Abstract
Mammary epithelial cells undergo changes in growth, invasion, differentiation, and dedifferentiation throughout much of adult hood, and most strikingly during pregnancy, lactation, and involution. Clusterin is a multifunctional glycoprotein that is involved in the differentiation and morphogenesis of epithelia, and that is important in the regulation of postnatal mammary gland development. However, the mechanisms that regulate clusterin expression are still poorly understood. Here, we show that clusterin is up-regulated twice during mouse mammary gland development, a first time at the end of pregnancy and a second time at the beginning of the involution. These points of clusterin up-regulation coincide with the dramatic phenotypic and functional changes occurring in the mammary gland. Using cell culture conditions that resemble the regulatory microenvironment in vivo, we determined that the factors responsible for the first up-regulation of clusterin levels can include the extracellular matrix component, laminin, and the lactogenic hormones, prolactin and hydrocortisone. On the other hand, the second and most dramatic up-regulation of clusterin can be due to the potent induction by TGF-β1, and this up-regulation by TGF-β1 is dependent on β1 integrin ligand-binding activity. Moreover, the level of expression of β-casein, a marker of mammary epithelial cell differentiation, was decreased upon treatment of cells with clusterin siRNA. Overall, these findings reveal several novel pathways for the regulation of clusterin expression during mammary gland development, and suggest that clusterin is a morphogenic factor that plays a key role during differentiation.
Keywords: Development, Pregnancy, Lactation, Involution, Lactogenic hormones, Extracellular matrix, Laminin, Integrins, TGF-β1
INTRODUCTION
Clusterin is a multifunctional secretory glycoprotein, which was discovered about 20 years ago from ram rete testis as a protein causing clustering of the erythrocyte (1,2). Besides the name of clusterin, this protein has many different names, including apolipoprotein J, SGP-2, glycoprotein III, and TRPM-2. Clusterin is found in all body fluids including plasma, milk, urine and cerebrospinal fluid as well as on the surface of cells lining body cavitities (3). Clusterin is highly conserved across the species showing 70–80% identity at the amino acid level among mammals. No homologs have been identified in fruit flies or yeast.
Clusterin is important for the regulation of differentiation and morphogenesis of epithelia and multiple experiments using clusterin antisense strategy have previously implied a key role of this glycoprotein in the regulation of epithelial cell phenotypes (4,5). Clusterin can modulate cell-cell and/or cell-matrix interactions, and has a variety of functions including transporting lipoproteins, the inhibition of complement-mediated cell lysis, regulation of survival/apoptosis, and tissue remodeling. It has been shown that clusterin is up-regulated in many different situations. For examples, in prostate regression after castration, in the brain neurodegeneration such as Alzheimer’s disease, in the response to injury and other stresses (heat shock or oxidative stress, etc.), and in the developmental remodeling (4–8). Clusterin can also interact with a wide range of molecules such as lipids, amyloid proteins, complement components, immunoglobulin, and TGF-β receptors (9). As the biological significance of clusterin is being slowly deciphered, particularly its role as a promoter or inhibitor of cell death, many questions remain unanswered, including the mechanisms for regulation of its expression.
The mammary gland undergoes striking changes in morphology and function during development, puberty and adult life, and is a useful model for dissect molecular mechanisms of tissue morphogenesis and function. During each menstrual cycle, and particularly during pregnancy, mammary epithelial cells undergo cycles of proliferation, invasion, differentiation, dedifferentiation, and cell death. During the first part of pregnancy, mammary epithelial cells proliferate, invade the surrounding stromal extracellular matrix (ECM), and form lobulo-alveolar structures that prepare the gland for lactation (10–12). During late pregnancy, prior to parturition, breast epithelial cells cease proliferation and invasion, and functionally differentiate into cells that express and secrete milk proteins. Throughout lactation, epithelial cells continue to express milk proteins (11). After weaning, the mammary gland undergoes involution, a phase of extensive remodeling characterized by degradation of ECM and epithelial cell death by apoptosis (13,14).
It has been shown that clusterin is up-regulated at the beginning of involution (14). However, the signals that regulate clusterin expression during mammary gland development are still poorly understood. The aim of this study is to elucidate some of these signaling events. We show that clusterin expression is, in fact, up-regulated twice during mammary gland development, a first time at the end of the pregnancy and a second time at the beginning of involution. Using cell culture models, we show that the first up-regulation of clusterin may be triggered by laminin, prolactin and hydrocortisone. On the other hand, the second up-regulation of clusterin appears to be dependent on the levels of hydrocortisone, TGF-β1, as well as β1 integrin ligand-binding activity. Using clusterin siRNA, we also determine that a decrease in clusterin levels triggers a reduction of β-casein expression, a marker of mammary epithelial cell differentiation.
MATERIALS AND METHODS
Mammary Gland Extracts
Mammary tissues were obtained from BALB/C female mice, purchased either from Simonsen Laboratories, Inc. (Gilroy, CA) or Harlan (San Diego, CA). To obtain mammary tissue during pregnancy, lactation and involution, 12-week-old virgin females were mated. In all cases, the fourth inguinal mammary glands were harvested. Virgin animals were sacrificed at 5, 7, 12, and 18 weeks of age; pregnant animals were sacrificed at 2, 5, 12, and 18 days after onset of pregnancy; lactating animals were sacrificed at 2, 7, 12, and 20 days after onset of lactation; and animals undergoing involution were sacrificed at 1, 2, 3, 5, and 6 days after weaning. Mammary glands were immediately frozen at −70 °C. Proteins were extracted using TriPure isolation reagents (Roche Molecular Biochemicals).
Cell Culture
SCp2-Id-1 cells were generated by transfecting SCp2 mouse mammary epithelial cells with the MMTV-Id-1 expression vector (15). Cells were grown in Dulbecco's modified Eagle's and Ham's F12 media (DME-F12 1:1) containing 5% fetal bovine serum, insulin (5 μg/ml, Sigma), and gentamicin (50 μg/ml; Invitrogen, CA) at 37 °C in humidified CO2 5% atmosphere. The derivation of EpH4 cells has been described previously (16). EpRas cells were obtained from Dr. Ernst Reichmann (Universitäts-Kinderspital Zürich, Zurich), and a non-malignant cell clone derived from Ras-transfected EpH4 cells (17). EpH4, EpRas, and NMuMG mouse mammary epithelial cell lines were cultured in DMEM-F12 medium containing 2% fetal bovine serum, insulin (5 μg/ml), and gentamicin (10 μg/ml). To induce differentiation, cells were plated with either 1.0–1.5% basement membrane components or 50–150 μg/ml laminin (Sigma) in DME-F12 lacking serum but containing lactogenic hormones (insulin, 5 μg/ml; hydrocortisone, 1.4x10−6M; prolactin, 5 μg/ml), as described (15). Basement membrane was supplied as growth factor reduced Matrigel from Collaborative Research. For serum starvation, cells were cultured in medium containing insulin and gentamicin for 2–3 days.
