Abstract
The binding of integrins to the extracellular matrix results in focal organization of the cytoskeleton and the genesis of intracellular signals that regulate vital neuronal functions. Recent evidence suggests that integrins modulate G-protein coupled receptor (GPCR) signaling in hippocampal neurons. In this study we evaluated the hypothesis that integrins regulate the mu opioid receptor in rat trigeminal ganglion neurons. For these studies, primary cultures of adult rat trigeminal ganglion neurons were used to demonstrate the colocalization of β1 and β3 integrins with mu opioid receptor in caveolin-1-rich membrane fractions, and at focal adhesions sites generated by integrin ligand binding. Furthermore, we show that the mu opioid receptor agonist, DAMGO ([D-Ala2,N-MePhe4,Gly-ol5]enkephalin), inhibits cAMP accumulation in response to PGE2 stimulation in bradykinin-primed, but not unprimed, cultured trigeminal ganglia neurons. Application of soluble GRGDS (Gly-Arg-Gly-Asp-Ser) peptides that bind specific integrins (i.e., RGD-binding integrins) completely abolished the DAMGO effect in bradykinin-primed trigeminal ganglia neurons, but did not alter bradykinin-mediated hydrolysis of phosphytidylinositol. Likewise, monospecific anti-β1 and anti-β3 integrin subunit antibodies blocked this DAMGO effect in bradykinin -primed trigeminal ganglia neurons. Indeed, application of anti-β1 integrin subunit actually reversed DAMGO signaling, resulting in increased cAMP accumulation in these cells. This suggests that the relative amounts of specific activated integrins at focal adhesions may govern signaling by the mu opioid receptor, perhaps by altering interactions with G proteins (e.g., Gαi vs. Gαs). Collectively, these data provide the first evidence that specific integrins regulate opioid receptor signaling in sensory neurons.
Keywords: signal transduction, focal adhesions, bradykinin, G-protein coupled receptor, DAMGO, sensory neurons
Cells interact with the extracellular matrix via heterodimeric (αβ) transmembrane receptors, termed integrins (Giancotti and Rouslahti, 1999). Integrins are expressed by virtually every cell type and are known to be involved in the regulation of several vital cell functions, including adhesion, migration, proliferation, and differentiation (Milam et al., 1991, Hynes, 1992, Curley et al., 1999, Giancotti and Rouslahti, 1999, Schlaepfer et al., 1999, Coppolino and Dedhar, 2000, Bouvard et al., 2001, Zamir and Geiger, 2001, Alenghat and Ingber, 2002, Martin et al., 2002, Miranti and Brugge, 2002). Eighteen α and eight β subunits have been identified forming at least 24 different αβ integrins (van der Flier and Sonnenberg, 2001, Miranti and Brugge, 2002). However, splice variants of integrins are known to exist that potentially increase the functional diversity of these important molecules (Melker and Sonnenberg, 1999).
Integrins composed of α4, α5, α8, αIIb, or αv subunits (ten of the known 24 integrins contain one of these α subunits) bind to molecules containing an arginine-glycine-aspartate (RGD) sequence (van der Flier and Sonnenberg, 2001). This RGD peptide sequence is exposed in intact fibronectin and vitronectin, and is contained as cryptic, or inaccessible, primary structure in some intact laminins and collagens. Integrins that are bound to domains on the extracellular matrix (or to RGD peptides bound to a solid substrate) initiate intracellular signals (i.e., “outside-in” signaling) (Coppolino and Dedhar, 2000). The application of soluble RGD-peptides to neuronal cultures evoked a rapid and substantial increase in the spontaneous discharges of parietal motor neurons (Wildering et al., 2002). Furthermore, administration of soluble RGD peptides, but not the inactive control peptide sequence (i.e., DGR), enhanced high-voltage-activated Ca++ currents in these neurons. Application of RGD peptides, or anti-integrin antibodies, but not inactive control peptides, have also been shown to block N-methyl-D-aspartate (NMDA)-mediated excitatory postsynaptic currents in hippocampal neurons (Chavis and Westbrook, 2001) suggesting that RGD-binding integrins are important in neurotransmission.
With respect to their potential significance in opioid receptor signaling, it is important to note that integrins may modulate G-protein coupled receptor (GPCR) signaling in the CNS (McPhee et al., 1998) as well as in other tissues (Della Rocca et al., 1999, Litvak et al., 2000, Short et al., 2000, Slack and Siniaia, 2005). In the present study, we addressed two major issues related to integrin regulation of sensory neurons. First, we characterized the expression pattern of integrin in sensory neurons, specifically trigeminal ganglion neurons. Second, we tested the hypothesis that the RGD class of integrins regulates the signaling of the GPCR mu opioid receptor (MOR) in trigeminal ganglion neurons.
EXPERIMENTAL PROCEDURES
Materials
Prostaglandin E2 was purchased from Cayman Chemicals (Ann Arbor, MI). Fetal bovine serum was from Gemini Bioproducts (Calabasas, CA). All other tissue culture reagents were purchased from GIBCO BRL (Grand Island, NY). All other compounds and chemicals (reagent grade) were purchased from Sigma-Aldrich (St. Louis, MO).
Animals
Adult male Sprague-Dawley rats (Charles River, Wilmington, MA, USA), weighing 250–300 gm, were used in this study. All animal study protocols were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio and conformed to federal guidelines. Animals were housed for one week prior to the experiment with food and water available ad libitum.
Rat trigeminal ganglia (TG) neuron cultures
Primary cultures of mature Sprague-Dawley (250–300 gm) rat trigeminal ganglion neurons were prepared using methods that we have adapted from others (Vasko et al., 1994). Fresh TG were washed with HBSS (Ca++, Mg++ free), digested with 3 mg/ml collagenase for 30 min at 37°C and centrifuged to pellet cells/tissue. The pellet was further digested with 0.1% trypsin (15 min) and 167 μg/mL DNase (10 min) at 37°C in the same solution. Cells were pelleted by centrifugation (5 min at 5000g) and resuspended in DMEM (high glucose) containing 250 ng/ml NGF (Harlan), 10% FBS, 1x Pen/Strep, 1x L-glutamine and the mitotic inhibitors: 7.5 μg/ml uridine and 17.5 μg/ml 5-fluoro-2′-deoxyuridine. After trituration to disrupt tissue, the cell suspension was seeded on poly-D-lysine-coated 0.17 mm glass inserts in 24 well plates or polylysine-coated 48 -well plates (3 TG per plate). Media was changed at 24h and then every 48h after plating. Cells were used after 5 or 6 days in culture (DIC) and serum and NGF were removed from the media 24 h before experimentation.
Immunohistochemistry, Immunocytochemistry and confocal fluorescence microscopy
Intact trigeminal ganglia were immediately placed in O.C.T. (Tissue-Tek) and frozen at −80°C. Tissue was cut in 20 μm thick sections and stored at −80°C. Sections were thawed and prepared for immunohistochemistry (IHC), as needed, using standard protocols. Neurons cultured on glass inserts were fixed for 1 h with fresh 4% paraformaldehyde at 4°C. After the blocking procedure, as detailed below (see Western blot), the following concentrations of monospecific antibodies were used for fluorescence microscopy: anti-MOR (1:500), anti-β1 (1:200) or anti-β3 (1:400) (Santa Cruz Biotechnology, Santa Cruz, CA) was applied for 2 h at room temperature. Following a series of washes with PBS, a secondary species-specific Alexa fluor (either 488 or 594) anti-IgG antibody (1:300) (Molecular Probes, Eugene, OR) was applied for 30 minutes at room temperature. After washing with PBS, fluorescent microscopy images were acquired at 10x, 20x or 40x using a Zeiss microscope, Axiocam camera, and Axiovision software.
