Abstract
Prion diseases are transmissible spongiform encephalopathies of humans and animals characterized by the accumulation of a proteinase-resistant isoform of the cellular prion-related protein (PrPc) within the central nervous system. In the present report we demonstrate for the first time the presence of PrPc on squamous epithelia of normal and diseased human skin and show that inflammatory cytokines regulate PrPc expression in cultured human keratinocytes (KCs). By immunohistochemistry, only little expression of PrPc, which was mainly confined to KCs, was detected in normal skin. In contrast, in inflammatory skin diseases including psoriasis and contact dermatitis, PrPc was strongly present on both KCs and infiltrating mononuclear cells. Strong PrPc expression was also observed in squamous cell carcinomas and viral warts whereas basal cell carcinomas were mostly negative. In mucous membranes of the upper digestive tract and the genital region, distinct PrPc expression by basal squamous epithelial cells was a constant feature. In tissue culture, primary KCs constitutively expressed PrPc mRNA and protein. Exposure of these cells to transforming growth factor (TGF)-α or interferon (IFN)-γ led to an increase of PrPc protein expression. The presence of PrPc on epithelial cells of skin and mucous membranes suggests that these cells represent possible first targets for peripheral infection with prions.
Cellular prion-related protein (PrPc) is a cell surface glycoprotein predominantly expressed within the central nervous system (CNS). 1,2 Accumulation of a modified proteinase-resistant isoform, ie, scrapie-associated PrP (PrPSc), in the CNS is the common pathogenic denominator for prion diseases, a group of neurodegenerative disorders of humans and animals. 1,2 The inoculation of prions is thought to trigger the continuous conversion of PrPc to PrPSc. 1-3 In the laboratory setting, prion diseases are transmitted by direct inoculation of infected tissue. 3 In humans, infection has primarily been related to dura grafts and the parenteral administration of pituitary extracts. 2 In contrast, the routes of transmission in the field are still not completely elucidated, and several different modes, such as the ingestion of contaminated food and peripheral inoculation, have been suggested. 4,5
Recently, the occurrence of a variant form of Creutzfeldt-Jakob disease (CJD) has raised the question of interspecies transmission of BSE from cows to humans. Whereas evidence suggests that BSE has spread to man 6 , no clear relationship between risk factors and disease occurrence has been established. 7 Scrapie of sheep is one of the longest known prion-associated disorders, but the transmission routes have not been clarified for this disease either. 2,8 Successful transmission of prion diseases via inoculation into the skin has been recently reported in rodents. 5 Several cell types found in the skin, including fibroblasts 9 and certain hemopoietic cells, 10,11 have been found to express PrPc; however, to the best of our knowledge, the skin compartment itself has otherwise not yet been studied for its possible involvement in prion diseases. Therefore, we set out to investigate the distribution of PrPc in human skin and mucous membranes.
Materials and Methods
Immunohistochemistry
Expression of PrPc was analyzed on sections derived from paraffin-embedded specimens collected for routine histopathology. After microwave treatment, immunostaining was performed using either anti-PrP monoclonal antibody (MAb) 3F4 (1:400; Chemicon, Temecula, CA) or 6H4 (1:300; Prionics AG, University of Zurich, Zurich, Switzerland 12 ) following a standard protocol. 13 Control stainings with irrelevant isotype-matched control MAbs, ie, IgG1 (Coulter, Hialeah, FL) for MAb 6H4 and IgG2a (Coulter) for MAb 3F4 in adequate concentrations were regularly included in our experimental procedures.