Reagents and Antibodies
TGF-β1 (R&D Systems, Inc.), EGF, bFGF and HGF (BD Biosciences), PDGF (Oncogene Research products), and TGF-α (Biosource) were purchased. For some experiments, cells were cultured with growth factor in the medium containing insulin and gentamicin for 2 days. The function-blocking integrin antibodies against α6 (GoH3) and β1 (Ha2/5 and 9EG7) subunits were purchased as azide- and endotoxin-free reagents from BD Biosciences.
RNA isolation and Northern Analysis
Total RNA was isolated and purified as described (18). RNA (15 μg) were electrophoresed through formaldehyde-agarose gels and transferred to a nylon membrane (Hybond-N; Amersham Biosciences). Membranes were hybridized with 32P-labeled probes prepared by random oligonucleotide priming (Amersham Biosciences), washed and exposed for autoradiography. The probe of clusterin was 450 bp partial mouse clusterin cDNA obtained by subtractive hybridization. Subtractive hybridization was performed between SCp2-Id-1 transfected cells and SCp2-control cells according to the manufacturer’s instructions (QIAGEN). The β-actin probe was purchased from CLONTECH and used as a loading control. 28S and 18S ribosomal RNA are shown as controls for RNA integrity and quantification.
Western Analysis
Cells were rinsed twice with PBS and lysed in 2% SDS, 5% glycerol, 62.5 mM Tris-HCl (pH6.8). Proteins were separated by SDS-polyacrylamide gel electrophoresis using readymade gels (Biowhittaler, Walkerville, MD) and blotted to Immobilion-P membranes (Amersham Biosciences). The membranes were blocked in 5% nonfat milk in TBST buffer (20 mM Tris-HCl (pH7.5), 100 mM NaCl, 0.1% (v/v) Tween-20), and probed with anti-clusterin antibody that recognizes a 40 kDa protein (M18; Santa Cruz Biotechnology), anti-E-cadherin antibody (clone 34; Transduction Laboratories, Lexington, KY), or anti-β1-integrin antibody (Transduction Laboratories, Lexington, KY). The membranes were washed and incubated with secondary antibody conjugated with horseradish peroxidase (Santa Cruz Biotechnology). Detection for the bound antibodies was achieved with ECL-Plus or ECL substrate (Amersham Biosciences), according to the manufacturer’s instructions.
siRNA Experiments
The siRNAs used in this study were supplied by Dharmacon Research Inc. (Lafayette, CO). The sequence of siRNA corresponding to the clusterin initiation site was: 5'-GCAGCAGAGUCUUCAUCAU-3'. The sequence of the negative control siRNA, a non-targeting siRNA used as a control for non-specific effects in mouse cells, was: 5'-CAGCGCUGACAACAGUUUCAU-3'. As a positive control, we used the GAPDH siRNA provided by Dharmacon Research Inc. Lipofectin, a cationic lipid (Invitrogen Life Technologies, Inc., Burlington, Ontario, Canada), was used to enhance transfection of cells with the siRNA. The EpH4 cells were treated with 60 nM siRNA after 20 min preincubation with 4 μg/ml lipofectin in serum free OPTI-MEM (Invitrogen). Four hours after starting the incubation, the medium containing siRNA and lipofectin was replaced with culture medium containing 1% Matrigel and lactogenic hormones as described above. Mammary epithelial cells were incubated in the differentiation medium for 2 or 3 consecutive days then harvested.
RESULTS
Isolation of partial clusterin cDNA by subtractive hybridization
The clusterin gene was detected in a screen for genes modulated by the transcriptional regulator and inhibitor of differentiation Id-1, using the mouse mammary epithelial cell line SCp2 (15). When induced to differentiate, SCp2 cells that ectopically expressed Id-1 showed loose cell-cell interactions. Using subtractive hybridization method, performed between SCp2-control cells and SCp2-Id-1 transfected cells as described previously (19), we isolated a cDNA fragment (450 bp). Partial sequencing of this cDNA fragment revealed that it corresponded to the mouse clusterin (also called apolipoprotein J, SGP-2, glycoprotein III, or TRPM-2) gene. Using this cDNA as a probe, a 1.9kb clusterin mRNA was detected in SCp2 cells (Fig. 1). We found a higher expression of the clusterin gene in serum-starved condition as compared with serum containing condition in SCp2-control cells. Moreover, the clusterin gene was preferentially down-regulated in serum-starved SCp2-Id-1 transfected cells, where ectopic expression of Id-1 could mimic some of the effects of the serum, compared with serum-starved SCp2-control cells (SCp2-ctl). These data suggested that loss of clusterin expression, associated with a decrease in cell-cell interaction here and in other cell types (2), was a consequence of the constitutive expression of Id-1.
Figure 1.
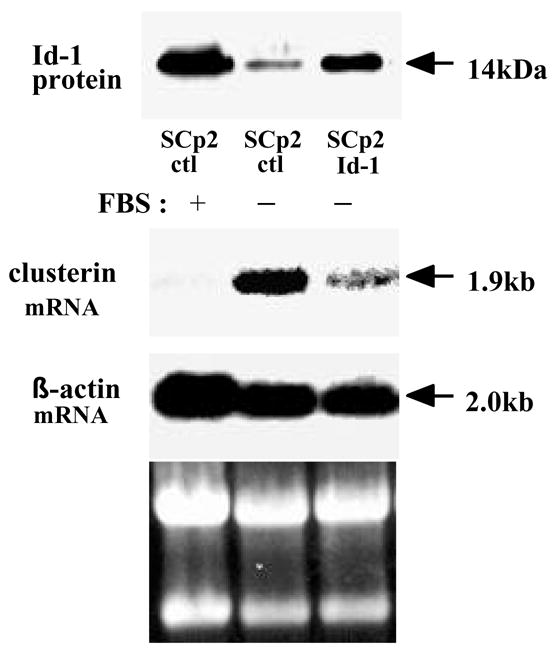
Clusterin expression in SCp2 mammary epithelial cells. SCp2-control cells were cultured in serum-containing (lane 1), or serum-starved conditions (lane 2). SCp2-Id-1 cells were cultured in serum-starved conditions (lane 3). Total RNA was extracted and Northern analysis was performed.