For colocalization studies, cultured TG cells (5 days in culture) were fixed and treated with antibodies against β1 integrin (Research Diagnostics Inc., Concord, MA), MOR (Immunostar, Hudson, WI) and phospho-Pyk2 (Calbiochem, San Diego, CA) for 2 h at room temperature. After washing 3X with PBS, the culture was incubated for 1 hr with a species-specific secondary antibody labeled with the Alexa fluorescent dyes AF 633, AF 488 and AF 546 (Molecular Probes, Eugene, OR), respectively. Finally, the insert was washed and coated with Biomeda’s anti-fade Gel/Mount (Foster City, CA) and sealed onto a coverslip. Sequential images at 0.22 μm intervals were obtained using confocal microscopy using lasers with fluorescent excitation wavelengths of 488, 543 and 633 nm, respectively, at the Core Optical Imaging Facility at UTHSCSA. Images were acquired sequentially to prevent bleed through from other channels.
Negative control antibody fluorescent stains for IHC and ICC were carried out using heat denatured integrin subunit monoclonal antibodies, followed by the usual species-specific secondary antibody. To test the specificity of the antibodies used, we preinubated the tissue or cell specimens with blocking peptides, when available, for 1 h at room temperature prior to addition of primary antibody. Blocking peptides were not available for anti-β1 and β3 antibodies; hence, we used heat-denatured forms of these antibodies. Blocking peptides for anti-MOR and anti- phospho-Pyk-2 (ESDI(p)YAEIP) were purchased from Immunostar (Hudson, WI) and Genemed Synthesis (South San Francisco, CA), respectively. The same parameters were used in the acquisition and optimization of test and control sample images.
Colocalization of targeted antigens, imaged by confocal microscopy, was evaluated quantitatively by CoLocalizer Pro 1.0 software (www.homepage.mac.com/colocalizerpro). The Manders Overlap Coefficient (MOC) was used to estimate the degree of colocalization (Manders et al., 1993). This coefficient indicates the degree of colocalization and its values are defined from 0 to 1. For example, a MOC value of 0.9 indicates that 90% of both components (i.e., signal from targeted antigens) overlap on the image.
SDS-PAGE/Western blot
Lysates from TG cultures were prepared by adding 0.3 ml of PBS-TDS lysis buffer (PBS (10 mM sodium phosphate, 138 mM NaCl, 2.7 mM KCl, pH 7.4), 1 % Triton X-100, 0.1 M deoxycholate and 1% SDS) and incubating for 20 min on ice. Cell lysis was verified by microscopy. The lysate was triturated briefly through a 21 g needle, aliquoted and stored at −80°C. Forty microliters of the lysate was mixed with 20 μl of sample buffer and subjected to SDS-PAGE/Western blots analysis. Samples were loaded on a 15% gradient gel (BioRad) and transferred to PVDF Immobilon membranes (BioRad). Membranes were then blocked in 5% non-fat milk in Tris-buffered saline with 0.1% Tween (TBST) for 1 h at room temperature. Anti-β1 integrin or anti-MOR antibodies were incubated with the membrane at 4°C overnight. Then, the membrane was incubated at room temperature for 1 h with peroxidase-linked species-specific anti-IgG antibodies. Following incubation with a chemiluminescent substrate, the bands were visualized by ECL (Amersham).
Isolation of integrin supramolecular complexes
Integrin supramolecular complexes were extracted from rat trigeminal ganglion neuron cultures in T-25 flasks after 5 days in culture using a method adopted from Green et al (Green et al., 1999). RGD or DGR peptides were coupled to 4.5 micron carboxyl-activated ferromagnetic beads (Spherotech, Libertyville, IL) as described by the manufacturer. Magnetic beads coated with DGR peptides served as negative control. Cells were resuspended in HBSS with 0.1 mM MnCl2. Substrate-coated magnetic beads were added to the cells and the flasks rocked for 15 min at 37°C. Cells were detached with a cell scraper in the presence of 4 ml of solution containing 0.1 mM MnCl2, 2 mM PMSF and 76 μl of protease inhibitor cocktail (Sigma) in PBS. Cells were pelleted by centrifugation at 2,500g for 5 min, resuspended in lysis buffer (20 mM HEPES, pH 7.5, 0.1 mM MnCl2, 250 mM sucrose, 25 mg/ml aprotinin, 25mg/ml leupeptin, 1 mM PMSF, and 10 mM CHAPS) and incubated with agitation for 10 min at room temperature. Lysed cells were loaded onto 2 ml μMac magnetic columns (Miltenyl Biotec, Auburn, CA) and the column washed with a solution containing 20 mM Tris and 0.1% Triton X-100 until the A280 was less than 0.001. Molecules bound to the magnetic beads via RGD were eluted in the same buffer supplemented with either 100 or 250 mM NaCl. Isolated proteins were analyzed by SDS-PAGE and Western blot.
Peptide coating of ferromagnetic beads
Six hundred micrograms of a 23-mer RGD containing peptide (PepTite-2000 from Integra, San Diego, CA) or the same amount of SDGRG (DGR) peptide (Sigma) were covalently crosslinked to 62 mg of 4.5 μM carboxyl ferromagnetic polystyrene beads (Spherotech, Libertyville, IL) using 10 mg of EDC (ethyl-3-(3-dimethylaminopropyl) carbodiimide) in 1.25 mL of 50 mM MES pH 5.0, for 2 h at room temperature. Beads were washed 3X in a micropure separator equipped with a 0.45 micron filter (Amicon) with PBS, stored at 4°C, and used within 6 days.
Measurement of cellular cAMP levels by radioimmunoassay (RIA)
MOR -mediated inhibition of PGE2-induced adenylyl cyclase activity was determined by measuring the amount of cAMP accumulated in the presence of the phosphodiesterase inhibitor, rolipram. Intracellular cAMP accumulation was measured by RIA as described previously (Berg et al., 1994). Briefly, TG cultures were washed twice with Hank’s balanced salt solution (HBSS) containing 10 mM HEPES and 4 mM sodium bicarbonate, pH 7.4 (wash buffer). Cells were pre-equilibrated in 250–500 μl wash buffer per well of 48 well plate for 30 min at 37 °C in 5% CO2. Where indicated, inhibitors were added during the pre-equilibration period. To determine MOR-mediated effects, cells were incubated with the phosphodiesterase inhibitor, rolipram (10 μM), along with DAMGO (1 μM; Peninsula Laboratories; Belmont, CA) (15 min, 37°C) followed by addition of the adenylyl cyclase activator, PGE2 (1 μM final concentration) and incubation for a further 15 min. Incubations were terminated by aspiration of the wash buffer and addition of 500 μl ice cold absolute ethanol. The ethanol extracts from individual wells were dried under a gentle air stream and reconstituted in 100 μl 50 mM sodium acetate, pH 6.2. The cAMP content of each 100 μl sample was determined by RIA.
Measurement of inositol phosphate (IP) accumulation
BK-stimulated inositol phosphate (IP) accumulation in TG cultures was measured as previously described (Berg et al., 1994, Berg et al., 1998). Cells, grown in 48-well plates, were labeled with 2 μCi/ml [3H]myo-inositol for 24 hr prior to experiments. After labeling, cells were rinsed twice with 0.25 ml HBSS that contained 20 mM HEPES and 20 mM LiCl and were pre-incubated in HBSS/LiCl at 37° C in room air for 30 min in the absence or presence of RDG peptide. BK was added to a final volume of 250 μl and cells were further incubated for 30 min. The incubation was terminated by the addition of 500 μl of ice cold 10 mM formic acid and total [3H]-IPs (i.e., IP1, IP2 and IP3) were separated with ion-exchange chromatography (Berridge et al., 1983) and measured with liquid scintillation spectrometry.
