Abstract
Different mutations in the microtubule-associated tau protein gene have recently been identified in several families with hereditary frontotemporal dementia and Parkinsonism (FTDP-17) linked to chromosome 17q21–22. Some families show neuronal and glial deposits containing hyperphosphorylated tau in several brain regions. We have investigated the presence of tau deposits by using a panel of anti-tau antibodies in three brains of a family with the P301L mutation (HFTD1) and in another family with the G272V mutation (HFTD2) of the tau gene. Numerous intracytoplasmic tau deposits in neurons, glial cells, and neurites were found in hippocampal formation, neocortex, and substantia nigra. These deposits in three patients from HFTD1 consisted of slender twisted filaments 15 nm wide with variable periodicity and a few straight filaments. Tau extracted from these filaments appeared as two major bands of 64 and 68 kd and a minor band of 72 kd that, after alkaline phosphatase treatment, proved to consist mainly of 4-repeat tau isoforms and one of the 3-repeat isoforms. In three patients from HFTD2 numerous Pick-like bodies were present. The conclusion is that the type and distribution of tau deposits in HFTD1 and HFTD2, the physical structure of filaments, and tau isoform composition in HFTD1 differ from Alzheimer’s disease and an FTDP-17 family with a V337M mutation in the tau gene.
Hereditary frontotemporal dementia and Parkinsonism linked to chromosome 17 (FTDP-17) refers to a group of neurodegenerative disorders often characterized by early behavioral changes accompanied by later cognitive and motor disturbances. 1 At least 13 families have been now identified. 1 Pathological changes consist of selective frontotemporal atrophy, neuronal loss, gliosis, and spongiosis in cortex and subcortical nuclei, sometimes with ballooned cells. Neuropathologically, several families with FTDP-17 show microtubule-associated protein tau deposits, which can be present in neurons and in glial cells. 2-9 Tau protein in adult brain consists of 6 isoforms produced by alternative splicing, containing 3 or 4 microtubule-binding repeats, with or without 29 or 58 amino acid insertions in the amino-terminal region. 10,11 In some FTDP-17 families tau deposits are similar to those found in Alzheimer’s disease (AD); they are mainly in neurons and consist of filaments similar to AD paired helical filaments (PHFs), which contain all 6 tau isoforms. 3,12 In contrast, other families show tau deposits in neurons and glial cells and they consist of filaments which are different from AD PHFs and contain mainly tau isoforms with 4 repeats. 4,5,7 Very recently, mutations in the microtubule-associated protein tau gene have been identified for several families with FTDP-17, strongly suggesting that tau is the gene responsible for FTDP-17. 13-15 We have investigated the presence of tau deposits in the two Dutch families 16 with mutations in the microtubule-binding domains of tau. 14 The mutation in HFTD1 is a proline to a leucine at position 301 (numbered from the longest tau isoform) in exon 10, whereas that in HFTD2 is a glycine to a valine at position 272 in exon 9. Both mutations appear at almost equivalent positions in two of the microtubule-binding domains and more specifically in the highly conserved PGGG motif. We describe here the characterization of tau deposits in these families and we discuss their relation to the tau gene mutations.
Materials and Methods
Materials
Paraffin-embedded sections of frontal, temporal, and parietal cortices, hippocampus, cerebellum, and different subcortical nuclei from 3 patients from HFTD1 (53, 66, and 76 years), and 3 patients from HFTD2 (54, 56, and 57 years) together with 2 AD patients (74 and 80 years) were used for immunohistochemistry. Fresh-frozen tissue from hippocampus, temporal cortex, and frontal cortex of the 3 patients from HFTD1, 2 AD patients (65 and 78 years) and 2 control subjects (68 and 72 years) was used for biochemical studies.