Western Blot Analysis
Cells were lysed in a buffer containing 1% Nonidet P-40 and 1% SDS buffer. SDS-polyacrylamide gel electrophoresis and Western transfer to nitrocellulose membranes were performed under standard conditions. For some experiments, gels were electrophoresed in duplicate under identical conditions; one was stained with Coomassie blue and the other was subjected to Western blot analysis to assure equal loading of protein. Membranes were reacted to MAb 3F4 or to an isotype-matched control MAb followed by horseradish-peroxidase-labeled sheep anti-mouse IgG (Amersham Life Science, Little Chalfont, UK). After rinsing in enhanced chemiluminescence reagent (Amersham), membranes were exposed to X-OMAT-AR film (Eastman Kodak, Rochester, NY). For deglycosylation experiments, PNGaseF (New England BioLabs, Schwalbach, Germany) was used according to the manufacturer’s instructions. For some experiments, protein glycosylation was inhibited with tunicamycin (5 μg/ml; Calbiochem, San Diego, CA) for 24 hours.
Cell Cultures
Human neonatal foreskin KCs (Clonetics, San Diego, CA) were cultured under low-Ca2+ conditions (0.15 mmol/L) in serum-free KC growth medium (KGM) consisting of KC basal medium (KBM) supplemented with human recombinant epidermal growth factor (0.1 ng/ml), bovine pituitary extract, insulin (5 μg/ml), hydrocortisone (0.5 μg/ml), and gentamicin/amphotericin B (50 μg/ml/50 ng/ml; all Clonetics) at 37°C in 5% CO2. Cells were routinely passaged at a confluence of 60% to 80%. All experiments were carried out between passages 2 and 5. For stimulation experiments, KCs were grown either in KBM alone or in KBM supplemented with interferon (IFN)-γ (1000 U/ml; Imukin, Bender-Med, Vienna, Austria), transforming growth factor (TGF)-α (100 ng/ml; Eubio, Vienna, Austria), interleukin (IL)-1β (4 pg/ml; Eubio), tumor necrosis factor (TNF)-α (50 U/ml; Biomol, Hamburg, Germany) or phorbol myristate acetate (100 nmol/L; Sigma Aldrich, Vienna, Austria). The epidermoid carcinoma cell line A431, the immortalized KC cell line HaCaT, and the melanoma cell line SkMEL28 were grown in RPMI-1640 supplemented with 10% fetal bovine serum and 1% l-glutamine (all GIBCO BRL, Gaithersburg, MD).
Northern Hybridization
For Northern blot analysis, total RNA was size fractionated in 1% agarose gels containing 1.48% formaldehyde and transferred to nylon membranes (Nytran, Schleicher and Schüll, Dassel, Germany) as described previously. 14 For hybridization, a mouse PrPc cDNA probe (kindly provided by A. Aguzzi 15 ) was used under high-stringency conditions.
Results
In normal skin, only little expression of PrPc was detectable by immunohistochemistry (Figure 1a) ▶ . Staining was confined mainly to epithelial cells (Figure 1a) ▶ and to sporadic mononuclear cells within the dermis. In squamous epithelium of mucous membranes of different sites, distinct constitutive PrPc expression was detected in basal but not suprabasal KCs (Figure 1b) ▶ . This pattern of expression was consistently observed in pharynx (n = 5, Figure 1b ▶ ), larynx (n = 3), esophagus (n = 2), vulva (n = 3), and the ectocervix (n = 3). In contrast to low PrPc expression in normal epidermis, this protein was strongly up-regulated in basal and suprabasal KCs up to the granular layer in eczema (n = 5, Figure 1c ▶ ) and psoriasis (n = 5, Figure 1d ▶ ) and adjacent to skin ulcerations (n = 4, Figure 1e ▶ ). In addition, dermal mononuclear cells were also distinctly positive for PrPc in these diseases (Figure 1, c and d ▶ , arrows). These infiltrating cells essentially comprise T lymphocytes and macrophages, 16 both of which are able to express PrPc. 10,11,17 In epithelial tumors, PrPc expression was most pronounced in common warts (n = 7, Figure 1f ▶ ) and squamous cell carcinomas (n = 6, Figure 1h ▶ ), whereas basal cell carcinomas (n = 4, Figure 1g ▶ ) were mostly negative. In all experiments, the replacement of anti-PrPc MAbs with the appropriate isotype-matched control MAbs resulted in no specific staining (not shown).