Statistics
Data were analyzed with two-way ANOVA or with one-way ANOVA followed by Tukey’s post-hoc test as specified using GraphPad InStat version 3.0a for Macintosh (GraphPad Software, San Diego, CA, USA). Experiments were repeated at least 3 times..
RESULTS
β1 and β3 integrin subunit expression by trigeminal ganglion neurons
Of the known RGD-binding integrins, eight belong to β1 or β3 integrin subfamilies (i.e., possess either a β1 or β3 subunit). In this study, β1 and β3 integrin subunits were detected in intact trigeminal ganglia by IHC methods (Figure 1) and in cultured trigeminal neurons using ICC methods (Figures 2 and 3). In addition, β1 and β3 integrin subunit expression was confirmed in cultured trigeminal neurons by Western blot analyses of cell lysates (Figure 4). In agreement with other reports, the molecular masses of these proteins were found to be approximately 115 kDa (β1) and 105 kDa (β3), although a smaller band at about 90 kDa was also seen in the latter.
Figure 1.
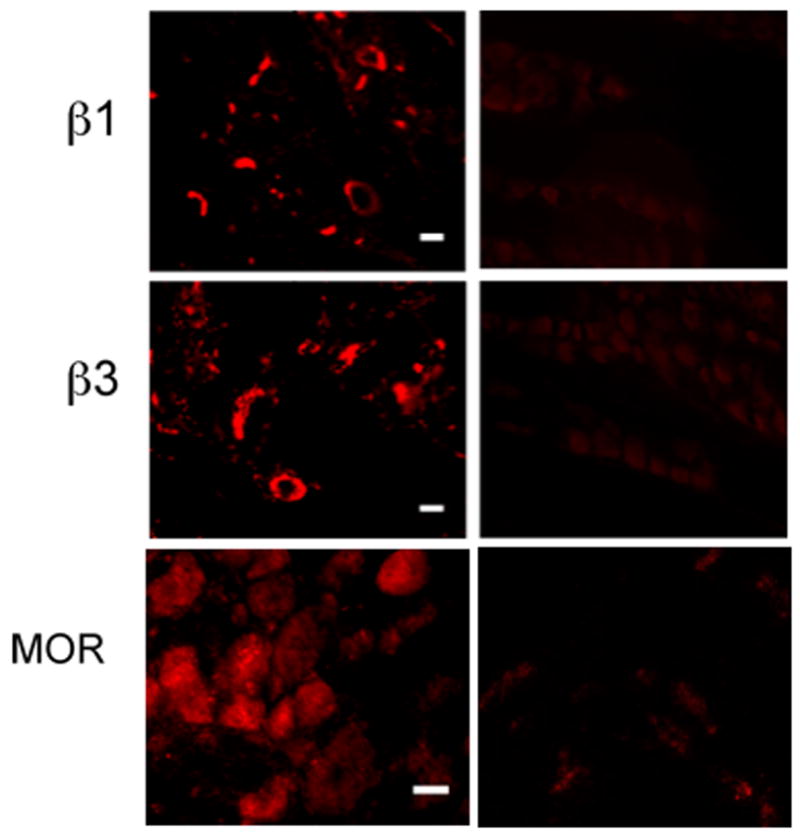
Immunohistochemistry of trigeminal ganglia sections. Left column shows the localization of the immunoreactive integrin subunits β1 and β3 at 20x and MOR at 10x in rat trigeminal ganglia revealed by IHC methods using fluorophore tagged antibodies. The scale bars represent 10 microns. The right column shows background staining (negative controls) using heat denatured primary antibodies for the integrins and a blocking peptide for MOR as detailed under Methods.
Figure 2.
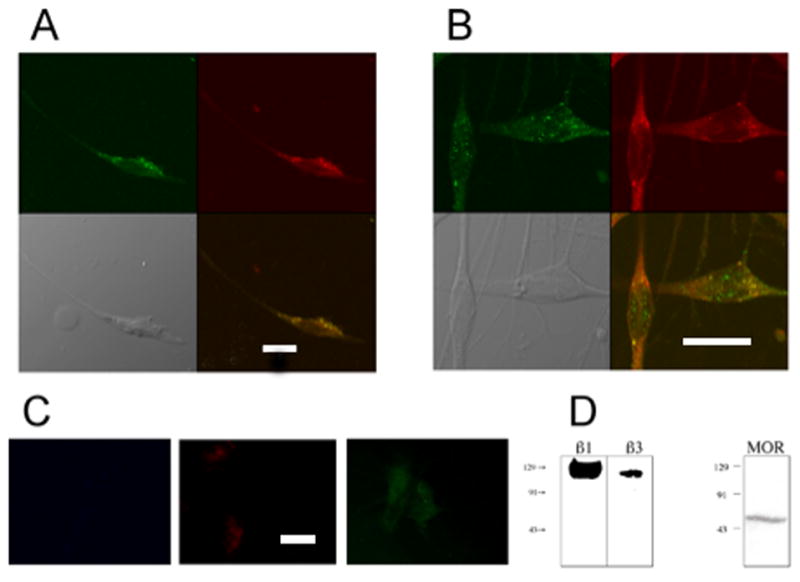
Colocalization of β1 and β3 integrin subunits with MOR. Colocalization of β1 or β3 integrin subunits (green cells, panels A, B, respectively) with MOR (red cells, panels A, B) in cultured rat trigeminal ganglion neurons (fifth day in culture) was examined using ICC methods with confocal imaging. Panels were acquired in sequential single wavelength channels to eliminate bleed through fluorescence. Colocalization is indicated by the yellow color seen in the neurons in the bottom right image in Panels A and B. Panel C shows fluorescence controls for β1, MOR and β3 acquired as described in the legend to fig 1. Scale bars in figs A and C are 10 microns, while that for fig B is 20 microns. Both β1 (A panels) and β3 (B panels) integrin subunits colocalized with MOR in cultured neurons (MOC 0.76 and 0.85, respectively). Western blot analyses (D) confirm expression of β1 (115 kDa), β3 (105 kDa), and MOR (60 kDa) by cultured trigeminal ganglion neurons.
Figure 3.
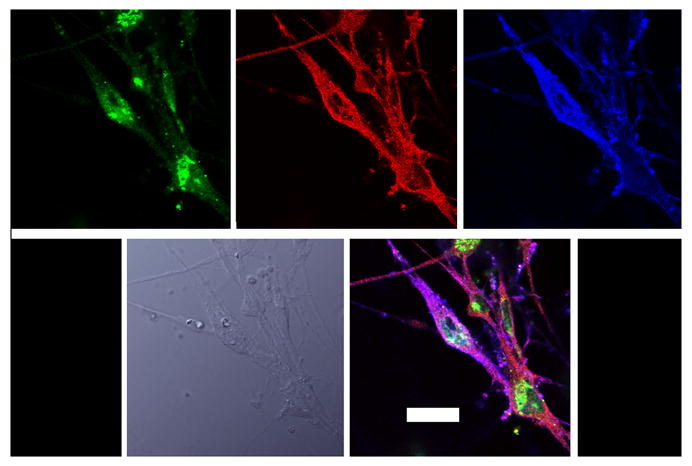
Colocalization of β1 and MOR with phosho-Pyk2. Cultured rat trigeminal ganglion neurons prepared as described in Methods were probed by conventional ICC methods with antibodies against β1 (blue) MOR (green) and p-Pyk2 (red)- in top row. The bottom left picture is a bright field image, and colocalization of all three proteins is indicated by the pink color in the bottom right panel. All confocal images were obtained at 60x in sequential channels, to eliminate fluorescence bleed through. The scale bar represents 10 microns.