Clusterin expression during mouse mammary gland development
We next examined clusterin expression during normal mouse mammary gland development in vivo using Northern analysis, to observe its regulation in relation to the different stages of mammary gland proliferation and differentiation (Fig. 2) as we previously determined for the expression of Zfp289 (19) and for the expression of ß-casein and Id-1 (20). Consistent with previous observations (14), we detected a strong and transient up-regulation of clusterin mRNA at the beginning of involution (between day 20 of lactation and day 1 of involution). In addition, we detected another up-regulation during the second part of pregnancy (between day 12 and day 18). The up-regulation during pregnancy was however weaker than the one detected at the beginning of involution. These changes in clusterin expression correlate with alterations in mammary gland architecture and function. Moreover, these changes correlate inversely with Id-1 expression. We previously determined that Id-1 was down-regulated during the second part of pregnancy (when clusterin expression increases) and was up-regulated at day 3 of involution (when clusterin expression decreases) (20).
Figure 2.
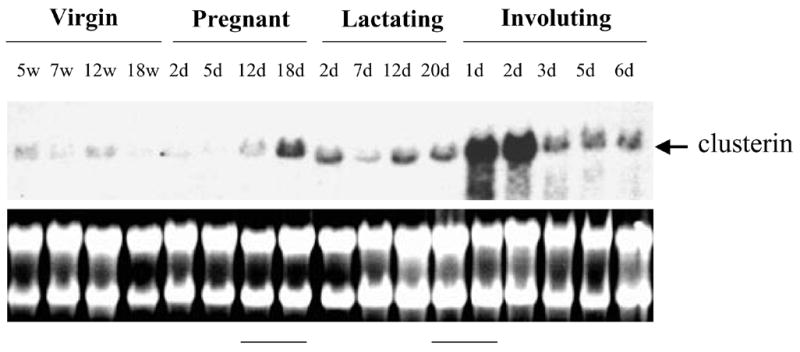
Clusterin expression during mouse mammary gland development in vivo. Total RNA was isolated from mammary glands at different stages of postnatal development and analyzed by Northern blot. Virgin mice: 5, 7, 12 and 18 weeks after birth; Pregnant mice: 2, 5, 12, and 18 d after onset of pregnancy; Lactating mice: 2, 7, 12, and 20 d after onset of lactation; Involuting mice: 1, 2, 3, 5 and 6 d after onset of involution.
Effect of extracellular matrix and lactogenic hormones on clusterin expression
The first up-regulation of clusterin that occurs at the end of pregnancy corresponds to the onset of lactogenesis. We therefore examined the role of known regulators of lactogenesis, including the extracellular matrix (Matrigel or laminin) and lactogenic hormones in this regulation. Culture of SCp2 cells with Matrigel, as well as with insulin, hydrocortisone and prolactin, represents a well-studied model to investigate the pathways of mammary epithelial cell differentiation (15). Clusterin mRNA was gradually increased during the course of treatment (Fig. 3A). When cultured with lactogenic hormones and with laminin, the major component of extracellular matrix that induces a faster mammary epithelial cell differentiation than Matrigel, clusterin expression was markedly increased within 12 hours (Fig. 3B).
Figure 3.
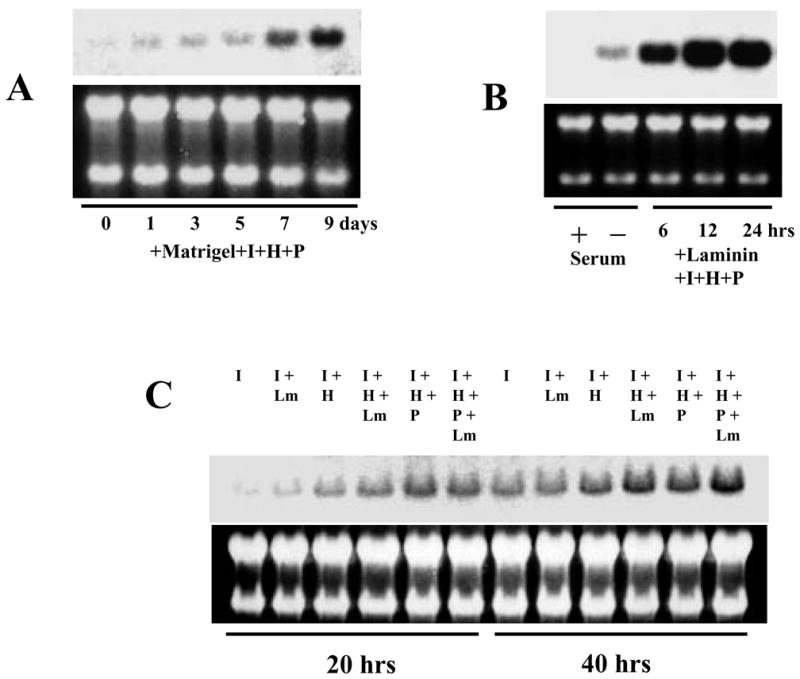
Effect of Matrigel, laminin and lactogenic hormones on clusterin expression. SCp2 cells were cultured and total RNA was extracted and analyzed on Northern blots. (A) Cells were cultured with 1.0% of Matrigel and RNA was extracted at 0, 1, 3, 5, 7 and 9 days. (B) Cells were cultured with (+) or without (−) serum or with 50 μg/ml of laminin and insulin, hydrocortisone and prolactin, and RNA was extracted at 6, 12 and 24 hrs. (C) Cells were cultured in the presence of different combinations of laminin, insulin, hydrocortisone and prolactin, and RNA was extracted after 20 and 40 hrs.
To determine the key factor responsible for clusterin upregulation, we tested different combinations of lactogenic factors for their ability to regulate clusterin expression. Each of the different factors, i.e. laminin (Lm), hydrocortisone (H) or prolactin (P), appeared to have a weak effect individually. However, the combination of these three factors had a strong effect on the induction of clusterin expression (Fig. 3C). We conclude that clusterin up-regulation occurring at the end of pregnancy might be due to lactogenic hormones, and supported by the surrounding extracellular matrix (particularly laminin).
Effect of TGF-β1 in mammary epithelial cells
At the beginning of involution, differentiated mammary epithelial cells stop producing milk protein and undergo apoptosis. Several regulatory factors are known to be induced at beginning of involution (21). One potent factor is TGF-β1 (14). TGF-β1 is detected in early involution and might act as a potential modulator of apoptotic cell death. TGF-β1 also acts as a growth inhibitor in the mouse mammary gland (22,23).