Figure 1.
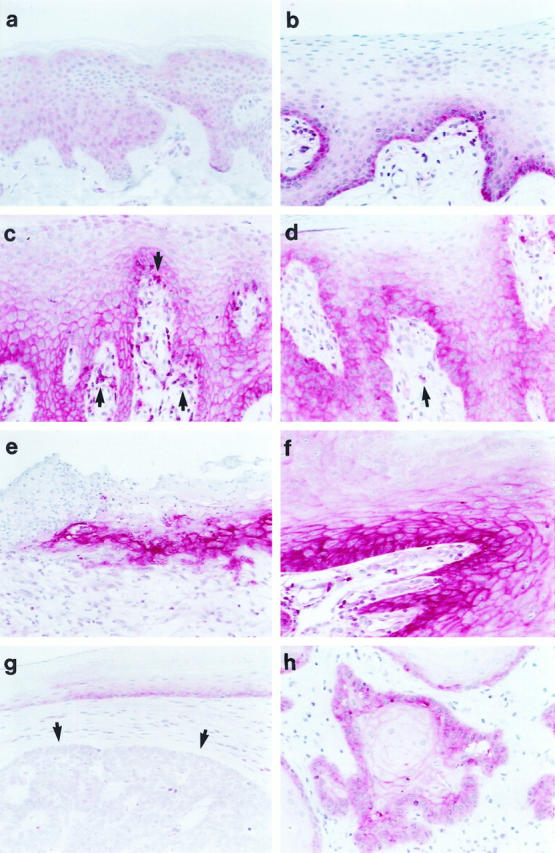
Human KCs in situ express PrPc. Immunohistochemical staining was performed on paraffin sections after microwave treatment using the anti-PrP MAb 3F4 (1:400). PrPc is weakly present in the cytoplasm of KCs of normal skin (a) but distinctly in basal cells of squamous epithelium of mucous membranes (pharynx; b). Besides a weak cytoplasmic staining, basal and suprabasal KCs up to the granular layer stain strongly for PrPc in a chicken-wire pattern characteristic for membrane proteins in eczema (c) and psoriasis (d), adjacent to ulcerations (e), and in common warts (f). In addition, mononuclear cells within the dermis and epidermis (c and d, arrows) express PrPc. Whereas basal cell carcinomas (g, arrows) showed weak to no PrPc expression, squamous cell carcinomas (h) stained distinctly for PrPc. Original magnification, ×200.
KCs in primary culture were positive for PrPc by both Western blotting (Figure 2a) ▶ and FACS analysis (data not shown). PrPc protein moieties ranged between 30 and 43 kd, corresponding to reports for other tissues. 11,18 In contrast to KCs in primary culture and to the melanoma cell line SkMEL28, little to no expression was detected in HaCaT and A431 epithelial cell lines (Figure 2a) ▶ . In keeping with the protein data were the data obtained by Northern hybridization with a PrPc cDNA probe. 15 A specific band of approximately 2.3 kb was present in RNA from epithelial cells in primary culture and in SkMEL28 cells but not in A431 cells (Figure 2b) ▶ . The expression of PrPc by KC in tissue culture was up-regulated by IFN-γ and transforming TGF-α but not by IL-1β or TNF- α (Figure 3a) ▶ . TGF-α also strongly induced the expression of PrPc in HaCaT (Figure 3a) ▶ but not in A431 cells (data not shown). Interestingly, stimulation with TGF-α resulted in strong expression of an additional band of approximately 23 kd, corresponding to nonglycosylated PrPc. 19 Deglycosylation experiments with either PNGase F or incubation of cells with tunicamycin confirmed that the molecular weight of the KC-derived PrPc protein backbone was indeed approximately 23 kd (Figure 3b) ▶ . Shorter exposure of the blot revealed that in lysates of tunicamycin-treated HaCaT cells this moiety consisted of two closely migrating bands (not shown).
