003-9861(83)90532-5. [DOI] [PubMed] [Google Scholar]
- 13.Quantock AJ, Meek KM, Chakravarti S. An x-ray diffraction investigation of corneal structure in lumican-deficient mice. Invest Ophthalmol Vis Sci. 2001;42:1750–1756. [PubMed] [Google Scholar]
- 14.Jester JV, Moller-Pedersen T, Huang J, et al. The cellular basis of corneal transparency: evidence for “corneal crystallins”. J Cell Sci. 1999;112:613–622. doi: 10.1242/jcs.112.5.613. [DOI] [PubMed] [Google Scholar]
- 15.Moller-Pedersen T, Vogel M, Li HF, Petroll WM, Cavanagh HD, Jester JV. Quantification of stromal thinning, epithelial thickness, and corneal haze after photorefractive keratectomy using in vivo confocal microscopy. Ophthalmology. 1997;104:360–368. doi: 10.1016/s0161-6420(97)30307-8. [DOI] [PubMed] [Google Scholar]
- 16.Jester JV, Lee YG, Li J, et al. In vivo confocal microscopy: measurement of corneal thickness and transparency in transgenic mice. Vision Res. 2001;41:1283–1290. doi: 10.1016/s0042-6989(00)00222-4. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Jester JV, Cavanagh HD, Black TD, Petroll WM. On-line 3-dimensional confocal imaging in vivo. Invest Ophthalmol Vis Sci. 2000;41:2945–2953. [PubMed] [Google Scholar]
- 18.Jester JV, Petroll WM, Cavanagh HD. Measurement of tissue thickness using confocal microscopy. Methods Enzymol. 1999;307:230–245. doi: 10.1016/s0076-6879(99)07016-0. [DOI] [PubMed] [Google Scholar]
- 19.Li HF, Petroll WM, Moller-Pedersen T, Maurer JK, Cavanagh HD, Jester JV. Epithelial and corneal thickness measurements by in vivo confocal microscopy through focusing (CMTF) Curr Eye Res. 1997;16:214–221. doi: 10.1076/ceyr.16.3.214.15412. [DOI] [PubMed] [Google Scholar]
- 20.Petroll WM, Boettcher K, Barry P, Cavanagh HD, Jester JV. Quantitative assessment of anteroposterior keratocyte density in the normal rabbit cornea. Cornea. 1995;14:3–9. [PubMed] [Google Scholar]
- 21.Cornuet PK, Blochberger TC, Hassell JR. Molecular polymorphism of lumican during corneal development. Invest Ophthalmol Vis Sci. 1994;35:870–877. [PubMed] [Google Scholar]
- 22.Coulombre AJ, Coulombre JL. The development of the structural and optical properties of the cornea. In: Smelser GK, editor. The Structure of the Eye. Academic Press; New York: 1961. pp. 405–420. [Google Scholar]
- 23.Siegler V, Quantock AJ. Two-stage compaction of the secondary avaian cornea during development. Exp Eye Res. 2002;74:427–431. doi: 10.1006/exer.2001.1162. [DOI] [PubMed] [Google Scholar]
- 24.Joyce NC, Harris DL, Zieske JD. Mitotic inhibition of corneal endothelium in neonatal rats. Invest Ophthalmol Vis Sci. 1998;39:2572–2583. [PubMed] [Google Scholar]
- 25.Stiemke MM, Mccartney MD, Cantu-Crouch D, Edelhauser HF. Maturation of the corneal endothelial tight junction. Invest Ophthalmol Vis Sci. 1991;32:2757–2765. [PubMed] [Google Scholar]
- 26.Piatigorsky J. Gene sharing in lens and cornea: facts and implications. Prog Retinal Eye Res. 1998;17:145–174. doi: 10.1016/s1350-9462(97)00004-9. [DOI] [PubMed] [Google Scholar]
- 27.Sax CM, Kays WT, Salamon C, Chervenak M, Yu YS, Piatigorsky J. Transketolase gene expression in the cornea is influenced by environmental factors and developmentally controlled events. Cornea. 1998;19:833–841. doi: 10.1097/00003226-200011000-00014. [DOI] [PubMed] [Google Scholar]
- 28.Quantock AJ, Meek KM, Ridgway AEA, Bron AJ, Thonar EJ-MA. Macular corneal dystrophy: reduction in both corneal thickness and collagen interfibrillar spacing. Curr Eye Res. 1990;9:393–398. doi: 10.3109/02713689008999628. [DOI] [PubMed] [Google Scholar]
- 29.Klintworth GK, Meyer R, Dennis R, et al. Macular corneal dystrophy: lack of keratan sulfate in serum and cornea. Ophthalmic Paedatr Genet. 1986;7:139–143. doi: 10.3109/13816818609004130. [DOI] [PubMed] [Google Scholar]
- 30.Midura RJ, Hascall VC, MacCallum DK, et al. Proteoglycan biosynthesis by human corneas from patients with types 1 and 2 macular corneal dystrophy. J Biol Chem. 1990;265:15947–15955. [PubMed] [Google Scholar]
- 31.Hassell JR, Newsome DA, Krachmer JH, Rodrigues MM. Macular corneal dystrophy: failure to synthesize a mature keratan sulfate proteoglycan. Proc Natl Acad. Sci USA. 1980;77:3705–3709. doi: 10.1073/pnas.77.6.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.SundarRaj N, Fite D, Belak R, et al. Proteoglycan distribution during healing of corneal stromal wounds in chick. Exp Eye Res. 1998;67:433–442. doi: 10.1006/exer.1998.0540. [DOI] [PubMed] [Google Scholar]