To determine whether TGF-β1 plays a role in the induction of clusterin expression, we treated four different mammary epithelial cell lines with TGF-β1. After 2 days treatment, clusterin was up-regulated in all the cell lines (Fig. 4A), particularly in mouse mammary epithelial EpH4 cells. Since clusterin expression in SCp2 cells was much lower than in EpH4 cells, we decided to focus on the later cell line to determine the role of TGF-β1 in the regulation of clusterin expression.
Figure 4.
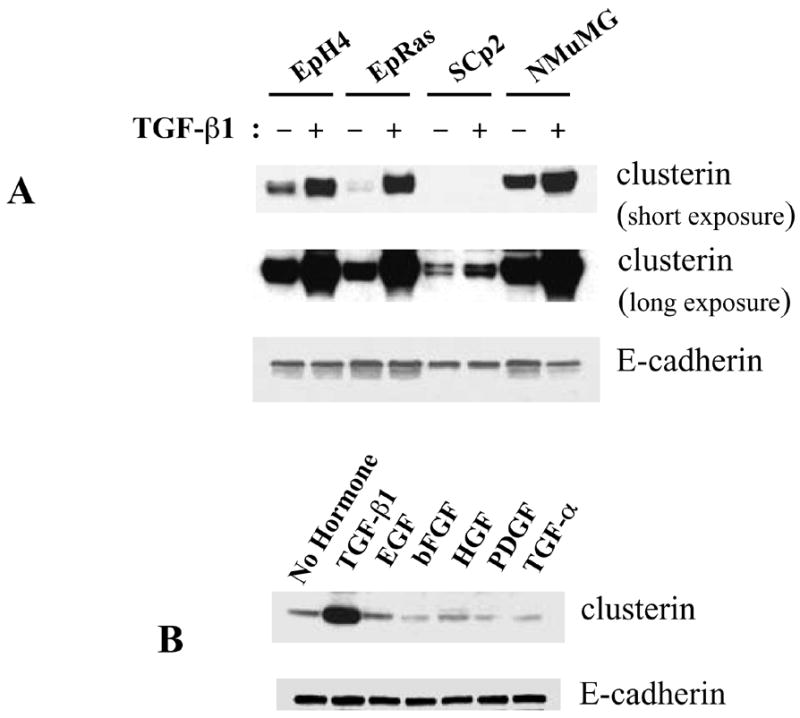
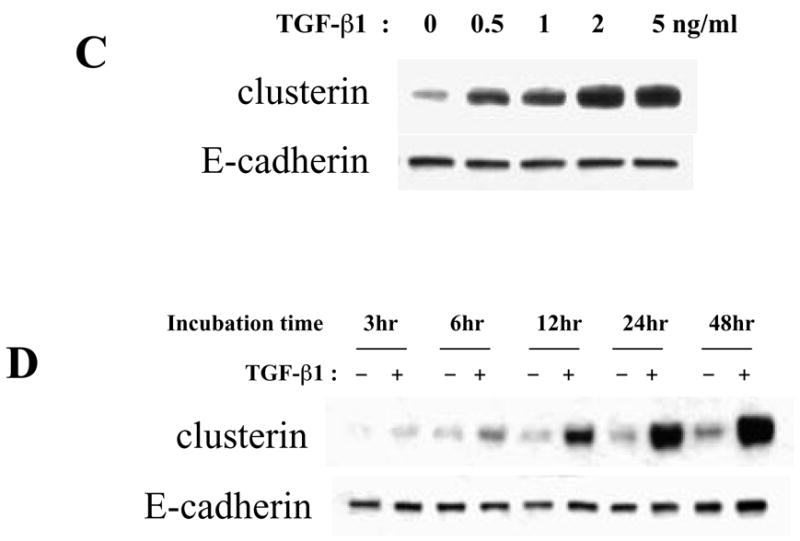
Effect of TGF-β1 on clusterin expression in mammary epithelial cell lines. Protein was extracted and analyzed on Western blots. (A) Mammary epithelial cell lines were cultured with 2 ng/ml of TGF-β1 for 2 days. Since clusterin expression in SCp2 was low compared to the other cell lines, here is shown a short as well as a long exposure of the same blot. (B) EpH4 cells were cultured with 2 ng/ml of TGF-β1 and, 40 ng/ml of EGF, bFGF, HGF, PDGF and TGF-α for 2 days. (C) EpH4 cells were cultured with 0, 0.5, 1, 2 or 5 ng/ml of TGF-β1 for 2 days. (D) EpH4 cells were cultured with or without 2 ng/ml of TGF-β1, and clusterin protein expression determined at 3, 6, 12, 24 and 48 hrs.
To determine whether clusterin was specifically induced by TGF-β1, we treated EpH4 cells with multiple growth factors. There was no up-regulation of clusterin upon treatment with EGF, bFGF, HGF, PDGF and TGF-α (Fig. 4B). Clusterin was specifically up-regulated by TGF-β1, at concentrations as low as 0.5 ng/ml (Fig. 4C) and within a few hours of treatment (Fig. 4D).
Effect of lactogenic hormones in TGF-β1 treated mammary epithelial cells
It was previously reported that the levels of prolactin and hydrocortisone are high during lactation, and that there is a dramatic drop of their expression at the transition from lactation to involution (24). We therefore determined the effects of these lactogenic hormones on clusterin expression in TGF-β1-treated EpH4 cells. Upon treatment with TGF-β1, clusterin was strongly induced when cells were cultured with or without Matrigel (Fig. 5). Paradoxically, clusterin up-regulation by TGF-β1 was suppressed by hydrocortisone (Fig. 5). Prolactin alone did not have major effect on clusterin expression. Since the degradation of basement membrane only starts after 2 to 3 days of involution (25), epithelial cells are still surrounded by extracellular matrix at the beginning of involution. Therefore, it is not surprising that clusterin is highly expressed when cells are treated with Matrigel and TGF-β1, in the absence of hydrocortisone and prolactin, conditions that resemble the beginning of involution when clusterin is strongly induced in the mammary gland.
Figure 5.