Figure 4.
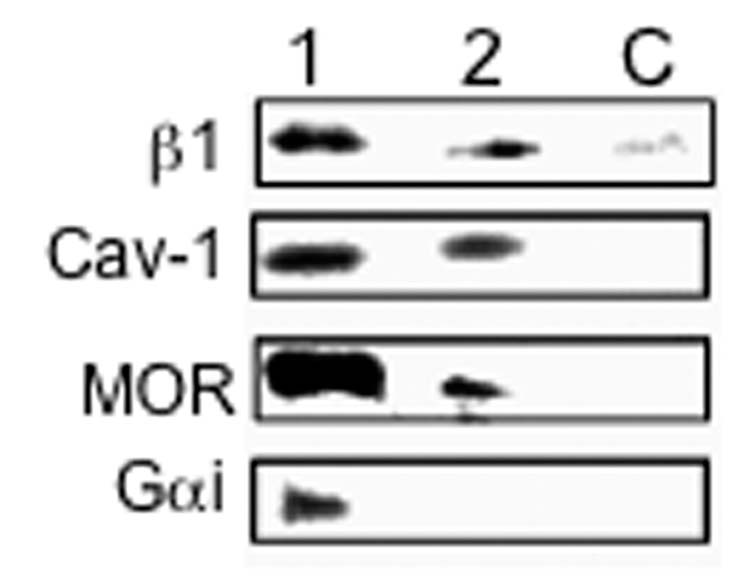
Presence of β1 integrin, MOR and Gαi in lipid rafts. RGD-binding integrin supramolecular complexes (SMC) were isolated from cultured rat trigeminal ganglion neurons as described in Methods. Associated molecules were identified by Western blot using monospecific antibodies. Lane 1 demonstrates MOR and Gαi in RGD-binding integrin SMCs isolated in 100 mM NaCl-containing buffer. Caveolin-1 (Cav-1), a component of lipid rafts, is also identified in these SMCs, as is the β1 integrin subunit. These molecules were not identified in negative controls (i.e., using DGR-coated ferromagnetic beads; C). Lane 2 depicts results of Western blot analyses of SMCs isolated in 250 mM NaCl-containing buffer. Under these more stringent conditions, Gαi is not detected in RGD-binding integrin SMCs from trigeminal ganglion neurons. However, MOR, Cav-1, and β1 are identified under these conditions indicating a stronger interaction of these molecules in these SMCs. These data indicate that MOR is closely associated with RGD-binding integrins (in this instance those of the β1 subfamily), possibly as components of lipid rafts that form adjacent focal adhesions of trigeminal ganglion neurons.
μ Opioid Receptor (MOR) is expressed by trigeminal ganglion neurons
MOR was detected in intact trigeminal ganglia and in cultured trigeminal ganglion neurons using standard IHC, ICC, and Western blot methods (Figures 1–4). Immunostaining of sectioned trigeminal ganglia with fluorophore-tagged antibodies revealed the presence of MOR in 41.2 % of all trigeminal ganglia cells (n=2573). MOR positive neurons had an average diameter of 25.4 microns (SD = 8.3), while that of unstained neurons was 31.8 microns (SD = 8.1). Cell diameters were measured with Axiovision 3.1 software from Carl Zeiss (Thornwood, NY). Western blot analyses of homogenized whole trigeminal ganglia or cultured trigeminal ganglion neurons revealed a major band at ~ 60kDa using the anti-MOR antibody (Santa Cruz Biotechnology, Santa Cruz, CA).
β1 and β3 integrin subunits co-localize with MOR in cultured trigeminal ganglion neurons; β1 also co-localizes with phospho-Pyk2
β1 and β3 integrin subunits were found to have a high degree of co-expression with MOR in cultured trigeminal ganglion neurons (MOC 0.76 and 0.85, respectively) (Figure 2). The phosphorylation of Pyk2 is a major neuronal signaling pathway for integrins (Li et al., 1996) and both β1 and MOR colocalized with phosphorylated Pyk2 in distinct subcellular regions of cultured trigeminal ganglion neurons as assessed by confocal microscopy (Figure 3). Collectively, these observations indicate that RGD-binding integrins are expressed by trigeminal ganglion neurons, and RGD-binding integrins colocalize with MOR in these neurons in distinct regions that are characteristic of focal adhesions.
MOR is isolated in RGD-binding integrin supramolecular complexes
Activated integrins organize supramolecular scaffolds consisting of cytoskeletal domains and associated receptors and signaling molecules that may contribute to the formation of specialized lipid microdomains referred to as “lipid rafts” (Leitinger and Hogg, 2002). Integrin supramolecular complexes (SMCs) were isolated from homogenates of cultured rat trigeminal ganglion neurons and associated molecules were identified by Western blot using monospecific antibodies (Green et al., 1999). Figure 4, lane 1 demonstrates MOR and Gαi in RGD-binding integrin SMCs isolated in 100 mM NaCl-containing buffer. Caveolin-1 (Cav-1), a component of lipid rafts (Baron and Coburn, 2004), is also identified in these SMCs, as is the β1 integrin subunit. These molecules were not identified in negative controls (i.e., using DGR-coated ferromagnetic beads; lane C). Lane 2 depicts results of Western blot analyses of SMCs isolated in 250 mM NaCl-containing buffer. Under these more stringent conditions, Gαi is not detected in RGD-binding integrin SMCs from trigeminal ganglion neurons. However, MOR, Cav-1, and β1 are identified under high ionic strength conditions indicating a stronger interaction of these molecules in SMCs. Collectively, these data indicate that MOR is closely associated with some RGD-binding integrins, possibly as components of lipid rafts that form adjacent focal adhesions of trigeminal ganglion neurons.
MOR agonists inhibit PGE2-stimulated adenylyl cyclase activity in bradykinin-primed trigeminal ganglion neurons
Application of PGE2 (1 μM) to TG cultures, in the presence of the phosphodiesterase inhibitor, rolipram, resulted in a ~120% increase in cAMP accumulation over basal levels (Figure 5). Treatment of TG cultures with BK (10 μM) for 15 min did not alter PGE2-stimulated cAMP accumulation or basal levels of cAMP. The MOR agonist, DAMGO (1 μM), did not inhibit PGE2-stimulated cAMP accumulation under control conditions, however, after exposure to BK for 15 min, DAMGO inhibited PGE2-stimulated cAMP levels.
Figure 5.