Immunohistochemistry
For immunohistochemistry sections were incubated with phosphorylation-dependent (AT8, AT180, AT270, and AT100 17 (E. Vanmechelen, Innogenetics]; PHF1 [P. Davies], 12 E8 18 (P. Seubert, Athena Neurosciences), and phosphorylation-independent anti-tau antibodies (BR133, BR134, BR304, BR189). 11 Polyclonal and monoclonal antibodies raised against amyloid Aβ (Ab 2332; V.M.-Y Lee) and anti-heparan sulfate antibody 10E4 19 (Seikagaku, Japan) were also used. Immunohistochemistry was performed as previously reported. 3
Tau Extraction, Dephosphorylation, and Immunoblotting
Sarkosyl-insoluble tau was extracted and dephosphorylated as previously described. 4,20 PHF-tau samples were run on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted onto Immobilon P (Millipore), incubated overnight at 4°C with the primary antibodies and stained using the biotin-avidin Vectastain system (Vector Laboratories). Soluble tau was extracted using 2.5% perchloric acid as previously described. 21
Electron Microscopy
Aliquots of sarkosyl-insoluble tau were processed as previously described for electron microscopy and for immunogold electron microscopy. 22
Results
Neuropathological examination of HFTD1 brains revealed severe neuronal loss in frontal and temporal cortex, gliosis, and a few ballooned cells as previously reported. 16 There was variable neuronal loss in the parietal cortex. Amyloid plaques and congophilic angiopathy were generally lacking in all cases, except for a number of congophilic vessels and some small senile plaques in the temporal cortex and gyrus parahippocampalis in one case. The subcortical white matter was severely gliotic. The hippocampus showed mild to moderate neuronal loss and gliosis, with a few neurofibrillary tangles visible by Bodian staining in the pyramidal layer of the hippocampus and gyrus parahippocampalis in all three brains. Substantia nigra showed severe loss of pigmented cells. The amygdala and overlying entorhinal cortex showed severe neuronal loss. Thalamus and caudate nucleus were normal or showed variable loss of neurons. No Pick bodies were observed.
The three brains from HFTD2 also showed severe loss of neurons and gliosis of the frontal and temporal cortex, and in less extent of the parietal cortex. Neuronal loss was also present in the hippocampus and gyrus parahippocampalis as previously reported. 16,23,24 Caudate nucleus was severely degenerated. Severe loss of pigmented cells occurred in the substantia nigra. A few Pick bodies were observed in one case. 16
Immunohistochemistry
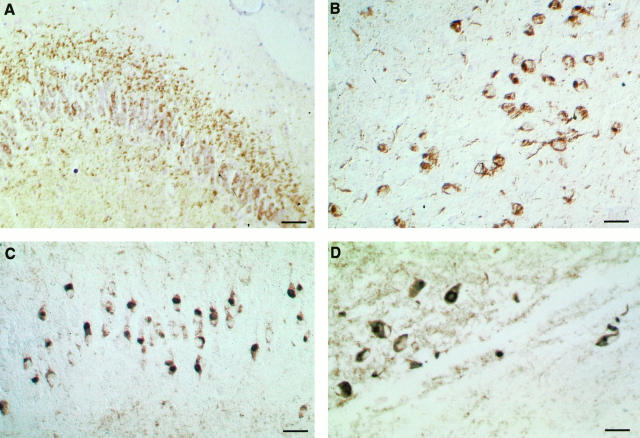
In both families phosphorylation-dependent and -independent anti-tau antibodies stained numerous deposits in the superficial layer 2–3 and deep layer 6 of the frontal, temporal, and parietal cortices, brain stem, gyrus cinguli, the granule cells of dentate gyrus (DG) including its inner molecular layer, cornu ammonis (CA) 1 and 2, entorhinal cortex, and substantia nigra in all cases examined (Figure 1) ▶ . These deposits were mainly of the pre-tangle type located in the perinuclear region and cell body and sometimes extending to the apical dendrites of neurons.
Figure 1.
Tau immunostaining in hippocampal formation and neocortex from affected members from HFTD1 (A) and (B) and HFTD2 (C) and (D). Tau deposits are stained by antibody AT8 in the inner molecular layer, granule cells of the dentate gyrus (A) and temporal cortex (B) from an affected member from HFTD1. Numerous Pick-like bodies and granule cells with a perinuclear ring are stained by antibody AT8 in dentate gyrus (C) and temporal cortex (D) from an affected member from HFTD2. Scale bars: 100 μm in A, 66 μm in B, 50 μm in C, and 40 μm in D.