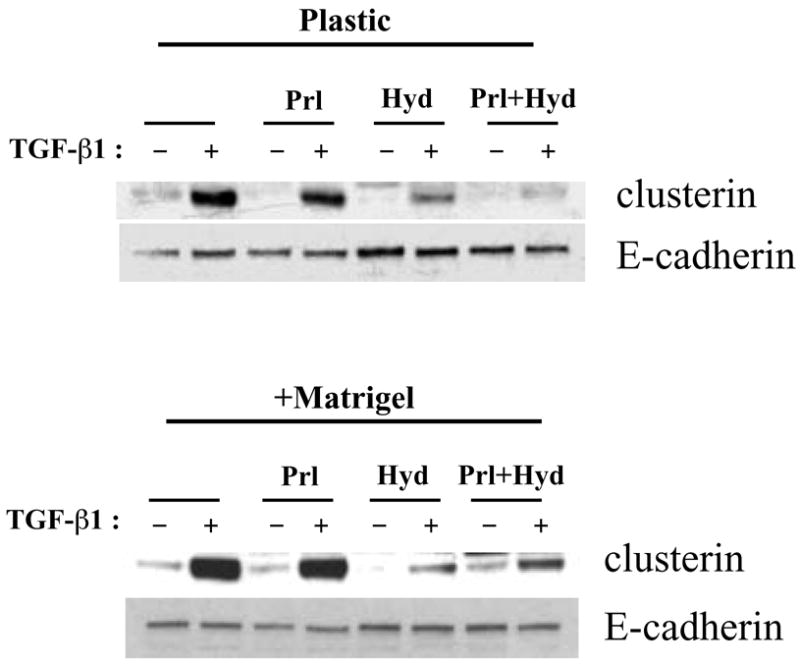
Effect of lactogenic hormones on clusterin expression in TGF-β1 treated cells. EpH4 cells were cultured with or without Matrigel and treated with or without 2 ng/ml of TGF-β1 for 2 days under the presence of prolactin, hydrocortisone, or both prolactin and hydrocortisone. Protein was extracted and analyzed by Western blotting.
Effect of β1 integrin function blocking antibody
Clusterin is highly expressed at day 1 and 2 of involution, and strongly down-regulated at day 3 (Fig. 2A). This down-regulation occurs despite the continued presence of TGF-β1, which was reported to peak after 5 to 6 days of involution (14). Therefore, we investigated the identity of the factors that suppress clusterin expression at the day 3 of the involution.
We hypothesized that disruption in extracellular matrix signaling contributes to clusterin down-regulation at day 3 of involution. To test this hypothesis, we used integrin function-blocking antibodies to block ECM signaling, and determined the effects on clusterin expression. Cells were treated with the function blocking antibody against β1 integrin or α6 integrin, as well as Matrigel and TGF-β1. The β1 integrin-blocking antibody potently suppressed clusterin expression, while there was no effect from the α6 integrin-blocking antibody (Fig. 6A). Since clusterin is a secretory protein, we also used conditioned medium to detect clusterin expression. As shown in Fig. 6B, clusterin levels in condition medium were also decreased by adding β1 integrin blocking antibody. Therefore, the dramatic down-regulation of clusterin expression observed at day 3 of involution could be a consequence of a disruption of ECM signaling through the degradation of the basement membrane.
Figure 6.
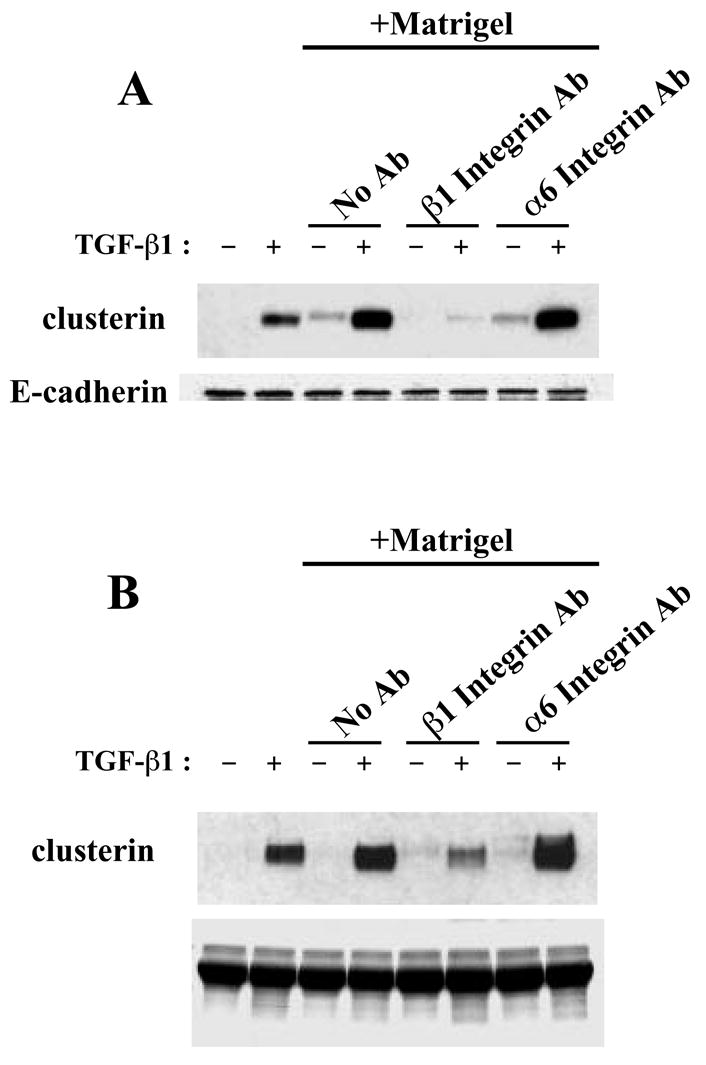
Effect of integrin function-blocking antibodies on clusterin expression. EpH4 cells were cultured with function-blocking antibodies against β1 integrin or α6 integrin in the presence of Matrigel, and with or without treatment of TGF-β1 for 3 days. (A) Western analysis using whole cell extracts. (B) Western analysis using condition medium.
Effect of a reduction of clusterin expression on mammary epithelial cell differentiation
A key role of clusterin in the regulation of epithelial cell phenotypes has been reported in many different cell types. The increase in clusterin levels, which we detected at the end of pregnancy (Fig. 2) and after treatment of cells with extracellular matrix and lactogenic hormones (Fig. 3), was either a necessary event for mammary epithelial cell differentiation and β-casein expression or was only correlative. To test for a direct role of clusterin, we treated mammary epithelial cells with either 60 nM of a negative control siRNA or a clusterin-specific siRNA and determined the effects on β-casein expression. Four hours after starting the incubation, the medium containing siRNA was replaced with culture medium containing Matrigel and lactogenic hormones. Clusterin protein expression was determined after 2 days of treatment and β-casein protein expression after 3 days. In the cells treated with the clusterin-specific siRNA, a marked decrease in β-casein expression was observed, relative to control-treated cells (Fig. 7). The level of decrease in β-casein expression was proportional to the level of clusterin down-regulation.