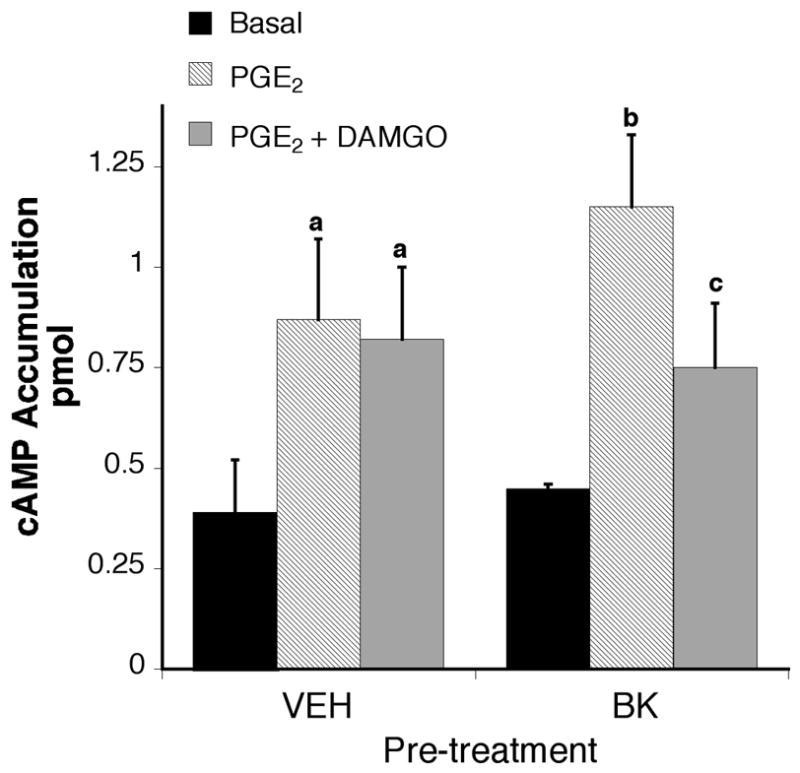
DAMGO inhibits PGE2-induced cAMP accumulation only in the presence of BK. TG primary cultures (5–6 DIC) were incubated with or without bradykinin (10 μM) for 15 min (37°C). After pretreatment, cells were incubated with the phosphodiesterase inhibitor, rolipram (10 μM) with or without the MOR agonist, DAMGO (1 μM) and incubated for 15 min (37 °C) followed by addition of PGE2 (1 μM) and further incubation for 15 min (37°C). Cellular cAMP levels were determined by RIA. Data shown are the mean ± SEM of 5 experiments. Data are expressed as the pmol cAMP accumulated per well. Statistical analysis by one-way ANOVA (F(5,70)=7.009), followed by Tukey’s post-test. A p < 0.05 relative to vehicle basal; b p < 0.05 relative to BK basal; c p < 0.05 compared with the BK-PGE2 condition.
Integrin blocking agents modulate MOR signaling in bradykinin-primed trigeminal ganglion neurons
To assess the role of RGD-binding integrins in the enhancement of MOR signaling by BK, integrin blocking peptides (soluble RGD peptides) and anti-integrin antibodies were used. MOR-mediated inhibition of PGE2-stimulated cAMP accumulation was blocked in the presence of soluble RGD peptides, but not inactive reverse sequence DGR peptides (Figure 6). However, RGD peptides did not block BK-mediated hydrolysis of phosphatidylinositol in TG cultures (Figure 7), suggesting that RGD peptides did not affect BK signaling or have nonspecific effects on cellular metabolism. . Similarly, MOR signaling in BK-primed TG cultures was blocked by monospecific antibodies directed against ß3 integrin subunits (Figure 8). Interestingly, MOR activation by DAMGO significantly enhanced PGE2-stimulated cAMP accumulation after pre-treatment with the anti-ß1 antibody, but not by a denatured anti-ß1 antibody.
Figure 6.
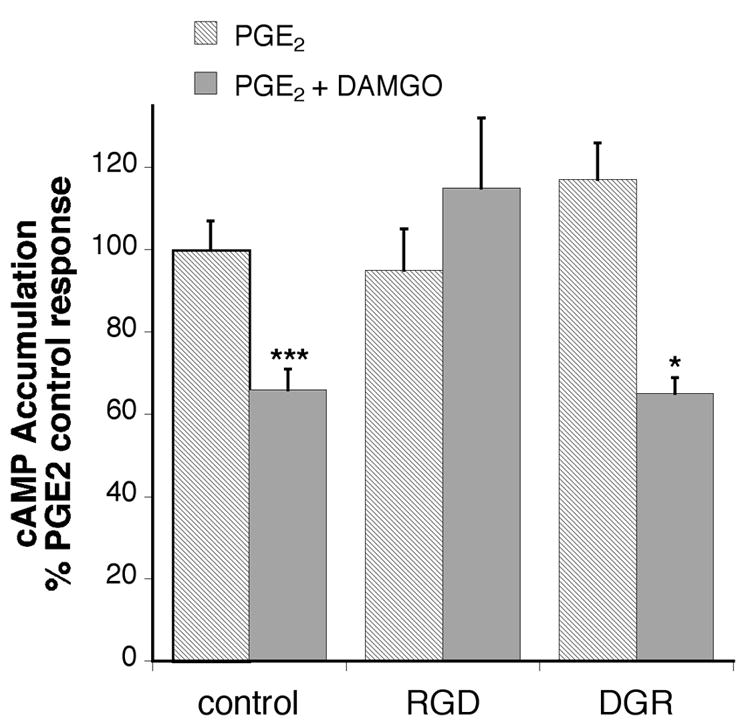
RGD prevents inhibition of cAMP accumulation by DAMGO in BK primed TG cells. TG primary cultures (5–6 DIC) were incubated with or without soluble RGD peptides or reverse (inactive) sequence DGR peptide for 30 min (37°C) followed by addition of bradykinin (10 μM) and further incubation for 15 min (37°C). After pretreatment, cells were incubated with the phosphodiesterase inhibitor, rolipram (10 μM) with or without the MOR agonist, DAMGO (1 μM) and incubated for 15 min (37 °C) followed by addition of PGE2 (1 μM) and further incubation for 15 min (37°C). Cellular cAMP levels were determined by RIA. Data shown are expressed as the percentage of PGE2 stimulation in the presence of bradykinin and are the mean ± SEM of 3–5 experiments The presence of peptides did not alter either basal or PGE2 stimulated cAMP levels. Statistical analysis by one-way ANOVA (F(5,46) = 9.287) followed by Tukey’s post-test. * p < 0.01 comparison between PGE2 and PGE2+DAMGO for each pretreatment condition
Figure 7.
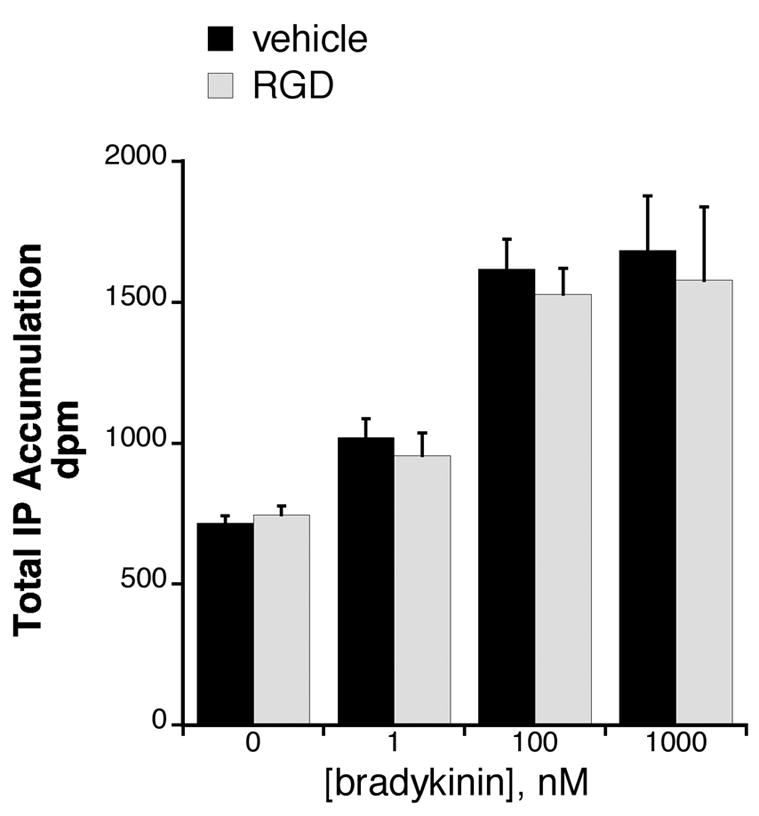
Lack of effect of RGD peptide on BK-mediated hydrolysis of phosphatidylinositol in TG cultures. TG cultures (5–6 DIC) were labeled with [3H]myo-inositol for 24 hr. After a brief wash, cells were incubated with or without soluble RGD peptide for 30 min (37°C) followed by addition of various concentrations of bradykinin for 30 min. Data represent the mean ± SEM of 3 experiments and were analyzed with two-way ANOVA. RGD treatment did not alter BK-mediated IP accumulation (F(1,3)=0.3662).