In HFTD1 numerous neuropil threads and glial fibrillary tangles were stained in both gray and white matter (Figure 1, A and B) ▶ . All anti-tau antibodies stained the deposits, although AT8 and PHF1 gave the most extensive staining. Antibody 12E8 stained some granulovacuolar structures around glial cells and neuronal cell bodies, whereas only a few neurons were positive. BR189 raised against the sequence encoded by the exon 3 of the tau gene stained neuronal cells and few glial cells weakly. In all 3 cases from HFTD1 anti-heparan sulfate antibody 10E4 showed punctate staining inside and outside the granule layer of the dentate gyrus, and staining of cell bodies in CA2, CA3, and CA4. In one case (76 years) numerous neuritic plaques containing β-amyloid were present in subiculum, hippocampus and temporal cortex.
In HFTD2 the granule cells in the DG showed various Pick-like inclusions, often with associated cytoplasmic and perinuclear tau staining (Figure 1, C and D) ▶ . Neurons in pre-tangle stage were present in CA4, whereas globose staining of neurons was often associated with Pick-like bodies in CA1, CA2, and subiculum, with occasional extracellular neurofibrillary tangles in CA2 in one case. Antibody 12E8 did not stain Pick-like bodies or perinuclear and cytoplasmic tau deposits; in a few cells it stained deposits in one side of the cytoplasm and in the DG some cells presented granular staining. The number of glial cells stained by anti-tau antibodies was much lower in HFTD2 compared to HFTD1. β-Amyloid deposits were absent.
Electron Microscopy
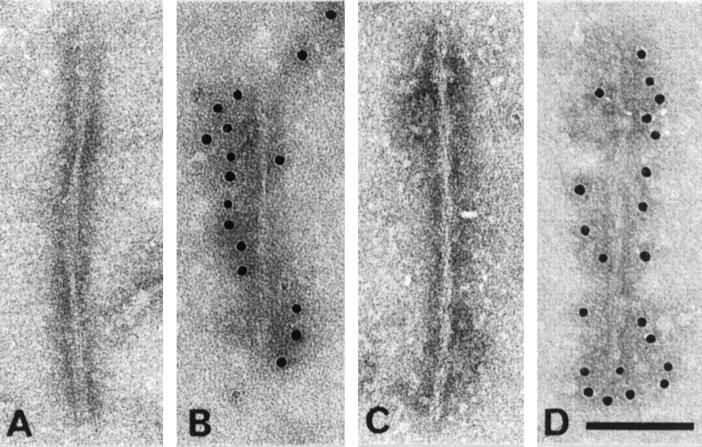
Electron microscopy of dispersed filament preparations from frontal and temporal cortices and hippocampal formation of three patients from HFTD1 showed tau-containing filaments structurally different from Alzheimer’s disease PHFs. The majority of these filaments were irregularly twisted ribbons of width about 15 nm and crossover spacing greater than 130 nm (Figure 2, A and B) ▶ . A minority consisted of straight filaments about 12 nm wide with a strongly stranded rope-like appearance (Figure 2, C and D) ▶ .
Figure 2.
Electron micrographs of sarkosyl-insoluble filaments from HFTD1. (A) and (B) show examples of twisted ribbons and (C) and (D) show examples of rope-like stranded filaments. (A) and (C) are unlabeled, whereas (B) and (D) have been labeled with phosphorylation-dependent anti-tau antibody AT8. Scale bar, 100 nm.