Figure 7.
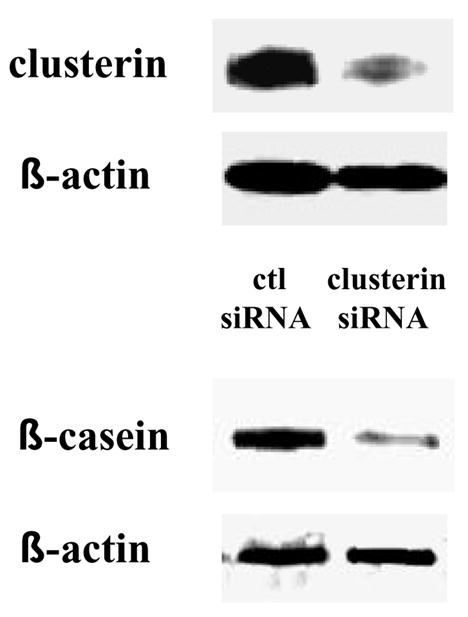
Effect of a reduction of clusterin protein expression on mammary epithelial cell differentiation. EpH4 cells were treated with either 60 nM of negative control siRNA or clusterin siRNA. Four hours after starting the incubation, the medium containing siRNA was replaced with culture medium containing 1% Matrigel and lactogenic hormones. Cells were incubated in the differentiation medium for 2 or 3 consecutive days then harvested. Clusterin expression was determined after day 2 of treatment and β-casein expression after day 3.
DISCUSSION
Clusterin is a multifunctional glycoprotein and is ubiquitously expressed and secreted in body fluids (1). It has been shown that clusterin is up-regulated under many different circumstances, such as developmental remodeling, brain neurodegeneration, and the response of injury or other stresses (4). Multiple experiments imply a key role of clusterin in the regulation of epithelial cell phenotypes. For example, the depletion of clusterin can lead to the programmed cell death in ovary (26), and its knock-down via antisense RNA in neoplastic epidermoid cells enhances proliferation (27). Clusterin cannot only suppress epithelial cell proliferation in vitro, but can also interfere with the promotion stage of skin carcinogenesis (27). Moreover, clusterin was also found to be important for the regulation of tubuloalveolar morphogenesis and alveolar epithelial cell differentiation in the adult rat mammary gland (28).
During mouse mammary gland development, we determined that clusterin expression increased during the second part of pregnancy. During these stages, mammary epithelial cells cease proliferation and functionally differentiate into cells that express and secrete milk proteins. The extracellular matrix component, laminin, as well as the lactogenic hormones, hydrocortisone and prolactin, are required for mammary epithelial cells to differentiate. Using mammary epithelial cells in culture, we determined that laminin, hydrocortisone and prolactin were necessary for the fully induction of clusterin expression, which may explain the induction of clusterin at the end of pregnancy.
The second up-regulation of clusterin, occurring at the beginning of involution, is very strong and transient. This induction has been previously reported (13). However, we now provide some possible pathways behind this dramatic up-regulation of clusterin expression. The increase of clusterin expression that occured at the beginning of involution (day 1 and 2) may be due increased levels of TGF-β1 coupled with low level of hydrocortisone, and the decrease of clusterin levels at day 3 of involution could be a consequence of diminished signaling through β1 integrins.
During mammary gland development, apoptosis occurs at the beginning of involution (14). Epithelial cells begin to undergo apoptosis at day 1 of involution and the number of apoptotic cells continues to increase during subsequent days. At day 5 of involution, apoptotic cells, detected by TUNEL, decrease because most of the epithelial cells already undergone cell death (13). TGF-β1 is induced in early involution and it has been thought that TGF-β1 might act as a potential modulator of apoptotic cell death in addition to regulating the expression of tissue remodeling enzymes (22). Since clusterin is up-regulated at the onset of involution and its level are increased by TGF-β1, it is possible that clusterin acts as an important mediator of TGF-β1 actions. It has already been proposed that clusterin acts as an anti-apoptotic agent, while some reports state that the function of clusterin is pro-apoptotic (4,5). It was also published that clusterin has anti-proliferative properties (29,30).
The up-regulation of clusterin by TGF-β1 that we detected in vitro may occur thought the induction of the c-fos protein. It has been previously reported that c-fos is one of the regulators of clusterin expression, that the levels of c-fos mRNA were increased at the beginning of involution and that AP-1 DNA binding activity was detectable at days 1 and 2, and dropped to low level at days 3 and 4 (31,32). Since clusterin promoter sequence possesses a consensus AP-1 binding site, we suggest that the up-regulation of clusterin by TGF-β1 may be modulated through the induction of c-fos.
We also determined that clusterin is regulated by the lactogenic hormone hydrocortisone. During mouse mammary gland, the levels of hydrocortisone are high during lactation, and drop at the beginning of involution. Upon daily treatment with hydrocortisone, involution in mice could be delayed up to 3 days (13). It has been also reported that hydrocortisone could inhibit TGF-β1 induction (33) and AP-1 DNA binding activity (34,35). Our results show that hydrocortisone strongly suppresses the up-regulation of clusterin by TGF-β1. Therefore, simultaneous with the reduction of hydrocortisone levels, TGF-β1 is induced and AP-1 DNA binding activity increases partially through the up-regulation of c-fos protein expression. As a consequence, clusterin expression is induced at the beginning of involution.
We attempted to determine why the inhibition of clusterin expression occurs at day 3 of involution, at a time ECM signaling through the degradation of the basement membrane is disrupted (25). Using a β1 integrin-blocking antibody, we found that β1 integrin ligand-binding activity was necessary for the regulation of clusterin expression by TGF-β1. Indeed a crosstalk between TGF-β1 and β1 integrin signaling has been previously demonstrated in mammary epithelial cells (36). To our knowledge, this is the first report showing that the levels of ligand-bound β1 integrin could affect clusterin expression. It has been reported that the perturbation of β1 integrin function in involuting mouse mammary gland could induce precocious dedifferentiation of the milk secretory epithelium (37). This implies that the function of β1 integrin is required for the correct initiation of involution. On the other hand, it has been reported that the level of ligand-bound β1 integrin declined when apoptosis of mammary epithelial cells began during involution (38). From all these observations, we speculate that the fall of ligand-bound β1 integrin levels leads to the drop in clusterin expression at day 3 of involution.