Figure 8.
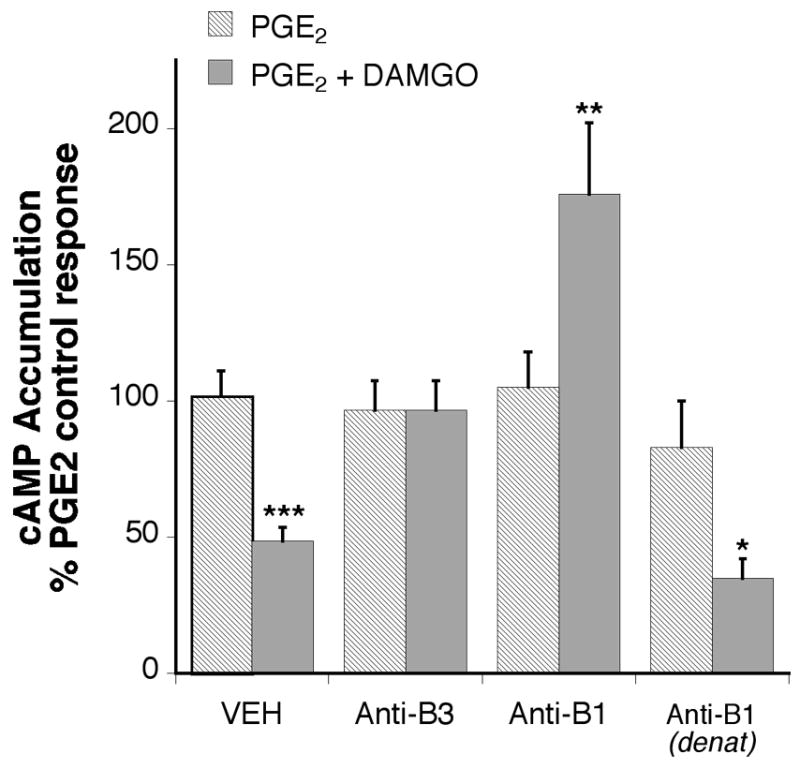
Integrin subtype antibodies reverse DAMGO inhibition of cAMP accumulation in BK primed TG cells. TG cultures were incubated with or without anti-integrin subtype antibodies, or denatured anti-β1 antibody (1:100) for 2 h followed by addition of bradykinin (10 μM) for 15 min. After pre-treatment, cells were incubated with the phosphodiesterase inhibitor, rolipram (10 μM) with or without the MOR agonist, DAMGO (1 μM) and incubated for 15 min followed by addition of PGE2 (1 μM) for 15 min. Cellular cAMP levels were measured with RIA. Data shown are expressed as the percentage of PGE2 stimulation and are the mean +/− SEM of 5 experiments. The presence of antibodies did not alter either basal or PGE2 stimulated cAMP levels. Statistical analysis by one-way ANOVA (F(7,60) = 14.38) followed by Tukey’s post-hoc test. ***p < 0.001, **p < 0.01 and *p < 0.05 effect of DAMGO compared to PGE2 alone for each condition.
DISCUSSION
This study provides the first evidence that RGD-binding integrins play an important role in the modulation of opioid signaling in trigeminal ganglion neurons. β1 and β3 integrins co-localize with MOR in these neurons. In addition, the subcellular distribution of MOR occurs at sites of focal adhesions as evidenced by colocalization with phosphorylated Pyk2, the activated form of Pyk2 found in these adhesions (Li et al., 1996). Furthermore, we provide evidence of at least an indirect linkage between RGD-binding integrins and MOR, associated with lipid rafts as evidence by colocalization with caveolin-1. Caveolin-1 is known to interact with cytoplasmic domains of some β integrin subunits and is a component of lipid rafts (Chapman et al., 1999).
BK pretreatment of trigeminal ganglion cultures significantly enhanced signaling by MOR in our studies. Interestingly, BK-mediated enhancement of MOR signaling was blocked by soluble RGD peptides and anti-integrin antibodies, but not by inactive, DGR peptides or heat-denatured anti-integrin antibodies. It has been reported that integrin activation can modify intracellular calcium levels (Coppolino et al., 1997), which in turn, can affect adenylyl cyclase and hence cAMP production. However, in our experiments both the blocking antibody and soluble RGD peptide have no effect in calcium levels as reported previously (Illario et al., 2003). Furthermore, neither the antibody nor peptide alone had an effect on basal or PGE2-stimulated cAMP levels in our assay.
Integrins do not contain catalytic subunits. Therefore, integrin signaling is dependent on molecular intermediates. When integrins bind their extracellular matrix ligands, they cluster by lateralization in the plasma membrane. By association with adaptor molecules, clustered integrins link with cytoskeletal elements (e.g., f-actin) and signal transduction elements (Petit and Thiery, 2000, Brunton et al., 2004, Grashoff et al., 2004). Hence, integrin ligands (i.e., extracellular matrix molecules), integrins, and cytoskeletal elements assemble into aggregates forming functional microdomains referred to as focal adhesions (Petit and Thiery, 2000). There is strong evidence to suggest that the formation of focal adhesions provide a mechanism by which a multitude of cytoplasmic signal transduction molecules, including members of the Ras/Erk, PI(3)K/Akt, and CAS/JNK pathways, are assembled (Giancotti and Rouslahti, 1999, Schlaepfer et al., 1999, Miranti and Brugge, 2002).
Several studies have suggested that integrins can regulate the function of GPCRs and their associated signaling components. For example, P2Y, a GPCR for ATP, signals thru MAPK via a mechanism that is dependent on integrin activation (Short et al., 2000). In addition, McPhee et al. have provided evidence that G-protein activated potassium channels (GIRKs) may directly interact with some integrins (McPhee et al., 1998). These interactions may regulate the plasma membrane distribution of GIRKs, as well as their functional coupling to the cytoskeleton (McPhee et al., 1998). However, more recent studies have failed to define a functional role for the observed GIRK-integrin interactions (Ivanina et al., 2000).