Immunoblotting of Sarkosyl-Insoluble Tau
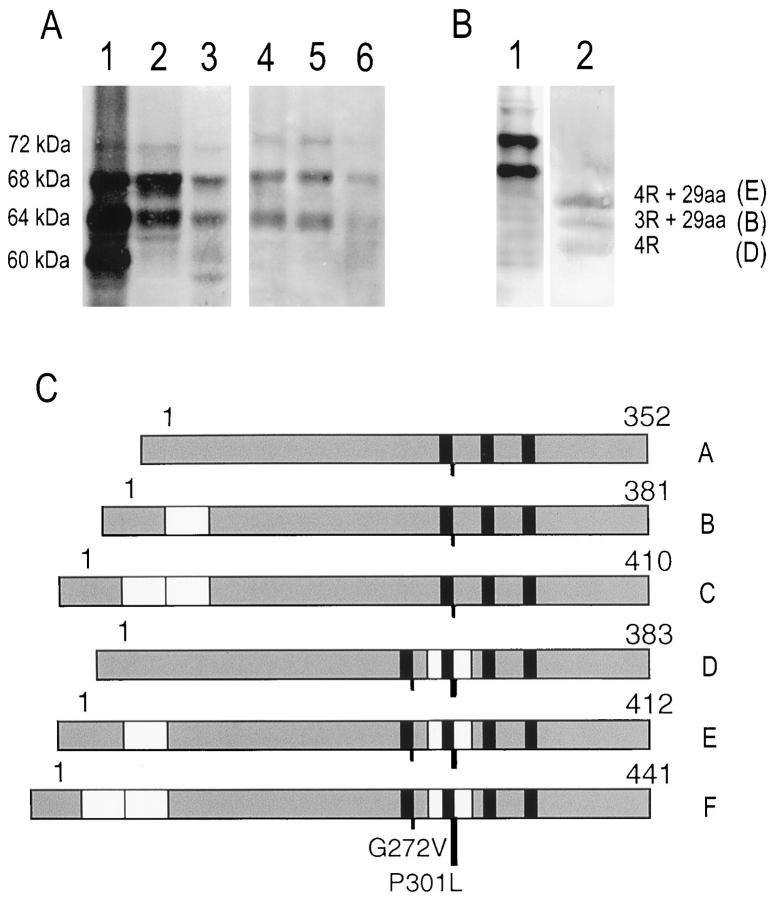
Sarkosyl-insoluble tau extracted from frontal and temporal cortices and hippocampal formation from 3 patients of HFTD1 appeared in immunoblots as two major bands of 64 and 68 kd and a minor band of 72 kd (Figure 3A) ▶ . After alkaline phosphatase treatment of sarkosyl-insoluble material, tau appeared as three major bands corresponding to the isoforms with 4 repeats and no amino-terminal insert, and to the isoforms with 3 and 4 repeats and the 29 amino acid amino-terminal insert (Figure 3B) ▶ . The pattern of soluble tau in HFTD1 was similar to that in control brains (data not shown).
Figure 3.
Immunoblots of sarkosyl-insoluble tau from affected members from HFTD1. (A) Immunoblots of sarkosyl-insoluble tau extracted from hippocampus from an Alzheimer’s disease patient (lane 1) and hippocampus (lanes 2, 3) and frontal cortex (lanes 4–6) from three affected members of HFTD1 stained by antiserum BR134. Tau bands of 60, 64, 68, and 72 kd are indicated. (B) Immunoblots of sarkosyl-insoluble tau from brain of an affected HFTD1 member, before (lane 1) and after (lane 2) alkaline phosphatase treatment, using antiserum BR133. Note that before alkaline phosphatase treatment two major bands of 64 and 68 kd and a minor band of 72 kd are present; after alkaline phosphatase treatment, three major bands are visible which correspond to tau isoforms with 4 repeats and no amino-terminal amino acid insert (4R) and both 4 and 3 repeat tau isoforms with 29-amino acid amino-terminal insert (4R + 29aa and 3R + 29aa). These are isoforms D, E and B respectively in panel (C). (C) Schematic representation of the 6 tau isoforms present in adult human brain showing the position of G272V and P301L mutations. The P301L mutation is in the alternatively spliced exon 10 and is present only in tau isoforms with 4 repeats, whereas G272V is present in all isoforms.