Figure 2.

KCs in primary culture express PrPc mRNA and proteins. a: Western blot analysis was performed with the 3F4 anti-PrP MAb. Lysates of KCs in primary culture (lane 1) were strongly positive for PrPc whereas in lysates of HaCaT (lane 2) and A431 cells (lane 3) little or no reactivity was detectable. The SkMEL28 melanoma cell line (lane 4) also strongly expressed PrPc. Equal protein loading was confirmed by Coomassie blue staining of a gel run in parallel under identical conditions (lower part of a). b: When total RNA was hybridized to a cDNA of mouse PrPc, a specific hybridization signal was detected at approximately 2.3 kb in primary cultured KCs (lane 2) and SkMEL28 melanoma cells (lane 3) but not in A431 cells (lane 1). The lower panel depicts the ethidium bromide staining of 18 S RNA.
Figure 3.
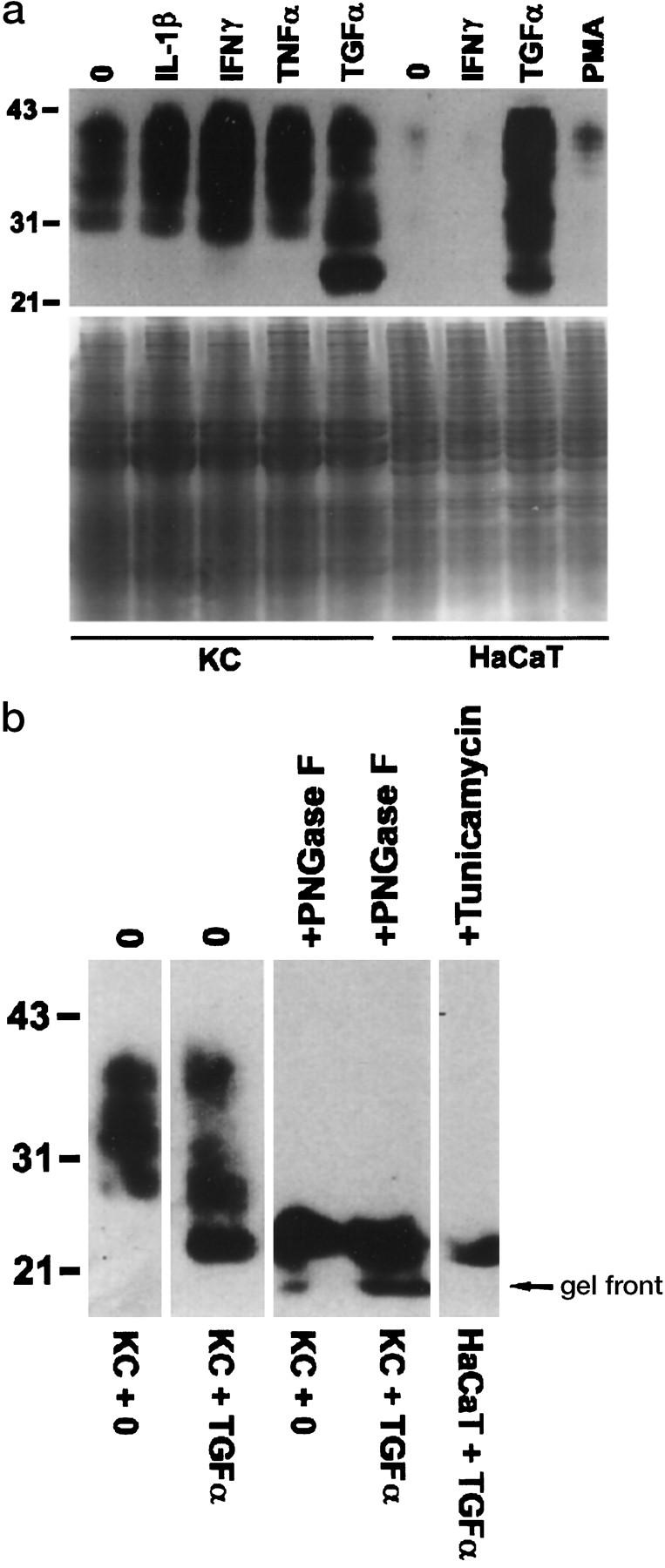
PrPc expression in human KCs is up-regulated by TGF-α and IFN-γ. a: In KCs in primary culture (left panel), PrPc was up-regulated after a 48-hour exposure to TGF-α (lane 5) and IFN-γ (lane 3) but not to IL-1β (lane 2 ) or TNF- α (lane 4). Lane 1 contains lysates of unstimulated KCs. In HaCaT cells (right panel), strong induction of PrPc was found 24 hours after exposure to TGF-α (lane 3), whereas incubation with IFN-γ (lane 2) and phorbol myristate acetate (lane 4) had only little or no effect as compared with unstimulated cells (lane 1). Equal protein loading was confirmed by Coomassie blue staining of a gel run in parallel under identical conditions (lower part of a). b: Deglycosylation of lysates of unstimulated (lanes 1 and 3) and TGF-α-stimulated KCs (lanes 2 and 4) with PNGase F (lanes 3 and 4) resulted in a band of approximately 23 kd. PrPc of the same size was detected after inhibition of protein glycosylation with tunicamycin in HaCaT cells (lane 5).