Finally, to determine if the upregulation of clusterin expression was required for mammary differentiation, we treated mammary epithelial cells with a clusterin small interfering RNA (siRNA). Using conditions that induced differentiation and milk secretion, i.e. extracellular matrix and lactogenic hormones, treatment of cells with clusterin siRNA induced a significant decrease in the levels of the milk protein β-casein. These data therefore suggest an important role of the glycoprotein clusterin as an inducer of mammary gland differentiation. An upregulation of clusterin expression was previously described in several tissues undergoing differentiation. For example, clusterin participates in the cytodifferentiation of pancreatic tissue, particularly the endocrine islet cells, and insulin cell differentiation was increased in a dose-dependent manner by treating duct cells in culture with clusterin (39).
In summary, our results reveal several novel pathways for the regulation of clusterin expression during mammary gland development and suggest that clusterin is a functionally important glycoprotein for mammary epithelial cell differentiation.
Acknowledgments
The authors wish to thank Drs. Jarnail Singh and Lynn Weir for helpful scientific discussions. This work was supported by grants from the California Breast Cancer Research Program (7WB-0026) and the National Institutes of Health-National Cancer Institute (RO1 CA82548) to PYD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blaschuk O, Burdzy K, Fritz IB. Purification and characterization of a cell-aggregating factor (clusterin), the major glycoprotein in ram rete testis fluid. J Biol Chem. 1983;258:7714–20. [PubMed] [Google Scholar]
- 2.Fritz IB, Burdzy K, Setchell B, Blaschuk O. Ram rete testis fluid contains a protein (clusterin) which influences cell-cell interactions in vitro. Biol Reprod. 1983;28:1173–88. doi: 10.1095/biolreprod28.5.1173. [DOI] [PubMed] [Google Scholar]
- 3.Jenne DE, Tschopp J. Clusterin: the intriguing guises of a widely expressed glycoprotein. Trends Biochem Sci. 1992;17:154–9. doi: 10.1016/0968-0004(92)90325-4. [DOI] [PubMed] [Google Scholar]
- 4.Jones SE, Jomary C. Clusterin. Int J Biochem Cell Biol. 2002;34:427–31. doi: 10.1016/s1357-2725(01)00155-8. [DOI] [PubMed] [Google Scholar]
- 5.Koch-Brandt C, Morgans C. Clusterin: a role in cell survival in the face of apoptosis? Prog Mol Subcell Biol. 1996;16:130–49. doi: 10.1007/978-3-642-79850-4_8. [DOI] [PubMed] [Google Scholar]
- 6.Wilson MR, Easterbrook-Smith SB. Clusterin is a secreted mammalian chaperone. Trends Biochem Sci. 2000;25:95–8. doi: 10.1016/s0968-0004(99)01534-0. [DOI] [PubMed] [Google Scholar]
- 7.Han BH, DeMattos RB, Dugan LL, Kim-Han JS, Brendza RP, Fryer JD, Kierson M, Cirrito J, Quick K, Harmony JA, Aronow BJ, Holtzman DM. Clusterin contributes to caspase-3-independent brain injury following neonatal hypoxia-ischemia. Nat Med. 2001;7:338–43. doi: 10.1038/85487. [DOI] [PubMed] [Google Scholar]
- 8.May PC, Lampert-Etchells M, Johnson SA, Poirier J, Masters JN, Finch CE. Dynamics of gene expression for a hippocampal glycoprotein elevated in Alzheimer's disease and in response to experimental lesions in rat. Neuron. 1990;5:831–9. doi: 10.1016/0896-6273(90)90342-d. [DOI] [PubMed] [Google Scholar]
- 9.Reddy KB, Karode MC, Harmony AK, Howe PH. Interaction of transforming growth factor beta receptors with apolipoprotein J/clusterin. Biochemistry. 1996;35:309–14. doi: 10.1021/bi951880a. [DOI] [PubMed] [Google Scholar]
- 10.Borst DW, Mahoney WB. Mouse mammary gland DNA synthesis during pregnancy. J Exp Zool. 1982;221:245–50. doi: 10.1002/jez.1402210216. [DOI] [PubMed] [Google Scholar]
- 11.Traurig HH. A radioautographic study of cell proliferation in the mammary gland of the pregnant mouse. Anat Rec. 1967;159:239–47. doi: 10.1002/ar.1091590213. [DOI] [PubMed] [Google Scholar]
- 12.Zwierzchowski L, Kleczkowska D, Niedbalski W, Grochowska I. Variation of DNA polymerase activities and DNA synthesis in mouse mammary gland during pregnancy and early lactation. Differentiation. 1984;28:179–85. doi: 10.1111/j.1432-0436.1984.tb00281.x. [DOI] [PubMed] [Google Scholar]
- 13.Lund LR, Romer J, Thomasset N, Solberg H, Pyke C, Bissell MJ, Dano K, Werb Z. Two distinct phases of apoptosis in mammary gland involution: proteinase-independent and -dependent pathways. Development. 1996;122:181–93. doi: 10.1242/dev.122.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strange R, Li F, Saurer S, Burkhardt A, Friis RR. Apoptotic cell death and tissue remodelling during mouse mammary gland involution. Development. 1992;115:49–58. doi: 10.1242/dev.115.1.49. [DOI] [PubMed] [Google Scholar]
- 15.Desprez PY, Hara E, Bissell MJ, Campisi J. Suppression of mammary epithelial cell differentiation by the helix-loop-helix protein Id-1. Mol Cell Biol. 1995;15:3398–404. doi: 10.1128/mcb.15.6.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reichmann E, Ball R, Groner B, Friis RR. New mammary epithelial and fibroblastic cell clones in coculture form structures competent to differentiate functionally. J Cell Biol. 1989;108:1127–38. doi: 10.1083/jcb.108.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oft M, Peli J, Rudaz C, Schwarz H, Beug H, Reichmann E. TGF-beta1 and Ha-Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumor cells. Genes Dev. 1996;10:2462–77. doi: 10.1101/gad.10.19.2462. [DOI] [PubMed] [Google Scholar]