Discussion
The two families discussed here, with coding mutations in exon 9 or exon 10 of the tau gene (Figure 3C) ▶ , showed numerous intracytoplasmic tau deposits in neurons, glial cells, and neurites in several brain regions including the hippocampal formation, neocortex, and substantia nigra. The deposits were stained by phosphorylation-dependent and -independent anti-tau antibodies. Electron microscopy of sarkosyl-insoluble material from HFTD1 showed slender twisted ribbons about 15 nm wide, similar to but narrower than those in multiple system tauopathy with presenile dementia (MSTD), 4 and also straight rope-like filaments similar to filaments seen occasionally in AD preparations (RA Crowther, unpublished observations). The filaments contained hyperphosphorylated tau, which migrated as two major bands of 64 and 68 kd and a minor band of 72 kd. Tau isoforms with 4 repeats with or without the 29-amino acid insert appeared together more abundant than the isoform with 3 repeats and the 29-amino acid insert.
In HFTD2, the first family described as familial Pick disease, 16,23,24 Pick body-like inclusions were observed in the granular layer of the DG and cortex, often associated with perinuclear and cytoplasmic staining. The presence of strong cytoplasmic tau staining surrounding the Pick-like inclusions and the absence of information about the characteristics of the filaments in the inclusions have led us to name these inclusions Pick-like bodies instead of Pick bodies, until more ultrastructural and biochemical data become available. Such inclusions have not been described in other FTDP-17 families and this is the first time a mutation in the tau gene appears linked to Pick-like bodies. In both families antibody 12E8 stained mainly granular structures, sometimes surrounded by a vacuole, and a few neurons, similar to the pattern observed in MSTD. 4 It did not stain the Pick body-like inclusions in HFTD2, suggesting that they are similar to those in Pick’s disease. 25,26
The two families have mutations in different microtubule-binding repeats of tau, so the population of tau molecules is affected in different ways. In HFTD1 the P301L mutation lies in exon 10, which is included in 4 repeat isoforms but not in 3 repeat isoforms (Figure 3C) ▶ . In HFTD2 the G272V mutation lies in exon 9 and therefore affects all 6 isoforms (Figure 3C) ▶ . In both cases it is likely that the binding to microtubules of those tau isoforms carrying a mutation will be affected, probably adversely. The balance between binding of 3 and 4 repeat isoforms will be changed in HFTD1, producing an effect similar to that created by a splicing mutation in MSTD, which favors production of 4 repeat isoforms 5,15 and leads to ribbon-like twisted filaments 4 as here. However, in MSTD the sarkosyl-insoluble material contains only 4 repeat isoforms, so perhaps in HFTD1 the 3 repeat tau isoform, found after alkaline phosphatase treatment, co-assembles in the filaments, or the mutated 4 repeat tau isoform prevents normal 3 repeat tau isoform from binding to microtubules by occupying binding places. The tau isoform with 3 repeats and 29-amino acid amino-terminal insert is abundant in human brain and does not contain the P301L mutation, so its presence in filaments shows that they contain both normal and mutated tau molecules.
In HFTD2, for which no fresh tissue is available, all isoforms are affected, as in Seattle family A 3,13 where filaments are of AD type, 3,12 so filaments in HFTD2 may be of AD type but the presence of Pick body-like structures may also give straight filaments. 27 Furthermore, it has been reported that Pick bodies contain only tau isoforms with 3 repeats, 26 so it remains to be seen if this is the case in the Pick body-like inclusions. However, in both families disturbance of tau binding may destabilize microtubules and lead to accumulation of tau in an inappropriate compartment, hyperphosphorylation, and assembly of abnormal filaments, resulting in cell death.
Acknowledgments
We thank Dr. M. Goedert for useful discussions and the Dutch Brain Bank for help in collecting brain specimens of HFTD1.
Footnotes
Address reprint requests to Dr. John C. van Swieten, Dijkzigt Hospital Rotterdam, Department of Neurology, Dr. Molenwaterplein 40, 3015 GD Rotterdam, The Netherlands. E-mail: vanswieten@neur.azr.nl.
Supported in part by the UK Medical Research Council (to RAC, MGS) and by The Royal Society of London (to MGS).