Discussion
In the present report we demonstrate for the first time that KCs in situ and in tissue culture produce PrPc mRNA and protein. KC- PrPc is up-regulated in inflammatory skin diseases and can be induced in cultured KCs by TGF-α and IFN-γ. As TGF-α is strongly expressed in psoriatic epidermis 20 and IFN-γ is produced at inflammatory sites by activated T lymphocytes, these cytokines may also in vivo be involved in the regulation of PrPc expression by KCs in an autocrine and paracrine manner.
In recent years, it has been found that PrPc is expressed not only by neurons but also by several non-neuronal tissues. 9-11,21 Functionally, PrPc in the CNS is involved in synaptic transmission in nerve cells, 22 in survival of Purkinje cells, 23 and in sleep regulation. 24 In both human and murine lymphocytes it plays a role in mitogen-induced lymphocyte activation. 10,17 Despite intensive research, the function of PrPc in other tissues, including the epidermis, is unknown. The fact that PrPc is a glycolipid-anchored cell surface glycoprotein 3 is suggestive for a role in signaling and/or adhesion. Recent data indeed indicate that at least two ligands, ie, a 37-kd laminin receptor precursor 25 and a 66-kd membrane protein, 26 exist that are able to bind to PrPc. However, the in vivo significance and possible functional implications of these findings remain to be determined.