Integrins may be involved in the organization of plasma membrane microdomains. Plasma membrane microdomains, typically defined by their resistance to detergent solubilization, are enriched with cholesterol and glycosphingolipids which are known to interact directly with integrins (Pande, 2000). Interestingly, the αvβ3 integrin expressed by human ovarian carcinoma cells (OV10) is linked to G proteins by an adaptor molecule known as integrin associated protein or CD47 (Green et al., 1999). Cholesterol depletion by methyl-β-cyclodextrin disrupts the linkage between the integrin and G proteins in these cells, an effect that was reversed by repletion of methyl-β-cyclodextrin extracted cholesterol (Green et al., 1999). This observation is particularly interesting for two reasons. First, it indicates that at least some integrin functions may be dependent on intact plasma membrane microdomains (Leitinger and Hogg, 2002). Second, in light of the fact that opioid receptors (MOR, kappa-OR (KOR), and delta-OR (DOR)) are G-protein coupled receptors, these studies provide the first evidence that some G proteins are directly coupled to specific integrins by molecular intermediates. Therefore, integrins could influence signaling by some G-protein coupled receptors, such as opioid receptors, by regulating the spatial distribution of G proteins in adjacent organized plasma membrane microdomains containing their coupled receptor (e.g., MOR, KOR, DOR). This may occur in trigeminal sensory neurons since our studies demonstrate a physical association between RGD-binding integrins, MOR, and Gαi in cultured trigeminal ganglion neurons. With this in mind, it is interesting to note our observation that the MOR agonist, DAMGO, enhanced PGE2-induced cAMP accumulation in bradykinin-primed trigeminal ganglion neurons after pretreatment with an anti-β1 antibody, but exerted no effect in neurons after pretreatment with an anti-β3 antibody. This observation suggests that effective coupling of G proteins to MOR in trigeminal ganglion neurons is regulated by specific integrins, and that the relative amounts of specific activated integrins at focal adhesions may govern G protein subunit composition (e.g., Gi vs. Gs) associated with competent opioid receptors. MOR has been shown to couple to a variety of G proteins (Connor and Christie, 1999), and while coupling to Gαs has been debated(Chalecka-Franaszek et al., 2000), recent evidence suggests that MOR can also activate Gαs (Szucs et al., 2004, Chakrabarti et al., 2005).
There is evidence that signaling of GPCRs is regulated by dynamic association of these receptors with G proteins at or near specialized membrane microdomains. For example, association with caveolae regulates the relative efficiency of coupling of the ß2-adrenergic receptor to Gαs vs. Gαi (Xiang et al., 2002). In our study, a MOR agonist (DAMGO) significantly decreased cAMP accumulation in PGE2 -stimulated bradykinin-primed trigeminal ganglion neurons, but significantly increased cAMP accumulation in the presence of β1 integrin antagonism. However, β1 integrin antagonism using a monospecific blocking antibody did not affect PGE2 signaling. Collectively, this data suggests that specific integrins differentially regulate MOR signaling by mechanisms that could involve effective coupling of either Gαi or Gαs to the receptor. Perhaps, then MOR (and possibly other GPCRs) signals could be inhibitory or stimulatory in different regions of a neuron dependent on specific local integrin-mediated interactions with the extracellular matrix and membrane microdomains.
In summary, these data suggest that the regulation of the spatial distribution of MOR and/or cognate signaling molecules by RGD-binding integrins may be critical for effective signaling in trigeminal ganglion neurons. This new information may be especially important in the context of inflammation and wound healing. Under these conditions, native integrin ligands (i.e., extracellular matrix molecules such as fibronectin and collagens) undergo extensive modifications by matrix degrading enzymes and free radicals. These modifications likely have a profound effect on integrin function, which could lead to altered opioid receptor function at sensory terminals innervating affected tissues. Therefore, our observations implicate such factors as extracellular matrix turnover as important modulators of primary sensory function and peripheral opioid responses.
Acknowledgments
We would like to thank Dr. Victoria Frolich from the UTHSCSA Core Optical Imaging Facility for helping with the acquisition of confocal microscopy images. The authors also would like to thank Drs. Ted Price, and Amol Patwardhan for helpful discussions, as well as Yong Cui, Ruben Gomez, Teresa Sanchez, and Michelle Silva for their excellent technical assistance. This study was funded by USPS grant DA PO1 016719.
Glossary
- β1
β3, beta 1 or beta 3 integrin subunits
- BK
bradykinin
- cAMP
cyclic-AMP
- Cav-1
caveolin-1
- CNS
central nervous system
- DAMGO
[D-Ala2,N-MePhe4,Gly-ol5]enkephalin
- DGR
abbreviation for peptide sequence SDGRG
- DIC
days in culture
- DMEM
Dulbecco’s modified Eagle’s media
- DOR
delta opioid receptor
- ECL
electrochemiluminescence
- GPCR
G-protein coupled receptor
- HBSS
Hank’s balanced salt solution
- ICC
immunocytochemistry
- IHC
immunohistochemistry
- KOR
kappa opioid receptor
- MOC
Manders overlap coefficient
- MOR
mu opioid receptor
- NGF
nerve growth factor
- NMDA
N-methyl-D-aspartate
- PBS-TDS
phosphate buffered saline-Tris. deoxycholate, sodium dodecyl sulfate
- PGE2
prostaglandin E2
- RGD
abbreviation for peptide sequence GRGDS
- RIA
radioimmunoassay
- SMC
supramolecular complex
- TBST
Tris buffered saline with Tween
- TG
trigeminal ganglia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alenghat FJ, Ingber DE. Mechanotransduction: all signals point to cytoskeleton, matrix, and integrins. Science’s STKE [Electronic Resource]: Signal Transduction Knowledge Environment. 20022002:PE6. doi: 10.1126/stke.2002.119.pe6. [DOI] [PubMed] [Google Scholar]
- Baron CB, Coburn RF. Smooth muscle raft-like membranes. Journal of Lipid Research. 2004;45:41–53. doi: 10.1194/jlr.M300402-JLR200. [DOI] [PubMed] [Google Scholar]
- Berg KA, Clarke WP, Sailstad C, Saltzman A, Maayani S. Signal transduction differences between 5-hydroxytryptamine type 2A and type 2C receptor systems. Molecular Pharmacology. 1994;46:477–484. [PubMed] [Google Scholar]
- Berg KA, Maayani S, Goldfarb J, Scaramellini C, Leff P, Clarke WP. Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Molecular Pharmacology. 1998;54:94–104. [PubMed] [Google Scholar]
- Berridge MJ, Dawsonk RM, Downes CP, Heslop JP, Irvine RF. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. l. Biochemistry Journal. 1983;212:473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvard D, Brakebusch C, Gustafsson E, Aszodi A, Bengtsson T, Berna A, Fassler R. Functional consequences of integrin gene mutations in mice. Circulation Research. 2001;89:211–223. doi: 10.1161/hh1501.094874. [DOI] [PubMed] [Google Scholar]
- Brunton VG, MacPherson IR, Frame MC. Cell adhesion receptors, tyrosine kinases and actin modulators: a complex three-way circuitry. Biochimica et Biophysica Acta. 2004;1692:121–144. doi: 10.1016/j.bbamcr.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Regec A, Gintzler AR. Biochemical demonstration of mu-opioid receptor association with Gs[alpha]: enhancement following morphine exposure. Molecular Brain Research. 2005;135:217. doi: 10.1016/j.molbrainres.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Chalecka-Franaszek E, Weems HB, Crowder AT, Cox BM, Cote TE. Immunoprecipitation of High-Affinity, Guanine Nucleotide-Sensitive, Solubilized μ-Opioid Receptors from Rat Brain. Coimmunoprecipitation of the G proteins G o, G i1, and G i3. Journal of Neurochemistry. 2000;74:1068–1078. doi: 10.1046/j.1471-4159.2000.0741068.x. [DOI] [PubMed] [Google Scholar]