References
- 1.Foster NL, Wilhelmsen K, Sima AAF, Jones MZ, D’Amato C, Gilman S, Spillantini MG, Lynch T, Mayeux RP, Gaskell Ph-C, Hulette C, Pericak-Vance MA, Welsh-Bohmer KA, Dickson DW, Heutink P, Kros J, van Swieten JC, Arwert F, Ghetti B, Murrell J, Lannfelt L, Hutton M, Phelps CH, Snyder DS, Oliver E, Ball MJ, Cummings JL, Miller BL, Katzman R, Reed L, Schelper RL, Lanska DJ, Brun A, Fink JK, Khul DE, Knopman DS, Wszolek Z, Miller CL, Bird TD, Lendon C, Elechi C: Frontotemporal dementia and parkinsonism linked to chromosome 17: a consensus statement. Ann Neurol 1997, 41:706-715 [DOI] [PubMed] [Google Scholar]
- 2.Sima AAF, Defendini R, Keohane C, D’Amato C, Foster NL, Parchi P, Gambetti M, Lynch T, Wilhelmsen KC: The neuropathology of chromosome 17-linked dementia. Ann Neurol 1996, 39:734-743 [DOI] [PubMed] [Google Scholar]
- 3.Spillantini MG, Crowther RA, Goedert M: Comparison of the neurofibrillary pathology in Alzheimer’s disease and familial presenile dementia with tangles. Acta Neuropathol 1996, 92:42-48 [DOI] [PubMed] [Google Scholar]
- 4.Spillantini MG, Goedert M, Crowther RA, Murrell J, Farlow MJ, Ghetti B: Familial multiple system tauopathy with presenile dementia: a disease with abundant neuronal and glial tau filaments. Proc Natl Acad Sci USA 1997, 94:4113-4118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spillantini MG, Bird TD, Ghetti B: Frontotemporal dementia and parkinsonism linked to chromosome 17: a new group of tauopathies. Brain Pathol 1998, 8:387-402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lendon CL, Lynch T, Norton J, McKeel DW, Busfield F, Craddock N, Chakraverty S, Gopalakrishnan G, Shears A, Grimmett W, Wilhelmsen C, Hansen L, Morris JC, Goate AM: Hereditary dysphasic dementia: a frontotemporal dementia linked to 17q21–22. Neurology 1998, 50:1546-1555 [DOI] [PubMed] [Google Scholar]
- 7.Reed L, Schmidt ML, Wszolek ZK, Balin BJ, Soontornniyomkij V, Lee VM-Y, Trojanowski JQ, Schelper RL: The neuropathology of a chromosome 17-linked autosomal dominant parkinsonism and dementia (“Pallido-ponto-nigral degeneration”). J Neuropathol Exp Neurol 1998, 57:588-601 [DOI] [PubMed] [Google Scholar]
- 8.Petersen RB, Tabaton M, Chen SG, Monari L, Richardson SL, Lynches T, Manetto V, Lanska DJ, Markesbery WR, Currier RD, Autilio-Gambetti L, Wilhelmsen KC, Gambetti P: Familial progressive subcortical gliosis: presence of prions and linkage to chromosome 17. Neurology 1995, 45:1062-1067 [DOI] [PubMed] [Google Scholar]
- 9.Reed LA, Grabowski ThJ, Schmidt ML, Morris JC, Goate A, Solodkin A, van Hoesen G, Schelper RL, Talbot CJ, Wragg MA, Trojanowski JQ: Autosomal dominant dementia with widespread neurofibrillary tangles. Ann Neurol 1997, 42:564-572 [DOI] [PubMed] [Google Scholar]
- 10.Goedert M, Spillantini MG, Potier MC, Ulrich J, Crowther RA: Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: differential expression of tau mRNAs in human brain. EMBO J. 1989, 8:393-399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA: Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 1989, 3:519-526 [DOI] [PubMed] [Google Scholar]
- 12.Sumi SM, Bird TD, Nochlin D, Raskind MA: Familial presenile dementia with psychosis associated with cortical neurofibrillary tangles and degeneration of the amygdala. Neurology 1992, 42:120-127 [DOI] [PubMed] [Google Scholar]
- 13.Poorkaj P, Bird Th, Wijsman E, Nemens E, Garruto RM, Anderson L, Andreadis A, Wiederholt WC, Raskind M, Schellenberg GD: Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol 1998, 43:815-825 [DOI] [PubMed] [Google Scholar]