As to the involvement of PrPc in the pathogenesis of prion diseases, it has been amply documented that its presence is the prerequisite for the development of transmissible spongiform encephalopathies. 1-3 Therefore, the clinically most relevant question is whether or not epithelia-associated PrPc plays a role in the acquisition of prion diseases. It is conceivable or even likely that if prions penetrate into the epidermis they would be able to start the conversion of KC-derived PrPc to PrPSc. Penetration is certainly possible via the broken skin barrier in eczema and ulcerations. As in these disorders KCs do strongly express PrPc, the scenario would be particularly favorable for a transmission to occur. After inoculation, the skin-associated lymphoid tissue could play a role in the further propagation of prions to lymphoid organs and to the CNS. 5 Infiltrating mononuclear cells in eczema and psoriasis, mainly composed of T lymphocytes and macrophages, 16 are PrPc positive (Figure 1c) ▶ . They thus represent appropriate targets for conversion of PrPc to PrPSc in the skin, in the course of primary infection, and could account for the spread of the altered protein to the lymphoid organs. Conversely, in infected individuals, skin-homing lymphocytes might encounter PrPSc in the lymphoid organs and transport it back to the skin at sites of inflammation. Lymphocyte-associated PrPSc could thus gain access to the epidermis causing the conversion of KC-associated PrPc. However, in contrast to other organs where PrPSc accumulates within cells and in intercellular spaces, 1-3 the epidermis is constantly self-renewing and sheds terminally differentiated cells. This mechanism would probably not allow that PrPSc accumulates intraepidermally and induces epidermal pathologies. On the other hand, such a mechanism might lead to the shedding of contaminated material from the body surface. Sheep affected by scrapie suffer from severe pruritus, prompting them to rub their hindquarters on any objects. 2 If PrPSc were present in the skin as argued above, it could readily be deposited on these objects and passed on to noninfected animals that use the same objects for rubbing.
Although there are only few hard data available on the route of transmission of prion diseases, it appears that the intimate contact with contaminated material, by direct inoculation or ingestion, is necessary. CJD is transmitted in the course of dura grafting or after parenteral administration of natural human growth hormone. 2 Infection by ingestion of prion-contaminated material has recently aroused great interest as a result of the BSE crisis where cows have been infected most likely after being fed meat and bone meals derived from scrapie-infected sheep. Kuru, a prion-mediated disease of the Fore people, was acquired during cannibalistic rites and the ingestion of organs of relatives. 3 In the light of our finding it is conceivable that, in addition to the gut, PrPc-expressing squamous epithelium of the upper gastrointestinal tract might constitute a target for primary generation of PrPSc in both BSE and kuru. Interestingly, in transmission of kuru, also contamination during the preparation of the ritual meals and peripheral inoculation has been suggested to be involved. 27 As inflammatory skin diseases were frequent among the Fore people, 27 epidermal PrPc might have indeed represented a relevant first target for prions in kuru. As to other human prion diseases, ie, CJD and its new variant, 7 there exists no epidemiological indication that acquisition of prions via the skin plays a role in their spread. Nevertheless, we believe that our data on the expression of PrPc in epithelial cells of skin and mucous membranes justify the inclusion of these organs in future studies on prion disease transmission and pathogenesis.
A potential further implication of our findings concerns the current practice of grafting of skin substitutes. Skin substitutes contain in vitro expanded keratinocytes and are routinely used for autologous and allogeneic grafting on surgical and burn wounds and chronic leg ulcers. 28 Growth of these cells mostly is carried out in medium supplemented with either fetal bovine serum or with bovine pituitary extracts. 29 If epithelial PrPc indeed constituted a possible target for prions, skin substitutes may represent a possible route for inoculation of bovine prions into humans. As currently there are no routine tests available to detect prion-contaminated material, our data emphasize the necessity to develop cell culture systems devoid of undefined animal proteins.
Acknowledgments
We thank Drs. H. Budka and A. Aguzzi and Mr. M. Rendl for helpful discussion and provision of reagents.
Footnotes
Address reprint requests to Dr. Erwin Tschachler, Division of Immunology, Allergy, and Infectious Diseases, Department of Dermatology, University of Vienna Medical School, Währinger Gürtel 18–20, A-1090 Vienna, Austria. E-mail: erwin.tschachler@akh-wien.ac.at.
Supported in part by the Ludwig Boltzmann-Institut for the Investigation of Infectious Venerodermatological Diseases.