- 18.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 19.Singh J, Itahana Y, Parrinello S, Murata K, Desprez PY. Molecular cloning and characterization of a zinc finger protein involved in Id-1-stimulated mammary epithelial cell growth. J Biol Chem. 2001;276:11852–58. doi: 10.1074/jbc.M006931200. [DOI] [PubMed] [Google Scholar]
- 20.Parrinello S, Lin CQ, Murata K, Itahana Y, Singh J, Krtolica A, Campisi J, Desprez PY. Id-1, ITF-2, and Id-2 comprise a network of helix-loop-helix proteins that regulate mammary epithelial cell proliferation, differentiation, and apoptosis. J Biol Chem. 2001;276:39213–9. doi: 10.1074/jbc.M104473200. [DOI] [PubMed] [Google Scholar]
- 21.Strange R, Friis RR, Bemis LT, Geske FJ. Programmed cell death during mammary gland involution. Methods Cell Biol. 1995;46:355–68. doi: 10.1016/s0091-679x(08)61935-4. [DOI] [PubMed] [Google Scholar]
- 22.Daniel CW, Robinson SD. Regulation of mammary growth and function by TGF-beta. Mol Reprod Dev. 1992;32:145–51. doi: 10.1002/mrd.1080320210. [DOI] [PubMed] [Google Scholar]
- 23.Daniel CW, Robinson S, Silberstein GB. The role of TGF-beta in patterning and growth of the mammary ductal tree. J Mammary Gland Biol Neoplasia. 1996;1:331–41. doi: 10.1007/BF02017389. [DOI] [PubMed] [Google Scholar]
- 24.Atwood CS, Ikeda M, Vonderhaar BK. Involution of mouse mammary glands in whole organ culture: a model for studying programmed cell death. Biochem Biophys Res Commun. 1995;207:860–7. doi: 10.1006/bbrc.1995.1265. [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Hernandez A, Fink LM, Pierce GB. Removal of basement membrane in the involuting breast. Lab Invest. 1976;34:455–62. [PubMed] [Google Scholar]
- 26.Zwain I, Amato P. Clusterin protects granulosa cells from apoptotic cell death during follicular atresia. Exp Cell Res. 2000;257:101–10. doi: 10.1006/excr.2000.4885. [DOI] [PubMed] [Google Scholar]
- 27.Thomas-Tikhonenko A, Viard-Leveugle I, Dews M, Wehrli P, Sevignani C, Yu D, Ricci S, el-Deiry W, Aronow B, Kaya G, Saurat JH, French LE. Myc-transformed epithelial cells down-regulate clusterin, which inhibits their growth in vitro and carcinogenesis in vivo. Cancer Res. 2004;64:3126–36. doi: 10.1158/0008-5472.can-03-1953. [DOI] [PubMed] [Google Scholar]
- 28.French LE, Soriano JV, Montesano R, Pepper MS. Modulation of clusterin gene expression in the rat mammary gland during pregnancy, lactation, and involution. Biol Reprod. 1996;55:1213–20. doi: 10.1095/biolreprod55.6.1213. [DOI] [PubMed] [Google Scholar]
- 29.Bettuzzi S, Scorcioni F, Astancolle S, Davalli P, Scaltriti M, Corti A. Clusterin (SGP-2) transient overexpression decreases proliferation rate of SV40-immortalized human prostate epithelial cells by slowing down cell cycle progression. Oncogene. 2002;21:4328–34. doi: 10.1038/sj.onc.1205594. [DOI] [PubMed] [Google Scholar]
- 30.Zhou W, Janulis L, Park II, Lee C. A novel anti-proliferative property of clusterin in prostate cancer cells. Life Sci. 2002;72:11–21. doi: 10.1016/s0024-3205(02)02183-5. [DOI] [PubMed] [Google Scholar]
- 31.Jin G, Howe PH. Transforming growth factor beta regulates clusterin gene expression via modulation of transcription factor c-Fos. Eur J Biochem. 1999;263:534–42. doi: 10.1046/j.1432-1327.1999.00533.x. [DOI] [PubMed] [Google Scholar]
- 32.Marti A, Jehn B, Costello E, Keon N, Ke G, Martin F, Jaggi R. Protein kinase A and AP-1 (c-Fos/JunD) are induced during apoptosis of mouse mammary epithelial cells. Oncogene. 1994;9:1213–23. [PubMed] [Google Scholar]
- 33.Wen FQ, Kohyama T, Skold CM, Zhu YK, Liu X, Romberger DJ, Stoner J, Rennard SI. Glucocorticoids modulate TGF-beta production. Inflammation. 2002;26:279–90. doi: 10.1023/a:1021412601538. [DOI] [PubMed] [Google Scholar]
- 34.Aljada A, Ghanim H, Mohanty P, Hofmeyer D, Tripathy D, Dandona P. Hydrocortisone suppresses intranuclear activator-protein-1 (AP-1) binding activity in mononuclear cells and plasma matrix metalloproteinase 2 and 9 (MMP-2 and MMP-9) J Clin Endocrinol Metab. 2001;86:5988–91. doi: 10.1210/jcem.86.12.8212. [DOI] [PubMed] [Google Scholar]
- 35.Jaggi R, Marti A, Guo K, Feng Z, Friis RR. Regulation of a physiological apoptosis: mouse mammary involution. J Dairy Sci. 1996;79:1074–84. doi: 10.3168/jds.S0022-0302(96)76461-5. [DOI] [PubMed] [Google Scholar]
- 36.Bhowmick NA, Zent R, Ghiassi M, McDonnell M, Moses HL. Integrin beta 1 signaling is necessary for transforming growth factor-beta activation of p38MAPK and epithelial plasticity. J Biol Chem. 2001;276:46707–13. doi: 10.1074/jbc.M106176200. [DOI] [PubMed] [Google Scholar]
- 37.Faraldo MM, Deugnier MA, Tlouzeau S, Thiery JP, Glukhova MA. Perturbation of beta1-integrin function in involuting mammary gland results in premature dedifferentiation of secretory epithelial cells. Mol Biol Cell. 2002;13:3521–31. doi: 10.1091/mbc.E02-02-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prince JM, Klinowska TC, Marshman E, Lowe ET, Mayer U, Miner J, Aberdam D, Vestweber D, Gusterson B, Streuli CH. Cell-matrix interactions during development and apoptosis of the mouse mammary gland in vivo. Dev Dyn. 2002;223:497–516. doi: 10.1002/dvdy.10070. [DOI] [PubMed] [Google Scholar]
- 39.Kim BM, Kim SY, Lee S, Shin YJ, Min BH, Bendayan M, Park IS. Clusterin induces differentiation of pancreatic duct cells into insulin-secreting cells. Diabetologia Jan. 2006;13:1–10. doi: 10.1007/s00125-005-0106-2. [DOI] [PubMed] [Google Scholar]