- Chapman HA, Wei Y, Simon DI, Waltz DA. Role of urokinase receptor and caveolin in regulation of integrin signaling. Thrombosis & Haemostasis. 1999;82:291–297. [PubMed] [Google Scholar]
- Chavis P, Westbrook G. Integrins mediate functional pre- and postsynaptic maturation at a hippocampal synapse. Nature. 2001;411:317–321. doi: 10.1038/35077101. [DOI] [PubMed] [Google Scholar]
- Connor M, Christie MJ. Opioid Receptor Signalling Mechanisms. Clinical and Experimental Pharmacology and Physiology. 1999;26:493–499. doi: 10.1046/j.1440-1681.1999.03049.x. [DOI] [PubMed] [Google Scholar]
- Coppolino MG, Dedhar S. Bi-directional signal transduction by integrin receptors. International Journal of Biochemistry & Cell Biology. 2000;32:171–188. doi: 10.1016/s1357-2725(99)00043-6. [DOI] [PubMed] [Google Scholar]
- Coppolino MG, Woodside MJ, Demaurex N, Grinstein S, St-Arnaud R, Dedhar S. Calreticulin is essential for integrin-mediated calcium signalling and cell adhesion. Nature. 1997;386:843–847. doi: 10.1038/386843a0. [DOI] [PubMed] [Google Scholar]
- Curley GP, Blum H, Humphries MJ. Integrin antagonists. Cell Mol Life Sci. 1999;56:427–441. doi: 10.1007/s000180050443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Rocca GJ, Maudsley S, Daaka Y, Lefkowitz RJ, Luttrell LM. Pleiotropic coupling of G protein-coupled receptors to the mitogen-activated protein kinase cascade. Role of focal adhesions and receptor tyrosine kinases. Journal of Biological Chemistry. 1999;274:13978–13984. doi: 10.1074/jbc.274.20.13978. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Rouslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Grashoff C, Thievessen I, Lorenz K, Ussar S, Fassler R. Integrin-linked kinase: integrin’s mysterious partner. Current Opinion in Cell Biology. 2004;16:565–571. doi: 10.1016/j.ceb.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Green J, Zhelesnyak A, Chung J, Lindberg F, Sarafati M, Frazier W, Brown E. Role of cholesterol in formation and functioning of signaling complex involving alpha v beta 3, integrin-associated protein (CD47) and heterotrimeric G proteins. Journal of Cell Biology. 1999;146:673–682. doi: 10.1083/jcb.146.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signalling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Illario M, Cavallo AL, Bayer KU, Di Matola T, Fenzi G, Rossi G, Vitale M. Calcium/calmodulin-dependent protein kinase II binds to Raf-1 and modulates integrin-stimulated ERK activation. Journal of Biological Chemistry. 2003;278:45101–45108. doi: 10.1074/jbc.M305355200. [DOI] [PubMed] [Google Scholar]
- Ivanina T, Neusch C, Li YX, Tong Y, Labarca C, Mosher DF, Lester HA. Expression of GIRK (Kir3.1/Kir3.4) channels in mouse fibroblast cells with and without beta1 integrins. FEBS Lett. 2000;466:327–332. doi: 10.1016/s0014-5793(99)01738-x. [DOI] [PubMed] [Google Scholar]
- Leitinger B, Hogg N. The involvement of lipid rafts in the regulation of integrin function. Journal of Cell Science. 2002;115:963–972. doi: 10.1242/jcs.115.5.963. [DOI] [PubMed] [Google Scholar]
- Li J, Avraham H, Rogers RA, Raja S, Avraham S. Characterization of RAFTK, a novel focal adhesion kinase, and its integrin-dependent phosphorylation and activation in megakaryocytes. Blood. 1996;88:417–428. [PubMed] [Google Scholar]
- Litvak V, Tian D, Shaul YD, Lev S. Targeting of PYK2 to focal adhesions as a cellular mechanism for convergence between integrins and G protein-coupled receptor signaling cascades. Journal of Biological Chemistry. 2000;275:32736–32746. doi: 10.1074/jbc.M004200200. [DOI] [PubMed] [Google Scholar]
- Manders E, Verbeek F, Aten J. Measurement of colocalization of objects in dual-colour confocal images. Journal of Microscopy. 1993;169:375–382. doi: 10.1111/j.1365-2818.1993.tb03313.x. [DOI] [PubMed] [Google Scholar]
- Martin KH, Slack JK, Boerner SA, Martin CC, Parsons JT. Integrin connections map: to infinity and beyond. Science. 2002;296:1652–1653. doi: 10.1126/science.296.5573.1652. [DOI] [PubMed] [Google Scholar]
- McPhee JC, Dang YL, Davidson N, Lester HA. Evidence for a functional interaction between integrins and G protein-activated inward rectifier K+ channels. Journal of Biological Chemistry. 1998;273:34696–34702. doi: 10.1074/jbc.273.52.34696. [DOI] [PubMed] [Google Scholar]
- Melker AAd, Sonnenberg A. Integrins: alternative splicing as a mechanism to regulate ligand binding and integrin signaling events. Bioessays. 1999;21:499–509. doi: 10.1002/(SICI)1521-1878(199906)21:6<499::AID-BIES6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Milam SB, Haskin C, Zardeneta G, Chen D, Magnuson VL, Klebe RJ, Steffenson B. Cell adhesion proteins in oral biology. Critical Reviews in Oral Biology & Medicine. 1991;2:451–491. doi: 10.1177/10454411910020040201. [DOI] [PubMed] [Google Scholar]
- Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Nature Cell Biology. 2002;4:83–90. doi: 10.1038/ncb0402-e83. [DOI] [PubMed] [Google Scholar]
- Pande G. The role of membrane lipids in the regulation of integrin functions. Current Opinion in Cell Biology. 2000;12:569–574. doi: 10.1016/s0955-0674(00)00133-2. [DOI] [PubMed] [Google Scholar]
- Petit V, Thiery JP. Focal adhesions: structure and dynamics. Biology of the Cell. 2000;92:477–494. doi: 10.1016/s0248-4900(00)01101-1. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Progress in Biophysics & Molecular Biology. 1999;71:435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- Short SM, Boyer JL, Juliano RL. Integrins regulate the linkage between upstream and downstream events in G protein-coupled receptor signaling to mitogen-activated protein kinase. Journal of Biological Chemistry. 2000;275:12970–12977. doi: 10.1074/jbc.275.17.12970. [DOI] [PubMed] [Google Scholar]
- Slack BE, Siniaia MS. Adhesion-dependent redistribution of MAP kinase and MEK promotes muscarinic receptor-mediated signaling to the nucleus. Journal of Cellular Biochemistry. 2005;95:366–378. doi: 10.1002/jcb.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szucs M, Boda K, Gintzler AR. Dual effects of DAMGO [D-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin and CTAP (D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2) on adenylyl cyclase activity: implications for mu-opioid receptor Gs coupling. Journal of Pharmacology & Experimental Therapeutics. 2004;310:256–262. doi: 10.1124/jpet.104.066837. [DOI] [PubMed] [Google Scholar]
- van der Flier A, Sonnenberg A. Function and interactions of integrins. Cell & Tissue Research. 2001;305:285–298. doi: 10.1007/s004410100417. [DOI] [PubMed] [Google Scholar]
- Vasko MR, Campbell WB, Waite KJ. Prostaglandin E2 enhances bradykinin-stimulated release of neuropeptides from rat sensory neurons in culture. Journal of Neuroscience. 1994;14:4987–4997. doi: 10.1523/JNEUROSCI.14-08-04987.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildering WC, Hermann PM, Bulloch AGM. Rapid neuromodulatory actions of integrin ligands. Journal of Neuroscience. 2002;22:2419–2426. doi: 10.1523/JNEUROSCI.22-07-02419.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Rybin VO, Steinberg SF, Kobilka B. Caveolar localization dictates physiologic signaling of beta 2-adrenoceptors in neonatal cardiac myocytes. Journal of Biological Chemistry. 2002;277:34280–34286. doi: 10.1074/jbc.M201644200. [DOI] [PubMed] [Google Scholar]
- Zamir E, Geiger B. Molecular complexity and dynamics of cell-matrix adhesions. Journal of Cell Science. 2001;114:3583–3590. doi: 10.1242/jcs.114.20.3583. [DOI] [PubMed] [Google Scholar]