References
- 1.Prusiner SB: Molecular biology of prion diseases. Science 1991, 252:1515-1522 [DOI] [PubMed] [Google Scholar]
- 2.Prusiner SB: Prions. Fields BN Knipe DM Howley PM eds. Fields Virology. 1996, :pp 2901-2950 Lippincott-Raven, Philadelphia [Google Scholar]
- 3.Aguzzi A, Weismann C: Prion research: the next frontiers. Nature 1997, 389:795-798 [DOI] [PubMed] [Google Scholar]
- 4.Wilesmith JW, Wells G, Cranwell MP, Ryan J: Bovine spongiform encephalopathy: epidemiological studies. Vet Rec 1988, 123:638-644 [PubMed] [Google Scholar]
- 5.Taylor DM, McConnel I, Fraser H: Scrapie infection can be established readily through skin scarification in immunocompetent but not immunodeficient mice. J Gen Virol 1996, 77:1595-1599 [DOI] [PubMed] [Google Scholar]
- 6.Bruce ME, Will RG, Ironside JW, McConnel I, Drummond D, Suttie A, McCardle L, Chree A, Hope J, Birkett C, Cousens S, Fraser H, Bostock CJ: Transmissions to mice indicate that ’new variant’ CJD is caused by the BSE agent. Nature 1997, 389:498-501 [DOI] [PubMed] [Google Scholar]
- 7.Will RG, Ironside JW, Zeidler M, Cousens SN, Estibeiro K, Alperovitch A, Poser S, Pocchiari M, Hofman A, Smith PG: A new variant of Creutzfeld-Jacob disease in the UK. Lancet 1996, 347:921-925 [DOI] [PubMed] [Google Scholar]
- 8.Wood J, Done SH, Pritchard GC, Wooldridge M: Natural scrapie in goats: case histories and clinical signs. Vet Rec 1992, 131:66-68 [DOI] [PubMed] [Google Scholar]
- 9.Meiner Z, Halimi M, Polakiewicz RD, Prusiner SB, Gabizon R: Presence of prion protein in peripheral tissues of Libyan Jews with Creutzfeld-Jakob disease. Neurology 1992, 42:1355-1360 [DOI] [PubMed] [Google Scholar]
- 10.Cashman NR, Loertscher R, Nalbantoglu J, Shaw I, Kascsak RJ, Bolton DC, Bendheim PE: Cellular isoform of the scrapie agent protein participates in lymphocyte activation. Cell 1990, 61:185-192 [DOI] [PubMed] [Google Scholar]
- 11.Diomede L, Sozzani S, Luini W, Algeri M, de Gioia L, Chiesa R, Lievens PMJ, Bugiani O, Forloni G, Tagliavini F, Salmona M: Activation effects of a prion protein fragment (PrP-(106–126)) on human leukocytes. Biochem J 1995, 320:563-570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korth C, Stierli B, Streit P, Moser M, Schaller O, Fischer R, Schulz-Schaeffer W, Kretzschmar H, Raeber A, Braun U, Ehrensperger F, Hornemann S, Glockshuber R, Riek R, Billeter M, Wuthrich K, Oesch B: Prion (PrPSc)-specific epitope defined by a monoclonal antibody. Nature 1997, 390:74-77 [DOI] [PubMed] [Google Scholar]
- 13.Pammer J, Plettenberg A, Weninger W, Diller B, Mildner M, Uthman A, Issing W, Stürzl M, Tschachler E: CD40 antigen is expressed by endothelial cells and tumor cells in Kaposi’s sarcoma. Am J Pathol 1996, 148:1387-1396 [PMC free article] [PubMed] [Google Scholar]
- 14.Weninger W, Uthman A, Pammer J, Pichler A, Ballaun C, Lang IM, Plettenberg A, Bankl HC, Stürzl M, Tschachler E: Vascular endothelial growth factor production in normal epidermis and in benign and malignant epithelial skin tumors. Lab Invest 1996, 75:647-657 [PubMed] [Google Scholar]