- 14.Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Kuei Che L, Norton J, Morris JC, Reed LA, Trojanowski J, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd P, Hayward N, Kwok DBJ, Schofield PR, Andreadis A, Snowden J, Craufurd A, Neary D, Owen F, Oostra BA, Hardy J, Goate A, van Swieten J, Mann D, Lynch T, Heutink P: Coding and splice-donor site mutations in tau cause inherited dementia (FTDP-17). Nature 1998, 393:702-705 [DOI] [PubMed] [Google Scholar]
- 15.Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, Ghetti B: Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci USA 1998, 95:7737-7741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heutink P, Stevens M, Rizzu P, Bakker E, Kros JM, Tibben A, Niermeijer MF, van Duijn CM, Oostra BA, van Swieten JC: Hereditary fronto-temporal dementia is linked to chromosome 17q21–22: a genetic and clinico-pathological study of three Dutch families. Ann Neurol 1997, 41:150-159 [DOI] [PubMed] [Google Scholar]
- 17.Mercken M, Vandermeeren M, Lübke U, Six J, Boons J, Vaan de Voorde, Martin JJ, Gheuens J: Monoclonal antibodies with selective specificity for Alzheimer tau are directed against phosphatase-sensitive epitopes. Acta Neuropathol 1992, 84:265-272 [DOI] [PubMed] [Google Scholar]
- 18.Seubert P, Mawal-Dewan M, Barbour R, Jakes R, Goedert M, Johnson GVW, Litersky JM, Shenk D, Lieberburg I, Trojanowski JQ, Lee M-Y: Detection of phosphorylated Ser262 in fetal tau, adult tau, and paired helical filament tau. J Biol Chem 1995, 270:18917-18922 [DOI] [PubMed] [Google Scholar]
- 19.David G, Bai XM, Van der Schueren B, Cassiman JJ, Van den Berghe H: Developmental changes in heparan sulphate expression: in situ detection with mAbs. J Cell Biol 1992, 119:961-975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goedert M, Spillantini MG, Cairns NJ, Crowther RA: Tau proteins of Alzheimer paired helical filaments: abnormal phosphorylation of all six brain isoforms. Neuron 1992, 8:159-168 [DOI] [PubMed] [Google Scholar]
- 21.Goedert M, Jakes R: Expression of separate isoforms of human tau: correlation with the tau pattern in brain and effects on tubulin polymerization. EMBO J 1990, 9:4225-4230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crowther RA: Straight and paired helical filaments in Alzheimer disease have a common structural unit. Proc Natl Acad Sci USA 1991, 88:2288-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanders J, Schenk VWD, Veen van P: A family with Pick’s disease. Verhandelingen de Koninklijke Nederlandse Akademie van Wetenschappen 1939, section 2, part 38, no 3
- 24.Schenk VWD: Re-examination of a family with Pick’s disease. Ann Hum Genet 1959, 23:325-333 [DOI] [PubMed] [Google Scholar]
- 25.Probst A, Tolnay M, Langui D, Goedert M, Spillantini MG: Pick’s disease: hyperphosphorylated tau protein segregates to the somatoaxonal compartment. Acta Neuropathol 1996, 92:588-596 [DOI] [PubMed] [Google Scholar]
- 26.Delacourte A, Sergeant N, Wattez A, Gauvreau D, Robitaille Y: Vulnerable neuronal subsets in Alzheimer’s and Pick’s disease are distinguished by their τ isoform distribution and phosphorylation. Ann Neurol 1998, 43:193-204 [DOI] [PubMed] [Google Scholar]
- 27.Kato S, Nakamura H: Presence of two different fibril subtypes in Pick bodies: an immunoelectron microscopic study. Acta Neuropathol 1990, 81:125-129 [DOI] [PubMed] [Google Scholar]