- 15.Moser M, Colello RJ, Pott U, Oesch B: Developmental expression of the prion protein gene in glial cells. Neuron 1995, 14:509-517 [DOI] [PubMed] [Google Scholar]
- 16.Christophers E, Mrowietz U: The inflammatory infiltrate in psoriasis. Clin Dermatol 1995, 13:131-135 [DOI] [PubMed] [Google Scholar]
- 17.Mabott NA, Brown KL, Manson J, Bruce ME: T-lymphocyte activation and the cellular form of the prion protein. Immunology 1997, 92:161-165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill AF, Zeidler M, Ironside J, Collinge J: Diagnosis of a new variant Creutzfeld-Jacob disease by tonsil biopsy. Nature 1997, 349:99-100 [DOI] [PubMed] [Google Scholar]
- 19.Haraguchi T, Fisher S, Olofsson S, Endo T, Groth D, Tarentino A, Borchelt DR, Teplow D, Hood L, Burlingame A, Lycke E, Kobata A, Prusiner SM: Asparagine-linked glycosylation of the scrapie and cellular prion proteins. Arch Biochem Biophys 1989, 274:1-13 [DOI] [PubMed] [Google Scholar]
- 20.Elder JT, Fisher GJ, Lindquist PB, Bennett GL, Pittelkow MR, Coffey RJ, Ellingsworth L, Derynck R, Voorhees JJ: Overexpression of transforming growth factor α in psoriatic epidermis. Science 1989, 243:811-814 [DOI] [PubMed] [Google Scholar]
- 21.Horiuchi M, Yamazaki N, Ikeda T, Ishiguro N, Shinagawa M: A cellular form of prion protein (PrPc) exists in many non-neuronal tissues in sheep. J Gen Virol 1995, 76:2583-2587 [DOI] [PubMed] [Google Scholar]
- 22.Colling SB, Collinge J, Jefferys JG: Hippocampal slices from prion protein null mice: disrupted Ca(2+)-activated K+ currents. Neurosci Lett 1996, 209:49-52 [DOI] [PubMed] [Google Scholar]
- 23.Sakaguchi S, Katamine S, Nishida N, Moriuchi R, Shigematsu T, Sugimoto T, Nakatani A, Kataoka Y, Houtani T, Shirabe S, Okada H, Hasegawa S, Miyamoto T, Noda T: Loss of cerebellar Purkinje cells in aged mice homozygous for a disrupted PrP gene. Nature 1996, 380:528-531 [DOI] [PubMed] [Google Scholar]
- 24.Tobler I, Gaus SE, Deboer T, Achermann P, Fischer M, Rülicke T, Moser M, Oesch B, McBride PA, Manson JC: Altered circadian rhythms and sleep in mice devoid of prion protein. Nature 1996, 380:639-642 [DOI] [PubMed] [Google Scholar]
- 25.Rieger R, Edenhofer F, Lasmezas CI, Weiss S: The human 37-kd laminin receptor precursor interacts with the prion protein in eukaryotic cells. Nature Med 1997, 3:1383-1388 [DOI] [PubMed] [Google Scholar]
- 26.Martins VR, Graner E, Garcia-Abreu J, de Souza SJ, Mercadante AF, Veiga SS, Zanata SM, Neto VM, Brentani RR: Complementary hydropathy identifies a cellular prion protein receptor. Nature Med 1997, 3:1376-1381 [DOI] [PubMed] [Google Scholar]
- 27.Gajdusek CD: Infectious amyloids. Fields BN Knipe DM Howley PM eds. Fields Virology. 1996, :pp 2851-2900 Lippincott-Raven, Philadelphia [Google Scholar]
- 28.Phillips TJ: New skin for old: developments in biological skin substitutes. Arch Dermatol 1998, 134:344-349 [DOI] [PubMed] [Google Scholar]
- 29.Leigh IM, Watt FM: The culture of human epidermal keratinocytes. Leigh IM Lane EB Watt FM eds. The Keratinocyte Handbook. 1984, :pp 43-52 Cambridge University Press, Cambridge, UK, [Google Scholar]


